Abstract
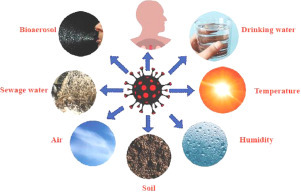
Viruses are biologically active parasites that only exist inside a host they are submicroscopic level. The novel coronavirus disease, or COVID-19, is generally caused by the SARS-CoV-2 virus and is comparable to severe acute respiratory syndrome (SARS). As a result of globalization, natural alterations or changes in the SARS-CoV-2 have created significant risks to human health over time. These viruses can live and survive in different ways in the atmosphere unless they reach another host body. At this stage, we will discuss the details of the transmission and detection of this deadly SARS-CoV-2 virus via certain environmental media, such as the atmosphere, water, air, sewage water, soil, temperature, relative humidity, and bioaerosol, to better understand the diffusion, survival, infection potential and diagnosis of COVID-19.
Keyword: COVID-19, Diagnosis, Environmental media, Structure, Transmission
Graphical abstract
Introduction
The COVID-19 disease originated in Wuhan, China (Chen et al., 2020; Lu et al., 2020). According to the World Health Organization (WHO), several cases of pneumonia were reported in Wuhan City, China (Zhu et al., 2020). The cause of this disease was not identified (Sohrabi et al., 2020; Anderson et al., 2020) until January 7, 2020, and the disease has spread very quickly since then. According to the Chinese government, the new type of coronavirus results in a condition called pneumonia (Li et al., 2020). Based on the sharp increase in the number of cases of COVID-19, by the end of January 2020, the WHO had confirmed that the recent outbreak of the disease represented an immediate health emergency that was not only a problem at the country level but could affect the world as a whole. As of March 26, 2020, the WHO had confirmed 416,686 cases of coronavirus and more than 18,589 deaths worldwide. The disease spreads quickly and has affected more than 197 countries around the world. As of May 4, 2020, according to a WHO report, 239,740 people have died due to coronavirus. Up to 24 January 2021 there are about 97,264,519 confirmed cases of COVID-19, including 2,107,554 deaths, reported to WHO (WHO, 2020c).
Respiratory disease due to SARS-CoV-2 is severe, and the disease is similar to the conditions reported for SARS-CoV (Xu et al., 2020a,b). The initial symptoms of coronavirus are fever, fatigue, dry cough, and acute fatigue. The disease appears to multiply at a high pace, and the leading cause of infection spread is considered to be the droplets that are released from the infected patient while coughing. Physicians, therefore, recommend the use of face masks to prevent the spread of this deadly infection (Chan et al., 2020; Li et al., 2020; Lai et al., 2020; Wang et al., 2020a,b; Jin et al., 2020; Anderson, 2020). Weather conditions favor the spread of this disease, and climate is expected to play a significant role in this disease, particularly in parts of Europe, the United States, and the West Nile (Epstein, 2001; Peiris et al., 2003a,b). Climate conditions generally affect SARS diseases. The spread of the SARS virus can be determined with the aid of optimum temperature, humidity, and wind speed (Yuan et al., 2006; Amuakwa Mensah et al., 2017). Conditions may change, and it has been observed that these conditions are linked to a particular pattern of death and fatality changes due to a disease called pneumonia (Bull, 1980; Wang et al., 2020a,b, Wiedinmyer et al., 2012). Numerous factors, such as population density, humidity, temperature, and other factors, determine the spread of COVID-19 (Dalziel et al., 2018). Experimental investigations are still very limited in regard to COVID-19 and climate change. For this reason, detailed and thorough studies are needed to control the spread of this disease.
There is currently an opportunity available for researchers to work together to identify environmental factors that could influence coronavirus transmission by 2019 to minimize its spread. Additionally, to take into account the long-range effects of COVID-19, researchers need to identify the various environmental conditions that may have an impact on how long the virus lives in multiple media. This review explains the structure and overview of COVID-19, its mode of spread, and the impact of ecological media on coronavirus or COVID-19.
Coronavirus
Coronaviruses belong to the Coronaviridae family under the Orthocoronavirinae subfamily, which is from the order of Nidovirales and within the kingdom of Riboviria (Gorbalenya et al., 2020). Approximately 200 novel coronaviruses have been identified in bats, as 35% of bat virome are composed of coronaviruses (Chen et al., 2014). The species is said to be zoonotic at the beginning of their diseases, while a minimal number of these species are likely to be pathogenic and cause infection (Schoeman and Fielding, 2019; Karakus et al., 2020). At present, almost seven human coVs (HCoVs) have been segregated. They are mainly known as NL63 (HCoV-NL63), which are human coronaviruses (229E (HCoV-229E) and are also considered to be coronavirus-causing viruses in humans. HCoV-HKU1, SARS-CoV, SARS-CoV-2, and Middle East Coronavirus Respiratory Syndrome (MERS-CoV) are all grouped under human coronavirus and belong to the beta coronavirus genus (Kawase et al., 2012; Keyaerts et al., 2004). Coronavirus strains that cause minute or mild illness in humans include HCoV-229E, HCoVNL63, HCoV-HKU1, and HCoV-OC43. Zoonotic viruses are called SARS-CoV and are grouped under the Miridae corona family, which is likely to infect humans and some species of animals (Lu et al., 2020). SARS-CoV-2 is generally a Sarbecovirus, which is a subgenus and resembles a human coronavirus with approximately a 96.2 percent homology sequence (Chan et al., 2020; Kannan et al., 2020). In the current circumstances, further research on COVID-19 (Table 1 ) is needed.
Table 1.
Important study with immediate, intermediate and long goals of COVID-19.
| S.No | Immediate Goals | Intermediate Goals | Long-Term Goals |
|---|---|---|---|
| 1 | Diagnostics: RNA assays, antibody and antigen assays, point of care detection | Diagnostics: Multiplex diagnostic platforms | Diagnostics: Prognostic markers |
| 2 | Therapeutics: Remdesivir, favipiravir, chloroquine, plasma therapy | Therapeutics: Intravenous immunoglobulin | Therapeutics: Innovative approaches, Cell based, RNAi |
| 3 | Vaccines: Development of animal Models | Vaccines: mRNA candidates and candidates viral vector | Vaccines: In activated and subunit candidates |
Structural assembly of SARS-CoV-2
Coronaviruses are single-stranded RNA viruses that are divided into four critical genera (Perlman and Netland, 2009). The coronavirus enters the host with the help of the envelope-anchored spike protein, which binds to the host receptor and then fuses to the host and viral membranes (Li, 2015). In particular, the distinct receptor-binding domain (RBD) of the SARS-CoV spike recognizes the host receptor angiotensin-converting enzyme-2 (ACE2) (Li, 2016; Li et al., 2003). The positive sense of the RNA genome is generally identified as + ssRNA, with a structure similar to 5′-cap3′ and poly-A tail (Chen, 2020). Inside these viruses, four vital structural proteins are present, namely, encased proteins (abbreviated as E), membrane proteins (referred to as M), spike proteins (abbreviated as N), and nucleocapsid proteins (Figure 1 ), which are considered vital for the proper regulation of viral construction (Schoeman and Fielding, 2019). N and S are the most vital proteins. N supports the virus to make capsid and proper viral arrangements, whereas S plays a specific role in the viral addition to the host cell (Siu et al., 2008; Walls et al., 2020; Huang et al., 2011; Lau et al., 2005). The large ectodomain, single transcoding anchor, and short intracellular tail are the three significant sections present in S proteins. The close trimeric and crowned frameworks in these subunits are responsible for naming coronaviruses (Zumla et al., 2016; Hoffmann et al., 2020; Howard et al., 2008).
Fig. 1.
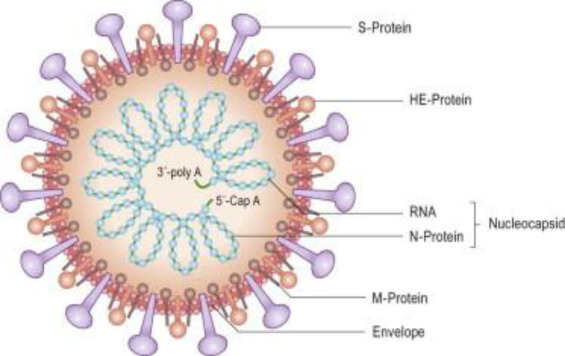
Coronavirus (Korsman S, Virology 2012 Pub Churchill Livingtone).
Duplication of SARS-CoV-2
Copying the RNA is essential when the SARS-CoV-2 virus enters a person's mass cells, as shown in Fig. 2 . Duplication of the virus is usually odd and, for endurance, inside the swarming body. Some serious steps are being taken to double the viral RNA. The apparatus is highly mandatory for the practice of duplication and is considered to be an open reading framework. They contain replicate 1 a and replicate 1 ab as two primary replicate genes, 5′-UUUAAAC-3′ as a slippery sequence, and pp la and pp lb as two main poly protein molecules (Leung et al., 2003a,b; Fehr and Perlman, 2015). Currently, Nsp 15 contains a key for duplication, but the resilient host system is also attacked during the virus duplication process (Young Chang et al., 2020; Kim et al., 2016).
Fig. 2.
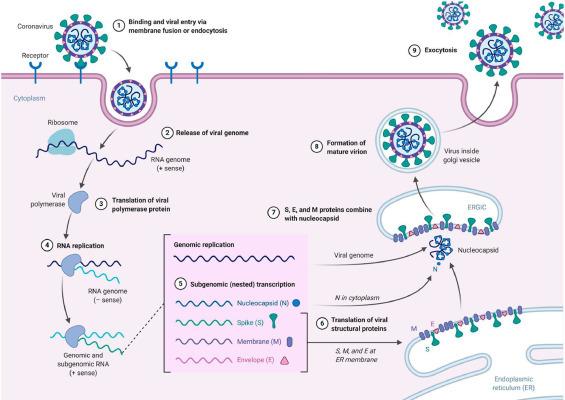
Duplication cycle of coronaviruses (Korsman S, Virology 2012 Pub Churchill Livingtone).
The COVID-19 transmission mode
The symptoms and the multiplication of viruses, especially among humans, are mentioned in Fig. 3 . Respiratory droplets and direct physical contact with the diseased person are the leading cause of viral infection (Lu et al., 2020). When a person coughs within 1 meter, droplet spread is likely to occur, especially if someone has a respiratory disorder, such as severe coughing or sneezing, flu, or sore throat. Thus, if a person has conjunctival, oral, and nasal infections, he or she is potentially exposed to respiratory infection. Respiratory air droplets may cause an infection to spread in public gatherings. Concerning fomites in the surrounding area, the spread occurs through the infected person's droplets (Chan et al., 2020). Therefore, the best way to protect oneself is to stay away from the sick person (Zhu et al., 2020). Studies often have not included quantitative analyses of the viral load as a whole.
Fig. 3.
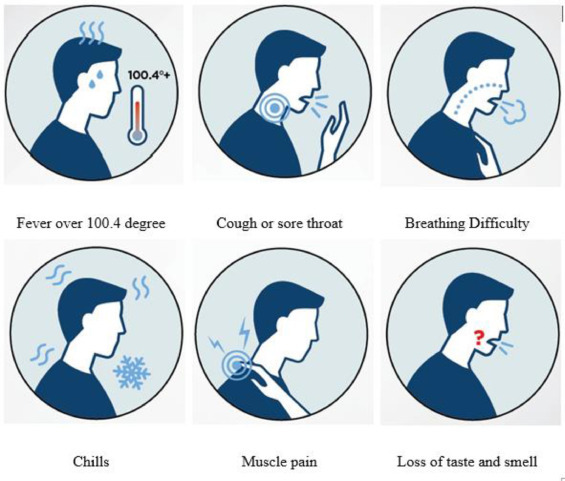
Symptoms of COVID-19 (Infographic by Sanford Health).
Although filters cause more harm to viruses than other strategies do, they are more effective in detecting viral loads in aerosols (Daniel et al., 2008). In China, aerosol particles were isolated from air samples collected from various cases in 2 hospitals and outdoors in Wuhan, and the genome of the virus was detectable in some aerosols but at deficient concentrations (Liu et al., 2020). Another report from a health center detected SARS-CoV-2 RNA in 35 percent of aerosol test samples from the intensive care unit and 12.5 percent of specimens from COVID-19. The spread of the virus is indicated by the reproduction number (R0), which is the average number of new infections caused by infectious people in a fully native population. If the R0 is higher than 1, the infected number is likely to increase; if the R0 is lower than 1, the spread may disappear. The WHO estimates the average reproduction number (R0) for COVID-19 is 1.95. In this review, we provide a brief explanation of how various environmental media interact with COVID-19.
Diagnosis of COVID-19
The test kit used to detect SARS-CoV-2 is generally an immune chromatographic assay along with a lateral flow, which may exhibit IgM and IgG antibodies in the human blood system or serum or inside plasma samples. In addition, the detection mechanism is comparable to that of SARS-CoV-2. The test kit consists of an antigen conjugated with SARS-CoV-2 chromatographs. If the samples contain any evidence of IgM and IgG, they should be bound to the antigenic conjugate and, thus, form an antigen-antibody-colored coronavirus complex. The specimen, therefore, contains IgG and IgM antibodies for SARS-CoV-2. The colored stripe will be seen as indicated in the test lined area (Fig. 4 ). The laboratory approach to COVID-19 monitoring has two pathways. The first way is to detect coronavirus directly and the body's adaptive immune response to coronavirus (Fig. 5 ). The stage of the evolution of COVID-19 determines the efficacy of the method. Nucleic acid test methods are a specific antibody rapid test accompanied by RT-PCR for the speed of diagnosis and disease progression monitoring. Coronaviruses mainly attack the human respiratory system and the lungs. The swab is collected from the upper respiratory tract of human beings. It can sometimes lead to false PCR negatives. The incubation period is, on average, five days. However, it takes 3 to 5 days for SARS-CoV antibodies to become visible once the disease has become symptomatic. The assessment consists of 158 confirmed cases outside of Wuhan, probably with a mean incubation period of 5.0 days (CI, 4.4 to 5.6 days), ranging from 2 to 14 days (Huang et al., 2020). These estimates are usually reliable, with estimates from 10 confirmed cases in China (cruel brooding period, 5.2 days; CI, 4.1 to 7.0 days) (Donnelly et al., 2003). We want our diagnostic tests to be accurate in identifying outpatients and not to falsely identify one as positive or negative for a condition. However, there is no test available with this kind of accuracy to avoid false-positive and false-negative results in countries, such as America, and China has used antibody-based testing.
Fig. 4.
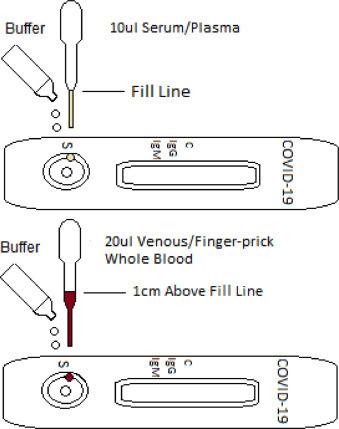
Illustration of Rapid Test Kit.
Fig. 5.
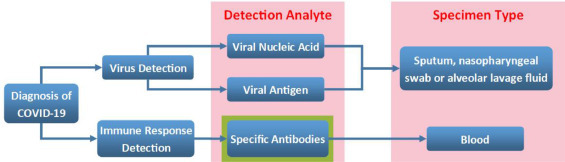
Laboratory diagnosis of COVID-19.
Pool testing is a screening algorithm that works on a polymerase chain reaction (RT-PCR) system. This method saves time, reduces costs, and reduces the workforce. In this method, two to 5 samples are tested in a single test. Each sample is tested separately when the results of the pool are positive. However, in areas with a low infection rate, the RT-PCR test is prescribed for use; the rate of positive cases is much lower than 2%. Pool testing is used to expand the laboratory test capacity. It can be used in a person without any symptoms. However, pool testing is prohibited in the case of persons who have had direct contact with positive cases. In severe cases viruses are studied in stools of patients, other laboratory investigations shows normal or low white blood corpuscles, in severe cases lymphopenia occurs (<1000 lymphocyte count), platelets count are mildly low or normal, elevated CRP, ESR, pro calcitonin is normal but in bacterial co-infection the level is increased.
The linkage of coronavirus and environmental media
Air
Regarding the transmission of droplets, airborne infectious spread is unlikely. As a result, the droplets of different nuclei transmit the germs and the particles, which are estimated to be < 5 μm in diameter, which are caused by the spread of air droplets that the diseased person produces during coughing. Particles may remain in the air for some time and may then travel a distance of 1 km and, thus, spread to people nearby (Ksiazek, 2003; Drosten, 2003; Buttler et al., 2004; Seto et al., 2003, Poutanen, 2003). On the other hand, the cultured virus was also obtained from a patient's stool specimen. However, to date, it has not been called an airborne disease. Airborne transmission is selected from the bead transmission because it refers to the proximity of the body's inner bead cores, which are ordinarily considered to be particles < 5 μm in diameter, that may remain in the air for long periods and be transmitted to others through more than 1 m of separation.
A recent publication in the New England Journal of Medicine describes the assessment of the diligence of viruses for COVID-19 (Van Doremalen et al., 2020a,b; Ong et al., 2020a,b). The accumulation of the SARS-CoV-2 on dust-loaded air and particulate matter may be included in the long-term transmission of the virus, especially for long-range viral transmission. As a result, the adsorption of the virus has been studied, and further investigations of COVID-19 disease in particulate matter have been conducted. They are needed to identify the COVID-19 particulate matter and understand how the virus is transmitted to air and particulate matter (Adhikari et al., 2019; Holshue et al., 2020).
In wuhan collected sample from ICU(15 patients) and general ward (24 patients) and the sample is analyzed by RT-PCR, results shows that SARS-COV-2 virus spread in air up to 4m from affected persons, this suggest that the release of SARS-COV-2 virus is not only by breathing but also by aerosolization of patients urine or feaces. (Chang et al., 2020) made study in Hong kong, the air and surface samples were collected with an SAS air sampler in the room of the first confirmed case it shows the absence of SARS-COV-2- RNA in the air sample which was collected from 10cm distance from patient chin during various respiratory activities. (Faridi et al., 2018) investigated the air samples which was collected from ICU about 2–5 m away from patients bed, the samples are tested by RT-PCR are negative for SARS-COV-2. The air samples are negative, but the samples taken from the surface shows the presence of virus which suggest that small viral droplet spread the disease (Ong et al., 2020a,b)
The droplets from the infected persons is settled down in the near fomites, which acts as a common route for infection. The SARS COV-2 virus can survive on in animated surface for certain period in papers it can survive up to 3 h, 2 days in treated wood, 4 days in glass and notes, and in stainless steel for 7 days, in outer layer of surgical mask the virus are detected even after 7 days (Chu et al., 2020). But the SARS COV-2 is non-infectious on copper surface after 4 hours, and after 24 h in cardboard (Van Doremalen et al., 2020a,b).
Drinking and sewage water
Medema et al. (2020) at the KWR Water Research Institute in Nieuwegein examined wastewater in seven Dutch cities and wastewater at Amsterdam Schiphol Airport. SARS-CoV-2 was identified in the sewage water. Viruses are present in the feces of infected persons; thus, wastewater should be examined for the discovery of pathogens. The location of the infection in sewage when the frequency of COVID-19 is low shows that sewage recognition should be a delicate device to detect the circulation of infection within the population.
In nature, coronavirus or COVID-19 is more prone to oxidation by oxidants, such as chlorine. Wang et al. (2005c) reported that coronavirus survives in dechlorinated tap water for two days and that washing or heating water to 20 °C is likely to kill the virus.
The survival rate of poliovirus is drastically similar and may remain in water at 4 °C. However, at a temperature of 23 °C, which is considered to be room temperature, the chances of survival of viruses are six times higher in any form of filtered or unfiltered water (Berchenko et al., 2017). In wastewater, therefore, we can say that coronavirus degrades very rapidly. The 99.9% decrease over the period of only two to three days is analogous to the data collected for the SARS-CoV-2 virus (Wang et al., 2005a, b).
The latest WHO report shows that there is no evidence of spread of corona virus through contaminated drinking water (Naddeo and Liu, 2020). Waste water acts as a significant source of virus infection whereas drinking water is very well protected against viruses. Hong kong SARS COV-2 outbreak was linked with leakage of sewage line (Peiris et al., 2003a,b). The SARS COV 2 virus are able to replicate in the human GIT and the infectious particles are identified in the stools of affected persons (Leung et al., 2003a,b). The sewage water becomes the major pathway for transmission the novel corona virus is detected in feces and urine of infected individuals (Quilliam et al., 2020). In Netherland the presence of SARS COV-2 in waste water is reported by binary RT-q PCR data (Ahmed et al., 2020).
Relative humidity and temperature
Inactivation may also occur when viral capsids are collected at the air-water interface of the solution, causing auxiliary damage (Thompson and Yates, 1999; Trouwborst et al., 1974). Drying up can also be a key supporter of surface inactivation (Abad et al., 1994), as the loss of water atoms triggers changes in the lipid layer stage, cross-linking, Maillard reactions, and peroxide formation (Cox, 1993). Infection inactivation on surfaces may involve drying and exchange at the air-water interface, each of which depends on its relative stickiness. Additionally, low relative humidity, oxidation, and Maillard reactions that occur during rapid drying can predominate. High temperatures at intemperate relative stickiness involve a synergistic effect on the inactivation of SARS-CoV functionality, whereas lower temperatures and low humidity help increase the survival of the infection on contaminated surfaces.
In this way, the natural conditions of nations, such as Malaysia, Indonesia, and Thailand, are not conducive to the increased survival of infection. In countries such as Singapore and Hong Kong, where air conditioning is heavily used, transmission occurs, for the most part, in well-heated situations, such as healing centers or inns (Chan et al., 2011). SARS-CoV can be maintained at least two weeks after drying under the conditions of temperature and humidity observed in an air-conditioned environment. Infection remains stable for three weeks at room temperature in a fluid environment> In any case, it is effectively killed by heat at 56 °C in 15 min, showing that SARS-CoV may be a steady-state infection that can be transmitted via indirect contact or fomites (WHO, 2003). Human coronaviruses may continue to be transmittable on surfaces for almost nine days. Surface sanitization with 62–71% ethanol or 0.1 percent sodium hypochlorite reduces surface coronavirus infectivity within 1 min of exposure. We expect to find a comparable effect on SARS-CoV-2 (Kampf et al., 2020).
Bat viruses, such as henipaviruses, filoviruses, and coronaviruses, are RNA viruses that are sufficiently complex to increased temperatures and adjusted pH, ultraviolet light, and desiccation levels (Sinclair et al., 2008; Fogarty et al., 2008; Piercy et al., 2010). Environmental conditions in nature may also be much lower for viral survival; the temperature, humidity, and microclimate under trees and in caves may also affect viral decay charges and, ultimately, the likelihood of spillover.
Ambient temperature is a significant aspect of coronavirus spread and level (Chan et al., 2011; Casanova et al., 2010; VanDoremalen et al., 2013). Earlier investigations have shown that temperature plays a vital role in the spread and survival of coronaviruses, such as MERS and SARS (Chan et al., 2011; Tan et al., 2005). Tan et al. (2005) found that the most exceptional probable temperature associated with SARS cases was 16 °C to 28 °C in Taiyuan (Cheng et al., 2020; Bi et al., 2007). A laboratory investigation reported that the virus was inactivated at 20 °C and then at 4 °C (Casanova et al., 2010). Further laboratory investigations have shown that coronavirus is present at 22 °C–25 °C on surfaces and that viability is lost at 38 °C.
The temperature standard was connected to COVID-19, with the lowest standard temperature of 26.1 °C and the highest temperature of 28.6 °C. This relationship is linked to the previous study of the association between climate transmission and respiratory syncytial infection (RSV) (Vandini et al., 2013). Temperature is also the ecological force of the outbreak of COVID-19 in China (Sohrabi et al., 2020).
The survival rate and half-life of SARS virus were compared with aerolization of 3 h at 21–23 °C and relative humidity of 65%. The results shows that the stability of SARS –COV-1 and SARS-COV-2 was similar (Van Doremalen et al., 2020a,b). At 40-60% of relative humidity the SARS –COV-2 is more stable (Smither et al., 2020) temperature plays an important role in the survival and transmission of corona viruses. The experiment was done with SARS-COV-2 aerosol produced by artificial saliva shows the inactivation of virus when it is simulated with sunlight. Sunlight simulated summer with high intensity shows about 90% reduction in the infectious concentration after 6 min, whereas in winter the low intensity sunlight simulated 90% reduction in the infectious concentration was observed after 19 min. The prevalence of covid-19 is decreased with increase in the temperature (Bhattacharjee, 2020).
Bioaerosol
Solid or liquid particles from aerosols are suspended in the air. As the patient coughs, the viruses are released into the air through droplets. The virus has been viable in aerosol particles for up to three hours; however, aerosols have been generated by the use of high-powered equipment that no longer reflects common human coughing conditions or a clinical setting where aerosol-generating strategies are employed. Even people who breathe or speak loudly and may spread the virus and develop bioaerosols (Tsang, 2003). The main focus of bioaerosol emission from humans carrying COVID-19, which aerosolizes individual particles, is the virus, which is categorized by its aerodynamic diameter. The source device of bioaerosols emitted by humans restricts the distribution of the size of the particles (Fig. 6 ). Normal inhalation results in particles between < 0.8 and 2.0 μm (Morawska et al., 2009). Two size distributions were noted, namely, 16–125 μm (Chao et al., 2009) and < 0.8 to 7.0 μm (Morawska et al., 2009), with a noise mean of 1.0 μm (Lai et al., 2011).
Fig. 6.
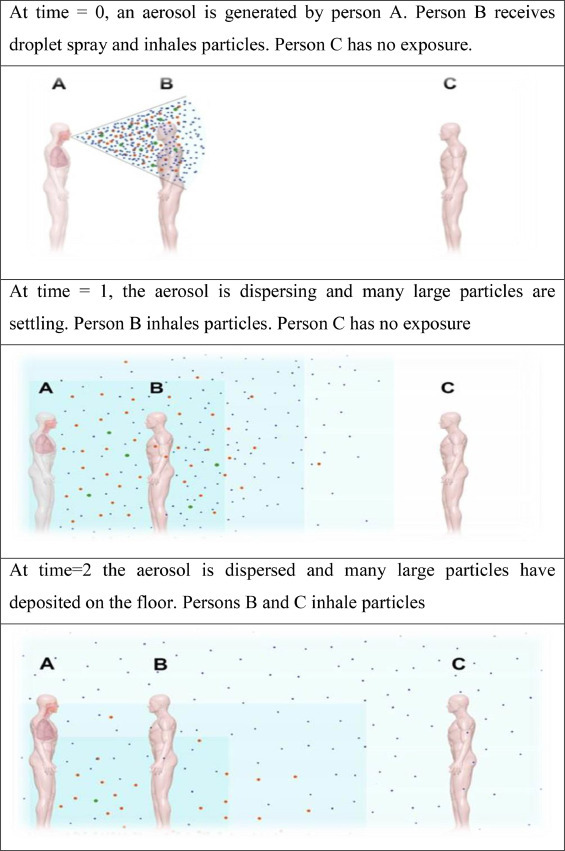
Bioaerosol dispersing with time (Lisa Brosseau, 2020).
Similarly, coughing also has a dual range of 0.6 to 16 μm (Morawska et al., 2009; Yang et al., 2007; Lindsley et al., 2010) and 40 to 125 μm. Sneezing contributes particles in the range of 7 to 125 μm. Although humans can only inhale particles < 100 μm, it should be determined whether the initial larger particles can undergo rapid evaporation, depending on the ecological relative humidity (Tang et al., 2006; Gralton et al., 2011). Aerosols may transmit the infection with COVID-19. In the clinical laboratory department in Wuhan, China, four clinical laboratory technicians reportedly were infected with coronavirus or COVID-19 and have not been shown to have been exposed to confirmed coronary artery disease. It was later found that they were infected by contact with blood samples, which were exposed to the air from aerosols, and the viruses were transmitted to the four laboratory technicians. When the virus spreads through aerosols, it is difficult to control the spread of the disease.
The droplet with the size of >60 μm can settle in the air quickly and causes the transmission of the infection from person to person through saliva. Small droplets with the range of diameter ≤ 60 μm causes short range transmission (distance between persons < 1 m) the long distance aerosol transmission is done by the droplet nuclei with the diameter of <10 μm (Xu et al., 2020a,b). Speaking and coughing of the infected individual produces both mixture of aerosol and droplets with the range of size which can travel upto 27 feet.
Soil
Viruses that are soil-adsorbed have a significant impact on their infection with host organisms (Chattopadhyay and Puls, 2000). A positive and negative charge of clay particles is capable of absorbing more than 90% of the viruses in clay minerals. In clay soil, the survival of poliovirus type 1 bacteriophages is longer than that of sandy soil (Straub et al., 1992). The aggregate and sorption of viruses in suspended sediments depend on the size and mineral nature of the soil particles. Herpesvirus, coronavirus, and influenza A and B are plant and animal pathogens that contain several structural characteristics of non-enveloped viruses (Nag et al., 2020). The sewage sludge and waste water contaminates the soil with SARS-COV-2. The virus as an envelope the survival of this virus in soil is just for few days (shorter period) there is no research studies till date to prove the survival of SARS-COV-2 in soil (Nunz-Delgado, 2020).
COVID-19 variants
The viruses constantly mutate and produces the new variants, some of the variants persists and some gets disappears, now a days COVID 19 virus gets multiple variants and spread globally. The variant B.1.1.7 was emerged in United Kingdom more prevalent in London and south east England with are easily spread with many number of mutation, variants 1.351 and P.I are seen in South Africa and Brazil respectively. More studies are needed to learn about these variants, their spread, therapies and vaccines to control the spread.
Conclusion
Recently, COVID-19 has developed into a life-threatening pandemic and is considered to be one of the most serious human infections caused by the virus. The WHO, through the processes of isolation, quarantine, public hand hygiene, social distancing, and sanitation, will carefully screen and provide information to the public around the world to reduce the extent of COVID-19. COVID-19 is mainly transmitted through bioaerosols, viruses that are released into the air through coughing and sneezing from the infected person. The virus once had the potential to maintain aerosol particles for up to 3 h, and recent research has reported that viruses are present in sewage water through the feces of contaminated persons. Viruses are more susceptible to oxidation in water media with the help of chlorine. In dechlorinated water, viruses can continue to exist for two days and are killed when heated at 20 °C. Increased temperatures at excessive relative humidity have an impact on the survival of SARS-CoV, whereas lower temperatures and lower humidity increase the survival of the virus on a nonhygienic surface. COVID-19 likely originated in bats, but contemporary theories suggest that an intermediate animal passed it on to humans. Studies are underway to help researchers recognize which animal transmitted the virus and how to control animal infections in the future. Genome sequencing learning helps us understand the spread of the virus across the country. Serological research is useful in the discovery of antibodies that the body makes after infection that act as a means of prevention and treatment. All research and development funders, manufacturers, and governments need to interact with each other to discover new vaccines for COVID-19. It is, therefore, time for all people to take part and fight COVID-19 by maintaining self-hygiene and social distancing.
Recommendations
-
•
Personal protection equipment, such as a face mask and gloves, repeated hand washing with proper sanitizing agents and travel screening is to be followed to avoid infection.
-
•
Quarantine, isolation, and social distancing, limiting interaction, improving ventillations (by nature or artificial) should be followed to control the virus spread effectively.
-
•
Equipment in hospitals should be installed, supervised, and refilled regularly, Well engineered sanitary plumbing is to be established to avoid the spread of infection in indoor environment.
-
•
Private and public health care facilities should be established and made available at all transport locations. Physical contact with the contaminated surfaces (route of transmission of virus) is to be avoided
-
•
The guidelines provide for the medical workers, research persons and health care workers is to be followed carefully., Childrens, old aged peoples and health care workers is to be protected by using various restriction methods, proper diagnostic methods is to be followed.
-
•
The probable route of transmission is to be considered and spread of virus between peoples and intermediate animals and reservoirs is to be perceived.
-
•
Safety precautions should be carried out by health workers who are working in front line, mild and severely infected patients should be kept separately.
Suggestions for future research
-
•
The research should made to detect the concentration of antibiotics in water (more use of antibiotics in COVID -19 treatment).
-
•
Detect the presence of SARS-COV2 virus in waste water and sludge.
-
•
Survival of virus in air in various humidity and temperature and probabilisation of droplet size and aerosolization.
-
•
Research should be made to reveal the impact of COVID 19 virus on bacteria and protozoa which are abundantly present in sewage water and destruction and inactivation of COVID 19 virus in sewage water.
-
•
Temperature and COVID 19 transmission research should be conducted.
-
•
Monitoring of water quality (the presence of E.coli) should be done.
-
•
Study should be made to know the dose response relation and minimal infectious dose.
-
•
Standardized protocol for quantification of SARS COV-2 in waste water is to be developed.
-
•
Development of Integration of climate change mitigation and air quality protection within nations.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Funding
There is no funding support for this review article.
Acknowledgments
The author would like to extend thanks to reviewers and the Editor-in-Chief for constructive comments and suggestions.
References
- Abad F., Pinto R., Bosch A. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 1994;60:3704–3710. doi: 10.1128/aem.60.10.3704-3710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari U., Chabrelie A., Weir M., Boehnke K., McKenzie E., Ikner L., Wang M., Wang Q., Young K., Haas C.N., Rose J., Mitchell J. A case study evaluating the risk of infection from Middle Eastern respiratory syndrome coronavirus (MERS-CoV) in a hospital setting through bioaerosols. Risk Anal. 2019;39:2608–2624. doi: 10.1111/risa.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Payyappat S., Cassidy M., Harrison N., Besley C. Sewage-associated marker genes illustrate the impact of wet weather overflows and dry weather leakage in urban estuarine waters of Sydney. Australia. Sci. Tot. Environ. 2020;705:135–390. doi: 10.1016/j.scitotenv.2019.135390. [DOI] [PubMed] [Google Scholar]
- Amuakwa Mensah F., Marbuah G., Mubanga M. Climate variability and infectious diseases nexus: evidence from Sweden. Infect. Dis. Modell. 2017;2:203–217. doi: 10.1016/j.idm.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchenko Y., Manor Y., Freedman L.S., Kaliner E., Grotto I., Mendelson E., Huppert A. Estimation of polio infection prevalence from environmental surveillance data. Sci. Transl. Med. 2017;9:67–86. doi: 10.1126/scitranslmed.aaf6786. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S. 2020. Statistical Investigation of Relationship Between Spread of Coronavirus Disease (COVID-19) and Environmental Factors Based on Study of Four Mostly Affected Places of China and Five Mostly Affected Places of Italy.https://arxiv.org/abs/2003.11277 arXiv2003.11277v1 [Preprint].[cited 2020 March 24]. Available from: [Google Scholar]
- Bi P., Wang J., Hiller J. Weather: driving force behind the transmission of severe acute respiratory syndrome in China? Intern. Med. J. 2007;37:550–554. doi: 10.1111/j.1445-5994.2007.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull G. The weather and deaths from pneumonia. Lancet. 1980;315:1405–1408. doi: 10.1016/s0140-6736(80)92666-5. [DOI] [PubMed] [Google Scholar]
- Butler M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736. (20) 30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Malik Peiris J.S., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS. Coronavirus Adv. Virol. 2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus. Med. Rev. 2020 doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.Y.H., Wan M.P., Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Li Y., Xie X. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J. Aerosol. Sci. 2009;40:122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S., Puls R.W. Forces dictating colloidal interactions between viruses and soil. Chemosphere. 2000;41:1279–1286. doi: 10.1016/s0045-6535(99)00519-6. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu B., Yang J., Jin Q. DBatVir: The database of bat-associated viruses. Database. bau021. 2014 doi: 10.1093/database/bau021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Liang H., Yuan X., Hu Y., Xu M., Zhao Y. Roles of meteorological conditions in COVID-19 transmission on a worldwide scale. medRxiv 2020. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio-Medica. 2020;91:10–17. [Google Scholar]
- Cheng V., Wong S.C., Chen J., Yip C., Chuang V., Tsang O. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K.W, Pan Y., Cheng S.M.S. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa029. (published online Jan 31.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. Roles of water molecules in bacteria and viruses. Origins Life Evol. Biosph. 1993;23:29–36. doi: 10.1007/BF01581988. [DOI] [PubMed] [Google Scholar]
- Dalziel B.D., Kissler S., Gog J.R., Viboud C., Bjornstad O.N., Metcalf C.J.E. Urbanization and humidity shape the intensity of influenza epidemics in U.S. cities. Science. 2018;362:75–79. doi: 10.1126/science.aat6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V., Sylvain M., Caroline D. Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 2008;72:413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C.A., Ghani A.C., Leung G.M., Hedley A.J., Fraser C., Riley S., Abu-Raddad L.J., Ho L.M., Thach T.Q., Chau P. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England J. Med. 2003;15:67–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Epstein P.R. West Nile virus and the climate. J. Urban Health. 2001;78:367–371. doi: 10.1093/jurban/78.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi S., Shamsipour M., Krzyzanowski M., Kunzli N., Amini H., Azimi F. Long-term trends and health impact of PM2. 5 and O3 in Tehran, Iran, 2006–2015. Environ. Int. 2018;114:37–49. doi: 10.1016/j.envint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. forecasting models using climate variables as predictors. Methods Mol. Biol. 2015:1–23. doi: 10.1371/journal.pntd.0005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty R., Halpin K., Hyatt A.D., Daszak P., Mungall B.A. Henipavirus susceptibility to environmental variables. Virus Res. 2008;132:140–144. doi: 10.1016/j.virusres.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E. 2020. Severe Acute Respiratory Syndrome-Related Coronavirus–the Species and its Viruses, a Statement of the Coronavirus Study Group. BioRxiv. [DOI] [Google Scholar]
- Gralton J., Tovey E., McLaws M.L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Krueger N., Mueller M.A., Drosten C., Pohlmann S. 2020. The Novel Coronavirus 2019 (2019-nCoV) Uses the SARS-Coronavirus Receptor ACE2 and the Cellular Protease TMPRSS2 for Entry into Target Cells. BioRxiv. [DOI] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. For the Washington State 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M.W., Travanty E.A., Jeffers S.A., Smith M., Wennier S.T., Thackray L.B., Holmes K.V. Aromatic amino acids in the juxtamembrane domain of severe acute respiratory syndrome coronavirus spike glycoprotein are important for receptordependent virus entry and cell-cell fusion. J. Virol. 2008;82:2883–2894. doi: 10.1128/JVI.01805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang Q. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hospital Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. doi:https://doi.org/10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S., Ali P.S.S., Sheeza A., Hemalatha K. COVID-19 (Novel Coronavirus 2019)–recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- Karakus U., Pohl M.O., Stertz S. Breaking the convention: sialoglycan variants, coreceptors, and alternative receptors for influenza a virus entry. J. Virol. 2020;94 doi: 10.1128/JVI.01357-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H. Extensive viable middle east respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin. Infect. Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsman S. Human coronaviruses. Virology. Pub. Churchill livingstone and therapeutic options. Nat. Rev. Drug Discov. 2012;15:327. doi: 10.1038/nrd.2015.37. [DOI] [Google Scholar]
- Ksiazek T.G. A novel coronavirus associated with severe acute respiratory syndrome. New Eng. J. Med. 2003;15:53–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.M., Bottomley C., McNerney R. Propagation of respiratory aerosols by the vuvuzela. PLOS One. 2011;6:20–26. doi: 10.1371/journal.pone.0020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Woo P.C.Y., Li K.S.M., Huang Y., Tsoi H.W., Wong B.H.L. Proceedings of the National Academy of Sciences of the United States of America. Vol. 102. 2005. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats; pp. 14040–14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K.S., Chan H.L.Y., Wu A.K.L., Lee N. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N., Yuen K.Y., Sung J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125(4):1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S., Lau E.H., Wong J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J. Virol. 2015;89:1954–1964. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.H., Moore M.J., Vasilieva N., Sui J.H., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley W.G., Blachere F.M., Thewlis R.E., Vishnu A., Davis K.A., Cao G., Palmer J.E., Clark K.E., Fisher M.A., Khakoo R. Measurements of airborne influenza virus in aerosol particles from human coughs. PLOS ONE. 2010;5:15–100. doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisa brosseau . 2020. Commentary: COVID-19 Transmission Messages Should Hinge on Science. march, 16. [Google Scholar]
- Liu Y, Ning Z., Chen Y. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. BioRxiv. 2020 doi: 10.1101/2020.03.08.982637. 2020. [DOI] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J. Med. Virol. 2020 doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G.J., Heijnen L., Elsinga G.S., Italiaander R., Brouwer- Hanzens A.J. MedRxiv; 2020. Presence of SARS-Coronavirus-2 in Sewage. – Preprint. [DOI] [PubMed] [Google Scholar]
- Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus. Environ. Sci. Water Res. Technol. 2020;6:1213–1216. doi: 10.1039/d0ew90015j. [DOI] [Google Scholar]
- Nag R., Whyte P., Markey B.K., Flaherty V., Bolton D., Fenton O., Richards K.G., Cummins E. Ranking hazards pertaining to human health concerns from land application of anaerobic digestate. Sci. Total Environ. 2020;710 doi: 10.1016/j.scitotenv.2019.136297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Delgado A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Am. Med. Assoc. 2020;323(16):10–12. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y., Group, H. U. S. S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L., Law K.I., Tang B.S.F., Hon T.Y.W., Chan C.S., Chan K.H. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy T., Smither S., Steward J., Eastaugh L., Lever M. The survival of filoviruses in liquids, on solidsubstrates and in a dynamic aerosol. J. Appl. Microbiol. 2010;109:1531–1539. doi: 10.1111/j.1365-2672.2010.04778.x. [DOI] [PubMed] [Google Scholar]
- Poutanen S.M. Identification of severe acute respiratory syndrome in Canada. New Eng. J. Med. 2003;15:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Quilliam R.S., Weidmann M., Moresco V., Purshouse H., O'Hara Z., Oliver D.M. COVID-19: the environmental implications of shedding SARS-CoV-2 in human faeces. Environ. Int. 2020;140 doi: 10.1016/j.envint.2020.105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virology. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto W.H., Tsang D., Yung R.W., Ching T.Y., Ng T.K., Ho M., Ho L.M., Peiris J.S. Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair R., Boone S.A., Greenberg D., Keim P., Gerba C.P. Persistence of category A select agents in the environment. Appl. Environ. Microbiol. 2008;74:555 563. doi: 10.1128/AEM.02167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu Y., Teoh K., Lo J., Chan C., Kien F., Escriou N., Tsao S., Nicholls J., Altmeyer R., Peiris J. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither S.J., Eastaugh L.S., Findlay J.S., Lever M.S. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg. Microbes Infect. 2020;9(1):14–15. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub T.M., Pepper I.L., Gerba C.P. Persistence of viruses in desert soils amended with anaerobically digested sewage sludge. Appl. Environ. Microbiol. 1992;58:636–641. doi: 10.1128/aem.58.2.636-641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Mu L., Huang J., Yu S., Chen B., Yin J. An initial investigation of the association between the SARS outbreak and weather: with the view of the environmental temperature and its variation. J. Epidemiol. Commun. Health. 2005;59:186–192. doi: 10.1136/jech.2004.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp.l Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Yates M. Bacteriophage inactivation at the air-water-solid interface in dynamic batch systems. Appl. Environ. Microbiol. 1999;65:1186. doi: 10.1128/aem.65.3.1186-1190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouwborst T., Kuyper S., de Jong J.C., Plantinga A. Inactivation of some bacterial and animal viruses by exposure to liquid-air interfaces. J. Gen. Virol. 1974;24:155–165. doi: 10.1099/0022-1317-24-1-155. [DOI] [PubMed] [Google Scholar]
- Tsang T. SARS- environmental issues. WHO Conference on Severe Acute Respiratory Syndrome (SARS); Kuala Lumpur, Malaysia; 2003. pp. 17–18. [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;0 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Munster V. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eurosurveillance. 2013;18:20590. doi: 10.2807/1560-7917.es2013.18.38.20590. http://www.eurosurveillance.org/ViewArticle.aspx? ArticleId=20590. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N., Morrism D., Bushmaker T. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandini S., Corvaglia L., Alessandroni R., Aquilano G., Marsico C., Spinelli M. Respiratory syncytial virus infection in infants and correlation with meteorological factors and air pollutants. Ital. J. Pediatr. 2013;39:1–10. doi: 10.1186/1824-7288-39-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W, Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Wu X.M., Xiao W.J., Zhu X.M., Gu C.Q., Yin J., Wei W., Yao. W., Liu C., Li J.F., Ou G.R., Wang M.N., Fang T.Y., Wang G.J., Qiu Y.H., Wu H.H., Chao F.H., Li J.W. Excretion and detection of SARS coronavirus and its nucleic acid from digestive system. World J. Gastroenterol. 2005;11:4390–4395. doi: 10.3748/wjg.v11.i28.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Zhen B., Kong Q.X., Song N., Xiao W.J. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Repor., 2003. “First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network,” http:www.who.int/csr/sars/survival
- Wiedinmyer C., Yoksas T., Thomson M.C. The role of weather in meningitis outbreaks in Navrongo, Ghana: a generalized additive modeling approach. J. Agric. Biol. Environ. Stat. 2012;17:442–460. doi: 10.1007/s13253-012-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2020. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) – the Kingdom of Saudi Arabia.https://www.who.int/csr/don/24-february-2020-mers-saudi-arabia/en/ [Google Scholar]
- Xu R., Cui B., Duan X., Zhang P., Zhou X., Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020;12:11. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Lee G.W.M., Chen C.M.W, Chih Cheng, Yu K.P. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol Med. 2007;20:484–494. doi: 10.1089/jam.2007.0610. [DOI] [PubMed] [Google Scholar]
- Yuan J., Yun H., Lan W. A climatologic investigation of the SARS-CoV outbreak in Beijing, China. Am. J. Infect. Control. 2006;34:234–236. doi: 10.1016/j.ajic.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. New Eng. J. Med. 2020;382:8. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chan J., Azhar E. Coronaviruses drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


