Abstract.
Sera from white-tailed deer (WTD, Odocoileus virginianus) hunter-harvested throughout New York State (NYS), 2007–2015, were tested by plaque reduction neutralization for antibodies against nine mosquito-borne viruses from the families Peribunyaviridae, Flaviviridae, and Togaviridae. Overall, 76.1% (373/490) of sampled WTD were seropositive against at least one virus, and 38.8% were exposed to multiple viruses. The seropositivity rate in adult WTD (78.0%) was significantly greater (P < 0.0001) than that in fawns (47.7%). Neutralizing antibodies against California serogroup viruses were most common in WTD sampled across all regions (67.1%), followed by the Bunyamwera serogroup (BUN) (37.6%). Jamestown Canyon and Cache Valley orthobunyaviruses were responsible for most California and BUN infections, respectively. Seroprevalence rates to West Nile virus were higher in samples originating from Long Island (LI) (19.0%) than in those originating from the central (7.3%), western (5.0%), and Hudson Valley (4.4%) regions of NYS. Antibodies to Eastern equine encephalitis virus were seen primarily in WTD from central NYS (5.1%), where annual enzootic activity occurs, but low rates were documented in western NYS (1.4%) and LI (1.7%). Low rates of Potosi and LaCrosse orthobunyavirus, and Highlands J virus antibodies were detected over the course of this investigation. St. Louis encephalitis virus (or a closely related virus) antibodies were detected in samples collected from central and western NYS, suggesting local virus transmission despite a lack of evidence from routine mosquito surveillance. Serologic results demonstrate the value of WTD in NYS as an indicator of arbovirus distribution and recent transmission on a relatively fine spatial scale.
INTRODUCTION
Serosurveys of white-tailed deer (WTD, Odocoileus virginianus) and other cervids have been used to detect enzootic transmission of arthropod-borne viruses (arboviruses) across the United States.1–7 White-tailed deer are a useful sentinel species to monitor arbovirus activity, because they have limited home ranges8; are fed upon frequently by hematophagous arthropods (ticks, mosquitoes, and midges) capable of transmitting a number of bacterial and viral pathogens9,10; and can be readily sampled in large numbers during hunting seasons.
Certain alphaviruses (Togaviridae), flaviviruses (Flaviviridae), and orthobunyaviruses (Peribunyaviridae) distributed throughout the United States are known to infect deer at varying rates, having been isolated from euthanized cervids with neurologic symptoms and animals found moribund, as illustrated herein. West Nile virus (WNV), a Flavivirus, and Eastern equine encephalitis virus (EEEV), an Alphavirus, have been isolated from deer and moose in several states.11–13 Recently, these viruses have been the source of continual epidemic/epizootic activity in New York State (NYS) since 199914 and 2003,15 respectively. Cache Valley orthobunyavirus (CVV), a Bunyamwera serogroup (BUN) orthobunyavirus, is a suspected human pathogen,16,17 and an important etiologic agent of livestock, causing spontaneous abortions and malformations in sheep and cattle.18 Cache Valley orthobunyavirus isolates have been documented from horses and from a dying caribou in the midwestern United States,19,20 and, more recently, CVV has been detected from a euthanized horse (NYS Department of Health [NYSDOH], unpublished data) and two humans in western NYS (WNY).21,22 Deer are routinely exposed to several other mosquito-borne viruses capable of causing disease in humans including St. Louis encephalitis virus (SLEV),23–25 and Jamestown Canyon (JCV) and LaCrosse orthobunyaviruses (LACV).5,23–31
The NYSDOH maintains an active arthropod-borne disease surveillance system originally established in the 1970s. Because of limitations in spatial coverage of mosquito surveillance and system design targeting certain viruses in particular areas (i.e., EEEV in central NYS [CNY], and WNV in WNY and New York City metropolitan area including Long Island [LI]), the current distribution of mosquito-borne viruses across NYS is not defined and is the impetus for this study. Field investigations involving vertebrate serosurveys have not been conducted in NYS since the 1970s,32–39 with the exception of comprehensive avian surveys for EEEV in CNY and WNV in and around New York City and Albany.40–43 Mammals had not been sampled as part of arbovirus surveillance in NYS for at least 25 years before the incursion of WNV. Therefore, we took advantage of the annual hunting seasons of WTD in NYS to document arbovirus exposure in a large mammalian species common in all parts of the state.
MATERIALS AND METHODS
Sample collection.
Deer sampling was conducted at a surveillance site for chronic wasting disease in Rome located in CNY, 2007–2009. We also sampled at privately operated venison processors in the Hudson Valley (HV) region and WNY, 2011–2013, and deer culls on LI in 2015. Blood samples were collected from each deer using a 5cc or 10cc 20-gauge syringe barrel to extract blood pooled in the abdominal or chest cavity of field-dressed deer. At the mandatory check station in CNY, NYS Department of Environmental Conservation personnel severed the head of each deer below the jaw line, providing a source of blood. The sample was transferred from the needleless syringe barrel to a 1.5-mL pre-labeled polypropylene skirted and externally threaded screw-cap tube with O-ring seal (USA Scientific, Ocala, FL). Tubes of blood were held in racks on ice until centrifuged. Within 24 hours of collection, blood samples were centrifuged for 5 minutes at 3,200 revolutions per minute to separate blood into clot and serum. Sera were transferred to sterile pre-labeled cryogenic 2 mL Nalgene screw-cap tubes and stored at −80°C. Abnormally appearing blood samples (i.e., obvious contamination, diluted samples from rinsing of body cavities) were excluded from analyses.
Recorded data included the date/time of blood collection, date/time of deer kill (determined by hunter interview at the time of sample collection), and county/town of kill. Sex was determined for all sampled deer. Deer age was assessed for some samples based on the extent of tooth eruption and wear estimates. Deer aged 1 year or younger were classified as fawns, and those older than 1 year were classified as adults.
Serology.
Deer sera were tested by plaque reduction neutralization tests (PRNTs). Samples were diluted in BA-1 {M199 medium with Hank’s salts, 1% bovine albumin, TRIS base (tris [hydroxymethyl] aminomethane), sodium bicarbonate, 2% fetal bovine serum, and antibiotics} and heat-inactivated at 56°C for 30 minutes. Sera were screened at a 1:10 dilution against EEEV, CVV, JCV, SLEV, and WNV. Sera that neutralized 50% (PRNT50) of the virus, as compared with the virus control well (no antibody), were then serially diluted 2-fold. Cross neutralizations were performed against related viruses of the same family, that is, EEEV and Highlands J virus (HJV), JCV and LACV, CVV and Potosi orthobunyavirus (POTV), WNV and SLEV.44,45 In addition, 10–20 deer samples from each year that had screened positive against a California serogroup (CAL) virus were randomly selected and screened against trivittatus orthobunyavirus (TVTV). Endpoint titers are expressed as the reciprocal of the dilution of serum that inhibited 90% (PRNT90) of the test virus inoculum. Plaque reduction neutralization tests 90 cutoffs were used to assure the most conservative interpretation of results. Antibody titers that were less than 1:20 were considered negative. Titers of 1:20 were considered equivocal and classified as exposure to virus serogroup or a virus order, that is, CAL, BUN, FLAVI (Flavivirus), or ALPHA (Alphavirus). Antibody titers greater than or equal to 1:40 were considered positive and specific. Eastern equine encephalitis virus, HJV, JCV, LACV, and WNV strains used for the PRNTs were isolated in NYS.42,46–49 Trivittatus orthobunyavirus and POTV were originally provided by the CDC.47,50,51 Cache Valley orthobunyavirus was provided by the National Institute of Allergy and Infectious Diseases.36,52 St. Louis encephalitis virus (Parton) was originally obtained from the Rockefeller Foundation Virus Laboratories (Table 1).35,53
Table 1.
Strain information for viruses used in plaque reduction neutralization test
| Virus | Strain | Source |
|---|---|---|
| Eastern equine encephalitis | 69-7836 | Pheasant, Orange Co., NY, 1969 |
| Highlands J | 78-33495 | Culiseta melanura, Suffolk Co., NY, 1978 |
| Jamestown Canyon | 78-30641 | Source unknown, NY |
| LaCrosse | 74-32813 | Aedes triseriatus, Albany Co., NY, 1974 |
| Trivittatus | Prototype | Aedes trivittatus, Bismarck, ND, 1948 |
| Cache valley | Prototype | Culiseta inornata, Cache valley, UT, 1956 |
| Potosi | 89-3380 | Aedes albopictus, Potosi, MO, 1989 |
| St. Louis encephalitis | Parton | Human. St. Louis, MO, 1933 |
| West Nile | 3100365 | Culex pipiens/restuans, Richmond Co., NY, 2000 |
Statistical analysis.
The proportions of seropositive deer by geographic region and age-group were compared using the chi-squared test of independence (α = 0.05) or, when noted, the Fisher’s exact test (α = 0.05), whenever expected cell values were ≤ 5. The Holm–Bonferroni adjustment was used to correct all P-values for multiple comparisons.
RESULTS
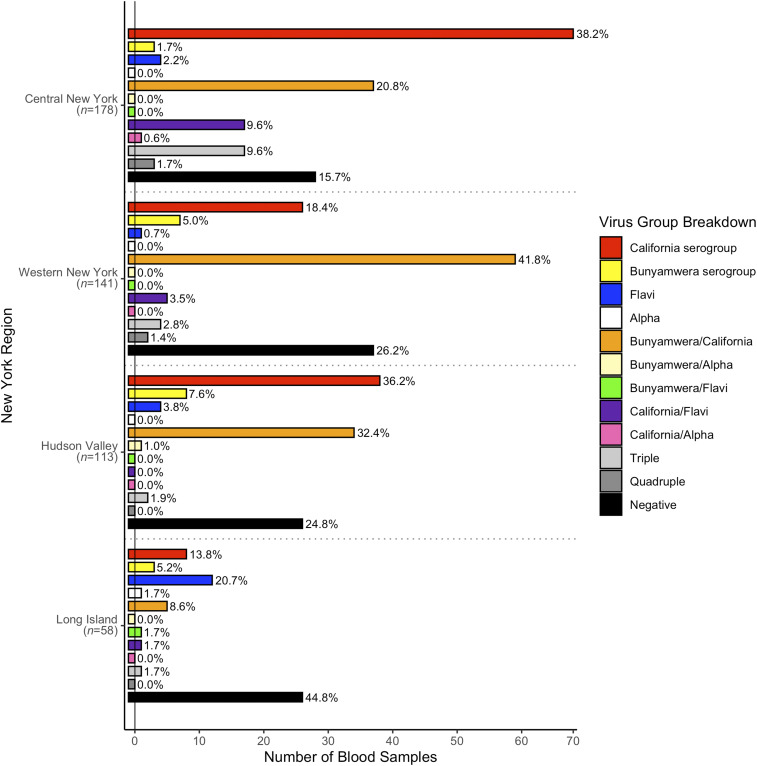
Overall, 490 WTD sera were tested for the presence of arboviral antibodies (Figure 1A, Table 2). Exposure to at least one mosquito-borne virus was noted in 76.1% (373/490) of sampled deer. Across regions, arbovirus seropositivity rates were 84.3% (CNY), 77.0% (HV), 73.8% (WNY), and 55.2% (LI). Overall, arbovirus seropositivity was significantly less common (χ2 = 14.61, P = 0.0020) on LI than in the other three regions. Among deer that were aged, the rate of seropositivity was significantly higher (χ2 = 22.97, P < 0.0001) in adults (78.0%, 224/287) than in fawns (47.7%, 31/65). The seropositivity rate among adult deer was highest in CNY (84.1%, 74/88), followed by WNY (80.6%, 100/124), HV (72.5%, 29/40), and LI (60.0%, 21/35). The overall seropositivity rate among adult deer was not significantly different (χ2 = 6.43, P = 0.1012) in LI compared with the overall rate of the other three regions.
Figure 1.
Arbovirus testing results by New York State region. (A) Number of bloods tested by region, (B) California serogroup (CAL) orthobunyaviruses, and (C) Bunyamwera serogroup orthobunyaviruses.
Table 2.
Sampling information of hunter-harvested white-tailed deer
| Region* | Sex | Age | Total | ||
|---|---|---|---|---|---|
| Fawn (≤ 1 year old) | Adult (> 1 year old) | Not assessed | |||
| CNY (2007–2009)† | Male | 20 | 55 | 51 | 126 |
| Female | 6 | 31 | 13 | 50 | |
| Total | 26 | 88 | 64 | 178§ | |
| WNY (2011–2013)‡ | Male | 10 | 92 | 1 | 103 |
| Female | 5 | 32 | 1 | 38 | |
| Total | 15 | 124 | 2 | 141 | |
| HV (2011–2013) | Male | 0 | 36 | 58 | 94 |
| Female | 1 | 4 | 14 | 19 | |
| Total | 1 | 40 | 72 | 113 | |
| LI (2015) | Male | 13 | 1 | 0 | 14 |
| Female | 10 | 34 | 0 | 44 | |
| Total | 23 | 35 | 0 | 58 | |
| State | Male | 43 | 184 | 110 | 337 |
| Female | 22 | 101 | 28 | 151 | |
| Total | 65 | 287 | 138 | 490 | |
CNY = Central New York; HV = Hudson Valley; LI = Long Island; WNY = Western New York.
Includes two deer, one each harvested in 2011 and 2013.
Includes one deer harvested in 2009.
Sex was not noted for two deer.
Alphavirus.
Overall seropositivity to an alphavirus across all regions sampled was 2.9% (Table 3). The rate of alphavirus seropositivity did not differ significantly (Fisher’s exact test, P = 1.0) between adult WTD (3.5%, 10/287) and fawns (1.5%, 1/65). Alphavirus seropositivity rate was highest in the CNY region, but was not significantly different from the rest of NYS (χ2 = 3.71, P = 0.3253). Of 14 seropositive deer, nine were harvested in CNY, three in WNY, and one each from HV and LI regions. Twelve deer were EEEV seropositive, and two deer were classified as nonspecific ALPHA positives. One of the ALPHA-positive deer had an EEEV antibody titer of 1:20 and HJV < 1:20. The other ALPHA-positive deer had an HJV titer of 1:20 and EEEV < 1:20. In CNY, EEEV seropositivity was 6.8% in 2007 and 5.3% in 2009. Eastern equine encephalitis virus seropositivity was not detected in 28 hunter-harvested deer in CNY in 2008. In WNY, one EEEV seropositive deer each was harvested in 2011 and 2013. One deer sampled in 2012 was ALPHA positive. In the HV region, one of 113 deer tested was seropositive to an alphavirus. This deer was harvested in Columbia County in 2012, and it was reactive against EEEV only at a low titer (1:20), so it was conservatively classified as ALPHA. Of the 58 deer tested from LI, one was positive for EEEV antibodies.
Table 3.
Alphavirus testing results
| Region* | Year | Number of samples tested | Number of positive samples (%) | |||
|---|---|---|---|---|---|---|
| EEEV | HJV | ALPHA | Total | |||
| CNY | 2007 | 73 | 5 (6.8) | 0 | 0 | 5 (6.8) |
| 2008 | 28 | 0 | 0 | 0 | 0 | |
| 2009 | 75 | 4 (5.3) | 0 | 0 | 4 (5.3) | |
| 2011 | 1 | 0 | 0 | 0 | 0 | |
| 2013 | 1 | 0 | 0 | 0 | 0 | |
| Total | 178 | 9 (5.1) | 0 (0.0) | 0 (0.0) | 9 (5.1) | |
| WNY | 2009 | 1 | 0 | 0 | 0 | 0 |
| 2011 | 39 | 1 (2.6) | 0 | 0 | 1 (2.6) | |
| 2012 | 50 | 0 | 0 | 1 (2.0)† | 1 (2.0) | |
| 2013 | 51 | 1 (2.0) | 0 | 0 | 1 (2.0) | |
| Total | 141 | 2 (1.4) | 0 (0.0) | 1 (0.7) | 3 (2.1) | |
| HV | 2011 | 10 | 0 | 0 | 0 | 0 |
| 2012 | 36 | 0 | 0 | 1 (2.8)‡ | 1 (2.8) | |
| 2013 | 67 | 0 | 0 | 0 | 0 | |
| Total | 113 | 0 (0.0) | 0 (0.0) | 1 (0.9) | 1 (0.9) | |
| LI | 2015 | 58 | 1 (1.7) | 0 | 0 | 1 (1.7) |
| State | 490 | 12 (2.4) | 0 (0.0) | 2 (0.4) | 14 (2.9) | |
EEEV = Eastern equine encephalitis virus; HJV = Highlands J virus.
CNY = Central New York; HV = Hudson Valley; LI = Long Island; WNY = Western New York..
One ALPHA-positive deer was EEEV < 1:20, HJV 1:20.
One ALPHA-positive deer was EEEV 1:20, HJV < 1:20.
Flavivirus.
Mosquito-borne flavivirus reactivity in WTD was 14.1% across all regions and years (Table 4). Flavivirus seropositivity was most common in LI, where the rate (25.9%) was significantly greater than that in WNY (7.8%) (χ2 = 10.27, P = 0.0163) and the HV region (5.3%) (χ2 = 13.18, P = 0.0037). No significant difference (χ2 = 0.39, P = 1.0) was observed between seropositivity rates in LI and CNY (20.8%). For all samples tested, WNV was responsible for 36 sero-reactors and SLEV for six, whereas 27 were classified as nonspecific FLAVI positive. Seroprevalence rates to WNV were higher on LI (19.0%) than in CNY (7.3%), WNY (5.0%), or HV (4.4%).
Table 4.
Flavivirus testing results
| Region* | Year | Number of samples tested | Number of positive samples (%) | |||
|---|---|---|---|---|---|---|
| West Nile virus | St. Louis encephalitis virus | FLAVI | Total | |||
| CNY | 2007 | 73 | 6 (8.2) | 1 (1.4) | 6 (8.2) | 13 (17.8) |
| 2008 | 28 | 3 (10.7) | 0 | 1 (3.6) | 4 (14.3) | |
| 2009 | 75 | 4 (5.3) | 2 (2.7) | 14 (18.7) | 20 (26.7) | |
| 2011 | 1 | 0 | 0 | 0 | 0 | |
| 2013 | 1 | 0 | 0 | 0 | 0 | |
| Total | 178 | 13 (7.3) | 3 (1.7) | 21 (11.8) | 37 (20.8) | |
| WNY | 2009 | 1 | 0 | 0 | 0 | 0 |
| 2011 | 39 | 0 | 0 | 0 | 0 | |
| 2012 | 50 | 5 (10.0) | 2 (4.0) | 1 (2.0) | 8 (16.0) | |
| 2013 | 51 | 2 (3.9) | 1 (2.0) | 0 | 3 (5.9) | |
| Total | 141 | 7 (5.0) | 3 (2.1) | 1 (0.07) | 11 (7.8) | |
| HV | 2011 | 10 | 1 (10.0) | 0 | 0 | 1 (10.0) |
| 2012 | 36 | 3 (8.3) | 0 | 1 (2.8) | 4 (11.1) | |
| 2013 | 67 | 1 (1.5) | 0 | 0 | 1 (1.5) | |
| Total | 113 | 5 (4.4) | 0 (0.0) | 1 (0.9) | 6 (5.3) | |
| LI | 2015 | 58 | 11 (19.0) | 0 | 4 (6.9) | 15 (25.9) |
| State | 490 | 36 (7.3) | 6 (1.2) | 27 (5.5) | 69 (14.1) | |
CNY = Central New York; HV = Hudson Valley, LI = Long Island; WNY = Western New York..
In the CNY region, overall flavivirus rates were 17.8%, 14.3%, and 26.7% for 2007, 2008, and 2009, respectively. Thirteen of 37 seropositive deer across all years were reactive to WNV, three to SLEV, and 21 deer were FLAVI reactive. One and two SLEV reactive deer were harvested during the 2007 and 2009 hunting seasons, respectively.
In the WNY region, flavivirus reactivity was 0%, 16.0%, and 5.9% in deer harvested in 2011, 2012, and 2013, respectively. West Nile virus-specific rates were 0%, 10.0%, and 3.9%; and SLEV specific rates were 0%, 4.0%, and 2.0% in 2011, 2012, and 2013, respectively. One FLAVI-classified deer was detected in 2012. Two SLEV seropositive deer were harvested in 2012, and one was harvested in 2013.
Flavivirus seropositive rates were lowest in the HV region. One of 10 (10.0%) sampled deer was WNV antibody positive in 2011. Three (8.3%) WNV seropositive and one (2.8%) FLAVI positive deer were detected from 36 tested in 2012. In 2013, one of 67 deer sampled was WNV antibody positive.
Long Island had the highest rate of flavivirus seropositivity, with 11 deer (19.0%) testing positive for WNV antibodies, and four were classified as FLAVI positive (6.9%). The rate of flavivirus seropositivity was not significantly different (χ2 = 4.74, P = 0.2062) between fawns (43.5%, 10/23) and adults (14.3%, 5/35) on LI.
Orthobunyavirus.
California serogroup
Overall, 66.9% of all deer sampled were antibody positive to at least one CAL orthobunyavirus (Table 5). California serogroup seropositivity was significantly more common (χ2 = 30.11, P < 0.0001) among adult WTD (69.7%, 200/287) than among fawns (32.3%, 21/65). Seropositivity rates were highest in CNY at 80.0%, followed by WNY (68.1%), HV (66.4%), and LI (25.9%), respectively (Figure 1B). The CAL seropositivity rate was significantly lower on LI among adults (χ2 = 18.27, P = 0.0003) but not fawns (χ2 = 7.48, P = 0.0624) when compared with overall rates in the other three regions. Jamestown Canyon virus accounted for the majority of CAL seropositive deer (81.7%, 268/328). Jamestown Canyon virus seropositivity was most common in CNY, where the rate (71.3%) was significantly greater than that in WNY (50.4%) (χ2 = 13.85, P = 0.0028), HV (52.2%) (χ2 = 10.16, P = 0.0163), and LI (19.0%) (χ2 = 47.30, P < 0.0001). Specific neutralizing antibodies against TVTV were not detected in any of the screened samples.
Table 5.
California serogroup testing results
| Region* | Year | Number of samples tested | Number of positive samples (%) | |||
|---|---|---|---|---|---|---|
| Jamestown Canyon orthobunyavirus | LaCrosse orthobunyavirus | California serogroup | Total | |||
| CNY | 2007 | 73 | 57 (78.1) | 0 | 2 (2.7) | 59 (80.8) |
| 2008 | 28 | 22 (78.6) | 0 | 2 (7.1) | 24 (85.7) | |
| 2009 | 75 | 46 (61.3) | 2 (2.7) | 10 (13.3) | 58 (77.3) | |
| 2011 | 1 | 1 (100.0) | 0 | 0 | 1 (100.0) | |
| 2013 | 1 | 1 (100.0) | 0 | 0 | 1 (100.0) | |
| Total | 178 | 127 (71.3) | 2 (1.1) | 14 (7.9) | 143 (80.3) | |
| WNY | 2009 | 1 | 0 | 0 | 1 (100.0) | 1 (100.0) |
| 2011 | 39 | 27 (69.2) | 1 (2.6) | 4 (10.3) | 32 (82.1) | |
| 2012 | 50 | 17 (34.0) | 0 | 13 (26.0) | 30 (60.0) | |
| 2013 | 51 | 27 (52.9) | 0 (0.0) | 6 (11.8) | 33 (64.7) | |
| Total | 141 | 71 (50.4) | 1 (0.7) | 24 (17.0) | 96 (68.1) | |
| HV | 2011 | 10 | 5 (50.0) | 0 | 1 (10.0) | 6 (60.0) |
| 2012 | 36 | 12 (33.3) | 0 | 6 (16.7) | 18 (50.0) | |
| 2013 | 67 | 42 (62.7) | 0 | 8 (11.9) | 50 (74.6) | |
| Total | 113 | 59 (52.2) | 0 (0.0) | 15 (13.3) | 74 (65.5) | |
| LI | 2015 | 58 | 11 (19.0) | 0 | 4 (6.9) | 15 (25.9) |
| State | 490 | 268 (54.7) | 3 (0.6) | 57 (11.6) | 328 (66.9) | |
CNY = Central New York; HV = Hudson Valley; LI = Long Island; WNY = Western New York.
In CNY, CAL seroprevalence rates varied from year to year but were greater than 75.0% in all years. In 2007, exposure to a CAL virus was 80.8%. The following year, 85.7% of harvested deer were seropositive, and in 2009, the rate was 77.3%. Jamestown Canyon virus was the source of infection for 127 of 143 CAL virus-infected CNY deer. Fourteen deer were classified as nonspecific CAL positive, and two deer were seropositive against LACV, both harvested in 2009.
California serogroup virus seroprevalence in WNY was 82.1%, 60.0%, and 64.7% during 2011, 2012, and 2013, respectively. Jamestown Canyon virus accounted for 71 of the 96 seropositive deer. LaCrosse orthobunyaviruses was responsible for one infection in 2011. Twenty-four deer were classified as CAL positive.
In the HV region, seroprevalence of CAL antibodies was variable; 60.0%, 50.0%, 74.6% across the 3 years sampled, 2011–2013. Similar to the other regions, JCV antibodies predominated. LaCrosse orthobunyaviruses antibody positive deer were not observed, although 15 of 74 seropositive deer were classified as CAL positive.
On LI, 11 deer (19.0%) were positive for JCV, and four (6.9%) CAL positives were recorded.
Bunyamwera serogroup
Overall, 38.0% of all deer sampled were positive for anti-BUN (CVV and POTV) antibodies (Table 6). Bunyamwera serogroup seropositivity rate was significantly higher (χ2 = 32.12, P < 0.0001) in adults (46.7%, 134/287) than in fawns (7.7%, 5/65). Antibody prevalence was highest in WNY deer (51.1%), followed by HV (38.8%), CNY (32.8%), and LI (15.5%) (Figure 1C). Cache Valley orthobunyavirus accounted for the majority of BUN–positive samples (67.7%, 126/186).
Table 6.
Bunyamwera serogroup testing results
| Region* | Year | Number of samples tested | Number of positive samples (%) | |||
|---|---|---|---|---|---|---|
| Cache Valley orthobunyavirus | Potosi orthobunyavirus | Bunyamwera serogroup | Total | |||
| CNY | 2007 | 73 | 22 (30.1) | 3 (4.1) | 1 (1.4) | 26 (35.6) |
| 2008 | 28 | 1 (3.6) | 1 (3.6) | 2 (7.1) | 4 (14.3) | |
| 2009 | 75 | 9 (12.0) | 8 (10.7) | 11 (14.7) | 28 (37.3) | |
| 2011 | 1 | 0 | 0 | 0 | 0 | |
| 2013 | 1 | 0 | 0 | 1 (100.0) | 1 (100.0) | |
| Total | 178 | 32 (18.0) | 12 (6.7) | 15 (8.4) | 59 (33.1) | |
| WNY | 2009 | 1 | 0 | 0 | 0 | 0 |
| 2011 | 39 | 19 (48.7) | 4 (10.3) | 3 (7.7) | 26 (66.7) | |
| 2012 | 50 | 19 (38.0) | 1 (2.0) | 5 (10.0) | 25 (50.0) | |
| 2013 | 51 | 16 (31.4) | 1 (2.0) | 4 (7.8) | 21 (41.2) | |
| Total | 141 | 54 (38.3) | 6 (4.3) | 12 (8.5) | 72 (51.1) | |
| HV | 2011 | 10 | 3 (30.0) | 0 | 1 (10.0) | 4 (40.0) |
| 2012 | 36 | 7 (19.4) | 3 (8.3) | 2 (5.6) | 12 (33.3) | |
| 2013 | 67 | 24 (35.8) | 4 (6.0) | 1 (1.5) | 29 (43.3) | |
| Total | 113 | 34 (30.1) | 7 (6.2) | 4 (3.5) | 45 (39.8) | |
| LI | 2015 | 58 | 6 (10.3) | 0 | 4 (6.9) | 10 (17.2) |
| State | 490 | 126 (25.7) | 25 (5.1) | 35 (7.1) | 186 (38.0) | |
CNY = Central New York; HV = Hudson Valley; LI = Long Island; WNY = Western New York.
In CNY, seroprevalence to BUN viruses varied by year from 2007 to 2009, at 35.6, 14.3, and 37.3%, respectively. Cache Valley orthobunyavirus was the etiologic agent responsible for antibody in 32 deer, and POTV in 12 deer. Fifteen deer were classified as nonspecific BUN positive.
In WNY, seroprevalence rates decreased year to year from 2011 to 2013. In 2011, 66.7% of 39 deer sampled were positive against at least one BUN virus. Seropositive rates decreased to 50.0% in 2012 and 41.2% in 2013. Cache Valley orthobunyavirus infections were most common in WNY in all years, compared with POTV, and accounted for 54 of 72 (75.0%) BUN–positive deer, whereas POTV accounted for six (8.3%). The source of the antibody could not be determined for the remaining 12 (16.7%) BUN-positive deer in WNY.
Bunyamwera serogroup rates in the HV region from 2011 to 2013 were 40.0%, 33.3%, and 43.3%, respectively. Similar to trends seen in the other regions, CVV antibody was more prevalent, accounting for 34 of 45 (75.6%) seropositive deer versus seven (15.6%) for POTV and four (8.9%) for BUN.
On LI, 10 BUN reactive deer were detected. Six (60.0%) deer were positive for CVV-specific antibody and four (40.0%) were classified as BUN positives.
“Poly”-infection.
Infection with more than one mosquito-borne virus or serogroup was apparent in 38.8% (190/490) of all deer sampled (Figure 2). The rates of poly-infection were: WNY (49.6%), CNY (42.1%), HV (32.7%), and LI (13.8%). The majority of poly-infections were to both California and BUN viruses (85.8%, 163/190). Poly-infection was significantly more common (χ2 = 15.13, P = 0.0016) in adults (45.6%, 131/287) than in fawns (18.5%, 12/65).
Figure 2.
Antibody rates to mosquito-borne viruses, including “poly”-infections, of white-tailed deer in New York State.
DISCUSSION
The earliest arbovirus investigations in NYS occurred in the late 1950s and early 1960s to assess prevalence and distribution of groups A (alphaviruses) and B (flaviviruses) viruses.35,36 Serosurveys were performed on sera collected from dairy herds, foxes, deer, raccoons, bats, and rodents. Results indicated widespread activity of flaviviruses, especially in raccoon and fox sera. Alphavirus activity was limited. Two of 70 deer sampled were found to have antibodies to an arbovirus. Both deer were exposed to an alphavirus, likely HJV, and both sera were collected in Seneca County, which lies along the border of the CNY and WNY regions sampled during our study. A more exhaustive deer serosurvey was conducted on 352 samples collected from nine NYS counties during 1959–1969. Seropositivity rates against the alphaviruses, determined by PRNTs, were low (0% for EEEV and 3.9% for HJV), and none of the samples originated from counties with recognized EEEV activity.39
Results from the current study indicate limited alphavirus exposure on a state-wide or regional scale (2.9%), and only in deer harvested from counties with historical or recent EEEV activity.41,54,55 The overall EEEV seropositivity rate found in NYS WTD, 2.4%, is lower than those recently reported for cervids harvested in Vermont (10.2% in deer, 29% in moose),56,57 Maine (7.1% in deer),58 Georgia (14% in deer),11 Florida (11.9%),59 and from historic reports in North Dakota (23% in deer).60 Eastern equine encephalitis virus seropositivity rates by county/site within those states were highly variable, and some rates were similar to those observed for the CNY-harvested deer in this study (5.1%), demonstrating the focal and episodic transmission dynamics of EEEV in the northeastern United States. The EEEV seropositivity rate in NYS was similar to those reported in harvested WTD in Alabama (2.5%), Indiana (2.2%), Louisiana (3.1%), Minnesota (1.7%), Mississippi (3.1%), New Jersey (3.4%), and Pennsylvania (2.9%).59 Since 2003, EEEV has persisted in the CNY region, with some years (2006, 2009–2011, and 2014–2015) experiencing increased levels of mammalian transmission as evidenced by human and equine fatalities and numerous mosquito isolates.54 Eastern equine encephalitis virus was isolated from brain tissue of two deer (one each 2008 and 2009) displaying neurologic symptoms in Oswego County (CNY), indicative of increased epizootic activity during those transmission seasons. Fatal EEEV infections in deer have been reported in Georgia and Michigan.11,13 Highlands J virus seropositivity was not detected during the current study, although previously reported in NYS deer.39
Evidence of group B (Flavivirus) activity was not observed in deer sampled during one of the earlier NYS studies; however, SLEV hemagglutination inhibition (HI) antibodies were recorded in 26 samples from other species.35 A number of these were determined to be Powassan virus (POWV) infections by PRNTs, but a handful were inconclusive, suggesting possible cross-reactivity with a virus closely related to SLEV. Results from the deer serosurvey published later indicated seven deer with serologic evidence of exposure to SLEV (or a SLEV-like virus).39 Screening tests were performed using the HI test, an assay with known limitations especially against flaviviruses.48 Two of the seven deer were negative to POWV by HI examination. The five remaining deer were reactive to both antigens. Plaque reduction neutralization testss confirmed three POWV and two SLEV infections. During the current study, six deer were considered specific SLEV reactors, three each in CNY and WNY. Four of the six deer had SLEV titers at least 4-fold higher than any other flavivirus tested (WNV and POWV). The remaining two deer had less than 4-fold differences in antibody titer to POWV (data not shown). Using a strict conservative interpretation, these two samples would be classified as FLAVI reactors. Considering the closest SLEV relative circulating in the United States is WNV (antibody titers to WNV were < 1:20), and POWV is distributed across NYS, one possible interpretation is that these samples represent dual infections with SLEV and POWV. Of note, SLEV has never been isolated in NYS, although multiple human cases were documented in 1975, which coincided with a large outbreak in the Mississippi and Ohio River valleys, and in 2003, a single confirmed case with no recent history of travel was detected in Ontario County (WNY). Similar observations were documented for deer sampled in New Jersey, where 11 samples were SLEV antibody positive, yet virus activity was not detected in mosquitoes or humans.24 In another study, 3.9% of WTD in New Jersey were seropositive for SLEV antibodies.59 The detection of SLEV (or SLEV-like) antibodies in NYS deer suggests a cryptic enzootic cycle, an alternative vector, or an unrecognized virus closely related to SLEV.
Compared with unvaccinated horses, clinical or fatal WNV infections of cervids are rare. In 2002, four captive reindeer at the National Animal Disease Center in Ames, Iowa, were determined to have succumbed to WNV infection.61 At the same farm, nine additional reindeer and six WTD were exposed to the virus but did not present with clinical signs. In Georgia, WNV RNA was detected in the brain of a euthanized deer that displayed neurologic symptoms in 2003.12 In 2012, WNV was isolated from a WTD brain originally submitted to the NYSDOH for rabies testing (unpublished data). A study by Farajollahi et al.24 observed WNV seroprevalence rates of 0.9% in hunter-harvested deer in New Jersey in 2001. A later study found 9.0% of New Jersey deer harvested between 2010 and 2016 were seropositive against WNV.59 In Iowa, before WNV introduction in the Midwest, flavivirus seroprevalence rates ranged from 2.2 to 3.2%. In 2002 and 2003, the period encompassing the WNV epizootic in the Midwest, seroprevalence rates increased to 7.9% and 8.5%, respectively.62 During the current study, WNV rates in NYS (7.3%) were similar to those reported in the Iowa study (6.0%), although obvious temporal relationships, mosquito species composition (enzootic and bridge vectors), and likely other transmission dynamics differ across the two states. Rates in the current study differed by region and year, reflecting local and annual variability in virus maintenance and transmission.
Despite continuous WNV and EEEV activity in NYS since at least 2003, deer exposure to these viruses is relatively low, especially compared with exposure rates to orthobunyaviruses. One likely explanation is that the mosquitoes responsible for enzootic transmission of WNV (Culex pipiens) and EEEV (Culiseta melanura) in NYS are predominantly ornithophilic.9,10,63,64 Recent studies have reported that both mosquito species take a small percentage of meals from mammals, including WTD,9,10 suggesting that bridge vectors are not necessary for transmission to these dead end hosts.
Consistent with prior published WTD serosurveys, seroprevalence rates to JCV and CVV in the current study are higher than rates to the alpha- and flaviviruses. Unlike WNV and EEEV, WTD have been implicated as an amplifying host for JCV and CVV,7,65–68 although many orthobunyaviruses are maintained by vertical transmission.69–73 Jamestown Canyon virus and CVV infection rates in the current study were 54.7% and 25.7%, respectively, but varied by region and year. Rates for the two viruses in the 1969 NYS study were 29% for JCV and 30.6% for CVV.37 Rates from the 1969 study were based on PRNTs using single viruses, whereas rates in the current study were the result of cross-neutralizations using JCV and LACV (CAL) and CVV and POTV (BUN). Determining the seroprevalence using lesser standards (i.e., single representatives of serogroups and lower cutoff for PRNT positivity) on the current samples would increase rates to 67.4% for JCV and 35.4% for CVV, or 1.2–1.4 times higher than the historical rates. The reason for this difference likely cannot be attributed to a single cause, but one plausible contributing factor is the expansion of the WTD population in the United States, including NYS, since the original study was published.74,75
Potosi orthobunyavirus was first isolated from Aedes albopictus mosquitoes collected in Missouri in 1989,50 and then subsequently detected in mosquitoes throughout the midwestern United States and the Carolinas. The first evidence of POTV in the northeastern United States was from mosquitoes collected in Connecticut in 2000.76 In 2003, POTV was first isolated in NYS from a pool of Ochlerotatus trivittatus (Aedes trivittatus) mosquitoes.77 Twenty-five deer during the course of this study were confirmed POTV antibody positive. Results of POTV neutralization in deer sera collected from each region of NYS during this study and 242 isolates of POTV from 19 counties in nine of the 16 years during mosquito surveillance since 2003 (NYSDOH, unpublished data) suggest widespread, episodic transmission of the virus in NYS. White-tailed deer are susceptible to infection with POTV and mount a viremia sufficient to infect mosquitoes.68
A single virus could not be implicated for observed antibody responses in a large proportion of seropositive deer despite the use of closely related, sympatric viruses in the cross-neutralization tests. This is especially true for deer with California and BUN antibodies. To determine CAL positivity, we included JCV and LACV in our testing strategy. Very few deer were confirmed LACV reactors (0.6%), consistent with other studies.1,26,28,60 The hosts of this virus are typically chipmunks and squirrels.78 We screened a number of JCV-, LACV-, and CAL-positive reactors against another CAL virus, TVTV, which has been routinely isolated during mosquito surveillance activities in NYS. All deer samples screened from our study were negative against TVTV. A study from Wisconsin in 1989 found no deer infected with TVTV, whereas an earlier study, also in Wisconsin, found only four of 587 deer with specific antibody to TVTV.4,28 Trivittatus orthobunyavirus is likely maintained transovarially in mosquitoes, but replicates in small mammal hosts as well, similar to LACV.79 It is possible that nonspecific CAL antibody positive deer may be the result of yet another CAL virus circulating in NYS. Both snowshoe hare and South River orthobunyaviruses have been isolated during NYS arbovirus surveillance (NYSDOH, unpublished data), and Keystone orthobunyavirus is known to circulate along the Atlantic coast.26,30 Alternatively, the nonspecific CAL and BUN positives may represent infections with two or more viruses, considering spatiotemporal overlap of multiple viruses and relative longevity of deer. Similar serologic results were observed in studies conducted in California, Indiana, North Carolina, Texas, and Wisconsin.4,5,26,68,80–82 The most common poly-infection we observed was with California and BUN viruses. This observation is not surprising, given the high overall rates of JCV, CVV, and POTV in each region sampled. Low antibody titers to JCV (and possibly other arboviruses) in fawns may represent maternally acquired antibodies.83
One of the limitations of the current study is the inability to compare temporal and regional seroprevalence rates due to inconsistent sampling. County of origin for harvested deer varied each year with LI sampled for only 1 year. Statistical comparisons of seroprevalence rate differences in sexes of deer were not considered because mostly bucks were sampled in the CNY, WNY, and HV regions, whereas LI samples were from a cull that targeted female deer, although other studies did not show a difference in seroprevalence rates to orthobunyaviruses between sexes.4,68
Positive serologic results in NYS WTD indicate recent, local virus transmission of several known human pathogens. Before 2009, only two human cases of EEEV infection were recognized in NYS, one in 1971 and the other in 1983.15,41,55 Since 2009, nine cases have been reported,55 four of which were fatal. It is unlikely that this is the result of improved diagnostics, considering that the NYSDOH has had an active arbovirus surveillance system originally designed for EEEV monitoring in the Oneida Lake (CNY) region since the 1970s,41,54,55 and physicians and local health departments have been aware of the disease. Conversely, the detection of human cases of JCV, CVV, and SLEV infection were likely the result of enhanced arboviral surveillance and newly developed testing methods enacted after the arrival of WNV in NYS in 1999.21,31 Hunter-harvested deer testing provides an efficient passive surveillance system to assess the presence of arthropod-borne viruses of human health significance on a local and regional scale. It can be an especially important tool in areas where active arboviral surveillance is lacking or to augment an established program. Testing can be made more practical, high-throughput, and biosafety level-2 compatible with the use of chimeric viruses and the development of serologic techniques such as microsphere immunofluorescence and enzyme-linked immunoassays to avoid working with infectious virus.
Acknowledgments:
We would like to express our sincere gratitude to the New York State Department of Environmental Conservation for their assistance with specimen collection at check stations and with aging of hunter-killed deer, and to the following deer processors for allowing us to obtain samples for this study at their facilities: J. Clough, F. Dennis, C. Conrad, D. Parshley, and P. Bouchard, and the staff of West End Deer Processing, J. Kreutziger, and the staff of Kreutziger Custom Butchering, and the owners and staff at the Buck and Doe Shop. The Wadsworth Center’s Tissue Culture Core maintained and provided BHK-21 and Vero cells for serology. We thank L. Meehan, B. O’Brien, and R. Lewis for assisting with specimen and data collection. We also extend thanks to B. Laniewicz for preliminary mapping and exploratory data analysis.
REFERENCES
- 1.Friend M, Halterman LG, 1967. Serologic survey of two deer herds in New York State. Bull Wildl Dis Assoc 3: 32–34. [Google Scholar]
- 2.Emmons RW, 1968. Serologic survey of a deer herd in California for arbovirus infections. Bull Wildl Dis Assoc 4: 78–80. [DOI] [PubMed] [Google Scholar]
- 3.Trainer DO, Hanson RP, 1969. Serologic evidence of arbovirus infections in wild ruminants. Am J Epidemiol 90: 354–358. [DOI] [PubMed] [Google Scholar]
- 4.Issel CJ, Trainer DO, Thompson WH, 1972. Serologic evidence of infections of white-tailed deer in Wisconsin with three California group arboviruses (La Crosse, trivittatus, and Jamestown Canyon). Am J Trop Med Hyg 21: 985–988. [DOI] [PubMed] [Google Scholar]
- 5.Issel CJ, Hoff GL, Trainer DO, 1973. Serologic evidence of infection of white-tailed deer in Texas with three California group arboviruses, (Jamestown Canyon, San Angelo, and Keystone). J Wildl Dis 9: 245–248. [DOI] [PubMed] [Google Scholar]
- 6.Merrell CL, Wright DN, 1978. A serologic survey of mule deer and elk in Utah. J Wildl Dis 14: 471–478. [DOI] [PubMed] [Google Scholar]
- 7.McLean RG, Kirk LJ, Shriner RB, Cook PD, Myers EE, Gill JS, Campos EG, 1996. The role of deer as a possible reservoir host of potosi virus, a newly recognized arbovirus in the United States. J Wildl Dis 32: 444–452. [DOI] [PubMed] [Google Scholar]
- 8.Rhoads CL, Bowman JL, Eyler B, 2010. Home range and movement rates of female exurban white-tailed deer. J Wildl Manage 74: 987–994. [Google Scholar]
- 9.Molaei G, Andreadis TG, 2006. Identification of avian- and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, U.S.A. J Med Entomol 43: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 10.Molaei G, Oliver J, Andreadis TG, Armstrong PM, Howard JJ, 2006. Molecular identification of blood-meal sources in Culiseta melanura and culiseta morsitans from an endemic focus of Eastern Equine Encephalitis virus in New York. Am J Trop Med Hyg 75: 1140–1147. [PubMed] [Google Scholar]
- 11.Tate CM, Howerth EW, Stallknecht DE, Allison AB, Fischer JR, Mead DG, 2005. Eastern equine encephalitis in a free-ranging white-tailed deer (Odocoileus virginianus). J Wildl Dis 41: 241–245. [DOI] [PubMed] [Google Scholar]
- 12.Miller DL, Radi ZA, Baldwin C, Ingram D, 2005. Fatal West Nile virus infection in a white-tailed deer (Odocoileus virginianus). J Wildl Dis 41: 246–249. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt SM, Cooley TM, Fitzgerald SD, Bolin SR, Lim A, Schaefer SM, Kiupel M, Maes RK, Hogle SA, O’Brien DJ, 2007. An outbreak of eastern equine encephalitis virus in free-ranging white-tailed deer in Michigan. J Wildl Dis 43: 635–644. [DOI] [PubMed] [Google Scholar]
- 14.Bernard KA, Kramer LD, 2001. West Nile virus activity in the United States, 2001. Viral Immunol 14: 319–338. [DOI] [PubMed] [Google Scholar]
- 15.Oliver J, Lukacik G, Kramer LD, Backenson PB, Sherwood JA, Howard JJ, 2016. Geography and timing of cases of eastern equine encephalitis in New York state from 1992 to 2012. Vector Borne Zoonotic Dis 16: 283–289. [DOI] [PubMed] [Google Scholar]
- 16.Sexton DJ, et al. 1997. Life-threatening Cache Valley virus infection. N Engl J Med 336: 547–549. [DOI] [PubMed] [Google Scholar]
- 17.Campbell GL, Mataczynski JD, Reisdorf ES, Powell JW, Martin DA, Lambert AJ, Haupt TE, Davis JP, Lanciotti RS, 2006. Second human case of Cache Valley virus disease. Emerg Infect Dis 12: 854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SI, Livingston CW, Jr., Edwards JF, Crandell RW, Shope RE, Shelton MJ, Collisson EW, 1990. Evidence that Cache Valley virus induces congenital malformations in sheep. Vet Microbiol 21: 297–307. [DOI] [PubMed] [Google Scholar]
- 19.Hoff GL, Spalatin J, Trainer DO, Hanson RP, 1970. Isolation of a bunyamwera group arbovirus from a naturally infected caribou. J Wildl Dis 6: 483–487. [DOI] [PubMed] [Google Scholar]
- 20.McLean RG, Calisher CH, Parham GL, 1987. Isolation of Cache valley virus and detection of antibody for selected arboviruses in Michigan horses in 1980. Am J Vet Res 48: 1039–1041. [PubMed] [Google Scholar]
- 21.Nguyen NL, Zhao G, Hull R, Shelly MA, Wong SJ, Wu G, St George K, Wang D, Menegus MA, 2013. Cache valley virus in a patient diagnosed with aseptic meningitis. J Clin Microbiol 51: 1966–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Qiu J, Snyder-Keller A, Wu Y, Sun S, Sui H, Dean AB, Kramer L, Hernandez-Ilizaliturri F, 2018. Fatal Cache Valley virus meningoencephalitis associated with rituximab maintenance therapy. Am J Hematol 93: 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nofchissey RA, et al. 2013. Seroprevalence of Powassan virus in New England deer, 1979–2010. Am J Trop Med Hyg 88: 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farajollahi A, Gates R, Crans W, Komar N, 2004. Serologic evidence of West Nile virus and St. Louis encephalitis virus infections in white-tailed deer (Odocoileus virginianus) from New Jersey, 2001. Vector Borne Zoonotic Dis 4: 379–383. [DOI] [PubMed] [Google Scholar]
- 25.Kokernot RH, Hayes J, Will RL, Tempelis CH, Chan DH, Radivojevic B, 1969. Arbovirus studies in the Ohio-Mississippi Basin, 1964–1967. II. St. Louis encephalitis virus. Am J Trop Med Hyg 18: 750–761. [DOI] [PubMed] [Google Scholar]
- 26.Nagayama JN, Komar N, Levine JF, Apperson CS, 2001. Bunyavirus infections in North Carolina white-tailed deer (Odocoileus virginianus). Vector Borne Zoonotic Dis 1: 169–171. [DOI] [PubMed] [Google Scholar]
- 27.Zamparo JM, Andreadis TG, Shope RE, Tirrell SJ, 1997. Serologic evidence of Jamestown Canyon virus infection in white-tailed deer populations from Connecticut. J Wildl Dis 33: 623–627. [DOI] [PubMed] [Google Scholar]
- 28.Murphy RK, 1989. Serologic evidence of arboviral infections in white-tailed deer from central Wisconsin. J Wildl Dis 25: 300–301. [DOI] [PubMed] [Google Scholar]
- 29.Boromisa RD, Grimstad PR, 1987. Seroconversion rates to Jamestown Canyon virus among six populations of white-tailed deer (Odocoileus virginianus) in Indiana. J Wildl Dis 23: 23–33. [DOI] [PubMed] [Google Scholar]
- 30.Watts DM, LeDuc JW, Bailey CL, Dalrymple JM, Gargan TP, 2nd, 1982. Serologic evidence of Jamestown Canyon and Keystone virus infection in vertebrates of the DelMarVa Peninsula. Am J Trop Med Hyg 31: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 31.Pastula DM, Hoang Johnson DK, White JL, Dupuis AP, 2nd, Fischer M, Staples JE, 2015. Jamestown Canyon virus disease in the United States-2000–2013. Am J Trop Med Hyg 93: 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srihongse S, Grayson MA, Bosler EM, 1979. California encephalitis complex virus isolations from mosquitoes collected in northeastern New York, 1976–1977. Mosq News 39: 73–76. [Google Scholar]
- 33.Srihongse S, Grayson MA, Deibel R, 1984. California serogroup viruses in New York State: the role of subtypes in human infections. Am J Trop Med Hyg 33: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 34.Srihongse S, Woodall JP, Grayson MA, Deibel R, 1980. Arboviruses in New York State; surveillance in arthropods and nonhuman vertebrates, 1972–1977. Mosquito News 40: 269–276. [Google Scholar]
- 35.Whitney E, 1963. Serologic evidence of group A and B arthropod-borne virus activity in New York State. Am J Trop Med Hyg 12: 417–424. [DOI] [PubMed] [Google Scholar]
- 36.Whitney E, 1965. Arthropod-borne viruses in New York state: serologic evidence of groups A, B, and Bunyamwera viruses in dairy herds. Am J Vet Res 26: 914–919. [PubMed] [Google Scholar]
- 37.Whitney E, Jamnback H, Means RG, Roz AP, Rayner GA, 1969. California virus in New York State. Isolation and characterization of California encephalitis virus complex from Aedes cinereus. Am J Trop Med Hyg 18: 123–131. [PubMed] [Google Scholar]
- 38.Whitney E, Jamnback H, Means RG, Watthews TH, 1968. Arthropod-borne-virus survey in St. Lawrence County, New York. Arbovirus reactivity in serum from amphibians, reptiles, birds, and mammals. Am J Trop Med Hyg 17: 645–650. [DOI] [PubMed] [Google Scholar]
- 39.Whitney E, Roz AP, Rayner GA, Deibel R, 1969. Serologic survey for arbovirus activity in deer sera from nine counties in New York State. Wildl Dis 5: 392–397. [DOI] [PubMed] [Google Scholar]
- 40.Howard JJ, Emord DE, Morris CD, 1983. Epizootiology of eastern equine encephalomyelitis virus in upstate New York, USA. V. Habitat preference of host-seeking mosquitoes (Diptera: Culicidae). J Med Entomol 20: 62–69. [DOI] [PubMed] [Google Scholar]
- 41.Howard JJ, Grayson MA, White DJ, Oliver J, 1996. Evidence for multiple foci of eastern equine encephalitis virus (Togaviridae:Alphavirus) in central New York State. J Med Entomol 33: 421–432. [DOI] [PubMed] [Google Scholar]
- 42.Howard JJ, Oliver J, Grayson MA, 2004. Antibody response of wild birds to natural infection with alphaviruses. J Med Entomol 41: 1090–1103. [DOI] [PubMed] [Google Scholar]
- 43.Komar N, 2001. West Nile virus surveillance using sentinel birds. Ann New York Acad Sci 951: 58–73. [DOI] [PubMed] [Google Scholar]
- 44.Lindsey HS, Calisher CH, Matthews JH, 1976. Serum dilution neutralization test for California group virus identification and serology. J Clin Microbiol 4: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE, 1989. Antigenic relationships between flaviviruses as determined by cross- neutralization tests with polyclonal antisera. J Gen Virol 70 (Pt 1): 37–43. [DOI] [PubMed] [Google Scholar]
- 46.Morris CD, Whitney E, Bast TF, Deibel R, 1973. An outbreak of eastern equine encephalomyelitis in upstate New York during 1971. Am J Trop Med Hyg 22: 561–566. [DOI] [PubMed] [Google Scholar]
- 47.Walker ED, Grayson MA, Edman JD, 1993. Isolation of Jamestown Canyon and snowshoe hare viruses (California serogroup) from Aedes mosquitoes in western Massachusetts. J Am Mosq Control Assoc 9: 131–134. [PubMed] [Google Scholar]
- 48.Deibel R, Srihongse S, Woodall JP, 1979. Arboviruses in New York State: an attempt to determine the role of arboviruses in patients with viral encephalitis and meningitis. Am J Trop Med Hyg 28: 577–582. [PubMed] [Google Scholar]
- 49.Dupuis AP, Marra PP, Kramer LD, 2003. Serologic evidence of West Nile virus transmission, Jamaica, West Indies. Emerg Infect Dis 9: 860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell CJ, Smith GC, Miller BR, 1990. Vector competence of Aedes albopictus for a newly recognized Bunyavirus from mosquitoes collected in Potosi, Missouri. J Am Mosq Control Assoc 6: 523–527. [PubMed] [Google Scholar]
- 51.Karabatsos N, 1985. International Catalog of Arboviruses 1985. San Antonio, TX: American Society of Tropical Medicine and Hygiene. [Google Scholar]
- 52.Holden P, Hess AD, 1959. Cache Valley virus, a previously undescribed mosquito-borne agent. Science 130: 1187–1188. [DOI] [PubMed] [Google Scholar]
- 53.Reisen WK, 2003. Epidemiology of St. Louis encephalitis virus. Adv Virus Res 61: 139–183. [DOI] [PubMed] [Google Scholar]
- 54.Oliver J, Lukacik G, Kokas J, Campbell SR, Kramer LD, Sherwood JA, Howard JJ, 2018. Twenty years of surveillance for eastern equine encephalitis virus in mosquitoes in New York State from 1993 to 2012. Parasit Vectors 11: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliver JO, et al. 2020. Spatial and temporal expansions of Eastern equine encephalitis virus and phylogenetic groups isolated from mosquitoes and mammalian cases in New York State from 2013 to 2019. Emerg Microbes Infect 9: 1638–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berl E, Eisen RJ, MacMillan K, Swope BN, Saxton-Shaw KD, Graham AC, Turmel JP, Mutebi JP, 2013. Serological evidence for eastern equine encephalitis virus activity in white-tailed deer, Odocoileus virginianus, in Vermont, 2010. Am J Trop Med Hyg 88: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mutebi JP, Swope BN, Saxton-Shaw KD, Graham AC, Turmel JP, Berl E, 2012. Eastern equine encephalitis in moose (Alces americanus) in northeastern Vermont. J Wildl Dis 48: 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mutebi JP, et al. 2011. Using wild white-tailed deer to detect eastern equine encephalitis virus activity in Maine. Vector Borne Zoonotic Dis 11: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen K, Wang E, Weaver SC, Wolf PC, Randall AR, Van Why KR, Travassos Da Rosa APA, Gidlewski T, 2017. Serologic evidence of various arboviruses detected in white-tailed deer (Odocoileus virginianus) in the United States. Am J Trop Med Hyg 97: 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoff GL, Issel CJ, Trainer DO, Richards SH, 1973. Arbovirus serology in North Dakota mule and white-tailed deer. J Wildl Dis 9: 291–295. [DOI] [PubMed] [Google Scholar]
- 61.Palmer MV, Stoffregen WC, Rogers DG, Hamir AN, Richt JA, Pedersen DD, Waters WR, 2004. West Nile virus infection in reindeer (Rangifer tarandus). J Vet Diagn Invest 16: 219–222. [DOI] [PubMed] [Google Scholar]
- 62.Santatella J, McLean R, Hall JS, Gill JS, Bowen RA, Hadow HH, Clark L, 2005. West Nile virus serosurveillance in Iowa white-tailed deer (1999–2003). Am .J Trop Med Hyg 73: 1038–1042. [PubMed] [Google Scholar]
- 63.Molaei G, Huang S, Andreadis TG, 2012. Vector-host interactions of Culex pipiens complex in northeastern and southwestern USA. J Am Mosq Control Assoc 28: 127–136. [DOI] [PubMed] [Google Scholar]
- 64.Ngo KA, Kramer LD, 2003. Identification of mosquito bloodmeals using polymerase chain reaction (PCR) with order-specific primers. J Med Entomol 40: 215–222. [DOI] [PubMed] [Google Scholar]
- 65.Issel CJ, Trainer DO, Thompson WH, 1972. Experimental studies with white-tailed deer and four California group arboviruses (La Crosse, Trivittatus, snowshoe hare, and Jamestown Canyon). Am J Trop Med Hyg 21: 979–984. [DOI] [PubMed] [Google Scholar]
- 66.Issel CJ, 1973. Isolation of Jamestown Canyon virus (a California group arbovirus) from a white-tailed deer. Am J Trop Med Hyg 22: 414–417. [DOI] [PubMed] [Google Scholar]
- 67.Watts DM, Tammariello RF, Dalrymple JM, Eldridge BF, Russell PK, Top FH, Jr., 1979. Experimental infection of vertebrates of the Pocomoke Cypress Swamp, Maryland with Keystone and Jamestown Canyon viruses. Am J Trop Med Hyg 28: 344–350. [DOI] [PubMed] [Google Scholar]
- 68.Blackmore CG, Grimstad PR, 1998. Cache Valley and Potosi viruses (Bunyaviridae) in white-tailed deer (Odocoileus virginianus): experimental infections and antibody prevalence in natural populations. Am J Trop Med Hyg 59: 704–709. [DOI] [PubMed] [Google Scholar]
- 69.Rosen L, 1987. Overwintering mechanisms of mosquito-borne arboviruses in temperate climates. Am J Trop Med Hyg 37: 69S–76S. [DOI] [PubMed] [Google Scholar]
- 70.Watts DM, Eldridge BF, 1975. Transovarial transmission of arboviruses by mosquitoes: a review. Med Biol 53: 271–278. [PubMed] [Google Scholar]
- 71.Beaty BJ, Thompson WH, 1976. Delineation of La Crosse virus in developmental stages of transovarially infected Aedes triseriatus. Am J Trop Med Hyg 25: 505–512. [DOI] [PubMed] [Google Scholar]
- 72.Thompson WH, Beaty BJ, 1978. Venereal transmission of La Crosse virus from male to female Aedes triseriatus. Am J Trop Med Hyg 27: 187–196. [DOI] [PubMed] [Google Scholar]
- 73.Turell MJ, LeDuc JW, 1983. The role of mosquitoes in the natural history of California serogroup viruses. Prog Clin Biol Res 123: 43–55. [PubMed] [Google Scholar]
- 74.Curtis PD, Sullivan KL, 2001. White-Tailed Deer. Wildlife Damage Management Fact Sheet Series. Cornell Cooperative Extension, Ithaca, NY: Cornell University, 6. [Google Scholar]
- 75.Batcheller GR, Riexinger P, 2011. Management Plan for White-tailed Deer in New York State 2012–2016. Conservation NYSDoE, ed. Albany, NY: New York State Department of Environmental Conservation, 1–59. [Google Scholar]
- 76.Armstrong PM, Andreadis TG, Anderson JF, Main AJ, 2005. Isolations of Potosi virus from mosquitoes (Diptera: Culicidae) collected in Connecticut. J Med Entomol 42: 875–881. [DOI] [PubMed] [Google Scholar]
- 77.Ngo KA, Maffei J, Dupuis AP, Kauffman EB, Backenson PB, Kramer LD, 2006. Isolation of bunyamwera serogroup viruses (Bunyaviridae, Orthobunyavirus) in New York state. J Med Entomol 43: 1004. [DOI] [PubMed] [Google Scholar]
- 78.Calisher CH, 1994. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev 7: 89–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watts DM, DeFoliart GR, Yuill TM, 1976. Experimental transmission of trivittatus virus (California virus group) by Aedes trivittatus. Am J Trop Med Hyg 25: 173–176. [DOI] [PubMed] [Google Scholar]
- 80.Boromisa RD, Grayson MA, 1990. Incrimination of Aedes provocans as a vector of Jamestown Canyon virus in an enzootic focus of northeastern New York. J Am Mosq Control Assoc 6: 504–509. [PubMed] [Google Scholar]
- 81.Campbell GL, Eldridge BF, Hardy JL, Reeves WC, Jessup DA, Presser SB, 1989. Prevalence of neutralizing antibodies against California and Bunyamwera serogroup viruses in deer from mountainous areas of California. Am J Trop Med Hyg 40: 428–437. [DOI] [PubMed] [Google Scholar]
- 82.Issel CJ, Hoff GL, Trainer DO, Thompson WH, 1970. Serologic evidence of bunyamwera group arbovirus infections in Wisconsin and Texas deer. J Wildl Dis 6: 479–482. [DOI] [PubMed] [Google Scholar]
- 83.Issel CJ, 1974. Maternal antibody to Jamestown Canyon virus in white-tailed deer. Am J Trop Med Hyg 23: 242–245. [DOI] [PubMed] [Google Scholar]