Abstract
Background: Apolipoprotein E (APOE) ɛ4 is associated with poor outcome following moderate to severe traumatic brain injury (TBI). There is a lack of studies investigating the influence of APOE ɛ4 on intracranial pathology following mild traumatic brain injury (MTBI). This study explores the association between APOE ɛ4 and MRI measures of brain age prediction, brain morphometry, and diffusion tensor imaging (DTI). Methods: Patients aged 16 to 65 with acute MTBI admitted to the trauma center were included. Multimodal MRI was performed 12 months after injury and associated with APOE ɛ4 status. Corrections for multiple comparisons were done using false discovery rate (FDR). Results: Of included patients, 123 patients had available APOE, volumetric, and DTI data of sufficient quality. There were no differences between APOE ɛ4 carriers (39%) and non-carriers in demographic and clinical data. Age prediction revealed high accuracy both for the DTI-based and the brain morphometry based model. Group comparisons revealed no significant differences in brain-age gap between ɛ4 carriers and non-carriers, and no significant differences in conventional measures of brain morphometry and volumes. Compared to non-carriers, APOE ɛ4 carriers showed lower fractional anisotropy (FA) in the hippocampal part of the cingulum bundle, which did not remain significant after FDR adjustment. Conclusion: APOE ɛ4 carriers might be vulnerable to reduced neuronal integrity in the cingulum. Larger cohort studies are warranted to replicate this finding.
Keywords: mild traumatic brain injury, APOE, brain-age gap, MRI
1. Introduction
Following mild traumatic brain injury (TBI) (MTBI), most individuals recover quickly; however, the rate of recovery varies considerably. Between 6% to 64% of patients experience long-term symptoms and disability [1,2,3]. This large heterogeneity in prognosis and clinical course following MTBI suggests that factors other than injury severity are important predictors of outcome. Some of the unexplained variance may arise from genetic differences in processes involved in neural repair or neurodegenerative mechanisms. Apolipoprotein E (APOE), having three common protein isoforms (ɛ2, ɛ3, and ɛ4), is involved in neuronal repair and plasticity [4] and carriers of the ɛ4 allele are at increased risk for neurological conditions such as Alzheimer’s disease (AD) [5] and poor outcome following moderate and severe TBI [6]. While the mechanisms by which the ɛ4 allele exerts an effect is unclear, APOE gene expression increases following brain injury [7], and it has been shown that the ɛ4 allele is less effective at promoting neuronal repair than the ɛ2 and ɛ3 alleles [4].
In addition to its effects on outcome after TBI, the APOE genotype may interact with TBI to increase the risk of developing neurodegenerative disorders later in life [8]. APOE ɛ4 carriers are at increased risk for cognitive impairment and chronic traumatic encephalopathy following head injury [9,10,11]. APOE ɛ4 has been shown to promote amyloid deposition in individuals with TBI [12] and the combination of MTBI and genetic risk for AD may play a role in the degeneration of structural brain integrity [13]. These results highlight the intuitive notion that clinical and neuropathological outcomes following MTBI are determined by complex interactions between injury characteristics and genetic factors.
The majority of prior studies assessing the risk of neurodegenerative diseases following TBI have focused either on the risk imparted by a TBI of any severity or on the risk imparted by moderate or severe TBI. Two systematic reviews considering the risk of dementia following MTBI [14,15] that included literature published from 1980 through 2012 identified only four qualifying studies [16,17,18,19]. These studies were limited by a relatively small number of MTBI patients, yet two reported a significant association between MTBI and dementia [17,18]. Meanwhile, in a large, prospective, population-based study of 6645 individuals aged 55 years or older who were free of dementia at baseline, Metha et al. [16] found that MTBI was not a major risk factor for AD. Moreover, brain trauma did not appear to increase the risk of AD in ɛ4 carriers. One study by Yue et al. [20] of a group of MTBI patients found that the APOE ɛ4 allele may confer an increased risk of impairment of six-month verbal memory. These authors also found that intracranial pathology was the driver of decreased verbal memory performance at six months. Finally, it has been reported reduced cortical thickness in AD-related brain regions in MTBI patients at increased genetic risk for AD [13].
In contrast to measures of brain volume and gross morphology, diffusion tensor imaging (DTI) provides measures of white matter coherence and structure. DTI measures are highly sensitive to age [21,22] and have been used to study associations between APOE status and brain white matter in healthy individuals [23] and after TBI, suggesting greater vulnerability to white matter abnormalities in ɛ4 carriers as compared with non-carriers following close-range blast exposure [24].
A critical goal of biomedical research is to establish indicators of neuropathology after TBI to identify actionable targets for treatment and improved outcome. These biomarkers are quantifiable characteristics of biological processes related to TBI and can be used as surrogates for the disease process. Recent efforts to develop neuroimaging-derived markers for brain health include brain-age prediction, which uses machine learning to predict age based on brain characteristics [25,26,27]. An individual’s estimated brain age can be compared to their chronological age to calculate deviation from normative age trajectories, often referred to as the brain-age gap (BAG). Such deviations have been associated with a range of clinical risk factors [28,29,30] as well as neurological and neuropsychiatric diseases [31,32,33,34]. Estimated brain age in TBI patients has been shown to be higher relative to their chronological age [35]. This discrepancy increases with more time since the injury, suggesting that TBI accelerates brain aging.
Brain-age prediction based on different brain imaging measures provides a window into different mechanisms of brain aging affecting brain gray and white matter. The successful identification of neurodegenerative markers in TBI patients would have important implications for the development of interventions to prevent TBI-induced neurodegeneration and planning of health service delivery such as individualized follow-ups and short- and long-term targeting impairments and disability. There is a lack of studies investigating the influence of APOE ɛ4 on intracranial neuropathology following MTBI and no investigations have conducted brain-age predictions among MTBI patients. Hence, by performing brain-age prediction based on sensitive MRI measures of brain morphometry and DTI measures of white matter architecture and coherence, we tested for associations between APOE ɛ4 status and brain-age gap in patients with MTBI at 12 months after injury. To increase the generalizability of our study, we used an independent training set for brain-age prediction. For comparison and transparency, we also report results from tests comparing ɛ4 carriers and non-carriers with regard to more conventional measures of brain morphometry and DTI measures.
2. Materials and Methods
2.1. Ethical Statement
All study protocols were approved by the Norwegian Regional Committee for Medical Research Ethics (2010/1899) (Oslo, Norway). Additionally, all participants provided written informed consent and all methods were carried out in accordance with the relevant guidelines and regulations of the Norwegian Regional Committee for Medical Research Ethics.
2.2. Participants and Procedure
Patients with acute MTBI admitted to Oslo University Hospital between September 2011 and September 2013 were included in this prospective cohort study and followed up for 12 months postinjury (2012–2014). Patients aged 16 to 65 years with a recent (<24 h) history of head trauma (hospitalization with an International Classification of Disease, 10th revision (ICD-10) diagnosis codes S06.0–S06.9), resulting in loss of consciousness (LOC) of less than 30 min, posttraumatic amnesia lasting (PTA) less than 24 h, and Glasgow Coma Scale score of 13 to 15 points were included. MTBI was defined using criteria from the American Congress of Rehabilitation Medicine (ACRM) [36]. Exclusion criteria for this study included the presence of severe mental illness (schizophrenia or bipolar disorder), progressive neurologic disease, ICD-10 diagnosis of substance dependence, contraindications for magnetic resonance imaging (MRI), and a lack of Norwegian language skills. We have previously reported brain volumetric and morphometric findings based on T1-weighted MRI data and DTI data in an overlapping sample [37,38,39], but not used brain-age prediction and not in relation to APOE status. Of the total sample of 168 patients at baseline, 134 (80%) patients returned for the 12-month follow-up visit, which included a clinical assessment and multimodal MRI; of these patients, 123 had available volumetric, APOE, and DTI data of sufficient quality. Thus, the final study population was 123 patients.
2.3. Biospecimen and Genotyping Procedures
The variants in exon 4 in APOE (GenBank: NM_000041)—that is, c.388T>C and c.526C>T—were analyzed by polymerase chain reaction and Sanger sequencing in DNA extracted from peripheral leukocytes. Primers were designed using Primer3Plus (Bioinformatics, Arlington, VA, USA) [40] and the polymerase chain reaction products were purified and Sanger-sequenced using an ABI 3730xl DNA analyzer and ABI BigDye terminator cycle-sequencing kits v3.1 (Thermo Fisher Scientific, Waltham, MA, USA). Sequences were analyzed with the DNA Sequencing Analysis software program (version 5.1; Applied Biosystems, Foster City, CA, USA) and the SeqScape software program (version 2.7; Thermo Fisher Scientific).
2.4. MRI Processing
3-Tesla MRI scans (GE Signa HDxt; GE Medical Systems, Chicago, IL, USA) data were obtained 12 months postinjury using two different head coils (Head/Neck/Spine (HNS) and 8HRBRAIN). Briefly, the protocol included a three-dimensional fast spoiled gradient-echo T1-weighted sequence used for morphometric assessments (repetition time (TR)/echo time (TE) ms/inversion time (Ti): 7.8/2.96/450 ms, flip angle (FA): 12° and spatial resolution: 1.0 × 1.0 × 1.2 mm). Acquisition parameters were optimized for increased gray/white matter contrast. In addition, a T2-weighted sequence and a T2 susceptibility-weighted angiography sequence were performed to depict hemorrhagic or other lesions. No major scanner upgrade occurred during the study period and the cortical reconstruction and segmentation processes are described in previous articles [37,38].
For diffusion-weighted imaging, a two-dimensional spin-echo whole-brain echo-planar imaging pulse with the following parameters was used: TR, 15 s; TE, 85 ms; FA, 90°; slice thickness, 2.5 mm; field of view, 240 × 240; acquisition matrix, 128 × 128; and in-plane resolution, 1.875 × 1.875. Additionally, 30 volumes with different gradient directions (b = 1000 s/mm2) and two b = 0 volumes with reversed phase-encode (blip up/down) were acquired. Image analyses and extraction of atlas-based regions of interests were completed using FMRIB Software Library (FSL) [41] and tract-based spatial statistics (TBSS) [42], as previously described [39]. We report on fractional anisotropy (FA) and mean diffusivity (MD), estimated using dtifit in FSL. FA and MD measure demyelination as a sign of white matter alteration [43] and have been used as markers of structural damage in some pathologies, e.g., mild cognitive impairment and Alzheimer’s disease [44]. Results from axial diffusivity (AD) and radial diffusivity (RD) are presented in the Supplementary Materials Table S1.
2.5. Brain-Age Prediction
In line with previous research [45,46], the Cambridge Centre for Ageing and Neuroscience (Cam-CAN) dataset (http://www.mrc-cbu.cam.ac.uk/datasets/camcan) was used as a training sample in the brain-age analyses. Briefly, this dataset includes information from healthy participants aged 18 to 87 years who were recruited through a research project funded by the Biotechnology and Biological Sciences Research Council, the UK Medical Research Council and University of Cambridge [47,48]. For more information, see http://www.cam-can.org. After quality control, MRI data from 622 participants were included in the training sample (age range: 18–87 years, mean age ± standard deviation: 54.17 ± 18.38 years).
The MRI features included 276 measures for DTI and 269 measures for the T1-weighted data, as described in Section 2.4. Age-prediction models were run using the XGBoost regression model, which is based on a decision-tree ensemble algorithm (https://xgboost.readthedocs.io/en/latest/python). XGboost includes advanced regularization to reduce overfitting [49] and incorporates a gradient-boosting framework where the final model is based on a collection of individual models (https://github.com/dmlc/xgboost). The parameters were set to a maximum depth of 3, number of estimators of 180, and learning rate of 0.1 based on a grid search with five folds for optimization. In addition, a grid search with nested cross-validation was performed to test for potential overfitting. The models were validated using five-fold cross-validation with 100 repetitions and the values of R2, root mean square error (RMSE), and mean absolute error (MAE) were calculated to evaluate model performance within the training sample.
Next, the models were applied to the sample of MTBI patients and R2, RMSE, and MAE values were calculated to evaluate prediction accuracy. The predicted age for each patient was derived using the Scikit Learn library (https://scikit-learn.org/stable/index.html). To adjust for a commonly observed age-bias (i.e., overestimated predictions for younger participants and underestimated predictions for older participants) [50,51], we applied a statistical correction by first fitting in the Cambridge Centre for Ageing and Neuroscience dataset training sample, where Y is the modelled predicted age as a function of chronological age () and α and β represent the slope and intercept, respectively. We then used the derived values of α and β to correct the predicted age in the MTBI sample using the equation corrected predicted age = predicted age + , before recalculating R2, RMSE, and MAE. BAG values for each participant were calculated using (corrected predicted age−chronological age).
2.6. Statistical Analyses
Descriptive statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (version 25; IBM Corporation, Armonk, NY, USA). Sample characteristics are presented as the group mean with standard deviation (SD). Differences between groups concerning continuous variables were tested using the Student’s t-test. The chi-squared test for contingency tables was conducted to detect group differences in categorical variables.
As the primary assessment, we performed multiple linear regression analysis to test for differences between APOE ɛ4 carriers and non-carriers in BAG based on T1-weighted and DTI data, while covarying for age, sex, and head coil. Because of the number of tests performed, we controlled the false discovery rate (FDR) using the Benjamini−Hochberg procedure [52]. Briefly, all p values were ordered from smallest to largest. The smallest p value was given a rank of i = 1, then next smallest i = 2, etc. We then compared each individual p value to its Benjamini−Hochberg critical value, (i/m)Q, where i is the rank, m is the total number of tests, and Q is the FDR you choose. The largest p value that has p < (i/m)Q is significant, and all p-values smaller than it are also significant. We present the raw p values and those significant at FDR = 0.1.
As follow-up analysis, we conducted additional linear regressions to test for group differences in individual MRI features, including intracranial volume, age, sex, and head coil in the models for volume measures and age, sex, and head coil for cortical thickness and DTI.
Regression results are presented as β coefficients, standard errors of β, p-values, and explained variance (R2).
2.7. Demographic and Clinical Assessment
We assessed key preinjury, acute, and postinjury variables. Acute clinical data were obtained from patient medical records and MRI and APOE assessments at 12 months of follow-up. Information regarding age, sex, and education level was obtained from clinical interviews.
The Glasgow Coma Scale (GCS) [53] assesses the conscious state of the patient at injury site or/and admission to the hospital and mild severity scores range from 13 to 15 points (from alert to well-orientated). The duration of posttraumatic amnesia was assessed in the emergency department and dichotomized into yes/unknown or no. The presence and duration of LOC were confirmed based on medical records and classified into no LOC or LOC/unknown. Causes of injury were obtained from patient medical records and classified as traffic accident, fall, violence, or other. Education was measured in years. Glasgow Outcome Scale-Extended (GOSE) was used as measure of global functions including independence, work, social and leisure activities, and participation in social life [54]. It is an 8-point ordinal scale reflecting good recovery (>7), moderate (5, 6) and severe (3, 4) disability, vegetative state (2), and death (1). Rivermead post-concussion symptoms questionnaire (RPQ) was used to assess cognitive, emotional and somatic symptoms [55]. Patient Health Questionnaire 9 (PHQ-9) was used to assess depressive symptoms [56]. Total score of 0–4 indicate no depression, 5–9 mild, 10–14 moderate, 15–19 moderately severe, and 20–27 severe depression, and the total score was used.
3. Results
3.1. Comparison Groups, Demographics, and Injury-Related Variables
The study population (n = 123 MTBI cases that met all inclusion criteria) was 61% men, with a mean age of 39 years and, on average, 15 years of education. When stratified by APOE ɛ4 status, the ɛ4+ and the ɛ4− groups did not differ significantly in any demographic or clinical variable (Table 1). APOE genotypes were distributed as follows: 1 case of ɛ2/ɛ2 (1%), 11 cases of ɛ2/ɛ3 (9%), 63 cases of ɛ3/ɛ3 (51%), 41 cases of ɛ3/ɛ4 (33%), 3 cases of ɛ2/ɛ4 (3%), and 4 cases of ɛ4/ɛ4 (3%). Overall, 39% of patients carried at least one APOE ɛ4 allele. Due to the relatively small sample size we were unable to test for differences between homozygote and heterozygote APOE ɛ4 carriers. There were no differences between the APOE ɛ4 groups between complicated or uncomplicated MTBI or in type of injury or location.
Table 1.
Demographics and injury related variables of apolipoprotein E (APOE) ɛ4 +/−mild traumatic brain injuries (MTBIs) n = 123.
| Variables | Overall | APOEɛ4(−) | APOEɛ4(+) | p-Value |
|---|---|---|---|---|
| (n = 123) | (n = 75) | (n = 48) | ||
| Age years, mean (SD) | 39.3 (14.0) | 40.7 (14.2) | 37.3 (13.6) | 0.19 |
| Gender (n, %) | ||||
| - male | 75 (61) | 45 (60) | 30 (62) | 0.78 |
| - female | 48 (39) | 30 (40) | 18 (38) | |
| Education (years) | 14.7 (2.8) | 14.9 (2.5) | 14.3 (3.3) | 0.33 |
| Mechanism of injury (n, %) | ||||
| - Traffic accidents | 52 (42) | 31 (41) | 21(43) | 0.63 |
| - Falls | 46 (37) | 31 (41) | 15 (31) | |
| - Violence | 13 (11) | 7 (10) | 6 (13) | |
| - Other | 12 (10) | 6 (8) | 6 (13) | |
| GCS | ||||
| 13 | 6 (5) | 4 (5) | 2 (4) | 0.55 |
| 14 | 29 (24) | 20(27) | 9 (19) | |
| 15 | 88 (71) | 51(68) | 37(77) | |
| LOC (n, %) | ||||
| - no | 25 (20) | 18 (24) | 7 (15) | 0.21 |
| - yes/unknown | 98 (80) | 57 (76) | 41(85) | |
| PTA (n, %) | ||||
| - no amnesia | 12 (10) | 9 (12) | 3 (6) | 0.29 |
| - yes/unknown | 111 (90) | 66 (88) | 45(94) | |
| Complicated | ||||
| -no | 67 (54) | 41 (55) | 26 (54) | 0.96 |
| - yes | 56 (46) | 34 (45) | 22 (46) | |
| Coil (n, %) | ||||
| -HNS | 37 (30) | 20 (27) | 17 (35) | 0.3 |
| -8HRBRAIN | 86 (70) | 55 (73) | 31 (65) |
GCS: Glasgow Coma Scale; LOC: loss of consciousness; PTA: Posttraumatic amnesia.: p-values: T-test for continuous variables; Chi square for categorical variables.
Table 2 presents means and standard deviation (SD) of GOSE, RPQ, and PHQ-9 assessed at 12 months follow-up for the two APOE groups. Results indicate overall good functional outcome (GOSE mean 7.2 (SD 0.82)), low symptom burden (RPQ mean 13.12 (SD 13.8)), and PHQ-9 mean 6.50 (SD 5.16). There were no significant differences between the APOE ɛ4+ and the ɛ4− groups.
Table 2.
Self-reported outcome measures of patients on APOE ɛ4 status.
| Variables | Overall (n = 123) |
APOEɛ4(−) (n = 75) |
APOEɛ4 (+) (n = 48) |
p-Value |
|---|---|---|---|---|
| RPQ total | ||||
| Mean (SD) | 13.12 (13.81) | 12.23 (13.54) | 14.52 (14.26) | 0.38 |
| GOSE | ||||
| Mean (SD) | 7.20 (.82) | 7.21 (.84) | 7.19 (.79) | 0.86 |
| PHQ 9 | ||||
| Mean (SD) | 6.50 (5.16) | 6.23 (4.83) | 6.94 (5.66) | 0.47 |
RPQ: Rivermead post-concussion symptoms questionnaire; GOSE: Glasgow Outcome Scale-Extended; PHQ-9: Patient Health Questionnaire 9; p-values: t-test for continuous variables. SD: standard deviation.
3.2. Brain-Age Prediction
The accuracies of the age prediction models are shown in Table 3. Within the training sample, the average R2 result presented mean ± standard deviation values of 0.82 ± 0.03 for the DTI model and 0.81 ± 0.03 for the model based on T1-weighted data. The results from the nested cross-validation approach for hyper-parameter optimization were similar to the main results, as shown in Table 3.
Table 3.
Brain age prediction.
| Model Performance—Training Sample (Cam-CAN) | ||||||
| Model | R2 | RMSE | MAE | R2nestedCV | RMSEnestedCV | MAEnestedCV |
| DTI | 0.82 ± 0.03 | 7.81 ± 0.48 | 6.17 ± 0.40 | 0.82 ± 0.03 | 7.75 ± 0.62 | 6.13 ± 0.52 |
| T1 | 0.81 ± 0.03 | 7.97 ± 0.50 | 6.26 ± 0.42 | 0.81 ± 0.02 | 8.07 ± 0.29 | 6.39 ± 0.21 |
| Model Performance—Test Sample (MTBI) | ||||||
| Model | R2 | RMSE | MAE | R2 corr | RMSE corr | MAE corr |
| DTI | 0.58 | 11.89 | 9.76 | 0.76 | 9.01 | 7.48 |
| T1 | 0.56 | 10.35 | 8.63 | 0.78 | 7.65 | 6.20 |
R2, root mean square error (RMSE), and mean absolute error (MAE) for the age prediction models within the training sample (the Cambridge Centre for Ageing and Neuroscience, Cam-CAN), and when applied to the test sample (MTBI patients). For the model validation within the training sample, means and standard deviations are provided based on 5-fold cross validations with 100 repetitions. NestedCV indicates the values based on nested cross-validation for hyperparameter optimization. For the test sample, R2, RMSE, and MAE are provided before and after age-bias correction (described in Section 2.5).
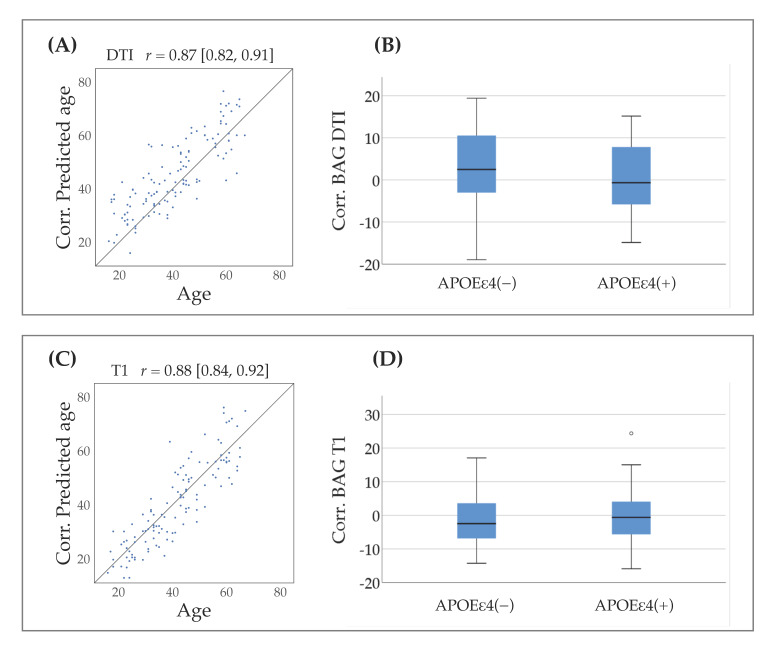
When the models were applied to the MTBI patients, the R2 values were 0.76 for the DTI model and 0.78 for the model based on T1-weighted data after age-bias correction was applied. The correlation between predicted and chronological age was r = 0.76 (95% confidence interval (CI): 0.68–0.83) for the DTI model and 0.74 (95% CI: 0.66–0.82) for the model based on T1-weighted data. Meanwhile, after age-bias correction, the correlations showed r = 0.87 (95% CI: 0.82–0.91) for the DTI model and r = 0.88 (95% CI: 0.84–0.92) for the model based on T1-weighted data, as shown in Figure 1.
Figure 1.
Brain-age prediction and BAG distributions per APOE group. (A) Chronoligcal age (x-axis) versus DTI-based corrected predicted age (y-axis; corrected for age-bias as described in Section 2.5). (B) The distribution of corrected DTI-based BAG estimated per APOE group (carriers versus non-carriers). (C) Chronological age (x-axis) versus T1-based corrected predicted age (y-axis). (D) The distribution of corrected T1-based BAG estimates per APOE group (carriers versus non-carriers). Abbreviations: Corr. = corrected, BAG = Brain-age gap.
3.3. Association between APOE Status and Brain-Age Gap
Table 4 summarizes the respective brain-age gaps in APOE ɛ4 carriers and non-carriers. Multiple regression analyses revealed no significant differences between the groups.
Table 4.
Multivariable regression of APOE ɛ4status and T1 and DTI brain age gap.
| Brain Age Gap | Comparison Groups | |||
|---|---|---|---|---|
| APOE-ɛ4(−) vs. APOE-ɛ4(+) | ||||
| B | SE | p-Value | R2 | |
| T1w-based: | 0.054 | |||
| -APOE ɛ4 | 0.790 | 1.404 | 0.58 | |
| -Age (per year) | 0.032 | 0.050 | 0.52 | |
| -Sex | 1.791 | 1.424 | 0.21 | |
| -Head coil | −3.328 | 1.508 | 0.03 | |
| DTI-based: | 0.119 | |||
| -APOE ɛ4 | −2.564 | 1.563 | 0.1 | |
| -Age (per year) | 0.131 | 0.056 | 0.02 | |
| -Sex | −3.614 | 1.586 | 0.02 | |
| -Head Coil | −3.425 | 1.679 | 0.04 | |
3.4. Association between APOE Status and Brain Morphometry
Table 5 summarizes the mean (SD) neuroanatomic volumes and cortical measures per region of interest (ROI) and Table 6 presents the mean value of cortical thickness per ROI at 12 months postinjury. Table 7 summarizes the results from the multiple regressions testing for group differences; no significant differences between groups were found.
Table 5.
APOE ɛ4 status on mean values of neuroanatomic volume measurements per region of interest (ROI).
| ROI | APOE-ɛ4(−) (n = 75) | APOE-ɛ4(+) (n = 48) | p-Value | ||
|---|---|---|---|---|---|
| Mean mm3 | SD mm3 | Mean mm3 | SD mm3 | ||
| Total ICV | 1.584767 | 152.613 | 1.571603 | 150.490 | 0.64 |
| Total gray volume | 662.282 | 66.495 | 664.349 | 64.095 | 0.87 |
| Cortex volume | 491.343 | 51.833 | 492.455 | 47.444 | 0.91 |
| L Accumbens area | 645 | 137 | 688 | 135 | 0.09 |
| R Accumbens area | 649 | 125 | 636 | 131 | 0.59 |
| L Amygdala | 1597 | 233 | 1607 | 195 | 0.81 |
| R Amygdala | 1843 | 249 | 1852 | 240 | 0.84 |
| Brainstem | 21,267 | 2108 | 20.485 | 2514 | 0.06 |
| L Caudate | 3815 | 573 | 3954 | 517 | 0.18 |
| R Caudate | 3999 | 629 | 4195 | 568 | 0.08 |
| CC-posterior | 990 | 147 | 982 | 168 | 0.78 |
| CC-mid-posterior | 432 | 91 | 402 | 89 | 0.08 |
| CC-central | 437 | 78 | 414 | 84 | 0.13 |
| CC-mid-anterior | 456 | 94 | 442 | 79 | 0.39 |
| CC-anterior | 905 | 144 | 890 | 141 | 0.58 |
| L Hippocampus | 4555 | 542 | 4480 | 478 | 0.44 |
| R Hippocampus | 4551 | 467 | 4576 | 446 | 0.78 |
| L Pallidum | 1337 | 296 | 1308 | 312 | 0.61 |
| R Pallidum | 1596 | 280 | 1558 | 291 | 0.47 |
| L Putamen | 60,441 | 892 | 6009 | 1009 | 0.84 |
| R Putamen | 5946 | 796 | 6009 | 856 | 0.68 |
| L Thalamus | 8612 | 1183 | 8603 | 1068 | 0.97 |
| R Thalamus | 6983 | 913 | 6975 | 900 | 0.96 |
| L lateral Ventricle | 9760 | 5507 | 10.717 | 6399 | 0.38 |
| R lateral Ventricle | 9068 | 6041 | 8983 | 4994 | 0.93 |
Abbreviations: ICV = intracranial volume, L = left, R = right, CC = corpus callosum, p-values are from the mean comparisons by the independent T-tests.
Table 6.
APOE ɛ4 status on the mean values of neuroanatomic thickness measurements per ROI.
| ROI | APOE-ɛ4(−) (n = 75) | APOE-ɛ4(+) (n = 48) | p-Value | ||
|---|---|---|---|---|---|
| Mean mm | SD mm | Mean mm | SD mm | ||
| L hemisphere, mean | 2.51 | 0.127 | 2.52 | 0.105 | 0.46 |
| R hemisphere, mean | 2.48 | 0.118 | 2.49 | 0.099 | 0.50 |
| L frontal | 2.51 | 0.145 | 2.51 | 0.126 | 0.95 |
| R frontal | 2.43 | 0.124 | 2.44 | 0.115 | 0.85 |
| L temporal | 2.95 | 0.151 | 2.97 | 0.136 | 0.45 |
| R temporal | 2.89 | 0.149 | 2.90 | 0.131 | 0.48 |
| L parietal | 2.29 | 0.132 | 2.30 | 0.139 | 0.67 |
| R parietal | 2.30 | 0.146 | 2.31 | 0.121 | 0.67 |
| L cingulate | 2.58 | 0.175 | 2.62 | 0.201 | 0.21 |
| R cingulate | 2.61 | 0.196 | 2.59 | 0.167 | 0.59 |
| L occipital | 2.04 | 0.111 | 2.05 | 0.100 | 0.55 |
| R occipital | 2.06 | 0.120 | 2.07 | 0.100 | 0.64 |
| L insula | 3.08 | 0.175 | 3.10 | 0.130 | 0.62 |
| R insula | 3.03 | 0.171 | 3.04 | 0.166 | 0.72 |
Abbreviations: L = left, R = right, p-values are from the mean comparisons by the independent T-tests.
Table 7.
Multivariable regression of APOE-ɛ4 status and neuroanatomic volume and thickness per ROI.
| ROI | Comparison Groups | |||
|---|---|---|---|---|
| APOE-ɛ4(−) vs. APOE-ɛ44(+) | ||||
| B | SE | p-Value | R2 | |
| Total ICV | −13698 | 23822 | 0.57 | 0.312 |
| Total gray volume | −1932.9 | 5208.5 | 0.71 | 0.825 |
| Cortex volume | −1125.3 | 4541.6 | 0.81 | 0.773 |
| L Accumbens area | 22.527 | 19.780 | 0.26 | 0.428 |
| R Accumbens area | −24.698 | 19.020 | 0.20 | 0.382 |
| L Amygdala | −6.604 | 33.963 | 0.85 | 0.334 |
| R Amygdala | 12.443 | 42.132 | 0.77 | 0.185 |
| Brain Stem | −710.59 | 306.40 | 0.02 | 0.512 |
| L Caudate | 95.763 | 77.147 | 0.22 | 0.468 |
| R Caudate | 135.66 | 83.163 | 0.11 | 0.492 |
| L Hippocampus | −91.780 | 74.287 | 0.22 | 0.434 |
| R Hippocampus | 9.805 | 67.792 | 0.89 | 0.399 |
| L Putamen | −143.81 | 124.25 | 0.25 | 0.516 |
| R Putamen | −53.235 | 97.863 | 0.59 | 0.606 |
| Left Thalamus | 34.182 | 170.03 | 0.84 | 0.384 |
| Right Thalamus | −25.139 | 125.03 | 0.84 | 0.475 |
| CC-posterior | 5.094 | 25.748 | 0.84 | 0.239 |
| CC-mid-poster | −30.256 | 16.590 | 0.07 | 0.089 |
| CC-central | −22.641 | 14.790 | 0.13 | 0.084 |
| CC-mid-anterior | −12.932 | 15.477 | 0.41 | 0.157 |
| CC-anterior | −7.022 | 24.002 | 0.77 | 0.216 |
| L Pallidum | −56.903 | 47.265 | 0.24 | 0.317 |
| R Pallidum | −6.0494 | 41.844 | 0.15 | 0.402 |
| L lateral Ventricle | 1669.5 | 945.24 | 0.08 | 0.287 |
| R lateral Ventricle | 555.08 | 933.86 | 0.55 | 0.246 |
| L hemisphere, mean thick | 0.006 | 0.019 | 0.75 | 0.304 |
| R hemisphere, mean thick | 0.005 | 0.018 | 0.80 | 0.279 |
| L frontal thick | −0.007 | 0.020 | 0.73 | 0.395 |
| R frontal thick | 0.001 | 0.019 | 0.95 | 0.266 |
| L temporal thick | 0.011 | 0.024 | 0.63 | 0.257 |
| R temporal thick | 0.008 | 0.024 | 0.73 | 0.235 |
| L parietal thick | −0.005 | 0.022 | 0.81 | 0.241 |
| R parietal thick | −0.001 | 0.023 | 0.96 | 0.208 |
| L cingulate thick | 0.024 | 0.027 | 0.37 | 0.424 |
| R cingulate thick | −0.029 | 0.028 | 0.30 | 0.351 |
| L occipital thick | 0.004 | 0.019 | 0.82 | 0.129 |
| R occipital thick | 0.002 | 0.020 | 0.94 | 0.129 |
| L insula thick | −0.004 | 0.026 | 0.89 | 0.266 |
| R insula thick | −0.007 | 0.028 | 0.81 | 0.231 |
Adjusted for total intracranial volume, sex, age, and head coil for volumes and adjusted for sex, age, and head coil for thickness. Abbreviations: L = left, R = right, CC = corpus callosum, Thick = thickness.
Multiple regressions revealed no significant between-group differences in total intracranial volume, left or right hemisphere thickness or total cortical volume. There was a nominally significant (p = 0.02) difference between APOE ɛ4 carriers as compared with non-carriers in brainstem volume, which did not remain after FDR adjustment.
3.5. Association between APOE and DTI Measures
Table 8 shows the results from ROI-based analyses. Multivariable regressions revealed lower FA in APOE ɛ4 carriers as compared with non-carriers in the hippocampal part of the cingulum bundle in the right hemisphere (p = 0.01), but this effect did not remain significant after FDR adjustment. No other significant group differences were found.
Table 8.
Multivariable regression of APOE-ɛ4 status and DTI (fractional anisotropy (FA) and mean diffusivity (MD)).
| ROI | Comparison Groups | |||
|---|---|---|---|---|
| APOE-ɛ4(−) vs. APOE-ɛ4(+) | ||||
| B | SE | p-Value | R2 | |
| FA-ATR L | 0.003 | 0.003 | 0.38 | 0.257 |
| FA-ATR R | 0.003 | 0.003 | 0.32 | 0.253 |
| FA-CG L | 0.001 | 0.004 | 0.87 | 0.317 |
| FA-CG R | 0.000 | 0.004 | 0.93 | 0.200 |
| FA-CING L | −0.011 | 0.005 | 0.03 | 0.214 |
| FA-CING R | −0.011 | 0.004 | 0.01 | 0.222 |
| FA-CST L | 0.001 | 0.003 | 0.83 | 0.253 |
| FA-CST R | −0.002 | 0.003 | 0.61 | 0.289 |
| FA-FMAJ | 0.002 | 0.003 | 0.59 | 0.244 |
| FA-FMIN | 0.004 | 0.004 | 0.26 | 0.430 |
| FA-IFOF L | 0.001 | 0.003 | 0.68 | 0.382 |
| FA-IFOF R | 0.004 | 0.003 | 0.24 | 0.315 |
| FA-ILF L | −0.001 | 0.003 | 0.79 | 0.348 |
| FA-ILF R | −0.001 | 0.003 | 0.86 | 0.274 |
| FA-SLF L | 0.002 | 0.003 | 0.44 | 0.296 |
| FA-SLF R | 0.002 | 0.003 | 0.41 | 0.328 |
| FA-SLFT L | 0.000 | 0.004 | 0.95 | 0.148 |
| FA-SLFT R | 0.002 | 0.003 | 0.60 | 0.277 |
| FA-UF L | 0.002 | 0.003 | 0.54 | 0.209 |
| FA-UF R | 0.004 | 0.003 | 0.18 | 0.206 |
| FA-CCBody | −0.005 | 0.006 | 0.41 | 0.249 |
| FA-CCGenu | 0.004 | 0.005 | 0.41 | 0.346 |
| FA-CCSplenium | 0.002 | 0.004 | 0.64 | 0.196 |
| FA-ws | 0.001 | 0.003 | 0.68 | 0.351 |
| MD-ATR L | 7.319 × 10−6 | 0.000 | 0.24 | 0.133 |
| MD-ATR R | 3.674 × 10−6 | 0.000 | 0.62 | 0.100 |
| MD-CG L | 8.128 × 10−8 | 0.000 | 0.99 | 0.033 |
| MD-CG R | −1.644 × 10−6 | 0.000 | 0.72 | 0.039 |
| MD-CING L | 1.377 × 10−5 | 0.000 | 0.08 | 0.215 |
| MD-CING R | 7.395 × 10−6 | 0.000 | 0.38 | 0.117 |
| MD-CST L | 4.134 × 10−6 | 0.000 | 0.36 | 0.132 |
| MD-CST R | 5.745 × 10−6 | 0.000 | 0.25 | 0.151 |
| MD-FMAJ | 1.775 × 10−7 | 0.000 | 0.97 | 0.362 |
| MD-FMIN | −2.931 × 10−6 | 0.000 | 0.66 | 0.191 |
| MD-IFOF L | −2.129 × 10−6 | 0.000 | 0.66 | 0.063 |
| MD-IFOF R | −5.288 × 10−6 | 0.000 | 0.29 | 0.100 |
| MD-ILF L | −2.673 × 10−6 | 0.000 | 0.60 | 0.060 |
| MD-ILF R | −5.404 × 10−6 | 0.000 | 0.29 | 0.066 |
| MD-SLF L | −7.339 × 10−7 | 0.000 | 0.86 | 0.029 |
| MD-SLF R | −1.968 × 10−6 | 0.000 | 0.65 | 0.051 |
| MD-SLFT L | −2.722 × 10−6 | 0.000 | 0.58 | 0.042 |
| MD-SLFT R | −5.847 × 10−6 | 0.000 | 0.29 | 0.154 |
| MD-UF L | −4.273 × 10−6 | 0.000 | 0.43 | 0.266 |
| MD-UF R | −2.661 × 10−6 | 0.000 | 0.64 | 0.207 |
| MD-CCBody | 5.241 × 10−6 | 0.000 | 0.46 | 0.100 |
| MD-CCGenu | −2.526 × 10−8 | 0.000 | 0.99 | 0.159 |
| MD-CCSplenium | 2.404 × 10−6 | 0.000 | 0.70 | 0.213 |
| MD-ws | 2.734 × 10−7 | 0.000 | 0.95 | 0.041 |
Adjusted for age, sex, and head coil. Abbreviations: fractional anisotropy (FA), mean diffusivity (MD), anterior thalamic radiation (ATR), cingulum (cingulate gyrus, CG), cingulum (hippocampus, CING), corticospinal tract (CST), forceps major (FMAJ), forceps minor (FMIN), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), superior longitudinal fasciculus (temporal part, SLFT), uncinate fasciculus (UF), corpus callosum (CC), whole skeleton (ws).
4. Discussion
The clinical outcome following MTBI is characterized by substantial individual variability and, while some patients suffer from long-term difficulties and decline, others experience only mild symptoms and a swift recovery. APOE ɛ4 is associated with an increased risk of unfavorable long-term (≥6 months) functional outcome [57] after TBI. Combining advanced brain imaging and information on genetic risk for neurodegenerative disorders may provide insight into the underlying pathophysiology related to functional impairment following MTBI.
APOE ɛ4 is the strongest genetic risk factor for AD and has also been associated with a greater risk for poor outcome following TBI. To date, the existing literature on APOE and TBI mostly covers functional outcome and there is a lack of studies on APOE in association with brain-structural changes and neurodegeneration following mild TBI. In this study, we combined different MRI approaches to study the associations between mild TBI, APOE ɛ4 status, and brain gray and white matter structure. In sum, our main analysis revealed no significant differences between APOE ɛ4 carriers and non-carriers in estimated brain-age gap, and follow-up analysis confirmed no significant differences in subcortical and cortical volumes, cortical thickness, or DTI metrics.
The lack of significant group differences may have several explanations. First, MRI was performed 12 months postinjury. The volume trajectory after MTBI is not known and available brain volumetric research focusing on MTBI is limited. A one-year interval between the injury and brain scan may be too short to identify long-term brain degeneration and atrophy, which may not happen until several years later [58]. The time point at which the brain begins atrophying after TBI and whether there are different long-term longitudinal brain changes for APOE ɛ4 carriers and non-carriers beyond the current interval of 12 months remain unclear. We did not observe differences in brain structures known to be involved in AD such as reductions in cortical thickness in the medial temporal lobe or hippocampus volumes. While APOE ɛ4 is the major genetic risk factor for late-onset AD [59], our results also underscore the need for powerful genome-wide and polygenic approaches to studying complex phenotypes such as clinical outcome and brain changes following TBI. Secondly, it could be that the measurements used in this study are not sensitive enough and that other objective markers of biological aging could provide additional information. For example, reduction in telomere length, a biomarker of cellular senescence and neurological health, is implicated in aging and neurodegenerative diseases (e.g., Alzheimer’s) [60] and has been associated with mild head injuries [61]. In a study by Li et al., they investigated other assessment of biological age like DNA methylation age estimator (DNAmAge), physiological age, cognitive function, functional aging index (FAI), and frailty index (FI) and found that the largest effects of mortality risk were seen for methylation age estimators and the FI [62]. There are also other biomarkers of interest when it comes to MTBI and aging pathobiology, like inflammatory biomarkers such as interferons, cytokines, and interleukins [63,64], and biomarkers for oxidative stress [65] which should be investigated further in future studies.
We did however find a difference in the brain-stem volume between the two APOE ɛ4 groups, albeit not a significant one. There is a lack of studies assessing mild TBI in this region and no studies have explored the association with APOE. Hemorrhagic lesions and axonal degeneration, however, can be seen in the brain stem after experimental mild to moderate neurotrauma [66]. Additionally, animal autopsy studies have shown that, even after very mild head trauma, microglia clusters indicative of axonal damage are most prominent in brain stem white matter [67]. Garman et al. [68] exposed body-shielded rats to a single blast exposure to examine neuropathological characterizations of the underlying brain tissue and found that, although neuronal and synaptic terminal degeneration improved over time, the primary pathology that persisted and worsened in the long-term was axonal injury and degeneration involving axons in the brain stems. Elsewhere, Delano-Wood et al. [69] found a link between the integrity of the brain stem’s white matter and injury severity. Brain-stem plaques were found in AD as well [70]. Taken together, these findings suggesting the brain stem as a possible vulnerable region in APOE ɛ4 (+) patients should be further examined.
DTI has been used to assess microstructural features of white matter [71]. Because of their length, axons are particularly vulnerable to mechanical injury and both clinical and experimental studies have repeatedly reported that mild, moderate, and severe TBI can cause axonal injury [72,73]. Postmortem case analysis has revealed diffuse axonal injury to be the most common kind of damage apparent after MTBI [74], while neuropathological features of dementia—for example, β-amyloid burden and neurofibrillary tangles—follow persistent axonal degeneration [75]. FA is the most commonly used DTI measure and is affected by many factors, including axonal degeneration, demyelination, disorganization, packing density, and other microstructural features, and should be interpreted as an indirect proxy of white matter integrity. Although not significant after FDR adjustment, we found lower FA in the left and right cingulum hippocampi in the APOE ɛ4 (+) group. Investigations of white matter connectivity changes in aging and AD have focused on the fornix and the cingulum as the major links between the limbic system and the rest of the brain. Specifically, the cingulum connects the cingulate and the parahippocampal gyri to the septal cortex and several studies have reported white matter changes in the cingulum in mild cognitive impairment and mild AD cases [76,77,78]. In a review and meta-analysis looking into the relationship between white-matter integrity and posttraumatic cognitive deficits after TBI, the fornix and the cingulum were particularly strongly associated with the memory domain [79]. Although not significant after FDR adjustment our results may suggest that APOE ɛ4 carriers are vulnerable to reduced neuronal integrity in the cingulum, which is associated with memory and, further, with mild cognitive impairment and AD; therefore, we will investigate the association between APOE ɛ4 and cognitive function in future work. In this study, e.g., proof of concept for the neuroimaging and APOE biomarkers, we will provide in-depth analysis of association between post-traumatic symptoms, cognitive function, MRI, and APOE in a subsequent publication.
5. Strengths and Limitations
The strengths of this study include its prospective recruitment from consecutive admissions to a single regional trauma center and the use of multimodal brain MRI. However, the study also has several limitations. First, the study population was drawn from neurosurgical admissions and therefore, most likely, includes cases on the more severe end of the MTBI spectrum. However, no evidence of an interaction between severity and APOE ɛ4 (+/−) status was found. Secondly, this study was limited by a relatively low power in particular for testing of differences between heterozygote and homozygote APOE ɛ4 carriers. Although our selective recruitment of patients without drug abuse, mental illness, or a lack of Norwegian language skills limits the generalizability of the results, the careful exclusion of these factors and control for confounding variables allows for greater confidence in drawing conclusions. Thirdly, because of the lack of a control group either without injury or with non-head injury, we were unable to determine whether the results are specific to MTBI. The cross-sectional design is also a limitation and longitudinal studies are needed to explore the time course of brain structural changes.
6. Conclusions
Our analyses revealed no significant associations between brain-age gap, volumetric measures, or cortical thickness and APOE ɛ4 status. The suggestive lower FA in the hippocampal part of the cingulum in APOE ɛ4 carriers suggests that this region could be vulnerable in APOE ɛ4 carriers following MTBI and it is therefore important to pursue this finding in larger cohort studies and meta-analyses.
Acknowledgments
The authors thank all the patients participated in the study.
Abbreviations
Axial diffusivity (AD), radial diffusivity (RD), anterior thalamic radiation (ATR), cingulum (cingulate gyrus, CG), cingulum (hippocampus, CING), corticospinal tract (CST), forceps major (FMAJ), forceps minor (FMIN), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), superior longitudinal fasciculus (temporal part, SLFT), uncinate fasciculus (UF), corpus callosum (CC), whole skeleton (ws).
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/3/418/s1, Table S1: Multivariable regression of APOEƐ4 status and DTI (AD and RD).
Author Contributions
Conceptualization: T.H., L.T.W., and N.A.; analysis and interpretation of data: T.H., L.T.W., A.-M.G.d.L., K.E., and N.A.; drafting of the manuscript: T.H., L.T.W., A.-M.G.d.L., E.H. and N.A.; critical revision of the manuscript for important intellectual content: T.H., L.T.W., E.H., and N.A.; statistical analysis: T.H., L.T.W., A.-M.G.d.L., and N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the Research Council of Norway, the South-Eastern Norway Regional Health Authority, and the European Research Council under the European Union’s Horizon 2020 research and Innovation program (ERCStG, Grant 802998).
Institutional Review Board Statement
All study protocols were approved by the Norwegian Regional Committee for Medical Research Ethics (2010/1899) (Oslo, Norway).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data in this study are available on request from the corresponding author. The data are not publicly available due to data privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dikmen S., Machamer J., Fann J.R., Temkin N.R. Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. 2010;16:401–411. doi: 10.1017/S1355617710000196. [DOI] [PubMed] [Google Scholar]
- 2.Polinder S., Cnossen M.C., Real R.G.L., Covic A., Gorbunova A., Voormolen D.C., Master C.L., Haagsma J.A., Diaz-Arrastis R., von Steinbügel N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018;9:1113. doi: 10.3389/fneur.2018.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigurdardottir S., Andelic N., Roe C., Jerstad T., Schanke A.K. Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: A prospective study. Brain Inj. 2009;23:489–497. doi: 10.1080/02699050902926309. [DOI] [PubMed] [Google Scholar]
- 4.Finnoff J.T., Jelsing E.J., Smith J. Biomarkers, genetics, and risk factors for concussion. J. Inj. Funct. Rehabil. 2011;3(Suppl. 2):S452–S459. doi: 10.1016/j.pmrj.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Bartzokis G., Lu P.H., Geschwind D.H., Edwards N., Mintz J., Cummings J.L. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: Implications for cognitive decline and dementia. Arch. Gen. Psychiatry. 2006;63:63–72. doi: 10.1001/archpsyc.63.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Samatovicz R.A. Genetics and brain injury: Apolipoprotein E. J. Head Trauma Rehabil. 2000;15:869–874. doi: 10.1097/00001199-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mahley R.W., Huang Y. Apolipoprotein e sets the stage: Response to injury triggers neuropathology. Neuron. 2012;76:871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Den Heuvel C., Thornton E., Vink R. Traumatic brain injury and Alzheimer’s disease: A review. Prog. Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 9.Liberman J.N., Stewart W.F., Wesnes K., Troncoso J. Apolipoprotein E epsilon 4 and short-term recovery from predominantly mild brain injury. Neurology. 2002;58:1038–1044. doi: 10.1212/WNL.58.7.1038. [DOI] [PubMed] [Google Scholar]
- 10.Mueller S.G., Weiner M.W. Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus. 2009;19:558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenihan M.W., Jordan B.D. The clinical presentation of chronic traumatic encephalopathy. Curr. Neurol. Neurosci. Rep. 2015;15:23. doi: 10.1007/s11910-015-0541-5. [DOI] [PubMed] [Google Scholar]
- 12.Nicoll J.A., Roberts G.W., Graham D.I. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat. Med. 1995;1:135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- 13.Hayes J.P., Logue M.W., Sadeh N., Spielberg J.M., Verfaellie M., Hayes S.M., Reagan A., Salat D.H., Wolf E.J., McGlinchey R.E., et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain J. Neurol. 2017;140:813–825. doi: 10.1093/brain/aww344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll L.J., Cassidy J.D., Peloso P.M., Borg J., von Holst H., Holm L., Paniak C., Pépin M. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;14(Suppl. 43):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 15.Godbolt A.K., Cancelliere C., Hincapié C.A., Marras C., Boyle E., Kristman V.L., Coronado V.G., Cassidy J.D. Systematic review of the risk of dementia and chronic cognitive impairment after mild traumatic brain injury: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 2014;95(Suppl. 3):S245–S256. doi: 10.1016/j.apmr.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Mehta K.M., Ott A., Kalmijn S., Slooter A.J., van Duijn C.M., Hofman A., Breteler M.M. Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology. 1999;53:1959–1962. doi: 10.1212/WNL.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 17.Graves A.B., White E., Koepsell T.D., Reifler B.V., van Belle G., Larson E.B., Raskind M. The association between head trauma and Alzheimer’s disease. Am. J. Epidemiol. 1990;131:491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- 18.Schofield P.W., Tang M., Marder K., Bell K., Dooneief G., Chun M., Sano M., Stern Y., Mayeux R. Alzheimer’s disease after remote head injury: An incidence study. J. Neurol. Neurosurg. Psych. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmes E., Østbye T., Steenhuis R.E. Incremental contribution of reported previous head injury to the prediction of diagnosis and cognitive functioning in older adults. Brain Inj. 2011;25:338–347. doi: 10.3109/02699052.2011.556104. [DOI] [PubMed] [Google Scholar]
- 20.Yue J.K., Robinson C.K., Burke J.F., Winkler E.A., Deng H., Cnossen M.C., Lingsma H.F., Ferguson A.R., McAllister T.W., Rosand J., et al. Apolipoprotein E epsilon 4 (APOE-ε4) genotype is associated with decreased 6-month verbal memory performance after mild traumatic brain injury. Brain Behav. 2017;7:e00791. doi: 10.1002/brb3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck D., de Lange A.G., Maximov I.I., Richard G., Andreassen O.A., Nordvik J.E., Westlye L.T. White matter microstructure across the adult lifespan: A mixed longitudinal and cross-sectional study using advanced diffusion models and brain-age prediction. NeuroImage. 2020;224:117441. doi: 10.1016/j.neuroimage.2020.117441. [DOI] [PubMed] [Google Scholar]
- 22.Westlye L.T., Walhovd K.B., Dale A.M., Bjørnerud A., Due-Tønnessen P., Engvig A., Grydeland H., Tamnes C.K., Ostby Y., Fjell A.M. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- 23.Westlye L.T., Reinvang I., Rootwelt H., Espeseth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79:1961–1969. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan D.R., Logue M.W., Wolf E.J., Hayes J.P., Salat D.H., Fortier C.B., Fonda J.R., McGlinchey R.E., Milberg W.P., Miller M.W. Close-Range Blast Exposure Is Associated with Altered White Matter Integrity in Apolipoprotein ɛ4 Carriers. J. Neurotrauma. 2019;36:3264–3273. doi: 10.1089/neu.2019.6489. [DOI] [PubMed] [Google Scholar]
- 25.Cole J.H., Franke K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 2017;40:681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Cole J.H., Poudel R.P.K., Tsagkrasoulis D., Caan M.W.A., Steves C., Spector T.D., Montana G. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage. 2017;163:115–124. doi: 10.1016/j.neuroimage.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 27.James H., Cole K.F., Cherbuin N. Biomarkers of Human Aging. Springer; Cham, Switzerland: 2019. [Google Scholar]
- 28.Smith S.M., Elliott L.T., Alfaro-Almagro F., McCarthy P., Nichols T.E., Douaud G., Miller K.L. Brain aging comprises many modes of structural and functional change with distinct genetic and biophysical associations. eLife. 2020;9:e52677. doi: 10.7554/eLife.52677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole J.H. Multimodality neuroimaging brain-age in UK biobank: Relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging. 2020;92:34–42. doi: 10.1016/j.neurobiolaging.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lange A.G., Anatürk M., Suri S., Kaufmann T., Cole J.H., Griffanti L., Zsoldos E., Jensen D.E.A., Filippini N., Singh-Manoux A., et al. Multimodal brain-age prediction and cardiovascular risk: The Whitehall II MRI sub-study. NeuroImage. 2020;222:117292. doi: 10.1016/j.neuroimage.2020.117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann T., van der Meer D., Doan N.T., Schwarz E., Lund M.J., Agartz I., Alnæs D., Barch D.M., Baur-Streubel R., Bertolino A., et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019;22:1617–1623. doi: 10.1038/s41593-019-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han L.K.M., Dinga R., Hahn T., Ching C.R.K., Eyler L.T., Aftanas L., Aghajani M., Aleman A., Baune B.T., Berger K., et al. Brain aging in major depressive disorder: Results from the ENIGMA major depressive disorder working group. Mol. Psychiatry. 2020:1–16. doi: 10.1038/s41380-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole J.H., Marioni R.E., Harris S.E., Deary I.J. Brain age and other bodily ‘ages’: Implications for neuropsychiatry. Mol. Psychiatry. 2019;24:266–281. doi: 10.1038/s41380-018-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole D.J., Raffel J., Friede D.T., Eshaghi D.A., Brownlee D.F.W.J., Chard D.D., Stefano. N.D., Enzinger C., Pirpamer L., Filippi M., et al. Longitudinal Assessment of Multiple Sclerosis with the Brain-Age Paradigm. Ann. Neurol. 2020;88:93–105. doi: 10.1002/ana.25746. [DOI] [PubMed] [Google Scholar]
- 35.Cole J.H., Leech R., Sharp D.J. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015;77:571–581. doi: 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mild Traumatic Brain Injury Committee. American Congress of Rehabilitation Medicine. Group HIISI Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. doi: 10.1097/00001199-199309000-00010. [DOI] [Google Scholar]
- 37.Hellstrøm T., Westlye L.T., Server A., Løvstad M., Brunborg C., Lund M.J., Nordhøy W., Andreassen O.A., Andelic N. Volumetric and morphometric MRI findings in patients with mild traumatic brain injury. Brain Inj. 2016;30:1683–1691. doi: 10.1080/02699052.2016.1199905. [DOI] [PubMed] [Google Scholar]
- 38.Hellstrøm T., Westlye L.T., Sigurdardottir S., Brunborg C., Soberg H.L., Holthe Ø., Server A., Lund M.J., Andreassen O.A., Andelic N. Longitudinal changes in brain morphology from 4 weeks to 12 months after mild traumatic brain injury: Associations with cognitive functions and clinical variables. Brain Inj. 2017;31:674–685. doi: 10.1080/02699052.2017.1283537. [DOI] [PubMed] [Google Scholar]
- 39.Hellstrøm T., Westlye L.T., Kaufmann T., Trung Doan N., Søberg H.L., Sigurdardottir S., Nordhøy W., Helseth E., Andreassen O.A., Andelic N. White matter microstructure is associated with functional, cognitive and emotional symptoms 12 months after mild traumatic brain injury. Sci. Rep. 2017;7:13795. doi: 10.1038/s41598-017-13628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 43.Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 44.Sexton C.E., Kalu U.G., Filippini N., Mackay C.E., Ebmeier K.P. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging. 2011;32:2322.e5–2322.e18. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Richard G., Kolskår K., Sanders A.M., Kaufmann T., Petersen A., Doan N.T., Sánches J.M., Alnæs D., Ulrichsen K.M., Dørum E.S., et al. Assessing distinct patterns of cognitive aging using tissue-specific brain age prediction based on diffusion tensor imaging and brain morphometry. PeerJ. 2018;6:e5908. doi: 10.7717/peerj.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richard G., Kolskår K., Ulrichsen K.M., Kaufmann T., Alnæs D., Sanders A.M., Dørum E.S., Sánches J.M., Petersen A., Ihle-Hansen H., et al. Brain age prediction in stroke patients: Highly reliable but limited sensitivity to cognitive performance and response to cognitive training. NeuroImage Clin. 2020;25:102159. doi: 10.1016/j.nicl.2019.102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shafto M.A., Tyler L.K., Dixon M., Taylor J.R., Rowe J.B., Cusack R., Calder A.J., Marslen-Wilson W.D., Duncan J., Dalgleish T., et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014;14:204. doi: 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor J.R., Williams N., Cusack R., Auer T., Shafto M.A., Dixon M., Tyler L.K., Cam-CAN. Henson R.N. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage. 2017;144:262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Tianqi G.C. XGBoost: A Scalable Tree Boosting System. ACM Digit. Libr. 2016:785–794. doi: 10.1145/2939672.2939785. [DOI] [Google Scholar]
- 50.de Lange A.G., Cole J.H. Commentary: Correction procedures in brain-age prediction. NeuroImage Clin. 2020;26:102229. doi: 10.1016/j.nicl.2020.102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith S.M., Vidaurre D., Alfaro-Almagro F., Nichols T.E., Miller K.L. Estimation of brain age delta from brain imaging. NeuroImage. 2019;200:528–539. doi: 10.1016/j.neuroimage.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 53.Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 54.Wilson J.T., Pettigrew L.E., Teasdale G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 55.King N.S., Crawford S., Wenden F.J., Moss N.E., Wade D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L., Bao Y., He S., Wang G., Guan Y., Ma D., Wu R., Wang P., Huang X., Tao S., et al. The Association Between Apolipoprotein E and Functional Outcome After Traumatic Brain Injury: A Meta-Analysis. Medicine. 2015;94:e2028. doi: 10.1097/MD.0000000000002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris T.C., de Rooij R., Kuhl E. The Shrinking Brain: Cerebral Atrophy Following Traumatic Brain Injury. Ann. Biomed. Eng. 2019;47:1941–1959. doi: 10.1007/s10439-018-02148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wightman D.P., Jansen I.E., Savage J.E., Shadrin A.A., Bahrami S., Rongve A., Børte S., Winsvold B.S., Drange O.K., Martinsen A.E., et al. Largest GWAS (N=1,126,563) of Alzheimer’s Disease Implicates Microglia and Immune Cells. medRxiv. 2020 doi: 10.1101/2020.11.20.20235275. [DOI] [Google Scholar]
- 60.Blasco M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 61.Symons G.F., Clough M., O’Brien W.T., Ernest J., Salberg S., Costello D., Sun M., Brady R.D., McDonald S.J., Wright D.K., et al. Shortened telomeres and serum protein biomarker abnormalities in collision sport atheletes regardless of concussion history and sex. J. Concussion. 2020;4:1–11. doi: 10.1177/2059700220975609. [DOI] [Google Scholar]
- 62.Li X., Ploner A., Wang Y., Magnusson P.K., Reynolds C., Finkel D., Pedersen N.L., Jylhävä J., Hägg S. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9:e51507. doi: 10.7554/eLife.51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Battista A.P., Churchill N., Rhind S.G., Richards D., Hutchison M.G. The relationship between symptom burden and systemic inflammation differs between male and female athletes following concussion. BMC Immunol. 2020;21:11. doi: 10.1186/s12865-020-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Brien W.T., Symons G.F., Bain J., Major B.P., Costello D.M., Sun M., Kimpton J.S., Chen Z., Brady R.D., Mychasiuk R., et al. Elevated serum interleukin-1β levels in male, but not female, collision sport athletes with a concussion history. J. Neurotrauma. 2020 doi: 10.1089/neu.2020.7479. [DOI] [PubMed] [Google Scholar]
- 65.Major B.P., McDonald S.J., O’Brien W.T., Symons G.F., Clough M., Costello D., Sun M., Brady R.D., Mccullough J., Aniceto R., et al. Serum Protein Biomarker Findings Reflective of Oxidative Stress and Vascular Abnormalities in Male, but Not Female, Collision Sport Athletes. Front. Neurol. 2020;11:549624. doi: 10.3389/fneur.2020.549624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Povlishock J.T., Becker D.P., Miller J.D., Jenkins L.W., Dietrich W.D. The morphopathologic substrates of concussion? Acta Neuropathol. 1979;47:1–11. doi: 10.1007/BF00698266. [DOI] [PubMed] [Google Scholar]
- 67.Browne K.D., Chen X.H., Meaney D.F., Smith D.H. Mild traumatic brain injury and diffuse axonal injury in swine. J. Neurotrauma. 2011;28:1747–1755. doi: 10.1089/neu.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garman R.H., Jenkins L.W., Switzer R.C., 3rd, Bauman R.A., Tong L.C., Swauger P.V., Parks S.A., Ritzel D.V., Dixon C.E., Clark R.S.B., et al. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- 69.Delano-Wood L., Bangen K.J., Sorg S.F., Clark A.L., Schiehser D.M., Luc N., Bondi M.W., Werhane M., Kim R.T., Bigler E.D. Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging Behav. 2015;9:500–512. doi: 10.1007/s11682-015-9432-2. [DOI] [PubMed] [Google Scholar]
- 70.Iseki E., Matsushita M., Kosaka K., Kondo H., Ishii T., Amano N. Distribution and morphology of brain stem plaques in Alzheimer’s disease. Acta Neuropathol. 1989;78:131–136. doi: 10.1007/BF00688200. [DOI] [PubMed] [Google Scholar]
- 71.Taylor W.D., Hsu E., Krishnan K.R., MacFall J.R. Diffusion tensor imaging: Background, potential, and utility in psychiatric research. Biol. Psychiatry. 2004;55:201–207. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Bramlett H.M., Dietrich W.D. Progressive damage after brain and spinal cord injury: Pathomechanisms and treatment strategies. Prog. Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- 73.Sharp D.J., Ham T.E. Investigating white matter injury after mild traumatic brain injury. Curr. Opin. Neurol. 2011;24:558–563. doi: 10.1097/WCO.0b013e32834cd523. [DOI] [PubMed] [Google Scholar]
- 74.Bigler E.D. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J. Int. Neuropsychol. Soc. JINS. 2004;10:794–806. doi: 10.1017/S1355617704105146. [DOI] [PubMed] [Google Scholar]
- 75.Scott G., Ramlackhansingh A.F., Edison P., Hellyer P., Cole J., Veronese M., Leech R., Greenwood R.J., Turkheimer F.E., Gentleman S.M., et al. Amyloid pathology and axonal injury after brain trauma. Neurology. 2016;86:821–828. doi: 10.1212/WNL.0000000000002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choo I.H., Lee D.Y., Oh J.S., Lee J.S., Lee D.S., Song I.C., Youn J.C., Kim S.G., Kim K.W., Jhoo J.H., et al. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging. 2010;31:772–779. doi: 10.1016/j.neurobiolaging.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 77.Firbank M.J., Blamire A.M., Krishnan M.S., Teodorczuk A., English P., Gholkar A., Harrison R., O’Brien J.T. Atrophy is associated with posterior cingulate white matter disruption in dementia with Lewy bodies and Alzheimer’s disease. NeuroImage. 2007;36:1–7. doi: 10.1016/j.neuroimage.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 78.Villain N., Desgranges B., Viader F., de la Sayette V., Mézenge F., Landeau B., Landeau B., Baron J.-C., Eustache F., Chételat G. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J. Neurosci. 2008;28:6174–61781. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J., Tian L., Zhang L., Cheng R., Wei R., He F., Li J., Luo B., Ye X. Relationship between white matter integrity and post-traumatic cognitive deficits: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2019;90:98–107. doi: 10.1136/jnnp-2017-317691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are available on request from the corresponding author. The data are not publicly available due to data privacy and ethical restrictions.