Abstract
Pathological angiogenesis is a hallmark of cancer; accordingly, a number of anticancer FDA-approved drugs act by inhibiting angiogenesis via different mechanisms. However, the development process of the most potent anti-angiogenics has met various hurdles including redundancy, multiplicity, and development of compensatory mechanisms by which blood vessels are remodeled. Moreover, identification of broad-spectrum anti-angiogenesis targets is proved to be required to enhance the efficacy of the anti-angiogenesis drugs. In this perspective, a proper understanding of the structure activity relationship (SAR) of the recent anti-angiogenics is required. Various anti-angiogenic classes have been developed over the years; among them, the heterocyclic organic compounds come to the fore as the most promising, with several drugs approved by the FDA. In this review, we discuss the structure–activity relationship of some promising potent heterocyclic anti-angiogenic leads. For each lead, a molecular modelling was also carried out in order to correlate its SAR and specificity to the active site. Furthermore, an in silico pharmacokinetics study for some representative leads was presented. Summarizing, new insights for further improvement for each lead have been reviewed.
Keywords: anticancer, heterocyclic, anti-angiogenics, structure–activity relationship, in silico pharmacokinetics, molecular modelling
1. Introduction
As the second leading cause of mortality globally, cancer has become the focus for extensive research [1,2]. Although cancer progression and metastasis consist of multiple, complex, interacting, and interdependent steps [3], angiogenesis plays an essential part of the tumor’s growth and metastasis [4], owing to the fact that growth beyond the size of l–2 mm3 requires tumors to develop an adequate blood supply [5,6]. Angiogenesis is the processes whereby new blood and lymphatic vessels form [7]. Angiogenesis and its induction remain a major hallmark of cancer as it flourishes nutrient-deprived tumors with oxygen and nutrients, thus routing tumor metastasis [8,9].
Under normal conditions, angiogenesis is essential for formation of a new vascular network to supply nutrients, oxygen, and immune cells, as well as to remove waste products [10]. This process is regulated by a balance between pro- and anti-angiogenic molecules, and once that delicate balance is disturbed [11], it could lead to various diseases, especially cancer [12]. Angiogenesis is a vital mediator of tumor development [13]. As tumors enlarge, diffusion distances from the current vascular supply rise, leading to hypoxia [14]. Continued expansion of a tumor mass needs new blood vessel formation to offer rapidly proliferating tumor cells with a suitable supply of oxygen and metabolites [15]. Without the proper blood supply, tumors cannot grow beyond a critical size or metastasize to another organ [16]. Therefore, targeting angiogenesis is one of the most effective ways to stop a tumor progression [17].
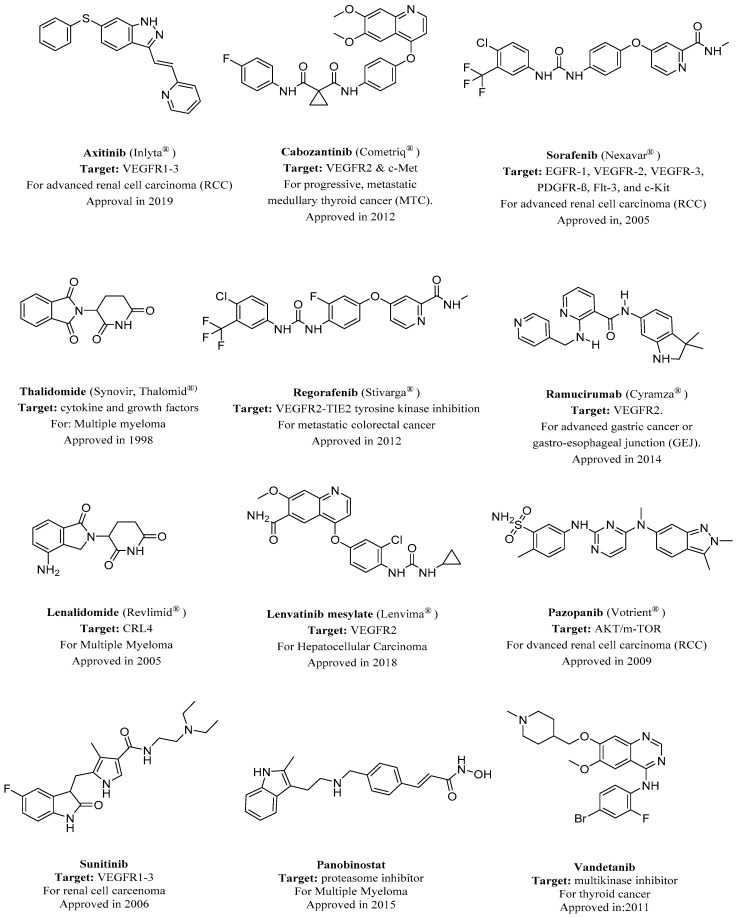
Most of the drugs that have been developed to combat angiogenesis have heterocyclics in their backbone as a common feature. This is demonstrated by heterocyclics occupying the major part of the number of FDA-approved anti-angiogenic drugs, as demonstrated in Figure 1. This domination of FDA-approved drugs is likely due to the wide availability of various heterocyclic fragments that have different potency, physicochemical properties, and lipophilicity. This versatility makes heterocyclic fragments a sound rational choice when developing new drugs or altering an existing one.
Figure 1.
FDA-approved angiogenesis inhibitors for treatment of cancer [18,19]. VEGFR, vascular endothelial growth factor receptor.
In order to develop more potent and targeted anti-angiogenics, a proper understanding of their structure–activity relationship is required. In this review, we discuss some of the most potent heterocyclic anti-angiogenics. In each class of heterocyclics, we illustrate the structure–activity relationship (SAR) of some promising leads that have been chosen thanks to their future potential for development into more potent anti-angiogenics as well as the availability of enough supporting data to predict their SAR. A molecular modeling was then conducted to illuminate how these leads interact with their active sites and which parts should be improved in order to obtain a more potent and specific drug candidate.
2. Nitrogen-Based Heterocycles
The number of anti-cancer candidates possessing a nitrogen heterocycle is an indicator of the structural significance of nitrogen-based heterocycles in the fight against cancer [20,21,22]. More than 75% of drugs approved by the FDA and currently available in the market are nitrogen-containing heterocyclic moieties [23]. One of the most important targets in angiogenesis inhibition is vascular endothelial growth factor receptors (VEGFRs).
Vascular endothelial growth factor is an important signaling protein involved in both vasculogenesis (the formation of the circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature) [24,25]. As its name implies, VEGF activity is restricted mainly to cells of the vascular endothelium [26]. The expression of VEGF is potentiated in response to hypoxia, by activated oncogenes, and by a variety of cytokines [27]. VEGF induces endothelial cell proliferation, promotes cell migration, and inhibits apoptosis [28]. VEGF induces angiogenesis as well as permeabilization of blood vessels, and plays a central role in the regulation of vasculogenesis [29,30].
Deregulated VEGF expression contributes to the development of solid tumors by promoting tumor angiogenesis and to the etiology of several additional diseases characterized by abnormal angiogenesis [31]. Consequently, inhibition of VEGF signaling abrogates the development of a wide variety of tumors [32]. All members of the VEGF family stimulate cellular responses by binding to tyrosine kinase receptors (the VEGFRs) on the cell surface, causing them to dimerize and become activated through transphosphorylation [33,34].
Several nitrogen-based heterocyclic drugs have been used to inhibit VEGF, for example, agents that inhibit the VEGFR tyrosine kinase such as the pyrrolidinone-based Sunitinib were approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor [35,36]. Another example is the phthalazine-based vatalanib, which is under investigation for the treatment of metastatic colorectal cancer and non-small cell lung cancer (NSCLC) [37,38]. Indole ring is one of the nitrogen-based heterocyclics that have been involved in VEGF inhibition [39]. It has demonstrated the ability to inhibit the proliferation, growth, and invasion of human cancer cells. Panobinostat is an indole-based drug approved in 2015 for the treatment of multiple myeloma [40].
Renhowe et al. synthesized a series of quinolin-2-one analogues that showed promising VEGFR-2 inhibition activity (Table 1) [41]. Upon examining the series of analogues synthesized by Renhowe et al., it was found that the free NH of the hydroquinolin-2-one scaffold (Figure 2), the quinolinone carbonyl, and the benzimidazole NH formed donor–acceptor motifs that would bind to the hinge region of the VEGFR-2, which could be implicated in tumor vasculature formation and maintenance [42,43]. The importance of a hydrogen donating group was found through substituting different groups at the C4 position, observing the potency increasing with (NH2 > OH > H). The incorporation of large basic amine at the C4 position such as an amino quinuclidine resulted in much more potent activity; however, it negatively affected the pharmacokinetics. Therefore, the NH2 substitution at C4 was maintained and subsequent efforts focused on modifying the substitution on both rings A and D to attain additional enzymatic affinity and to improve physicochemical properties.
Table 1.
In vitro inhibition of vascular endothelial growth factor receptor 2 (VEGFR-2) by the quinolin-2-one analogues (1–10) [41].
| Compound | Y | R1 | R2 | VEGFR-2 (IC50 μM) | PDGFRβ (IC50 μM) |
|---|---|---|---|---|---|
| 1 | OH | H | H | 0.24 | 0.020 |
| 2 | NH2 | H | H | 0.058 | 0.010 |
| 3 | NHMe | H | H | 0.22 | 0.030 |
| 4 |
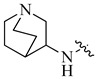
|
H | H | 0.17 | 0.0002 |
| 5 | H |
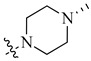
|
H | 0.017 | 0.0003 |
| 6 | NH2 |
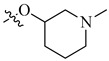
|
H | 0.005 | 0.0001 |
| 7 | NH2 | H | Me | 0.22 | 0.030 |
| 8 | NH2 | H |
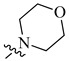
|
0.057 | 0.005 |
| 9 | NH2 | H |
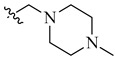
|
0.027 | 0.002 |
| 10 | NH2 | H |
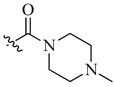
|
0.026 | 0.0009 |
Figure 2.
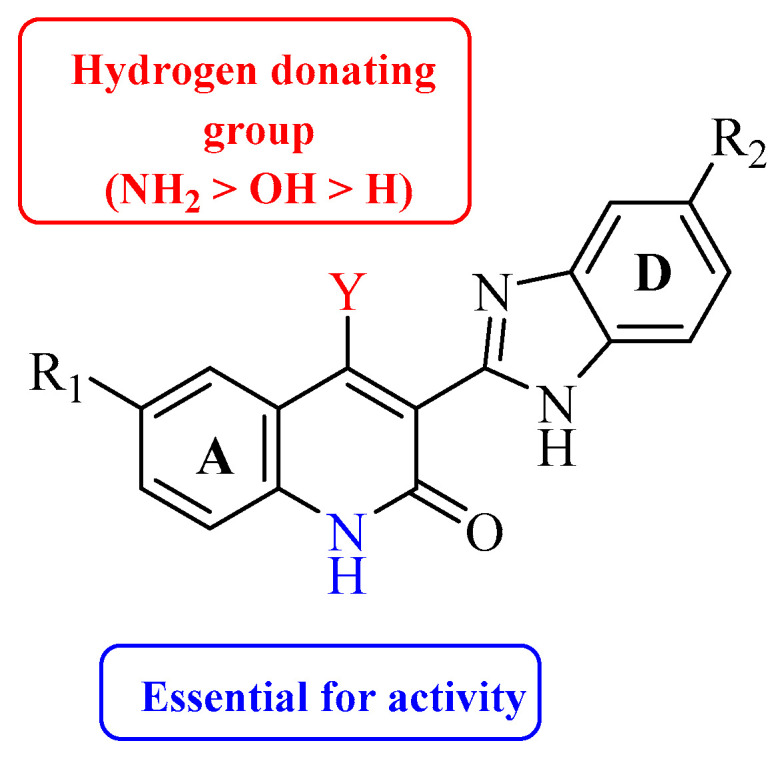
Structure–activity relationship study of 3-benzimidazol-2- ylhydroquinolin-2-one derivatives.
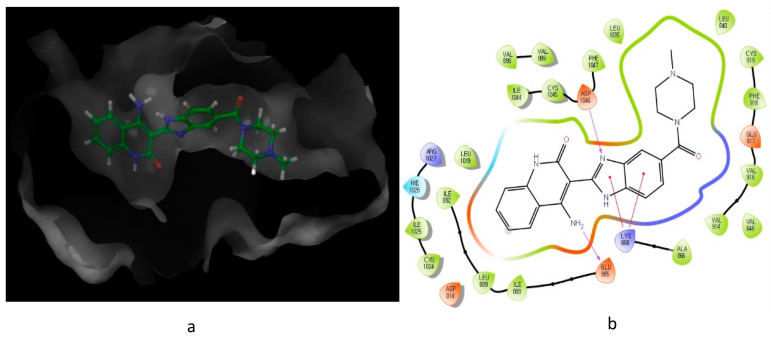
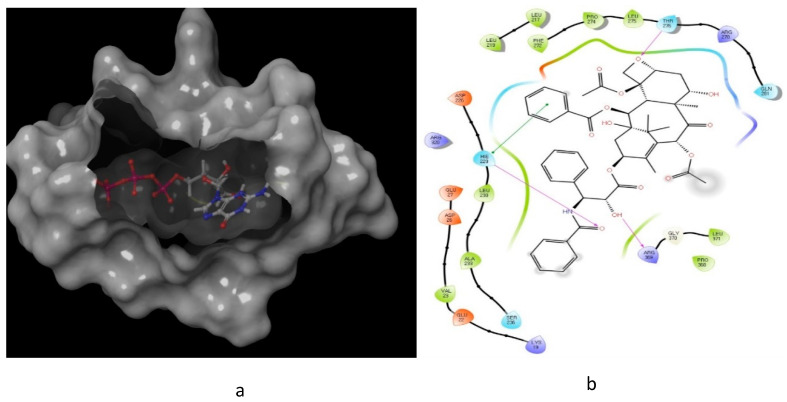
As illustrated in Figure 3, the interaction of compound 10 with VEGFR-2 receptor active site (IC50 = 0.026 μM) was subjected to docking studies using Schrodinger maestro. The modelling simulation shows a significant docking score of −9.058, along with the formation of two hydrogens bonds as well as two π–cation bonds. These interactions were found to be energetically significant as all the formed bonds among the donor and acceptor atoms are within about 3.7 Å.
Figure 3.
Predicted interaction of compound 10 (imidazole-scaffold) with active site residues of VEGFR-2. (a) 3D structural view of ligand inside the receptor’s active site. (b) Ligand interaction diagram; violet lines represent hydrogen bonds and red lines represent π–cation interactions.
Although VEGF is an important pathway in angiogenesis, several other targets are also involved in angiogenesis. Matrix metalloproteinases (MMPs) are one such target involved in angiogenesis [44]. MMPs are implicated in early steps of tumor evolution including stimulation of cell proliferation and modulation of angiogenesis [45]. During angiogenesis, new vessels develop from present endothelial lined vessels to encourage the degradation of the vascular basement membrane and remodel the extracellular matrix (ECM), followed by endothelial cell migration, proliferation, and formation of the new generation of matrix components [46,47].
Matrix metalloproteinases participate in the disruption, tumor neovascularization, and successive metastasis, while tissue inhibitors of metalloproteinases (TIMPs) downregulate the activity of these MMPs [48]. Therefore, MMPs, through the modulation of the balance between pro- and anti-angiogenic factors, can directly or indirectly mediate the angiogenic response [49].
The relation between MMP overexpression in tumor and cancer progression has encouraged the progress of preclinical trials with a series of inhibitors designed to block the proteolytic activity of these enzymes.
Thus far, no FDA-approved MMP inhibitors for the treatment of cancer have emerged. However, several leads have been developed; for example, Becker et al. reported new piperidine α-sulfone hydroxamates with potent matrix metalloproteinase inhibition activity [50]. The α- and β-sulfone derivatives showed a difference in the inhibitory activity toward the metalloproteinases. The β-sulfone hydroxamate showed potency for the targeted MMPs and selectivity for MMP-1, but generally exhibited poor oral bioavailability. In addition, some β-sulfones with α-hydrogens can undergo β-elimination. On the other hand, the α-sulfones possess both potency and selectivity and provide an improvement in oral exposure demonstrated by a higher Cmax value and bioavailability relative to the β-sulfones.
On further investigation, they concluded that α-sulfone derivatives are two to four times more potent and bioavailable than the β-sulfones. The higher bioavailability and Cmax may be due to greater steric bulk around the hydroxamate, protecting it from the usual modes of hydroxamate metabolism including N-O bond cleavage, hydrolysis, and glucuronidation. The findings of Becker et al. can be explained through the comparison of the different activities of α- and β-sulfone derivatives, as demonstrated in Table 2.
Table 2.
Comparing α- and β-sulfone derivatives for their matrix metalloproteinase 1 (MMP-1) activity [50].
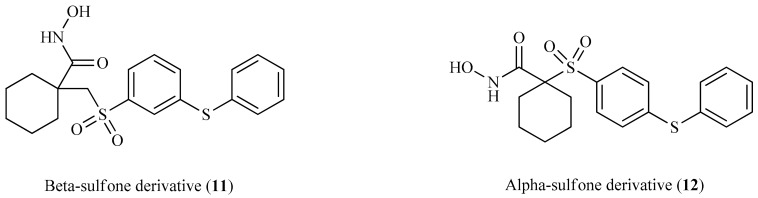
| ||||||
| Ki (nM) | ||||||
| Compound | MMP-1 | MMP-2 | MMP-3 | MMP-9 | MMP-13 | Cmax |
| 11 (β-sulfone) | 800 | 0.4 | 17.5 | 1 | 0.6 | 1372 |
| 12 (α-sulfone) | 435 | ˂0.1 | 18.1 | 0.3 | 0.15 | 3119 |
Looking at the interaction of the α- and β-sulfone derivatives (Figure 4 and Figure 5) with the active site residues of MMP-2 [51], the difference in potency can be elucidated because of the fact that the α-derivative showed a docking score of −7.054, while its counterpart, the β-derivative, exhibited a docking score of −5.22. This difference in the docking scores as well as forming different interactions with the active site could explain why the α-sulfone derivative would be a more promising lead for further development.
Figure 4.
Predicted interaction of α-sulfone (12) derivative active site residues of matrix metalloproteinase (MMP) [51,52]. (a) 3D structural view of ligand inside the receptor’s active site. (b) Ligand interaction diagram; violet lines represent hydrogen bonds and green lines and grey line represent metal interactions.
Figure 5.
Predicted interaction of β-sulfone (11) derivative active site residues of MMP. (a) 3D structural view of ligand inside the receptor’s active site. (b) Ligand interaction diagram; violet lines represent hydrogen bonds, green lines represent π–π interactions, and grey line represent metal interactions.
3. Heterocyclic Sulfonamides
Sulfonamides significance stems from the fact that they constitute a significant class of drugs with various types of pharmacological effects including antitumor, anti-carbonic anhydrase, and diuretic activity [53,54,55,56]. There are many reports of a multitude of structurally novel heterocyclic sulfonamide derivatives that have been stated to display significant antitumor activity [57,58]. Although the mechanism by which they combat cancer may vary, the majority of anti-cancer sulfonamides act on angiogenesis through several pathways.
As of today, there are no FDA-approved heterocyclic sulfonamide anti-angiogenics in the market, but there are several promising leads being developed. One of such leads is E7820, which is now in phase II clinical trial for colorectal cancer treatment through the inhibition of integrin α2 [59]. Integrins are transmembrane receptors that are central to the biology of many human pathologies, where they act through the mediation of the cell-extracellular matrix and cell–cell interaction [60]. This mediative action is carried out through the transduction of information from the extracellular environment to modulate cell responses, together with adhesion, spreading, migration, growth signaling, survival signaling, secretion of proteases, and invasion [3,61,62]. Numerous studies report that the increased levels of integrin α2 facilitate the spread of cancer [63,64,65,66]. Thus, inhibitors of integrin α2, such as that shown in Figure 6, will slow the spread of cancer and its metastasis.
Figure 6.
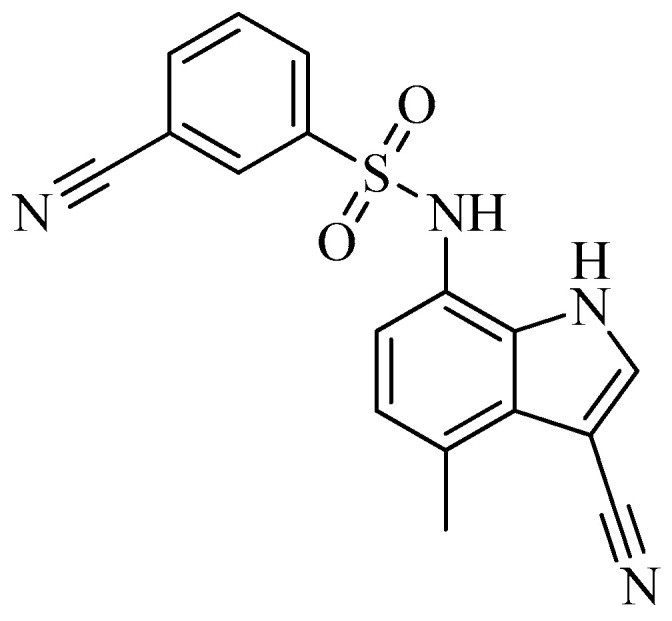
Chemical structure of the heterocyclic sulfonamide anti-angiogenic E7820.
Hypoxia inducible factors (HIFs) are another promising anti-angiogenic inhibitor [67]. This is because of the requirement of an adequate oxygen supply for the macroscopic tumor to grow [68]. This need is fulfilled through tumor angiogenesis, which results from an increased synthesis of angiogenic factors and a decreased synthesis of anti-angiogenic factors [49,69].
The shift of the balance between pro- and anti-apoptotic factors due to the metabolic adaptation of tumor cells to decrease the oxygen availability by increasing glucose transport and glycolysis to promote survival is prominent in hypoxia [69,70]. In this regard, HIF-1, which is induced by many factors, is mainly implicated in tumor angiogenesis.
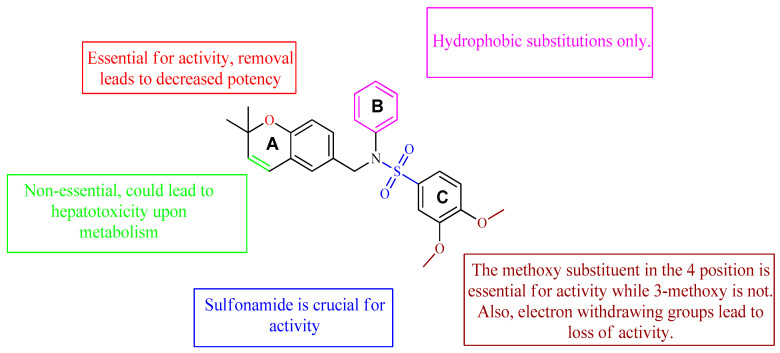
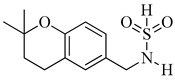
A class of heterocyclics based on the lead sulfonamide hits identified by Gerguson et al. showed potent activity against hypoxia (Table 3) [71,72]. It was found that the oxygen atom in ring A was essential for the activity, while the double bond in the same ring resulted in increased hepatotoxicity, leading to the conclusion that the double bond should be removed. Furthermore, the sulfonamide moiety as well as the hydrophobic substitutions in ring B increased the activity significantly. In ring C, the methoxy substituent at para position is essential for activity, while meta methoxy is not. Additionally, upon replacing the para methoxy with electron withdrawing groups, the activity was lost, demonstrating that only electron donating groups should occupy this position. This SAR is summarized in Figure 7.
Table 3.
In vitro inhibition of hypoxia inducible factor 1 (HIF-1) transcriptional activity in cell-based HRE reporter assay by the benzenesulfonamide analogues (13–18) [72].
| |||
| Compound | R1 | R2 | LN229-HRE-Lux (IC50 μM) |
| 13 |
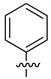
|
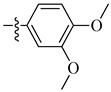
|
0.6 μM |
| 14 |
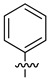
|
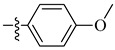
|
0.6 μM |
| 15 |
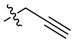
|
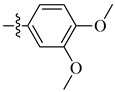
|
1.3 μM |
| 16 |
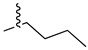
|
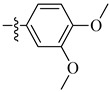
|
3.3 μM |
| 17 |
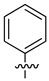
|
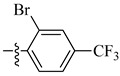
|
>25 μM |
| 18 |
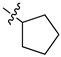
|
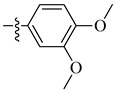
|
0.5 μM |
Figure 7.
Structure–activity relationship study of the benzenesulfonamide hits and its analogues [71,72].
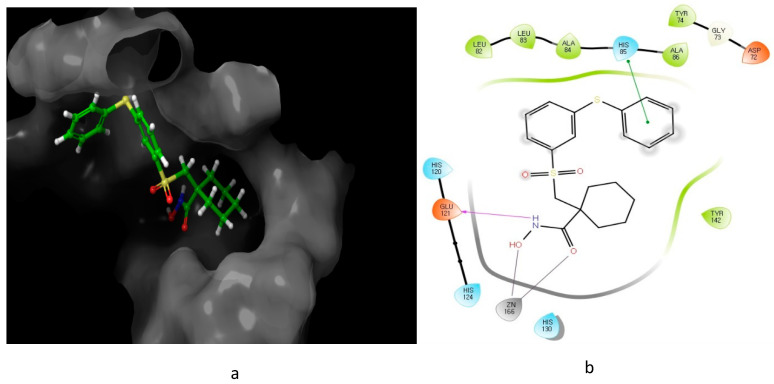
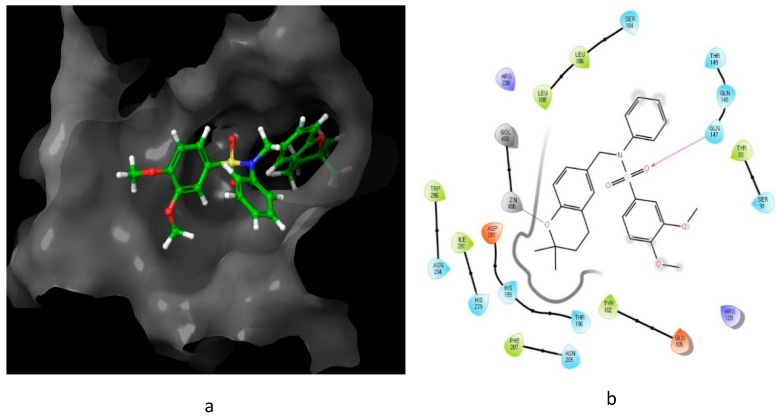
The interaction of compound 13, possessing IC50 of 0.6 μM, with HIF-1 active site residue, is demonstrated in Figure 8. It showed better binding energy and inhibition constant as compared with N-((2,2-dimethylchroman-6-yl) methyl) sulfonic amide, with docking scores of −6.85 and −5.4, respectively. This could be owing to the ability of the sulfonamide derivative (13) to form a hydrogen bond and metal interaction with the active site residue.
Figure 8.
Predicted interaction of compound 13 with hypoxia-inducible factor active site residue. (a) 3D structural view of the sulfonamide ligand inside the receptor’s active site. (b) Ligand interaction diagram; violet lines represent hydrogen bonds and grey lines represent metal interaction.
4. Oxygen-Based Heterocycles
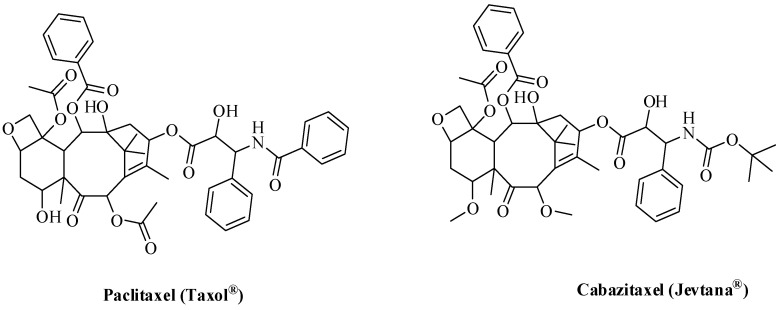
About 15% of all FDA-approved drugs are oxygen-based heterocycles [73]. To date, there are several FDA-approved oxygen-based anti-angiogenic cancer drugs. Some of these drugs (Figure 9) are based on the oxetane ring such as paclitaxel (PTX, Taxol®) and cabazitaxel (Jevtana) acting via microtubule-targeting [74].
Figure 9.
FDA-approved oxygen-based anti-angiogenics.
Microtubules are cytoskeletal elements that are necessary for many functions including intracellular transport, motility, morphogenesis, and cell division [75,76]. α–β tubulin heterodimers make up the microtubules by assembling in sequence to form the protofilaments of the tube [77]. This microtubule polymerization leads to a characteristic heterogeneity among the two ends of the microtubule, giving rise to different kinetics of the addition and subtraction of heterodimers at the two ends [78]. At the ‘plus’ end, the kinetics of the polymerization and depolymerization are faster than those at the other end, the slower so-called ‘minus’ end. Inside the cell, microtubules are attached by their minus ends at the microtubule-organizing center, placing their plus ends to the cell border [79]. The plus ends constantly grow and shorten, which is a property vital for various cellular processes including cell division [80].
Throughout mitosis, normal cells could arrest owing to interference in the dynamic properties of microtubules, where microtubules continuously polymerize and rapidly depolymerize, making a ‘pulling device’ for the duplicated chromosomes [81]. MTAs (microtubule-targeting agents) act through the stabilization of microtubules against depolymerization [82,83,84]. One of the most promising microtubule targeting agents is paclitaxel. In a study conducted by Ganesh et al., the cytotoxicity of some paclitaxel derivatives was measured as illustrated in Table 4 [85].
Table 4.
Cytotoxic activity of some paclitaxel analogues (19–24) [85].
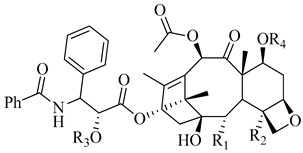
| |||||
| Compound | R1 | R2 | R3 | R4 | IC50 PC3 (nM) |
| 19 |
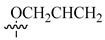
|
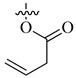
|
H | H | 550 |
| 20 |
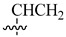
|
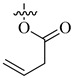
|
H | H | 320 |
| 21 |
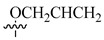
|
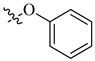
|
H | H | 128 |
| 22 |
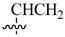
|
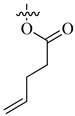
|
H | H | 55 |
| 23 |
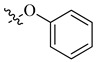
|
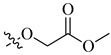
|
H | H | 53 |
| 24 |
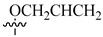
|
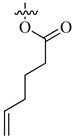
|
H | H | 4600 |
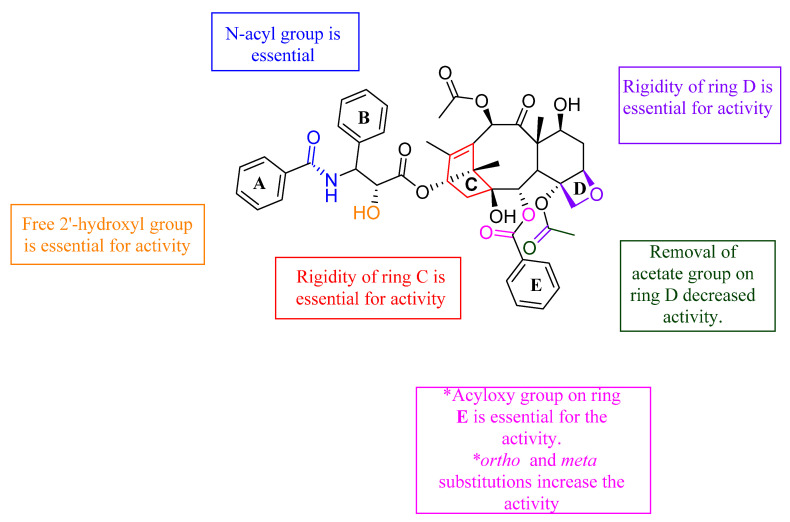
From the study of Ganesh et al., it was found that any changes to rings C and D led to loss of activity, indicating the importance of their structural rigidity. The following groups were found to be essential for activity: the N-acyl group, the free 2′-hydroxy group, and the acyloxy group substitution on ring E. In addition, the ortho and meta substitutions on ring E increased the activity. On the other hand, when the acetate group on ring D was removed, the activity decreased significantly. The SAR of the above-mentioned paclitaxel analogues is illustrated in Figure 10.
Figure 10.
The interaction of paclitaxel with microtubule active site (Figure 11) further illuminated this SAR, where paclitaxel showed a very high binding affinity with the active site (docking score of −9.48) [87]. Furthermore, paclitaxel formed three hydrogen bonds and one π–π interaction within the active site, showing a remarkable ability to interact with the microtubule active site.
Figure 11.
Predicted interaction of paclitaxel with microtubule active site residue [88,89]. (a) 3D structural view of the sulfonamide ligand inside the receptor’s active site. (b) Ligand interaction diagram; violet lines represent hydrogen bonds and green lines represent π–π interactions.
5. Drug Likeness and Absorption Distribution Metabolism Excretion (ADME) Prediction of the Chosen Inhibitors
Computational approaches have become an essential part of interdisciplinary drug discovery research. Understanding the science behind computational tools and their opportunities is essential to make a real outcome on drug discovery at different stages. If applied in a scientifically meaningful way, computational methods improve the ability to identify and evaluate potential drug molecules [90,91,92,93,94,95]. A good antagonistic interaction of inhibitors with a receptor protein or enzyme does not assure the capability of an inhibitor as a drug; consequently, absorption distribution metabolism excretion (ADME) analysis is important in the drug development [96]. ADME is based on Lipinski’s rule of five and assists in the approval of inhibitors for biological systems.
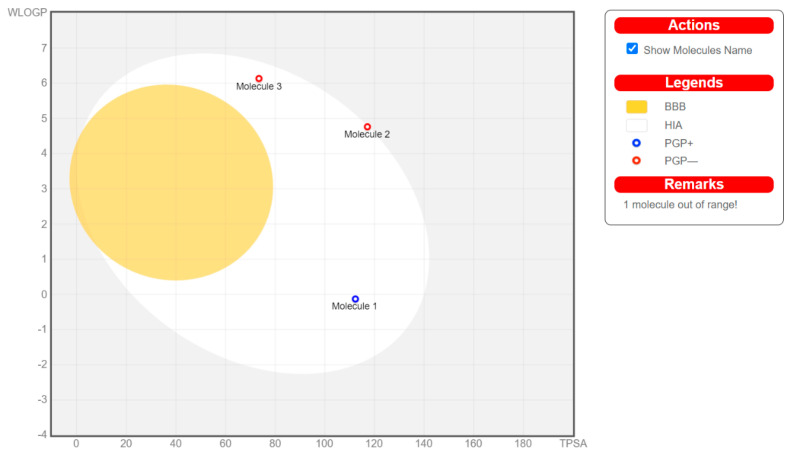
One of the major causes of the failure of most medicines in clinical experiments is having poor ADME characteristics and unfavorable toxicology [97]. Apart from efficacy and toxicity, various drug development failures are due to poor pharmacokinetics and bioavailability [98]. Gastrointestinal absorption and brain access are two pharmacokinetic behaviors crucial to be evaluated at various stages of the drug discovery [99]. Therefore, four leads belonging to each class were subjected to an in silico pharmacokinetic study using the SwissADME server (compound 10 as representative for nitrogen-based drugs (referred as molecule 1 in the BOILED-Egg chart), compound 12 as a representative for MMPIs (molecule 2), compound 13 as a representative for the heterocyclic sulfonamides (molecule 3), and paclitaxel as a representative for oxygen-based heterocycles (molecule 4)). The pharmacokinetic study is demonstrated in Figure 12 using the brain or intestinal estimated permeation method (BOILED-Egg).
Figure 12.
Brain or intestinal estimated permeation method (BOILED-Egg) chart for compounds 10, 12, 13, and paclitaxel.
Upon looking at the BOILED-Egg chart, we can see that, even though the four leads exert their pharmacological action through different mechanisms, three compounds (10, 12, and 13) were found to obey Lipinski’s rule of five, indicating their absorbance in the GIT if taken orally, but avoiding any side effects resulting from passing the blood–brain barrier (BBB). However, compound 10 is predicted to be expelled from the central nervous system by P-glycoprotein (Pgp), resulting in a possible poor bioavailability. Even though paclitaxel showed promising biological activity, it fails to obey Lipinski’s rule of five, leading to poor oral bioavailability.
6. Conclusions
Throughout this review, several leads were investigated for SAR, molecular interaction, and in silico pharmacokinetics study. The following outlines were concluded, aiming to develop more active analogues of the discussed leads. Compound 10 and its derivatives would benefit from both a modification to prevent its efflux by the PGP as well as modifications to increase its interaction with the VEGFR-2 active site. Both compounds 12 and 13 and their derivatives show good drug-likeness properties, but would benefit from further isosteric changes to their structures to increase their interactions with their respective active site. Paclitaxel and its derivatives have shown the highest binding potential to MTA active site residue, with docking scores around −9.5. However, they all violated Lipinski’s rule of five, leading to poor bioavailability. Therefore, further modification to their chemical structure is highly needed.
Author Contributions
Conceptualization, K.L.; methodology, H.N. and A.E.; software, K.L.; validation, H.N. and A.E.; formal analysis, H.N. and A.E.; investigation, A.E.; resources, K.L.; data curation, A.E.; writing—original draft preparation, H.N. and A.E.; writing—review and editing, A.E., K.L.; visualization, H.N.; supervision, K.L.; project administration, A.E.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the national Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2018R1A5A2023127).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D.J. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A.J. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Hamidi H., Ivaska J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Drug Discov. 2018;17:31–46. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mashreghi M., Azarpara H., Bazaz M.R., Jaafari M.R., Masoudifar A., Mirzaei H., Jaafari M.R. Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J. Cell. Physiol. 2018;233:2949–2965. doi: 10.1002/jcp.26049. [DOI] [PubMed] [Google Scholar]
- 5.Ellis L., Fidler I. Angiogenesis and metastasis. Eur. J. Cancer. 1996;32:2451–2460. doi: 10.1016/S0959-8049(96)00389-9. [DOI] [PubMed] [Google Scholar]
- 6.Schättler H., Ledzewicz U. Optimal Control for Mathematical Models of Cancer Therapies. Volume 42 Springer; Berlin/Heidelberg, Germany: 2015. [Google Scholar]
- 7.McColl B.K., Stacker S.A., Achen M.G. Molecular regulation of the VEGF family–inducers of angiogenesis and lymphangiogenesis. J. Pathol. Microbiol. Immunol. 2004;112:463–480. doi: 10.1111/j.1600-0463.2004.apm11207-0807.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed N., Escalona R., Leung D., Chan E., Kannourakis G. Tumour microenvironment and metabolic plasticity in cancer and cancer stem cells: Perspectives on metabolic and immune regulatory signatures in chemoresistant ovarian cancer stem cells. Semin. Cancer Biol. 2018;53:265–281. doi: 10.1016/j.semcancer.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Fantozzi A., Gruber D.C., Pisarsky L., Heck C., Kunita A., Yilmaz M., Meyer-Schaller N., Cornille K., Hopfer U., Bentires-Alj M., et al. VEGF-Mediated Angiogenesis Links EMT-Induced Cancer Stemness to Tumor Initiation. Cancer Res. 2014;74:1566–1575. doi: 10.1158/0008-5472.CAN-13-1641. [DOI] [PubMed] [Google Scholar]
- 10.Chung A.S., Lee J., Ferrara N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat. Rev. Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 11.Otrock Z.K., Mahfouz R.A., Makarem J.A., Shamseddine A.I. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol. Dis. 2007;39:212–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Fidler I.J. Angiogenesis and cancer metastasis. Cancer J. 2000;6:S134–S141. [PubMed] [Google Scholar]
- 13.Nussenbaum F., Herman I.M. Tumor Angiogenesis: Insights and Innovations. J. Oncol. 2010;2010:1–24. doi: 10.1155/2010/132641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewhirst M.W., Secomb T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer. 2017;17:738–750. doi: 10.1038/nrc.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronca R., Benkheil M., Mitola S., Struyf S., Liekens S. Tumor angiogenesis revisited: Regulators and clinical implications. Med. Res. Rev. 2017;37:1231–1274. doi: 10.1002/med.21452. [DOI] [PubMed] [Google Scholar]
- 16.Wang D., Sun H., Wei J., Cen B., Dubois R.N. CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res. 2017;77:3655–3665. doi: 10.1158/0008-5472.CAN-16-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyake M., Goodison S., Lawton A., Gomes-Giacoia E., Rosser C. Angiogenin promotes tumoral growth and angiogenesis by regulating matrix metallopeptidase-2 expression via the ERK1/2 pathway. Oncogene. 2015;34:890–901. doi: 10.1038/onc.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Abd A.M., AlAmoudi A.J., Abdel-Naim A.B., Neamatallah T.A., Ashour O.M. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies—A review. J. Adv. Res. 2017;8:591–605. doi: 10.1016/j.jare.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zirlik K., Duyster J. Anti-Angiogenics: Current Situation and Future Perspectives. Oncol. Res. Treat. 2018;41:166–171. doi: 10.1159/000488087. [DOI] [PubMed] [Google Scholar]
- 20.Akhtar J., Khan A.A., Ali Z., Haider R., Yar M.S. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017;125:143–189. doi: 10.1016/j.ejmech.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Kang S., Lee J.M., Jeon B., Elkamhawy A., Paik S., Hong J., Oh S.-J., Paek S.H., Lee C.J., Hassan A.H., et al. Repositioning of the antipsychotic trifluoperazine: Synthesis, biological evaluation and in silico study of trifluoperazine analogs as anti-glioblastoma agents. Eur. J. Med. Chem. 2018;151:186–198. doi: 10.1016/j.ejmech.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 22.Elkamhawy A., Park J.-E., Cho N.-C., Sim T., Pae A.N., Roh E.J. Discovery of a broad spectrum antiproliferative agent with selectivity for DDR1 kinase: Cell line-based assay, kinase panel, molecular docking, and toxicity studies. J. Enzym. Inhib. Med. Chem. 2016;31:158. doi: 10.3109/14756366.2015.1004057. [DOI] [PubMed] [Google Scholar]
- 23.Kerru N., Gummidi L., Maddila S., Gangu K.K., Jonnalagadda S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules. 2020;25:1909. doi: 10.3390/molecules25081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng F.-W., Liu D.-K., Zhang Q.-W., Xu Y.-G., Shi L. VEGFR-2 inhibitors and the therapeutic applications thereof: A patent review (2012–2016) Expert Opin. Ther. Patents. 2017;27:987–1004. doi: 10.1080/13543776.2017.1344215. [DOI] [PubMed] [Google Scholar]
- 25.Cheng K., Liu C.-F., Rao G.-W. Anti-angiogenic Agents: A Review on Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) Inhibitors. Curr. Med. Chem. 2020 doi: 10.2174/0929867327666200514082425. [DOI] [PubMed] [Google Scholar]
- 26.Pandey A.K., Singhi E.K., Arroyo J.P., Ikizler T.A., Gould E.R., Brown J., Beckman J.A., Harrison D.G., Moslehi J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor–Associated Hypertension and Vascular Disease. Hypertension. 2018;71:e1–e8. doi: 10.1161/HYPERTENSIONAHA.117.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farina A.R., Cappabianca L., Sebastiano M., Zelli V., Guadagni S., Mackay A.R. Hypoxia-induced alternative splicing: The 11th Hallmark of Cancer. J. Exp. Clin. Cancer Res. 2020;39:1–30. doi: 10.1186/s13046-020-01616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X., Hui Y., Hua Y., Huang L., Wang L., Peng F., Tang C., Liu D., Song J., Wang F. EG-VEGF silencing inhibits cell proliferation and promotes cell apoptosis in pancreatic carcinoma via PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2019;109:762–769. doi: 10.1016/j.biopha.2018.10.125. [DOI] [PubMed] [Google Scholar]
- 29.Schafer C.M., Gurley J.M., Kurylowicz K., Lin P.K., Chen W., Elliott M.H., Davis G.E., Bhatti F., Griffin C.T. An inhibitor of endothelial ETS transcription factors promotes physiologic and therapeutic vessel regression. Proc. Natl. Acad. Sci. USA. 2020;117:26494–26502. doi: 10.1073/pnas.2015980117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. doi: 10.1096/fasebj.13.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Viallard C., Larrivée B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 32.Rosen L.S. Clinical Experience with Angiogenesis Signaling Inhibitors: Focus on Vascular Endothelial Growth Factor (VEGF) Blockers. Cancer Control. 2002;9:36–44. doi: 10.1177/107327480200902S05. [DOI] [PubMed] [Google Scholar]
- 33.Schlessinger J. Cell Signaling by Receptor Tyrosine Kinases. Cell. 2000;103:211–225. doi: 10.1016/S0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 34.Park S.A., Jeong M.S., Ha K.-T., Jang S.B. Structure and function of vascular endothelial growth factor and its receptor system. BMB Rep. 2018;51:73–78. doi: 10.5483/BMBRep.2018.51.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzer R.J., Hutson T.E., Cella D., Reeves J., Hawkins R., Guo J., Nathan P., Staehler M., De Souza P., Merchan J.R., et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich M.C., Maki R.G., Corless C.L., Antonescu C.R., Harlow A., Griffith D., Town A., McKinley A., Ou W.-B., Fletcher J.A., et al. Primary and Secondary Kinase Genotypes Correlate With the Biological and Clinical Activity of Sunitinib in Imatinib-Resistant Gastrointestinal Stromal Tumor. J. Clin. Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scagliotti G.V., Govindan R. Targeting Angiogenesis with Multitargeted Tyrosine Kinase Inhibitors in the Treatment of Non-Small Cell Lung Cancer. Oncology. 2010;15:436–446. doi: 10.1634/theoncologist.2009-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Z., Zhang Q., Luo W. Angiogenesis inhibitors as therapeutic agents in cancer: Challenges and future directions. Eur. J. Pharmacol. 2016;793:76–81. doi: 10.1016/j.ejphar.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 39.Liu J.-C., Narva S., Zhou K., Zhang W. A Review on the Antitumor Activity of Various Nitrogenous-based Heterocyclic Compounds as NSCLC Inhibitors. Mini Rev. Med. Chem. 2019;19:1517–1530. doi: 10.2174/1389557519666190312152358. [DOI] [PubMed] [Google Scholar]
- 40.Garnock-Jones K.P. Panobinostat: First global approval. Drugs. 2015;75:695–704. doi: 10.1007/s40265-015-0388-8. [DOI] [PubMed] [Google Scholar]
- 41.Renhowe P.A., Pecchi S., Shafer C.M., Machajewski T.D., Jazan E.M., Taylor C., Antonios-McCrea W., McBride C.M., Frazier K., Wiesmann M., et al. Design, Structure−Activity Relationships and in Vivo Characterization of 4-Amino-3-benzimidazol-2-ylhydroquinolin-2-ones: A Novel Class of Receptor Tyrosine Kinase Inhibitors. J. Med. Chem. 2009;52:278–292. doi: 10.1021/jm800790t. [DOI] [PubMed] [Google Scholar]
- 42.Kubo H., Fujiwara T., Jussila L., Hashi H., Ogawa M., Shimizu K., Awane M., Sakai Y., Takabayashi A., Alitalo K.J.B. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. J. Am. Soc. Hematol. 2000;96:546–553. [PubMed] [Google Scholar]
- 43.McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5:3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 44.Stetler-Stevenson W.G. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J. Clin. Investig. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gialeli C., Theocharis A.D., Karamanos N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 46.Spuul P., Daubon T., Pitter B., Alonso F., Fremaux I., Kramer I., Montañez E., Génot E. VEGF-A/Notch-Induced Podosomes Proteolyse Basement Membrane Collagen-IV during Retinal Sprouting Angiogenesis. Cell Rep. 2016;17:484–500. doi: 10.1016/j.celrep.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.González-Avila G., Sommer B., Mendoza-Posada D.A., Ramos C., Garcia-Hernandez A.A., Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. 2019;137:57–83. doi: 10.1016/j.critrevonc.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Baeriswyl V., Christofori G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009;19:329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Becker D.P., Villamil C.I., Barta T.E., Bedell L.J., Boehm T.L., DeCrescenzo G.A., Freskos J.N., Getman D.P., Hockerman S., Heintz R.J., et al. Synthesis and Structure−Activity Relationships of β- and α-Piperidine Sulfone Hydroxamic Acid Matrix Metalloproteinase Inhibitors with Oral Antitumor Efficacy. J. Med. Chem. 2005;48:6713–6730. doi: 10.1021/jm0500875. [DOI] [PubMed] [Google Scholar]
- 51.Solution Structure and Backbone Dynamics of the Catalytic Domain of Matrix Metalloproteinase-2 Complexed with a Hydroxamic Acid Inhibitor. [(accessed on 1 November 2020)]; doi: 10.1016/s0167-4838(02)00307-2. Available online: http://www.rcsb.org/structure/1HOV. [DOI] [PubMed]
- 52.Feng Y., Likos J.J., Zhu L., Woodward H., Munie G., McDonald J.J., Stevens A.M., Howard C.P., De Crescenzo G.A., Welsch D., et al. Solution structure and backbone dynamics of the catalytic domain of matrix metalloproteinase-2 complexed with a hydroxamic acid inhibitor. Biochimica et Biophysica Acta. 2002;1598:10–23. doi: 10.1016/S0167-4838(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 53.Gulçin I., Taslimi P. Sulfonamide inhibitors: A patent review 2013-present. Expert Opin. Ther. Patents. 2018;28:541–549. doi: 10.1080/13543776.2018.1487400. [DOI] [PubMed] [Google Scholar]
- 54.Awadallah F.M., Bua S., Mahmoud W.R., Nada H.H., Nocentini A., Supuran C.T. Inhibition studies on a panel of human carbonic anhydrases with N1-substituted secondary sulfonamides incorporating thiazolinone or imidazolone-indole tails. J. Enzym. Inhib. Med. Chem. 2018;33:629–638. doi: 10.1080/14756366.2018.1446432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Sanea M.M., Elkamhawy A., Paik S., Bua S., Lee S.H., AbdelGawad M.A., Roh E.J., Eldehna W.M., Supuran C.T. Synthesis and biological evaluation of novel 3-(quinolin-4-ylamino)benzenesulfonamides as carbonic anhydrase isoforms I and II inhibitors. J. Enzym. Inhib. Med. Chem. 2019;34:1457–1464. doi: 10.1080/14756366.2019.1652282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Sanea M.M., Elkamhawy A., Paik S., Lee K., El Kerdawy A.M., Abbas B.S.N., Roh E.J., Eldehna W.M., Elshemy H.A., Bakr R.B., et al. Sulfonamide-based 4-anilinoquinoline derivatives as novel dual Aurora kinase (AURKA/B) inhibitors: Synthesis, biological evaluation and in silico insights. Bioorg. Med. Chem. 2020;28:115525. doi: 10.1016/j.bmc.2020.115525. [DOI] [PubMed] [Google Scholar]
- 57.Owa T., Nagasu T. Novel sulphonamide derivatives for the treatment of cancer. Expert Opin. Ther. Patents. 2000;10:1725–1740. doi: 10.1517/13543776.10.11.1725. [DOI] [Google Scholar]
- 58.Supuran C.T. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin. Drug Discov. 2020;15:671–686. doi: 10.1080/17460441.2020.1743676. [DOI] [PubMed] [Google Scholar]
- 59.Funahashi Y., Sugi N.H., Semba T., Yamamoto Y., Hamaoka S., Tsukahara-Tamai N., Ozawa Y., Tsuruoka A., Nara K., Takahashi K. Sulfonamide derivative, E7820, is a unique angiogenesis inhibitor suppressing an expression of integrin α2 subunit on endothelium. Cancer Res. 2002;62:6116–6123. [PubMed] [Google Scholar]
- 60.Pan L., Zhao Y., Yuan Z., Qin G. Research advances on structure and biological functions of integrins. SpringerPlus. 2016;5:1–11. doi: 10.1186/s40064-016-2502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hytönen V.P., Wehrle-Haller B. Mechanosensing in cell–matrix adhesions–Converting tension into chemical signals. Exp. Cell Res. 2016;343:35–41. doi: 10.1016/j.yexcr.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 62.Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20:457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- 63.Mizejewski G.J. Role of integrins in cancer: Survey of expression patterns. Proc. Soc. Exp. Boil. Med. 1999;222:124–138. doi: 10.1046/j.1525-1373.1999.d01-122.x. [DOI] [PubMed] [Google Scholar]
- 64.Lu X., Lu D., Scully M., Kakkar V. The Role of Integrins in Cancer and the Development of Anti-Integrin Therapeutic Agents for Cancer Therapy. Perspect. Med. Chem. 2008;2:57–73. doi: 10.1177/1177391X0800200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yousefi H., Vatanmakanian M., Mahdiannasser M., Mashouri L., Alahari N.V., Monjezi M.R., Ilbeigi S., Alahari S.K. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene. 2021:1–21. doi: 10.1038/s41388-020-01588-2. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimura K., Meckel K.F., Laird L.S., Chia C.Y., Park J.-J., Olino K.L., Tsunedomi R., Harada T., Iizuka N., Hazama S., et al. Integrin α2 Mediates Selective Metastasis to the Liver. Cancer Res. 2009;69:7320–7328. doi: 10.1158/0008-5472.CAN-09-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan C., De Noronha R.G., Devi N.S., Jabbar A.A., Kaluz S., Liu Y., Mooring S.R., Nicolaou K., Wang B., Van Meir E.G. Sulfonamides as a new scaffold for hypoxia inducible factor pathway inhibitors. Bioorg. Med. Chem. Lett. 2011;21:5528–5532. doi: 10.1016/j.bmcl.2011.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatzikirou H., Basanta D., Simon M., Schaller K., Deutsch A. ‘Go or Grow’: The key to the emergence of invasion in tumour progression? Math. Med. Biol. 2012;29:49–65. doi: 10.1093/imammb/dqq011. [DOI] [PubMed] [Google Scholar]
- 69.Lu J., Tan M., Cai Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–164. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bacon A., Harris A.L. Hypoxia-inducible factors and hypoxic cell death in tumour physiology. Ann. Med. 2004;36:530–539. doi: 10.1080/07853890410018231. [DOI] [PubMed] [Google Scholar]
- 71.Ferguson J., De Los Santos Z., Devi N., Van Meir E., Zingales S.K., Wang B. Examining the structure-activity relationship of benzopyran-based inhibitors of the hypoxia inducible factor-1 pathway. Bioorg. Med. Chem. Lett. 2017;27:1731–1736. doi: 10.1016/j.bmcl.2017.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhattarai D., Xu X., Lee K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016): A “structure–activity relationship” perspective. Med. Res. Rev. 2018;38:1404–1442. doi: 10.1002/med.21477. [DOI] [PubMed] [Google Scholar]
- 73.Delost M.D., Smith D.T., Anderson B.J., Njardarson J.T. From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem. 2018;61:10996–11020. doi: 10.1021/acs.jmedchem.8b00876. [DOI] [PubMed] [Google Scholar]
- 74.Han X., Chen J., Jiang M., Zhang N., Na K., Luo C., Zhang R., Sun M., Lin G., Zhang R., et al. Paclitaxel–Paclitaxel Prodrug Nanoassembly as a Versatile Nanoplatform for Combinational Cancer Therapy. ACS Appl. Mater. Interfaces. 2016;8:33506–33513. doi: 10.1021/acsami.6b13057. [DOI] [PubMed] [Google Scholar]
- 75.Nogales E. Structural insights into microtubule function. Ann. Rev. Biophys. Biomol. Struct. 2001;30:397–420. doi: 10.1146/annurev.biophys.30.1.397. [DOI] [PubMed] [Google Scholar]
- 76.Petrášek J., Schwarzerová K. Actin and microtubule cytoskeleton interactions. Curr. Opin. Plant. Biol. 2009;12:728–734. doi: 10.1016/j.pbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Knossow M., Campanacci V., Khodja L.A., Gigant B. The Mechanism of Tubulin Assembly into Microtubules: Insights from Structural Studies. iScience. 2020;23:9. doi: 10.1016/j.isci.2020.101511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vemu A., Atherton J., Spector J.O., Moores C.A., Roll-Mecak A. Tubulin isoform composition tunes microtubule dynamics. Mol. Biol. Cell. 2017;28:3564–3572. doi: 10.1091/mbc.e17-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin M., Akhmanova A. Coming into Focus: Mechanisms of Microtubule Minus-End Organization. Trends Cell Biol. 2018;28:574–588. doi: 10.1016/j.tcb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 80.Janke C., Magiera M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020;21:307–326. doi: 10.1038/s41580-020-0214-3. [DOI] [PubMed] [Google Scholar]
- 81.Checchi P.M., Nettles J.H., Zhou J., Snyder J.P., Joshi H.C. Microtubule-interacting drugs for cancer treatment. Trends Pharmacol. Sci. 2003;24:361–365. doi: 10.1016/S0165-6147(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 82.Banerjee S., Hwang D.-J., Li W., Miller D.D. Current Advances of Tubulin Inhibitors in Nanoparticle Drug Delivery and Vascular Disruption/Angiogenesis. Molecules. 2016;21:1468. doi: 10.3390/molecules21111468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bates D., Eastman A. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs. Br. J. Clin. Pharmacol. 2017;83:255–268. doi: 10.1111/bcp.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasquier E., Andre N., Braguer D. Targeting Microtubules to Inhibit Angiogenesis and Disrupt Tumour Vasculature:Implications for Cancer Treatment. Curr. Cancer Drug Targets. 2007;7:566–581. doi: 10.2174/156800907781662266. [DOI] [PubMed] [Google Scholar]
- 85.Ganesh T., Yang C., Norris A., Glass T., Bane S., Ravindra R., Banerjee A., Metaferia B., Thomas S.L., Giannakakou P., et al. Evaluation of the Tubulin-Bound Paclitaxel Conformation: Synthesis, Biology, and SAR Studies of C-4 to C-3‘ Bridged Paclitaxel Analogues. J. Med. Chem. 2007;50:713–725. doi: 10.1021/jm061071x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Żwawiak J., Zaprutko L.J. A brief history of taxol. J. Med. Sci. 2014;83:47–52. [Google Scholar]
- 87.Dynamic and Asymmetric Fluctuations in the Microtubule Wall Captured by High-Resolution Cryoelectron Microscopy. [(accessed on 1 October 2020)]; doi: 10.1073/pnas.2001546117. Available online: http://www.rcsb.org/structure/6WVM. [DOI] [PMC free article] [PubMed]
- 88.Debs G.E., Cha M., Liu X., Huehn A.R., Sindelar C.V. Dynamic and asymmetric fluctuations in the microtubule wall captured by high-resolution cryoelectron microscopy. Proc. Nat. Acad. Sci. USA. 2020;117:16976–16984. doi: 10.1073/pnas.2001546117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.6WVM—High Curvature Lateral Interaction within a 13-Protofilament, Taxol Stabilized Microtubule. [(accessed on 18 January 2021)]; Available online: http://www.rcsb.org/structure/6WVM.
- 90.Jürgen B.J.F. Computer-aided drug discovery. F1000Res. 2015;4:630. doi: 10.12688/f1000research.6653.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elkamhawy A., Kim N.Y., Hassan A.H., Park J.-E., Paik S., Yang J.-E., Oh K.-S., Lee B.H., Lee M.Y., Shin K.J., et al. Thiazolidine-2,4-dione-based irreversible allosteric IKK-β kinase inhibitors: Optimization into in vivo active anti-inflammatory agents. Eur. J. Med. Chem. 2020;188:111955. doi: 10.1016/j.ejmech.2019.111955. [DOI] [PubMed] [Google Scholar]
- 92.Da Silva V.B., Resende J., Rodrigues P.F., Bononi F.C., Benevenuto C.G., Taft C.A. Computer-aided drug design and ADMET predictions for identification and evaluation of novel potential farnesyltransferase inhibitors in cancer therapy. J. Mol. Graph. Model. 2010;28:513–523. doi: 10.1016/j.jmgm.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 93.Elkamhawy A., Paik S., Kim H.J., Park J.-H., Londhe A.M., Lee K., Pae A.N., Park K.D., Roh E.J. Discovery of N-(1-(3-fluorobenzoyl)-1H-indol-5-yl)pyrazine-2-carboxamide: A novel, selective, and competitive indole-based lead inhibitor for human monoamine oxidase B. J. Enzym. Inhib. Med. Chem. 2020;35:1568–1580. doi: 10.1080/14756366.2020.1800666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elkamhawy A., Kim N.Y., Hassan A.H., Park J.-E., Yang J.-E., Elsherbeny M.H., Paik S., Oh K.-S., Lee B.H., Lee M.Y., et al. Optimization study towards more potent thiazolidine-2,4-dione IKK-β modulator: Synthesis, biological evaluation and in silico docking simulation. Bioorg. Chem. 2019;92:103261. doi: 10.1016/j.bioorg.2019.103261. [DOI] [PubMed] [Google Scholar]
- 95.Elkamhawy A., Paik S., Hassan A.H., Lee Y.S., Roh E.J. Hit discovery of 4-amino- N -(4-(3-(trifluoromethyl)phenoxy)pyrimidin-5-yl)benzamide: A novel EGFR inhibitor from a designed small library. Bioorg. Chem. 2017;75:393–405. doi: 10.1016/j.bioorg.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 96.Zhang P., Xu S., Zhu Z., Xu J. Multi-target design strategies for the improved treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019;176:228–247. doi: 10.1016/j.ejmech.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 97.Di L., Kerns E.H. Drug-Like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization. Academic Press; Cambridge, MA, USA: 2015. [Google Scholar]
- 98.Daina A., Zoete V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alavijeh M.S., Chishty M., Qaiser M.Z., Palmer A.M. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRX. 2005;2:554–571. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]