Abstract
Purpose of review
Experimental pre-clinical models of recovery of consciousness (ROC) and anesthesia emergence are crucial for understanding the neuronal circuits restoring arousal during coma emergence. Such models can also potentially help to better understand how events during coma emergence facilitate or hinder recovery from brain injury. Here we provide an overview of current methods used to assess ROC/level of arousal in animal models. This exposes the need for objective approaches to calibrate arousal levels. We outline how correlation of measured behaviors and their reestablishment at multiple stages with cellular, local and broader neuronal networks, gives a fuller understanding of ROC.
Recent findings
Animals emerging from diverse coma like-states share a dynamic process of cortical and behavioral recovery that reveals distinct states consistently sequenced from low to high arousal level and trackable in non-human primates and rodents. Neuronal activity modulation of layer V-pyramidal neurons and neuronal aggregates within the brainstem and thalamic nuclei play critical roles at specific stages to promote restoration of a conscious state.
Summary
A comprehensive, graded calibration of cortical, physiological, and behavioral changes in animal models is undoubtedly needed to establish an integrative framework. This approach reveals the contribution of local and systemic neuronal circuits to the underlying mechanisms for recovering consciousness.
Keywords: levels of arousal, recovery of consciousness, emergence from anesthesia, righting reflex, cortico-motor regimens
Introduction:
Neuronal systems evoke waking from sleep, as well as states reflecting deeply impaired arousal such as coma and general anesthesia, through ascending projections to the cortex and descending projections through the spinal cord[1]. Activation of these systems via several specific brainstem and thalamic nuclei, posterior hypothalamus, and basal forebrain generates cortical activation, increased muscle tone, sensory-motor responsiveness, and behavioral activity[1, 2]. Despite the major advances in assessing levels of consciousness using combined techniques that explore activity within these brain areas in association with behavioral responses, there is still a significant gap in our understanding of the neuronal circuits driving forebrain arousal and how restoration of arousal regulation facilitates recovery from brain injuries. In humans, recovery of consciousness (ROC) is rigorously assessed including examination of cortical activity [3, 4], spontaneously emitted behavior and response to stimuli [5, 6]. Yet, detailed underlying mechanisms supporting these changes remain unknown. Moreover, lacking this information ultimately limits the ability of physicians to accurately establish level of awareness, develop treatments, assess prognosis, and estimate timelines for recovery from coma [7].
Although animal models are necessary to elucidate these unknown mechanisms, the progress in assessing ROC/levels of arousal in rodents and non-human primates has been surprisingly modest compared to quantitative behavioral assessment employed in the evaluation of patients[8, 9]. Animal studies of anesthesia reversal, survival sepsis and recovery from traumatic brain injury typically arbitrarily define awakening with emergence of reflexive responses, including reaction to painful stimuli, recovery of the righting reflex, RR [10-13] and a variety of behavioral scales[14-18] that do not set operationally clear cut-offs for conscious awareness. Human quantitative behavioral scales, particularly those discriminating coma and related disorders of consciousness have developed robust, operationally defined behavioral signs that demarcate conscious behaviors[9]. However, both animal and human studies exclusively using these methods only examine downstream events, and do not integrate more direct measures of processes constituting the cortical arousal process [19]. Associations between EEG patterns and other neuronal outputs are typically not tracked and correlated, meaning that objective parameters for staging anesthesia emergence or ROC in model systems lack a quantifiable, precise and reproducible foundation. There is, therefore, a pressing need for objective approaches that systematically calibrate arousal levels in animal models while they recover from a low arousal state, and to correlate measured behaviors with the reestablishment of integrative function at different levels across the nervous system (cellular, local microcircuit, and larger neuronal networks).
Having objective metrics will also allow meaningful comparison across studies,and will help prioritize preclinical results that are more likely to successfully translate from bench to bedside. This would ultimately improve testing of treatments, and aid accurate diagnosis in disorders of consciousness, sepsis and brain injury.
Conventional methods for evaluating arousal in animal models
Current studies continue to actively and primarily use the righting reflex (RR), currently seen as a gold standard for assessing arousal/ROC in rodents. The RR is assessed by placing the animal on its back and measuring the time it takes for the animal to return to a prone posture. This reflex relies on vestibular inputs that sense head movement, suggesting that environmental awareness is returning to the animal. When positive, RR is seen as a mark of restored consciousness[20], leading to its broad use in studies following anesthesia reversal[11, 21-24], those assessing the median effective concentration for sedatives [25, 26], and in brain injury research[16, 18]. However, the righting reflex is also retained in comatose-like rodents[27]. Prior studies indicate that RR lacks cortical involvement [28], and is preserved after rodent precollicular transections (absence of the telencephalon) [29]. In short, the righting reflex does not accurately reflect cortical arousal processes during recovery of consciousness.
Recently Gao et al. (Gao S, Calderon D.P, unpublished data) demonstrated that RR events are casually linked to brain states with either a predominance of theta, alpha frequencies or reemergence of higher beta and gamma frequencies. Despite these widely separated and distinct patterns of cortical activity, the measured RR was similar in each state. Thus, consistent with the experimental studies, RR events can be disaggregated from wakeful patterns of cortical activation, making the observation of recovery of RR alone, an imprecise measurement of arousal level.
Despite the lack of a tight intrinsic link between RR and shifts in corticothalamic activity, when combined with cortical and behavioral changes, RR is often used as the primary metric of recovery of arousal. Studies applying RR as a de facto standard show an increase in the theta/delta ratio[11, 21, 23, 30], reduced burst suppression ratio [31], or modest behavioral[32] and cortical changes after stimulation[22]. Moreover, these studies typically overlook emergence of beta and gamma oscillations as well as purposeful movements and posture, all consistent characteristics of arousal in humans[33], non-human primates[17] and rodents (Gao S, Calderon D.P, unpublished data). As a result, such investigations of restoration of consciousness only sample a restricted range of recovered arousal.
An example of this approach is a study in which authors argue that cholinergic stimulation of frontal cortex while rodents are deeply anesthetized restores consciousness[11]. The study defined full ROC as the restoration of the RR, increased theta/delta cortical ratio, and uncoordinated limb mobility. Since cortical gamma connectivity remained suppressed despite carbachol-induced wakefulness, and changes in slow oscillations correlated with EEG activation rather than behavior, a follow-up study[34] concluded that the role of EEG measures in monitoring consciousness needs to be reevaluated. However, the investigation followed an insufficient operational behavioral approach and in depth cortical and behavioral changes would be needed for a complete description. The cortical state of animals was thus ultimately unclear, further supporting the idea that more combined analyses are needed for clear conclusions to be drawn about ROC in any study. Unequivocally, described features are distinct from the broad increase of the beta and gamma frequency power associated with the recovery of complex behaviors in a wakeful rodent state. Only a handful of studies in rodents have included a thorough examination of responses to olfactory and sensory stimuli and analysis of cortical activity including higher frequency ranges [10, 18, 35].
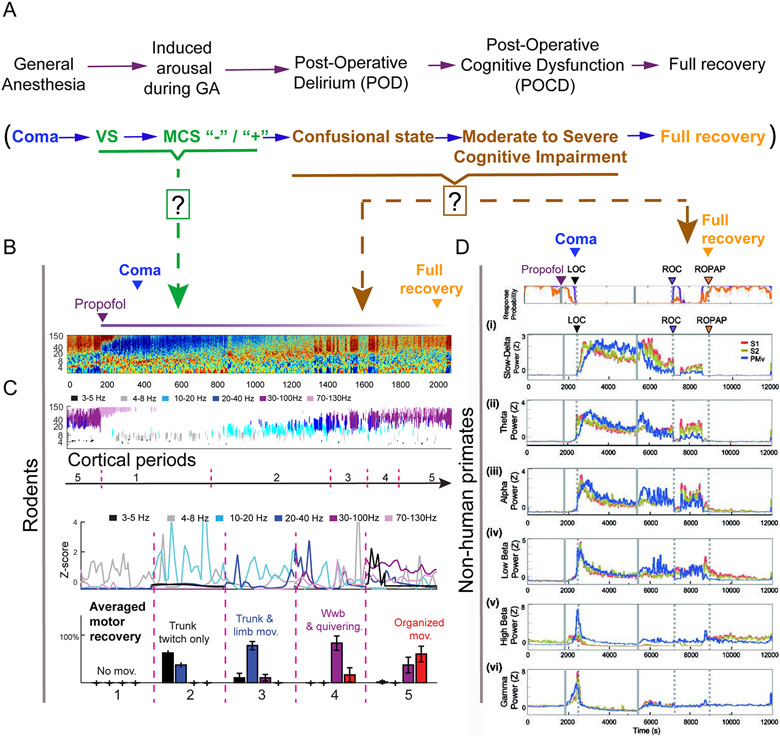
Recently, our group[35] showed that the restoration of full mobility including grooming and response to external stimuli was associated with increased cortical activity, despite high anesthetic concentration exposure. While modulating neuronal activity in the anterior portion of the nucleus gigantocellularis (aNGC) with GABAa antagonists in animals exposed to isoflurane (1.25-1.5%), we discovered a robust cortical activation associated with motor arousal. The aNGC is an aggregate of glutamatergic neurons present at the medullary level of the brainstem with strong ascending and descending projections to the brain and spinal cord[35]. We found that behavior during awakening in our model progresses through distinct phases starting with subtle trunk movements, followed by limb protraction and retraction and alternation, and finally pelvic lifting, touch down of limbs on the surface, and organized movements. These features are accompanied by changes in cortical activity frequency bands (cortical periods) that are consistently seen during recovery from a range of mechanistically distinct anesthetics (propofol recovery; Fig 1c). as well as hypoglycemic coma (Gao S, Calderon D.P, unpublished data). We demonstrated that animals emerging from diverse coma like-states share a common dynamic process of cortical and motor arousal that is consistently sequenced(Gao S, Calderon D.P, unpublished data from low to high arousal level, and that occur at higher anesthetic concentration 48.6±10 minutes before the righting reflex. These results suggest that our combined cortico-motor analysis is consistent and trackable across multiple conditions.
Figure 1. Detailed cortico-motor regimens in rodents and non-human primates while emerging from propofol-induced anesthesia.
(A) Schematic depicting the behavioral and physiological phases during emergence from anesthesia (top panel) compared to recovery from coma (bottom panel). Arrows indicate graded levels of recovery modeled in rodents and nonhuman-primates. Question marks denote unexplored cortical and behavioral arousal states that remain to be identified in animal models. (B) Representative trace of motor cortex LFP during propofol bolus injection (15mg/Kg; purple line) and normalized spectrogram of LFP. (C) Segmented cortical periods and averaged density estimation per cortical state for each period (500 s interval) and averaged percentage distribution of motor behavior while emerging from propofol (n=5). Gao S and Calderon DP, unpublished data. (d) Change of power in different cortical bands in somatosensory cortex (S1 (red) and S2, (brown) versus ventral promotor area, PMv, (blue). Bands shown are slow-delta [0.5–4 Hz, (i)], theta [4–8 Hz, (ii)], alpha [8–12 Hz, (iii)], low beta [12–18 Hz, (iv)], high beta [18–25 Hz, (v)], low gamma [25–34 Hz, (vi)]. Power was normalized using z-scores. Propofol was infused for 60 min (1800–5400 s, grey solid lines. LOC (loss of consciousness) is shown with a black arrow and dotted lines, ROC (recovery of consciousness) with a purple arrow and dotted lines, and ROPAP (return of preanesthetic performance) with an orange arrow and dotted lines. Reproduced with permission from[38].
Similar to rodent studies, studies of recovery from general anesthesia in non-human primates exhibit a similarly limited calibration of behavioral and cortical changes as subjects recover consciousness[17] demonstrated that electrical stimulation of central lateral thalamus could induce arousal in anesthetized macaques linked to reliable change in local and cross-regional cortical activity measured at both the population and single neuron level. However, the customized quantitative behavioral arousal index developed in this study included few features consistent with human metrics of consciousness that separate non-reflexive movements from those observed in comatose patients and those in a vegetative state[36]. The behavioral arousal state measured during stimulation rarely reached the top of the ten point scale used, and the distinct wakeful state observed in the study[17] varied between being consistent with a vegetative state and the initial transition into a minimally conscious state marked by the appearance of non-reflexive movements [36]. Ishizawa and colleagues reported a full range of behavioral recovery from general anesthesia and concomitant physiological correlates in non-human primates, providing detailed cortical frequency changes during loss and recovery of consciousness while exposing macaques to different injectable anesthetics[37-39], (Fig. 1D). ROC was defined using specific probability thresholds of the subject engaging in a pretrained behavioral task. The distinction between performance engagement and actual performance in these studies reveals several distinct states that evolve beyond the initial definition of ROC used; ROC in these experiments was defined as the first-time task engagement exceeding a probability of 30%. Among several interesting observations, regimes of near total engagement with very low performance completion appeared at the initial ROC, and reaction times after recovery of pre-anesthetic task performance levels remained slow compared with pre-infusion wakeful measurements. Most interestingly, and comparable to effects seen in awake animals after withdrawal of effective CL stimulation[40], periods of marked and sustained drops of both task engagement and performance appeared post-anesthetic infusion. Physiological cortical changes associated with ROC included return of beta power and concurrent decline of slow delta oscillations in both pre-motor and somatosensory regions. Interestingly, only increased beta power correlated with return to baseline task performance levels, similar to changes observed in awake monkeys after return of performance during CL stimulation[40]. Such a comprehensive description of cortical physiological and behavioral changes is undoubtedly needed to establish a graded calibration of the level of arousal and to facilitate comparisons of ROC as defined across different models (Fig. 1). Interestingly, only increased beta power correlated with return to baseline task performance levels, similar to changes observed in awake monkeys after return of performance during central lateral thalamic (CL) stimulation[40]. Moreover, this approach may uncover novel biomarkers that can accurately track both recovery of consciousness from anesthesia and brain injury.
Linking specific neuronal cell types, local circuit, and large-scale circuit interactions to behavioral recovery from coma
The arousal systems consist of millions of neurons residing in various brain regions and employing multiple neurotransmitters to restore and maintain a state of wakefulness[19, 41]. Despite this broad pleuripotentiality of arousal pathways and mechanisms, several observations suggest a specific causal role for particular cell types and neuronal aggregates in modulation of the conscious state[1, 2, 33]. Recent work suggests the exciting potential to link behavioral recovery of consciousness to integrative changes at multiple levels of neuronal scale (intracellular, cellular, local network and broader networks; Fig. 2) to better understand recovery after brain injuries or during emergence from anesthesia.
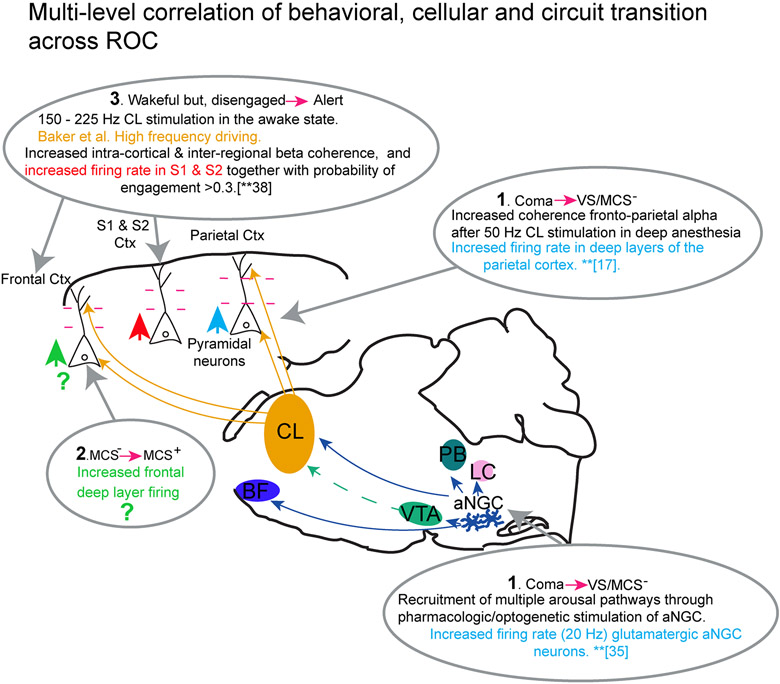
Figure 2. Integrating physiological changes at the cellular and circuit level with phases of behavioral recovery from coma-like behaviors.
The schematic displays the powerful potential for linking functional changes at the cellular level (neuronal cell types), local circuits, and broader networks involving multiple neuronal pathways located in the brainstem, thalamus and cortex with behavioral stages of recovery of consciousness.
At the cortical level, changes in activity at the ensemble level, columnar level, and inter-regional microcircuit highlight two important themes: 1) the potential integrative role of Layer V cortical pyramidal neurons, and 2) the fragmentation and coalescence of cortical ensembles. As noted above, initial emergence of wakefulness and non-reflexive behaviors with stimulation of the central lateral nucleus of the thalamus in anesthetized macaques are associated with increased deep-layer firing rates in parietal cortex and the emergence of strong correlation of field potentials across the deep and superficial layers of frontal and parietal cortex (studies of Redinbaugh et al. [17]). These data support a view of the emergence from anesthesia beginning with increased background synaptic activity emanating from deep-layer cortical pyramidal neurons driving overall increases in background synaptic activity and cortico-cortical communication. It is likely that Layer V pyramidal neurons play a key role in integrating such shifts in columnar activity[36, 42]. In a combined optogenetic stimulation and electrophysiological recording study, Suzuki and Larkum (2020)[43] directly demonstrated that three different types of general anesthesia decoupled information between the dendritic and somatic compartments within the Layer V pyramidal cell. While action potentials could be driven at wakeful levels within the dendritic compartment, outflow from the somatic compartment was suppressed. The anesthetics produced an as yet uncharacterized change in the intracellular propagation of dendritic activity to the soma and loss of evoked fast spiking seen in wakefulness[43]. Taken together with the Redinbaugh et al. results, these findings suggest a future linkage of specific stages of restoration of activity within Layer V pyramidal neurons and levels of behavioral recovery. While initial restoration of coherence of local field potential activity may emerge with initial behavioral evidence of consciousness, at the far range of modulation of attentive wakeful behaviors, integrative electrical properties of Layer V pyramidal neurons have been proposed to play a role in response to central lateral thalamic nucleus stimulation[40].
A wide range of changes in firing patterns within Layer V neurons and integration of cross-regional activity likely characterizes the sharp behavioral distinctions seen in recovery after brain injury[36]; similar phenomena are likely to correlate emergence from anesthesia[44]
While unknown, many human studies indicate that restoration of interactive behaviors characterizing the transition from minimally conscious state to confusional state and higher levels of cognitive function involve restoration of frontal lobe activity[45, 46]; these levels of recovery likely restore deep layer firing in frontal regions and baseline features of the resting power spectrum and coherence spectrum of inter-regional and cross-regional (coherence) [36]. At the opposite end of the spectrum, high frequency stimulation (150Hz-220Hz) of central lateral thalamus facilitates performance of demanding cognitive tasks[40]. This effect is also likely linked to the key role of the Layer V pyramidal neuron and those in Layers II/III which demonstrate high frequency thresholds for dendritic electrogenesis[36, 40, 42] supporting integrative properties of the Layer V neurons[47]. Such effects may be important to restoring higher level aspects of cognitive function after coma.
In addition to thalamocortical neural interactions, brainstem neurons play a pivotal role during awakening from anesthesia and coma. Chemogenetic and optogenetic stimulation of glutamatergic neurons in the parabrachial nucleus produces cortical activation while animals are deeply anesthetized and accelerates the process of emergence to consciousness[48]. Similarly, chemogenic stimulation of noradrenergic neurons from the locus coeruleus produces cortical activation, but does not trigger behavioral activation during ongoing anesthetic exposure[49], even though they diffusely innervate the cerebral cortex and the spinal cord. Conversely, optogenetic stimulation of dopaminergic neurons from the ventral tegmental area (VTA) restores righting during light anesthesia, without significant electroencephalographic changes[22]. Nevertheless, optogenetic stimulation of orexinergic terminals in the VTA activates cortical activity and shortens the time to emergence from anesthesia[31].
Our group has also recently demonstrated[35] that optogenetic stimulation of glutamatergic neurons in the anterior portion of the nucleus gigantocellularis restores cortical activity, organized movements, and behavioral reactivity to noxious and chemical stimuli in the context of two coma models: deep anesthesia and hypoglycemic coma. aNGC stimulation also produces activation of noradrenergic (locus coeruleus), glutamatergic (parabrachial nucleus), posterior hypothalamus and basal forebrain nuclei (ventral pathway), as well as thalamic nuclei (dorsal pathway) [35]. aNGC stimulation recruits multiple neurophysiological routes to robustly recover awakening from a coma state. Stimulation of aNGC may be a possible approach for restoring wakefulness after brain injury.
Conclusions:
-As experimental pre-clinical models of ROC and anesthesia emergence reveal the specific contributions of neuronal cell types and their impact on cerebral microcircuits and mesocircuits, greater precision in characterizing behavioral states will aid the interpretation of these observations.
-A wide range of relatively stable, altered levels of consciousness typify recovery from coma in humans; quantitative assessments of such behavioral states have driven significant changes in understanding diagnosis, prognosis and natural history[45]. Moreover, linking behaviors to underlying mechanisms will expand our model of recovery of consciousness.
-Bringing calibrated and graded behavioral testing together with the remarkable depth of new experimental work in this area will yield crucial advances and insights. Fig. 2 suggests an integrative framework, organizing some of the measured physiological changes at the cellular and circuit level reviewed above. Phases of behavioral changes during emergence from coma along with predictions are based on observations in human studies of recovery of consciousness. Recent work has demonstrated that functional reafferentation of activity within the cortical columns of frontal and parietal cortex by stimulation of the central lateral thalamus[17] can counteract a broad uncoupling of the dendritosomatic compartments of the Layer V pyramidal cells[43]. The initial effects of increased deep layer firing in parietal cortex, and restoration of coherence of population activity in the alpha and gamma frequencies, correlates with a minimal restoration of behaviors and eye-opening at the boundaries of restoring wakeful states seen in vegetative and minimally conscious states.
-The studies reviewed above show great promise for more graded finely calibrated studies of recovery from brain injuries and anesthesia in animals to correlate such distinctions with simultaneous measurements at the cellular and circuit level.
Key points:
Combined cortico-motor regimens objectively quantify the reemergence of integrative function in animal models.
Identification of neuronal populations is crucial for the restoration of consciousness.
Linking behaviors to underlying cellular mechanisms will expand our model of recovery of consciousness.
Acknowledgements:
Financial support and sponsorship. This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS). NS094655 awarded to DPC and NS095554 awarded to NDS. James S. McDonnell Foundation awarded to NDS
Footnotes
Conflict of interest:
None
References:
- [1].Jones BE. Arousal systems. Front Biosci 2003; 8:s438–451. [DOI] [PubMed] [Google Scholar]
- [2].Kelz MB, Garcia PS, Mashour GA, Solt K. Escape From Oblivion: Neural Mechanisms of Emergence From General Anesthesia. Anesth Analg 2019; 128:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schiff ND, Nauvel T, Victor JD. Large-scale brain dynamics in disorders of consciousness. Curr Opin Neurobiol 2014; 25:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Forgacs PB, Frey HP, Velazquez A et al. Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Ann Clin Transl Neurol 2017; 4:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Giacino JT, Katz DI, Schiff ND et al. Comprehensive Systematic Review Update Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch Phys Med Rehabil 2018; 99:1710–1719. [DOI] [PubMed] [Google Scholar]
- [6].Giacino JT, Katz DI, Schiff ND et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch Phys Med Rehabil 2018; 99:1699–1709. [DOI] [PubMed] [Google Scholar]
- [7].Provencio JJ, Hemphill JC, Claassen J et al. The Curing Coma Campaign: Framing Initial Scientific Challenges-Proceedings of the First Curing Coma Campaign Scientific Advisory Council Meeting. Neurocrit Care 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sherer M, Nakase-Thompson R, Yablon SA, Gontkovsky ST. Multidimensional assessment of acute confusion after traumatic brain injury. Archives of physical medicine and rehabilitation 2005; 86:896–904. [DOI] [PubMed] [Google Scholar]
- [9].Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004; 85:2020–2029. [DOI] [PubMed] [Google Scholar]
- [10].Kortelainen J, Jia X, Seppanen T, Thakor N. Increased electroencephalographic gamma activity reveals awakening from isoflurane anaesthesia in rats. Br J Anaesth 2012; 109:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[11].Pal D, Dean JG, Liu T et al. Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr Biol 2018; 28:2145–2152 e2145.This study demonstrates that stimulation of the prefrontal prelimbic cortex in deeply anesthetized rats increases cortical activity, restoration of righting reflex, and recovered mobility.
- [12].Pal D, Hambrecht-Wiedbusch VS, Silverstein BH, Mashour GA. Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br J Anaesth 2015; 114:979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brook B, Harbeson D, Amenyogbe N et al. Robust health-score based survival prediction for a neonatal mouse model of polymicrobial sepsis. PLoS One 2019; 14:e0218714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Esteves M, Almeida AM, Silva J et al. MORPhA Scale: Behavioral and electroencephalographic validation of a rodent anesthesia scale. J Neurosci Methods 2019; 324:108304. [DOI] [PubMed] [Google Scholar]
- [15].Mansouri MT, Fidler JA, Meng QC et al. Sex effects on behavioral markers of emergence from propofol and isoflurane anesthesia in rats. Behav Brain Res 2019; 367:59–67. [DOI] [PubMed] [Google Scholar]
- [16].Yarnell AM, Barry ES, Mountney A et al. The Revised Neurobehavioral Severity Scale (NSS-R) for Rodents. Curr Protoc Neurosci 2016; 75:9 52 51–59 52 16. [DOI] [PubMed] [Google Scholar]
- **[17].Redinbaugh MJ, Phillips JM, Kambi NA et al. Thalamus Modulates Consciousness via Layer-Specific Control of Cortex. Neuron 2020; 106:66–75 e12.This study demonstrates that the electrical stimulation of centrolateral thalamus in anesthetized macaques produces restoration of behavioral arousal associated with increased deep-layer firing rates and higher inter-regional coherence of LFPs across the parietal and frontal cortex.
- *[18].Pais-Roldan P, Edlow BL, Jiang Y et al. Multimodal assessment of recovery from coma in a rat model of diffuse brainstem tegmentum injury. Neuroimage 2019; 189:615–630.This study developed a behavioral coma scale to characterize multiple neurological states while rodents acutely recovered from coma induced by brainstem injury.
- [19].Calderon DP, Kilinc M, Maritan A et al. Generalized CNS arousal: An elementary force within the vertebrate nervous system. Neurosci Biobehav Rev 2016; 68:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wasilczuk AZ, Maier KL, Kelz MB. The Mouse as a Model Organism for Assessing Anesthetic Sensitivity. Methods Enzymol 2018; 602:211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Muindi F, Kenny JD, Taylor NE et al. Electrical stimulation of the parabrachial nucleus induces reanimation from isoflurane general anesthesia. Behav Brain Res 2016; 306:20–25. [DOI] [PubMed] [Google Scholar]
- [22].Taylor NE, Van Dort CJ, Kenny JD et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Solt K, Van Dort CJ, Chemali JJ et al. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 2014; 121:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vanini G, Bassana M, Mast M et al. Activation of Preoptic GABAergic or Glutamatergic Neurons Modulates Sleep-Wake Architecture, but Not Anesthetic State Transitions. Curr Biol 2020; 30:779–787 e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol 2006; 147 Suppl 1:S72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McKinstry-Wu AR, Wasilczuk AZ, Harrison BA et al. Analysis of stochastic fluctuations in responsiveness is a critical step toward personalized anesthesia. Elife 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fuller PM, Sherman D, Pedersen NP et al. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 2011; 519:933–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wenzel DG, Lal H. The relative reliability of the escape reaction and righting-reflex sleeping times in the mouse. J Am Pharm Assoc Am Pharm Assoc 1959; 48:90–91. [DOI] [PubMed] [Google Scholar]
- [29].Bignall KE. Ontogeny of levels of neural organization: the righting reflex as a model. Exp Neurol 1974; 42:566–573. [DOI] [PubMed] [Google Scholar]
- [30].Kenny JD, Chemali JJ, Cotten JF et al. Physostigmine and Methylphenidate Induce Distinct Arousal States During Isoflurane General Anesthesia in Rats. Anesth Analg 2016; 123:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li J, Li H, Wang D et al. Orexin activated emergence from isoflurane anaesthesia involves excitation of ventral tegmental area dopaminergic neurones in rats. Br J Anaesth 2019; 123:497–505. [DOI] [PubMed] [Google Scholar]
- [32].Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology 2011; 115:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reshef ER, Schiff ND, Brown EN. A Neurologic Examination for Anesthesiologists: Assessing Arousal Level during Induction, Maintenance, and Emergence. Anesthesiology 2019; 130:462–471. [DOI] [PubMed] [Google Scholar]
- [34].Pal D, Li D, Dean JG et al. Level of Consciousness Is Dissociable from Electroencephalographic Measures of Cortical Connectivity, Slow Oscillations, and Complexity. J Neurosci 2020; 40:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[35].Gao S, Proekt A, Renier N et al. Activating an anterior nucleus gigantocellularis subpopulation triggers emergence from pharmacologically-induced coma in rodents. Nat Commun 2019; 10:2897.This study found a previously unidentified pathway that utilizes activation of the anterior portion of the nucleus gigantocellularis originating in the medullary reticular formation to restore arousal in two coma models, anesthesia and hypoglycemic coma, by engaging multiple arousal pathways.
- [36].Schiff ND. Central Lateral Thalamic Nucleus Stimulation Awakens Cortex via Modulation of Cross-Regional, Laminar-Specific Activity during General Anesthesia. Neuron 2020; 106:1–3. [DOI] [PubMed] [Google Scholar]
- [37].Ballesteros JJ, Huang P, Patel SR et al. Dynamics of Ketamine-induced Loss and Return of Consciousness across Primate Neocortex. Anesthesiology 2020; 132:750–762. [DOI] [PubMed] [Google Scholar]
- **[38].Patel SR, Ballesteros JJ, Ahmed OJ et al. Dynamics of recovery from anaesthesia-induced unconsciousness across primate neocortex. Brain 2020; 143:833–843.This study applied a detailed description of cortical frequency changes during loss and recovery of consciousness while exposing macaques to injected anesthetics. Recovery of consciousness was defined by combining cortical changes (distinctive return of beta and the concurrent decline of slow oscillations) with behavior (probability of the subject engaging in a pretrained behavioral task)
- [39].Ballesteros JJ, Briscoe JB, Ishizawa Y. Neural signatures of alpha2-Adrenergic agonist-induced unconsciousness and awakening by antagonist. Elife 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Baker JL, Ryou JW, Wei XF et al. Robust modulation of arousal regulation, performance, and frontostriatal activity through central thalamic deep brain stimulation in healthy nonhuman primates. J Neurophysiol 2016; 116:2383–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pfaff DW. Brain arousal and information theory: neural and genetic mechanisms. Harvard University Press; 2006. [Google Scholar]
- [42].Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 1999; 398:338–341. [DOI] [PubMed] [Google Scholar]
- **[43].Suzuki M, Larkum ME. General Anesthesia Decouples Cortical Pyramidal Neurons. Cell 2020; 180:666–676 e613.This study demonstrated that general anesthesia decouples information flow, tested using optogenetic stimulation between layer V pyramidal neuron dendrites and their cell bodies.
- [44].Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med 2010; 363:2638–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gerrard P, Zafonte R, Giacino JT. Coma Recovery Scale-Revised: evidentiary support for hierarchical grading of level of consciousness. Arch Phys Med Rehabil 2014; 95:2335–2341. [DOI] [PubMed] [Google Scholar]
- [46].Fridman EA, Schiff ND. Neuromodulation of the conscious state following severe brain injuries. Curr Opin Neurobiol 2014; 29:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bernander O, Douglas RJ, Martin KA, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A 1991; 88:11569–11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang TX, Xiong B, Xu W et al. Activation of Parabrachial Nucleus Glutamatergic Neurons Accelerates Reanimation from Sevoflurane Anesthesia in Mice. Anesthesiology 2019; 130:106–118. [DOI] [PubMed] [Google Scholar]
- [49].Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A 2014; 111:3859–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]