Abstract
Plants are regularly exposed to biotic and abiotic stresses that adversely affect agricultural production. Omics has gained momentum in the last two decades, fueled by statistical methodologies, computational capabilities, mass spectrometry, nucleic-acid sequencing, and peptide-sequencing platforms. Functional genomics—especially metabolomics, transcriptomics, and proteomics—have contributed substantially to plant molecular responses to stress. Recent progress in reverse and forward genetics approaches have mediated high-throughput techniques for identifying stress-related genes. Furthermore, web-based genetic databases have mediated bioinformatics techniques for detecting families of stress-tolerant genes. Gene ontology (GO) databases provide information on the gene product’s functional features and help with the computational estimation of gene function. Functional omics data from multiple platforms are useful for positional cloning. Stress-tolerant plants have been engineered using stress response genes, regulatory networks, and pathways. The genome-editing tool, CRISPR-Cas9, reveals the functional features of several parts of the plant genome. Current developments in CRISPR, such as de novo meristem induction genome-engineering in dicots and temperature-tolerant LbCas12a/CRISPR, enable greater DNA insertion precision. This review discusses functional omics for molecular insight and CRISPR-Cas9-based validation of gene function in crop plants. Omics and CRISPR-Cas9 are expected to garner knowledge on molecular systems and gene function and stress-tolerant crop production.
Keywords: plant stress, abiotic stress, biotic stress, omics, CRISPR-Cas9, crop stress tolerance
1. Introduction
Abiotic stresses, such as drought, salinity, temperature extremes, and climate change, are major considerations for scientists. The development of high-yielding varieties exposed to stress depends on direct selection for yield stability in multiple locations. Germplasm development with tolerance to biotic and abiotic factors is important for sustainable crop production [1,2]. The molecular term “omics” suggests a comprehensive assessment of numerous molecules [3]. Omics approaches offer a holistic view of the molecules that make up a cell or organism to identify genes (genomics), metabolites (metabolomics), mRNA (transcriptomics), and proteins (proteomics) in a non-biased biological context. Globally, web-based databases are an important resource for plant genomics, specifically detecting stress-reactive genes [4]. Functional genomics has helped to detect stress-related genes in crops [5,6]. Accessibility to the whole genome sequence of numerous plant species and recent developments in genomic approaches promise to deliver methods for locating stress-responsive genes at the genome-wide level. For complex trait loci, genome-wide association studies have identified stress-responsive genes and their favorable alleles. The advancement of genetic databases has enabled bioinformatics tools to identify stress-resistant gene families in various plant species using synteny and homology.
For targeted genome editing, three methods are currently available: transcription-activator-like effector nucleases (TALEN), clustered regularly interspaced short palindromic repeats (CRISPR), and zinc finger nuclease (ZFN). In cells, CRISPR-Cas9 is a cheap, easy, fast, and effective system for gene knockout [7]. For effective genome engineering, CRISPR-Cas9 has been used in animals, plants, and bacteria [8,9,10,11]. Furthermore, CRISPR-Cas9 has been used for high-throughput screening of genes, gene knockout, chromosomal loci live-cell labeling, endogenous gene expression, and single-stranded RNA (ssRNA) edition. The application of CRISPR-Cas9 for studying the function of a gene has generated disease models. However, several queries and challenges need to be addressed. CRISPR-Cas9 will likely enhance our comprehension of disease activity and its management. For targeted genome engineering, detecting programmable nucleases that produce cuts in double-strands has radically changed molecular biology; ZFNs pioneered this success, with TALEN extending the genome modifying capacity [12]. Globally, CRISPR-Cas9 received recognition from researchers for its visible benefits over TALEN and ZFN [13], being its (1) ease of designing target, (2) ability to create mutations by inserting the guided RNA and Cas9 protein, and (3) multiplexing ability to target several genes at one time [14,15]. Omics and CRISPR-Cas9 technology are poised to identify stress tolerance genes, molecular insight, and genome engineering to generate stress tolerance in crops. Developing and improving modern technologies to modify plant genomes and accumulate sufficiently large volumes of experimental molecular biological data will help create new schemes and approaches to improve economically valuable traits in plants and develop new varieties of important crops.
2. Multi-Omics Technology
2.1. Genomics
In plants, functional genomics has identified several genes that control abiotic and biotic stress reactions [16,17]. Some genes have been engineered to develop stress (biotic and abiotic) resistance in crop plants [18,19,20,21,22]. Numerous new candidate genes have been discovered from wild crop relative genomics for stress (abiotic and biotic) tolerance in crops [2,23]. For example, a high-density buckwheat complete genome sequencing genomic map, Hi-C online accessible sequencing data, and fosmid DNA libraries [17]. The authors also detected whole-genome duplication, identified numerous candidate genes for drought, cold stress, and heavy metal stress resistance, and predicted nearly 33,500 genes. Another study identified 33 transcription factors (TFs) of the tea plant using the transcriptomic and genomic database (http://planttfdb.cbi.pku.edu.cn/), which were classified into four groups (HD-Zip I to IV) after analyzing common motifs and domains [24]. A protein interaction was found. The results highlighted the diverse expression of Cshdz genes to salinity, drought, high and low temperature, and the association between Cshdz genes and resistant plants. In Solanum americanum, integrated RenSeq and genetic mapping were used to locate the genetic locus that confers resistance against late blight [25]. In wheat, MutRenSeq, a new version of RenSeq, was used to isolate R genes that confer resistance against stem rust [26]. Genome-wide analysis with ChIP-seq identified 21 ABA-associated TFs and their broad regulatory network [27]. Furthermore, a novel family of TFs was identified in Arabidopsis that was functionally involved in salt reactions and ABA. Genotyping by sequencing (GBS) is a newly discovered genomics technology for inspecting plant genetic diversity at a whole-genome level. An F2 population of Brassica olearacea was used to develop a high-density genetic map covering 879.9 cM, genotyped by 4103 single nucleotide polymorphisms (SNPs) [28]. The authors detected two major quantitative trait loci (QTLs) that confer resistance against clubroot resistance. The integration of high-throughput phenotyping and functional genomics delivers new approaches for crop improvement systems.
2.2. Transcriptomics
RNA profiling—realized recently using microarrays, gene expression, digital profiling, RNA sequencing, and serial analysis of gene expression [29]—can identify multiple stress resistance-related candidate genes, inferring relevant gene functions. The available online databases provide whole genome-wide transcriptomics data for plant stress reactions [30,31]. In Arabidopsis, transcriptomic analysis under drought and heat stress identified nearly 770 unchanged transcripts with 53 dissimilar specific proteins [32]. These findings were confirmed in sunflower [33]. Furthermore, combined heat and drought upregulated stress cytosolic ascorbate peroxidase1 (APX1) [34]. In chickpea, serial analysis of gene expression (SAGE) and next-generation sequencing (NGS) approaches were used to analyze the total transcriptome of drought- and salt-stressed plants [33,34]. Similarly, the subtractive cDNA suppression hybridization method was used in stressed chickpea plants [35]. A comparative microarray approach provided information on functional genes and pathways crosstalk in multiple stress transcriptomic studies in cotton [36]. In maize, RNA sequencing was performed to understand the adverse effects of cold, drought, salt stress, and heat stress [37]. Li et al. documented differentially expressed genes associated with signaling pathways, transcription, and metabolism [38]. RNA gel blot and microarray combined approaches verified that DREB2A, a transcription factor, controls the expression level of drought and cold stress genes [38]. Serial analysis of gene expression (SAGE) has been used extensively in plants to study gene-related responses against stresses. For example, in rice, from 5921 expressed genes, almost 10,122 tags were analyzed. Of 50,519 tags by global gene expression, 15,131 tags were similar to distinctive transcripts [39]. The integration of RNA-seq and bulked segregant analysis, called BSR-seq, has the power to enhance stress resistance in plants. For instance, Bra019409 and Bra019410 were possible candidate genes for clubroot resistance in Brassica rapa [40,41]. RNA-seq-mediated gene expression analysis could accelerate plant breeding by garnering knowledge on host-P interactions and identifying stress-related genes.
2.3. Proteomics
The qualitative and quantitative study of total proteins expressed in a cell, tissue, or organism is known as proteomics [42]. In the context of plant stress tolerance, entire proteomes are studied; however, numerous studies have investigated the cell wall proteome, organellar proteome, proteogenome, nuclear proteome, and phosphoproteome [43]. Several forms of mass spectrometry were used recently to profile the proteome in response to abiotic stresses [42,44,45]. Mass spectrometry for proteomics provides extensive proteome information when used in plant stress reactions and genome-wide studies. Proteome profiles can be compared to identify the function of particular proteins in biotic- and abiotic-induced stress signaling and differentially expressed stress-resistant proteins. Furthermore, phosphorylation group proteins play an important role in abiotic stresses [42,46]. A study on a proteome matrix in water-stressed rice identified signaling proteins and reactive oxygen species [47]. Various studies have used proteomics to highlight heavy metal stress in Brassica juncea [48], Glycine max [49], Linum usitatissimum [50], and Arabidopsis thaliana [51]. Heidarvand and Maali-Amiri (2013) comprehensively studied the proteomic profile of chickpea exposed to cold stress [52]. The phosphoproteome of wheat leaves has also been studied [53]. Several isoforms of S-adenosylmethionine in soybean under flooding and drought have been identified [54]. In tomato, signaling nuclear proteins with crosstalk chloroplast proteins were reported in drought-stressed plants [55]. Another study used tandem MS and two-dimensional gel electrophoresis (2-DE) approaches in waterlogged barley regimes to reveal the proteome profile [43]. The authors noted that sensitive barley genotypes had reduced photosynthetic performance and total biomass. Differentially expressed proteins in roots and leaves were associated with antioxidants and energy metabolism [43]. In Eriobotrya japonica, RNA-seq with isobaric tags relative absolute quantification (iTRAQ) was used to understand the cold tolerance mechanism [56]. The results revealed 1210 differentially expressed genes (DEGs) and 300 differentially expressed proteins (DEPs); of 3620 genes, only 27 shared both DEPs and DEGs. Kyoto encyclopedia of genes and genomes (KEGG) analysis predicted that biosynthesis of secondary metabolites and metabolic pathways were common. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) validation showed that gene expression of phenylalanine ammonia-lyase, anthocyanin synthase, and NADP-D-sorbitol-6-phosphate dehydrogenase was consistent with the transcriptome profile. Lou et al. suggested that these three genes play an important role in cold tolerance. Proteomics is a new technology for identifying proteins and pathways linked to the plant stress response and plant physiology. Moreover, proteomics enhances the understanding of stress-related proteins applied to molecular biology for crop improvement.
2.4. Metabolomics
Metabolomics is a high-throughput assessment of all metabolites in an organism. For exogenous and endogenous metabolites, scientists use non-targeted and targeted techniques [57]. Metabolites—including organic acids, peptides, secondary metabolites, steroids, hormones, ketones, vitamins, aldehydes, amino acids, and lipids—generate extensive data compared to transcriptomics and proteomics [58]. Advances in liquid chromatography–mass spectrometry (LC-MS), gas chromatography–mass spectrometry (GC-MS), direct injection mass spectrometry (DIMS), nuclear magnetic resonance (NMR), and high-performance liquid chromatography (HPLC) with other metabolomic approaches have further clarified stress tolerance processes and metabolite profiling [59]. There are almost 250,000 metabolites in plants; the concentration and total number are considerably higher in stressed than non-stressed environments [60]. The detection of valid metabolomic markers will enhance stress tolerance in plants [59,61]. Numerous researchers have documented metabolic profiles under stress environments in plants [62,63,64,65]. For example, drought-stressed Arabidopsis thaliana accumulated various metabolites containing proline, gamma-aminobutyrate (GABA), raffinose oligosaccharides, and others in the tricarboxylic acid cycle. Furthermore, activation of stress metabolic pathways and transcriptional regulation was dependent on abscisic acid (ABA) [66]. The superoxide dismutase gene was engineered into Populus plants, and data processing generated information on reactive oxygen species (ROS) metabolism [67]. Feng et al. (2013) reported a reduction in glycolysis-related sugar levels in salt-stressed barley leaves [68]. Shen et al. (2016) studied drought stress in chickpea varieties, which increased branched-chain amino acids and allantoin and decreased glucosamine, aspartic acid, and aromatic amino acids [8]. In Arabidopsis, transcription factor genes, Myb28 and Myb29, particularly for aliphatic GSL production and biosynthetic gene expression, unknown genes, and regulatory networks were estimated by integrating metabolic profiling and transcriptome data [69]. Furthermore, overexpression of these TFs in Arabidopsis produced industrial GSLs. Functional genomics, metabolomics, transcriptomics, and proteomics open a new direction for decoding secondary metabolism. We suggest that omics approaches from multiple platforms could provide molecular insight and enhance stress resistance through plant breeding. Table 1 summarizes some available databases and their URLs.
Table 1.
Accessible genome level databases.
| Name | Species | Database Resource | URL |
|---|---|---|---|
| TAIR | Mainly for Arabidopsis thaliana | Whole genome | http://www.arabidopsis.org |
| 1001genomes | Arabidopsis thaliana | Whole genome | http://www.1001genomes.org |
| Phytozome | Numerous | Whole genome | http://www.phytozome.net |
| NCBI | Numerous | Whole genome | http://www.ncbi.nlm.nih.gov |
| Cottongen | Gossypium spp. | Whole genome and breeding | http://www.cottongen.org |
| Soybean breeders toolbox | Glycine max | Whole genome | http://www.soybase.org |
| MaizeGDB | Zea mays | Whole genome | http://www.maizegdb.org |
| RAP-DB | Oryza sativa | Whole genome | http://rapdb.dna.affrc.go.jp |
| PlantGDB | Numerous | Whole genome | http://www.plantgdb.org |
| IWGSC | Triticum aestivum | Whole genome | http://www.wheatgenome.org |
| Gramene | Numerous | Whole genome | http://www.gramene.org |
| Ensemblplants | Numerous | Whole genome | http://plants.ensembl.org |
| KEGG | Numerous | Whole genome | http://www.genome.jp/kegg/genome/plant.html |
| Graingenes | Numerous | Whole genome | http://wheat.pw.usda.gov/GG2/index.shtm |
| PMN | Numerous | Metabolomics | http://www.plantcyc.org |
| CSB.DB | Arabidopsis thaliana | Metabolomics | http://csbdb.mpimp-golm.mpg.de/csbdb/gmd/gmd.html |
| PRIMe | Arabidopsis thaliana | Metabolomics | http://prime.psc.riken.jp/lcms/ms2tview/ms2tview.html |
| AFGN | Arabidopsis thaliana | Gene expression | https://www.deutsche-botanische-gesellschaft.de/en/about-us/afgn |
| OryzaExpress | Oryza sativa | Gene expression | http://plantomics.mind.meiji.ac.jp/OryzaExpress/ |
| RGAP | Oryza spp. | Gene expression | http://rice.plantbiology.msu.edu |
| CottonFGD | Gossypium spp. | Gene expression | http://www.cottonfgd.org |
| Genevestigator | Numerous | Gene expression | http://genevestigator.com |
| TriFLDB | Triticum aestivum | Gene expression | https://bigd.big.ac.cn/databasecommons/database/id/3452 |
| BAR | Numerous | Gene expression | http://bar.utoronto.ca/welcome.htm |
| NOBLE | Medicago truncatula | Gene expression | http://mtgea.noble.org/v2 |
| Uniprot | Numerous | Proteomics | http://www.uniprot.org/proteomes/ |
| RICE PROTEOME | Oryza sativa | Proteomics | http://gene64.dna.affrc.go.jp/RPD |
| Proteomics database | Arabidopsis thaliana | Proteomics | http://proteomics.arabidopsis.info |
| SUBA | Arabidopsis thaliana | Proteomics | http://www.suba.bcs.uwa.edu.au/ |
| AGRIS | Arabidopsis thaliana | Transcription factor | http://arabidopsis.med.ohio-state.edu |
| PlantTFDB | Numerous | Transcription factor | http://planttfdb.gao-lab.org/ |
| LegumeTFDB | Lotus japonicas, Medicago truncatula, Glycine max | Transcription factor | http://legumetfdb.psc.riken.jp |
| Grassius | Zea mays, Oryza sativa, Sorghum bicolor | Transcription factor | http://grassius.org/ |
| TRIM | Oryza sativa | Mutants | http://trim.sinica.edu.tw |
| RMD | Oryza spp. | Mutants | http://rmd.ncpgr.cn/ |
| ABRC | Arabidopsis thaliana | Mutants | http://abrc.osu.edu |
| NASC | Arabidopsis thaliana | Mutants | http://arabidopsis.org.uk/home.html |
| Fox Hunting | Numerous | Mutants | http://nazunafox.psc.database.riken.jp |
| SIGnAL | Arabidopsis thaliana | Mutants | http://signal.salk.edu |
3. CRISPR Technology
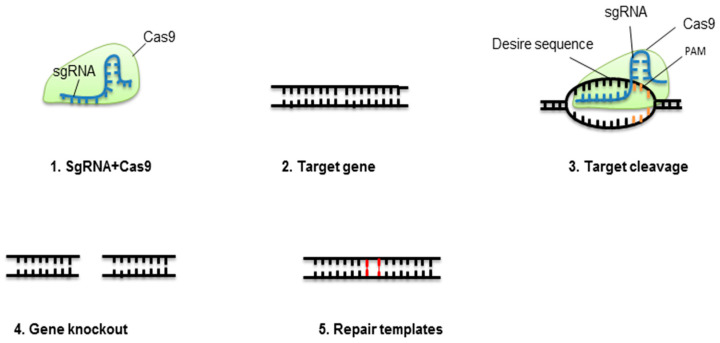
Due to its robust success, CRISPR-Cas9 is becoming a potential tool for genetically enhancing desirable crop traits, i.e., disease resistance, nutrient content, adaptation to multiple stresses, plant architecture, and yield. In some cases, a specific trait can be improved by negative regulatory gene knockout. Rice grain weight improved with gene modification of some QTL [70]. Maize grain yield under drought increased with genome engineering of the ARGOS8 locus [71]. In woody plants, CRISPR-Cas9 produced mutants in the first transgenic generation; this is significant as woody plant breeding is difficult due to their long lifespan [72,73]. Another study knocked out the OsGAN1 gene in rice and verified that it regulates root length and plant height [74]. Similarly, OsABCG26 gene knockout verified that this gene regulates pollen exine and anther cuticle, and OsTCD10 had a substantial role in chloroplasts of cold-stressed rice [75,76]. Figure 1 summarizes the principles of CRISPR-Cas9.
Figure 1.
Concept of CRISPR-Cas9-mediated gene elimination. Single guide RNA (sgRNA) containing crRNA and tracrRNA fixes to Cas9 protein. This complex will break at a specific target of the double-stranded DNA molecule. The nonhomologous end-joining pathway (NHEJ) will repair the cleaved location.
3.1. CRISPR-Cas9 Genome Engineering to Biotic Stress Tolerance
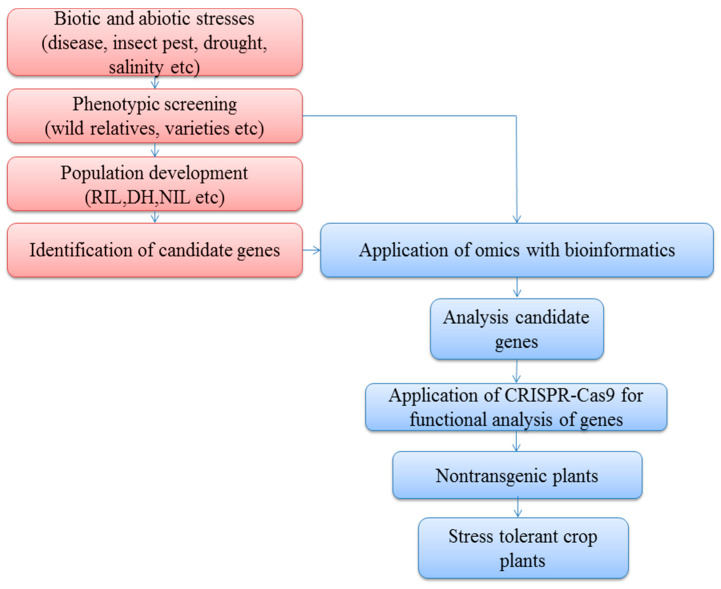
Genome editing by CRISPR-Cas9 has been used effectively in several crops, including cotton, maize, rice, and wheat. However, most genome engineering studies have targeted biotic stresses, such as diseases. In wheat, the CRISPR-Cas9 method was used successfully to knock out all three EDR1 homologs to create plants (Taedr1) with increased tolerance to powdery mildew [77]. In Arabidopsis, the knockout of susceptible gene EDR1 increased resistance to powdery mildew [78]. Recessive resistance genes, eIF (eukaryotic translation initiation factor), have been detected in several dissimilar hosts, with eIF (iso) 4E and eIF4E genes used with CRISPR-Cas9 to form virus-resistant plants in Arabidopsis and cucumber, respectively [79,80]. CsLOB1 is a susceptible gene of the citrus canker (causative agent; Xanthomonascitri); CRISPR-Cas9 was used to edit this gene to develop resistant grapefruit plants [81,82]. Additionally, a negative resistance function MLO gene, responsible for powdery mildew susceptibility, was mutated successfully by Cas9 knockouts to enhance resistance against powdery mildew in tomato and wheat [83,84,85]. The application of CRISPR-Cas9 as an antivirus tool cleaved beet severe curly top virus, which decreased the viral infection [86,87]. The rice tungro spherical virus (RTSV), linked to the negatively controlled susceptible eIF4G gene, was eliminated using CRISPR-Cas9 to develop resistant rice varieties [88]. From CRISPR-Cas9, the loss of function VvWRKY52 gene produced resistance against Botrytis cinerea in grape (Vitis vinifera) [89]. Furthermore, CRISPR-Cas9 has been used to interrupt multiple virus genomes, including CLCuK0V, TYLCSV, and TYLCV [90]. For cucumber mosaic virus and tobacco mosaic virus, a technology to modify RNA virus genomes has been advanced from sgRNA and FnCas9. Hence, molecular immunity to RNA viruses was mediated by sgRNA/FnCas9 expression in Arabidopsis and tobacco [91]. CRISPR-Cas9 successfully targeted OsERF922 against blast fungus resistance in rice [92]. Plant ethylene-responsive factors (ERFs) can control tolerance against various stresses because they are involved in the ethylene (cytokinin) pathway [93]. When taken together, these reports deliver robust indications that CRISPR-Cas9 can enhance biotic stress resistance in plants. Figure 2 summarizes omics and CRISPR-Cas9 strategies for stress-tolerant crop production.
Figure 2.
Omics and CRISPR-Cas9 strategies for garnering knowledge on molecular systems and gene function as the main objective for producing stress-tolerant crop plants. RIL: Recombinant inbred line, DH: Doubled haploid, NIL: Near isogenic line.
3.2. CRISPR-Cas9 Genome Engineering to Abiotic Stress Tolerance
Abiotic stress tolerance mediated by various genes is a complex trait. There are major interactions and crosstalk among components of metabolic, regulatory, and signaling pathways [94,95]. CRISPR-Cas9-mediated genome editing can be used to modify almost any sequence (depending on accessibility to the protospacer adjacent motif, PAM site) to reveal its function in the genome. Molecular breeders have discovered numerous abiotic-stress-resistant T genes and engineered them into crop plants. CRISPR-Cas9-generated mitogen-activated protein kinases3 (slmapk3) gene mutants increased the defense response to drought in tomato (Solanum lycopersicum) [96]. CRISPR-Cas9 was used to generate mutants in rice to understand the mechanism of stress-ABA-activated protein kinase2 [97]. In Arabidopsis under cold stress, CRISPR-Cas9 was used to generate mutants (cbfs double and triple mutants) to determine the role of C-repeat binding factors [98]. In maize, the CRISPR-Cas9 approach was used to increase the expression level of the ARGOS8 gene (negatively regulate ethylene response) to develop drought tolerance; the promoter of ARGOS8 changed into GOS2. These mutants had enhanced grain yields under drought conditions in the field [69]. Moreover, overexpressing TaCP and SPCP2 increased drought tolerance in Arabidopsis [99,100,101]. Plants overexpressing the melatonin biosynthesis genes were identified as abiotic stress-tolerant [102,103]. In hybrid rice, targeted editing of the TMS5 gene led to the rapid formation of temperature-sensitive breeding lines [104]. Plant breeding activities may have reduced T gene alleles after selecting yield-related genes during domestication programs [105]. Breeders have developed stress-tolerant crops with gene function information [3]. The above examples show that CRISPR-Cas9 can modify/eliminate genes to mediate resistance against numerous abiotic stresses, e.g., salinity, drought, extreme temperatures, heavy metals, and nutrient deficiencies [106,107] (Table 2).
Table 2.
CRISPR-Cas9 application for crop improvement.
| Species | Traits | Target Genes | Reference |
|---|---|---|---|
| Abiotic stresses | |||
| Rice | Improved resistance to arsenic stress | ARM1 | [108] |
| Depletion of Cd into grain | LCT1 | [109] | |
| Depletion of Cd into grain | Nramp5 | [107] | |
| Drought tolerance | SAPK2 | [97] | |
| Tomato | Drought tolerance | SIMAPK3 | [96] |
| Maize | Drought tolerance | ARGOS8 | [69] |
| Arabidopsis | Cold tolerance | CBF1 CBF2 | [99] |
| Biotic stresses | |||
| Arabidopsis | Resistance to turnip mosaic virus | eIF (iso)4E | [80] |
| Wheat | Improved resistance to powdery mildew | TaMLO | [85] |
| Improved resistance to powdery mildew | EDR1 | [77] | |
| Rice | Increased resistance to blast fungus | OsERF922 | [92] |
| Increased resistance to tungro spherical virus | eIF4G | [88] | |
| Barley | Improved resistance to fungal pathogens | MORC1 | [110] |
| Orange | Improved resistance to citrus canker | CsLOB1 | [111] |
| Tomato | Improved resistance to powdery mildew | Mlo1 | [84] |
| Anthocyanin biosynthesis | ANT1 | [112] | |
| Grape | Improved resistance to Botrytis cinerea | WRKY52 | [89] |
| Cucumber | Virus resistance | eIF4E | [79] |
4. Conclusions and Perspectives
High-throughput verification of experimental platforms is required to reveal gene functions in plants. Innovative efforts include plant phenotyping (http://www.lemnatec.com), metabolomics, and enzyme assay (http://www.biolog.com) platforms [113]. However, the prediction of gene function based on networks is an active research area but limited in plant science. We need additional data, easy access to tools and data, improved data analysis, and high-throughput verification from experiments to achieve a network-based gene function identification goal. Omics technologies, databases, and bioinformatics tools primarily provide information on candidate genes, biosynthetic pathways, proteins, master regulators, biological networks, and cross talk, especially on the plant stress response.
The CRISPR-Cas9-mediated genome editing system has fundamentally influenced gene function research and, ultimately, crop improvement [114,115]. The plant genome engineering approach has no ethical issues. CRISPR-Cas9 mutants are generated with greater efficiency and specificity than TALEN and ZFN. Hence, the CRISPR-Cas9-mediated genome editing system has great potential for practical research. Various CRISPR-Cas9 platforms have been developed for plant genome engineering but require advanced targets for specificity and efficiency.
Moreover, gene replacement and DNA part knock-in is a challenge [116]. Cas9 variants, gene repression, and activation domains can control target gene expression [117]. Hence, this system could be used to develop climate-resilient crops.
A toolbox based on CRISPR-Cas9 has been established for gene repression and activation in plants [118]. CRISPR-Cas9 could be adapted for new approaches, e.g., epigenomic regulation, chromatin imaging, and RNA cleavage [40,41,119]. Given its versatility, simplicity, efficiency, and flexibility, the future of functional genomics is likely to depend on the CRISPR-Cas9 system. Omics and CRISPR have provided a snapshot for improving an organism’s functioning and interactions at the cell and tissue level by depicting and measuring biomolecules.
Author Contributions
M.K.R. prepared the article outline and wrote the omics section; M.A., S.R., and S.I. wrote the CRISPR section; S.M. and M.A.K. reviewed the article; K.H.M.S. conceived the basic idea and reviewed and improved the article. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manavalan L.P., Guttikonda S.K., Phan Tran L.-S., Nguyen H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009;50:1260. doi: 10.1093/pcp/pcp082. [DOI] [PubMed] [Google Scholar]
- 2.Razzaq M.K., Rauf S., Khurshid M., Iqbal S., Bhat J.A., Farzand A., Riaz A., Xing G., Gai J. Pollen viability an index of abiotic stresses tolerance and methods for the improved pollen viability. Pak. J. Agric. Res. 2019;32 doi: 10.17582/journal.pjar/2019/32.4.609.624. [DOI] [Google Scholar]
- 3.Hasin Y., Seldin M., Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:1. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karahalil B. Overview of systems biology and omics technologies. Curr. Med. Chem. 2016;23:4221. doi: 10.2174/0929867323666160926150617. [DOI] [PubMed] [Google Scholar]
- 5.Sharma M., Pandey G.K. Genomics and functional genomics of stress-mediated signaling in plants: Volume I. Curr. Genom. 2017;18:467. doi: 10.2174/138920291806170929123912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh B., Salaria N., Thakur K., Kukreja S., Gautam S., Goutam U. Functional genomic approaches to improve crop plant heat stress tolerance. F1000Research. 2019;8 doi: 10.12688/f1000research.19840.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan N., Bano A., Rahman M.A., Rathinasabapathi B., Babar M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2019;42:115. doi: 10.1111/pce.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Z., Zhang B., Ding W., Liu X., Yang D.-L., Wei P., Cao F., Zhu S., Zhang F., Mao Y. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013;23:1229. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang W., Zhou H., Bi H., Fromm M., Yang B., Weeks D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013;41:e188. doi: 10.1093/nar/gkt780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J.-F., Norville J.E., Aach J., McCormack M., Zhang D., Bush J., Church G.M., Sheen J. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013;31:688. doi: 10.1038/nbt.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan Q., Wang Y., Li J., Zhang Y., Chen K., Liang Z., Zhang K., Liu J., Xi J.J., Qiu J.-L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013;31:686. doi: 10.1038/nbt.2650. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasegaran S., Carroll D. Origins of programmable nucleases for genome engineering. J. Mol. Biol. 2016;428:963. doi: 10.1016/j.jmb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao Y., Zhang H., Xu N., Zhang B., Gou F., Zhu J.-K. Application of the CRISPR–Cas system for efficient genome engineering in plants. Mol. Plant. 2013;6:2008. doi: 10.1093/mp/sst121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X., Zhang Q., Zhu Q., Liu W., Chen Y., Qiu R., Wang B., Yang Z., Li H., Lin Y. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015;8:1274. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Malzahn A., Lowder L., Qi Y. Plant genome editing with TALEN and CRISPR. Cell Biosci. 2017;7:21. doi: 10.1186/s13578-017-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P., Su L., Gao H., Jiang X., Wu X., Li Y., Zhang Q., Wang Y., Ren F. Genome-wide characterization of bHLH genes in grape and analysis of their potential relevance to abiotic stress tolerance and secondary metabolite biosynthesis. Front. Plant Sci. 2018;9:64. doi: 10.3389/fpls.2018.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Li X., Ma B., Gao Q., Du H., Han Y., Li Y., Cao Y., Qi M., Zhu Y. The tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant. 2017;10:1224. doi: 10.1016/j.molp.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Chen S., Jiang J., Li H., Liu G. The salt-responsive transcriptome of Populussimonii × Populusnigra via DGE. Gene. 2012;504:203. doi: 10.1016/j.gene.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Gilliham M., Able J.A., Roy S.J. Translating knowledge about abiotic stress tolerance to breeding programmes. Plant J. 2017;90:898. doi: 10.1111/tpj.13456. [DOI] [PubMed] [Google Scholar]
- 20.Le D.T., Nishiyama R., Watanabe Y., Tanaka M., Seki M., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.-S.P. Differential gene expression in soybean leaf tissues at late developmental stages under drought stress revealed by genome-wide transcriptome analysis. PLoS ONE. 2012;7:e49522. doi: 10.1371/journal.pone.0049522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiry A.A., Chavez Dulanto P.N., Reynolds M.P., Davies W.J. How can we improve crop genotypes to increase stress resilience and productivity in a future climate? A new crop screening method based on productivity and resistance to abiotic stress. J. Exp. Bot. 2016;67:5593. doi: 10.1093/jxb/erw330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao D., Zhang X., Zhao X., Liu C., Wang C., Zhang Z., Zhang C., Wei Q., Wang Q., Yan H. Transcriptome analysis reveals salt-stress-regulated biological processes and key pathways in roots of cotton (Gossypium hirsutum L.) Genomics. 2011;98:47. doi: 10.1016/j.ygeno.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Rauf S., Warburton M., Naeem A., Kainat W. Validated markers for sunflower (Helianthus annuus L.) breeding. Oilseeds Fats Crop. Lipids. 2020;27 doi: 10.1051/ocl/2020042. [DOI] [Google Scholar]
- 24.Shen W., Li H., Teng R., Wang Y., Wang W., Zhuang J. Genomic and transcriptomic analyses of HD-Zip family transcription factors and their responses to abiotic stress in tea plant. Genomics. 2019;111:1142. doi: 10.1016/j.ygeno.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Witek K., Jupe F., Witek A.I., Baker D., Clark M.D., Jones J.D. Accelerated cloning of a potato late blight resistance gene using RenSeq and SMRT sequencing. Nat. Biotechnol. 2016;34:656. doi: 10.1038/nbt.3540. [DOI] [PubMed] [Google Scholar]
- 26.Steuernagel B., Periyannan S.K., Hernandez Pinzon I., Witek K., Rouse M.N., Yu G., Lagudah E.S. Rapid cloning of disease resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 2016;34:652. doi: 10.1038/nbt.3543. [DOI] [PubMed] [Google Scholar]
- 27.Song L., Huang S.S.C., Wise A., Castanon R., Nery J.R., Chen H., Ecker J.R. A transcription factor hierarchy defines an environmental stress response network. Science. 2016;354:6312. doi: 10.1126/science.aag1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J., Izzah N.K., Choi B., Joh H.J., Lee S., Perumal S., Seo J., Ahn K., Jo E.J., Choi G.J. Genotyping by sequencing map permits identification of clubroot resistance QTLs and revision of the reference genome assembly in cabbage (Brassica oleracea L.) DNA Res. 2015;23:29. doi: 10.1093/dnares/dsv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leisner C.P., Yendrek C.R., Ainsworth E.A. Physiological and transcriptomic responses in the seed coat of field-grown soybean (Glycine max L. Merr.) to abiotic stress. BMC Plant Biol. 2017;17:242. doi: 10.1186/s12870-017-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Xu Y., Huang B. Lipidomic reprogramming associated with drought stress priming-enhanced heat tolerance in tall fescue (Festuca arundinacea) Plant Cell Environ. 2019;42:947. doi: 10.1111/pce.13405. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Yao C., Fu S., Xuan H., Wen S., Liu C., Li F., Liu A., Bi S., Zhang S., et al. Stress2TF: A manually curated database of TF regulation in plant response to stress. Gene. 2018;638:36. doi: 10.1016/j.gene.2017.09.067. [DOI] [PubMed] [Google Scholar]
- 32.Rizhsky L., Liang H., Shuman J., Shulaev V., Davletova S., Mittler R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134:1683. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewezi T., Léger M., Gentzbittel L. A comprehensive analysis of the combined effects of high light and high temperature stresses on gene expression in sunflower. Ann. Bot. 2008;102:127. doi: 10.1093/aob/mcn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koussevitzky S., Suzuki N., Huntington S., Armijo L., Sha W., Cortes D., Shulaev V., Mittler R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008;283:34197. doi: 10.1074/jbc.M806337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain D., Chattopadhyay D. Analysis of gene expression in response to water deficit of chickpea (Cicer arietinum L.) varieties differing in drought tolerance. BMC Plant Biol. 2010;10:24. doi: 10.1186/1471-2229-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y.-N., Shi D.-Q., Ruan M.-B., Zhang L.-L., Meng Z.-H., Liu J., Yang W.-C. Transcriptome analysis reveals crosstalk of responsive genes to multiple abiotic stresses in cotton (Gossypium hirsutum L.) PLoS ONE. 2013;8:e80218. doi: 10.1371/journal.pone.0080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li P., Cao W., Fang H., Xu S., Yin S., Zhang Y., Lin D., Wang J., Chen Y., Xu C. Transcriptomic profiling of the maize (Zea mays L.) leaf response to abiotic stresses at the seedling stage. Front. Plant Sci. 2017;8:290. doi: 10.3389/fpls.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H., Qin F. Genome wide association study reveals natural variations contributing to drought resistance in crops. Front. Plant Sci. 2017;8:1110. doi: 10.3389/fpls.2017.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbings J.G., Cook B.P., Dufault M.R., Madden S.L., Khuri S., Turnbull C.J. Global transcript analysis of rice leaf and seed using SAGE technology. Plant Biotechnol. J. 2003;1:271. doi: 10.1046/j.1467-7652.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 40.Hilton I.B., D’Ippolito A.M., Vockley C.M., Thakore P.I., Crawford G.E., Reddy T.E., Gersbach C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015;33:510. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kearns N.A., Pham H., Tabak B., Genga R.M., Silverstein N.J., Garber M., Maehr R. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nature Methods. 2015;12:401. doi: 10.1038/nmeth.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luan H., Shen H., Pan Y., Guo B., Lv C., Xu R. Elucidating the hypoxic stress response in barley (Hordeum vulgare L.) during waterlogging: A proteomics approach. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-27726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagami H., Sugiyama N., Ishihama Y., Shirasu K. Shotguns in the front line: Phosphoproteomics in plants. Plant Cell Physiol. 2012;53:118. doi: 10.1093/pcp/pcr148. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu S., Kamal A.H., Hossain Z. Wheat proteomics: Proteome modulation and abiotic stress acclimation. Front. Plant Sci. 2014;5:684. doi: 10.3389/fpls.2014.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao S., Guo T., Aebersold R. Mass spectrometry-based proteomic quest for diabetes biomarkers. Biochimica et Biophysica Acta (BBA) Proteins Proteom. 2015;1854:519. doi: 10.1016/j.bbapap.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Yin X., Komatsu S. Quantitative proteomics of nuclear phosphoproteins in the root tip of soybean during the initial stages of flooding stress. J. Proteom. 2015;119:183. doi: 10.1016/j.jprot.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Pandey A., Rajamani U., Verma J., Subba P., Chakraborty N., Datta A., Chakraborty S., Chakraborty N. Identification of extracellular matrix proteins of rice (Oryza sativa L.) involved in dehydration-responsive network: A proteomic approach. J. Proteome Res. 2010;9:3443. doi: 10.1021/pr901098p. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez S., Berla B.M., Sheffield J., Cahoon R.E., Jez J.M., Hicks L.M. Comprehensive analysis of the Brassica juncea root proteome in response to cadmium exposure by complementary proteomic approaches. Proteomics. 2009;9:2419. doi: 10.1002/pmic.200800478. [DOI] [PubMed] [Google Scholar]
- 49.Hossain Z., Hajika M., Komatsu S. Comparative proteome analysis of high and low cadmium accumulating soybeans under cadmium stress. Amino Acids. 2012;43:2393. doi: 10.1007/s00726-012-1319-6. [DOI] [PubMed] [Google Scholar]
- 50.Hradilová J., Řehulka P., Řehulková H., Vrbová M., Griga M., Brzobohatý B. Comparative analysis of proteomic changes in contrasting flax cultivars upon cadmium exposure. Electrophoresis. 2010;31:421. doi: 10.1002/elps.200900477. [DOI] [PubMed] [Google Scholar]
- 51.Semane B., Dupae J., Cuypers A., Noben J.-P., Tuomainen M., Tervahauta A., Kärenlampi S., Van Belleghem F., Smeets K., Vangronsveld J. Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J. Plant Physiol. 2010;167:247. doi: 10.1016/j.jplph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Heidarvand L., Maali-Amiri R. Physio-biochemical and proteome analysis of chickpea in early phases of cold stress. J. Plant Physiol. 2013;170:459. doi: 10.1016/j.jplph.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M., Lv D., Ge P., Bian Y., Chen G., Zhu G., Li X., Yan Y. Phosphoproteome analysis reveals new drought response and defense mechanisms of seedling leaves in bread wheat (Triticum aestivum L.) J. Proteom. 2014;109:290. doi: 10.1016/j.jprot.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y.S., Yao H.Y., Xue H.W. Lipidomic profiling analysis reveals the dynamics of phospholipid molecules in Arabidopsis thaliana seedling growth. J. Integr. Plant Biol. 2016;58:890. doi: 10.1111/jipb.12481. [DOI] [PubMed] [Google Scholar]
- 55.Tamburino R., Vitale M., Ruggiero A., Sassi M., Sannino L., Arena S., Costa A., Batelli G., Zambrano N., Scaloni A. Chloroplast proteome response to drought stress and recovery in tomato (Solanum lycopersicum L.) BMC Plant Biol. 2017;17 doi: 10.1186/s12870-017-0971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lou X., Wang H., Ni X., Gao Z., Iqbal S. Integrating proteomic and transcriptomic analyses of loquat in response to cold stress. Gene. 2018;677:57. doi: 10.1016/j.gene.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 57.Frederich M., Pirotte B., Fillet M., De Tullio P. Metabolomics as a challenging approach for medicinal chemistry and personalized medicine. J. Med. Chem. 2016;59:8649. doi: 10.1021/acs.jmedchem.5b01335. [DOI] [PubMed] [Google Scholar]
- 58.Dos Santos V.S., Macedo F.A., Do Vale J.S., Silva D.B., Carollo C.A. Metabolomics as a tool for understanding the evolution of Tabebuias ensulato. Metabolomics. 2017;13:72. doi: 10.1007/s11306-017-1209-8. [DOI] [Google Scholar]
- 59.Parida A.K., Panda A., Rangani J. Plant Metabolites and Regulation under Environmental Stress. Elsevier; Amsterdam, The Netherlands: 2018. Metabolomics-guided elucidation of abiotic stress tolerance mechanisms in plants; p. 89. [Google Scholar]
- 60.Kim H.K., Choi Y.H., Verpoorte R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010;5:536. doi: 10.1038/nprot.2009.237. [DOI] [PubMed] [Google Scholar]
- 61.Vickers N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017;27:R713. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 62.Muthuramalingam P., Jeyasri R., Selvaraj A., Pandian S.K., Ramesh M. Integrated transcriptomic and metabolomic analyses of glutamine metabolism genes unveil key players in Oryza sativa (L.) to ameliorate the unique and combined abiotic stress tolerance. Int. J. Biol. Macromol. 2020;164:222. doi: 10.1016/j.ijbiomac.2020.07.143. [DOI] [PubMed] [Google Scholar]
- 63.Muthuramalingam P., Krishnan S.R., Pandian S., Mareeswaran N., Aruni W., Pandian S.K., Ramesh M. Global analysis of threonine metabolism genes unravel key players in rice to improve the abiotic stress tolerance. Sci. Rep. 2018;8:9270. doi: 10.1038/s41598-018-27703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lakshmanan M., Cheung C., Mohanty B., Lee D.-Y. Modeling rice metabolism: From elucidating environmental effects on cellular phenotype to guiding crop improvement. Front. Plant Sci. 2016;7:1795. doi: 10.3389/fpls.2016.01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jwa N.-S., Agrawal G.K., Tamogami S., Yonekura M., Han O., Iwahashi H., Rakwal R. Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem. 2006;44:261. doi: 10.1016/j.plaphy.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Urano K., Maruyama K., Ogata Y., Morishita Y., Takeda M., Sakurai N., Suzuki H., Saito K., Shibata D., Kobayashi M. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009;57:1065. doi: 10.1111/j.1365-313X.2008.03748.x. [DOI] [PubMed] [Google Scholar]
- 67.Srivastava V., Obudulu O., Bygdell J., Löfstedt T., Rydén P., Nilsson R., Ahnlund M., Johansson A., Jonsson P., Freyhult E. OnPLS integration of transcriptomic, proteomic and metabolomic data shows multi-level oxidative stress responses in the cambium of transgenic hipI-superoxide dismutase Populus plants. BMC Genom. 2013;14:893. doi: 10.1186/1471-2164-14-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen Q., Fu L., Dai F., Jiang L., Zhang G., Wu D. Multi-omics analysis reveals molecular mechanisms of shoot adaption to salt stress in Tibetan wild barley. BMC Genom. 2016;17:889. doi: 10.1186/s12864-016-3242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirai M.Y., Sugiyama K., Sawada Y., Tohge T., Obayashi T., Suzuki A., Goda H. Omics based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA. 2007;104:6478. doi: 10.1073/pnas.0611629104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu R., Yang Y., Qin R., Li H., Qiu C., Li L., Wei P., Yang J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. Yi Chuanxuebao. 2016;43:529. doi: 10.1016/j.jgg.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Shi J., Gao H., Wang H., Lafitte H.R., Archibald R.L., Yang M., Hakimi S.M., Mo H., Habben J.E. ARGOS 8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017;15:207. doi: 10.1111/pbi.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan D., Liu T., Li C., Jiao B., Li S., Hou Y., Luo K. Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Sci. Rep. 2015;5:12217. doi: 10.1038/srep12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai C.-J., Xue L.-J. CRISPRing into the woods. GM Crop. Food. 2015;6:206. doi: 10.1080/21645698.2015.1091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma X., Feng F., Wei H., Mei H., Xu K., Chen S., Li T., Liang X., Liu H., Luo L. Genome-wide association study for plant height and grain yield in rice under contrasting moisture regimes. Front. Plant Sci. 2016;7:1801. doi: 10.3389/fpls.2016.01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang Z., Chen Z., Yan W., Xie G., Lu J., Wang N., Lu Q., Yao N., Yang G., Xia J. An ABC transporter, OsABCG26, is required for anther cuticle and pollen exine formation and pollen-pistil interactions in rice. Plant Sci. 2016;253:21. doi: 10.1016/j.plantsci.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Wu L., Wu J., Liu Y., Gong X., Xu J., Lin D., Dong Y. The rice pentatricopeptide repeat gene TCD10 is needed for chloroplast development under cold stress. Rice. 2016;9 doi: 10.1186/s12284-016-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y., Bai Y., Wu G., Zou S., Chen Y., Gao C., Tang D. Simultaneous modification of three homoeologs of Ta EDR 1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017;91:714. doi: 10.1111/tpj.13599. [DOI] [PubMed] [Google Scholar]
- 78.Frye C.A., Tang D., Innes R.W. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc. Natl. Acad. Sci. USA. 2001;98:373. doi: 10.1073/pnas.98.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chandrasekaran J., Brumin M., Wolf D., Leibman D., Klap C., Pearlsman M., Sherman A., Arazi T., Gal-On A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016;17:1140. doi: 10.1111/mpp.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pyott D.E., Sheehan E., Molnar A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016;17:1276. doi: 10.1111/mpp.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu Y., Zhang J., Jia H., Sosso D., Li T., Frommer W.B., Yang B., White F.F., Wang N., Jones J.B. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA. 2014;111:E521. doi: 10.1073/pnas.1313271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jia H., Zhang Y., Orbović V., Xu J., White F.F., Jones J.B., Wang N. Genome editing of the disease susceptibility gene Cs LOB 1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017;15:817. doi: 10.1111/pbi.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Humphry M., Consonni C., Panstruga R. mlo-based powdery mildew immunity: Silver bullet or simply non-host resistance? Mol. Plant Pathol. 2006;7:605. doi: 10.1111/j.1364-3703.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 84.Nekrasov V., Wang C., Win J., Lanz C., Weigel D., Kamoun S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-00578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Cheng X., Shan Q., Zhang Y., Liu J., Gao C., Qiu J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014;32:947. doi: 10.1038/nbt.2969. [DOI] [PubMed] [Google Scholar]
- 86.Chaparro-Garcia A., Kamoun S., Nekrasov V. Boosting plant immunity with CRISPR/Cas. Genome Biol. 2015;16:254. doi: 10.1186/s13059-015-0829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji X., Zhang H., Zhang Y., Wang Y., Gao C. Establishing a CRISPR-Cas-like immune system conferring DNA virus resistance in plants. Nat. Plants. 2015;1:15144. doi: 10.1038/nplants.2015.144. [DOI] [PubMed] [Google Scholar]
- 88.Macovei A., Sevilla N.R., Cantos C., Jonson G.B., Slamet-Loedin I., Čermák T., Voytas D.F., Choi I.R., Chadha-Mohanty P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol. J. 2018;16:1918. doi: 10.1111/pbi.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X., Tu M., Wang D., Liu J., Li Y., Li Z., Wang Y., Wang X. CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol. J. 2018;16:844. doi: 10.1111/pbi.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaidi S.S.-E.-A., Tashkandi M., Mansoor S., Mahfouz M.M. Engineering plant immunity: Using CRISPR/Cas9 to generate virus resistance. Front. Plant Sci. 2016;7:1673. doi: 10.3389/fpls.2016.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang T., Zheng Q., Yi X., An H., Zhao Y., Ma S., Zhou G. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol. J. 2018;16:1415. doi: 10.1111/pbi.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang F., Wang C., Liu P., Lei C., Hao W., Gao Y., Liu Y.-G., Zhao K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE. 2016;11:e0154027. doi: 10.1371/journal.pone.0154027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jung J., Won S.Y., Suh S.C., Kim H., Wing R., Jeong Y., Hwang I., Kim M. The barley ERF-type transcription factor HvRAF confers enhanced pathogen resistance and salt tolerance in Arabidopsis. Planta. 2007;225:575. doi: 10.1007/s00425-006-0373-2. [DOI] [PubMed] [Google Scholar]
- 94.Garg R., Verma M., Agrawal S., Shankar R., Majee M., Jain M. Deep transcriptome sequencing of wild halophyte rice, Porteresia coarctata, provides novel insights into the salinity and submergence tolerance factors. DNA Res. 2014;21:69. doi: 10.1093/dnares/dst042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mickelbart M.V., Hasegawa P.M., Bailey-Serres J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015;16:237. doi: 10.1038/nrg3901. [DOI] [PubMed] [Google Scholar]
- 96.Wang L., Chen L., Li R., Zhao R., Yang M., Sheng J., Shen L. Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J. Agric. Food Chem. 2017;65:8674. doi: 10.1021/acs.jafc.7b02745. [DOI] [PubMed] [Google Scholar]
- 97.Lou D., Wang H., Liang G., Yu D. OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front. Plant Sci. 2017;8:993. doi: 10.3389/fpls.2017.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jia Y., Ding Y., Shi Y., Zhang X., Gong Z., Yang S. The cbfs triple mutants reveal the essential functions of CBF s in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345. doi: 10.1111/nph.14088. [DOI] [PubMed] [Google Scholar]
- 99.Chen H.-J., Su C.-T., Lin C.-H., Huang G.-J., Lin Y.-H. Expression of sweet potato cysteine protease SPCP2 altered developmental characteristics and stress responses in transgenic Arabidopsis plants. J. Plant Physiol. 2010;167:838. doi: 10.1016/j.jplph.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Liu H., Hu M., Wang Q., Cheng L., Zhang Z. Role of papain-like cysteine proteases in plant development. Front. Plant Sci. 2018;9:1717. doi: 10.3389/fpls.2018.01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zang Q.-W., Wang C.-X., Li X.-Y., Guo Z.-A., Jing R.-L., Zhao J., Chang X.-P. Isolation and characterization of a gene encoding a polyethylene glycol-induced cysteine protease in common wheat. J. Biosci. 2010;35:379. doi: 10.1007/s12038-010-0043-1. [DOI] [PubMed] [Google Scholar]
- 102.Antoniou C., Chatzimichail G., Xenofontos R., Pavlou J.J., Panagiotou E., Christou A., Fotopoulos V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017;62:e12401. doi: 10.1111/jpi.12401. [DOI] [PubMed] [Google Scholar]
- 103.Byeon Y., Back K. Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotoninmethyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxic conditions. J. Pineal Res. 2016;60:348. doi: 10.1111/jpi.12317. [DOI] [PubMed] [Google Scholar]
- 104.Zhou H., He M., Li J., Chen L., Huang Z., Zheng S., Zhu L., Ni E., Jiang D., Zhao B. Development of commercial thermo-sensitive genic male sterile rice accelerates hybrid rice breeding using the CRISPR/Cas9-mediated TMS5 editing system. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep37395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rauf S., da Silva J.T., Khan A.A., Naveed A. Consequences of plant breeding on genetic diversity. Int. J. Plant Breed. 2010;4:1–21. [Google Scholar]
- 106.Abdelrahman M., Al-Sadi A.M., Pour-Aboughadareh A., Burritt D.J., Tran L.-S.P. Genome editing using CRISPR/Cas9–targeted mutagenesis: An opportunity for yield improvements of crop plants grown under environmental stresses. Plant Physiol. Biochem. 2018;131:31. doi: 10.1016/j.plaphy.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 107.Tang L., Mao B., Li Y., Lv Q., Zhang L., Chen C., He H., Wang W., Zeng X., Shao Y. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang F.-Z., Chen M.-X., Yu L.-J., Xie L.-J., Yuan L.-B., Qi H., Xiao M., Guo W., Chen Z., Yi K. OsARM1, an R2R3 MYB transcription factor, is involved in regulation of the response to arsenic stress in rice. Front. Plant Sci. 2017;8:1868. doi: 10.3389/fpls.2017.01868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu H.P., Liu S.M., Xu S.L., Chen W.Y., Zhou X., Tan Y.Y., Huang J.Z., Shu Q.Y. CRISPR-S: An active interference element for a rapid and inexpensive selection of genome-edited, transgene-free rice plants. Plant Biotechnol. J. 2017;15:1371. doi: 10.1111/pbi.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumar N., Galli M., Ordon J., Stuttmann J., Kogel K.H., Imani J. Further analysis of barley MORC 1 using a highly efficient RNA-guided Cas9 gene-editing system. Plant Biotechnol. J. 2018;16:1892. doi: 10.1111/pbi.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng A., Chen S., Lei T., Xu L., He Y., Wu L., Yao L., Zou X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene Cs LOB 1 promoter in citrus. Plant Biotechnol. J. 2017;15:1509. doi: 10.1111/pbi.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Čermák T., Baltes N.J., Čegan R., Zhang Y., Voytas D.F. High-frequency, precise modification of the tomato genome. Genome Biol. 2015;16:232. doi: 10.1186/s13059-015-0796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fiehn O. Functional Genomics. Springer; Berlin/Heidelberg, Germany: 2002. Metabolomics—The link between genotypes and phenotypes; p. 155. [PubMed] [Google Scholar]
- 114.Belhaj K., Chaparro-Garcia A., Kamoun S., Patron N.J., Nekrasov V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015;32:76. doi: 10.1016/j.copbio.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 115.Ma X.L., Liu Y.G. CRISPR/Cas9-based genome editing systems and the analysis of targeted genome mutations in plants. Hereditas. 2016;38:118. doi: 10.16288/j.yczz.15-395. [DOI] [PubMed] [Google Scholar]
- 116.Ma X., Zhu Q., Chen Y., Liu Y.G. CRISPR/Cas9 platforms for genome editing in plants: Developments and applications. Mol. Plant. 2016;9:961. doi: 10.1016/j.molp.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 117.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., Stern-Ginossar N., Brandman O., Whitehead E.H., Doudna J.A., et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lowder L.G., Zhang D., Baltes N.J., Paul J.W., 3rd, Tang X., Zheng X., Voytas D.F., Hsieh T.F., Zhang Y., Qi Y. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiol. 2015;169:971. doi: 10.1104/pp.15.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Connell M.R., Oakes B.L., Sternberg S.H., East-Seletsky A., Kaplan M., Doudna J.A. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature. 2014;516:263. doi: 10.1038/nature13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.