Abstract
Background
Quercetin has potential value in treating cardiovascular diseases, but it is not suitable for clinical application due to its own water solubility. The limitation of quercetin can be distinctly ameliorated by delivering it with nanocarriers.
Objective
To determine the effect of quercetin-loaded mesoporous silica nanoparticles (Q-MSNs) on myocardial ischemia-reperfusion injury in rats and its mechanism.
Methods
Q-MSNs were synthesized, and the morphology of Q-MSNs and MSNs was characterized by transmission electron microscopy and dynamic light scattering technique, respectively. Healthy rats were enrolled and randomly divided into a sham operation control group, an ischemia-reperfusion (IR) group, an IR+Q group, an IR+Q-MSNs group, and an MSNs group (each n = 10). Rats in the sham operation group were not treated with ischemia reperfusion, but given normal perfusion meantime. Rats in the sham operation control group, IR group, and MSNs group were given normal saline for 10 days before ischemia reperfusion, and rats in the IR+Q group and IR+Q-MSNs group were given drugs by gavage for 10 days before ischemia reperfusion. Primary myocardial cells were sampled from SD neonatal rats to construct hypoxia/reoxygenation myocardial cell models. The myocardial cells were assigned to a control group, IR group, quercetin (Q) group, Q-MSNs group, and MSNs group. Except for the control group, all the other groups were treated with hypoxia/reoxygenation. Cells in the Q group were treated with quercetin (10 μM, 20 μM, 40 μM) for 24 h in advance and then treated with measures to cause hypoxia-reoxygenation injury. Cells in the Q-MSNs group were treated with the same concentration of loaded quercetin and the same method used for the Q group. The myocardial apoptosis, myocardial infarction, ventricular remodeling, hemodynamic indexes, physiological and biochemical indexes, and JAK2/STAT3 pathway expression of each group were detected, and the apoptosis, viability, oxidative stress, and JAK2/STAT3 pathway expression of primary myocardial cells in each group were also detected.
Results
Quercetin significantly activated the JAK2/STAT3 pathway in vivo and in vitro, and MSNs intensified the activation. Compared with quercetin, Q-MSNs were more effective in inhibiting cell apoptosis and oxidative stress, reducing myocardial infarction size, improving ventricular remodeling and cardiac function-related biochemical indexes, and promoting the recovery of cardiac blood flow.
Conclusion
Q-MSNs can significantly enhance the activation effect of quercetin on JAK2/STAT3 pathway, thus enhancing its protection on the heart of MIRI rats.
Keywords: myocardial ischemia-reperfusion injury, quercetin-loaded mesoporous silica nanoparticles, JAK2/STAT3 pathway, cardiovascular diseases
Introduction
Cardiovascular diseases are the main cause of death and disability worldwide,1,2 and irregular daily routine,3 long-time lack of exercise,4 obesity,5 anxiety,6 and stress7 are all significant factors for a higher risk of cardiovascular diseases. The incidence rate and mortality rate of these diseases are increasing annually, posing a serious impact on human health. Ischemia-reperfusion can effectively reduce myocardial infarction size and improve myocardial function by quickly restoring myocardial blood supply, so it is a preferred treatment strategy for cardiovascular diseases.8,9 However, this strategy is not perfect, because it may cause further injury or necrosis of myocardial cells during operation, which is called myocardial ischemia-reperfusion injury (MIRI).10 Improperly treated MIRI may induce angina pectoris or myocardial infarction due to its hindering of the normal energy metabolism of the heart,11,12 so MIRI is also the main cause of poor prognosis of cardiovascular diseases. How to effectively ameliorate MIRI has become an important issue in the treatment of cardiovascular diseases.
Cardiovascular disease may be alleviated by diet adjustment,13 and fruits and vegetables have potential value in treating such diseases.14 Flavonoids are natural compounds widely found in plant-based foods including fruits and vegetables.15 Flavonoids have antioxidant, anti-inflammatory and anti-cancer effects.16 One study has revealed that a high intake of flavonoids is closely related to a decrease in the risk of cardiovascular diseases-related death.17 Therefore, exploring the mechanism of flavonoids on cardiovascular diseases may help promote the development of treatment for these diseases. Flavonoids can be classified into flavones, flavonols, and flavanones according to their chemical structure, oxidation degree and cyclic pyran ring substitution mode.16 Quercetin is a typical flavonoid compound,18 belonging to flavonol, with potential value in treating cardiovascular diseases. Quercetin can participate in regulating cell proliferation, migration, autophagy and other biological functions through AMPK pathway, Akt/PκB pathway, and MAPK/ERK pathway, thus exerting antioxidant, anti-inflammatory and anticancer effects.19,20 It has been verified that quercetin has strong myocardial protection in MIRI by regulating inflammation, oxidative stress and cardiomyocyte apoptosis,21–24 but its mechanism of action and appropriate dosage still need further exploration. In addition, with low water solubility, short half-life, and poor transferability and bioavailability,25 quercetin is restricted in clinical application for the treatment of cardiovascular diseases. Nanocarriers are a powerful tool to improve the bioavailability and pharmacokinetics of compounds at this point,26–28 which can effectively overcome the above defects of quercetin, and thus improve the efficacy of quercetin in disease treatment.29–34
Mesoporous silica nanoparticles (MSNs) are porous materials with the advantages of a stable structure, rich surface chemical properties, and high dispersibility. The porous structure of MSNs (including size, morphology, and surface function) is beneficial to the drug delivery and release in vivo.35 Loading quercetin with MSNs may help to improve the absorption and release of quercetin in organisms.36 In this study, we constructed ischemia-reperfusion models in rats and a hypoxia/reoxygenation (H/R) model in primary rat myocardial cells to explore the potential molecular mechanism of ischemia-reperfusion. We also adopted MSNs to load quercetin to study the protective effect and possible application value of quercetin-loaded mesoporous silica nanoparticles (Q-MSNs) on myocardial ischemia-reperfusion.
Methods
Q-MSNs and Characterization of Their Physicochemical Properties
Synthesis of MSNs: Ammonia solution (0.3 mL 25 wt.% solution) and cetyltrimethylammonium bromide (CTAB) solution (1 mL 0.15 m solution) were added into 9 mL water, during which the water was stirred constantly. After 5–10 min, the water was mixed with 0.5–2.0 mL 3-aminopropyltriethoxysilane (APTES) solution and 0.5–2.0 mL tetraethyl orthosilicate (TEOS) solution dissolved in ethanol, separately. After continuous stirring for 5 h, ethanol was added to the reaction mixture, and the precipitate was washed with ethanol and ammonium nitrate aqueous solution, separately. Quercetin-loaded nanoparticles: MSNs were dispersed in quercetin (20 mg/mL) solution, stirred at 37 °C for 24 h, and then centrifuged and washed with brine to obtain Q-MSNs. The potential and particle size-related parameters of Q-MSNs were measured by the dynamic light scattering (DLS) method. The drug loading capacity and loading efficiency of Q-MSNs were calculated via a UV spectrophotometer. Drug loading capacity = Wt/Ws and loading efficiency = Wt/Wo, where Wt is the mass of quercetin encapsulated in nanoparticles; Wo is the initial dosage of quercetin; and Ws is the total mass of MSNs after freeze-drying.
Study on Drug Release Kinetics in vitro
Phosphate buffer saline (PBS) solution supplemented with Q-MSNs was shaken in a constant-temperature shaker at 37 °C in the dark. At 0.5 h, 1 h, 2 h, 4 h, 6 h, 9 h, 12 h, and 24 h after shake, the solution was centrifuged to collect the supernatant, and the release amount of BTZ in the supernatant was determined. Finally, the concentration of quercetin in the supernatant was determined by a UV spectrophotometer, on which the in vitro release profile was drawn.
Myocardial Cell Models of H/R
A total of 15–20 Sprague-Dawley (SD) neonatal rats (1–4 days old) were enrolled and sterilized, and their hearts were separated, cut into pieces, trypsinized, filtered by a screen mesh, and then centrifuged to obtain primary myocardial cells. The myocardial cells were assigned to a control group, IR group, quercetin (Q) group, Q-MSNs group, and MSNs group. Cells in the control group were not treated with H/R, but incubated at 5% CO2 and 37°C. When cells in the IR group grew close to the condition of confluence during incubation at 5% CO2 and 37 °C, they were incubated with simulated hypoxia solution saturated with gas mixture containing 95% N2 and 5% CO2 in advance in a device (37 °C) which kept the balance of 95% N2 and 5% CO2 for 3 h for hypoxia, followed by incubation with simulated reperfusion solution at 95% O2, 5% CO2 and 37 °C for 2 h for reoxygenation. Cells in the Q group were treated with quercetin (10 μM, 20 μM, and 40 μM) for 24 h in advance, and then treated with measures to cause H/R injury. Cells in the Q-MSNs group were treated with the same concentration of loaded quercetin and the same method used for the Q group. The research was carried out based on the proposals in the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal study was reviewed and approved by Cangzhou Central Hospital.
Rat Models of Myocardial Ischemia Reperfusion
Collected healthy SD male rats (Weight: 250–300 mg; age: 8–10 weeks) were fasted for 12 h before the animal experiment, and then anesthetized with pentobarbital sodium through intraperitoneal injection. Subsequently, each rat was fixed on an experimental board in a supine position, and then treated to make skin preparation on the neck and chest part. Afterwards, the rat was disinfected with iodophor, and draped with an aseptic hole-towel, and needle-shaped electrocardiogram (ECG) electrodes were inserted under the skin of rat’s limbs, which were connected with the electrocardiograph to monitor and record the ECG changes. The skin of the rat’s neck was cut open with sterile scissors to fully expose the trachea, and then the rat was given tracheal intubation with an indwelling needle, and connected with a rodent ventilator to assist the breathing of the rat through artificial ventilation at pressure of 3 kPa and frequency of 70 times/min. Then, the right common carotid artery of the rat was bluntly dissected, and the distal end was ligated. The proximal common carotid artery was clamped with a micro-arterial clamp, and a V-shaped incision was cut. A polyethylene catheter filled with 1% heparin solution was inserted into the right common carotid artery incision and sent to the left ventricle. The catheter was fixed, connected with a pressure transducer and a multi-channel physiological signal analytical system to record related indexes of the cardiac function. Thorax was opened at the fifth intercostal space beside the left sternum, and the pericardium was separated to fully expose the heart. The left coronary artery is located between the left atrial appendage and pulmonary artery, so a curved needle was used for threading at a site 1–2 mm below the left atrial appendage and upper 1/3 of the left anterior descending coronary artery (note that the threading depth and width should not exceed 1 mm and 3 mm, respectively). The left anterior descending coronary artery was ligated with a slipknot. The operation was successful if the color of the anterior wall at the distal end of ligation turned purple and ECG monitoring showed that the ST segment of ECG leads I, II and AVL was raised by 0.2 m. Ventilation and indoor air were kept with the rodent ventilator, and the rats’ temperature was kept steady by a heating pad.
The rats were randomly divided into a sham operation control group, an ischemia-reperfusion (IR) group, IR+Q (40 μmol/L) group, IR+Q-MSNs (40 μmol/L) group, and MSNs group (each n = 10). Rats in the sham operation control group were not treated with ischemia reperfusion, but given normal perfusion at the same time. Rats in the sham operation control group, IR group, and MSNs group were given normal saline for 10 days before ischemia reperfusion, and rats in the IR+Q group and IR+Q-MSNs group were given drugs by gavage for 10 days before ischemia reperfusion. The rats were injected intravenously with 5 mg/kg Q-MSNs or MSNs. Rats were euthanized according to humanitarianism after ischemia-reperfusion for 4 h, and myocardial tissues and blood were sampled. The humane endpoint of animal experiment was that rats quickly lost 15–20% of their original weight or lost their appetite, remained weak after anesthesia recovery, or had persistent self-harm behavior or severe organ system failure, and the experimental end point was that the rates were given humanitarian euthanasia after their cardiac dynamics and LDH activity were determined.
Determination of Apoptosis, Oxidative Stress, and Cell Viability
Cell apoptosis was determined using annexin V/PI staining and Tunel staining, and cell viability was detected using an MTT assay. The annexin V/PI apoptosis detection kit was purchased from BD Biosciences, CellQuest software (BD Biosciences) for apoptosis rate analysis. The MTT kit was purchased from Abcam Company, and the optical density was detected at the peak of 570 nm. In addition, the level of reactive oxygen species (ROS) in cells was determined by staining with a 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) probe. The final concentration of DCFH-DA was 25 μM, and the incubation time was 30 min. Fluorescence was detected by a FLUOstarOPTIMA microplate reader with excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Western Blotting
Myocardial tissues or primary myocardial tissues were prepared into homogenate, and the expression of JAK signaling pathway (JAK2 and STAT3) and apoptosis-related proteins (Caspase-3, Bax, Bim, Bid, and Bcl-2) were determined by a Western blotting assay. The above protein antibodies were all purchased from the Abcam Company.
Myocardial Infarction and Ventricular Remodeling
The myocardial infarction of myocardial cells was measured by the Evans Blue method. Myocardial ischemia area was recorded as area at risk (AAR)/left ventricle (LV) and myocardial infarction size was recorded as an area of necrosis (AN)/AAR. Myocardial tissues of the rats were stained using the hematoxylin-eosin (HE) staining and masson’s trichrome staining.
Physiological and Biochemical Indicators
The left ventricular diastolic pressure (LVDP), left ventricular end diastolic pressure (LVEDP), and the maximum rising and falling rates of left ventricular internal pressure (±dp/dtmax) were determined using a pressure transducer, and the activity of lactate dehydrogenase (LDH) in coronary effluent was determined using a kit (Nanjing Jiancheng Bioengineering Institute). In addition, the levels of superoxide dismutase (SOD), human catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR) were determined using an automatic biochemical analyzer. The activities of Ca2+-ATPase and sodium (Na)-kalium (K) pump in sarcoplasmic reticulum of myocardium were measured by an enzyme coupling assay.
Statistical Analyses
In this study, measurement data were expressed as the mean ± standard deviation, and compared between groups using the one-way ANOVA. Post hoc pairwise comparison was carried out using Dunnett’s multiple comparisons test, and 95% was adopted as the confidence interval. P<0.05 indicates a significant difference. The experiment was repeated three times.
Results
Physicochemical Characteristics and Drug Release Kinetics of Q-MSNs
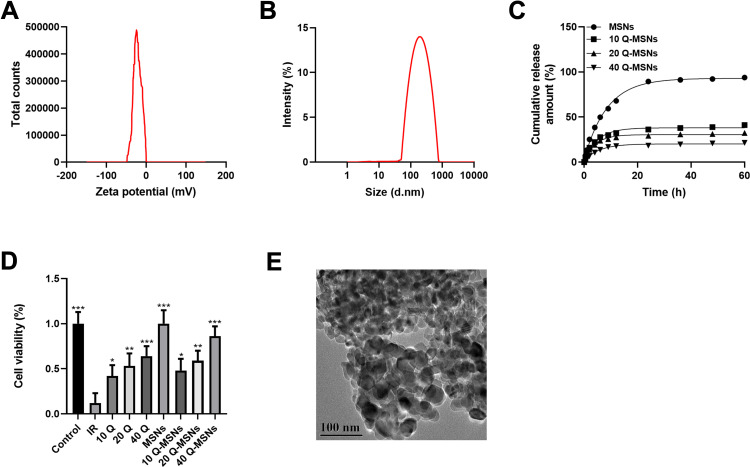
The maximum loading capacity and loading efficiency of Q-MSNs were (8.11±0.21)% and (89.47±1.28)%, respectively. Figure 1 shows the physicochemical characteristics and drug release kinetics of Q-MSNs. Figure 1A and B show the potential characterization and particle size characterization obtained using the damped-least-squares (DLS) method. The particle size of Q-MSNs was about 100–150 nm. Figure 1C showed the drug release kinetics of each group of Q-MSNs in vitro. The release effect of 40Q-MSNs was better. Figure 1D shows the effect of quercetin on the cell state of myocardial cells during H/R injury. Figure 1E shows the electron micrograph of Q-MSNs. There was inhibition on cell death in both Q group and Q-MSNs group. At the same concentration, Q-MSNs had a better effect, and among different concentrations of Q-MSNs, 40Q-MSNs had better effect.
Figure 1.
Physicochemical characterization and drug release kinetics of Q-MSNs. (A) Potential characterization of Q-MSNs. (B) Particle size characterization of Q-MSNs. The particle size was about 100–150 nm. (C) Drug release kinetics in vitro. (D) Effect of quercetin on the cell state of myocardial cells during hypoxia/reoxygenation injury. (E) Q-MSNs electron micrograph, with a scale of 100 nm. There was inhibition on cell death in both Q group and Q-MSNs group. At the same concentration, Q-MSNs had a better effect, and among different concentrations of Q-MSNs, 40Q-MSNs had a better effect.
Notes: Primary myocardial cells were sampled from SD neonatal rats at 8–15 days old, and were treated with quercetin or quercetin loaded mesoporous silica nanoparticles after hypoxia/reoxygenation treatment. Each experiment was repeated 3 times. Compared with the IR group, *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: MSNs, mesoporous silica nanoparticles; Q-MSNs, quercetin-loaded mesoporous silica nanoparticles; Q group, quercetin group; 10Q, 10 μmol/L quercetin; 20Q, 20 μmol/L quercetin; 40Q, 40 μmol/L quercetin; IR, ischemia reperfusion.
Effect of Q-MSNs on Apoptosis and Oxidative Stress of H/R Myocardial Cells
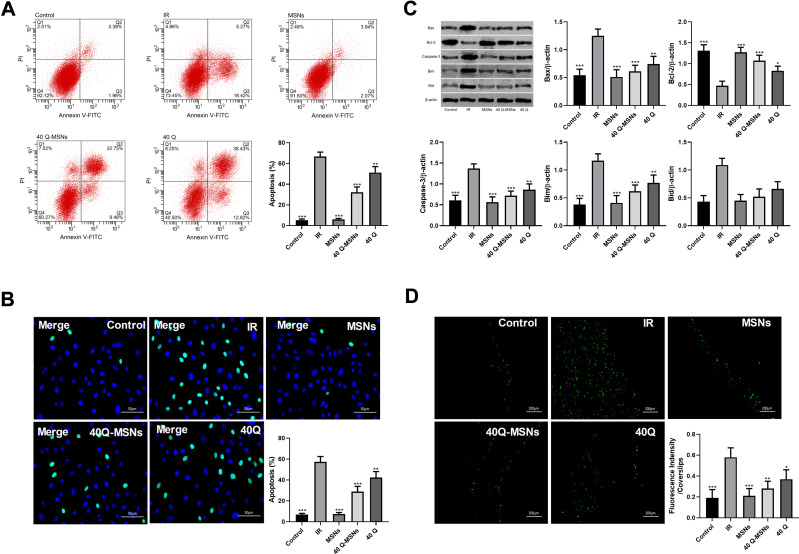
This study constructed ischemia-reperfusion models by inducing myocardial cells through H/R, and adopted annexin V/PI staining and Tunel staining to detect cell apoptosis in each group, Western blotting to detect apoptosis-related proteins in cells, and staining method with DCFH-DA probe to determine the level of ROC. Figure 2A and B show that there was inhibition on cell apoptosis in both the Q group and Q-MSNs group. At the same concentration, the inhibitory effect in the 40Q-MSNs group was better, and silica nanoparticles alone had no effect on apoptosis. Figure 2C show that there was regulation on apoptosis-related proteins in cells in both the Q group and Q-MSNs group, and at the same concentration, the regulation effect in the 40Q-MSNs group was better. Figure 2D shows that there was inhibition on the production of ROS in cells in both Q group and Q-MSNs group, and at the same concentration, the inhibitory effect in the 40Q-MSNs group was better.
Figure 2.
Effect of Q-MSNs on apoptosis and oxidative stress of hypoxia/reoxygenation myocardial cells. (A) Cell apoptosis in each group determined by annexin V/PI staining. There was inhibition on cell apoptosis in both Q group and Q-MSNs group. At the same concentration, the inhibitory effect in the 40Q-MSNs group was better, and silica nanoparticles alone had no effect on apoptosis. (B) Cell apoptosis in each group determined by Tunel staining. There was inhibition on cell apoptosis in both Q group and Q-MSNs group. At the same concentration, the inhibitory effect in the 40Q-MSNs group was better. (C) Apoptosis-related proteins in cells determined by Western blotting.There was regulation on apoptosis-related proteins in cells in both the Q group and Q-MSNs group, and at the same concentration, the regulation effect in the 40Q-MSNs group was better. (D) Reactive oxygen species (ROS) in each group. There was inhibition on the production of ROS in cells in both Q group and Q-MSNs group, and at the same concentration, the inhibitory effect in the 40Q-MSNs group was better.
Notes: Primary myocardial cells were sampled from SD neonatal rats at 8–15 days old, and their apoptosis and ROS level were determined after hypoxia/reoxygenation treatment. Each experiment was repeated 3 times. Compared with the IR group, *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: MSNs, mesoporous silica nanoparticles without quercetin; 40Q-MSNs, 40 μmol/L quercetin-loaded mesoporous silica nanoparticles; 40Q, 40 μmol/L quercetin; IR, ischemia reperfusion.
Effect of Q-MSNs on JAK Pathway in H/R Myocardial Cells
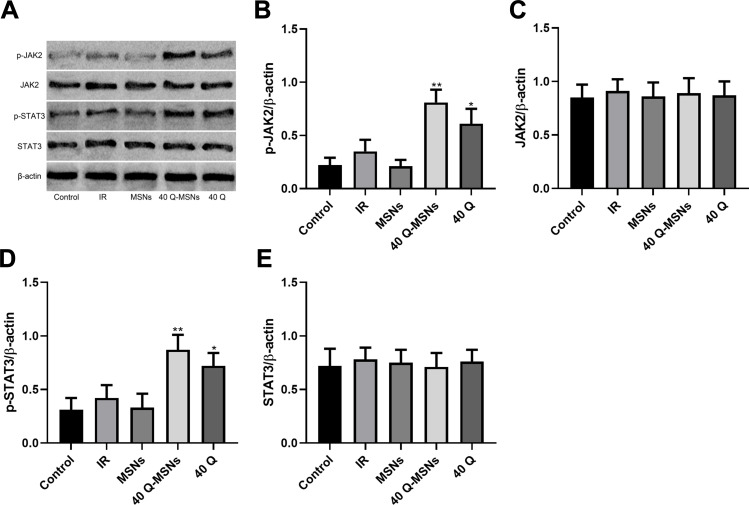
JAK pathway included JAK2 and STAT3 proteins. The expression and phosphorylation levels of the two proteins were detected. Figure 3 shows that both quercetin and Q-MSNs exerted no effect on the total protein expression of JAK2 and STAT3, but could promote the phosphorylation of them, and at the same concentration, the promotion effect of 40Q-MSNs was better. These results suggest that Q-MSNs may regulate H/R myocardial cells through the JAK pathway.
Figure 3.
Effect of Q-MSNs on the JAK pathway in hypoxia/reoxygenation myocardial cells. (A) Protein bands in Western blotting. (B–E) Effect of Q-MSNs on JAK pathway proteins (JAK2 and STAT3) in H/R myocardial cells. Both quercetin and Q-MSNs exerted no effect on the total protein expression of JAK2 and STAT3, but can promote the phosphorylation of them, and at the same concentration, the promotion effect of 40Q-MSNs was better.
Notes: Primary myocardial cells were sampled from SD neonatal rats at 8–15 days old, and their protein level was determined using Western blot after hypoxia/reoxygenation treatment. Each experiment was repeated 3 times. Compared with the IR group, *p<0.05, **p<0.01.
Abbreviations: JAK2, janus kinase 2; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylation of signal transducer and activator of transcription 3; MSNs, mesoporous silica nanoparticles without quercetin; 40Q-MSNs, 40 μmol/L quercetin-loaded mesoporous silica nanoparticles; 40Q, 40 μmol/L quercetin; IR, ischemia reperfusion.
Effect of Q-MSNs on Myocardial Tissues of Ischemia-Reperfusion Rats
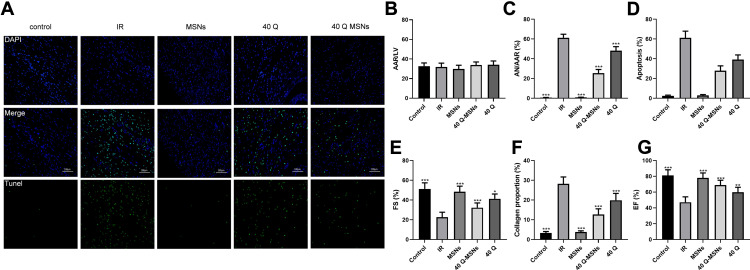
Figure 4 shows the effect of Q-MSNs on myocardial tissues of ischemia-reperfusion rats. Figure 4A shows the cell apoptosis in myocardial tissues of rats determined by TUNEL staining. Figure 4B–D shows the myocardial infarction determined by the Evans Blue staining. Both quercetin and Q-MSNs exerted no effect on myocardial ischemia area, but could reduce the myocardial infarction area, and at the same concentration, 40Q-MSNs had a better inhibitory effect. AAR: area at risk; AN: area of necrosis: LV: left ventricle; AAR/LV: myocardial ischemia area; AN/AAR: myocardial infarction area. Figure 4E–G shows myocardial tissues of the rats stained using the hematoxylin-eosin (HE) staining and masson’s trichrome staining, and determined FS and ES. 40Q-MSNs reduced myocardial remodeling and improved myocardial function after reperfusion.
Figure 4.
Effect of Q-MSNs on myocardial tissues of ischemia-reperfusion rats. (A) Cell apoptosis in myocardial tissues of rats determined by TdT-mediated dUTP nick labeling staining. (B–D), Myocardial infarction determined by the Evans Blue staining. Both quercetin and Q-MSNs exerted no effect on myocardial ischemia area,but can reduce the myocardial infarction area, and at the same concentration, 40Q-MSNs had better inhibitory effect. (E–G) Myocardial tissues of the rats stained using the hematoxylin-eosin staining and masson’s trichrome staining, and determined FS and ES. 40Q-MSNs reduced myocardial remodeling and improved myocardial function after reperfusion.
Notes: There were 10 rats in each group. Compared with the IR group, *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: AAR, area at risk; AN, area of necrosis; LV, left ventricle; AAR/LV, myocardial ischemia area; AN/AAR, myocardial infarction area; MSNs, mesoporous silica nanoparticles without quercetin; 40Q-MSNs, 40 μmol/L quercetin-loaded mesoporous silica nanoparticles; 40Q, 40 μmol/L quercetin; IR, ischemia reperfusion.
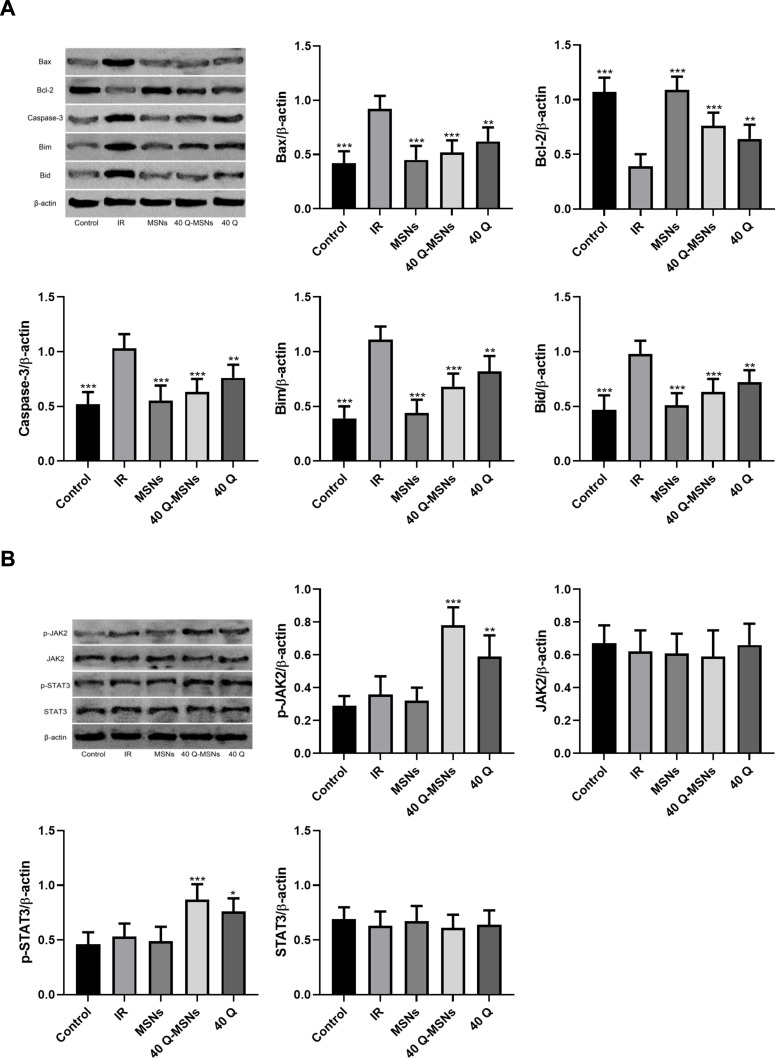
Effect of Q-MSNs on Related Myocardial Proteins in Ischemia-Reperfusion Rats
Figure 5 shows the effect of Q-MSNs on myocardial proteins in ischemia-reperfusion rats. It was found that both quercetin and Q-MSNs regulated apoptosis-related protein, and exerted no influence on the total protein expression of JAK2 and STAT3, but could promote the phosphorylation of them, and at the same concentration, 40Q-MSNs had better regulation effect.
Figure 5.
Effect of Q-MSNs on myocardial proteins in ischemia-reperfusion rats. (A) Effect of Q-MSNs on apoptosis-related proteins in myocardial tissues of ischemia-reperfusion rats. Both quercetin and Q-MSNs regulated apoptosis-related proteins, and at the same concentration, 40Q-MSNs had better regulation effect. (B) Effect of Q-MSNs on the JAK pathway in myocardial tissues of ischemia-reperfusion rats. Both quercetin and Q-MSNs exerted no effect on the total protein expression of JAK2 and STAT3, but can promote the phosphorylation of them, and at the same concentration, 40Q-MSNs had a better promotion effect. There were 10 rats in each group.
Notes: Compared with the IR group, *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: JAK2, janus kinase 2; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylation of signal transducer and activator of transcription 3; MSNs, mesoporous silica nanoparticles without quercetin; 40Q-MSNs, 40 μmol/L quercetin-loaded mesoporous silica nanoparticles; 40Q, 40 μmol/L quercetin; IR, ischemia reperfusion.
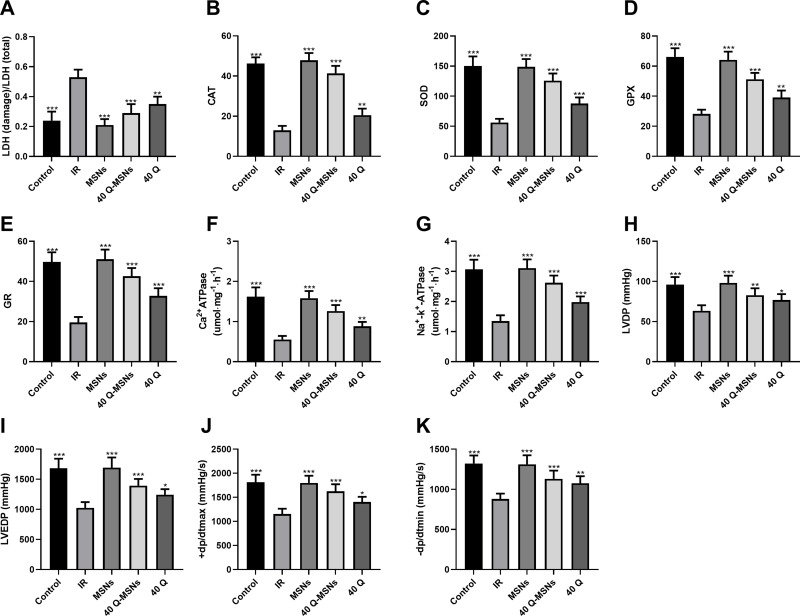
Effect of Q-MSNs on Biochemical Indexes of Ischemia-Reperfusion Rats
Figure 6 shows the effect of Q-MSNs on cardiac hemodynamics, biochemical indexes, Ca2+-ATPase and Na-K pump in ischemia-reperfusion rats. Ca2+-ATPase and Na-K pump are important ATPases that regulate myocardial contraction and relaxation. Understanding the activities of these two ATPases is helpful to understand the changes of myocardial relaxation after ischemia-reperfusion. It was found that 40Q-MSNs had the strongest effect on improving the above indexes.
Figure 6.
Effect of Q-MSNs on cardiac hemodynamics, biochemical indexes, Ca2+-ATPase and Na-K pump in ischemia-reperfusion rats. (A–E) Effect of Q-MSNs on biochemical indexes including LDH, SOD, CAT, GPX, and GR in ischemia-reperfusion rats. Compared with the IR group, SOD, CAT, GPX and GR in the 40Q-MSNs group and 40Q group increased, while LDH in them decreased. (F and G) Effect of Q-MSNs on the activities of Ca2+-ATPase and Na-K pump in the myocardium of ischemia-reperfusion rats. Compared with the IR group, the activities of Ca2+-ATPase and NA+-K+-ATPase in the 40Q-MSNs group and the 40Q group increased. (H–K) Effect of Q-MSNs on cardiac hemodynamics in ischemia-reperfusion rats. Compared with the IR group, LVEDP, +dp/dtmax, and -dp/dtmin in the 40Q-MSNs group and the 40Q group decreased. There were 10 rats in each group. MSNs, mesoporous silica nanoparticles without quercetin.
Notes: Compared with the IR group, *p<0.05, **p<0.01, and ***p<0.001.
Abbreviations: 40Q-MSNs, 40 μmol/L quercetin-loaded mesoporous silica nanoparticles; 40Q, 40 μmol/L quercetin; IR, ischemia reperfusion; LVDP, left ventricular diastolic pressure; LVEDP, left ventricular end diastolic pressure; +dp/dtmax, the maximum rising rates of left ventricular internal pressure; -dp/dtmax, the maximum falling rates of left ventricular internal pressure; LDH, the activity of lactate dehydrogenase; SOD, superoxide dismutase; CAT, human catalase; GPX, glutathione peroxidase; GR, glutathione reductase.
Discussion
MIRI gives rise to obvious myocardial apoptosis and harmful oxidative stress reaction, which seriously damages the normal function of the cardiovascular system and slows down the recovery of cardiac function of patients with cardiovascular diseases. Therefore, it is urgent to understand the molecular mechanism of MIRI and develop appropriate interventions or treatment strategies. Previous studies have confirmed that nanoparticles are helpful to promote myocardial tissue repair,37,38 so nanoparticle therapy in MIRI has captured more and more attention. During the development and progression of MIRI, many signal pathways change their activities to initiate their regulation on apoptosis and oxidative stress of downstream myocardial cells.10,39–41 Therefore, MIRI can be better treated by finding crucial drugs that can be adopted to regulate a certain signaling pathway. The JAK2/STAT3 pathway is an essential part in the development of MIRI. Increasing its activity can strongly inhibit myocardial apoptosis and oxidative damage, and thus promote cardiac protection.42–45 In this study, we constructed ischemia-reperfusion male rats and H/R myocardial cells, and treated the cells and rats with Q-MSNs to study the improvement of MSNs on the delivery of quercetin and the regulation mechanism of quercetin and JAK2/STAT3 pathway on MIRI.
According to Figure 1, quercetin released slower when being loaded on MSNs, which would help quercetin play a more effective role in organisms. In addition, 40 mol/L quercetin loaded on nanoparticles demonstrated the strongest effect on improving the cell activity in MIRI. Therefore, this study would explore the effect of 40 μmol/L quercetin loaded on nanoparticles on MIRI.
Apoptosis and oxidative stress are typical cell phenotypes of MIRI. According to Figure 2, MIRI could give rise to obvious apoptosis and oxidative stress in myocardial cells, up-regulated apoptosis-related proteins including Bax, Bim, Bid, and Caspase 3, and down-regulated Bcl-2. Both direct treatment with quercetin and treatment with quercetin loaded on nanoparticles could alleviate the above damage, and the latter had a better effect. Although quercetin has considerable anti-inflammatory and anti-apoptosis effects, its low water solubility limits its absorption and release in organisms.46 According to the results of this study, the porous structure of MSNs was used to load quercetin, which effectively promoted the absorption and release of quercetin in myocardial cells and significantly improved the therapeutic effect of qquercetin. These results indicate that Q-MSNs can effectively enhance the inhibitory effect of quercetin on apoptosis of myocardial cells and oxidative stress. Some studies have revealed that quercetin affects melanoma and glioma by regulating JAK2/STAT3 pathway,31,32 but the roles of quercetin and JAK2/STAT3 pathway in MIRI are still under investigation. Therefore, we carried out a Western blotting assay to determine the effect of quercetin on JAK2/STAT3 pathway in MIRI. According to Figure 3, both direct treatment with quercetin and treatment with quercetin loaded on nanoparticles could significantly improve the phosphorylation of JAK2 and STAT3, and the later had a better effect, which implies that quercetin loaded on nanoparticles can effectively improve the activation effect of quercetin on JAK2/STAT3 pathway. JAK2/STAT3 pathway is a signal pathway involving biological functions such as cell cycle, apoptosis, proliferation, and differentiation, etc. Absorption of quercetin results in changes in intracellular JAK2 level, thus regulating downstream STAT3 phosphorylation level and finally causing corresponding changes in the above functions.47,48 In addition, JAK2/STAT3 pathway is involved in regulating apoptosis and oxidative stress in MIRI, so quercetin can inhibit apoptosis and oxidative stress by activating JAK2/STAT3 pathway in H/R myocardial cells, and quercetin loaded on MSNs can effectively improve the myocardial protection of quercetin.
Although Q-MSNs have an amazing protective effect on H/R myocardial cells, whether it has a similar effect in MIRI rats is still unclear. In order to answer the question, we studied the effect of Q-MSNs on MIRI rats. According to Figures 4–6, Q-MSNs could significantly reduce myocardial infarction area and improve ventricular remodeling and biochemical indexes of cardiac function in MIRI rats by activating JAK2/STAT3 pathway.
The above results indicate that quercetin can improve the apoptosis degree and oxidative stress level of H/R myocardial cells by regulating the JAK2/STAT3 pathway, and quercetin loaded on nanoparticles can effectively improve the utilization of quercetin by cells. It is worth mentioning that 40 mol/L quercetin loaded on nanoparticles performed best in therapeutic effect. These results suggest that Q-MSNs have a therapeutic value for MIRI in vivo or in vitro. The JAK2/STAT3 pathway is not an independent signal pathway, and there are many regulatory factors in its upstream. Does quercetin regulate JAK2/STAT3 pathway via a certain medium? Quercetin can play a better role when its loading amount is 40 mol/L, but is 40 mol/L the best dose? These questions are worth discussing in future research. MSNs are ideal drug carriers because of their large surface area and adjustable pore size. However, this study has not studied the removal of MSNs. Therefore, we hope to carry out relevant experiments and discussions on the removal of MSNs in vivo in future research.
To sum up, we have verified that Q-MSNs can improve the bioavailability and therapeutic effect of quercetin, and quercetin can inhibit myocardial apoptosis and oxidative stress by activating the JAK2/STAT3 pathway in MIRI, reduce myocardial infarction area, improve ventricular remodeling and biochemical indexes of cardiac function, and promote the recovery of cardiac blood flow.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Maddison R, Rawstorn JC, Shariful Islam SM, et al. mHealth interventions for exercise and risk factor modification in cardiovascular disease. Exerc Sport Sci Rev. 2019;47(2):86–90. doi: 10.1249/JES.0000000000000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salas-Salvado J, Becerra-Tomas N, Garcia-Gavilan JF, Bullo M, Barrubes L. Mediterranean diet and cardiovascular disease prevention: what do we know? Prog Cardiovasc Dis. 2018;61(1):62–67. doi: 10.1016/j.pcad.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–1411. doi: 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter S, Hartman Y, Holder S, Thijssen DH, Hopkins ND. Sedentary behavior and cardiovascular disease risk: mediating mechanisms. Exerc Sport Sci Rev. 2017;45(2):80–86. doi: 10.1249/JES.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 5.Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107. doi: 10.1016/j.metabol.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 6.Allgulander C. Anxiety as a risk factor in cardiovascular disease. Curr Opin Psychiatry. 2016;29(1):13–17. doi: 10.1097/YCO.0000000000000217 [DOI] [PubMed] [Google Scholar]
- 7.Kivimaki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15(4):215–229. doi: 10.1038/nrcardio.2017.189 [DOI] [PubMed] [Google Scholar]
- 8.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65(14):1454–1471. doi: 10.1016/j.jacc.2015.02.032 [DOI] [PubMed] [Google Scholar]
- 9.Penela P, Inserte J, Ramos P, Rodriguez-Sinovas A, Garcia-Dorado D, Mayor F. Degradation of GRK2 and AKT is an early and detrimental event in myocardial ischemia/reperfusion. EBioMedicine. 2019;48:605–618. doi: 10.1016/j.ebiom.2019.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y, Wu N, Xia F, Liu S, Jia D. Long noncoding RNA GAS5 regulates myocardial ischemia-reperfusion injury through the PI3K/AKT apoptosis pathway by sponging miR5325p. Int J Mol Med. 2020;45(3):858–872. doi: 10.3892/ijmm.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conklin DJ, Guo Y, Nystoriak MA, et al. TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2019;316(4):H889–H899. doi: 10.1152/ajpheart.00106.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Wang Y, Xiao ZY, Hou DN, Li DB, Zhang XP. Effect of propofol on myocardial ischemia/reperfusion injury in rats through JAK/STAT signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(14):6330–6338. doi: 10.26355/eurrev_201907_18456 [DOI] [PubMed] [Google Scholar]
- 13.Xu Z, Steffen LM, Selvin E, Rebholz CM. Diet quality, change in diet quality and risk of incident CVD and diabetes. Public Health Nutr. 2020;23(2):329–338. doi: 10.1017/S136898001900212X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alissa EM, Ferns GA. Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr. 2017;57(9):1950–1962. doi: 10.1080/10408398.2015.1040487 [DOI] [PubMed] [Google Scholar]
- 15.Rees A, Dodd GF, Spencer JPE. The effects of flavonoids on cardiovascular health: a review of human intervention trials and implications for cerebrovascular function. Nutrients. 2018;10(12):1852. doi: 10.3390/nu10121852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liskova A, Koklesova L, Samec M, et al. Flavonoids in cancer metastasis. Cancers(Basel). 2020;12(6):1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Je Y. Flavonoid intake and mortality from cardiovascular disease and all causes: a meta-analysis of prospective cohort studies. Clin Nutr ESPEN. 2017;20:68–77. doi: 10.1016/j.clnesp.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Patel RV, Mistry BM, Shinde SK, Syed R, Singh V, Shin HS. Therapeutic potential of quercetin as a cardiovascular agent. Eur J Med Chem. 2018;155:889–904. doi: 10.1016/j.ejmech.2018.06.053 [DOI] [PubMed] [Google Scholar]
- 19.Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian S. Autophagy as a molecular target of quercetin underlying its protective effects in human diseases. Arch Physiol Biochem. 2019;1–9. doi: 10.1080/13813455.2019.1671458 [DOI] [PubMed] [Google Scholar]
- 20.Najafi M, Tavakol S, Zarrabi A, Ashrafizadeh M. Dual role of quercetin in enhancing the efficacy of cisplatin in chemotherapy and protection against its side effects: a review. Arch Physiol Biochem. 2020;1–15. doi: 10.1080/13813455.2020.1773864 [DOI] [PubMed] [Google Scholar]
- 21.Bartekova M, Radosinska J, Pancza D, Barancik M, Ravingerova T. Cardioprotective effects of quercetin against ischemia-reperfusion injury are age-dependent. Physiol Res. 2016;65(Suppl 1):S101–107. doi: 10.33549/physiolres.933390 [DOI] [PubMed] [Google Scholar]
- 22.Dong LY, Chen F, Xu M, Yao LP, Zhang YJ, Zhuang Y. Quercetin attenuates myocardial ischemia-reperfusion injury via downregulation of the HMGB1-TLR4-NF-kappaB signaling pathway. Am J Transl Res. 2018;10(5):1273–1283. [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J, Lu L, Liu Y, et al. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J Cell Biochem. 2019;120(6):9747–9757. doi: 10.1002/jcb.28255 [DOI] [PubMed] [Google Scholar]
- 24.Wan L, Xia J, Ye D, Liu J, Chen J, Wang G. Effects of quercetin on gene and protein expression of NOX and NOS after myocardial ischemia and reperfusion in rabbit. Cardiovasc Ther. 2009;27(1):28–33. doi: 10.1111/j.1755-5922.2009.00071.x [DOI] [PubMed] [Google Scholar]
- 25.Vinayak M, Maurya AK. Quercetin loaded nanoparticles in targeting cancer: recent development. Anticancer Agents Med Chem. 2019;19(13):1560–1576. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad N, Ahmad R, Alam MA, Samim M, Iqbal Z, Ahmad FJ. Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int J Biol Macromol. 2016;88:320–332. doi: 10.1016/j.ijbiomac.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 27.Ahmad N, Alam MA, Ahmad R, Umar S, Ahmad FJ. Improvement of oral efficacy of irinotecan through biodegradable polymeric nanoparticles through invitro and invivo investigations. J Microencapsul. 2018;35(4):327–343. doi: 10.1080/02652048.2018.1485755 [DOI] [PubMed] [Google Scholar]
- 28.Ahmad N, Ahmad R, Ahmad FJ, et al. Poloxamer-Chitosan-based Naringenin nanoformulation used in brain targeting for the treatment of Cerebral Ischemia. Saudi J Biol Sci. 2020;27(1):500–517. doi: 10.1016/j.sjbs.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugazio E, Gastaldi L, Brunella V, et al. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int J Pharm. 2016;511(1):446–454. doi: 10.1016/j.ijpharm.2016.07.024 [DOI] [PubMed] [Google Scholar]
- 30.Sapino S, Ugazio E, Gastaldi L, et al. Mesoporous silica as topical nanocarriers for quercetin: characterization and in vitro studies. Eur J Pharm Biopharm. 2015;89:116–125. doi: 10.1016/j.ejpb.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Wang JJ, Chen XL, et al. The JAK2/STAT3 and mitochondrial pathways are essential for quercetin nanoliposome-induced C6 glioma cell death. Cell Death Dis. 2013;4:e746. doi: 10.1038/cddis.2013.242 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Cao HH, Tse AK, Kwan HY, et al. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem Pharmacol. 2014;87(3):424–434. doi: 10.1016/j.bcp.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 33.Sarkar A, Ghosh S, Chowdhury S, Pandey B, Sil PC. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochim Biophys Acta. 2016;1860(10):2065–2075. doi: 10.1016/j.bbagen.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Liu J, Wei T, et al. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale. 2016;8(9):5126–5138. doi: 10.1039/C5NR08966B [DOI] [PubMed] [Google Scholar]
- 35.Manzano García M, Vallet Regí M. Progress report on mesoporous silica nanoparticles for drug delivery. Adv Funct Mater. 2019;1902634. [Google Scholar]
- 36.Ahmad N, Ahmad R, Alrasheed RA, et al. Quantification and evaluations of catechin hydrate polymeric nanoparticles used in brain targeting for the treatment of epilepsy. Pharmaceutics. 2020;12(3):203. doi: 10.3390/pharmaceutics12030203 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Chen F, Zhao ER, Hableel G, et al. Increasing the efficacy of stem cell therapy via triple-function inorganic nanoparticles. ACS Nano. 2019;13(6):6605–6617. doi: 10.1021/acsnano.9b00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemaster JE, Chen F, Kim T, Hariri A, Jokerst JV. The development of a trimodal contrast agent for acoustic and magnetic particle imaging of stem cells. ACS Appl Nano Mater. 2018;1(3):1321–1331. doi: 10.1021/acsanm.8b00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sulaiman D, Li J, Devarajan A, et al. Paraoxonase 2 protects against acute myocardial ischemia-reperfusion injury by modulating mitochondrial function and oxidative stress via the PI3K/Akt/GSK-3beta RISK pathway. J Mol Cell Cardiol. 2019;129:154–164. doi: 10.1016/j.yjmcc.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 40.Li P, Lin N, Guo M, Huang H, Yu T, Zhang L. REDD1 knockdown protects H9c2 cells against myocardial ischemia/reperfusion injury through Akt/mTORC1/Nrf2 pathway-ameliorated oxidative stress: an in vitro study. Biochem Biophys Res Commun. 2019;519(1):179–185. doi: 10.1016/j.bbrc.2019.08.095 [DOI] [PubMed] [Google Scholar]
- 41.Ding S, Liu D, Wang L, Wang G, Zhu Y. Inhibiting microRNA-29a protects myocardial ischemia-reperfusion injury by targeting SIRT1 and suppressing oxidative stress and NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther. 2020;372(1):128–135. doi: 10.1124/jpet.119.256982 [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Che G, Di Z, Sun W, Tian J, Ren M. Calycosin-7-O-beta-D-glucoside attenuates myocardial ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway via the regulation of IL-10 secretion in mice. Mol Cell Biochem. 2020;463(1–2):175–187. doi: 10.1007/s11010-019-03639-z [DOI] [PubMed] [Google Scholar]
- 43.Chen B, Yang L, Chen J, et al. Inhibition of Connexin43 hemichannels with Gap19 protects cerebral ischemia/reperfusion injury via the JAK2/STAT3 pathway in mice. Brain Res Bull. 2019;146:124–135. doi: 10.1016/j.brainresbull.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 44.Das A, Salloum FN, Durrant D, Ockaili R, Kukreja RC. Rapamycin protects against myocardial ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. J Mol Cell Cardiol. 2012;53(6):858–869. doi: 10.1016/j.yjmcc.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Duan W, Jin Z, et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res. 2013;55(3):275–286. doi: 10.1111/jpi.12070 [DOI] [PubMed] [Google Scholar]
- 46.Huang C, Chen T, Zhu D, Huang Q. Enhanced tumor targeting and radiotherapy by quercetin loaded biomimetic nanoparticles. Front Chem. 2020;8:225. doi: 10.3389/fchem.2020.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashrafizadeh M, Rafiei H, Mohammadinejad R, Afshar EG, Farkhondeh T, Samarghandian S. Potential therapeutic effects of curcumin mediated by JAK/STAT signaling pathway: a review. Phytother Res. 2020;34(8):1745–1760. doi: 10.1002/ptr.6642 [DOI] [PubMed] [Google Scholar]
- 48.Ashrafizadeh M, Zarrabi A, Orouei S, et al. STAT3 pathway in gastric cancer: signaling, therapeutic targeting and future prospects. Biology (Basel). 2020;9(6):126. doi: 10.3390/biology9060126 [DOI] [PMC free article] [PubMed] [Google Scholar]