Abstract
Neuronal intracellular chloride ([Cl−]i) is a key determinant in γ-aminobutyric acid type A (GABA)ergic signaling. γ-Aminobutyric acid type A receptors (GABAARs) mediate both inhibitory and excitatory neurotransmission, as the passive fluxes of Cl− and HCO3− via pores can be reversed by changes in the transmembrane concentration gradient of Cl−. The cation–chloride co-transporters (CCCs) are the primary systems for maintaining [Cl−]i homeostasis. However, despite extensive electrophysiological data obtained in vitro that are supported by a wide range of molecular biological studies on the expression patterns and properties of CCCs, the presence of ontogenetic changes in [Cl−]i—along with the consequent shift in GABA reversal potential—remain a subject of debate. Recent studies showed that the β3 subunit possesses properties of the P-type ATPase that participates in the ATP-consuming movement of Cl− via the receptor. Moreover, row studies have demonstrated that the β3 subunit is a key player in GABAAR performance and in the appearance of serious neurological disorders. In this review, we discuss the properties and driving forces of CCCs and Cl−, HCO3−ATPase in the maintenance of [Cl−]i homeostasis after changes in upcoming GABAAR function. Moreover, we discuss the contribution of the β3 subunit in the manifestation of epilepsy, autism, and other syndromes.
Keywords: GABAA receptors; β3 subunit; cation–chloride co-transporters; Cl−, HCO3−ATPase; chloride homeostasis; neurodegenerative diseases
1. Introduction
Intracellular chloride ([Cl−]i) and bicarbonate ([HCO3−]i) concentrations are pivotal parameters that control neuronal inhibition and excitation; their effect depends on neuronal specialization and the level of development [1,2,3,4]. γ-Aminobutyric acid type A receptors (GABAARs) are ionotropic receptors that mediate inhibitory or excitatory neurotransmission, as the net flux of Cl− and HCO3− via pores can be reversed by modest changes in the transmembrane concentration gradient of Cl− [5,6,7]. Indeed, in mature neurons, a low [Cl−]i renders the reversal potential for GABA (EGABA) more hyperpolarized than the membrane potential (EM) [8]. The interaction of GABA with synaptic or extrasynaptic GABAARs leads to Cl− influx into the neurons and the hyperpolarization of EM [9,10,11]. Under certain circumstances (for example, massive activation), GABAAergic signaling can be switched from fast hyperpolarization to long-term depolarization of the EM [12,13,14]. Such paroxysmal depolarizing shifts in the EM during seizures induce Cl− accumulation or the efflux of HCO3− through GABAAR channels [15,16]. However, in immature and primary sensory neurons—in contrast to mature cells—the elevated [Cl−]i is more negative than Cl− equilibrium (ECl−), which renders EGABA more depolarized than the EM [17,18]. Here, GABAAR activation leads to Cl− efflux from the neurons, depolarizing the EM [19,20]. Thus, EGABA is largely determined by the electrochemical gradient of Cl−. Nevertheless, [Cl−]i homeostasis is determined by various Cl− conductive systems that may become activated in response to membrane-potential changes (ClC-channels), intracellular Ca2+-activated channels (such as anoctamin channels), pH-sensitive Cl− channels (SLC4 and SLC26), and second-active cation–chloride co-transporters (CCCs) [1,6]. However, although Cl− conductive channels may assist in maintaining [Cl−]i, they cannot generate an electrochemical ionic gradient [21]; electroneutral CCCs play this role first. According to widely accepted continuity, in mature neurons, the K+/Cl− cotransporters (KCCs) and (initially) the KCC2 pump Cl− out of cells, which is required for the generation of a rapid GABAAR-mediated hyperpolarization of the EM [22,23]. The Na+/K+/Cl− cotransporter (NKCC1) accumulates Cl− in immature and primary sensory neurons, thereby promoting slow, depolarizing GABAAR responses [19]. Generally, there is a dramatic transient shift in GABAergic signaling during development that is associated with the expression levels of CCCs—NKCC1 is downregulated while KCC2 is upregulated [24]. However, while the role of these systems in maintaining ionic homeostasis has been established, their dominant role in the rapid recovery of [Cl−]i after changes in upcoming GABA activity remains in doubt, as recent studies have demonstrated [5,25].
Functional GABAARs are heteropentameric, consisting of five individual subunits encoded by 19 genes that have been characterized and grouped according to their amino acid similarity and named α1-6, β1-3, γ1-3, δ, ε, θ, π, and p1-3 [26,27]. These GABAAR subunits are assembled to have a high level of heterogeneity with the general stoichiometry of the 2α, 2β, and 1γ subunits [28]. In several less-abundant subtypes, the γ subunit can be replaced by a δ, ε, or π subunit; the β subunit can be replaced by a θ subunit. The majority of GABAARs expressed in the human central nervous system (CNS) are α1β2γ2; however, the α3β3γ2 and α2β3γ2 receptor isoforms are also common [29]. Growing evidence suggests that the native assembly of a functional GABAAR requires the inclusion of the β subunit [28,30,31]. Furthermore, data from the literature identify the unique role of the β3 subunit in GABAAR-mediated inhibition and demonstrate the set of functional properties that distinguish β3 from other β subunits [31,32]. Progress in understanding the role of the β3 subunit in GABAAR function has been facilitated by approaches from biochemistry, molecular biology, electrophysiology, and optic genetics [30,31,32,33,34]. Recently, it was reported that the β3 subunit determines the P-type Cl−, HCO3−ATPase (EC 3.6.3.11, Cl−-transporting ATPase) [35] that uses the energy of ATP hydrolysis for vanadate-sensitive Cl− transport against an electrochemical gradient [36,37]. The GABAARs have been identified as key players in processes such as sleep, anxiety, and anesthesia. They may contribute to major disorders of the CNS, including epilepsy, autism, Parkinson’s disease (PD), and Alzheimer’s disease (AD) [38,39,40]. In this review, we discuss achievements in elucidating the role of CCCs and the GABAAR β3 subunit in GABAergic neurotransmission. Furthermore, we consider the exceptional role of the β3 subunit in the manifestation of some neurological disorders.
2. CCCs and GABAAR Activity
2.1. Role of KCC2
The maintenance of low [Cl−]i is typically attributed to KCC2, which appears to be the primary Cl− extruder in mature neurons [23]. Of the nine KCCs that exist in various cell types, KCC2 is the only one that is expressed in the CNS [41,42,43]. KCC2 is encoded by the solute carrier family 12 member 5 (SLC12A5) gene. There are two major isoforms of KCC2: in immature mouse neurons, KCC2a and KCC2b are present in low levels, whereas KCC2b is the major isoform in the adult brain [44]. KCC2 pumps Cl− against its gradient by utilizing the energy electrochemical K+ gradient, whose maintenance involves Na+, K+ ATPase performance [43,44,45,46,47]. KCC2′s net transport activity depends on the chemical gradients of K+ and Cl−; it is inhibited by the diuretic furosemide (1–2 mM) [48,49,50]. Furosemide produces a greater increase in [Cl−]i in adults versus P10 cells, demonstrating that KCC2 activity increases with age [51]. KCC2 has been extensively studied in the context of its influence on GABAAR-mediated inhibition because wherever Cl− influx occurs, the transmembrane Cl− gradient is depleted [52]. In particular, the expression of neuron-specific KCC2 is required for the generation of the inwardly directed Cl− electrochemical gradient in CA1 pyramidal neurons of the rat hippocampus [53], as well as other types of neurons [54,55,56]. The hypothesis that KCC2 is necessary for fast Cl−-dependent hyperpolarizing inhibition has been supported by results from Northern blot analyses of hippocampal pyramidal neurons [57]. However, despite extensive electrophysiological evidence and a wide range of molecular biological studies on the expression patterns and properties of KCC2 [43,58], ontogenetic changes in [Cl−]i and the consequent shift in EGABA remain a subject of debate [59,60].
Indeed, accumulating data on the kinetic properties of KCC2 have called into question their dominant role in the fast recovery of [Cl−]i since the time constant is several minutes or longer [48,56,61], while the recovery of GABAAR conductance after desensitization occurs at a time constant of approximately several seconds [5,23]. For example, the range of experiences in the various neurons has shown that, after furosemide-induced [Cl−]i changes, the KCC2-mediated extrusion of ~5 mM Cl− requires over 5 min [48,56]. These findings are consistent with observations in rat central neurons, indicating that, in contrast to the recovery of EGABA, the recovery of [Cl−]i after changes is slow (a time constant of ~30 min) [61]. In particular, in different neurons, the recovery of GABAAR-mediated conductance after desensitization occurs with a time constant of no more than 13 s [51,62]. Additionally, in vivo experiments in mouse pyramidal neurons demonstrate that the activity-dependent [Cl−]i increase of 12 mM that occurs after an epileptic seizure is recovered within less than 30 s [4,63].
2.2. Role of NKCC1
Immature neurons express high levels of NKCC1, which increases [Cl−]i by using the energy gradient for Na+ and K+ produced by the Na+, K+ ATPase [22,64,65]. Earlier studies have demonstrated the essential role of NKCC1 in the transport of Na+, K+, and Cl− into the cell in various electroneutral stoichiometries, which is selectively blocked by low micromolar concentrations of bumetanide [66,67,68]. Specifically, some evidence supports the involvement of NKCC1 in maintaining high [Cl−]i and restoring it after changes [19,69,70,71]. For example, in immature rat neocortical neurons, Cl− accumulation by NKCC1 made up ~37% of the total Cl− transport [66]. In another study, NKCC1 transcripts were detected in practically all thalamus neurons; however, the authors failed to provide evidence for a major contribution of NKCC1 to neuronal Cl− uptake in these neurons [25]. In addition, in chick embryonic motoneurons, it has been found that Cl− accumulation is only partly mediated by NKCC1 [72]. The authors investigated the possible existence of other major Cl− accumulators or anion exchangers that may be responsible for this high [Cl−]i. The low efficacy of NKCC1 in Cl− uptake has also been observed in sensory neurons. In particular, the use of specific blockers (loop diuretics) of CCCs has indicated that the contribution of NKCC1 to the maintenance of [Cl−]i in these neurons accounts for only 23–36% of total Cl− accumulation [73,74]. Moreover, substantial Cl− accumulation persists even in mice lacking NKCC1, while DIDS (a stilbene derivative), which blocks various Cl−/HCO3− transporters and exchangers, reduced Cl− uptake significantly. However, as noted by the authors, the common Cl−/HCO3− exchanger (AE2) is unlike the candidate, since the EM in olfactory sensory neurons was found to have a normal amplitude in AE2 knockout mouse pups [74]. The molecular identity of the DIDS-sensitive Cl−/HCO3− transporter in these neurons was not established.
NKCC1 has been extensively studied in the context of its influence on GABAAergic synaptic transmission. Specifically, in hippocampal pyramidal neurons, in situ hybridization and immunohistochemical results demonstrate greater NKCC1 expression levels during the first postnatal week than in later ages, a temporal sequence that mirrors the changes in GABA responses [75]. Indeed, current studies demonstrate that, in many developing neurons, NKCC1 can contribute to Cl− accumulation, which generates depolarizing GABAergic currents [66,71,72,76]. By reducing NKCC1-mediated Cl− accumulation, bumetanide shifts EGABA to negative potentials, resulting in more effective inhibition [31,77,78]. Specifically, the pharmacological action by bumetanide led to a negative shift in EGABA in high-[Cl−]i neurons, in which NKCC1 mRNA was expressed, while it had no effect on low-[Cl−]i cells, in which NKCC1 mRNA expression was not detected [79,80]. However, although the molecular properties and mechanism regulation of NKCC1 are well-studied, few reports address the kinetics of NKCC1-dependent Cl− uptake. In particular, in immature rat neocortical neurons and in human neuroblastoma cells, bumetanide-sensitive NKCC1-mediated Cl− uptake has been observed in the time interval of 5–15 min [66,68]. In addition, Gonzalez-Islas et al. showed in chick embryonic motoneurons that Cl− recovery after depletion showed both NKCC1-independent (1–10 min) and NKCC1-dependent (10–50 min) phases [72]. However, the restoration of [Cl−]i and GABAAR-mediated Cl− conductance after changes had occurred was observed over a period of 3–10 s [71].
2.3. CCCs and Neurological Disorders
GABAergic neurotransmission is exceptional in that the polarity of its actions very much depends upon [Cl−]i that is highly hesitant, leading to depolarizing and even excitatory actions under some conditions [17,24]. As noted above, most mature neurons in the CNS actively extrude Cl− and thus support a low [Cl−]i [1,3], while a return to an immature state in terms of [Cl−]i occurs after seizures, spinal cord and brain injuries, massive stimulation, and other pathological conditions [39]. Over the past two decades, the mechanisms underlying the higher [Cl−]i accumulation in immature and pathological neurons were explained by the varied efficacy of CCCs [18,45]. Indeed, based on their developmental, cellular, and subcellular patterns of functional expression, CCCs have turned out to be highly diversified factors in ensuring GABAAergic signaling [23]. Disrupting the expression or regulation of NKCC1 or KCC2 during development can change the normal excitation/inhibition balance (E/I), which is critical for proper neuronal circuit development and function [22,23]. Interestingly, even while bumetanide and furosemide were unable to counteract the initial changes in [Cl−]i, they entirely prevented the secondary rise in [Cl−]i during reoxygenation in hippocampal slices from adult mice [69], while the [Cl−]i recovery by NKCC1 was observed over 45 min after in vitro ischemia. Kilb et al. demonstrated that bumetanide did not attenuate low-Mg2+-induced epileptiform activity, although it did enhance kainate-induced ictal-like events over 60 min [81]. Another study found that bumetanide has no effect on low-seizure activity in neonatal neurons (P5); however, it plays a significant role in progressive Cl− accumulation induced by recurrent seizures [78]. In addition, a residual increase in the GABAAR-mediated current in the presence of bumetanide indicates that a persistent elevation of [Cl−]i in epileptic neurons is only partially mediated by NKCC1 [72].
The contribution of KCC2 dysfunction and subsequent increases in [Cl−]i is also considered as one of the major factors in several neurological disorders. In particular, a downregulation of KCC2 associated with epileptiform activity or after an injury has been observed in several in vivo and in vitro studies [57,62,63]. Rivera et al. found that sustained interictal-like activity in hippocampal slices downregulates the mRNA and protein expression of KCC2 in CA1 pyramidal neurons, which leads to a reduced capacity for neuronal Cl− extrusion [57]. Moreover, it has been shown that even a robust downregulation of KCC2 activity does not abolish inhibitory postsynaptic potentials (IPSPs) if cellular Cl− regulation is challenged by a Cl− load [58] and, besides, that this may take place at a low rate. Finally, Ferrini et al. recently uncovered a gradient in Cl− extrusion capacity via the superficial dorsal horn of the spinal cord (laminae I-II), which remains concealed under a low Cl− load [11]. Under a high Cl− load or a heightened synaptic drive, low Cl− extrusion occurred via the expression of KCC2. However, it is important to note that altering [Cl−]i in a time scale of seconds via slowly desensitizing or non-desensitizing GABAARs may cause the collapse of the Cl− gradient and contribute to the induction or maintenance of pathological conditions (for example, epilepsy) [51,63,64].
In conclusion, important progress in understanding the molecular mechanisms maintaining chloride homeostasis has been achieved, including a definition of a contribution of specific CCCs in GABAergic signaling. Moreover, these studies reveal the dual nature of GABA action, and [Cl−]i homeostasis determines the ambivalent behavior of GABAAR activity. However, its short-term switching and the rapid recovery of GABAAR-mediated Cl− conductance after [Cl−]i changes are difficult to explain simply through the driving force of secondary active cotransporters. The expression of CCCs may be necessary for more delayed and long-term processes for maintaining neuronal [Cl−]i homeostasis, although CCCs are not the decisive factor in short-term and minor changes. In this regard, the existence of an alternative Cl− transport system is necessary to explain the rapid maintenance of Cl− homeostasis.
3. Role of GABAAR/Cl−, HCO3− ATPase
Historically, it has been believed that, unlike the plasmalemmal ATPases, which utilize the potential energy of ATP hydrolysis for the active transport of ions against their electrochemical gradients [82], the GABAARs are passively permeable to anions [83,84]. However, early research suggested that GABAAR function can involve both ATP-binding [85,86] and ATP-hydrolysis processes [87]. Specifically, electrophysiological studies have identified the presence of atypical GABAARs that participate in Cl−-transport against an electrochemical gradient in mammalian vestibular Deiters’ neurons [88,89]. It has been suggested by the authors that these “receptors” are GABA-activated chloride extrusion pumps, where the energy for chloride extrusion is provided by ATP in a phosphorylation step within the extrusion cycle. A recent study has directly shown that GABAAR can operate as a P-type ATPase that transports Cl− ions by consuming ATP energy [35,90,91]. Indeed, it was found that β3 (unlike the α and γ subunits) is catalytic, and determines the hydrolysis of ATP [35]. Activation of the ATPase requires the presence of 5 mM Cl− and 25 mM HCO3− (Cl−, HCO3−ATPase) in the experience medium (20 mM Hepes–Tris pH 7.3, 2 mM Mg2+-ATP), although other anions can also stimulate the enzyme in the following range: Cl− ≥ Br− > I− ≥ F− [92,93]. Nonetheless, the presence of a physiological concentration of HCO3− is an absolutely necessary condition for both the stabilization of ATPase activity and ATP-dependent Cl− transport via membranes; Na+ and K+ have no effect. ATP in the presence of Mg2+ is the most effective hydrolyzed nucleotide (ATP > UTP > CTP > ADP). Other divalent cations can replace Mg2+ in the following order: Mg2+ > Mn2+ > Co2+ > Al2+ > Cd2+ [92,93]. The β3-containing GABAAR ensembles can catalyze the auto-phosphorylation of a high-energy aspartate residue during ATP hydrolysis; this is inhibited by micromolar concentrations of γ-phosphate analogs such as o-vanadate or metal fluoride complexes [35,90]. The enzyme is sensitive to low concentrations of stilbene-derivative compounds (SITS and DIDS) and to the loop diuretic furosemide; it also has low sensitivity to another diuretic, bumetanide [91,94]. An important property of this enzyme is the regulation of its catalytic properties by GABAAergic ligands (benzodiazepines and barbiturates) and its inhibition by specific GABAAR blockers—bicuculline or picrotoxin [35,92,93]. Furthermore, the GABAAR-coupled Cl−, HCO3− ATPase and its Cl−-transporting performance are highly sensitive to phenol and phenol derivatives, which distinguishes it from other transport ATPases [90].
GABAAR is permeable to two physiological anions: Cl− and HCO3− [4,7,13,14,15]. Under physiological conditions, Cl− is the primary charge carrier across the receptor pore. However, under such conditions (as example, massive activation), HCO3− ions that flow in the opposite direction (at an HCO3−/Cl− ratio of 0.2/0.3) [83,84] can contribute to the net current via GABAAR, depending on both pH and [Cl−]i [4,5]. The Cl−, HCO3− ATPase functions with anions at a ratio of ~5:1 (HCO3− to Cl−), indicating a difference in the pumping processes and passive channel conductance, while this ATPase displays a diverse number of properties in its dependence on specificity neurons. For instance, HCO3− ions do not play a key role in the activation of the Cl−ATPase in the primary sensory neurons, but solely in its stabilization, unlike mature neurons, where they play an important role in GABAAR-mediated depolarization and in Cl−, HCO3− ATPase activation [87]. Thus, the role of the ATPase in bicarbonate transport and in membrane hyperpolarization must be clarified in future research. Furthermore, it seems necessary to establish the properties and roles of the ATPase in immature neurons. In addition, the β3 subunit, reconstituted into proteoliposomes, participates in ATP-dependent Cl−-transport over a short period (5–30 s) and then reaches a plateau [35]. To investigate this, we observed the inhibition of ion-pumping processes by γ-phosphate analogs. Thus, dependent on changes in [Cl−]i, the enzyme begins to pump anions into and out of the cell during this time period to reestablish anionic electrochemical gradients for upcoming GABA activity; therefore, this activity may have important physiological significance. Recent research confirms that this ATPase plays an important role in maintaining [Cl−]i levels and shows that enzymatic activity is elevated during seizures [90] and returns to baseline levels when seizures cease. In addition, there is some evidence to support the theory that rapid activity-induced elevation of [Cl−]i, via the GABAA receptor, contributes to certain disorders (for example, epileptic activity) [4,16].
Thus, β3-containing GABAARs possess bifunctional properties—that is, they can operate in two different modes: as a GABAA-gated Cl−-channel or as an anion-transporting P-type ATPase. This indicates that this enzyme not only maintains chloride homeostasis in various neurons, but can also facilitate a change in GABAergic neurotransmission from excitation to inhibition, which is crucial in the CNS (Figure 1). However, it should be noted that the kinetic properties of Cl−, HCO3− ATPase have only been observed in vitro, which may not fully reflect in vivo studies. Therefore, until conclusive in vivo evidence is presented to confirm this phenomenon, its importance and relevance will remain in question.
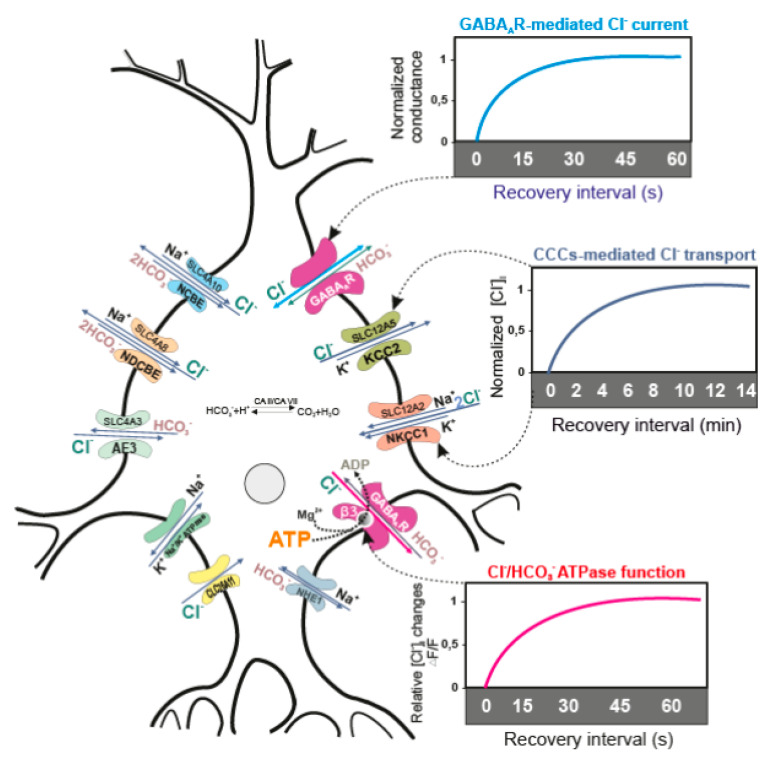
Figure 1.
Performance of transporters and recovery of the receptor function. Neuronal [Cl−]i is maintained mainly by neutral active CCCs (KCC2 and NKCC1), and less by Cl− channels (SLC26 family) and exchangers (SLC4 family—AE3, NCBE or NDCBE). CCCs move the Cl− ions by utilizing the energy electrochemical Na+ and K+ gradient, whose maintenance involves Na+, K+ATPase. CCCs need at least 5 min to recover [Cl−]i [48,57,68,69,70,71], while the GABAAR-mediated conductance may recover during approximately 10–15 s after [Cl−]i changes [51,61]. After purification and reconstitution into proteoliposomes, the β3-containing GABAAR homopentamer participates in ATP-consuming Cl− transport for a short period (~30 s) and then reaches a plateau [35]. The bottom panel shows the changes of [Cl−]i after application of Mg2+-ATP in the experimental medium containing proteoliposomes with embedded GABAAR/Cl−, HCO3−ATPase, as well as the fluorescent dye for chloride.
4. Role of the β3 Subunit in GABAAR Function
All the GABAARs without a β subunit are inactive, but many studies have noted the crucial importance of the β3 subunit in the functional properties of GABAARs. These properties distinguish β3 from both the β1/β2 subunits and the α and γ subunits [31,95]. In particular, the chimeric β3—in contrast to α2 and γ2 subunits—determines the ionic selectivity of recombinant GABAAR [32]. Moreover, the β3 and (to a lesser extent) β1 subunits can form functional homomeric ion channels in the various cells that are not only modulated by GABA, but also inhibited by picrotoxin and activated by pentobarbital [96,97]. While all five subunits are involved in the overall design of the pore, only the two β3 subunits are responsible for pore formation; this implies the importance of features that are absent in the β3 M1–M2 loop or the M2 domain, but present in both the α2 and γ2 counterparts [98]. Through a chimeric approach, four amino acids (glycine 171, lysine 173, glutamate 179, and arginine 180) in the N-terminal domain of the β3 subunit have been identified as mediating the functional cell-surface expression of this subunit, unlike β2, which is retained within the endoplasmic reticulum. In addition, immunofluorescence studies focusing on the β3 subunit have shown that (in contrast to homomeric α1, β2, or γ2L subunits) this protein can access the cell surface via homomeric expression [99].
Recently, it was found that the crystallized human GABAAR β3 homopentamer channel forms a closed gate at the base of the pore, representative of a desensitized state [100]. Furthermore, the β3 subunit is the molecular target for insecticides and phenol derivatives; other subunits (α1, α6, or γ2) differentially modulate binding to counter compound-dependent specificity and selective influence [90,101]. Moreover, a β3 subunit in the contrast to other β subunits is required alone or with another subunit (α1 or γ2) for the assembly of the [3H]ethynylbicycloorthobenzoate binding site. The β subunits are the primary substrates for various kinases in neurons. However, the regulation of GABAAR β subunits—as distinct from that of other subunits—may vary depending on the type of kinase [97]. Specifically, β3 is basally phosphorylated at the S408/S409 amino acid residue and is distinct from the β2 subunit in that it is phosphorylated by calcium/calmodulin-dependent kinase [102,103].
Using both CRISPR/Cas9 and optogenetic approaches, it was found that the presence of the β subunits (β1, β2, and β3) is absolutely necessary for the native assembly of a functional GABAAR [31]. While the knockout of both β1 and β2 did not change the inhibitory synaptic transmission, the presence of β3 alone was sufficient to maintain proper inhibitory transmission in the hippocampus. Indeed, when β3 is knocked out, either alone or in combination with another β isoform, inhibitory currents are depressed. The role of β3 was observed to be even more critical in the absence of β1 and β2 or all endogenous β subunits where expression of β3 alone is sufficient to maintain or restore inhibitory currents, respectively. In addition, the expression of β3 in the β1–β2 subunit knockout can fully restore responses to control levels [31]. This confirms that, out of the three β subunits, β3 is the most important for proper inhibitory transmission. It is possible that this is precisely due to the presence of Cl−, HCO3−ATPase in the structure of the β3 subunit and that it is able to maintain the GABA-mediated inhibitory currents. Further studies should show what other β subunits have the Cl−, HCO3−ATPase activity and affirm the uniqueness of the β3 subunit. The uniqueness of the β3 subunit also indicates its kinetic properties. In particular, results show faster kinetics in the β3 knockout GABAARs and confirm that the β3 subunit preferentially associates with α2 and α3 subunits to mediate slower IPSP decay kinetics and, therefore, longer-lasting inhibition. Indeed, it has been shown that the β3 subunit preferentially associates with the α2/α3 subunits; this is distinct from β2, which couples mainly with α1 [26,27,28,29] (Figure 2).
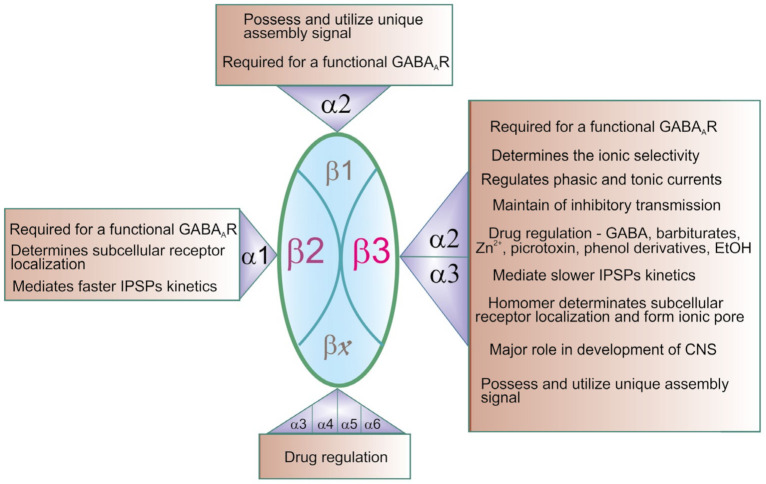
Figure 2.
Contribution of the β subunits in heterogeneity and functional properties of γ-aminobutyric acid type A receptors (GABAARs). In the central nervous system (CNS), the majority of expressed GABAARs are β2-containing (α1β2γ2) and β3-containing (α2β3γ2 and α3β3γ2) ensembles [27,28]. The β1-containing subtypes are in fewer in number. The β2-containing subtypes demonstrated a low range of properties. The β3-containing ensembles appear to have diverse properties (including maintaining properties of the inhibitory transmission and the determination of the ionic selectivity). The βx-containing ensembles are fewer in number and insufficiently defined.
Moreover, the decay kinetics of GABAAR depend on the identity of the particular α subunit isoform expressed, with α1-containing receptors having faster kinetics than α2/α3-containing receptors [30,104,105], while both the β3 subunit and α subunit determine the GABA potency [106,107]. In addition, the essential role of the β3 subunit in mediating spiny projection neuron tonic currents has been demonstrated using conditional β3 subunit knockout (β3f/fDrd2) mice [34]. The GABAAR β3 subunit gene (GABRB3) is known to play a major role in the development of the CNS, being the major β isoform in multiple regions in the prenatal and neonatal brain [106]. A developmental deficit of GABAAR function affects neurogenesis and maturation of the neuronal network [108].
5. The β3 Subunit and Human Diseases
5.1. Epilepsy
Epilepsy is a group of neurological disorders characterized by recurrent epileptic seizures with a range of etiologies and comorbidities [108]. A number of studies have demonstrated that GABRB3 gene mutations are associated with a broad phenotypic spectrum of epilepsies, and that reduced GABAAR function, causing GABAAergic disinhibition, represents the relevant disease mechanism [109,110,111,112]. In particular, the Epi 4K consortium has identified four de novo mutations in the GABRB3 in children with epileptic encephalopathies [113]. Research has found that mutations in GABAAR subunit genes (in particular, GABRB3) are associated with idiopathic epilepsy, including childhood absence epilepsy, juvenile myoclonic epilepsy, and other syndromes [109,110,111,114]. As an example, the GABRB3 mutation G32R, which is associated with childhood absence epilepsy, alters the expression of α1β3γ2L GABAAR, as well as channel-gating [109]. Janve et al. also demonstrated that the epileptic encephalopathy de novo gene GABRB3 (D120N, E180G, and Y302C) impairs GABAAR function [111]. In addition, the GABAAR coupling junction and pore GABRB3 mutations are linked to early-onset epileptic encephalopathy [115,116]. In the hippocampus of patients with temporal lobe epilepsy, expression of GABAAR β subunits (including the β3 subunit) was increased [117]. Interestingly, it was found that hyperglycosylation [118] and reduced GABA currents can alter receptor expression and channel-gating of mutated GABRB3 polypeptides to reduce childhood absence epilepsy [109].
The GABAAR β3 subunit is widely expressed in immature and adult brains in circuits involved in seizure generation, such as the cortex, hippocampus, and thalamic reticular nucleus [119]. Furthermore, it was found that mutant residues are part of conserved structural domains, such as the Cys-loop (L170R) and the M2-M3 loop (A305V), which form the GABA binding/channel-gating coupling junction and the channel pore (T288N), which are functionally coupled during receptor activation. In addition, mice lacking the β3 subunits exhibit thalamic disinhibition, a major reduction in GABAAR expression, and seizures that are associated with learning and memory deficits, poor motor skills on a repetitive task, hyperactivity, and a disturbed rest–activity cycle [120]—all features characteristic of children affected by this neurological disorder [110,111,112,113,114,116]. Null-β3 mice produce fewer functional GABAARs; pharmacological evidence indicates that other β subunits do not compensate for the absence of β3 [120]. Moreover, in model epilepsy, there is a modulation of the expression of the GABAAR β1 and β3 subunits; as a result, Cl− extrusion is impaired, and GABAAR-mediated depolarization appears [121,122,123,124]. These data highlight the importance of the β3 subunit in the appearance of epilepsy (Figure 3).
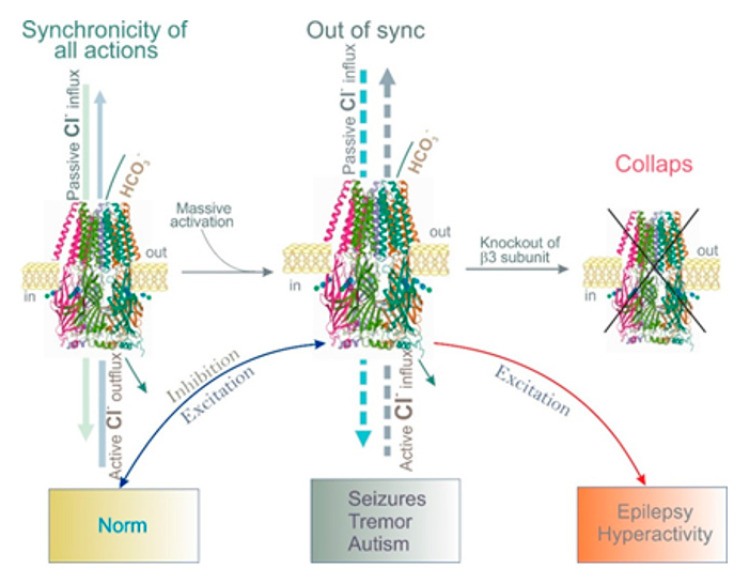
Figure 3.
Role of the β3 subunit in dissipated and generated Cl− gradients in norm and pathology. (Left) Schematic representation showing synchronicity work of passive and active Cl− fluxes via human GABAAB3R homopentamer (Protein Data Bank, 4COF) [93]. (Middle) Impaired synchronicity of multidirectional Cl− fluxes across receptor pores in certain circumstances (massive activation by GABA or other ligands). (Right) Collapse of Cl− moving via receptor pores lacking the β3 subunit.
5.2. Autism
Autism spectrum disorders (ASDs) are a group of complex disorders of neurodevelopment characterized by repetitive behaviors and difficulties with social interaction and verbal and nonverbal communication [125]. With ASD, deficits in social cognition and related cognitive functions may result from reduced synchronization between brain regions [126]. A possible explanation for ASDs is the disturbance of the delicate balance between excitation and inhibition in the developing brain, which may profoundly impact neurobehavioral phenotypes. An imbalance between excitation and inhibition may result from an increase in glutamatergic signaling (excitatory) or reduced inhibitory GABAAergic signaling [127,128,129]. Evidence connecting GABAARs in the etiology of ASD was first provided by genetic studies revealing submicroscopic abnormalities in the chromosomal locus 15q11–q13, which contains the GABRB3, GABRA5, and GABRG3 genes encoding the GABAAR β3, α5, and γ3 subunits, respectively [126,130,131]. In particular, the single-nucleotide polymorphism present in GABRB3 (rs2081648 and rs1426217) genes demonstrates significant associations between ASD and age- and gender-frequency-matched typically developing controls [127]. Locus 15q 11–13 duplications have been observed in ASD patients; association studies in idiopathic autism patients have found significant evidence for a susceptibility allele in the GABRB3 gene [131]. In addition, GABRB3 rs2081648 polymorphisms are associated with symptom-based deficits in social interaction and in sensorimotor and somatosensory coordination, visual response, imitation, activity level, and adaptability [132]. Although a mutation in the GABRB3 gene was associated with a 3–6 times greater risk of ASD with epilepsy [127], the mutation of other GABAAR subunits, including the β1 and α4 subunits, has also been coupled with ASD within various ethnic groups [133]. In addition, knockout mice for GABRA5 and GABRG3 have a normal phenotype, while GABRB3 knockouts have severe neurological abnormalities, hypersensitivity to tactile stimuli, and defects in social and exploratory behavior [120,126]. The expression of GABAAR subunits (including the ASD-relevant GABRB3) was also altered in the forebrain of young and adult Engrailed-2 knockout mice [134]. In particular, the GABAARs are reduced in various brain regions, with GABRB3 significantly altered in the parietal cortex and cerebellum. Overall, β3-containing GABAARs might be important players in the etiology of ASD pathology (Figure 3).
5.3. Alzheimer’s Disease (AD)
AD is a progressive neurodegenerative disorder that leads to the loss of cognitive functions such as executive function, learning, and memory [135]. AD is associated with a widespread loss of synapse density and continuous degeneration of cholinergic and glutamatergic pathways. Although the disruption of excitatory pathways is broadly accepted, inhibitory GABAergic pathways are generally thought to be well preserved in AD [135], while, with regard to progressive dementia, AD is characterized by an increased incidence of seizure activity. This was originally believed to be a secondary process occurring as a result of neurodegeneration. However, recent research has suggested that alterations in the E/I balance occur in AD and may be a primary mechanism contributing to the cognitive decline seen with AD [136]. Using immunohistochemistry and laser-scanning confocal microscopy, brain region-specific and cell layer-specific alterations were found in the expressions of the β2 and β3 subunits in the human hippocampus, entorhinal cortex, and superior temporal gyrus in AD cases [137,138]. In particular, the expression of three GABAAR subtypes was altered—α2 was upregulated, while the α5 and β3 subunits were downregulated [139]. Moreover, lower levels of GABAAR β2 mRNA in the prefrontal cortex of the AD brain [138]—along with lower levels of β3 mRNAs in the AD hippocampus [135]—suggest that some GABAAR subtypes may have an altered functional profile in AD. This contrasts with the β1 subunit, which was well-preserved.
5.4. Parkinson’s Disease (PD)
PD is the most common neurodegenerative movement disorder. Its clinical manifestations include motor symptoms (bradykinesia, rigidity, postural instability, and resting tremors) and a variety of nonmotor symptoms (particularly sleep and behavioral disturbances) [140,141]. It is generally thought that bradykinesia results from the loss of dopaminergic neurons in the substantia nigra pars compacta and subsequent striatal dopamine depletion [142]. However, it has recently been suggested that GABA plays a modulatory role in the pathophysiology of PD that is independent of dopaminergic medication [143,144,145]. In addition, the GABAAR/Cl−, HCO3−ATPase from rat brain is involved in the phenol-induced manifestations of both head-twitching and tremors [94]. Moreover, an interaction between dopamine and GABA was established in the basal ganglia [146]. In particular, dopamine in the absence of GABA can directly modulate recombinant GABAARs via binding with the β3 subunit [147]. In this regard, clarification of the role of the β3 subunit in the appearance of PD may be of particular interest.
6. Concluding Remarks
Since the 1970s, GABAARs have been considered as passively permeable, ligand-regulated Cl− channels that—after binding to GABA—mediate either hyperpolarization or depolarization of transmembrane potential. Among all subunits, an exclusive role in GABAAR functioning has been determined only for the β3 subunit. Research data on the properties of GABAARs highlight that the β3 subunit is an independent structure that can singly form an ion pore; importantly, it is also a key polypeptide that facilitates inhibitory neurotransmission. Moreover, recent findings indicate that β3-containing GABAARs are P-type ATPases, allowing us to review them as bifunctional systems. Other β subunits may also possess ATPase activity. These properties of GABAARs necessitate a special look at their role in neurological disorders—for instance, the violation of passive GABAAR-mediated Cl− currents or ATP-dependent Cl− pumping changes chloride homeostasis. In summary, advances in understanding how GABAARs operate have drawn a much more complex picture than what existed previously. New methods (particularly optic genetic methods) and approaches (including the use of genetically modified animals) provide multiple avenues for future research to elucidate the mechanisms of GABAAR function and reveal how its dysfunction leads to disease. We believe that the GABAAR β3 subunit may serve as a primary pharmacotherapeutic target. We hope that this paper will foster interest in clarifying the posttranslational mechanisms of its regulation.
Author Contributions
Conceptualization, S.A.M.; writing—original draft preparation, S.A.M.; writing—review and editing, S.G.M. and A.A.K.; project administration, S.G.M. and A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rahmati: N., Hoebeek F.E., Peter S., De Zeeuw C.I. Chloride Homeostasis in Neurons with Special Emphasis on the Olivocerebellar System: Differential Roles for Transporters and Channels. Front. Cell. Neurosci. 2018;12:101. doi: 10.3389/fncel.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruffin V.A., Salameh A.I., Boron W.F., Parker M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014;5:43. doi: 10.3389/fphys.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raimondo J.V., Richards B.A., Woodin M.A. Neuronal chloride and excitability—The big impact of small changes. Curr. Opin. Neurobiol. 2017;43:35–42. doi: 10.1016/j.conb.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Sato S.S., Artoni P., Landi S., Cozzolino O., Parra R., Pracucci E., Trovato F., Szczurkowska J., Luin S., Arosio D., et al. Simultaneous two-photon imaging of intracellular chloride concentration and pH in mouse pyramidal neurons in vivo. Proc. Natl. Acad. Sci. USA. 2017;114:8770–8779. doi: 10.1073/pnas.1702861114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombardi A., Jedlicka P., Luhmann H.J., Kilb W. Interactions between Membrane Resistance, GABA-A Receptor Properties, Bicarbonate Dynamics and Cl--Transport Shape Activity-Dependent Changes of Intracellular Cl- Concentration. Int. J. Mol. Sci. 2019;20:1416. doi: 10.3390/ijms20061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hübner C.A., Holthoff K. Anion transport and GABA signaling. Front. Cell. Neurosci. 2013;7:177. doi: 10.3389/fncel.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staley K.J., Proctor W.R. Modulation of mammalian dendritic GABAA receptor function by the kinetics of Cl− and HCO3− transport. J. Physiol. Lond. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller A.L., Taube J.S., Schwartzkroin P.A. Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-aminobutyric acid in rabbit hippocampus studied in vitro. J. Neurosci. 1984;4:860–867. doi: 10.1523/JNEUROSCI.04-03-00860.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: A personal journey. Neuroscience. 2014;279:187–219. doi: 10.1016/j.neuroscience.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Müller W., Misgeld U., Lux H.D. gamma-Aminobutyric acid-induced ion movements in the guinea pig hippocampal slice. Brain Res. 1989;484:184–191. doi: 10.1016/0006-8993(89)90361-2. [DOI] [PubMed] [Google Scholar]
- 11.Ferrini F., Perez-Sanchez J., Ferland S., Lorenzo L.E., Godin AG., Plasencia-Fernandez I., Cottet M., Castonguay A., Wang F., Salio C., et al. Differential chloride homeostasis in the spinal dorsal horn locally shapes synaptic metaplasticity and modality-specific sensitization. Nat. Commun. 2020;11:3935. doi: 10.1038/s41467-020-17824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørensen A.T., Ledri M., Melis M., Ledri L.N., Andersson M., Kokaia M. Altered Chloride Homeostasis Decreases the Action Potential Threshold and Increases Hyperexcitability in Hippocampal Neurons. eNeuro. 2018;4:ENEURO.0172-17.2017. doi: 10.1523/ENEURO.0172-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staley K.J., Soldo B.L., Proctor W.R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 14.Perkins K.L., Wong R.K. Ionic basis of the postsynaptic depolarizing GABA response in hippocampal pyramidal cells. J. Neurophysiol. 1996;76:3886–3894. doi: 10.1152/jn.1996.76.6.3886. [DOI] [PubMed] [Google Scholar]
- 15.Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 16.Isomura Y., Sugimoto M., Fujiwara-Tsukamoto Y., Yamamoto-Muraki S., Yamada J., Fukuda A. Synaptically activated Cl--accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J. Neurophysiol. 2003;90:2752–2756. doi: 10.1152/jn.00142.2003. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Ari Y., Khalilov I., Kahle K.T., Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- 18.Rivera C., Voipio J., Kaila K., Voipio J., Kaila K. Two developmental switches in GABAergic signalling: The K+–Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada J., Okabe A., Toyoda H., Kilb W., Luhmann H.J., Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 2004;557:829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balena T., Woodin M.A. Coincident pre- and postsynaptic activity downregulates NKCC1 to hyperpolarize E(Cl) during development. Eur. J. Neurosci. 2008;27:2402–2412. doi: 10.1111/j.1460-9568.2008.06194.x. [DOI] [PubMed] [Google Scholar]
- 21.Ratté S., Prescott S.A. ClC-2 channels regulate neuronal excitability, not intracellular chloride levels. J. Neurosci. 2011;31:15838–15843. doi: 10.1523/JNEUROSCI.2748-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaila K., Price T.J., Payne J.A., Puskarjov M., Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyon N., Vinay L., Prescott S.A., De Koninck Y. Chloride Regulation: A Dynamic Equilibrium Crucial for Synaptic Inhibition. Neuron. 2016;89:1157–1172. doi: 10.1016/j.neuron.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M., Fukuda A. Development and regulation of chloride homeostasis in the central nervous system. Front. Cell. Neurosci. 2015;9:371. doi: 10.3389/fncel.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt T., Ghaffarian N., Philippot C., Seifert G., Steinhäuser C., Pape H.C., Blaesse P. Differential regulation of chloride homeostasis and GABAergic transmission in the thalamus. Sci. Rep. 2018;8:13929. doi: 10.1038/s41598-018-31762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieghart W., Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 27.Sigel E., Steinmann M.E. Structure, function, and modulation of GABAA receptors. J. Biol. Chem. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michels G., Moss S.J. GABAA receptors: Properties and trafficking. Crit. Rev. Biochem. Mol. Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- 29.Nutt D. GABAA Receptors: Subtypes, Regional Distribution, and Function. J. Clin. Sleep Med. 2006;2:S7–S11. doi: 10.5664/jcsm.26525. [DOI] [PubMed] [Google Scholar]
- 30.Connolly C.N., Wooltorton J.R., Smart T.G., Moss S.J. Subcellular localization of gamma-aminobutyric acid type A receptors is determined by receptor beta subunits. Proc. Natl. Acad. Sci. USA. 1996;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen Q.A., Nicoll R.A. The GABAA Receptor β Subunit Is Required for Inhibitory Transmission. Neuron. 2018;98:718–725. doi: 10.1016/j.neuron.2018.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen M.L., Timmermann D.B., Johansen T.H., Schousboe A., Varming T., Ahring P.K. The beta subunit determines the ion selectivity of the GABAA receptor. J. Biol. Chem. 2002;277:41438–41447. doi: 10.1074/jbc.M205645200. [DOI] [PubMed] [Google Scholar]
- 33.Currin C.B., Trevelyan A.J., Akerman C.J., Raimondo J.V. Chloride dynamics alter the input-output properties of neurons. PLoS Comput. Biol. 2020;16:e1007932. doi: 10.1371/journal.pcbi.1007932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen M.J., Yasuda R.P., Vicini S. GABAA Receptor β3 Subunit Expression Regulates Tonic Current in Developing Striatopallidal Medium Spiny Neurons. Front. Cell. Neurosci. 2011;5:15. doi: 10.3389/fncel.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menzikov S.A., Zaichenko D.M., Moskovtsev A.A., Morozov S.G., Kubatiev A.A. Ectopic GABAA receptor β3 subunit determines Cl−/HCO3−-ATPase and chloride transport in HEK 293FT cells. FEBS J. 2020 doi: 10.1111/febs.15359. [DOI] [PubMed] [Google Scholar]
- 36.Gerencser G.A., Zhang J. Existence and nature of the chloride pump. Biochim. Biophys. Acta. 2003;1618:133–139. doi: 10.1016/j.bbamem.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Clausen J.D., Bublitz M., Arnou B., Olesen C., Andersen J.P., Mølle J.V., Nissen P. Crystal Structure of the Vanadate-Inhibited Ca2+-ATPase. Structure. 2016;24:617–623. doi: 10.1016/j.str.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Gong P., Hong H., Perkins E.J. Ionotropic GABA receptor antagonism-induced adverse outcome pathways for potential neurotoxicity biomarkers. Biomark. Med. 2015;11:1225–1239. doi: 10.2217/bmm.15.58. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y.S., Yoon B.E. Altered GABAergic Signaling in Brain Disease at Various Stages of Life. Exp. Neurobiol. 2017;26:122–131. doi: 10.5607/en.2017.26.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braat S., Kooy R.F. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron. 2015;86:1119–1130. doi: 10.1016/j.neuron.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 41.Payne J.A. Functional characterization of the neuronal-specific K-Cl cotransporter: Implications for [K+]o regulation. Am. J. Physiol. 1997;273:1516–1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 42.Williams J.R., Sharp J.W., Kumari V.G., Wilson M., Payne J.A. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J. Biol. Chem. 1999;274:12656–12664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]
- 43.Gillen C.M., Brill S., Payne J.A., Forbush B. Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J. Biol. Chem. 1996;271:16237–16244. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- 44.Uvarov P., Ludwig A., Markkanen M., Soni S., Hübner CA., Rivera C., Airaksinen M.S. Coexpression and heteromerization of two neuronal K-Cl cotransporter isoforms in neonatal brain. J. Biol. Chem. 2009;284:13696–13704. doi: 10.1074/jbc.M807366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blaesse P., Airaksinen M.S., Rivera C., Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Payne J.A., Stevenson T.J., Donaldson L.F. Molecular Characterization of a Putative K-Cl Cotransporter in Rat Brain. J. Biol. Chem. 1996;271:16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 47.Chamma I., Chevy Q., Poncer J.C., Lévi S. Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell. Neurosci. 2012;6:5. doi: 10.3389/fncel.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakazu Y., Uchida S., Nakagawa T., Akaike N., Nabekura J. Reversibility and cation selectivity of the K+-Cl- cotransport in rat central neurons. J. Neurophysiol. 2000;84:281–288. doi: 10.1152/jn.2000.84.1.281. [DOI] [PubMed] [Google Scholar]
- 49.Williams J.R., Payne J.A. Cation transport by the neuronal K+-Cl- cotransporter KCC2: Thermodynamics and kinetics of alternate transport modes. Am. J. Physiol. Cell Physiol. 2004;287:919–931. doi: 10.1152/ajpcell.00005.2004. [DOI] [PubMed] [Google Scholar]
- 50.Zhu L., Lovinger D., Delpire E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J. Neurophysiol. 2005;93:1557–1568. doi: 10.1152/jn.00616.2004. [DOI] [PubMed] [Google Scholar]
- 51.Cordero-Erausquin M., Coull J.A., Boudreau D., Rolland M., De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: Impact of chloride extrusion capacity. J. Neurosci. 2005;25:9613–9623. doi: 10.1523/JNEUROSCI.1488-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mercado A., Broumand V., Zandi-Nejad K., Enck A.H., Mount D.B. A C-terminal domain in KCC2 confers constitutive K+-Cl- cotransport. J. Biol. Chem. 2006;281:1016–1026. doi: 10.1074/jbc.M509972200. [DOI] [PubMed] [Google Scholar]
- 53.Viitanen T., Ruusuvuori E., Kaila K., Voipio J. The K+-Cl− cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J. Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera C., Voipio J., Payne J.A., Ruusuvuori E., Lahtinen H., Lamsa K., Pirvola U., Saarma M., Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 55.DeFazio R.A., Hablitz J.J. Chloride accumulation and depletion during GABAA receptor activation in neocortex. Neuroreport. 2001;12:2537–2541. doi: 10.1097/00001756-200108080-00049. [DOI] [PubMed] [Google Scholar]
- 56.DeFazio R.A., Keros S., Quick M.W., Hablitz J.J. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera C., Voipio J., Thomas-Crusells J., Li H., Emri Z., Sipilä S., Payne J.A., Minichiello L., Saarma M., Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gulyás A.I., Sík A., Payne J.A., Kaila K., Freund T.F. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 2001;13:2205–2217. doi: 10.1046/j.0953-816x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang C., Shimizu-Okabe C., Watanabe K., Okabe A., Matsuzaki H., Ogawa T., Mori N., Fukuda A., Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res. Dev. Brain Res. 2002;139:59–66. doi: 10.1016/S0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- 60.Tillman L., Zhang J. Crossing the Chloride Channel: The Current and Potential Therapeutic Value of the Neuronal K+-Cl- Cotransporter KCC2. Biomed. Res. Int. 2019:8941046. doi: 10.1155/2019/8941046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karlsson U., Druzin M., Johansson S. Cl− concentration changes and desensitization of GABAA and glycine receptors. J. Gen. Physiol. 2011;138:609–626. doi: 10.1085/jgp.201110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M., Wang J., Jiang J., Zheng X., Justice N.J., Wang K., Ran X., Li Y., Huo Q., Zhang J., et al. APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. eLife. 2017;6:e20142. doi: 10.7554/eLife.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin X., Huguenard J.R., Prince D.A. Impaired Cl- extrusion in layer V pyramidal neurons of chronically injured epileptogenic neocortex. J. Neurophysiol. 2005;93:2117–2126. doi: 10.1152/jn.00728.2004. [DOI] [PubMed] [Google Scholar]
- 64.Hyde T.M., Lipska B.K., Ali T., Mathew S.V., Law A.J., Metitiri O.E., Straub R.E., Ye T., Colantuoni C., Herman M.M., et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Medina I., Chudotvorova I. GABA neurotransmission and neural cation-chloride co-transporters: Actions beyond ion transport. Crit. Rev. Neurobiol. 2006;18:105–112. doi: 10.1615/CritRevNeurobiol.v18.i1-2.110. [DOI] [PubMed] [Google Scholar]
- 66.Achilles K., Okabe A., Ikeda M., Shimizu-Okabe C., Yamada J., Fukuda A., Luhmann H.J., Kilb W. Kinetic properties of Cl- uptake mediated by Na+-dependent K+-2Cl- cotransport in immature rat neocortical neurons. J. Neurosci. 2007;27:8616–8627. doi: 10.1523/JNEUROSCI.5041-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isenring P., Jacoby S.C., Payne J.A., Forbush B. Comparison of Na-K-Cl cotransporters. NKCC1, NKCC2, and the HEK cell Na-K-Cl cotransporter. J. Biol. Chem. 1998;273:11295–11301. doi: 10.1074/jbc.273.18.11295. [DOI] [PubMed] [Google Scholar]
- 68.Sun D., Murali S.G. Stimulation of Na+-K+-2Cl- cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am. J. Physiol. 1998;275:772–779. doi: 10.1152/ajpcell.1998.275.3.C772. [DOI] [PubMed] [Google Scholar]
- 69.Pond B.B., Berglund K., Kuner T., Feng G., Augustine G.J., Schwartz-Bloom R.D. The chloride transporter Na+-K+-Cl- cotransporter isoform-1 contributes to intracellular chloride increases after in vitro ischemia. J. Neurosci. 2006;26:1396–1406. doi: 10.1523/JNEUROSCI.1421-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hannaert P., Alvarez-Guerra M., Pirot D., Nazaret C., Garay R. Rat NKCC2/NKCC1 cotransporter selectivity for loop diuretic drugs. Naunyn-Schmiedebergs Arch. Pharmacol. 2002;365:193–199. doi: 10.1007/s00210-001-0521-y. [DOI] [PubMed] [Google Scholar]
- 71.Brumback A.C., Staley K.J. Thermodynamic regulation of NKCC1-mediated Cl- cotransport underlies plasticity of GABAA signaling in neonatal neurons. J. Neurosci. 2008;28:1301–1312. doi: 10.1523/JNEUROSCI.3378-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez-Islas C., Chub N., Wenner P. NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. J. Neurophysiol. 2009;101:507–518. doi: 10.1152/jn.90986.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaneko H., Putzier I., Frings S., Kaupp U.B., Gensch T. Chloride Accumulation in Mammalian Olfactory Sensory Neurons. J. Neurosci. 2004;24:7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nickell W.T., Kleene N.K., Kleene S.J. Mechanisms of neuronal chloride accumulation in intact mouse olfactory epithelium. J. Physiol. 2007;583:1005–1020. doi: 10.1113/jphysiol.2007.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plotkin M.D., Snyder E.Y., Hebert S.C., Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 1997;33:781–795. doi: 10.1002/(SICI)1097-4695(19971120)33:6<781::AID-NEU6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 76.Nardou R., Yamamoto S., Chazal G., Bhar A., Ferrand N., Dulac O., Ben-Ari Y., Khalilov I. Neuronal chloride accumulation and excitatory GABA underlie aggravation of neonatal epileptiform activities by phenobarbital. Brain. 2011;134:987–1002. doi: 10.1093/brain/awr041. [DOI] [PubMed] [Google Scholar]
- 77.Nardou R., Ben-Ari Y., Khalilov I. Bumetanide, an NKCC1 antagonist, does not prevent formation of epileptogenic focus but blocks epileptic focus seizures in immature rat hippocampus. J. Neurophysiol. 2009;101:2878–2888. doi: 10.1152/jn.90761.2008. [DOI] [PubMed] [Google Scholar]
- 78.Dzhala V.I., Kuchibhotla K.V., Glykys J.C., Kahle K.T., Swiercz W.B., Feng G., Kuner T., Augustine G.J., Bacskai B.J., Staley K.J. Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J. Neurosci. 2010;30:11745–11761. doi: 10.1523/JNEUROSCI.1769-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazarati A., Shin D., Sankar R. Bumetanide inhibits rapid kindling in neonatal rats. Epilepsia. 2009;50:2117–2122. doi: 10.1111/j.1528-1167.2009.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rheims S., Ryvlin P., Scherer C., Minotti L., Hoffmann D., Guenot M., Mauguière F., Benabid AL., Kahane P. Analysis of clinical patterns and underlying epileptogenic zones of hypermotor seizures. Epilepsia. 2008;49:2030–2040. doi: 10.1111/j.1528-1167.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 81.Kilb W., Sinning A., Luhmann H.J. Model-specific effects of bumetanide on epileptiform activity in the in-vitro intact hippocampus of the newborn mouse. Neuropharmacology. 2007;53:524–533. doi: 10.1016/j.neuropharm.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 82.Kühlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 83.Fatima-Shad K., Barry P.H. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proc. Biol. Sci. 1993;253:69–75. doi: 10.1098/rspb.1993.0083. [DOI] [PubMed] [Google Scholar]
- 84.Bormann J., Hamill O.P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and -aminobutyric acid in mouse cultured spinal neurons. J. Physiol. Lond. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li G., Yang K., Zheng C., Liu Q., Chang Y., Kerrigan J.F., Wu J. Functional rundown of gamma-aminobutyric acid (A) receptors in human hypothalamic hamartomas. Ann. Neurol. 2011;69:664–672. doi: 10.1002/ana.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu J., Wang Y.T. Allosteric modulation of GABAA receptors by extracellular ATP. Mol. Brain. 2014;24:6. doi: 10.1186/1756-6606-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stelzer A., Kay A.R., Wong R.K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988;241:339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- 88.Rapallino M.V., Cupello A., Hydén H. An electrogenic ionic pump derived from an ionotropic receptor: Assessment of a candidate. Cell Mol. Neurobiol. 1999;19:681–690. doi: 10.1023/A:1006944820946. [DOI] [PubMed] [Google Scholar]
- 89.Cupello A. Neuronal transmembrane chloride electrochemical gradient: A key player in GABAA receptor activation physiological effect. Amino Acids. 2003;24:335–346. doi: 10.1007/s00726-002-0350-4. [DOI] [PubMed] [Google Scholar]
- 90.Menzikov S.A., Morozov S.G. Involvement of brain GABAAR-coupled Cl-/HCO3--ATPase in phenol-induced the head-twitching and tremor responses in rats. Neurotoxicology. 2019;71:122–131. doi: 10.1016/j.neuro.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Menzikov S. Dual character of GABA action on Cl--transport by the reconstituted Cl-/HCO3--ATPase from rat brain. J. Recept. Signal Transduct. Res. 2018;38:307–310. doi: 10.1080/10799893.2018.1494741. [DOI] [PubMed] [Google Scholar]
- 92.Menzikov S.A. Neuronal multifunctional ATPase. Biophys. Rev. Lett. 2013;8:213–227. doi: 10.1142/S1793048013300065. [DOI] [Google Scholar]
- 93.Menzikov S. Biochemical properties of the sensitivity to GABAAergic ligands, Cl-/HCO3--ATPase isolated from fish (Cyprinus carpio) olfactory mucosa and brain. Fish Physiol. Biochem. 2018;44:583–597. doi: 10.1007/s10695-017-0455-z. [DOI] [PubMed] [Google Scholar]
- 94.Menzikov S., Menzikova O. Interaction of pentobarbital with GABAergic drugs acting on the Cl--ATPase activity of the plasma membranes from bream brain (Abramis brama L.) Neurosci. Lett. 2002;334:161–164. doi: 10.1016/S0304-3940(02)01076-5. [DOI] [PubMed] [Google Scholar]
- 95.Zezula J., Slany A., Sieghart W. Interaction of allosteric ligands with GABAA receptors containing one, two, or three different subunits. Eur. J. Pharm. 1996;301:207–214. doi: 10.1016/0014-2999(96)00066-0. [DOI] [PubMed] [Google Scholar]
- 96.Davies P.A., Kirkness E.F., Hales T.G. Modulation by general anaesthetics of rat GABAA receptors comprised of α1β3 and β3 subunits expressed in human embryonic kidney 293 cells. Br. J. Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fisher J.L., Macdonald R.L. Single channel properties of recombinant GABAA receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse L929 cells. J. Physiol. 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor P.M., Thomas P., Gorrie G.H., Connolly C.N., Smart T.G., Moss S.J. Identification of amino acid residues within GABAA receptor beta subunits that mediate both homomeric and heteromeric receptor expression. J. Neurosci. 1999;19:6360–6371. doi: 10.1523/JNEUROSCI.19-15-06360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hannan S., Smart T.G. Cell surface expression of homomeric GABAA receptors depends on single residues in subunit transmembrane domains. J. Biol. Chem. 2018;293:13427–13439. doi: 10.1074/jbc.RA118.002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller P.S., Aricescu A.R. Crystal structure of a human GABAA receptor. Nature. 2014;512:270–275. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ratra G.S., Kamita S.G., Casida J.E. Role of human GABAA receptor beta3 subunit in insecticide toxicity. Toxicol. Appl. Pharmacol. 2001;172:233–240. doi: 10.1006/taap.2001.9154. [DOI] [PubMed] [Google Scholar]
- 102.Nakamura Y., Darnieder L.M., Deeb T.Z., Moss S.J. Regulation of GABAARs by phosphorylation. Adv. Pharmacol. 2015;72:97–146. doi: 10.1016/bs.apha.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Houston C.M., Hosie A.M., Smart T.G. Distinct regulation of beta2 and beta3 subunit-containing cerebellar synaptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 2008;28:7574–7584. doi: 10.1523/JNEUROSCI.5531-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bollan K., King D., Robertson L.A., Brown K., Taylor P.M., Moss S.J., Connolly C.N. GABAA receptor composition is determined by distinct assembly signals within alpha and beta subunits. J. Biol. Chem. 2003;278:4747–4755. doi: 10.1074/jbc.M210229200. [DOI] [PubMed] [Google Scholar]
- 105.Gingrich K.J., Roberts W.A., Kass R.S. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: Implications for structure-function relations and synaptic transmission. J. Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramadan E., Fu Z., Losi G., Homanics G.E., Neale J.H., Vicini S. GABAA receptor beta3 subunit deletion decreases alpha2/3 subunits and IPSC duration. J. Neurophysiol. 2003;89:128–134. doi: 10.1152/jn.00700.2002. [DOI] [PubMed] [Google Scholar]
- 107.Chiodi C.G., Baptista-Hon D.T., Hunter W.N., Hales T.G. Amino acid substitutions in the human homomeric β3 GABAA receptor that enable activation by GABA. J. Biol. Chem. 2019;294:2375–2385. doi: 10.1074/jbc.RA118.006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaila K., Ruusuvuori E., Seja P., Voipio J., Puskarjov M. GABA actions and ionic plasticity in epilepsy. Curr. Opin. Neurobiol. 2014;26:34–41. doi: 10.1016/j.conb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 109.Gurba K.N., Hernandez C.C., Hu N., Macdonald R.L. GABRB3 mutation, G32R, associated with childhood absence epilepsy alters α1β3γ2L γ-aminobutyric acid type A (GABAA) receptor expression and channel gating. J. Biol. Chem. 2012;287:12083–12097. doi: 10.1074/jbc.M111.332528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Absalom N.L., Ahring P.K., Liao V.W., Balle T., Jiang T., Anderson L.L., Arnold J.C., McGregor I.S., Bowen M.T., Chebib M. Functional genomics of epilepsy-associated mutations in the GABAA receptor subunits reveal that one mutation impairs function and two are catastrophic. J. Biol. Chem. 2019;294:6157–6171. doi: 10.1074/jbc.RA118.005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janve V.S., Hernandez C.C., Verdier K.M., Hu N., Macdonald R.L. Epileptic encephalopathy de novo GABRB mutations impair γ-aminobutyric acid type A receptor function. Ann. Neurol. 2016;79:806–825. doi: 10.1002/ana.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Møller R.S., Wuttke T.V., Helbig I., Marini C., Johannesen K.M., Brilstra E.H., Vaher U., Borggraefe I., Talvik I., Talvik T., et al. Mutations in GABRB3: From febrile seizures to epileptic encephalopathies. Neurology. 2017;88:483–492. doi: 10.1212/WNL.0000000000003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Epi4K Consortium. Epilepsy Phenome/Genome Project. Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E., Epstein M.P., Glauser T., et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Macdonald R.L., Kang J.Q., Gallagher M.J. Mutations in GABAA receptor subunits associated with genetic epilepsies. J. Physiol. 2010;588:1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hernandez C.C., Zhang Y., Hu N., Shen D., Shen W., Liu X., Kong W., Jiang Y., Macdonald R.L. GABAA Receptor Coupling Junction and Pore GABRB3 Mutations are Linked to Early-Onset Epileptic Encephalopathy. Sci. Rep. 2017;7:15903. doi: 10.1038/s41598-017-16010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Papandreou A., McTague A., Trump N., Ambegaonkar G., Ngoh A., Meyer E., Scott R.H., Kurian M.A. GABRB3 mutations: A new and emerging cause of early infantile epileptic encephalopathy. Dev. Med. Child. Neurol. 2016;58:416–420. doi: 10.1111/dmcn.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pirker S., Schwarzer C., Czech T., Baumgartner C., Pockberger H., Maier H., Hauer B., Sieghart W., Furtinger S., Sperk G. Increased Expression of GABAA Receptor β-Subunits in the Hippocampus of Patients with Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2003;62:820–834. doi: 10.1093/jnen/62.8.820. [DOI] [PubMed] [Google Scholar]
- 118.Tanaka M., Olsen R.W., Medina M.T., Schwartz E., Alonso M.E., Duron R.M., Castro-Ortega R., Martinez-Juarez I.E., Pascual-Castroviejo I., Machado-Salas J., et al. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am. J. Hum. Genet. 2008;82:1249–1261. doi: 10.1016/j.ajhg.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hörtnagl H., Tasan R.O., Wieselthaler A., Kirchmair E., Sieghart W., Sperk G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience. 2013;236:345–372. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Homanics G.E., DeLorey T.M., Firestone L.L., Quinlan J.J., Handforth A., Harrison N.L., Krasowski M.D., Rick C.E., Korpi E.R., Mäkelä R., et al. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc. Natl. Acad. Sci. USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moghbelinejad S., Rashvand Z., Khodabandehloo F., Mohammadi G., Nassiri-Asl M. Modulation of the Expression of the GABAA Receptor β1 and β3 Subunits by Pretreatment with Quercetin in the KA Model of Epilepsy in Mice: -The Effect of Quercetin on GABAA Receptor Beta Subunits. J. Pharmacopunct. 2016;19:163–166. doi: 10.3831/KPI.2016.19.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khom S., Hintersteiner J., Luger D., Haider M., Pototschnig G., Mihovilovic M.D., Schwarzer C., Hering S. Analysis of β-Subunit-dependent GABAA Receptor Modulation and Behavioral Effects of Valerenic Acid Derivatives. J. Pharmacol. Exp Ther. 2016;357:580–590. doi: 10.1124/jpet.116.232983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khazipov R., Valeeva G., Khalilov I. Depolarizing GABA and developmental epilepsies. CNS Neurosci. Ther. 2015;21:83–91. doi: 10.1111/cns.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sperk G. Changes in GABAA receptors in status epilepticus. Epilepsia. 2007;48:2382. doi: 10.1111/j.1528-1167.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.American Psychiatric Association . Diagnostic and Statistical Mannal of Mental Disorders: DSM-V. American Psychiatric Association; Arlington, TX, USA: 2013. [Google Scholar]
- 126.Sesarini C.V. GABAergic neurotransmission alterations in autism spectrum disorders. Neurotransmitter. 2015;2:e1052. [Google Scholar]
- 127.Rubenstein J.L., Merzenich M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cellot G., Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front. Pediatr. 2014;2:70. doi: 10.3389/fped.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pizzarelli R., Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011:297153. doi: 10.1155/2011/297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.DeLorey T.M. GABRB3 gene deficient mice: A potential model of autism spectrum disorder. Int. Rev. Neurobiol. 2005;71:359–382. doi: 10.1016/s0074-7742(05)71015-1. [DOI] [PubMed] [Google Scholar]
- 131.Delahanty R., Kang J., Brune C., Kistner E.O., Courchesne E., Cox N.J., Cook E.H., Jr., Macdonald R.L., Sutcliffe J.S. Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism. Mol. Psychiatry. 2011;16:86–96. doi: 10.1038/mp.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang S., Guo X., Dong X., Han Y., Gao L., Su Y., Dai W., Zhang X. GABAA receptor subunit gene polymorphisms predict symptom-based and developmental deficits in Chinese Han children and adolescents with autistic spectrum disorders. Sci. Rep. 2017;7:3290. doi: 10.1038/s41598-017-03666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Collins A.L., Ma D., Whitehead P.L., Martin E.R., Wright H.H., Abramson R.K., Hussman J.P., Haines J.L., Cuccaro M.L., Gilbert J.R., et al. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Provenzano G., Gilardoni A., Maggia M., Pernigo M., Sgadò P., Casarosa S., Bozzi Y. Altered Expression of GABAergic Markers in the Forebrain of Young and Adult Engrailed-2 Knockout Mice. Genes (Basel) 2020;11:384. doi: 10.3390/genes11040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rissman R.A., Mobley W.C. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer’s disease. J. Neurochem. 2011;117:613–622. doi: 10.1111/j.1471-4159.2011.07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Y., Sun H., Chen Z., Xu H., Bu G., Zheng H. Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Front. Aging Neurosci. 2016;8:31. doi: 10.3389/fnagi.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kwakowsky A., Calvo-Flores G.B., Govindpani K., Waldvogel H.J., Faull R.L. Gamma-aminobutyric acid A receptors in Alzheimer’s disease: Highly localized remodeling of a complex and diverse signaling pathway. Neural Regen. Res. 2018;13:1362–1363. doi: 10.4103/1673-5374.235240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kwakowsky A., Calvo-Flores Guzmán B., Pandya M., Turner C., Waldvogel H.J., Faull R.L. GABAA receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018;145:374–392. doi: 10.1111/jnc.14325. [DOI] [PubMed] [Google Scholar]
- 139.Limon A., Reyes-Ruiz J.M., Miledi R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. USA. 2012;109:10071–10076. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Błaszczyk J.W. Parkinson’s Disease and Neurodegeneration: GABA-Collapse Hypothesis. Front. Neurosci. 2016;10:269. doi: 10.3389/fnins.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gironell A. The GABA Hypothesis in Essential Tremor: Lights and Shadows. Tremor. Other. Hyperkinet. Mov. (NY) 2014;4:254. doi: 10.5334/tohm.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Calon F., Morissette M., Rajput A.H., Hornykiewicz O., Bédard P.J., Di Paolo T. Changes of GABA receptors and dopamine turnover in the postmortem brains of parkinsonians with levodopa-induced motor complications. Mov. Disord. 2003;18:241–253. doi: 10.1002/mds.10343. [DOI] [PubMed] [Google Scholar]
- 143.van Nuland A.J.M., den Ouden H.E.M., Zach H., Dirkx M.F.M., van Asten J.J.A., Scheenen T.W.J., Toni I., Cools R., Helmich R.C. GABAergic changes in the thalamocortical circuit in Parkinson’s disease. Hum. Brain Mapp. 2020;41:1017–1029. doi: 10.1002/hbm.24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daniele A., Panza F., Greco A., Logroscino G., Seripa D. Can a Positive Allosteric Modulation of GABAergic Receptors Improve Motor Symptoms in Patients with Parkinson’s Disease? The Potential Role of Zolpidem in the Treatment of Parkinson’s Disease. Parkinsons Dis. 2016:2531812. doi: 10.1155/2016/2531812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paris-Robidas S., Brochu E., Sintes M., Emond V., Bousquet M., Vandal M., Pilote M., Tremblay C., Di Paolo T., Rajput A.H., et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135:105–116. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 146.Mograbi K.M., de Castro A.C., de Oliveira J.A., Sales P.J., Covolan L., Del Bel E.A., de Souza A.S. Effects of GABAa receptor antagonists on motor behavior in pharmacological Parkinson’s disease model in mice. Physiol. Rep. 2017;5:e13081. doi: 10.14814/phy2.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoerbelt P., Lindsley T.A., Fleck M.W. Dopamine directly modulates GABAA receptors. J. Neurosci. 2015;35:3525–3536. doi: 10.1523/JNEUROSCI.4390-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]