Abstract
The surfaces of grapes are covered by different yeast species that are important in the first stages of the fermentation process. In recent years, non-Saccharomyces yeasts such as Torulaspora delbrueckii, Lachancea thermotolerans, Metschnikowia pulcherrima, and Pichia kluyveri have become popular with regard to winemaking and improved wine quality. For that reason, several manufacturers started to offer commercially available strains of these non-Saccharomyces species. P. kluyveri stands out, mainly due to its contribution to wine aroma, glycerol, ethanol yield, and killer factor. The metabolism of the yeast allows it to increase volatile molecules such as esters and varietal thiols (aroma-active compounds), which increase the quality of specific varietal wines or neutral ones. It is considered a low- or non-fermentative yeast, so subsequent inoculation of a more fermentative yeast such as Saccharomyces cerevisiae is indispensable to achieve a proper fermented alcohol. The impact of P. kluyveri is not limited to the grape wine industry; it has also been successfully employed in beer, cider, durian, and tequila fermentation, among others, acting as a promising tool in those fermentation processes. Although no Pichia species other than P. kluyveri is available in the regular market, several recent scientific studies show interesting improvements in some wine quality parameters such as aroma, polysaccharides, acid management, and color stability. This could motivate yeast manufacturers to develop products based on those species in the near future.
Keywords: Pichia kluyveri, thiols, higher alcohols, esters, fatty acids, wine, P. anomala, P. fermentans, P. guilliermondii, P. kudriavzevii, P. membranifaciens
1. Introduction
Usually, indigenous non-Saccharomyces yeasts are present in grape musts in greater numbers than Saccharomyces cerevisiae, the yeast that dominates wine fermentation when the ethanol content gets over 4%. That is why must fermentation is not naturally a single-species process. Grapes contain diverse yeast species that will define the final fermented product [1]. When wine science began its development, non-Saccharomyces yeasts were often seen as pernicious, associated with microbial-related problems due to their regular presence in spoiled wines. Currently, science is clarifying the role they perform, and several non-Saccharomyces yeasts are considered to have a positive role in the wine industry [2,3,4].
Different non-Saccharomyces yeast species have typically been evaluated for their influence on the overall quality of wine (Table 1) [5,6]. Some of these attributes are highly strain dependent, and there must be a proper selection process in order to select the most appropriate strains to improve wine quality at the industrial level.
Table 1.
The main non-Saccharomyces yeast species of oenological importance and their influence on wine fermentation.
| Species | Oenological Impact |
|---|---|
| Torulospora delbrueckii | Increased esters and thiols; acetic acid consumption [7,8] |
| Lachancea thermotolerans | Increased L-lactic acid, glycerol, and 2-phenyl-ethanol [9,10,11] |
| Metschnikowia pulcherrima | Increased esters, terpenes, thiols, and aromatic complexity [12] |
| Schizosaccharomyces pombe | Increased deacidification by L-malic acid degradation [13] |
| Candida zemplinina | Increased glycerol and succinic acid; decreased acetic acid and higher alcohols [2] |
| Hanseniaspora spp. | Increased acetate esters and terpenes; biogenic amine adsorption [2] |
| Hansenula anomala | Low C6 alcohols; increased higher alcohols and acetate and ethyl esters [2] |
| Zygosaccharomyces bailii | Increased polysaccharides; acetic acid [2] |
| Pichia guillermondii | Increased color stability and 4-ethyl-phenol production [2] |
| Pichia kluyveri | Increased varietal thiols and esters [2] |
The growing interest in P. kluyveri is reflected in the number of scientific publications regarding this species. According to the PubMed® database, over a period of 10 years (2009–2019), 33 publications were related to P. kluyveri and wine, of which 14 were in the last two years (2018 and 2019). Despite the growing interest, it is still far from the interest shown for other wine-related yeast species. In the same period, 1503 works on S. cerevisiae and wine, 114 on Metschnkowia spp., 116 on T. delbrueckii, and 75 on L. thermotolerans were published.
Different Pichia species that have been found in must fermentations and are considered to be wine-related are included in the non-Saccharomyces group: P. fermentans, P. membranifaciens, P. occidentalis, P. terricola, P. manshurica, P. kudriavzevii, and P. kluyveri. The frequency of isolation of Pichia species from grapes is lower than that of S. cerevisiae (28%) and other non-Saccharomyces such as Hanseniaspora uvarum (44%). The frequency varies from 0.12% for P. occidentalis up to 4.7% for P. anomala. Other reported Pichia species usually isolated from grapes are P. manshurica (2.81%), P. menbranifaciens (0.98%), and P. kudriavzevii (0.85%) [14]. Those lower frequencies justify the lack of commercial strains compared to other species, making it difficult to make a proper selection.
Among the wine-related Pichia species, P. kluyveri is the most studied and is the only one commercially available in the yeast market currently. P. kluyveri is characterized by its ability to improve the composition of aromatic compounds such as thiols, terpenes, and fruity esters. Currently, there are only two commercial starters based on P. kluyveri: WLP605 (Vintner’s Harvest®, Yakima, WA, USA), which is advertised as increasing rose petal and floral aromas, contributing to improve the overall bouquet of wine, and FROOTZEN® (Hansen®, Hoersholm, Denmark), which is advertised as increasing varietal and thiolic aromas [4,15]. Both are indicated for use in sequential fermentation, first with P. kluyveri, and 48 h later with a S. cerevisiae strain, which will properly end the alcoholic fermentation.
Pichia species show multilateral buds for asexual reproduction, whereas sexual reproduction is characterized by unconjugated asci; the conjugation occurs between a parent cell and its bud or between independent cells. Asci may be persistent or deliquescent, with usually one to four and more rarely five to eight ascospores. The ascospores are rough or smooth and spherical to hat-shaped, and sometimes they present equatorial or subequatorial ledges. The cell shape is spherical to ovoid and occasionally may appear as pseudohyphae. Pichia spp. can ferment glucose but rarely other sugar molecules. The genus assimilates some sugars and is not able to assimilate nitrate as a nitrogen nutrient. The genus produces coenzyme Q-7 [16]. The last genus revision described 20 accepted species, among which Pichia membranifaciens is considered the type species [16]; only a few of them are considered positive in winemaking.
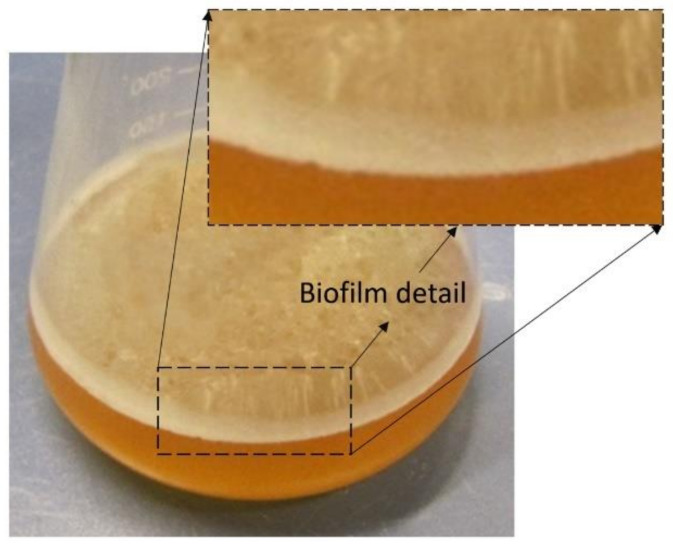
As far as P. kluyveri is concerned, the cells are slightly ovoid and about 2–10 µm, and it is very difficult to distinguish their shape from the shape of S. cerevisiae or S. ellipsoideus cells. Its ability to produce a film during its development in must is very characteristic and allows us to easily distinguish the species among other yeasts (Figure 1). The ability to distinguish this film formation is very useful at the industrial scale to quickly evaluate implantation success when using commercial products that contain P. kluyveri. This species is able to produce pseudohyphae in plate cultures but not in liquid fermentation. It can also produce hat-shaped ascospores. The species only ferments glucose and shows growth in liquid media containing glucose, ethanol, or glycerol. Like other Pichia species, P. kluyveri resists high osmotic pressure, presenting optimal growth in 10% NaCl or 5% glucose [16]. It has been usually isolated from rooted fruit and green parts of plants, being widely distributed in all type of ecosystems [17].
Figure 1.
Film produced by Pichia kluyveri over grape must at the beginning of alcoholic fermentation.
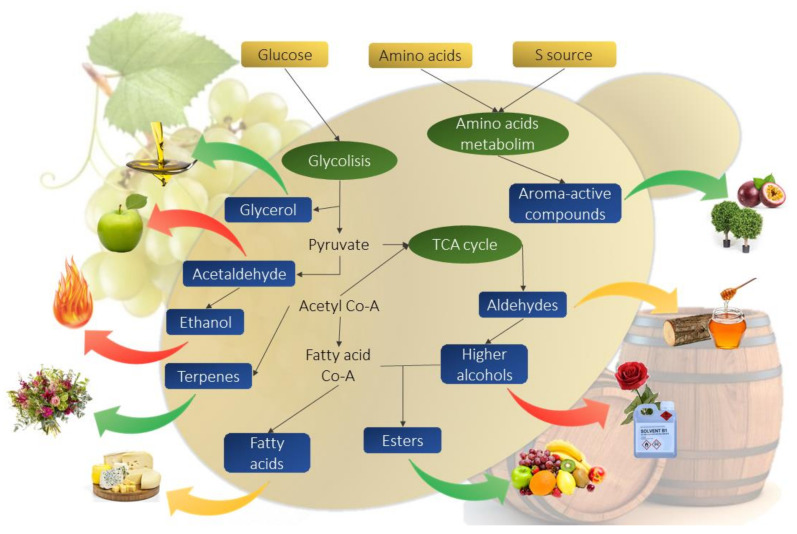
This review gathered all available information related to P. kluyveri and its influence on must fermentation considering that wine is a product obtained due to yeast metabolism. Despite that P. kluyveri presents a more oxidative metabolism than S. cerevisiae, Figure 2 shows the most common metabolic routes in P. kluyveri under fermentation conditions and its sensorial influence on wine. Additionally, this review also examined other Pichia species that are attracting increasing oenological interest.
Figure 2.
Diagram of main metabolic processes under fermentation conditions in P. kluyveri. Arrow color indicates variation compared to S. cerevisiae metabolism (red: decreased; yellow: no difference; green: increased). Adapted from [18].
2. P. kluyveri Impact on Different Wine Quality Parameters
2.1. Ethanol
P. kluyveri is only able to ferment up to 4–5% (v/v) in ethanol, consuming only glucose and leaving fructose [19]. This fermentation capacity is insufficient to produce regular wines or sparkling base wines but is enough to produce other beverages such as beer of about 3.2% (v/v) [20] or tequila base [21].
The ethanol yield of P. kluyveri is 22% lower than that of S. cerevisiae, producing 0.36 g of ethanol per gram of sugar. Most Pichia species have lower yields than P. kluyveri, such as P. fermentans (0.04 g), P. membranifaciens (0.08 g), P. terrricola (0.19 g), and P. kudriavzevii (0.33 g). However, some Pichia species have been shown to have a higher ethanol yield; for example, P. holstii yields around 0.43 g of ethanol per gram of sugar [22].
Since P. kluyveri is unable to ferment fructose and consume the full amount of glucose present in grape juice, it must be combined with fermentative yeast such as S. cerevisiae to completely ferment sugars and achieve the desired quality parameters. Sequential fermentation involving P. kluyveri and S. cerevisiae resulted in lower final ethanol content than S. cerevisiae controls. The difference increased in fermentations when P. kluyveri was present for a longer time during the winemaking process. Sequential inoculation at 48 h resulted in a lower ethanol content of 0.16% (v/v) [23], while another sequential inoculation at 96 h resulted in 0.25% (v/v) [24].
The ethanol reduction is due to the oxidative metabolism of non-Saccharomyces species that consume glucose without ethanol formation [25]. The sugar that is not converted into ethanol is transformed into other compounds, such as glycerol or acids [23]. Among those species, P. kluyveri is the second most efficient among 23 studied species, after M. pulcherrima. When it was employed in sequential fermentation, the ethanol content was reduced between 3 and 22% [22,25,26].
With regard to the fermentation kinetics, coinoculation of P. kluyveri and S. cerevisiae in a 9:1 ratio presumed a final delay of 3 days in alcoholic fermentation compared to the S. cerevisiae control [27]. In that study, P. kluyveri cells were detected during the first 9 days in an alcoholic fermentation that lasted for 23 days at 14 °C. The sequential inoculation strategy allowed the detection of P. kluyveri until 6 days after S. cerevisiae inoculation, which occurred 8 days later than the P. kluyveri inoculation [28]. However, other studies reported a fast to immediate decrease after S. cerevisiae inoculation [23], which reinforces the importance of selecting a compatible S. cerevisiae partner that allows the virtues of P. kluyveri to be increased during alcoholic fermentation.
2.2. Glycerol
Glycerol concentration is higher in sequential fermentation involving P. kluyveri than in S. cerevisiae controls, and the effect increases when P. kluyveri ferments longer. A sequential inoculation of 48 h resulted in an increase in glycerol of 0.33 g/L [23], while another inoculation of 96 h resulted in a higher increase of 1.3 g/L [24]. Other studies reported a decrease of about 48% in coinoculation [27]. This difference could be explained by possible strain variability similar to that reported for other non-Saccharomyces species. Although some studies reported positive significant increases in final glycerol concentration related to P. kluyveri performance, other non-Saccharomyces such as C. zemplinina are much more efficient for this purpose, able to produce a final glycerol concentration up to 15 g/L [5].
2.3. Organic Acids
P. kluyveri does not notably influence wine organic acids as other specific non-Saccharomyces do. It is reported to slightly consume malic acid in a concentration of about 0.1 g/L [24]. However, that is not enough to significantly influence the pH or achieve malic acid microbiological stability [29]. All studies involving P. kluyveri have reported nonsignificant statistical differences in acetic acid production between P. kluyveri sequential fermentation and S. cerevisiae control [23,24,27].
One study reported increments of some acids derived from the tricarboxylic acid cycle under sequential fermentation involving P. kluyveri: α-ketoglutaric acid (24%), oxalic acid (50%), and succinic acid (300%) [30]. In this study, the control was a T. delbrueckii strain and the fermentative product was durian wine. There are no available data yet for grape wine compared to S. cerevisiae control, so further studies must be performed on this topic, as similar results could occur with grape juice fermentation. Succinic acid concentration in wine usually varies from 0.5 to 1 g/L [31], so final concentrations up to 5 g/L by sequential inoculations reported for P. kluyveri could be an interesting alternative to wine acidification. As those concentrations are over the average value for wine, it is probable that they significantly influence its sensorial properties. While citric, L-lactic, L-malic, and L-tartaric acids are described as sour and astringent from a sensorial point of view, succinic acid is described as sour, salty, and bitter. However, the study does not include a sensory analysis to corroborate the possible influence of succinic acid on the final flavor [30].
2.4. Aroma Compounds
P. kluyveri species showed a remarkable ability to release 3-sulfanylhexan-1-ol acetate (3_SHA) compared to other Saccharomyces and non-Saccharomyces species [27]. 3-SHA is a pleasant volatile molecule that produces desired aromas in wine described as passionfruit or box tree. A study reported that sequential fermentation involving P. kluyveri and S. cerevisiae reached notably higher final concentrations of 3-SHA than the control fermented only by S. cerevisiae. The increases varied from 10 to 72% depending on the initial inoculation ratio between S. cerevisiae and P. kluyveri. The optimum reported initial ratio was 1:9 and the final 3-SHA concentration varied from 55 to 72% higher than the S. cerevisiae control depending on the P. kluyveri strain that performed the fermentation. The increase of 3-sulfanylhexan-1-ol (3-SH) was about 40% for the 1:9 ratio inoculation. Statistically significant differences in thiol release by P. kluyveri were reported to depend on the S. cerevisiae strain employed to properly end the alcoholic fermentation [27]. Those results suggest that the S. cerevisiae partner must be carefully selected to optimize the final total thiol concentration released during alcoholic fermentation when working together with the selected P. kluyveri strain. As significant strain variability regarding thiol release is reported for P. kluyveri [27], selecting yeast strains with high β-lyase activity, similar to other non-Saccharomyces species such as T. delbrueckii or M. pulcherrima, could optimize P. kluyveri thiol release activity [32].
P. kluyveri reduces the content of total higher alcohols under sequential fermentation by about 15% [23], with each higher alcohol affected in a different range (e.g., hexanol, –50%; 2-phenyl-ethanol, −20%; and butanol, −20%). Other studies reported the same results, with variations in different ranges [33] ((Z)-4-decen-1-ol, −9%; (E)-4-decen-1-ol, −8%; 1-decanol, −4%; 1-hexanol, −28%; 1-nonanol, −12%; 2-hepten-1-ol, −32%; 2-methyl-3-buten-1,2-diol, −14%; 3-octanol, −11%; 5-nonanol, −20%; and cyclooctanemethanol,α,α,-dimethyl, −12%). A similar effect was previously observed in other non-Saccharomyces such as Torulaspora, Lacchancea, and Metschnikowia [5]. Other studies observed an increase in higher alcohols of around 25% and great variation among them (e.g., hexanol, +12% and 2-phenyl-ethanol, +25%) [24]. The latest biotechnology techniques for producing varietal wines tend to reduce as much as possible the production of higher alcohols to values below 350 mg/L because they mask the varietal aroma compounds [34]. The final total higher alcohol concentration reported for sequential fermentation between P. kluyveri and S. cerevisiae was always below 350 mg/L, varying from 176 [23] to 254 mg/L [24].
Different studies report a higher production of total esters for sequential fermentation involving P. kluyveri than S. cerevisiae controls. A study on the presence of different enzymatic activities of oenological impact [35] reported that all studied strains of P. kluyveri presented esterase activity, which catalyzes the formation of esters. The highest increase was 25% for 2-phenyl ethyl acetate [23] and 50% [24] for a longer sequential inoculation. Another study reported further increases up to 60% in red wine [33]. The compound 2-phenyl-ethyl acetate is a desirable aromatic compound that increases the perception of aromas such as rose or floral when it appears in concentrations over 0.25 mg/L [34]. The yeast strains employed to ferment neutral varieties such as Airen and Ugni blanc, which did not possess high levels of molecules such as terpenes or thiols, are selected to enhance the final fruity ester concentration. On the contrary, yeast strains employed to ferment varieties with strong varietal characteristics such as Verdejo, sauvignon blanc, and Muscat are selected to produce lower levels of esters in order to not mask the varietal aromas [34].
Several studies reported no effect of P. kluyveri on the final concentration of total terpenes compared to S. cerevisiae control [23,24,33]. This is mainly because β-glucosidase activity is reported to be not common in P. kluyveri strains [35]. Other factors that could affect this phenomenon are the initial sterilization of the grape juice and the performance of varieties with low terpene content. Indeed, differences in specific terpenes are reported. It was reported that P. kluyveri sequential fermentation had higher levels of linalool oxide and hotrienol by about double and 40%, respectively, while the concentration of nerol was lower by about 10% [23].
Total fatty acid content was not influenced by P. kluyveri [23] or even decreased, and decanoic acid was the most affected, with decreased concentration by around 18% [24]. These results agree with a report showing the absence of lipase enzymes in P. kluyveri species [35]. Specific fatty acids such as isovaleric acid stood out due to an increase of around 25% compared to S. cerevisiae control [24]. The production of isovaleric acid should be taken into account for strain selection, as concentrations over 50 mg/L produce undesirable aromas such as rancid cheese [34]. This phenomenon has been previously reported for other non-Saccharomyces species such as T. delbrueckii [8].
One study reported lower production of acetaldehyde by nearly 40% compared to S. cerevisiae control [23], although the final values were far below the olfactory threshold of 125 mg/L and related to undesirable oxidative descriptors. This additional effect could increase the impact of other varietal aroma compounds such as thiols and terpenes, as they are less masked for this significant aromatic compound that produces oxidative aromas. Table 2 summarizes the main aroma compounds influenced by P. kluyveri.
Table 2.
Main aroma compounds influenced by P. kluyveri, chemical structure, aromatic descriptor, and perception threshold.
| Group | Aroma Compound | Structure | Odor Descriptor | Perception Threshold (ng/L) | Reference |
|---|---|---|---|---|---|
| Higher alcohols | 2-methyl butanol |
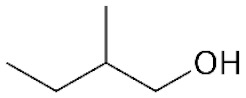
|
Harsh, nail polish remover | 30,000 | [36] |
| 3-methyl butanol |
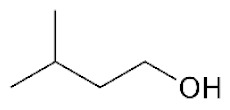
|
Harsh, nail polish remover | 30,000 | [36] | |
| 2-phenyl ethanol |
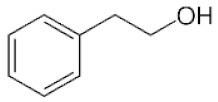
|
Rose | 10,000 | [36] | |
| Esters | 2-phenyl-ethyl acetate |
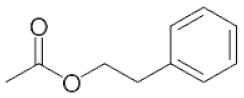
|
Rose, raspberry | 250 | [36] |
| 2-methyl-butyl acetate |
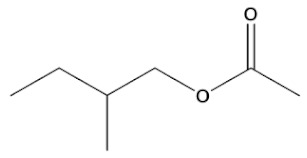
|
Banana | 5 | [36] | |
| Terpenes | Linalool |
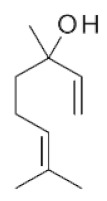
|
Flowery, fruity | 6 for white varieties 15 for red varieties |
[34] |
| Hotrienol |
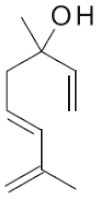
|
Faintly flowery, elderflower | 110 | [34] | |
| Thiols | 3-SHA |
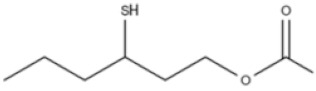
|
Passionfruit, box tree | 4 | [34] |
| 3-SH |
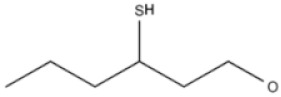
|
Grapefruit, citrus peel | 60 | [34] |
Some studies have reported some off-odor compounds in sequential fermentation with P. kluyveri compared to S. cerevisiae control. Some studies reported an increase in fatty acid content, such as 3-methyl-butanoic acid (isovaleric acid) and phenylamine, which are linked to undesirable aromas, such as cheese, sweaty feet, or off-putting sourness [24,28]. P. kluyveri has been reported to produce higher levels of H2S by about 50% in sequential fermentation, although the final value was below the fault threshold [24].
2.5. Amino Acids
Only one study reported the final content of amino acids in sequential fermentation with P. kluyveri compared to conventional fermentation by S. cerevisiae [23]. P. kluyveri sequential fermentation produced about 10% less final threonine than S. cerevisiae pure fermentation. There were no differences in ornithine, while the concentration of other studied amino acids was always higher for P. kluyveri sequential fermentation. Slight increases varying from 5% to 15% were reported for asparagine, alanine, leucine, and glycine, while higher increases of about 50% were reported for aspartic acid, arginine, phenylalanine, isoleucine, lysine, serine, and tyrosine. Increased final amino acid content is usually related to less nutrient nitrogen consumption or higher cellular release.
There are no scientific reports on P. kluyveri as a biogenic amine producer [37]. The increased final concentration of tyrosine and lysine could evolve to tyramine and cadaverine if they were decarboxylated by lactic bacteria during malolactic fermentation or an undesirable contamination process [5]. The 50% higher final concentration of phenylalanine than S. cerevisiae control could explain the higher isovaleric acid production observed in other studies [23,28]. Isovaleric acid can be produced from other amino acids due to aromatic-L-amino-acid decarboxylase enzyme [28].
3. Sensory Impact in Regular Wine Fermentation
Only one scientific study performed a sensorial evaluation of P. kluyveri fermentation, analyzing 17 parameters. The study reported sequential fermentation involving P. kluyveri to show a better overall impression by about 30% compared to S. cerevisiae control [23]. The better aromatic quality was related to a higher fruity character [27], mainly peach, apricot, citrus, and grapefruit, which could be related to an increase in thiol release (mainly 3-SHA) during the alcoholic fermentation. That study also reports higher Riesling grape varietal typicity commonly related to those descriptors. Other studies showed no increases from a sensorial point of view of those descriptors compared to S. cerevisiae control [28]. The differences can be related to the absence of thiol precursors or to yeast strain variability.
Although 3-SH appears in higher concentrations than 3-SHA, the corresponding perception threshold is 60 and 4 ng/L. For that reason, 3-SHA has a 15 times higher impact on wine than 3-SH, justifying its higher importance. The specific aroma descriptors of 3-SHA are passionfruit, box tree, and boxwood, which describe the typicity of some of the best sauvignon blanc in the world [34].
4. Killer Factor and Its Influence on Wine Ecology
Wine spoilage associated with different non-Saccharomyces strains is a major concern for winemakers. It is therefore important to develop a reliable and effective procedure to inhibit the presence of such yeasts. To achieve this purpose, sulfur dioxide (SO2) is widely used, but the antimicrobial effect of this compound is affected by the wine composition, and it can cause allergic reactions in wine consumers. Alternatives for biologically controlling its abundance are therefore actively sought [38]. This section of the review is focused on describing the killer toxins produced by P. kluyveri, which are antimicrobial compounds that have shown potential for inhibiting different spoilage yeasts in wine.
Several yeast genera and species can produce and secrete toxic proteins that inhibit many sensitive filamentous fungi and yeasts. The ability to produce killer toxins is strain-dependent, usually related to infection by virus-like particles of the Totiviridae family. However, in the Pichia genus, several toxins have been described to be either associated with cytoplasmic genetic elements, such as dsDNA virus-like elements, or chromosomally encoded [39].
Pichia is one of the most prolific yeast genera in the production of different kinds of killer toxins. P. acaciae, P. inositovora, P. anomala, P. kluyveri, and P. membranifaciens, which are widespread in nature and in wine-related environments, have been identified as killer yeasts and several of their toxins have been characterized. Most of these toxins are outside the scope of the present review, but their characteristics and potential applications have been previously reviewed [39].
As far as P. kluyveri is concerned, two killer toxins have been described. The first killer toxin described for P. kluyveri 1002 is a 19.0 kDa glycoprotein that is chromosomally inherited, showing its mayor activity in pH from 3.8 to 4.0. Its mechanism of action is based on membrane permeabilization [40]. The second killer toxin, Pkkp, has a greater molecular mass (54.0 kDa) and higher optimum pH value (4.0–4.5) and its primary receptor is in the cell wall; its mechanism of action has not been elucidated [41].
The first killer toxin of P. kluyveri has an antimycotic effect on sensitive cells. It is produced in the exponential growth phase and allows an advantage in must fermentation, although it is associated with a cost. The cost comes from a delay in reaching the exponential growth phase compared to the non-killer phenotype [42].
Sulfur dioxide, the most popular antimicrobial control agent in winemaking, is starting to come under legal restrictions; consequently, newly developed alternatives are starting to be used [38]. The utilization of killer yeasts such as P. kluyveri has been proposed as an interesting alternative to biologically controlling the initial level of undesirable microorganisms. For example, a Pkkp-producer strain has been demonstrated to be active against a wide variety of food and beverage spoilage yeasts such as Brettanomyces/Dekkera bruxellensis [41]. However, this killer toxin can inhibit S. cerevisiae in beverages, so as a biocontrol agent in winemaking, it should be used carefully [41]. It must be considered that when killer yeasts are used as antimicrobial agents in fermentations conducted using S. cerevisiae, a resistant S. cerevisiae strain must be used in order to ensure that the alcoholic fermentation in sequential inoculations is finished [40]. P. kluyveri has been found to be a killer species against low fermentative or oxidative yeasts such as Candida bodinii, C. patagonica, and Geotrichum silvicol, which develop a surface biota in incompletely filled barrels or vats [43].
Other Pichia toxins, such as P. membranifaciens killer toxin (PMKT), show synergistic interaction when used in combination with food antimicrobials such as potassium metabisulphite [44]. This characteristic has been proposed to reduce preservative concentration in foods and beverages; unfortunately, this seems to be an unusual characteristic because it is not observed for Pkkp [41].
5. Influence of Other Pichia Species on Winemaking
5.1. Pichia Guilliermondii
Although most applications of Pichia species in winemaking are related to improvements in aroma composition (Table 3), P. guilliermondii is applied to improve wine color properties [45]. P. guilliermondii is the yeast species that shows the most hydroxycinnamate decarboxylase enzymatic activity. Hydroxycinnamate decarboxylase produces pyranoanthocyanin adducts, which are the most stable colored compounds in wine chemistry [46]. These compounds are responsible for maintaining the color of wine during long aging periods. In other yeast genera, such as Saccharomyces, enzymatic activity is a strain-dependent characteristic. The maximum enzymatic activity reported for Saccharomyces is about 15% [45], while the maximum reported for P. guilliermondii is up to 90%. These differences in enzymatic activity allow P. guilliermondii to produce higher content of pyranoanthocyanin adducts, from 6 to 10 times higher than S. cerevisiae controls. Another hydroxycinnamate decarboxylase application is to capture ethylphenol precursors such as the p-coumaric acid [47]. This allows wines to be stabilized against ethylphenol deviations. P. guilliermondii has been also reported to reduce the final ethanol concentration by around 2% compared to the S. cerevisiae control [25]. As far as volatile compounds are concerned, esters are the most affected; P. guilliermondii reduces their concentration by about half under sequential inoculations with S. cerevisiae compared to standard control [48].
Table 3.
Summary of main Pichia species applications in winemaking.
| Teleomorph | Synonym | Impact on Wine Fermentation |
|---|---|---|
| Pichia kluyveri | Hansenula kluyveri | Increased levels of varietal thiols (3-MHA) [2] |
| Pichia fermentans | Candida lambica | Increased levels of higher alcohols, glycerol, and polysaccharides [2] |
| Pichia membranifaciens | Candida valida | Increased levels of esters [4] |
| Pichia terricola | Issatchenkia terricola | Increased β-glucosidase activity [63]; malic acid degradation [64] |
| Pichia kudriavzevii | Candida krusei. Issatchenkia orientalis | Malic acid degradation [17]; increased 2-pheyl-ethanol production [65] |
| Pichia manshurica | - | Spoilage yeast; increased volatile phenols and other off-odors [66] |
| Pichia guilliermondi | Candida guilliermondi, Meyerozyma guilliermondi | Increased color stability [29] |
| Pichia anomala | Candida anomala, Hansenula anomala, Wickerhanomyces anomalus | Increased levels of higher alcohols, acetates, and ethyl esters [2] |
| Pichia pastoris | Komagataella pastoris, K. pseudopastoris, K. phafii | Inhibition of protein haze formation in white wines (genetically modified strain) [67] |
5.2. Pichia Kudriavzevii
P. kudriavzevii, the anamorph of Candida krusei, is widely distributed in natural substrates such us soil, fruits, and spontaneous fermentations [17]. It is an interesting tool for wine pH management, since it can significantly reduce malic acid (−40%) during alcoholic fermentation of wine and increase the pH by around 0.3 units [49,50]. Malic acid is unstable from a microbiological point of view and must be removed before bottling to avoid undesirable second fermentations in red wines. The classical method to remove malic acid to use lactic acid bacteria during malolactic fermentation. That process, especially in warm viticulture areas, can result in deviations such as a loss of color or production of harmful compounds such as biogenic amines or ethyl carbamate. For that reason, interest in studying yeasts such as P. kudriavzevii or S. pombe, which are able to remove malic acid, has increased during the last years [13,29]. As well as other Pichia species, P. kudriavzevii influences the complexity of wine aromas under sequential fermentation with S. cerevisiae. It increases acetate esters (by around 30%, up to 65 mg/L) and higher alcohols (by around 20%, up to 240 mg/L), and decreases fatty acids (by around 40%, up to 60 mg/L) and C6-alcohols (by around 10%, up to 1.7 mg/L) [51]. Another use of this species is to reduce the ethanol content of wines (by around 30%) [17,22].
5.3. Pichia fermentans
P. fermentans has been described as a moderate ethanol producer, reaching values of around 5% (v/v) and producing huge quantities of polysaccharides (up to 278 mg/mL, around 40%) and ethyl acetate (up to 256 mg/mL, almost 6 times higher) compared to S. cerevisiae control [52]. Under sequential fermentation with S. cerevisiae, it increased the ethanol content by around 1% (v/v) and increased most of the volatile molecules (principally thiols, acetates, and esters) in a large range [53]. P. fermentans has been described as low acetic acid producer in different combinations and conditions; the species does not exceed the acid levels reached in fermentation with only S. cerevisiae. In a two-day fermentation, it allowed a considerable increase of aromatic compounds such as acetaldehyde (70%) and glycerol (by around 1000 times) [54,55]. In a recent study using a P. fermentans strain as inoculum for pilot scale fermentation in 1000 L, a slight decrease in ethanol yield was observed compared to S. cerevisiae control (12.80% v/v compared to 13.00% v/v), and an increase in volatile acidity (20%) and volatile compounds such as acetaldehyde (10%) and 2-methyl-1-propanol (53%) [56].
5.4. Pichia anomala
P. anomala is the greatest ethanol producer among Pichia species, producing up to 7% v/v [57]. This ability could allow the production of lower-alcohol beverages such as beer or even sparkling base wine. It is also reported to slightly increase higher alcohols, acetate, and ethyl esters, with the total content increased by around 15%, 20%, and 15%, respectively [58]. Nevertheless, its main potential could be as a biocontrol agent. It can effectively inhibit the development of spoilage molds in substrates such as malt for beer production. This antagonistic effect is due to the secretion of killer toxins; up to five killer toxins have been reported in P. anomala [38,59].
5.5. Pichia membranifaciens
P. membranifaciens has been reported to increase the overall quality parameters of terpenic varieties such as Muscat [60] and the content of polysaccharides (around 40%) compared to S. cerevisiae controls [57]. By contrast, its ethanol production is almost nil (up to 0.9% v/v) under single fermentation conditions and it increases acetic acid concentration by around four times compared to S. cerevisiae control [57]. With these characteristics, P. membranifaciens may play an important role by maintaining the microbial stability of must by producing killer toxins [38], which has been described as effective against spoilage molds such as Botrytis cinerea [61] and yeasts such as Brettanomyces/Dekkera bruxellensis [62].
6. Proposed Selection Parameters for P. kluyveri
One of the main problems in selecting non-Saccharomyces species based on their oenological aptitude is getting a representative collection of yeast strains of a specific species. This is due to the greater presence of other non-Saccharomyces in grapes such as the Hanseniaspora genus, which represents about 50% of the yeast isolates from grapes and is the predominant genus in fermenting grape juice when ethanol goes over 4% [1]. Previous studies solved this problem by developing specific selective media such as those described for the Schizosaccharomyces genus [13] or incubating at higher temperatures as in the case of L. thermotolerans [11]. Using those methodologies, it is relatively easy to obtain a representative collection of strains without great effort or financial investment. To date, there has not been selective media described for P. kluyveri. So, the most efficient way to find high numbers of strains of this species is based on looking for the most appropriate substrate where this species is predominant. P. kluyveri is among the predominant species in olives and coffee beans, reaching an isolation frequency of about 17% and 80%, respectively [52,53], while the frequency in grapes is below 1%.
As P. kluyveri shows different killer factors, the selected strains cannot have an antagonist effect on the fermentative S. cerevisiae strain, as this second species is needed to properly end any fermentation process in combination with P. kluyveri. That is why any negative effect on Saccharomyces would not compensate for other quality improvement.
The main application of P. kluyveri in winemaking is based on its ability to release thiols and produce fruity or floral esters. Due to this, the first criterion of selection must be the presence of enzymes such as β-lyase and glycosidase, which enhance varietal aromas to increase the quality of terpenic varieties such as Muscat and thiol varieties such as sauvignon blanc, riesling, and Verdejo [34]. This selection process has previously been successful for other non-Saccharomyces species such as T. delbrueckii [7], M. pulcherrima [68], and L. thermotolerans. Additionally, the selection of strains able to produce high concentrations of fruity esters, such as isoamyl and octanoate acetate, or floral esters, such as 2-penyl-ethyl acetate, would be of great interest in the making of wines from neutral grape varieties such as Airén and Ugni blanc, where varietal aromas have a low impact on the final bouquet.
The film formation of other species, such as specific flor-film S. cerevisiae strains, serves to protect the wine against oxidation in sherry winemaking [69,70,71,72]. As P. kluyveri is not a great fermenter, the formation of the film must be as quick as possible in order to avoid undesirable oxidative aromas and preserve the desired aromatic compounds generated by this strain that are especially sensitive to oxidation, such as thiols.
P. kluyveri is not considered a high fermentative yeast and always needs to be combined with a more fermentative partner such as Saccharomyces. We must establish the selection threshold as the maximum reported 5% (v/v) in order to facilitate fermentation as much as possible and allow P. kluyveri to work longer before S. cerevisiae inoculation [19]. Additionally, the objective ethanol yield must be about 0.36 ethanol grams per sugar gram in order to achieve wines with lower final ethanol content than those fermented only by S. cerevisiae. Glycerol production must also be considered in the selection process, as combined fermentations involving P. kluyveri are commonly described as containing higher concentrations of glycerol than S. cerevisiae controls. Although there are no reported data regarding SO2 resistance for P. kluyveri, most commercial non-Saccharomyces are especially sensitive to this additive, and one P. kluyveri manufacturer recommends reducing its dosage to the lowest amount possible. The selection of strains with higher resistance would increase the range of applications in winemaking. Currently, the use of P. kluyveri is limited to very healthy grapes that do not require higher doses of SO2. Despite studies reporting no significant differences in acetic acid production between fermentations involving P. kluyveri and S. cerevisiae controls, this parameter must be included in the selection process, as high strain variability, similar to that described for other non-Saccharomyces, could exist for this species [5].
The production of higher alcohols must be controlled to be as low as possible in order to avoid any masking effect over the varietal aromas to increase their impact as much as possible. There must be the same objective for fatty acids, especially isovaleric acid, which is described as having higher levels in fermentations involving P. kluyveri. Additionally, while P. kluyveri is described as producing less acetaldehyde than other non-Saccharomyces, the parameter must be verified during the selection process to avoid undesirable oxidative aromas.
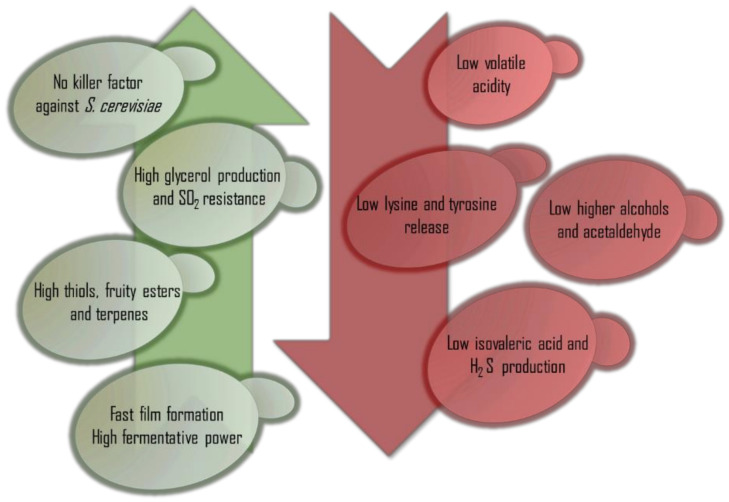
The release of amino acids such as tyrosine and lysine, which are able to evolve to biogenic amines such as tyramine and cadaverine, must be reduced as much as possible during the selection process [73]. Figure 3 summarizes the proposed P. kluyveri selection parameters.
Figure 3.
Summary of proposed Pichia kluyveri selection parameters.
7. Conclusions
P. kluyveri improves wine quality parameters such as thiol, fruity ester, and terpene concentrations, mainly in sequential fermentation. Additionally, clear patterns such as lower ethanol and higher glycerol production, and higher 2-phenyl ethyl acetate and lower hexanol production are observed in all research works. Other parameters such as malic acid and acetaldehyde reduction and methanol increase must be more deeply studied, as they are only reported once. Currently, only two strains are commercially available, so further strains should be selected to increase the market offerings. Possible occasional undesirable effects such as increased isovaleric acid, phenethylamine, H2S, or biogenic amine precursors, which seem to be strain-dependent, must be carefully studied in the future. Another technological factor of interest is the presence of killer toxins, which can enable the implantation of selected S. saccharomyces strains.
Due to its potential, P. kluyveri has generated interest in other fermentation products such as beer, cider, and cocoa in order to improve quality parameters related to sensory perception. Although P. kluyveri is the only Pichia species available on the yeast market, others such as P. fermentans, P. guilliermondii, P. kudriavzevii, P. anomala, and P. membranifaciens are being studied for winemaking purposes and new commercial strains could be available in the near future.
Author Contributions
J.V., D.M., F.C., A.S., and S.B. contributed to the scientific literature revision, text writing, and editing. Figure design: J.V. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by Ossian Vides y Vinos S. L under the framework of project FPA1720300120/IDI-20170703 (Centre for the Development of Industrial Technology, Spain) and Bodegas Pago de Carraovejas SLU under the framework of projects IDI-20180269 and IDI-20140448 (Centre for the Development of Industrial Technology, Spain). Javier Vicente developed this work under a contract (PEJ-2019-AI/BIO-12459) from Complutense University of Madrid in the framework of the Youth Improvement Initiative (Education and Research Counseling from Community of Madrid and European Social Fund).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Belda I., Ruiz J., Alastruey-Izquierdo A., Navascues E., Marquina D., Santos A. Unraveling the Enzymatic Basis of Wine “Flavorome”: A Phylo-Functional Study of Wine Related Yeast Species. Front. Microbiol. 2016;7:12. doi: 10.3389/fmicb.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolly N.P., Varela C., Pretorius I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014;14:215–237. doi: 10.1111/1567-1364.12111. [DOI] [PubMed] [Google Scholar]
- 3.Ciani M., Comitini F., Mannazzu I., Domizio P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010;10:123–133. doi: 10.1111/j.1567-1364.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 4.Petruzzi L., Capozzi V., Berbegal C., Corbo M.R., Bevilacqua A., Spano G., Sinigaglia M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017;8:995. doi: 10.3389/fmicb.2017.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benito Á., Calderón F., Benito S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation. 2019;5:54. doi: 10.3390/fermentation5030054. [DOI] [Google Scholar]
- 6.Ciani M., Capece A., Comitini F., Canonico L., Siesto G., Romano P. Yeast interactions in inoculated wine fermentation. Front. Microbiol. 2016;7:555. doi: 10.3389/fmicb.2016.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belda I., Navascués E., Marquina D., Santos A., Calderon F., Benito S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015;99:1911–1922. doi: 10.1007/s00253-014-6197-2. [DOI] [PubMed] [Google Scholar]
- 8.Benito S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018;102:3081–3094. doi: 10.1007/s00253-018-8849-0. [DOI] [PubMed] [Google Scholar]
- 9.Blanco P., Rabuñal E., Neira N., Castrillo D. Dynamic of Lachancea thermotolerans Population in Monoculture and Mixed Fermentations: Impact on Wine Characteristics. Beverages. 2020;6:36. doi: 10.3390/beverages6020036. [DOI] [Google Scholar]
- 10.Porter T.J., Divol B., Setati M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Int. Food Res. J. 2019;119:378–389. doi: 10.1016/j.foodres.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Benito S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018;102:6775–6790. doi: 10.1007/s00253-018-9117-z. [DOI] [PubMed] [Google Scholar]
- 12.Vicente J., Ruiz J., Belda I., Benito-Vázquez I., Marquina D., Calderón F., Santos A., Benito S. The Genus Metschnikowia in Enology. Microorganisms. 2020;8:1038. doi: 10.3390/microorganisms8071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benito S. Combined Use of Lachancea thermotolerans and Schizosaccharomyces pombe in Winemaking: A Review. Microorganisms. 2020;8:655. doi: 10.3390/microorganisms8050655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puig-Pujol A., Ferrando N., Capdevila F., Ocete R., Revilla E. Yeast biodiversity from Vitis vinifera L., subsp. sylvestris (Gmelin) Hegi to face up the oenological consequences of climate change. BIO Web Conf. EDP Sci. 2016;7:02026. doi: 10.1051/bioconf/20160702026. [DOI] [Google Scholar]
- 15.Roudil L., Russo P., Berbegal C., Albertin W., Spano G., Capozzi V. Non-Saccharomyces commercial starter cultures: Scientific trends, recent patents and innovation in the wine sector. Recent Pat. Food Nutr. Agric. 2019;10:1–13. doi: 10.2174/2212798410666190131103713. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzman C., Fell J.W., Boekhout T. The Yeasts: A Taxonomic Study. Elsevier; Amsterdam, The Netherlands: 2011. [Google Scholar]
- 17.Ciani M., Canonico L., Oro L., Comitini F. Biotechnological Progress and Beverage Consumption. Elsevier; Amsterdam, The Netherlands: 2020. Footprint of nonconventional yeasts and their contribution in alcoholic fermentations; pp. 435–465. [Google Scholar]
- 18.Pretorius I.S. Conducting wine symphonics with the aid of yeast genomics. Beverages. 2016;2:36. doi: 10.3390/beverages2040036. [DOI] [Google Scholar]
- 19.Contreras A., Hidalgo C., Henschke P.A., Chambers P.J., Curtin C., Varela C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014;80:1670–1678. doi: 10.1128/AEM.03780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez A., Boekhout T., Gojkovic Z., Katz M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018;124:389–402. doi: 10.1002/jib.512. [DOI] [Google Scholar]
- 21.Amaya-Delgado L., Herrera-López E., Arrizon J., Arellano-Plaza M., Gschaedler A. Performance evaluation of Pichia kluyveri, Kluyveromyces marxianus and Saccharomyces cerevisiae in industrial tequila fermentation. World J. Microb. Biotechnol. 2013;29:875–881. doi: 10.1007/s11274-012-1242-8. [DOI] [PubMed] [Google Scholar]
- 22.Contreras A., Hidalgo C., Schmidt S., Henschke P., Curtin C., Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Benito S., Hofmann T., Laier M., Lochbühler B., Schüttler A., Ebert K., Fritsch S., Röcker J., Rauhut D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015;241:707–717. doi: 10.1007/s00217-015-2497-8. [DOI] [Google Scholar]
- 24.Dutraive O., Benito S., Fritsch S., Beisert B., Patz C.-D., Rauhut D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation. 2019;5:79. doi: 10.3390/fermentation5030079. [DOI] [Google Scholar]
- 25.Varela J., Varela C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019;56:88–96. doi: 10.1016/j.copbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Röcker J., Strub S., Ebert K., Grossmann M. Usage of different aerobic non-Saccharomyces yeasts and experimental conditions as a tool for reducing the potential ethanol content in wines. Eur. Food Res. Technol. 2016;242:2051–2070. doi: 10.1007/s00217-016-2703-3. [DOI] [Google Scholar]
- 27.Anfang N., Brajkovich M., Goddard M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009;15:1–8. doi: 10.1111/j.1755-0238.2008.00031.x. [DOI] [Google Scholar]
- 28.Whitener M.E.B., Stanstrup J., Panzeri V., Carlin S., Divol B., Du Toit M., Vrhovsek U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics. 2016;12:53. doi: 10.1007/s11306-016-0962-4. [DOI] [Google Scholar]
- 29.Benito S. The impacts of Schizosaccharomyces on winemaking. Appl. Microbiol. Biotechnol. 2019;103:4291–4312. doi: 10.1007/s00253-019-09827-7. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y., Voon M.K.W., Chua J.-Y., Huang D., Lee P.-R., Liu S.-Q. The effects of co-and sequential inoculation of Torulaspora delbrueckii and Pichia kluyveri on chemical compositions of durian wine. Appl. Microbiol. Biotechnol. 2017;101:7853–7863. doi: 10.1007/s00253-017-8527-7. [DOI] [PubMed] [Google Scholar]
- 31.Waterhouse A.L., Sacks G.L., Jeffery D.W. Understanding Wine Chemistry. John Wiley & Sons; Hoboken, NJ, USA: 2016. [Google Scholar]
- 32.Belda I., Ruiz J., Navascués E., Marquina D., Santos A. Improvement of aromatic thiol release through the selection of yeasts with increased β-lyase activity. Int. J. Food Microbiol. 2016;225:1–8. doi: 10.1016/j.ijfoodmicro.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Whitener M.B., Stanstrup J., Carlin S., Divol B., Du Toit M., Vrhovsek U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017;23:179–192. doi: 10.1111/ajgw.12269. [DOI] [Google Scholar]
- 34.Ruiz J., Kiene F., Belda I., Fracassetti D., Marquina D., Navascues E., Calderon F., Benito A., Rauhut D., Santos A., et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019;103:7425–7450. doi: 10.1007/s00253-019-10008-9. [DOI] [PubMed] [Google Scholar]
- 35.Escribano R., González-Arenzana L., Garijo P., Berlanas C., López-Alfaro I., López R., Gutiérrez A.R., Santamaría P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017;54:1555–1564. doi: 10.1007/s13197-017-2587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina A.M., Guadalupe V., Varela C., Swiegers J.H., Pretorius I.S., Agosin E. Differential synthesis of fermentative aroma compounds of two related commercial wine yeast strains. Food Chem. 2009;117:189–195. doi: 10.1016/j.foodchem.2009.03.116. [DOI] [Google Scholar]
- 37.Landete J., Ferrer S., Pardo I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control. 2007;18:1569–1574. doi: 10.1016/j.foodcont.2006.12.008. [DOI] [Google Scholar]
- 38.Capece A., Pietrafesa R., Siesto G., Romano P. Biotechnological Approach Based on Selected Saccharomyces cerevisiae Starters for Reducing the Use of Sulfur Dioxide in Wine. Microorganisms. 2020;8:738. doi: 10.3390/microorganisms8050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belda I., Ruiz J., Alonso A., Marquina D., Santos A. The biology of Pichia membranifaciens killer toxins. Toxins. 2017;9:112. doi: 10.3390/toxins9040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Middelbeek E., Stumm C., Vogels G. Effects of Pichia kluyveri killer toxin on sensitive cells. Antonie Van Leeuwenhoek. 1980;46:205–220. doi: 10.1007/BF00444075. [DOI] [PubMed] [Google Scholar]
- 41.Labbani F.-Z.K., Turchetti B., Bennamoun L., Dakhmouche S., Roberti R., Corazzi L., Meraihi Z., Buzzini P. A novel killer protein from Pichia kluyveri isolated from an Algerian soil: Purification and characterization of its in vitro activity against food and beverage spoilage yeasts. Antonie van Leeuwenhoek. 2015;107:961–970. doi: 10.1007/s10482-015-0388-4. [DOI] [PubMed] [Google Scholar]
- 42.Pintar J., Starmer W.T. The costs and benefits of killer toxin production by the yeast Pichia kluyveri. Antonie van Leeuwenhoek. 2003;83:89–97. doi: 10.1023/A:1014215200360. [DOI] [PubMed] [Google Scholar]
- 43.Sangorrín M.P., Lopes C.A., Jofré V., Querol A., Caballero A.C. Spoilage yeasts from Patagonian cellars: Characterization and potential biocontrol based on killer interactions. World J. Microbiol. Biotechnol. 2008;24:945–953. doi: 10.1007/s11274-007-9557-6. [DOI] [Google Scholar]
- 44.Alonso A., Belda I., Santos A., Navascués E., Marquina D. Advances in the control of the spoilage caused by Zygosaccharomyces species on sweet wines and concentrated grape musts. Food Control. 2015;51:129–134. doi: 10.1016/j.foodcont.2014.11.019. [DOI] [Google Scholar]
- 45.Benito S., Morata A., Palomero F., González M., Suárez-Lepe J. Formation of vinylphenolic pyranoanthocyanins by Saccharomyces cerevisiae and Pichia guillermondii in red wines produced following different fermentation strategies. Food Chem. 2011;124:15–23. doi: 10.1016/j.foodchem.2010.05.096. [DOI] [Google Scholar]
- 46.Benito Á., Calderón F., Benito S. The combined use of Schizosaccharomyces pombe and Lachancea thermotolerans—Effect on the anthocyanin wine composition. Molecules. 2017;22:739. doi: 10.3390/molecules22050739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benito S., Palomero F., Morata A., Calderon F., Suarez-Lepe J.A. A method for estimating Dekkera/Brettanomyces populations in wines. J. Appl. Microbiol. 2009;106:1743–1751. doi: 10.1111/j.1365-2672.2008.04137.x. [DOI] [PubMed] [Google Scholar]
- 48.García M., Esteve-Zarzoso B., Cabellos J.M., Arroyo T. Sequential Non-Saccharomyces and Saccharomyces cerevisiae Fermentations to Reduce the Alcohol Content in Wine. Fermentation. 2020;6:60. doi: 10.3390/fermentation6020060. [DOI] [Google Scholar]
- 49.Del Monaco S.M., Barda N.B., Rubio N.C., Caballero A.C. Selection and characterization of a Patagonian Pichia kudriavzevii for wine deacidification. J. Appl. Microbiol. 2014;117:451–464. doi: 10.1111/jam.12547. [DOI] [PubMed] [Google Scholar]
- 50.Del Mónaco S.M., Rodriguez M.E., Lopes C.A. Pichia kudriavzevii as a representative yeast of North Patagonian winemaking terroir. Int. J. Food Microbiol. 2016;230:31–39. doi: 10.1016/j.ijfoodmicro.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Luan Y., Zhang B.-Q., Duan C.-Q., Yan G.-L. Effects of different pre-fermentation cold maceration time on aroma compounds of Saccharomyces cerevisiae co-fermentation with Hanseniaspora opuntiae or Pichia kudriavzevii. LWT. 2018;92:177–186. doi: 10.1016/j.lwt.2018.02.004. [DOI] [Google Scholar]
- 52.Domizio P., Liu Y., Bisson L., Barile D. Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. japonicus: Quantification and characterization. Food Microbiol. 2017;61:136–149. doi: 10.1016/j.fm.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong C.-L., Li A.-H., Su J., Wang X.-C., Chen C.-Q., Tao Y.-S. Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S. cerevisiae. LWT. 2019;109:83–92. doi: 10.1016/j.lwt.2019.03.101. [DOI] [Google Scholar]
- 54.Clemente-Jimenez J., Mingorance-Cazorla L., Martínez-Rodríguez S., Heras-Vázquez F.L., Rodríguez-Vico F. Influence of sequential yeast mixtures on wine fermentation. Int. J. Food Microbiol. 2005;98:301–308. doi: 10.1016/j.ijfoodmicro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Godoy L., Acuña-Fontecilla A., Catrileo D. Winemaking-Stabilization, Aging Chemistry and Biochemistry. IntechOpen; London, UK: 2020. Formation of aromatic and flavor compounds in wine: A perspective of positive and negative contributions of non-Saccharomyces yeasts. [Google Scholar]
- 56.Romani C., Lencioni L., Bartolini A.B., Ciani M., Mannazzu I., Domizio P. Pilot Scale Fermentations of Sangiovese: An Overview on the Impact of Saccharomyces and Non-Saccharomyces Wine Yeasts. Fermentation. 2020;6:63. doi: 10.3390/fermentation6030063. [DOI] [Google Scholar]
- 57.Domizio P., Romani C., Lencioni L., Comitini F., Gobbi M., Mannazzu I., Ciani M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011;147:170–180. doi: 10.1016/j.ijfoodmicro.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Cañas P.M.I., García-Romero E., Manso J.M.H., Fernández-González M. Influence of sequential inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the quality of red wines. Eur. Food Res. Technol. 2014;239:279–286. doi: 10.1007/s00217-014-2220-1. [DOI] [Google Scholar]
- 59.Laitila A., Sarlin T., Raulio M., Wilhelmson A., Kotaviita E., Huttunen T., Juvonen R. Yeasts in malting, with special emphasis on Wickerhamomyces anomalus (synonym Pichia anomala) Antonie van Leeuwenhoek. 2011;99:75–84. doi: 10.1007/s10482-010-9511-8. [DOI] [PubMed] [Google Scholar]
- 60.Viana F., Gil J.V., Genovés S., Vallés S., Manzanares P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008;25:778–785. doi: 10.1016/j.fm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 61.Santos A., Marquina D. Killer toxin of Pichia membranifaciens and its possible use as a biocontrol agent against grey mould disease of grapevine. Microbiology. 2004;150:2527–2534. doi: 10.1099/mic.0.27071-0. [DOI] [PubMed] [Google Scholar]
- 62.Santos A., Mauro M.S., Bravo E., Marquina D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiology. 2009;155:624–634. doi: 10.1099/mic.0.023663-0. [DOI] [PubMed] [Google Scholar]
- 63.González-Pombo P., Fariña L., Carrau F., Batista-Viera F., Brena B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011;46:385–389. doi: 10.1016/j.procbio.2010.07.016. [DOI] [Google Scholar]
- 64.Wen L., Wang L., Wang G. Degradation of L-malic and citric acids by Issatchenkia terricola. J. Food Sci. 2011;32:220–223. [Google Scholar]
- 65.Martínez-Avila O., Sánchez A., Font X., Barrena R. 2-Phenylethanol (rose aroma) production potential of an isolated Pichia kudriavzevii through solid-state fermentation. Process Biochem. 2020;93:94–103. doi: 10.1016/j.procbio.2020.03.023. [DOI] [Google Scholar]
- 66.Perpetuini G., Tittarelli F., Battistelli N., Suzzi G., Tofalo R. Contribution of Pichia manshurica strains to aroma profile of organic wines. Eur. Food Res. Technol. 2020;246:1405–1417. doi: 10.1007/s00217-020-03499-8. [DOI] [Google Scholar]
- 67.Schmidt S.A., Tan E.L., Brown S., Nasution U.J., Pettolino F., Macintyre O.J., Lopes M.D.B., Waters E.J., Anderson P.A. Hpf2 glycan structure is critical for protection against protein haze formation in white wine. J. Agric. Food Chem. 2009;57:3308–3315. doi: 10.1021/jf803254s. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz J., Belda I., Beisert B., Navascues E., Marquina D., Calderon F., Rauhut D., Santos A., Benito S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018;102:8501–8509. doi: 10.1007/s00253-018-9255-3. [DOI] [PubMed] [Google Scholar]
- 69.Guijo S., Mauricio J., Salmon J.-M., Ortega J. Determination of the relative ploidy in different Saccharomyces cerevisiae strains used for fermentation and “flor” film ageing of dry sherry-type wines. Yeast. 1997;13:101–117. doi: 10.1002/(SICI)1097-0061(199702)13:2<101::AID-YEA66>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Muñoz M., Cordero-Bueso G., Benítez-Trujillo F., Martínez S., Pérez F., Cantoral J.M. Rethinking about flor yeast diversity and its dynamic in the “criaderas and soleras” biological aging system. Food Microbiol. 2020;92:103553. doi: 10.1016/j.fm.2020.103553. [DOI] [PubMed] [Google Scholar]
- 71.Cordero-Bueso G., Ruiz-Muñoz M., González-Moreno M., Chirino S., Bernal-Grande M.D.C., Cantoral J.M. The Microbial Diversity of Sherry Wines. Fermentation. 2018;4:19. doi: 10.3390/fermentation4010019. [DOI] [Google Scholar]
- 72.Peinado R.A., Mauricio J.C. Wine Chemistry and Biochemistry. Springer; Berlin/Heidelberg, Germany: 2009. Biologically aged wines; pp. 81–101. [Google Scholar]
- 73.Benito A., Calderon F., Benito S. Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics. Molecules. 2016;21:1744. doi: 10.3390/molecules21121744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.