Abstract
This study was performed to investigate the distribution of phenolic compounds in the peanut skins of various cultivars, as well as their antioxidant and anti‐inflammatory effect (Arachishypogaea L. cv. K‐Ol, cv. Sinpalkwang, cv. Daan, cv. Heuksaeng) and extraction solvent. The major components of red peanut cultivars (K‐Ol, Sinpalkwang, and Daan) were identified as proanthocyanidin, catechin, gallic acid, coumaric acid, and hesperidine, whereas the major components of black peanut cultivar (Heuksaeng) were identified as anthocyanin, ferulic acid, and quercetin. The DPPH and ABTS radical scavenging activities, and FRAP values were the highest in Daan followed by Sinpalkwng, K‐Ol, and Heuksang. Furthermore, the skin extracts of red peanuts effectively improved cell viability, reactive oxygen species generation, MDA concentration, and antioxidant enzyme activity (GR, GPx, CAT, and superoxide dismutase) in oxidative stress‐induced HepG2 cells, and reduced the expression of pro‐inflammatory factors (NO, TNF‐α, IL‐6, and IL‐1β) in LPS‐stimulated RAW 264.7 macrophages. These results suggest that red peanut skin extracts could effectively mediate physiological activity and provide valuable information for the use of peanut byproducts as functional food materials.
Keywords: anti‐inflammatory effect, antioxidant effects, cultivar, peanut skin, polyphenols
This study was performed to investigate the distribution of phenolic compounds in the peanut skins of various cultivars, as well as their antioxidant and anti‐inflammatory effect (Arachishypogaea L. cv. K‐Ol, cv. Sinpalkwang, cv. Daan, cv. Heuksaeng) and extraction solvent. The skin extracts of red peanuts effectively improved cell viability, reactive oxygen species generation, MDA concentration, and antioxidant enzyme activity (GR, GPx, CAT, and superoxide dismutase) in oxidative stress‐induced HepG2 cells, and reduced the expression of pro‐inflammatory factors (NO, TNF‐α, IL‐6, and IL‐1β) in LPS‐stimulated RAW 264.7 macrophages.

1. INTRODUCTION
An increase in the levels of cellular reactive oxygen species (ROS) above the levels tolerated by a cell's defenses results in oxidative stress (Ghosh & Myers, 1998). The high levels of ROS alter redox homeostasis and cause oxidative stress, which damages cellular proteins, lipids, and DNA. Oxidative stress has been recognized to be involved in the pathogenesis of aging and several degenerative diseases, such as cardiovascular and inflammatory diseases, diabetes, and cancer (Halliwell & Gutteridge, 1999). Furthermore, inflammatory response is a complex response to local injury or infection and involves various immune cells and numerous mediators (Kindt et al., 2007). While acute inflammation is essential for combating infection and tissue repair, excessive and uncontrolled inflammation is often associated with chronic diseases, such as metabolic disorders, atherosclerosis, and certain types of cancer (Chakrabarti et al., 2014). The role of food polyphenolics in the prevention of cardiovascular diseases and certain types of cancer is well recognized. Byproducts of seeds (Galali et al., 2020), such as the skins of brazil nut (John & Shahidi, 2010), almond (Wijeratne et al., 2006), and peanuts (Levy et al., 2017; Sarnoski et al., 2012) have been reported to be rich sources of polyphenolics and antioxidants.
Peanut (Arachishypogaea L.) is a critical oil crop that is widely cultivated in many countries, and it is an important food material that is consumed worldwide (Oldoni et al., 2016). Even though peanut skin is a small portion of the total seed, it was discarded 7.5 × 105 tons a year as a by‐product. But peanut skin contains several abundant polyphenolic compounds, such as flavonoids, phenolic acids, procyanidins, and anthocyanins, in approximately 90–150 mg/g of the dried skin (Nepote et al., 2010). The Rural Development Administration (RDA) of Korea conducted research on the development of peanut varieties with various functional characteristics such as Sinpalkwang (Pae, Hwang, Kim, et al., 2016), Daan (Pae, Lee, et al., 2017), K‐Ol (Pae, Hwang, Lee, et al., 2016), and Heuksaeng (Pae, Kim, et al., 2017). Sinpalkwang and Daan could be cultivated easily in high yields. Additionally, K‐Ol was found to comprise greater than 80% of oleic acid among the typical fatty acid composition, and Heuksaeng had a characteristic black color and high containing anthocyanin content. These developed peanut cultivars have different characteristics and phenolic compound profiles in peanut skin, whereas there is a lack of investigation on antioxidant and anti‐inflammatory effect in the HepG2 cell and RAW 264.7 macrophages.
Therefore, the objective of this study is to (a) investigate the composition of phenolic compounds in the skins of four peanut cultivars, as well as their antioxidant and anti‐inflammatory effects on tert‐butyl hydroperoxide (TBHP)‐induced oxidative stress in HepG2 cells and LPS‐stimulated RAW 264.7 macrophages and (b) evaluate the correlation between the phenolic compounds and the antioxidant and anti‐inflammatory characteristics to identify marker compounds in peanut skin.
2. MATERIALS AND METHODS
2.1. Sample preparation and extraction
Four types of peanut cultivars (Arachishypogaea L.) cv. Sinpalkwang, cv. K‐Ol, cv. Daan, and cv. Heuksaeng were used in this study. Two peanut cultivars, cv. Sinpalkwang and K‐Ol, were grown at the National Institute of Crop Science and Foundation of Agriculture Technology Commercialization & Transfer in Jeonju, South Korea, respectively, during the 2018 crop season. The other two peanut cultivars, cv. Daan and Heuksaeng, were grown at EM Food in Gochang, South Korea, during the 2018 crop season. The powdered samples (4 g) were extracted thrice with 80% ethanol (80 ml), 80% methanol (80 ml), 80% acetone (80 ml), and distilled water (80 ml) at room temperature for 1 hr using an ultrasonic bath. The extracts were then filtered and concentrated using a rotary evaporator under vacuum and were then freeze‐dried and stored at −20°C in an ultralow temperature freezer.
2.2. Determination of phenolic compounds
The total polyphenol and flavonoid levels were measured according to the method reported by Dewonto et al (Dewanto et al., 2002). The results are expressed as milligrams of gallic acid and catechin equivalents per gram of peanut skin (mg GAE/g peanut skin, mg CE/g peanut skin). Total proanthocyanidin levels were measured using the vanillin‐sulfuric acid method (Takahama et al., 2010). The results are expressed as milligrams of catechin equivalents per gram of the peanut skin (mg CE/g peanut skin). Total anthocyanin content was determined based on a reported pH differential method; these results were expressed in milligrams of cyaniding‐3‐glucose equivalents per gram of peanut skin (mg C3GE/g peanut skin) (Tchabo et al., 2015). The phenolic acid and anthocyanin composition of each extract was determined by high‐performance liquid chromatography (HPLC), as described by Kim et al. (2016) and Choung (2008), respectively.
2.3. Antioxidant activity
The radical scavenging activities of 1,1‐Diphenyl‐2‐picrylhydrazyl (DPPH) and 2,2‐azinobis (3‐ethyl benzothiazoline)‐6‐sulfonic acid (ABTS) were measured according to the method reported by Choi et al. (2006). Both radical scavenging activities were expressed as the Trolox‐equivalent antioxidant capacities (TEAC), as mg TE/g extract residue (ER). Also, the method of Benzie and Strain was used with modifications to measure the ferric‐reducing antioxidant power assay (Benzie & Strain, 1996); results are presented as mM Fe2 + equivalents.
2.4. Protective effect in oxidative‐damaged HepG2 cell
Human hepatoma HepG2 cells were obtained from the Korean Cell Line Bank (Seoul, Korea). The cell line was maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 50 μg/ml streptomycin at 37°C in an incubator with a 5% CO2 atmosphere. Human hepatoma HepG2 cell death was measured using a 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazoliumbromide (MTT) assay (Ishiyama et al., 1996). To determine cytoprotective effects against oxidative stress, HepG2 cells were seeded in a 96‐well plate at a density of 1.5 × 104 cells/well. After 24 hr, the culture medium (200 ml/well) was replaced with an FBS‐free medium (200 ml/well) containing various concentrations of extracts, after 12 hr, the culture medium containing the extract was discarded, and the cells were treated for 3 hr with the medium (200 ml/well) containing 200 mmol/L of TBHP to induce oxidative stress. We then evaluated the protective effect of extracts using the MTT assay. Intracellular ROS levels were quantified with a DCFH‐DA fluorescent probe, as previously described (Wang & Joseph, 1999). The fluorescence intensity, corresponding to the intracellular ROS generation, was measured with a fluorescence spectrophotometer (Perkin‐Elmer, Norwalk, CT, USA) for 2 hr at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. To determine lipid peroxidation and antioxidant enzyme activity, the cells were harvested and lysed for 10 s using a Vibra‐Cell VCX 750 sonicator (Sonics&Materials, Inc., Newtown, CT, USA). The lysates were centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants were used for protein and lipid peroxidation, and antioxidant enzyme assay according to the Ham et al (Ham et al., 2015).
2.5. Anti‐inflammation activity in LPS‐induced RAW 264.7 cell
RAW 264.7 macrophages were obtained from the Korean Cell Line Bank (Seoul, Korea). The cell line was maintained in DMEM, supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 50 μg/mL streptomycin at 37°C in an incubator with a 5% CO2 atmosphere. RAW 264.7 cell death was measured using a 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazoliumbromide (MTT) assay20. Cytotoxicity was calculated as a percentage of control cell viability. RAW 264.7 cells (5 × 104 cells/well) were seeded in 96‐well plates and incubated for 6 hr at 37°C. Cells were treated with or without LPS (0.5 μg/ml) and the indicated concentrations of soybean protein extract for 24 hr. Then, the concentration of NO, TNF‐α, IL‐6, and IL‐1β in the medium was measured using a Griess Reagent System (Promega, Madison, WI, USA) and Cymax TM Mouse cytokine ELISA assay kit (Ab PRONTIER, Seoul, Korea), respectively (Srisook et al., 2006).
2.6. Statistical analysis
All data are expressed as means ± standard deviations. Significant differences among treatments were determined by one‐way analysis of variance using Duncan's multiple range test, with SAS ver. 9.2 software (SAS Institute). The significance level was set to 0.05.
3. RESULTS AND DISCUSSION
3.1. Composition of phenolic compounds in peanut skin extracts
Plant phenolic compounds are currently among the most studied phytochemicals because of their biological functions, including antioxidant and anti‐inflammatory characteristics (Koleckar et al., 2008). The total polyphenol contents (TPC), total flavonoid contents (TFC), total proanthocyanidin contents (TPAC), and total anthocyanin contents (TAC) of the peanut skin according to cultivars and extraction solvents are shown in Table 1. Among the extraction solvents tested, 80% methanol and 80% acetone were found to be the most efficient solvent systems for extracting the polyphenolic compounds when compared with all other solvent systems used, and the levels of the TPCs ranged from 122.17 to 166.29 mg GAE/g peanut skin. The TFC results tended to be similar to TPC results. The recovery of the phenolic contents in different samples is influenced by the polarity of extraction solvents and the solubility of the particular compound in the solvent used for the extraction process (Sulaiman et al., 2011). When considering the effect of the cultivar on the TPC and TFC, the highest TPC was observed for the Daan cultivar in the case of the peanut skin extracted with 80% ethanol, 80% acetone, and distilled water. In contrast, the highest TPC, which was significantly higher than that of others, was observed for Heuksaeng (166.29 mg GAE/g peanut skin) and Daan (166.20 mg GAE/g peanut skin) in the case of the peanut skin extracted with 80% acetone and methanol, respectively. The high polyphenol content of the Heuksaeng cultivar was related to the anthocyanin content of the black pigment peanut cultivar. Also, these results are in close agreement with those reported by Larrauri et al. (2016) They observed that the extraction yield of the roasted and blanched peanut skin with 70% ethanol and distilled water ranged from 9.8% to 18.0%, and total the polyphenol contents of these extracts ranged from 295 to 672 mg GAE/g.
TABLE 1.
Total polyphenol content (mg GAE/g), total flavonoid content (mg CE/g), total proanthocyanidin content (mg CE/g), and total anthocyanin contents (mg C3GE/g) of peanut skin extracts according to cultivars and extraction solvent
| Cultivar | Solvent | TPC (mg GAE/g) | TFC (mg CE/g) | TPAC (mg CE/g) | TAC (mg C3GE/g) |
|---|---|---|---|---|---|
| K‐Ol | 80% EtOH | 104.90 ± 2.45Db | 77.64 ± 2.49Cc | 23.93 ± 0.27Bb | 4.45 ± 0.13Cc |
| 80% MeOH | 122.17 ± 3.98Da | 83.38 ± 2.08Cb | 30.03 ± 3.94Ba | 7.43 ± 0.16Cb | |
| 80% Aceton | 125.79 ± 4.52Da | 90.14 ± 3.02Ca | 32.79 ± 1.99Ca | 8.69 ± 0.37Da | |
| Water | 58.11 ± 1.50Cc | 40.48 ± 1.07Cd | 10.65 ± 0.85Cc | 1.47 ± 0.10Cd | |
| Sinpalkwang | 80% EtOH | 112.22 ± 4.99Cc | 84.16 ± 4.24Bb | 25.71 ± 1.25Bc | 4.48 ± 0.04Cd |
| 80% MeOH | 131.83 ± 2.18Cb | 98.42 ± 2.24Ba | 34.02 ± 0.43Bb | 7.85 ± 0.35Cb | |
| 80% Aceton | 139.97 ± 4.22Ca | 101.17 ± 2.83Ba | 43.66 ± 1.37Ba | 9.56 ± 0.12Ca | |
| Water | 52.73 ± 0.55Dd | 33.25 ± 0.93Dc | 13.03 ± 0.81Bd | 1.52 ± 0.10Cc | |
| Daan | 80% EtOH | 130.68 ± 0.42Ac | 91.14 ± 1.11Ac | 38.62 ± 1.15Ab | 5.88 ± 0.17Bb |
| 80% MeOH | 166.20 ± 2.13Aa | 129.24 ± 2.32Aa | 47.92 ± 0.51Aa | 12.16 ± 0.21Ba | |
| 80% Aceton | 158.26 ± 0.74Bb | 109.69 ± 2.43Ab | 48.91 ± 0.57Aa | 12.51 ± 0.41Ba | |
| Water | 66.20 ± 2.15Bd | 52.77 ± 0.42Ad | 20.07 ± 0.58Ac | 2.17 ± 0.11Bb | |
| Heuksaeng | 80% EtOH | 123.65 ± 2.21Bc | 49.90 ± 0.44Dc | 8.68 ± 2.03Cb | 13.85 ± 0.43Ac |
| 80% MeOH | 149.21 ± 2.89Bb | 63.48 ± 0.53Db | 10.5 ± 1.95Cab | 16.91 ± 0.12Ab | |
| 80% Aceton | 166.29 ± 1.34Aa | 73.25 ± 0.67Da | 13.09 ± 2.57Da | 23.31 ± 0.45Aa | |
| Water | 118.19 ± 2.45Ad | 42.49 ± 0.68Bd | 9.12 ± 1.20Cb | 17.47 ± 0.29Ab |
Different capital letters and small letter in the same items indicate a significant difference (p < .05) among different cultivars and extraction solvent, respectively.
Abbreviations: TAC, Total anthocyanin content; TFC, Total flavonoid content; TPAC, Total proanthocyanidin content; TPC, Total polyphenol content.
Since the pigments of the peanut skin play an important role in physiological effects, there has been growing attention on the composition and medicinal use of peanut skin (Tatsuno et al., 2012). Therefore, we analyzed the pigment characteristics, such as UV absorption, total proanthocyanidin content (TPAC), and total anthocyanin content (TAC) of the peanut skin extracts of the various cultivars (Figure 1, Table 1). Peanut seeds have several skin colors, including red and black (Chukwumah et al., 2009). K‐Ol, Sinpalkwng, and Daan are red‐pigmented peanut cultivar, whereas Heuksaeng is a black‐pigmented peanut cultivar. These differences in the colors of the peanuts affected the UV spectra, TPAC, and TAC of the peanut skin extracts. As shown in Table 1, the total proanthoyanidin content was the highest in Daan (20.07–48.91 mg CE/g peanut skin) and was generally higher in the red peanut cultivars than those in the black ones. Meanwhile, the total anthocyanin contents were highest in Heuksang (13.85–23.31 mg C3GE/g peanut skin). Furthermore, the variations in the individual phenolics and anthocyanin profiles of the peanut skins according to their cultivar are shown in Table 2. Among the individual phenolics, the major compounds in the red peanut cultivars were catechin and ferulic acid, which accounted for approximately 85% of the total phenolics. However, in the case of the black peanut cultivar, ferulic acid was the major phenolics and accounted for approximately 75% of the total phenolics content. Moreover, anthocyanins, such as delpinidin‐3‐glucoside (1.88 mg/g), cyanidin‐3‐sorporoside (3.16 mg/g), and cyanidin‐3‐sambubitoside (1.26 mg/g), were only detected in black cultivars. Various studies have reported the phenolic compounds and anthocyanin according to the cultivar and skin color. Yu et al. (2005) reported that compounds found in red peanut skin are considered potent antioxidants, particularly, phenolic acid (ferulic acid, coumaric acid, and chlorogenic acid) and flavonoid (epicatechin, catechin, and Resveratrol). Also, Zhaoet al. (2017) found that the main anthocyanins were cyanidin‐3‐O‐sophoroside(C3So) and cyanidin‐3‐O‐sambubioside (C3Sa), which accounting forabout 90% of total anthocyanins in black peanut skin (BPS), and thereare also low content of Cyanidin‐3‐O‐glucosylrutinoside, Cyanidin‐3‐O‐xylosylrutinoside, etc. These differences in the peanut skin extracts depending on the color of the cultivar are believed to affect their antioxidant and anti‐inflammatory characteristics in the TBHP‐induced HepG2 cell and LPS‐stimulated RAW 264.7 macrophage tests.
FIGURE 1.

The wavelength scan (300–800 nm) of peanut skin extracts according to cultivars
TABLE 2.
Phenolic acid contents (μg/g peanut skin) and anthocyanin contents (mg/g peanut skin) of peanut skins, according to their cultivar
| Cultivar | ||||
|---|---|---|---|---|
| K‐Ol | Sinpalkwang | Daan | Heuksaeng | |
| phenolics (μg/g) | ||||
| Catehin | 845.87 ± 14.96b | 931.93 ± 25.70ab | 1,234.45 ± 64.12a | 166.74 ± 7.90c |
| Gallic acid | 55.30 ± 1.80b | 65.42 ± 3.28ab | 130.01 ± 14.53a | 35.54 ± 2.25c |
| Coumaric acid | 60.73 ± 0.50b | 34.53 ± 1.56c | 129.38 ± 9.69a | 4.95 ± 0.11d |
| Ferulic acid | 559.16 ± 15.59c | 730.57 ± 19.15b | 763.22 ± 127.38b | 1,055.15 ± 13.20a |
| Hesperidin | 8.82 ± 0.68b | 8.99 ± 0.07b | 12.75 ± 0.78a | 2.74 ± 0.07c |
| Quercetin | 60.30 ± 0.67c | 36.68 ± 2.38d | 94.55 ± 2.97b | 132.91 ± 1.45a |
| Total | 1,590.19 ± 1.08c | 1,808.11 ± 28.47b | 2,364.37 ± 159.57a | 1,398.05 ± 15.17d |
| Anthocyanin (mg/g) | ||||
| Delpinidin‐3‐glucoside | ND | ND | ND | 1.88 ± 0.36 |
| Cyanidin‐3‐soporoside | ND | ND | ND | 3.16 ± 0.37 |
| Cyanidin‐3‐sambubioside | ND | ND | ND | 1.26 ± 0.28 |
| Cyanidin | ND | ND | 0.17 ± 0.01 | ND |
| Peonidin | ND | ND | 0.06 ± 0.01 | ND |
| Total | ND | ND | 0.23 ± 0.00 | 6.3 ± 1.00 |
Different small letters in the same item indicates a significant difference (p < .05) among different cultivars.
Abbreviation: ND, Not detected.
3.2. Effect of peanut skin extracts on radical scavenging activity and reducing power
The effects of the cultivar and extraction solvents on the peanut skin extract's ABTS, DPPH radical scavenging activities, and reducing power (FRAP) are presented in Figure 2. The ABTS, DPPH radical scavenging activities, and FRAP values were highest in Daan (462.63–565.05 mg AAE/g extract, 178.80–228.92 mg AAE/g extract, and 278.38–451.29 μM/g extract), and were generally higher in the red peanut cultivars than the black peanut ones. Interestingly, the total polyphenol contents were not significantly different between Daan and Heuksang (Table 1), whereas the radical scavenging activity and reducing power was significantly higher in Daan than that in Heuksang (p < .05). These results are related to the difference in compositions of the phenolics that make up the total polyphenols. Muselík et al. (2007) reported in vitro antioxidant activity of isolated catechins, procyanidins, anthocyanins, and pyranoanthocyanins by using four methods that had different mechanisms. They concluded that procyanidins were among the in vitro tested groups and were the ones which showed higher antioxidant capacity, followed by catechins, anthocyanins, and pyranoanthocyanins. The antioxidant activity of the tested series of polyphenolic compounds correlated with the number of aromatic hydroxyl groups in the aqueous phase assays (Adhikari et al., 2019). In this study, we postulated that red peanut cultivars, containing a high amount of catechins and proanthocyanidins, which have a relatively large number of aromatic hydroxyl groups, showed higher antioxidant activity than the black peanut cultivar composed of anthocyanins.
FIGURE 2.

(a) ABTS (mg AAE/g extract), (b) DPPH (mg AAE/g extract) scavenging activity, and (c) FRAP (μM/g extract) of peanut skin extracts according to cultivars and extraction solvent. Different capital letters and small letter in the same items indicate a significant difference (p < .05) among different cultivars and extraction solvent, respectively
3.3. Effect of peanut skin extract on endogenous antioxidant defense system and biomarker of oxidative stress in TBHP‐induced HepG2 cell
The hepatoprotective effects of the peanut skin extracts on oxidative stress‐induced HepG2 cell were investigated to determine protective effects, ROS inhibition, malondialdehyde (MDA) concentration, and antioxidant enzyme activity (glutathione reductase; GR, glutathione peroxidase; GPx, SOD, catalase; CAT). Before measuring the cytoprotective effects of the peanut skin extract on TBHP‐induced HepG2 cells, the cytotoxicity was estimated by MTT assay Figure 3a). Next, we evaluated the cytoprotective effects of the peanut skin extracts of the four different cultivars against TBHP‐induced oxidative damage (Figure 3b). These results demonstrate that the treatment with 500 μM TBHP significantly decreased cell viability by 53.68% compared to that in control cells. However, pretreatment of the peanut skin extract at 2.5–10 μg/ml significantly increased cell viability in a dose‐dependent manner, regardless of the identity of the cultivar. Especially, the highest protective effects from the TBHP‐induced stress in the HepG2 cells were observed in the peanut skin extracts of Daan and Sinpalkwng; these extracts increased cell viability to 101.19% and 95.97%, respectively, at a concentration of 10 μg/ml.
FIGURE 3.
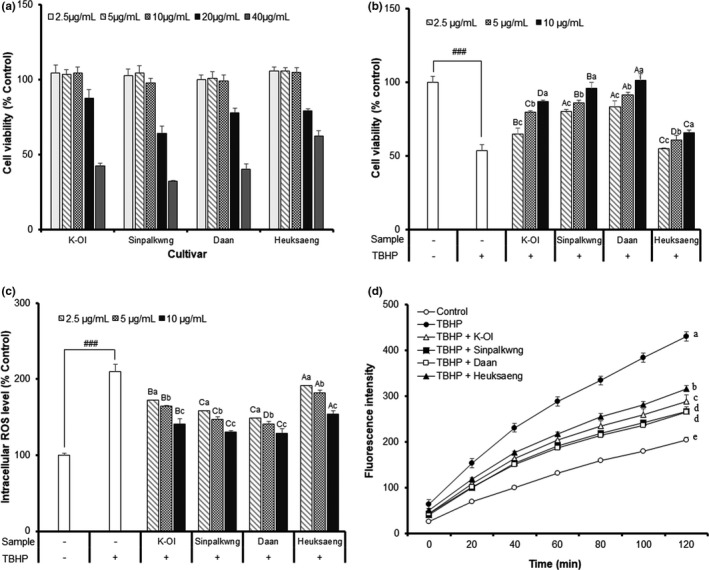
Effect of peanut skin extracts of different cultivars on (a) cell viability, (b) hepatoprotective effect, (c) intracellular ROS level, (d) fluorescence intensity for ROS in TBHP‐induced HepG2 cell. Different capital letters and small letter in the same items indicate a significant difference (p < .05) among different cultivars and extraction solvent, respectively
After evaluating the protective effects of the peanut skin extracts against oxidative stress, the levels of the ROS were analyzed in the TBHP‐induced HepG2 cells (Figure 3c,d). Accumulation of the cellular ROS is known to be a significant cause of intracellular damage and causes age‐related and metabolic diseases (Halliwell et al., 1992). Therefore, determination of the ROS released from the cell provides a good index of oxidative damage of the cell (Alía et al., 2006). As shown in Figure 3c,d, the TBHP treatment rapidly stimulated oxidative cellular stress and damage, elevating the release of ROS in HepG2 cell by 210.01% compared to the control cell without TBHP. However, the pretreatment of peanut skin extracts at 2.5–10 μg/ml significantly decreased the intracellular ROS level in the TBHP‐induced HepG2 cell in a dose‐dependent manner. These extracts of Daan and Sinpalkwng reduced the ROS level from 210.01% for the TBHP‐induced cellular oxidative stress to 130.18% and 129.19%, respectively, at a concentration of 10 μg/ml.
Next, the intracellular MDA concentration in the TBHP‐induced HepG2 cell protein was analyzed as a lipid peroxidation index (Suttnar et al., 2001). Additionally, the intracellular antioxidant enzymes play critical roles in the defense mechanism against oxidative damage (Alía et al., 2006). Oxidative stress (TBHP, 1 mM) treatment in HepG2 cells stimulated a significant increase of MDA concentration and the enzyme activities of CAT, SOD, GR, and GPx (Table 3). However, these increases were reversed by pretreatment with the peanut skin extracts at 10 μg/ml. Among them, the peanut skin extracts of Daan and Sinpalkwang cultivars were the most effective in reversing the increase in the MDA concentration and activities of CAT, SOD, GR, and GPx. Martín et al. (2010) reported that the pretreatment of epicatechin decreased the activity of the antioxidant enzymes against the TBHP‐induced oxidative damage.
TABLE 3.
Effect of peanut skin extracts of cultivars on lipid peroxidation and antioxidant enzyme activities in HepG2 cells
| Sample | 1MDA | 2GR | 3GPx | 4CAT | 5SOD |
|---|---|---|---|---|---|
| Control | 0.37 ± 0.05 | 4.78 ± 0.24 | 28.22 ± 1.09 | 11.49 ± 1.19 | 2.7 ± 0.13 |
| TBHP | 0.80 ± 0.03*** | 13.39 ± 0.79*** | 78.59 ± 3.63*** | 29.75 ± 1.67*** | 10.26 ± 0.40*** |
| TBHP + KME 10 | 0.58 ± 0.02b | 9.25 ± 1.16a | 30.98 ± 1.50b | 15.86 ± 0.60b | 3.93 ± 0.09b |
| TBHP + SME 10 | 0.49 ± 0.01c | 6.39 ± 0.66b | 25.48 ± 1.06c | 10.74 ± 0.35c | 2.98 ± 0.05d |
| TBHP + DME 10 | 0.45 ± 0.02d | 6.13 ± 0.39b | 20.19 ± 1.07d | 9.41 ± 0.46d | 3.21 ± 0.02c |
| TBHP + HME 10 | 0.63 ± 0.02a | 10.49 ± 0.33a | 38.79 ± 1.43a | 19.51 ± 0.90a | 4.86 ± 0.16a |
Lipid peroxidation of 1malondialdehyde (MDA, nmol mg protein) and antioxidant enzyme activity of 2glutathione reductase (GR, μmol min mg−1 protein), 3glutathione peroxidase (GPx, μmol min mg−1 protein), 4catalase (CAT, μmol min mg−1 protein), and 5SOD, unit mg‐1 protein) were evaluated in HepG2 cells treated for 6 hr with the samples, followed by treatment for 4 hr with 500 μM TBHP. Values are means ± SD (n = 3).
Significant at ***p < .001
Various studies have reported the antioxidant properties of peanut skin phenolics, mainly due to their radical scavenging ability (Bodoira et al., 2017). However, no study has sought to identify the effect of peanut skin extracts on bioprotective effects against oxidative stress in the HepG2 cells. Hepatoprotective effects of proanthocyanidin and catechin from various plants against oxidative stress have been investigated in many studies. These protective effects against oxidative stress are in consistent with the other in vitro studies carried out with various cell types (Praphasawat et al., 2011). Pretreatment of the HepG2 cells with the oligomeric procyanidin fraction could reduce the decrease in cell viability induced by TBHP by reducing ROS generation and malondialdehyde formation (Kim et al., 2013). Furthermore, it has been reported that the MDA levels were reduced by various plant polyphenols such as catechin, epicatechin, proanthocyanidin, following oxidative stress. It has also been reported that flavonoids have the ability to interact with bio‐membranes and protect them from free radicals38, and that procyanidins trigger the upregulation of a battery of antioxidant and detoxification enzymes, which boost cellular defenses against oxidative stress (Saija et al., 1995). From the results of this study, we postulate that the red peanut cultivars, which contain high amounts of catechins and proanthocyanidins, possess the ability to impart strong protection against oxidative damage in HepG2 cells, by modulating ROS production, MDA generation, and antioxidant enzyme activities.
3.4. Effect of peanut skin extracts on nitric oxide (NO) production and pro‐inflammatory cytokine secretion in LPS‐stimulated RAW 264.7 macrophages
The anti‐inflammatory effect of the peanut skin extracts was evaluated by measuring the levels of nitric oxide (NO), and the secretion of the pro‐inflammatory cytokines, such as TNF‐α, IL‐6, and IL‐1β. Before evaluating the anti‐inflammatory activities of the peanut skin extracts on the LPS‐stimulated RAW 264.7 cells, the cytotoxicity was estimated by MTT assay (Figure 3a). The effects of peanut skin extracts on the inhibition of NO production are presented in Figure 3b. NO production following LPS‐treatment for 24 hr (39.74 μM) significantly increased (p < .001) compared with that noted in the unstimulated RAW 264.7 cells (7.93 μM). However, the peanut skin extract (40 μg/ml) reduced the NO level by 70, 84, 81, and 30%, compared to the LPS‐stimulated controls of K‐Ol, Sinpalkwng, Daan, and Heuksang cultivar, respectively. Furthermore, TNF‐α, IL‐6, and IL‐1β are important pro‐inflammatory cytokines that are linked to the pathogenesis of many infectious and inflammatory diseases, including cancer (Chakrabarti et al., 2014). As shown in Figure 3c, LPS‐stimulated RAW 264.7 cells markedly up‐regulated TNF‐α, IL‐6, and IL‐1β production. The concentrations of TNF‐α, IL‐6, and IL‐1β in the media of the untreated cells (21.46, 1.46 ng/ml, not detected) were significantly increased by 446.85, 72.83, and 1.99 ng/ml after LPS stimulation, respectively. However, the peanut skin extract (40 μg/ml) decreased the TNF‐α, IL‐6, and IL‐1β levels by 19%–41%, 35%–88%, and 11%–38%, respectively, compared to the LPS‐stimulated control. Especially, peanut skin extracts of the Daan cultivar were most effective in reducing the concentration of TNF‐α, IL‐6, and IL‐1β, and these levels decreased by 264.15, 8.46, and 1.23 ng/ml, respectively. As a protective effect against HepG2 cells, the most effective inhibition of the major inflammatory factor (NO, TNF‐α, IL‐6, and IL‐1β) on the LPS‐stimulated RAW 264.7 cells was observed from the peanut skin extracts of Daan and Sinpalkwng. Anti‐inflammatory effects of proanthocyanidin polymers isolated from various plants, including peanut skin, have been reported in many studies. Tatsuno et al. (2012) reported that the proanthocyanidin polymers isolated from peanut skin inhibit the LPS‐induced cytokine (TNF‐α, IL‐6) production, which is attributable to the components of lower condensation. Therefore, we also conclude that the red peanut cultivars, which contain high amounts of proanthocyanidins, effectively enhance the anti‐inflammatory activities by modulating the major inflammatory factors (NO, TNF‐α, IL‐6, and IL‐1β) in LPS‐stimulated RAW264.7 macrophages (Figure 4).
FIGURE 4.

Effect peanut skin extracts of different cultivars on (a) Cell viability, (b) Nitric oxide concentration in LPS‐stimulated RAW 264.7 macrophages. Different capital letters and small letter in the same items indicate a significant difference (p < .05) among different cultivars and extraction solvent, respectively
3.5. Correlation analysis
The correlation between the functional compounds and key factors on the antioxidant and anti‐inflammatory characteristics of peanut skin extracts was analyzed for various cultivars with different colors, and is expressed by the value of the correlation coefficient (R) presented in Table 4. In general, major functional compounds of the red peanut cultivars, such as flavonoids, proanthocyanidins, catechin, gallic acid, coumaric acid, and hesperidin, showed a significant positive correlation with radical scavenging activities, FRAP, and hepatoprotective effects. In contrast, they exhibited a negative correlation with biomarkers (MDA, GR, GPx, CAT, and SOD) for oxidative damage in HepG2 cells and inflammatory factors (NO, TNF‐α, IL‐6, IL‐1β) for LPS‐stimulated RAW 264.7 macrophages. In other words, flavonoids, proanthocyanidins, catechin, gallic acid, coumaric acid, and hesperidin exhibited relatively higher positive correlations with antioxidant and anti‐inflammatory capacities compared to TPC and anthocyanins. These results suggested that the functional compounds (proanthocyanidin, catechin, and so on) of red peanuts are more effective in protecting HepG2 cells against oxidative damage and the control of inflammation factors in RAW 264.7 macrophages than the functional compounds (anthocyanin) of black peanuts (Figure 5).
TABLE 4.
Correlation analysis of biological activity and functional compounds in peanut skins
| Functional compound | Antioxidant activity | Protective effect on TBHP‐induced HepG2 | Anti‐inflammatory effect on LPS‐induced RAW 264.7 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABTS | DPPH | FRAP | Protective effect | ROS | MDA | GR | GPx | CAT | SOD | NO | TNF‐α | IL‐6 | IL‐1β | |
| Total polyphenol | −0.266 | −0.389 | 0.034 | −0.050 | 0.137 | −0.018 | 0.067 | 0.088 | 0.069 | 0.287 | 0.444 | 0.179 | 0.413 | 0.340 |
| Total flavonoid | 0.830** | 0.747** | 0.933** | 0.913** | −0.811** | −0.924** | −0.850** | −0.899** | −0.905** | −0.765** | −0.656** | −0.822** | −0.704* | −0.761** |
| Total proanthocyanidin | 0.977** | 0.954** | 0.926** | 0.897** | −0.847** | −0.884** | −0.831** | −0.98** | −0.939** | −0.913** | −0.924** | −0.942** | −0.942** | −0.940** |
| Total anthocyanin | −0.809** | −0.880** | −0.584* | −0.655* | 0.676* | 0.573 | 0.606* | 0.691* | 0.677* | 0.805** | 0.890** | 0.742** | 0.891** | 0.846** |
| Phenolics | ||||||||||||||
| Catehin | 0.983** | 0.965** | 0.904** | 0.907** | −0.827** | −0.890** | −0.837** | −0.952** | −0.910** | −0.893** | −0.920** | −0.933** | −0.954** | −0.953** |
| Gallic acid | 0.812** | 0.715** | 0.891** | 0.829** | −0.707* | −0.870** | −0.756** | −0.869** | −0.820** | −0.661* | −0.641* | −0.804** | −0.695* | −0.714** |
| Coumaric acid | 0.792** | 0.702* | 0.815** | 0.711** | −0.542 | −0.754** | −0.615* | −0.828** | −0.712** | −0.580* | −0.626* | −0.735** | −0.694* | −0.691* |
| Ferulic acid | −0.622* | −0.692* | −0.358 | −0.423 | 0.478 | 0.343 | 0.384 | 0.491 | 0.452 | 0.569 | 0.726** | 0.571 | 0.722** | 0.636* |
| Hesperidin | 0.964** | 0.930** | 0.902** | 0.864** | −0.763** | −0.864** | −0.818** | −0.947** | −0.885** | −0.843** | −0.864** | −0.911** | −0.918** | −0.913** |
| Quercetin | −0.632* | −0.747** | −0.402 | −0.534 | 0.626* | 0.423 | 0.523 | 0.501 | 0.555 | 0.753** | 0.784** | 0.589* | 0.752** | 0.727** |
| Anthocyanin | ||||||||||||||
| Delphinidin‐3‐glucoside | −0.912** | −0.951** | −0.735** | −0.777** | 0.762** | 0.717** | 0.720** | 0.822** | 0.795** | 0.871** | 0.934** | 0.843** | 0.954** | 0.920** |
| Cyanidin‐3‐sorporoside | −0.912** | −0.950** | −0.735** | −0.776** | 0.761** | 0.717** | 0.721** | 0.819** | 0.793** | 0.869** | 0.930** | 0.840** | 0.956** | 0.919** |
| Cyanidin‐3‐sambubioside | −0.912** | −0.950** | −0.735** | −0.776** | 0.760** | 0.717** | 0.721** | 0.818** | 0.792** | 0.868** | 0.927** | 0.839** | 0.956** | 0.919** |
Significant at **p < .01, *p < .05.
FIGURE 5.

Effect of peanut skin extracts of different cultivars on (a) TNF‐α, (b)IL‐6, and (c) IL‐1β concentration in LPS‐stimulated RAW 264.7 macrophages. Different capital letters and small letter in the same items indicate a significant difference (p < .05) among different cultivars and extraction solvent, respectively
4. CONCLUSION
This study was investigated the composition and distribution of phenolic compounds of peanut cultivars (Sinpalkwang, K‐Ol, Daan, Heuksaeng.), as well as the antioxidant and anti‐inflammatory effects of the skins of the four peanut cultivars. The total polyphenol contents were not significantly different between the Daan of red peanut cultivars and Heukasang of the black cultivar. In contrast, there is difference in the content of individual phenolics that made up the total polyphenol content. The total flavonoid content, total proanthocyanidin content, catechin, gallic acid, coumaric acid, and hesperidin were generally higher in red peanut cultivars (Sinpalkwng, K‐Ol, Daan) than in black peanut cultivars (Heukasang). Meanwhile, the anthocyanidin, ferulic acid, and quercetin contents were generally higher in black peanut cultivars than in red peanut cultivars. The ABTS and DPPH radical scavenging activities, and FRAP values were the highest in Daan, followed by Sinpalkwng, K‐Ol, and Heuksang, and were generally higher in red peanut cultivars than in black ones. In addition, our results show that the skin extracts of red peanut modulated cell viability, ROS generation, MDA concentration, and antioxidant enzyme activity (GR, GPx, CAT, and SOD) in oxidative stress‐induced HepG2 cells, and inhibited pro‐inflammatory factors (NO, TNF‐α, IL‐6, and IL‐1β) in LPS‐stimulated RAW 264.7 cells. Therefore, this study provides valuable information on the application of functional materials for improving the utilization of phenolic compounds extracted from peanut skins, depending on the different cultivars, as effective physiological agents.
6. CONFLICT OF INTEREST
The authors declare no conflict of interest.
7. ETHICAL APPROVAL
Neither animal nor human testing was involved in this study.
5. ACKNOWLEDGMENTS
This work was carried out with the support of the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01430301) Rural Development Administration, Republic of Korea.
Kim MY, Kim H‐J, Lee Y‐Y, et al. Antioxidant and anti‐inflammatory effects of Peanut (Arachishypogaea L.) skin extracts of various cultivars in oxidative‐damaged HepG2 cells and LPS‐induced raw 264.7 macrophages. Food Sci Nutr.2021;9:973–984. 10.1002/fsn3.2064
REFERENCES
- Adhikari, B. , Dhungana, S. K. , Ali, M. W. , Adhikari, A. , Kin, I. D. , & Shin, D. H. (2019). Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. Journal of the Saudi Society of Agricultural Sciences, 18(4), 347–442. 10.1016/j.jssas.2018.02.004 [DOI] [Google Scholar]
- Alía, M. , Mateos, R. , Ramos, S. , Lecumberri, E. , Bravo, L. , & Goya, L. (2006). Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). European Journal of Nutrition, 45(1), 19–28. 10.1007/s00394-005-0558-7 [DOI] [PubMed] [Google Scholar]
- Benzie, I. F. , & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- Bodoira, R. , Rossi, Y. , Montenegro, M. , Maestri, D. , & Velez, A. (2017). Extraction of antioxidant polyphenolic compounds from peanut skin using water‐ethanol at high pressure and temperature conditions. The Journal of Supercritical Fluids, 128, 57–65. 10.1016/j.supflu.2017.05.011 [DOI] [Google Scholar]
- Chakrabarti, S. , Jahandideh, F. , & Wu, J. (2014). Food‐derived bioactive peptides on inflammation and oxidative stress. BioMed Research International, 2014, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. , Lee, S. M. , Chun, J. , Lee, H. B. , & Lee, J. (2006). Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chemistry, 99(2), 381–387. 10.1016/j.foodchem.2005.08.004 [DOI] [Google Scholar]
- Choung, M. (2008). Optimal HPLC condition for simultaneous determination of anthocyanins in black soybean seed coats. Korean Journal of Crop Science, 53(4), 359–368. [Google Scholar]
- Chukwumah, Y. , Walker, L. T. , & Verghese, M. (2009). Peanut skin color:A biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars. International Journal of Molecular Sciences, 10(11), 4941–4952. 10.3390/ijms10114941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanto, V. , Wu, X. , & Liu, R. H. (2002). Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry, 50(17), 4959–4964. 10.1021/jf0255937 [DOI] [PubMed] [Google Scholar]
- Galali, Y. , Omar, Z. A. , & Sajadi, S. M. (2020). Biologically active components in by‐products of food processing. Food Science & Nutrition, 8(7), 3004–3022. 10.1002/fsn3.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, J. , & Myers, C. E. (1998). Inhibition of arachidonate 5‐lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 95, 13182–13187. 10.1073/pnas.95.22.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell, B. , & Gutteridge, J. M. C. (1999). Free radicals in biology and medicine (3rd ed.). Oxford University Press. [Google Scholar]
- Halliwell, B. A. R. R. Y. , Gutteridge, J. M. , & Cross, C. E. (1992). Free radicals, antioxidants, and human disease: Where are we now? Journal of Laboratory and Clinical Medicine, 119(6), 598–620. [PubMed] [Google Scholar]
- Ham, H. , Yoon, S. W. , Kim, I. H. , Kwak, J. , Lee, J. S. , Jeong, H. S. , & Lee, J. (2015). Protective effects of unsaponifiable matter from rice bran on oxidative damage by modulating antioxidant enzyme activities in HepG2 cells. LWT ‐ Food Science and Technology, 61(2), 602–608. 10.1016/j.lwt.2014.12.047 [DOI] [Google Scholar]
- Ishiyama, M. , Tominaga, H. , Shiga, M. , Sasamoto, K. , Ohkura, Y. , & Ueno, K. A. (1996). Combined assay of cell viability and in vitro cytotoxicity with a highly water‐soluble tetrazolium salt, neutral red and crystal violet. Biological and Pharmaceutical Bulletin, 19, 1518–1520. [DOI] [PubMed] [Google Scholar]
- John, J. A. , & Shahidi, F. (2010). Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). Journal of Functional Foods, 2(3), 196–209. 10.1016/j.jff.2010.04.008 [DOI] [Google Scholar]
- Kim, M. Y. , Jang, G. Y. , Lee, Y. , Li, M. , Ji, Y. M. , Yoon, N. , Lee, S. H. , Kim, K. M. , Lee, J. , & Jeong, H. S. (2016). Free and bound form bioactive compound profiles in germinated black soybean (Glycine max L.). Food Science and Biotechnology, 25, 1551–1559. 10.1007/s10068-016-0240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Choi, Y. , Ham, H. , Jeong, H. S. , & Lee, J. (2013). Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert‐butyl hydroperoxide‐induced oxidative damage in HepG2 cells. Food Chemistry, 137, 136–141. 10.1016/j.foodchem.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Kindt, T. J. , Goldsby, R. A. , Osborne, B. A. , & Kuby, J. (2007). Kuby Immunology (6th ed.). W.H. Freeman. [Google Scholar]
- Koleckar, V. , Kubikova, K. , Rehakova, Z. , Kuca, K. , Jun, D. , Jahodar, L. , & Opletal, L. (2008). Condensed and hydrolysable tannins as antioxidants influencing the health. Mini‐Reviews in Medicinal Chemistry, 8(5), 436–447. [DOI] [PubMed] [Google Scholar]
- Larrauri, M. , Zunino, M. P. , Zygadlo, J. A. , Grosso, N. R. , & Nepote, V. (2016). Chemical characterization and antioxidant properties of fractions separated from extract of peanut skin derived from different industrial processes. Industrial Crops and Products, 94, 964–971. [Google Scholar]
- Levy, J. , Boyer, R. R. , Neilson, A. P. , O'Keefe, S. F. , Chu, H. S. S. , Williams, R. C. , & Goodrich, K. M. (2017). Evaluation of peanut skin and grape seed extracts to inhibit growth of foodborne pathogens. Food Science & Nutrition, 5(6), 1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín, M. A. , Ramos, S. , Mateos, R. , Izquierdo‐Pulido, M. , Bravo, L. , & Goya, L. (2010). Protection of human HepG2 cells against oxidative stress by the flavonoid epicatechin. Phytotheraphy Research, 24(4), 503–509. [DOI] [PubMed] [Google Scholar]
- Muselík, J. , García‐Alonso, M. , Martín‐López, M. P. , Žemlička, M. , & Rivas‐Gonzalo, J. C. (2007). Measurement of antioxidant activity of wine catechins, procyanidins, anthocyanins and pyranoanthocyanins. International Journal of Molecular Sciences, 8(8), 797–809. [Google Scholar]
- Nepote, V. , Grosso, N. R. , & Guzmán, C. A. (2010). Optimization of extraction of phenolic antioxidants from peanut skins. Journal of the Science of Food and Agriculture, 85(1), 33–38. [Google Scholar]
- Oldoni, T. L. C. , Melo, P. S. , Massarioli, A. P. , Moreno, I. A. M. , & Bezerra, R. M. N. (2016). Bioassay‐guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chemistry, 192, 306–312. [DOI] [PubMed] [Google Scholar]
- Pae, S. B. , Hwang, C. D. , Kim, S. U. , Lee, M. H. , Shim, K. B. , & Park, C. H. (2016). A new large grain and high‐yielding Virginia type peanut cultivar ‘Sinpalkwang’. Korean Journal of Breeding Science, 48, 66–71. [Google Scholar]
- Pae, S. B. , Hwang, C. D. , Lee, M. H. , Kim, S. U. , Oh, K. W. , & Lee, B. K. (2016). A high oleate peanut variety ‘K‐Ol’. Korean Journal of Breeding Science, 48, 313–318. [Google Scholar]
- Pae, S. , Kim, S. , Lee, M. , Hwang, C. , Oh, K. , Jung, C. , & Lee, Y. (2017). A peanut variety 'Heuksaeng' with short stem and purple testa. Korean Journal of Breeding Science, 49, 103–108. [Google Scholar]
- Pae, S. B. , Lee, M. H. , Kim, S. U. , Hwang, C. D. , Oh, K. W. , Jung, C. S. , & Lee, Y. H. (2017). A Shinpung typed large grain and high‐yielding peanut variety 'Daan'. Korean Journal of Breeding Science, 49, 224–229. [Google Scholar]
- Praphasawat, R. , Klungsupya, P. , Muangman, T. , Laovitthayanggoon, S. , Arunpairojana, V. , & Himakoun, L. (2011). Antimutagenicity and antioxidative DNA damage properties of oligomeric proanthocyanidins from Thai grape seeds in TK6 cells. Asian Pacific Journal of Cancer Prevention, 12(5), 1317–1321. [PubMed] [Google Scholar]
- Saija, A. , Scalese, M. , Lanza, M. , Marzullo, D. , Bonina, F. , & Castelli, F. (1995). Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radical Bioloty and Medicine, 19(4), 481–486. 10.1016/0891-5849(94)00240-K [DOI] [PubMed] [Google Scholar]
- Sarnoski, P. J. , Johnson, J. V. , Reed, K. A. , Tanko, J. M. , & O’Keefe, S. F. (2012). Separation and characterisation of proanthocyanidins in Virginia type peanut skins by LC–MSn. Food Chemistry, 131(3), 927–939. 10.1016/j.foodchem.2011.09.081 [DOI] [Google Scholar]
- Srisook, K. , Han, S. S. , Choi, H. S. , Li, M. H. , Ueda, H. , Kim, C. , & Cha, Y. N. (2006). CO from enhanced HO activity or from CORM‐2 inhibits both O2− and NO production and downregulates HO‐1 expression in LPS‐stimulated macrophages. Biochemical Pharmacology, 71(3), 307–318. 10.1016/j.bcp.2005.10.042 [DOI] [PubMed] [Google Scholar]
- Sulaiman, S. F. , Sajak, A. A. B. , Ooia, K. L. , Supriatno, & Seow, E. M. (2011). Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. Journal of Food Composition and Analysis, 24, 506–515. 10.1016/j.jfca.2011.01.020 [DOI] [Google Scholar]
- Suttnar, J. , Mášová, L. , & Dyr, J. E. (2001). Influence of citrate and EDTA anticoagulants on plasma malondialdehyde concentrations estimated by high‐performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications, 751(1), 193–197. 10.1016/S0378-4347(00)00453-9 [DOI] [PubMed] [Google Scholar]
- Takahama, U. , Tanaka, M. , & Hirota, S. (2010). Proanthocyanidins in buckwheat flour can reduce salivary nitrite to nitric oxide in the stomach. Plant Foods for Human Nutrition, 65, 1–7. 10.1007/s11130-009-0144-x [DOI] [PubMed] [Google Scholar]
- Tatsuno, T. , Jinno, M. , Arima, Y. , Kawabata, T. , Hasegawa, T. , Yahagi, N. , Takano, F. , & Ohta, T. (2012). Anti‐inflammatory and anti‐melanogenic proanthocyanidin oligomers from peanut skin. Biological and Pharmaceutical Bulletin, 35(6), 909–916. 10.1248/bpb.35.909 [DOI] [PubMed] [Google Scholar]
- Tchabo, W. , Ma, Y. , Engmann, F. N. , & Zhang, H. (2015). Ultrasound‐assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Industrial Crops and Products, 63, 214–225. 10.1016/j.indcrop.2014.09.053 [DOI] [Google Scholar]
- Wang, H. , & Joseph, J. A. (1999). Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biology and Medicine, 27, 612–616. [DOI] [PubMed] [Google Scholar]
- Wijeratne, S. S. , Abou‐Zaid, M. M. , & Shahidi, F. (2006). Antioxidant polyphenols in almond and its coproducts. Journal of Agricultural and Food Chemistry, 54(2), 312–318. 10.1021/jf051692j [DOI] [PubMed] [Google Scholar]
- Yu, J. , Ahmedna, M. , & Goktepe, I. (2005). Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chemistry, 90(1–2), 199–206. 10.1016/j.foodchem.2004.03.048 [DOI] [Google Scholar]
- Zhao, Z. , Wu, M. , Zhan, Y. , Zhan, K. , Chang, X. , Yang, H. , & Li, Z. (2017). Characterization and purification of anthocyanins from black peanut (Arachis hypogaea L.) skin by combined column chromatography. Journal of Chromatography A, 1519, 74–82. 10.1016/j.chroma.2017.08.078 [DOI] [PubMed] [Google Scholar]


