Abstract
Lithium has been the most important mood stabilizer used for the treatment of bipolar disorder and prophylaxis of manic and depressive episodes. Despite long use in clinical practice, the exact molecular mechanisms of lithium are still not well identified. Previous experimental studies produced inconsistent results due to different duration of lithium treatment and using animals without manic-like or depressive-like symptoms. Therefore, we aimed to analyze the gene expression profile in three brain regions (amygdala, frontal cortex and hippocampus) in the rat model of mania and depression during chronic lithium administration (2 and 4 weeks). Behavioral changes were verified by the forced swim test, open field test and elevated maze test. After the experiment, nucleic acid was extracted from the frontal cortex, hippocampus and amygdala. Gene expression profile was done using SurePrint G3 Rat Gene Expression whole transcriptome microarrays. Data were analyzed using Gene Spring 14.9 software. We found that chronic lithium treatment significantly influenced gene expression profile in both mania and depression models. In manic rats, chronic lithium treatment significantly influenced the expression of the genes enriched in olfactory and taste transduction pathway and long non-coding RNAs in all three brain regions. We report here for the first time that genes regulating olfactory and taste receptor pathways and long non-coding RNAs may be targeted by chronic lithium treatment in the animal model of mania.
Keywords: manic-like behavior, depressive-like behavior, lithium, animal model, transcriptome, brain
1. Introduction
Bipolar disorder (BD) is a severe, recurrent psychiatric condition characterized by episodes of mania or hypomania and depression. Mania presents with hyperactivity, disinhibited behavior and inflated self-esteem. On the other hand, during depression patients demonstrate anhedonia and loss of motivation and interest in usual activities [1].
Pharmacological treatment of BD includes mostly the use of mood stabilizers. The first drug fulfilling criteria for the mood stabilizer such as preventing manic and depressive episodes was lithium. Its antimanic actions in the acute episode were first discovered by Cade et al. in animal model studies [2]. Its antidepressant potential was reported in the 1970s [3,4] and later it was recommended as an augmentation of antidepressants in the treatment-resistant depression and an effective long-term prophylaxis of recurrences in mood disorders (as recently reviewed by Rybakowski [5]).
Although lithium has been for decades in clinical use, the molecular mechanism of action and normotymic potential of lithium are still not well identified. Moreover, absence of suitable animal model of bipolar disorder impedes studies on lithium action. Previous studies focused on identifying target genes and molecular pathways of lithium indicated its role in reducing inositol signaling [6], phosphorylation of AKT [7] and inhibition of GSK-3β (glycogen synthase kinase 3) signaling [6,8]. The latter two may lead to the changes in the activation of WNT pathway. These findings were possible to discover using pharmacological and pharmacogenetic studies, which showed involvement of lithium in multiple cellular signaling pathways as reviewed previously [9,10].
Animal studies of the brain transcriptome during lithium administration showed that inconsistent results are mainly due to the different duration of lithium administration (1 week, two weeks and 6 weeks), analysis of the whole brain tissue (whole brain homogenates) and the use of different animals (rat or mouse) [11,12]. Moreover, these studies used animals treated with lithium, but without manic-like or depressive-like symptoms, so it is still not known if therapeutic action of lithium in manic and depressive behavior induces changes in the gene expression of similar or different pathways depending on the baseline condition and the brain region.
Therefore, we hypothesized that chronic lithium treatment exerts its therapeutic effect by inducing changes in the brain transcriptome specific for mania and depression. The aim of this study was to investigate if chronic lithium administration in rats presenting manic-like behavior (induced by amphetamine) or depressive like behavior (induced by chronic mild stress) influences specific gene expression profiles in different brain regions (amygdala, frontal cortex and hippocampus).
2. Results
2.1. Behavioral Changes in Amphetamine-Exposed and Stress-Exposed Animals (Models of Mania and Depression)
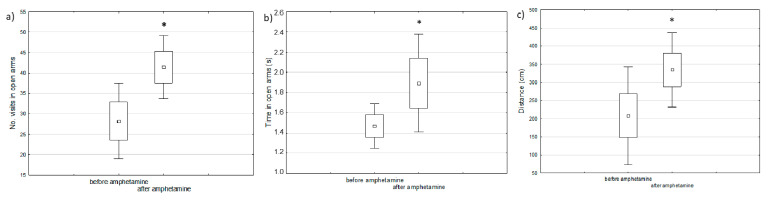
Manic-like behavior was observed after one week of amphetamine injections using the elevated maze test as compared to the behavior of the same animals before starting the experiment (baseline) (Figure 1).
Figure 1.
The comparison of behavioral changes before and after amphetamine injection in the elevated maze test: (a) number of visits in open arms, (b) time spent in open arms, (c) distance passed by the animals (paired t-test, * defines p < 0.05).
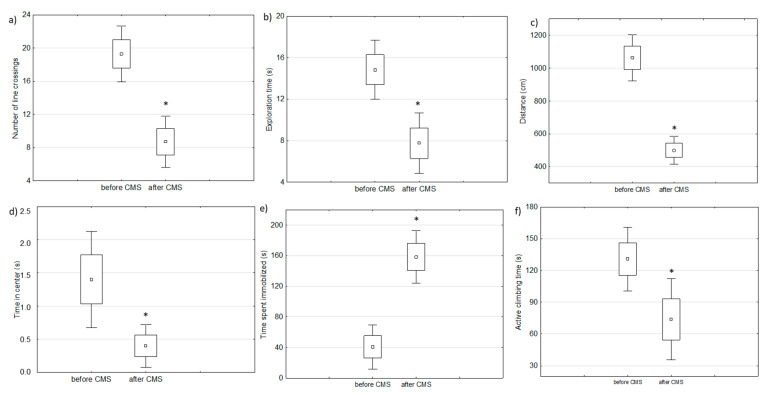
Two-week chronic mild stress protocol resulted in depressive-like behavior measured by behavioral tests (FST and OFT) in analyzed rats as compared to the behavior of the same animals before starting the experiment (baseline) (Figure 2).
Figure 2.
The results of behavioral changes after chronic mild stress protocol: (a–d) are parameters measured in an open filed test and (e,f) are parameters measured by a forced swim test (paired t-test, * defines p < 0.05).
2.2. Brain Transcriptome Changes in the Model of Mania and Depression
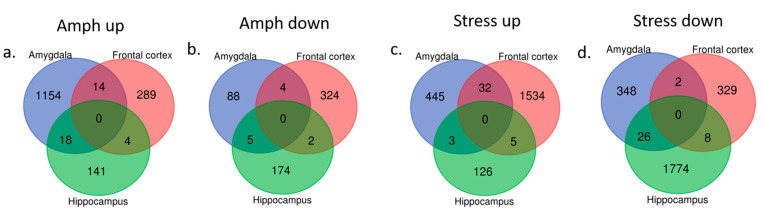
Significant changes in the behavior following chronic mild stress protocol or amphetamine corresponded to specific gene expression profiles in three brain regions. In rats presenting manic-like behavior, we found 1283 genes differentially expressed in the amygdala, 637 genes in the frontal cortex and 344 genes in the hippocampus as compared to the control group (Figure 3a,b). The altered genes in the amygdala were mainly downregulated and significantly enriched in 23 Gene Ontology (GO) terms including olfactory receptor activity and chemical response to stimuli (Table 1). The genes differentially expressed in the frontal cortex and hippocampus were not significantly enriched in any GO terms or pathways.
Figure 3.
Differentially expressed genes (up- and downregulated) in three analyzed brain regions (amygdala, frontal cortex and hippocampus) in amphetamine-exposed (a,b, respectively) and stress-exposed rats (c,d, respectively) as compared to the control group.
Table 1.
Gene Ontology (GO) terms enriched from significantly downregulated genes between rats with manic-like behavior and control rats in the amygdala.
| GO Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 117 |
| GO:0007600 | sensory perception | 0.000 | 103 |
| GO:0051606 | detection of stimulus | 0.000 | 88 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 84 |
| GO:0038023 | signaling receptor activity | 0.000 | 120 |
| GO:0060089 | molecular transducer activity | 0.000 | 120 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 81 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 79 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 111 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 93 |
| GO:0004871 | signal transducer activity | 0.000 | 126 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 82 |
| GO:0004984 | olfactory receptor activity | 0.000 | 74 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 74 |
| GO:0007608 | sensory perception of smell | 0.000 | 75 |
| GO:0007165 | signal transduction | 0.000 | 201 |
| GO:0003008 | system process | 0.000 | 123 |
| GO:0050877 | nervous system process | 0.000 | 106 |
| GO:0023052 | signaling | 0.000 | 209 |
| GO:0007154 | cell communication | 0.000 | 212 |
| GO:0050896 | response to stimulus | 0.001 | 287 |
| GO:0051716 | cellular response to stimulus | 0.004 | 238 |
| GO:0005833 | hemoglobin complex | 0.019 | 5 |
In rats with depressive-like behavior, we found 856 differentially expressed genes (either up- or downregulated) in the amygdala, 1910 genes in the frontal cortex and 1942 genes in the hippocampus as compared to the control rats (Figure 3c,d).
The downregulated genes in the amygdala were enriched mainly in locomotor behavior and blood circulation (8 GO terms), whereas the upregulated genes were involved, among others, in animal organ morphogenesis, response to stimuli and signal transduction (8 GO terms) (Table 2). In the frontal cortex and hippocampus the differentially expressed genes (either upregulated or downregulated) were not significantly enriched in any GO terms or pathways.
Table 2.
GO terms significantly enriched from differentially expressed genes between rats with depressive-like behavior and control group in the amygdala.
| GO Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| Downregulated | |||
| GO:0007610 | behavior | 0.002 | 26 |
| GO:0007188 | adenylate cyclase-modulating G-protein coupled receptor signaling pathway | 0.002 | 12 |
| GO:0007187 | G-protein coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger | 0.003 | 12 |
| GO:0044459 | plasma membrane part | 0.004 | 53 |
| GO:0003013 | circulatory system process | 0.004 | 17 |
| GO:0008015 | blood circulation | 0.004 | 17 |
| GO:0007626 | locomotory behavior | 0.004 | 14 |
| GO:0007193 | adenylate cyclase-inhibiting G-protein coupled receptor signaling pathway | 0.037 | 7 |
| Upregulated | |||
| GO:0023052 | signaling | 0.000 | 102 |
| GO:0007154 | cell communication | 0.000 | 102 |
| GO:0007165 | signal transduction | 0.000 | 95 |
| GO:0038023 | signaling receptor activity | 0.001 | 55 |
| GO:0060089 | molecular transducer activity | 0.001 | 55 |
| GO:0004888 | transmembrane signaling receptor activity | 0.001 | 52 |
| GO:0004871 | signal transducer activity | 0.006 | 57 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.006 | 49 |
| GO:0003008 | system process | 0.006 | 57 |
| GO:0051606 | detection of stimulus | 0.017 | 37 |
| GO:0050896 | response to stimulus | 0.022 | 126 |
| GO:0004930 | G-protein coupled receptor activity | 0.028 | 40 |
| GO:0009593 | detection of chemical stimulus | 0.028 | 34 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.045 | 34 |
| GO:0009653 | anatomical structure morphogenesis | 0.050 | 45 |
2.3. Brain Transcriptome Changes during Chronic Lithium Administration
2.3.1. Two-Week Lithium Administration
Lithium administration for two weeks resulted in significant changes in both, stress-exposed and manic-like rats in all analyzed brain regions. In rats presenting manic-like behavior and receiving lithium for two weeks, we found 4041 differentially expressed genes in the amygdala (all upregulated), 978 genes in the frontal cortex and 386 genes in the hippocampus. In the amygdala upregulated genes were enriched in 40 GO terms, mainly olfactory receptor activity, taste receptor and bitter taste receptor (Table 3). In the frontal cortex, most genes were significantly downregulated and involved in 11 GO terms, including olfactory receptor activity (Table 3). Genes differentially expressed in the hippocampus were not enriched in any GO terms.
Table 3.
Differentially expressed genes enriched in GO terms in amphetamine-exposed rats after two weeks of lithium treatment.
| GO Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| Amygdala upregulated | |||
| GO:0038023 | signaling receptor activity | 0.000 | 367 |
| GO:0060089 | molecular transducer activity | 0.000 | 367 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 352 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 344 |
| GO:0007600 | sensory perception | 0.000 | 310 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 299 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 285 |
| GO:0051606 | detection of stimulus | 0.000 | 281 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 273 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 268 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 264 |
| GO:0007608 | sensory perception of smell | 0.000 | 256 |
| GO:0004984 | olfactory receptor activity | 0.000 | 247 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 247 |
| GO:0004871 | signal transducer activity | 0.000 | 379 |
| GO:0050877 | nervous system process | 0.000 | 330 |
| GO:0003008 | system process | 0.000 | 363 |
| GO:0031224 | intrinsic component of membrane | 0.000 | 640 |
| GO:0016021 | integral component of membrane | 0.000 | 627 |
| GO:0007165 | signal transduction | 0.000 | 509 |
| GO:0042221 | response to chemical | 0.000 | 507 |
| GO:0044425 | membrane part | 0.000 | 687 |
| GO:0005886 | plasma membrane | 0.000 | 521 |
| GO:0005549 | odorant binding | 0.000 | 68 |
| GO:0071944 | cell periphery | 0.000 | 528 |
| GO:0023052 | signaling | 0.000 | 523 |
| GO:0007154 | cell communication | 0.000 | 533 |
| GO:0032501 | multicellular organismal process | 0.000 | 669 |
| GO:0050896 | response to stimulus | 0.000 | 731 |
| GO:0004252 | serine-type endopeptidase activity | 0.000 | 44 |
| GO:0051716 | cellular response to stimulus | 0.000 | 597 |
| GO:0008236 | serine-type peptidase activity | 0.000 | 44 |
| GO:0017171 | serine hydrolase activity | 0.000 | 44 |
| GO:0008527 | taste receptor activity | 0.000 | 15 |
| GO:0050912 | detection of chemical stimulus involved in sensory perception of taste | 0.001 | 17 |
| GO:0050913 | sensory perception of bitter taste | 0.002 | 16 |
| GO:0033038 | bitter taste receptor activity | 0.002 | 13 |
| GO:0001580 | detection of chemical stimulus involved in sensory perception of bitter taste | 0.004 | 15 |
| GO:0004175 | endopeptidase activity | 0.005 | 61 |
| GO:0050909 | sensory perception of taste | 0.007 | 19 |
| Frontal cortex downregulated | |||
| GO:0038023 | signaling receptor activity | 0.022 | 80 |
| GO:0060089 | molecular transducer activity | 0.022 | 80 |
| GO:0007608 | sensory perception of smell | 0.022 | 52 |
| GO:0004984 | olfactory receptor activity | 0.022 | 50 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.022 | 50 |
| GO:0009593 | detection of chemical stimulus | 0.026 | 52 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.026 | 51 |
| GO:0051606 | detection of stimulus | 0.029 | 55 |
| GO:0007606 | sensory perception of chemical stimulus | 0.033 | 54 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.033 | 52 |
| GO:0004888 | transmembrane signaling receptor activity | 0.036 | 72 |
Rats presenting depressive-like behavior showed differentially expressed 1650 genes in the amygdala, 481 genes in the frontal cortex and 304 genes in the hippocampus. The significantly altered genes in the amygdala were mainly downregulated and were involved in 206 GO terms, mainly associated with extracellular processes e.g., extracellular matrix organization (top 20 were listed in Table 4). In the frontal cortex differentially expressed genes were not significantly enriched in any GO terms between rats receiving lithium and control group. In the hippocampus, altered genes were significantly enriched in two GO terms, antigen processing and antigen binding (Table 4).
Table 4.
Differentially expressed genes enriched in GO terms in stress-exposed rats after two weeks of lithium treatment.
| GO Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| Amygdala (top 20 out of 206) | |||
| GO:0005578 | proteinaceous extracellular matrix | 0.000 | 41 |
| GO:0031012 | extracellular matrix | 0.000 | 34 |
| GO:0005576 | extracellular region | 0.000 | 117 |
| GO:0044421 | extracellular region part | 0.000 | 108 |
| GO:0005615 | extracellular space | 0.000 | 104 |
| GO:0044420 | extracellular matrix component | 0.000 | 19 |
| GO:0009888 | tissue development | 0.000 | 62 |
| GO:0030198 | extracellular matrix organization | 0.000 | 21 |
| GO:0009653 | anatomical structure morphogenesis | 0.000 | 68 |
| GO:0043062 | extracellular structure organization | 0.000 | 21 |
| GO:0007275 | multicellular organism development | 0.000 | 112 |
| GO:0030199 | collagen fibril organization | 0.000 | 11 |
| GO:0072001 | renal system development | 0.000 | 24 |
| GO:0005581 | collagen trimer | 0.000 | 13 |
| GO:0032502 | developmental process | 0.000 | 122 |
| GO:0048513 | animal organ development | 0.000 | 87 |
| GO:0048646 | anatomical structure formation involved in morphogenesis | 0.000 | 39 |
| GO:0009887 | animal organ morphogenesis | 0.000 | 41 |
| GO:0048731 | system development | 0.000 | 104 |
| Hippocampus | |||
| GO:0002474 | antigen processing and presentation of peptide antigen via MHC class I | 0.024 | 4 |
| GO:0042605 | peptide antigen binding | 0.024 | 4 |
2.3.2. Four-Week Lithium Administration
In rats presenting manic-like behavior and receiving lithium for 4 weeks, we observed 2831 genes differentially expressed in the amygdala, 1964 genes in the frontal cortex and 7412 genes in the hippocampus. In the amygdala, all genes were downregulated after 4 weeks on lithium and were enriched mainly in olfactory receptor activity and bitter taste (Table 5). In the frontal cortex, downregulated genes were enriched in 31 GO terms including, e.g., olfactory receptor activity (Table 5). In the hippocampus significantly upregulated genes were enriched in 57 GO terms (mainly response to chemical stimuli, bitter taste and olfactory receptor activity) (Table 5).
Table 5.
Differentially expressed genes enriched in GO terms in amphetamine-exposed rats after four weeks of lithium treatment.
| GO Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| Amygdala downregulated | |||
| GO:0038023 | signaling receptor activity | 0.000 | 228 |
| GO:0060089 | molecular transducer activity | 0.000 | 228 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 212 |
| GO:0007608 | sensory perception of smell | 0.000 | 149 |
| GO:0004984 | olfactory receptor activity | 0.000 | 144 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 144 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 159 |
| GO:0007600 | sensory perception | 0.000 | 186 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 147 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 151 |
| GO:0051606 | detection of stimulus | 0.000 | 157 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 147 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 172 |
| GO:0004871 | signal transducer activity | 0.000 | 237 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 196 |
| GO:0050877 | nervous system process | 0.000 | 199 |
| GO:0031224 | intrinsic component of membrane | 0.000 | 451 |
| GO:0016021 | integral component of membrane | 0.000 | 441 |
| GO:0003008 | system process | 0.000 | 220 |
| GO:0044425 | membrane part | 0.002 | 488 |
| GO:0005886 | plasma membrane | 0.003 | 365 |
| GO:0023052 | signaling | 0.007 | 366 |
| GO:0071944 | cell periphery | 0.008 | 369 |
| GO:0007165|GO:0023033 | signal transduction | 0.009 | 346 |
| GO:0007154 | cell communication | 0.011 | 373 |
| GO:0005549 | odorant binding | 0.020 | 40 |
| Frontal cortex downregulated | |||
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 154 |
| GO:0004984 | olfactory receptor activity | 0.000 | 140 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 140 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 145 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 143 |
| GO:0007608 | sensory perception of smell | 0.000 | 142 |
| GO:0007600 | sensory perception | 0.000 | 171 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 144 |
| GO:0051606 | detection of stimulus | 0.000 | 148 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 183 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 154 |
| GO:0038023 | signaling receptor activity | 0.000 | 190 |
| GO:0060089 | molecular transducer activity | 0.000 | 190 |
| GO:0050877 | nervous system process | 0.000 | 177 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 172 |
| GO:0004871 | signal transducer activity | 0.000 | 197 |
| GO:0003008 | system process | 0.000 | 195 |
| GO:0031224 | intrinsic component of membrane | 0.000 | 340 |
| GO:0016021 | integral component of membrane | 0.000 | 334 |
| GO:0071944 | cell periphery | 0.000 | 287 |
| GO:0005886 | plasma membrane | 0.000 | 281 |
| GO:0042221 | response to chemical | 0.000 | 269 |
| GO:0044425 | membrane part | 0.000 | 364 |
| GO:0032501 | multicellular organismal process | 0.001 | 352 |
| GO:0005549 | odorant binding | 0.001 | 34 |
| GO:0036156 | inner dynein arm | 0.006 | 4 |
| GO:0007165 | signal transduction | 0.006 | 252 |
| GO:0004252 | serine-type endopeptidase activity | 0.019 | 22 |
| GO:0023052 | signaling | 0.030 | 261 |
| GO:0007154 | cell communication | 0.030 | 267 |
| GO:0008236 | serine-type peptidase activity | 0.043 | 23 |
| Hippocampus downregulated | |||
| GO:0001580 | detection of chemical stimulus involved in sensory perception of bitter taste | 0.000 | 1394 |
| GO:0001594 | trace-amine receptor activity | 0.000 | 1323 |
| GO:0003008 | system process | 0.000 | 1316 |
| GO:0004252 | serine-type endopeptidase activity | 0.000 | 1313 |
| GO:0004866 | endopeptidase inhibitor activity | 0.000 | 1212 |
| GO:0004867 | serine-type endopeptidase inhibitor activity | 0.000 | 1119 |
| GO:0004869 | cysteine-type endopeptidase inhibitor activity | 0.000 | 1105 |
| GO:0004871 | signal transducer activity | 0.000 | 1083 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 1062 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 1053 |
| GO:0004984 | olfactory receptor activity | 0.000 | 1037 |
| GO:0005179 | hormone activity | 0.000 | 888 |
| GO:0005549 | odorant binding | 0.000 | 877 |
| GO:0005886 | plasma membrane | 0.000 | 877 |
| GO:0007154 | cell communication | 0.000 | 850 |
| GO:0007165 | signal transduction | 0.000 | 845 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 835 |
| GO:0007600 | sensory perception | 0.000 | 791 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 769 |
| GO:0007608 | sensory perception of smell | 0.000 | 740 |
| GO:0008527 | taste receptor activity | 0.000 | 720 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 697 |
| GO:0010466 | negative regulation of peptidase activity | 0.000 | 684 |
| GO:0010951 | negative regulation of endopeptidase activity | 0.000 | 675 |
| GO:0016020 | membrane | 0.000 | 673 |
| GO:0016021 | integral component of membrane | 0.000 | 652 |
| GO:0016503 | pheromone receptor activity | 0.000 | 644 |
| GO:0017171 | serine hydrolase activity | 0.000 | 644 |
| GO:0019236 | response to pheromone | 0.000 | 173 |
| GO:0023052 | signaling | 0.000 | 1413 |
| GO:0030414 | peptidase inhibitor activity | 0.000 | 1507 |
| GO:0030545 | receptor regulator activity | 0.000 | 41 |
| GO:0031224 | intrinsic component of membrane | 0.000 | 41 |
| GO:0032501 | multicellular organismal process | 0.000 | 1583 |
| GO:0033038 | bitter taste receptor activity | 0.000 | 27 |
| GO:0038023 | signaling receptor activity | 0.000 | 24 |
| GO:0042221 | response to chemical | 0.000 | 1644 |
| GO:0042742 | defense response to bacterium | 0.000 | 29 |
| GO:0044425 | membrane part | 0.000 | 1726 |
| GO:0048018 | receptor ligand activity | 0.000 | 26 |
| GO:0050789 | regulation of biological process | 0.000 | 26 |
| GO:0050794 | regulation of cellular process | 0.000 | 32 |
| GO:0050877 | nervous system process | 0.000 | 57 |
| GO:0050896 | response to stimulus | 0.000 | 54 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 54 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 13 |
| GO:0050909 | sensory perception of taste | 0.000 | 40 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.001 | 90 |
| GO:0050912 | detection of chemical stimulus involved in sensory perception of taste | 0.001 | 57 |
| GO:0050913 | sensory perception of bitter taste | 0.001 | 94 |
| GO:0051606 | detection of stimulus | 0.001 | 63 |
| GO:0051716 | cellular response to stimulus | 0.002 | 60 |
| GO:0060089 | molecular transducer activity | 0.010 | 23 |
| GO:0061134 | peptidase regulator activity | 0.011 | 54 |
| GO:0061135 | endopeptidase regulator activity | 0.014 | 49 |
| GO:0065007 | biological regulation | 0.025 | 28 |
| GO:0071944 | cell periphery | 0.043 | 53 |
Four weeks of lithium treatment in stress-exposed rats, we observed that in the amygdala 778 genes were differentially expressed, in the frontal cortex 779 genes and in the hippocampus 307 genes. In the amygdala the genes were mainly upregulated and enriched in 17 GO terms, e.g., olfactory receptor activity (Table 6), in the frontal cortex altered genes were grouped in 24 GO terms (including nucleosome assembly, DNA-protein complex) and in the hippocampus differentially expressed genes were involved in extracellular matrix/space/region and collagen fibril organization (two upregulated GO terms) (Table 6).
Table 6.
Differentially expressed genes enriched in GO terms in stress-exposed rats after four weeks of lithium treatment.
| GO Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| Amygdala upregulated | |||
| GO:0038023 | signaling receptor activity | 0.000 | 63 |
| GO:0060089 | molecular transducer activity | 0.000 | 63 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 59 |
| GO:0007600 | sensory perception | 0.000 | 54 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 43 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 45 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 43 |
| GO:0051606 | detection of stimulus | 0.000 | 45 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 43 |
| GO:0007608 | sensory perception of smell | 0.000 | 41 |
| GO:0004984 | olfactory receptor activity | 0.000 | 40 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 40 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 56 |
| GO:0004871 | signal transducer activity | 0.002 | 63 |
| GO:0003008 | system process | 0.003 | 63 |
| GO:0050877 | nervous system process | 0.003 | 55 |
| GO:0004930 | G-protein coupled receptor activity | 0.003 | 46 |
| Frontal cortex upregulated | |||
| GO:0000786 | nucleosome | 0.000 | 5 |
| GO:0006334 | nucleosome assembly | 0.000 | 5 |
| GO:0044815 | DNA packaging complex | 0.000 | 5 |
| GO:0045653 | negative regulation of megakaryocyte differentiation | 0.000 | 3 |
| GO:0031497 | chromatin assembly | 0.000 | 5 |
| GO:0006333 | chromatin assembly or disassembly | 0.000 | 5 |
| GO:0034728 | nucleosome organization | 0.000 | 5 |
| GO:0006323 | DNA packaging | 0.000 | 5 |
| GO:0065004 | protein-DNA complex assembly | 0.000 | 5 |
| GO:0006335 | DNA replication-dependent nucleosome assembly | 0.000 | 3 |
| GO:0034723 | DNA replication-dependent nucleosome organization | 0.000 | 3 |
| GO:0032993 | protein-DNA complex | 0.000 | 5 |
| GO:0071824 | protein-DNA complex subunit organization | 0.000 | 5 |
| GO:0000788 | nuclear nucleosome | 0.000 | 3 |
| GO:0006336 | DNA replication-independent nucleosome assembly | 0.000 | 3 |
| GO:0045652 | regulation of megakaryocyte differentiation | 0.000 | 3 |
| GO:0034724 | DNA replication-independent nucleosome organization | 0.000 | 3 |
| GO:0071103 | DNA conformation change | 0.001 | 5 |
| GO:0051290 | protein heterotetramerization | 0.002 | 3 |
| GO:0051291 | protein heterooligomerization | 0.006 | 4 |
| GO:0030492 | hemoglobin binding | 0.008 | 2 |
| GO:0006352 | DNA-templated transcription. initiation | 0.018 | 3 |
| GO:0045638 | negative regulation of myeloid cell differentiation | 0.018 | 3 |
| GO:0000785 | chromatin | 0.047 | 5 |
| Hippocampus upregulated | |||
| GO:0005578 | proteinaceous extracellular matrix | 0.022 | 10 |
| GO:0031012 | extracellular matrix | 0.009 | 13 |
2.3.3. Molecular Pathways in the Brain during Chronic Lithium Treatment
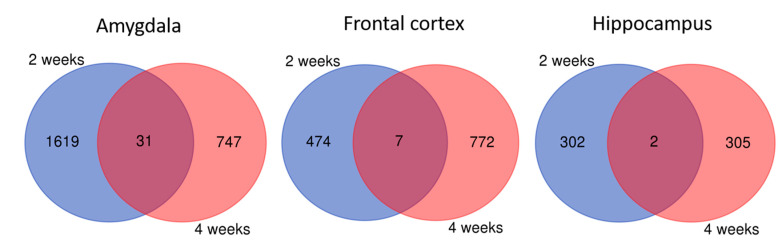
When we combined together gene sets differentially expressed in rats with depressive-like behavior receiving lithium for two and four weeks, we observed only a few shared genes between these two time points either in the amygdala or frontal cortex and hippocampus as compared to the control rats (Figure 4). These genes were not enriched in any GO terms and pathways.
Figure 4.
Genes differentially expressed in rats depressive-like after 2 weeks and 4 weeks on lithium treatment in the amygdala, frontal cortex and hippocampus. Shared genes between these time points were indicated by purple color.
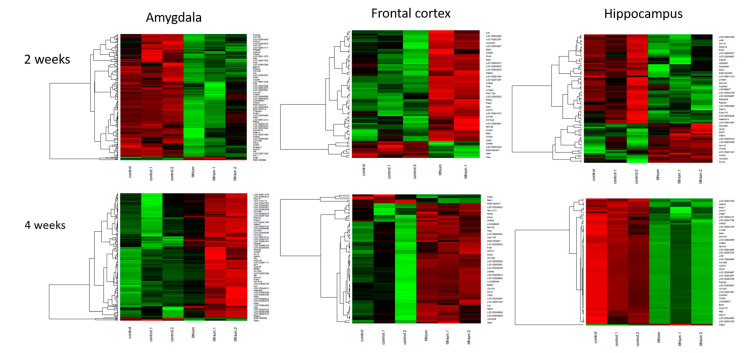
In rats with manic-like behavior, we observed that chronic lithium treatment (2 and 4 weeks) significantly influenced the expression of the genes from the olfactory and taste transduction pathway (mainly different olfactory and taste receptors) and long non-coding RNAs in all three brain regions in rats receiving lithium as compared to water-receiving animals (Figure 5). Gene-set enrichment analysis showed that the shared genes between 2 and 4 weeks showed different gene expression profile: those upregulated after 2-week lithium treatment were downregulated after 4 weeks in the amygdala and frontal cortex. In the hippocampus, a 4-week lithium treatment upregulated all the genes.
Figure 5.
Gene-set enrichment analysis indicated that the genes from olfactory transduction pathway are influenced by chronic lithium administration in manic-like rats in analyzed brain areas (amygdala, frontal cortex and hippocampus).
2.4. Brain Transcriptome Changes in Lithium Responders
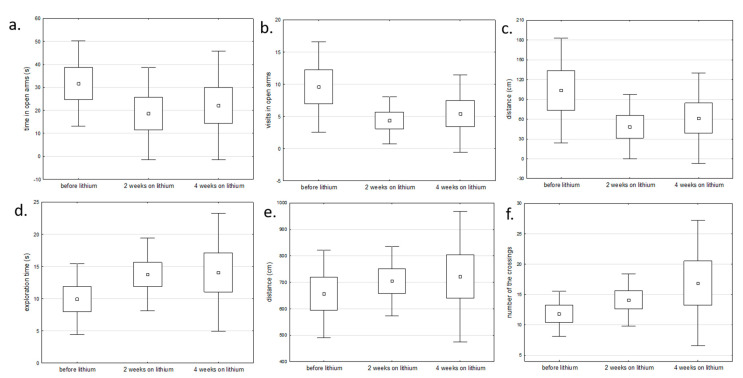
We also compared the gene expression profile before and after chronic lithium administration (2 and 4 weeks) in lithium responders. These rats showed changes in behavioral tests (open field test and elevated maze test) corresponding to reduced manic or depressive symptoms, e.g., reduced number of visits and time spent in open arms in manic rats after lithium and increased exploration time and number of line crossing in depressive rats after lithium (Figure 6).
Figure 6.
The comparison of behavioral changes before and after lithium administration in: (a–c) rats with manic-like behavior using elevated maze test and (d–f) rats with depressive-like behavior using open field test (one-way analysis of variance).
Gene expression analysis in rats responding to lithium showed that in after chronic lithium administration gene expression was mainly upregulated in the amygdala and frontal cortex, but downregulated in the hippocampus in manic rats responding to lithium as compared to rats before lithium treatment. Interestingly, depressive rats responding to lithium showed the opposite trend, with mostly downregulated gene expression in the amygdala and frontal cortex, but upregulated in the hippocampus as compared to depressive rats before lithium. These genes were significantly enriched in GO terms related i.a. to olfactory receptor pathway, sensory perception of smell, G-protein coupled receptor and detection of stimulus (Table 7 and Table 8).
Table 7.
Differentially expressed genes enriched in GO terms in amphetamine-exposed rats responding to chronic lithium in comparison to rats before lithium.
| Go Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| upregulated in the amygdala | |||
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 232 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 220 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 216 |
| GO:0051606 | detection of stimulus | 0.000 | 232 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 220 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 252 |
| GO:0007608 | sensory perception of smell | 0.000 | 210 |
| GO:0004984 | olfactory receptor activity | 0.000 | 205 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 205 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 291 |
| GO:0007600 | sensory perception | 0.000 | 261 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 293 |
| GO:0038023 | signaling receptor activity | 0.000 | 306 |
| GO:0060089 | molecular transducer activity | 0.000 | 306 |
| GO:0004871 | signal transducer activity | 0.000 | 317 |
| GO:0050877 | nervous system process | 0.000 | 275 |
| GO:0003008 | system process | 0.000 | 306 |
| GO:0031224 | intrinsic component of membrane | 0.000 | 556 |
| GO:0016021 | integral component of membrane | 0.000 | 548 |
| GO:0007165 | signal transduction | 0.000 | 436 |
| GO:0032501 | multicellular organismal process | 0.000 | 596 |
| GO:0007154 | cell communication | 0.000 | 464 |
| GO:0023052 | signaling | 0.000 | 452 |
| GO:0044425 | membrane part | 0.000 | 596 |
| GO:0005886 | plasma membrane | 0.000 | 447 |
| GO:0042221 | response to chemical | 0.000 | 429 |
| GO:0071944 | cell periphery | 0.000 | 455 |
| GO:0030414 | peptidase inhibitor activity | 0.000 | 35 |
| GO:0005549 | odorant binding | 0.001 | 50 |
| GO:0004252 | serine-type endopeptidase activity | 0.001 | 34 |
| GO:0004866 | endopeptidase inhibitor activity | 0.001 | 32 |
| GO:0061135 | endopeptidase regulator activity | 0.003 | 32 |
| GO:0061134 | peptidase regulator activity | 0.005 | 35 |
| GO:0010466 | negative regulation of peptidase activity | 0.011 | 38 |
| GO:0008236 | serine-type peptidase activity | 0.019 | 34 |
| GO:0017171 | serine hydrolase activity | 0.025 | 34 |
| GO:0050896 | response to stimulus | 0.026 | 631 |
| GO:003024 | carbohydrate binding | 0.041 | 38 |
| GO:0010951 | negative regulation of endopeptidase activity | 0.044 | 35 |
| GO:0004869 | cysteine-type endopeptidase inhibitor activity | 0.046 | 15 |
| upregulated in the frontal cortex | |||
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 44 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 49 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 49 |
| GO:0038023 | signaling receptor activity | 0.001 | 51 |
| GO:0060089 | molecular transducer activity | 0.001 | 51 |
| GO:0004871 | signal transducer activity | 0.003 | 53 |
| downregulated in the hippocampus | |||
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 87 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 85 |
| GO:0038023 | signaling receptor activity | 0.000 | 89 |
| GO:0060089 | molecular transducer activity | 0.000 | 89 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 68 |
| GO:0004871| | signal transducer activity | 0.000 | 92 |
| GO:0007600 | sensory perception | 0.003 | 67 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.007 | 54 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.008 | 52 |
| GO:0051606 | detection of stimulus | 0.009 | 56 |
| GO:0007606 | sensory perception of chemical stimulus | 0.011 | 55 |
| GO:0009593 | detection of chemical stimulus | 0.011 | 52 |
| GO:0003008 | system process | 0.011 | 86 |
| GO:0004984 | olfactory receptor activity | 0.014 | 49 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.014 | 49 |
| GO:0007608 | sensory perception of smell | 0.031 | 49 |
| GO:0050877 | nervous system process | 0.071 | 71 |
Table 8.
Differentially expressed genes enriched in GO terms in stress-exposed rats responding to chronic lithium treatment in comparison to rats before lithium.
| Go Accession | GO Term | Corr p | No. Genes |
|---|---|---|---|
| downregulated in the amygdala | |||
| GO:0044425 | membrane part | 0.000 | 1150 |
| GO:0032501| | multicellular organismal process | 0.000 | 1107 |
| GO:0031224 | intrinsic component of membrane | 0.000 | 1098 |
| GO:0016021 | integral component of membrane | 0.000 | 1090 |
| GO:0007154 | cell communication | 0.000 | 941 |
| GO:0023052 | signaling | 0.000 | 930 |
| GO:0007165 | signal transduction | 0.000 | 909 |
| GO:0042221 | response to chemical | 0.000 | 895 |
| GO:0071944 | cell periphery | 0.000 | 881 |
| GO:0005886 | plasma membrane | 0.000 | 874 |
| GO:0004871 | signal transducer activity | 0.000 | 773 |
| GO:0038023 | signaling receptor activity | 0.000 | 762 |
| GO:0060089 | molecular transducer activity | 0.000 | 762 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 749 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 736 |
| GO:0003008 | system process | 0.000 | 720 |
| GO:0050877 | nervous system process | 0.000 | 683 |
| GO:0007600 | sensory perception | 0.000 | 667 |
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 652 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 632 |
| GO:0051606 | detection of stimulus | 0.000 | 593 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 586 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 578 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 578 |
| GO:0007608 | sensory perception of smell | 0.000 | 565 |
| GO:0004984 | olfactory receptor activity | 0.000 | 556 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 556 |
| GO:0005549 | odorant binding | 0.000 | 164 |
| GO:0051716 | cellular response to stimulus | 0.000 | 1009 |
| GO:0050896 | response to stimulus | 0.000 | 1174 |
| GO:0016503 | pheromone receptor activity | 0.000 | 48 |
| GO:0019236 | response to pheromone | 0.000 | 47 |
| GO:0016020 | membrane | 0.000 | 1232 |
| GO:0050789 | regulation of biological process | 0.000 | 1346 |
| GO:0065007 | biological regulation | 0.000 | 1417 |
| GO:0050794 | regulation of cellular process | 0.000 | 1286 |
| GO:0033038 | bitter taste receptor activity | 0.000 | 19 |
| GO:0008527 | taste receptor activity | 0.000 | 20 |
| GO:0001580 | detection of chemical stimulus involved in sensory perception of bitter taste | 0.000 | 21 |
| GO:0050909 | sensory perception of taste | 0.000 | 27 |
| GO:0050912 | detection of chemical stimulus involved in sensory perception of taste | 0.000 | 22 |
| GO:0050913 | sensory perception of bitter taste | 0.000 | 21 |
| GO:0030414 | peptidase inhibitor activity | 0.000 | 47 |
| GO:0004866 | endopeptidase inhibitor activity | 0.000 | 44 |
| GO:0061135 | endopeptidase regulator activity | 0.001 | 44 |
| GO:0004252 | serine-type endopeptidase activity | 0.001 | 45 |
| GO:0008227 | G-protein coupled amine receptor activity | 0.003 | 22 |
| GO:0017171 | serine hydrolase activity | 0.003 | 49 |
| GO:0008236 | serine-type peptidase activity | 0.004 | 48 |
| GO:0061134 | peptidase regulator activity | 0.006 | 47 |
| GO:0004867 | serine-type endopeptidase inhibitor activity | 0.016 | 25 |
| GO:0019373 | epoxygenase P450 pathway | 0.030 | 10 |
| GO:0010466 | negative regulation of peptidase activity | 0.037 | 50 |
| downregulated in the frontal cortex | |||
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 50 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 45 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 43 |
| GO:0038023 | signaling receptor activity | 0.000 | 61 |
| GO:0060089 | molecular transducer activity | 0.000 | 61 |
| GO:0007600 | sensory perception | 0.000 | 51 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 42 |
| GO:0007608 | sensory perception of smell | 0.000 | 41 |
| GO:0004984 | olfactory receptor activity | 0.000 | 40 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 40 |
| GO:0051606 | detection of stimulus | 0.000 | 44 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 42 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 56 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.001 | 54 |
| GO:0003008 | system process | 0.001 | 63 |
| GO:0004871 | signal transducer activity | 0.002 | 62 |
| GO:0050877 | nervous system process | 0.002 | 54 |
| GO:0016021 | integral component of membrane | 0.006 | 112 |
| GO:0031224 | intrinsic component of membrane | 0.007 | 113 |
| upregulated in the hippocampus | |||
| GO:0004930 | G-protein coupled receptor activity | 0.000 | 118 |
| GO:0007606 | sensory perception of chemical stimulus | 0.000 | 108 |
| GO:0050907 | detection of chemical stimulus involved in sensory perception | 0.000 | 102 |
| GO:0009593 | detection of chemical stimulus | 0.000 | 103 |
| GO:0050906 | detection of stimulus involved in sensory perception | 0.000 | 104 |
| GO:0004984 | olfactory receptor activity | 0.000 | 98 |
| GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 0.000 | 98 |
| GO:0051606 | detection of stimulus | 0.000 | 105 |
| GO:0007608 | sensory perception of smell | 0.000 | 98 |
| GO:0004888 | transmembrane signaling receptor activity | 0.000 | 129 |
| GO:0038023 | signaling receptor activity | 0.000 | 135 |
| GO:0060089 | molecular transducer activity | 0.000 | 135 |
| GO:0007186 | G-protein coupled receptor signaling pathway | 0.000 | 127 |
| GO:0007600 | sensory perception | 0.000 | 115 |
| GO:0004871 | signal transducer activity | 0.000 | 136 |
| GO:0050877 | nervous system process | 0.000 | 119 |
| GO:0003008 | system process | 0.000 | 127 |
| GO:0016021 | integral component of membrane | 0.000 | 217 |
| GO:0031224 | intrinsic component of membrane | 0.000 | 219 |
| GO:0007165 | signal transduction | 0.000 | 173 |
| GO:0023052 | signaling | 0.000 | 178 |
| GO:0044425 | membrane part | 0.000 | 225 |
| GO:0007154 | cell communication | 0.000 | 179 |
| GO:0042221 | response to chemical | 0.000 | 167 |
| GO:0071944 | cell periphery | 0.000 | 174 |
| GO:0005886 | plasma membrane | 0.000 | 171 |
| GO:0032501 | multicellular organismal process | 0.000 | 214 |
| GO:0005549 | odorant binding | 0.000 | 25 |
| GO:0051716 | cellular response to stimulus | 0.000 | 193 |
3. Discussion
The main observation of this study shows that the brain transcriptome is influenced by chronic lithium administration. The important finding is that the differentially expressed genes specific for the mania model are involved in olfactory receptor transduction and taste receptor pathways and show altered expression of long non-coding RNA genes.
Comparing depressive-like and manic-like animals, we found that in the depression model, the gene expression profile is more region-specific (different pathways regulated upon lithium in the amygdala, frontal cortex and hippocampus) and time-dependent (2 weeks versus 4 weeks). In the model of mania, on the other hand, lithium significantly affected olfactory receptor and bitter taste receptor pathways, independent of the brain region or time of lithium administration suggesting their role in antimanic action. The genes from these pathways also showed significantly altered expression in “manic” rats before lithium administration. Our observation is consistent with the previous human studies in bipolar disorder patients that presented several olfactory and gustatory dysfunctions [13] and the sensory enhancement and dysregulation of taste were often reported during an acute manic episode in BD patients [14].
Previous animal studies of lithium showed that mice chronically fed with lithium accumulated it mainly in neurogenic brain regions including olfactory bulb [15]. Similarly, chronic lithium effects in the neurogenic brain region (hippocampus) were also observed in human BD studies showing that lithium influenced hippocampal volume and structural plasticity in BD patients [16,17,18]. These findings suggest that chronic lithium treatment targets olfactory bulb and alters associated pathways (e.g., olfactory transduction).
On the contrary, in “depressive” rats we observed the upregulated expression of genes regulating olfactory receptor activity after 4-week lithium administration in the amygdala, which is consistent with the previous observations that bipolar patients during depressive episode experienced sensory blunting and olfactory deficits [14]. Thus chronic lithium treatment seems to restore olfactory function in rats presenting depressive-like behavior. Moreover, previous study showed that olfactory bulbectomy induced depressive-like behavior in rodents [19], in particular when taking into account the close anatomical links between the olfactory system and the brain circuits involved in memory [20] and emotion [21]. The recent studies reported the presence of sensory changes during mood swings in BD [22] and that olfactory assessment may be useful to screen unipolar and bipolar depression [23]. These genes involved in olfactory receptors activity were also differentially expressed in either manic and depressive rats responding to chronic lithium treatment and showed the opposite effect that also depended on tissue: in the amygdala they were upregulated after chronic lithium in mania, but downregulated in depression; in the hippocampus they showed downregulated expression in mania, but upregulated in depression after chronic lithium. This opposite gene expression profiles between amygdala and hippocampus after chronic lithium treatment may be further supported by results of the recent study that showed the opposite temporal profiles of protein expression between amygdala and hippocampal neurons in long-term response to acute stress [24].
Our findings from the animal model indicating the involvement of the taste receptor pathway were further supported by the observations in our clinical sample of bipolar and unipolar patients showing altered expression of taste receptor genes in peripheral blood leukocytes during depressive episode (Dmitrzak-Węglarz et al., under review). However, this pathway was not previously reported in the chronic lithium mechanism of action.
Another pathway significantly influenced by chronic lithium treatment in our animal mania model was bitter taste receptor pathway. Recent transcriptome study by Lee et al. [25] showed altered taste receptors gene expression during manic episodes. These genes included bitter taste receptors, TAS2R5 and TAS2R3, that were suggested as potential markers specific for the manic state [25]. These receptors were previously found downregulated (together with olfactory receptors) in the dorsolateral prefrontal cortex of schizophrenia postmortem brain tissues [26] and this downregulation influenced altered cognition. The taste alteration was also related to psychosocial and cognitive performance in bipolar patients [13]. However, the previous studies did not investigate the effects of mood stabilizers on taste-related gene expression. Our observation that chronic lithium influences the regulation of the olfactory and taste receptors’ pathway is supported by the previous case studies reporting long lasting impaired taste (dysgeusia) and smell (hyposmia) in lithium users: cluster headache patient [27] and bipolar patient [28].
Our study revealed that a number of differentially expressed genes influenced by chronic lithium treatment in the brain were long non-coding RNAs. The paper by ConLiGen showed significant associations for two long non-coding RNAs (lncRNAs) localized on chromosome 21 with lithium prophylactic efficacy supporting their involvement in modulating a clinical response [29]. Several studies reported that lncRNAs expression was dysregulated in psychiatric conditions, including BD [30] whereas Lee et al. found recently that lncRNAs were the predominant category showing upregulation during an acute manic episode [25]. Here, we report for the first time that lncRNAs expression is altered by chronic lithium treatment and suggest they may be regulators of antimanic lithium action.
Comparing the 2-week and 4-week gene expression profile, we found that the longer the treatment was, the more differentially expressed genes were observed, independent of the brain region. A previous study comparing microarrays from rat brains after 7 and 42 days of lithium treatment showed that, although plasma Li concentration reached therapeutic levels after 2 days of treatment, it required 2 weeks to reach therapeutic levels in the brain [11]. This finding suggested that the long-term lithium treatment and associated gene expression profile changes better represent the downstream Li effects, which are more relevant to its clinical effect. The other report in the rat fed for 21 days with lithium led to significant changes in the transcriptome in the frontal cortex [31].
To investigate the chronic lithium effect in the brain that mimics changes during the episode of depression or mania, we aimed to model mania and depression separately as the animal model of bipolar disorder does not exist [32]. Animal models are relevant platforms to evaluate the intracellular mechanisms in the brain associated with the pathogenesis of psychiatric disorders and drug studies, including lithium discovery [2,33,34]. In our study, we applied a chronic mild stress (CMS) protocol, the most extensively validated [35], to induce depressive-like behavior. Moreover, the CMS protocol was also suitable to model recurrent depression (a hallmark of bipolar disorder) [36]. The choice of amphetamine to mimic mania was supported by the previous preclinical studies that used repeated intraperitoneal injections of psychostimulants, such as amphetamine, to mimic acute manic episodes in rodents, including hyperactivity and risk-taking behavior due to increased sustained dopamine efflux [37,38,39,40,41,42]. Moreover, these behaviors were reversed by the administration of mood stabilizers, including lithium [40,43]. This chronic amphetamine-induced mania model showed recently good face, predictive and construct validity in modeling mania in Wistar rats [44,45,46,47].
4. Materials and Methods
4.1. Experimental Animals
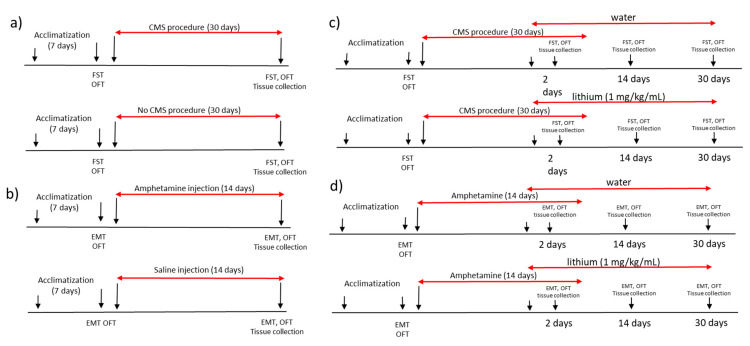
All experimental procedures were performed in agreement with 3R rule and the study was approved by local ethical committee Poznan University of Life Sciences, Poland (agreement no. 22/2017, 23 June 2017). We used male Wistar rats with the baseline weight of 180 ± 10 g. The rats were housed five animals per cage with food and water available ad libitum and were maintained in a 12 h light/dark cycle (lights on at 7:00 a.m.) at a temperature of 22 ± 1 °C. All experiments were performed at the same time each day to avoid circadian variations. The animals were kept for one week of acclimatization and then were randomly divided into experimental groups with 5 animals in each group (amphetamine-exposed and control group, chronic mild stress-exposed and non-stressed rats; amphetamine-exposed rats receiving lithium or water and stress-exposed rats receiving lithium or water). After the acclimatization period, animals underwent baseline behavioral tests. The experimental design was shown in Figure 7.
Figure 7.
The experimental design of the study showing the (a) procedure of inducing manic-like behavior, (b) procedure of inducing depressive-like behavior, (c) lithium administration in rats with manic-like behavior and (d) lithium administration in animals showing depressive-like behavior. CMS—chronic mild stress protocol; EMT—elevated maze test, FST—forced swim test, OFT—open field test.
4.2. Animal Model of Mania
Manic-like behavior was induced by daily intraperitoneal (i.p) injections of dextroamphetamine (d-AMPH) 2 mg/kg for 2 weeks as described previously [37,42]. The control group were animals receiving daily intraperitoneal injection with saline (0.9% NaCl, 1 mL/kg) for 14 days. After seven days, the behavior of all animals was assessed (Figure 7).
4.3. Animal Model of Depression
Animal model of depression was developed by the chronic mild stress protocol (CMS) as described previously [48]. To induce depressive behavior the animals were exposed for 4 weeks to different stress stimuli according to the CMS protocol described by Papp [49]. The control group were rats kept in standard conditions for 4 weeks that were not exposed to CMS. After two weeks of the start of the experiment, behavior of all animals was assessed (Figure 7).
4.4. Lithium Administration
The animals for the lithium study, after one week of amphetamine injections or two weeks of the chronic mild stress protocol and behavioral assessment, were randomly divided into lithium treated amphetamine-exposed group (n = 15) and amphetamine-exposed control group (rats receiving water, n = 15) and lithium-treated stress-exposed group (n = 15) and a stress-exposed control group (rats receiving water, n = 15). The animals were receiving the daily lithium solution in the dose of 1 mg/kg body mass or the same amount of water into the mouth with a syringe [50]. To minimize the side effects of lithium on kidney function, a saline bottle was provided in all lithium and control cages, as previously reported [51]. To assess the short term and long term lithium effects on brain transcriptome, the animals (n = 15 at each time in each group) were sacrificed by decapitation without euthanasia at two time points of chronic lithium administration, 14 and 30 days (long-term effect) after the first dose. The each control group (for stress-exposed and amphetamine-exposed rats) consisted of animals receiving water (Figure 7).
4.5. Behaviour Assessment
All the behavioral tests were performed during the light phase (between 08:00 a.m. and 12:00 p.m.), at room temperature in a separate quiet room. A blind evaluator assessed all behavioral parameters. To assess depressive-like or manic-like behavior we used a forced-swim test, open field test and elevated maze test at baseline and after induction of stress-induced depression or amphetamine-induced mania.
A forced swim test was performed to measure despair behavior and the hopelessness in the animal [52,53]. Each rat was placed in a glass cylinder 40 cm tall filled with water to a depth of 30 cm (24 ± 1 °C) so the rats could not support themselves by touching the bottom. Two swimming sessions were conducted: a 15-min pretest followed by a 5-min test 24 h later. For each animal, we recorded time spent immobilized (no additional activity other than that required to keep the rat’s head above the water) and active climbing time (upward-directed movements of the forepaws along the side of the swim chamber. The data were analyzed using the video tracking system (Videomot2, AnimaLab, Poznan, Poland). The water in the cylinders was replaced after each trial to remove urine or feces and to avoid confounding results.
Open field test was performed to evaluate the spontaneous locomotory activity in a new environment and exploration [54,55,56]. The rat was placed in the middle of the plastic box measuring 100 cm in diameter with 50-cm walls, divided into 25 squares of 20 × 20-cm size on the bottom (AnimaLab, Poland) and allowed to explore it for 5 min. We recorded the following parameters: time spent immobilized, time spent in center, total distance and exploration time and analyzed the data with the video tracking system (Videomot2, AnimaLab, Poland). After the end of each test, the open field was cleaned to remove any remaining materials (such as feces, urine or smell) that could interfere with the test results.
The elevated maze test was used to test the anxiety level and risk-taking behavior in the rats [42]. The apparatus consisted of two opposite open arms, two opposite closed arms and a central platform connecting the four arms. The maze was propped up 50 cm away from the ground by the maze feet. The test room was maintained with constant temperature, humidity and illumination. The rats were placed in the central platform facing one of the open arms. The number of entries into the open and closed arms and the total time spent in each arm during a 5-min exploration period were recorded by the video tracking system (Videomot2, AnimaLab, Poznań, Poland). After the end of each test, the open field was cleaned to remove any remaining materials (such as feces, urine or smell) that could interfere with the test results.
4.6. Microarray-Based Gene Expression Analysis
The different regions of brain tissue (amygdala, hippocampus, prefrontal cortex and hypothalamus) were collected immediately after decapitation (without anesthesia). The skull was opened, and the cerebral content was excised and rapidly dissected on a chilled Petri dish. The frontal cortex, hippocampus and amygdala were isolated and cleaned from the subcortical structures and white matter, immediately snap frozen in liquid nitrogen and kept frozen in −80 °C for further processing. Frozen tissues were used for RNA extraction using Nucleospin RNA/Protein kit (Macherey Nagel, Dylan, Germany). RNA integrity (RIN) was assessed using Tape Station 2200 (Agilent) and RNA concentration was measured using fluorimeter (Quantus, Promega). Total RNA from amygdala, hippocampus and frontal cortex in the starting amount of 50 ng was used for microarray experiments. We used SurePrint G3 Rat Gene Expression v2 Microarray Kit in format 8 × 60 k and one-color Low Input Quick Amp Labeling Kit (Agilent Technologies, Cedar Creek, TX, USA) to analyze gene expression profile following the standard protocol provided by the manufacturer. The hybridization signals were detected with SureScan Dx Microarray Scanner (Agilent Technologies, Santa Clara, CA, USA). The images obtained after scanning were analyzed with Agilent Feature extraction software v.12.0.3.1.
4.7. Statistical Analysis
Behavioral data were analyzed using a t-test for paired samples after checking normality of data distribution using the Shapiro–Wilk test. A cut-off value p < 0.05 was regarded as significant. Data were shown as mean ± SEM in figures and text if not otherwise stated. For behavioral measurements and gene expression data, 5 animals per experimental group were sufficient to achieve power of 80% (as calculated by the G-power calculator available at https://stats.idre.ucla.edu/other/gpower). To conform to the 3R’s rule, we included the minimal required number of animals (n = 5) in the study.
Bioinformatic analysis of gene expression data was analyzed with the use of Gene Spring 14.9 software (Agilent Technologies, Santa Clara, CA, USA). To identify differentially expressed genes, moderated t-statistics from the empirical Bayes method on normalized fluorescence signal was used for the fold of change (FC) calculation. FC was calculated in relation to the adequate control group. The list of significantly differentially expressed transcripts (p < 0.05 and FC > 2.0) was generated using statistical filtering (moderated t-test with multiple test correction FDR). Then, separate lists of up- and downregulated genes were used for functional analysis to identify Gene Ontology (GO) terms and pathways significantly enriched by the set of differentially expressed genes, either upregulated or downregulated (p < 0.05). Gene Ontology and pathway analyses were done in Gene Spring.
To identify the genes and pathways shared during lithium administration, we used Venn diagrams using a tool freely available at: http://bioinformatics.psb.ugent.be/webtools/Venn. For shared genes, we used G:Profiler tool (available at: https://biit.cs.ut.ee/gprofiler/gost) to perform functional enrichment analysis (gene set enrichment analysis) including GO and pathways from KEGG Reactome and Wiki Pathways [57]. Heat maps were drawn using the Heatmapper tool available at: http://www.heatmapper.ca.
5. Conclusions
We reported here for the first time that genes regulating olfactory and taste receptor pathways and long non-coding RNAs, that were implicated in BD pathogenesis, might be targeted by chronic lithium treatment in animals presenting manic-like behavior. Further functional studies of these pathways are warranted to elucidate the exact therapeutic molecular mechanism of lithium.
Acknowledgments
We would like to thank the staff of the Department of Medical Genetics, Poznan University of Medical Sciences for access to the laboratory equipment for microarrays experiments.
Author Contributions
D.S. participated in the study design and conceptualization, performed animal experiments, data interpretation and drafted the manuscript; P.C., P.A.K., E.P.-O., M.S. (Maciej Sassek), P.Z., E.B. and W.L. performed animal experiments, analyzed the data and revised the manuscript; K.S. and J.N. performed molecular analysis and revised the manuscript; M.S. (Magdalena Socha) and E.B.-O. optimized the microarray experiment and revised the manuscript; J.P. participated in the study design and supervised the experiments; J.T.-H. acquired the funding and supervised the experiments, L.N. and J.K.R. supervised the experiments and revised the manuscript, A.S. participated in the study design, administered the project, performed microarray experiments, analyzed and interpreted the data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Centre, Poland, grant number 2016/21/B/NZ5/00148.
Institutional Review Board Statement
The study was approved by local ethical committee at Poznan University of Life Sciences, Poland (agreement no. 22/2017, 23 June 2017).
Data Availability Statement
The detailed data used to support the findings of this study are available from the corresponding author upon written request.
Conflicts of Interest
The authors declare no conflict of interest. The funding body had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Pub.; Washington, DC, USA: 2013. [Google Scholar]
- 2.Cade J.F. Lithium salts in the treatment of psychotic excitement. Med. J. Aust. 1949;2:349–352. doi: 10.5694/j.1326-5377.1949.tb36912.x. [DOI] [PubMed] [Google Scholar]
- 3.Rybakowski J. Lithium carbonate in endogenous depression. Psychiatr. Pol. 1972;6:547–550. [PubMed] [Google Scholar]
- 4.Mendels J. Lithium in the treatment of depression. Am. J. Psychiatry. 1976;133:373–378. doi: 10.1176/ajp.133.4.373. [DOI] [PubMed] [Google Scholar]
- 5.Rybakowski J.K. Lithium-past, present, future. Int J. Psychiatry Clin. Pr. 2020;24:330–340. doi: 10.1080/13651501.2020.1775855. [DOI] [PubMed] [Google Scholar]
- 6.Harwood A.J. Lithium and bipolar mood disorder: The inositol-depletion hypothesis revisited. Mol. Psychiatry. 2005;10:117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- 7.Chalecka-Franaszek E., Chuang D.M. Lithium activates the serine/threonine kinase akt-1 and suppresses glutamate-induced inhibition of akt-1 activity in neurons. Proc. Natl. Acad. Sci. USA. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Sarno P., Li X., Jope R.S. Regulation of akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/S0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 9.Severino G., Squassina A., Costa M., Pisanu C., Calza S., Alda M., Del Zompo M., Manchia M. Pharmacogenomics of bipolar disorder. Pharmacogenomics. 2013;14:655–674. doi: 10.2217/pgs.13.51. [DOI] [PubMed] [Google Scholar]
- 10.Alda M. Lithium in the treatment of bipolar disorder: Pharmacology and pharmacogenetics. Mol. Psychiatry. 2015;20:661–670. doi: 10.1038/mp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosetti F., Seemann R., Bell J.M., Zahorchak R., Friedman E., Rapoport S.I., Manickam P. Analysis of gene expression with cdna microarrays in rat brain after 7 and 42 days of oral lithium administration. Brain Res. Bull. 2002;57:205–209. doi: 10.1016/S0361-9230(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 12.McQuillin A., Rizig M., Gurling H.M. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mrna to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharm. Genom. 2007;17:605–617. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- 13.Kazour F., Richa S., Desmidt T., Lemaire M., Atanasova B., El Hage W. Olfactory and gustatory functions in bipolar disorders: A systematic review. Neurosci. Biobehav. Rev. 2017;80:69–79. doi: 10.1016/j.neubiorev.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Parker G., Paterson A., Romano M., Granville Smith I. Suprasensory phenomena in those with a bipolar disorder. Australas. Psychiatry. 2018;26:384–387. doi: 10.1177/1039856218762306. [DOI] [PubMed] [Google Scholar]
- 15.Zanni G., Michno W., Di Martino E., Tjarnlund-Wolf A., Pettersson J., Mason C.E., Hellspong G., Blomgren K., Hanrieder J. Lithium accumulates in neurogenic brain regions as revealed by high resolution ion imaging. Sci. Rep. 2017;7:40726. doi: 10.1038/srep40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertolino A., Frye M., Callicott J.H., Mattay V.S., Rakow R., Shelton-Repella J., Post R., Weinberger D.R. Neuronal pathology in the hippocampal area of patients with bipolar disorder: A study with proton magnetic resonance spectroscopic imaging. Biol. Psychiatry. 2003;53:906–913. doi: 10.1016/S0006-3223(02)01911-X. [DOI] [PubMed] [Google Scholar]
- 17.Colla M., Schubert F., Bubner M., Heidenreich J.O., Bajbouj M., Seifert F., Luborzewski A., Heuser I., Kronenberg G. Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Mol. Psychiatry. 2009;14:696–704. doi: 10.1038/mp.2008.26. [DOI] [PubMed] [Google Scholar]
- 18.Giakoumatos C.I., Nanda P., Mathew I.T., Tandon N., Shah J., Bishop J.R., Clementz B.A., Pearlson G.D., Sweeney J.A., Tamminga C.A., et al. Effects of lithium on cortical thickness and hippocampal subfield volumes in psychotic bipolar disorder. J. Psychiatr. Res. 2015;61:180–187. doi: 10.1016/j.jpsychires.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poretti M.B., Rask-Andersen M., Kumar P., Rubiales de Barioglio S., Fiol de Cuneo M., Schioth H.B., Carlini V.P. Ghrelin effects expression of several genes associated with depression-like behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;56:227–234. doi: 10.1016/j.pnpbp.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Savic I., Gulyas B., Larsson M., Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/S0896-6273(00)81209-X. [DOI] [PubMed] [Google Scholar]
- 21.Anderson A.K., Christoff K., Stappen I., Panitz D., Ghahremani D.G., Glover G., Gabrieli J.D., Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 22.Parker G., Paterson A., Romano M., Graham R. Altered sensory phenomena experienced in bipolar disorder. Am. J. Psychiatry. 2017;174:1146–1150. doi: 10.1176/appi.ajp.2017.16121379. [DOI] [PubMed] [Google Scholar]
- 23.Kazour F., Richa S., Abi Char C., Surget A., Elhage W., Atanasova B. Olfactory markers for depression: Differences between bipolar and unipolar patients. PLoS ONE. 2020;15:e0237565. doi: 10.1371/journal.pone.0237565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madan J.S., Gupta K., Chattarji S., Bhattacharya A. Hippocampal and amygdalar cell-specific translation is similar soon after stress but diverge over time. Hippocampus. 2018;28:441–452. doi: 10.1002/hipo.22845. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y.C., Chao Y.L., Chang C.E., Hsieh M.H., Liu K.T., Chen H.C., Lu M.L., Chen W.Y., Chen C.H., Tsai M.H., et al. Transcriptome changes in relation to manic episode. Front. Psychiatry. 2019;10:280. doi: 10.3389/fpsyt.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansoleaga B., Garcia-Esparcia P., Pinacho R., Haro J.M., Ramos B., Ferrer I. Decrease in olfactory and taste receptor expression in the dorsolateral prefrontal cortex in chronic schizophrenia. J. Psychiatr. Res. 2015;60:109–116. doi: 10.1016/j.jpsychires.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 27.De Coo I.F., Haan J. Long lasting impairment of taste and smell as side effect of lithium carbonate in a cluster headache patient. Headache. 2016;56:1201–1203. doi: 10.1111/head.12872. [DOI] [PubMed] [Google Scholar]
- 28.Terao T., Watanabe S., Hoaki N., Hoaki T. Strange taste and mild lithium intoxication. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.05.2011.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou L., Heilbronner U., Degenhardt F., Adli M., Akiyama K., Akula N., Ardau R., Arias B., Backlund L., Banzato C.E.M., et al. Genetic variants associated with response to lithium treatment in bipolar disorder: A genome-wide association study. Lancet. 2016;387:1085–1093. doi: 10.1016/S0140-6736(16)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akula N., Barb J., Jiang X., Wendland J.R., Choi K.H., Sen S.K., Hou L., Chen D.T., Laje G., Johnson K., et al. Rna-sequencing of the brain transcriptome implicates dysregulation of neuroplasticity, circadian rhythms and gtpase binding in bipolar disorder. Mol. Psychiatry. 2014;19:1179–1185. doi: 10.1038/mp.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatemi S.H., Reutiman T.J., Folsom T.D. The role of lithium in modulation of brain genes: Relevance for aetiology and treatment of bipolar disorder. Biochem. Soc. Trans. 2009;37:1090–1095. doi: 10.1042/BST0371090. [DOI] [PubMed] [Google Scholar]
- 32.Kato T., Kasahara T., Kubota-Sakashita M., Kato T.M., Nakajima K. Animal models of recurrent or bipolar depression. Neuroscience. 2016;321:189–196. doi: 10.1016/j.neuroscience.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Beyer D.K.E., Freund N. Animal models for bipolar disorder: From bedside to the cage. Int. J. Bipolar Disord. 2017;5:35. doi: 10.1186/s40345-017-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan A., Einat H. Questioning the predictive validity of the amphetamine-induced hyperactivity model for screening mood stabilizing drugs. Behav. Brain Res. 2019;362:109–113. doi: 10.1016/j.bbr.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Willner P. Reliability of the chronic mild stress model of depression: A user survey. Neurobiol. Stress. 2017;6:68–77. doi: 10.1016/j.ynstr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remus J.L., Jamison D., Johnson J.D. An animal model of recurrent depression: Sensitized depression-like behavior when rats are re-exposed to chronic mild stress. Brain Behav. Immun. 2013;32:e4–e5. doi: 10.1016/j.bbi.2013.07.026. [DOI] [Google Scholar]
- 37.Frey B.N., Martins M.R., Petronilho F.C., Dal-Pizzol F., Quevedo J., Kapczinski F. Increased oxidative stress after repeated amphetamine exposure: Possible relevance as a model of mania. Bipolar Disord. 2006;8:275–280. doi: 10.1111/j.1399-5618.2006.00318.x. [DOI] [PubMed] [Google Scholar]
- 38.Szabo S.T., Machado-Vieira R., Yuan P., Wang Y., Wei Y., Falke C., Cirelli C., Tononi G., Manji H.K., Du J. Glutamate receptors as targets of protein kinase c in the pathophysiology and treatment of animal models of mania. Neuropharmacology. 2009;56:47–55. doi: 10.1016/j.neuropharm.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feier G., Valvassori S.S., Varela R.B., Resende W.R., Bavaresco D.V., Morais M.O., Scaini G., Andersen M.L., Streck E.L., Quevedo J. Lithium and valproate modulate energy metabolism in an animal model of mania induced by methamphetamine. Pharm. Biochem. Behav. 2013;103:589–596. doi: 10.1016/j.pbb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z., Wang Y., Tan H., Bharti V., Che Y., Wang J.F. Chronic treatment with mood stabilizer lithium inhibits amphetamine-induced risk-taking manic-like behaviors. Neurosci. Lett. 2015;603:84–88. doi: 10.1016/j.neulet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Valvassori S.S., Resende W.R., Dal-Pont G., Sangaletti-Pereira H., Gava F.F., Peterle B.R., Carvalho A.F., Varela R.B., Dal-Pizzol F., Quevedo J. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (hpa) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017;19:246–258. doi: 10.1111/bdi.12503. [DOI] [PubMed] [Google Scholar]
- 42.Valvassori S.S., Gava F.F., Dal-Pont G.C., Simoes H.L., Damiani-Neves M., Andersen M.L., Boeck C.R., Quevedo J. Effects of lithium and valproate on erk/jnk signaling pathway in an animal model of mania induced by amphetamine. Heliyon. 2019;5:e01541. doi: 10.1016/j.heliyon.2019.e01541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valvassori S.S., Tonin P.T., Varela R.B., Carvalho A.F., Mariot E., Amboni R.T., Bianchini G., Andersen M.L., Quevedo J. Lithium modulates the production of peripheral and cerebral cytokines in an animal model of mania induced by dextroamphetamine. Bipolar Disord. 2015;17:507–517. doi: 10.1111/bdi.12299. [DOI] [PubMed] [Google Scholar]
- 44.Menegas S., Dal-Pont G.C., Cararo J.H., Varela R.B., Aguiar-Geraldo J.M., Possamai-Della T., Andersen M.L., Quevedo J., Valvassori S.S. Efficacy of folic acid as an adjunct to lithium therapy on manic-like behaviors, oxidative stress and inflammatory parameters in an animal model of mania. Metab. Brain Dis. 2020;35:413–425. doi: 10.1007/s11011-019-00503-3. [DOI] [PubMed] [Google Scholar]
- 45.Varela R.B., Resende W.R., Dal-Pont G.C., Gava F.F., Nadas G.B., Tye S.J., Andersen M.L., Quevedo J., Valvassori S.S. Role of epigenetic regulatory enzymes in animal models of mania induced by amphetamine and paradoxical sleep deprivation. Eur J. Neurosci. 2020 doi: 10.1111/ejn.14922. [DOI] [PubMed] [Google Scholar]
- 46.Valvassori S.S., Tonin P.T., Dal-Pont G.C., Varela R.B., Cararo J.H., Garcia A.F., Gava F.F., Menegas S., Soares J.C., Quevedo J. Coadministration of lithium and celecoxib reverses manic-like behavior and decreases oxidative stress in a dopaminergic model of mania induced in rats. Transl. Psychiatry. 2019;9:297. doi: 10.1038/s41398-019-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bristot G., Ascoli B.M., Scotton E., Gea L.P., Pfaffenseller B., Kauer-Sant’Anna M. Effects of lithium on inflammatory and neurotrophic factors after an immune challenge in a lisdexamfetamine animal model of mania. Braz. J. Psychiatry. 2019;41:419–427. doi: 10.1590/1516-4446-2017-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willner P., Muscat R., Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992;16:525–534. doi: 10.1016/S0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 49.Papp M. Models of affective illness: Chronic mild stress in the rat. Curr. Protoc. Pharm. 2012;57 doi: 10.1002/0471141755.ph0509s57. [DOI] [PubMed] [Google Scholar]
- 50.Atcha Z., Rourke C., Neo A.H., Goh C.W., Lim J.S., Aw C.C., Browne E.R., Pemberton D.J. Alternative method of oral dosing for rats. J. Am. Assoc. Lab. Anim. Sci. 2010;49:335–343. [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G., Rajkowska G., Du F., Seraji-Bozorgzad N., Manji H.K. Enhancement of hippocampal neurogenesis by lithium. J. Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 52.Porsolt R.D., Bertin A., Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharm. 1977;229:327–336. [PubMed] [Google Scholar]
- 53.Willner P., Bergman J., Vanderschuren L., Ellenbroek B. Pharmacological approaches to the study of social behaviour. Behav. Pharm. 2015;26:501–504. doi: 10.1097/FBP.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 54.Jindal A., Mahesh R., Bhatt S. Etazolate rescues behavioral deficits in chronic unpredictable mild stress model: Modulation of hypothalamic-pituitary-adrenal axis activity and brain-derived neurotrophic factor level. Neurochem. Int. 2013;63:465–475. doi: 10.1016/j.neuint.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Belovicova K., Bogi E., Csatlosova K., Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017;10:40–43. doi: 10.1515/intox-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun H.L., Zhou Z.Q., Zhang G.F., Yang C., Wang X.M., Shen J.C., Hashimoto K., Yang J.J. Role of hippocampal p11 in the sustained antidepressant effect of ketamine in the chronic unpredictable mild stress model. Transl. Psychiatry. 2016;6:e741. doi: 10.1038/tp.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raudvere U., Kolberg L., Kuzmin I., Arak T., Adler P., Peterson H., Vilo J. G:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The detailed data used to support the findings of this study are available from the corresponding author upon written request.