Abstract
Simple Summary
Over the last decade, immune checkpoint inhibitors (ICIs) targeting programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) have dramatically changed the therapeutic algorithm of several hematological and solid tumors. Of note, these agents have been also investigated in biliary tract cancer (BTC), reporting controversial results so far; in this setting, the role of ICIs is still to be established, and available data on immunotherapy in BTC patients are mainly limited to sub-analyses of basket trials and small single-arm studies. A crucial challenge is represented by the lack of validated predictive biomarkers, that could help identify responders to immunotherapy, a high unmet need in these immunologically “cold” malignancies where ICIs are still looking for their niche.
Abstract
Biliary tract cancer (BTC) represents the second most frequently diagnosed primary liver cancer worldwide following hepatocellular carcinoma, and the overall survival of patients with unresectable disease remains poor. In recent years, the advent of immune checkpoint inhibitors (ICIs) has revolutionized the therapeutic landscape of several malignancies with these agents, which have also been explored in advanced BTC, as monotherapy or in combination with other anticancer agents. However, clinical trials evaluating ICIs in BTC have shown conflicting results, and the clinical benefit provided by immunotherapy seems limited to a small subgroup of BTC patients. Thus, the identification of reliable predictors of the response to immunotherapy represents a significant challenge in this setting. This review provides an overview of the available evidence on the biomarkers predictive of the response to ICIs in patients with advanced BTC, especially focusing on programmed death-ligand 1 (PD-L1), tumor mutational burden (TMB), microsatellite instability (MSI), and other emerging biomarkers.
Keywords: predictive biomarkers, PD-L1, TMB, immunotherapy, immune checkpoint inhibitors, biliary tract cancer, cholangiocarcinoma
1. Introduction
Biliary tract cancers (BTCs) encompass a group of aggressive, rare, and heterogeneous tumors arising in the bile duct system, comprising gallbladder cancer (GBC), ampulla of Vater cancer (AVC), and cholangiocarcinoma (CCA) [1,2]. CCA is classically divided into extrahepatic cholangiocarcinoma (eCCA), originating outside the liver and further subclassified into distal (dCCA) and perihilar cholangiocarcinoma (pCCA), and intrahepatic cholangiocarcinoma (iCCA), occurring within the liver parenchyma [3,4]. Of note, this classification—based on the anatomical location of BTCs within the biliary tree—mirrors remarkable differences in terms of tumor biology, molecular features, epidemiology, prognosis, and therapeutic approaches [5].
BTC represents the second most frequent hepatobiliary tumor following hepatocellular carcinoma (HCC), accounting for approximately 3% of all gastrointestinal malignancies worldwide [6]. Although BTCs have been traditionally considered rare tumors, their overall incidence has seen a remarkable increase over recent decades in most Western countries [7]. Radical surgery remains the only potentially curative treatment option, but unfortunately, most patients with BTC are diagnosed with advanced disease; moreover, a non-negligible proportion of BTCs initially considered resectable are subsequently found to be unresectable during exploratory laparotomy [8,9]. Additionally, even following surgical resection with negative tumor margins, distant and locoregional recurrence rates are high. In BTC patients with metastatic disease, systemic treatments represent the only potential therapeutic option. More than ten years after the publication of the landmark ABC-02 phase III trial, the combination of gemcitabine plus cisplatin remains the current standard of care in treatment-naïve patients [10,11]. According to the results of this study, the gemcitabine–cisplatin combination showed superior median overall survival (OS) compared to gemcitabine monotherapy (11.7 months versus 8.2 months, respectively; hazard ratio (HR), 0.64; 95% confidence interval (CI), 0.52–0.80; p < 0.001), with the ABC-02 establishing gemcitabine–cisplatin as the reference doublet [10,11]. Nonetheless, the limited survival benefit provided by systemic treatments has highlighted the need for more effective medical therapies in this setting [12].
The last decade has registered important advances in the understanding of the tumor biology of BTCs, as witnessed by the parallel development of novel treatment options and genomic sequencing, which has paved the way toward the identification of several possible therapeutic targets [13,14,15]. In fact, molecularly targeted therapies have been tested in BTC patients harboring specific druggable alterations, especially in iCCAs where agents targeting isocitrate dehydrogenase (IDH) mutations and fibroblast growth factor receptor (FGFR) aberrations have entered into clinical practice [16,17,18,19,20,21,22]; in addition, following the results observed in several hematological and solid malignancies, immune checkpoint inhibitors (ICIs) have been explored and are currently being investigated in BTC (Table 1) [23,24,25]. However, most BTC patients receiving ICIs as a monotherapy or in combination with other anticancer agents do not achieve response, and the mechanisms behind the variations in the response to immunotherapy in this setting have been poorly studied [26]. Based on these premises, the identification of biomarkers able to predict responses to ICIs and the understanding of resistance mechanisms in non-responders represent high unmet needs.
Table 1.
Ongoing phase I to III clinical trials evaluating immune checkpoint inhibitors in biliary tract cancer patients with advanced disease.
| NCT Name | Phase | Setting (Type of BTC) | Arm A | Arm B | Agents Description | Primary Outcomes | Estimated Enrollment |
|---|---|---|---|---|---|---|---|
| NCT04066491 | II/III | First-line (iCCA, eCCA, GBC) | Bintrafusp alfa (M7824) plus CisGem | Placebo plus CisGem | Bintrafusp alfa: first-in-class bifunctional fusion protein composed by a PD-L1 antibody fused with 2 extracellular domains of TGF-β receptor | DLTs OS |
512 |
|
NCT03875235 (TOPAZ-1) |
III | First-line (iCCA, eCCA, GBC) | Durvalumab plus CisGem | Placebo plus CisGem | Durvalumab: PD-L1 inhibitor | OS | 757 |
| NCT03260712 | II | First-line (iCCA, eCCA, GBC) | Pembrolizumab plus CisGem | Pembrolizumab: PD-1 antibody | PFS at 6 months | 50 | |
| NCT04300959 | II | First-line (iCCA, eCCA, GBC) | Anlotinib plus sintilimab plus CisGem | CisGem | Anlotinib: TKI inhibiting PDGFR, FGFR, VEGFR and c-KIT kinase Sintilimab: PD-1 antibody |
12-month OS rate | 80 |
| NCT03046862 | II | First-line (AVC, iCCA, eCCA, GBC) | Durvalumab plus tremelimumab plus CisGem | Durvalumab: PD-L1 inhibitor Tremelimumab: anti-CTLA-4 agent |
ORR | 31 | |
| NCT03796429 | II | First-line (iCCA, eCCA, GBC) | Toripalimab plus S-1 plus gemcitabine | Toripalimab: PD-1 antibody | PFS OS |
40 | |
| NCT04172402 | II | First-line (AVC, iCCA, eCCA, GBC) | Nivolumab plus S-1 plus gemcitabine | Nivolumab: PD-1 antibody | ORR | 48 | |
| NCT04027764 | II | First-line (iCCA, eCCA, GBC) | Toripalimab plus S-1 plus albumin paclitaxel | Toripalimab: PD-1 antibody | ORR | 30 | |
| NCT03478488 | III | First-line (iCCA, eCCA, GBC) | KN035 plus GEMOX | GEMOX | KN035: PD-L1 inhibitor | OS | 390 |
| NCT04191343 | II | First-line (iCCA, eCCA, GBC) | Toripalimab plus GEMOX | Toripalimab: PD-1 antibody | ORR | 20 | |
|
NCT04003636 (KEYNOTE-966) |
III | First-line (iCCA, eCCA, GBC) | Pembrolizumab plus CisGem | Placebo plus CisGem | Pembrolizumab: PD-1 antibody | PFS OS |
788 |
| NCT03937895 | I/IIA | First- or later-line (iCCA, eCCA, GBC) | Pembrolizumab plus allogenic NK cell (SMT-NK) | Pembrolizumab: PD-1 antibody SMT-NK: allogenic natural killer cell |
DLTs ORR |
40 | |
| NCT03639935 | II | Maintenance after platinum-based first-line chemotherapy (iCCA, eCCA, GBC) | Nivolumab plus rucaparib | Nivolumab: PD-1 antibody Rucaparib: PARP inhibitor |
Proportion of patients alive and without radiological or clinical progression at 4 months | 35 | |
| NCT03785873 | Ib/II | Second-line (iCCA, eCCA, GBC) | Nivolumab plus 5-FU plus NalIri | Nivolumab: PD-1 antibody | DLTs PFS |
40 | |
| NCT04298021 | II | Second-line (AVC, iCCA, eCCA, GBC) | AZD6738 (ceralasertib) plus durvalumab | AZD6738 plus olaparib | AZD6738: ATR and ATM inhibitor Durvalumab: PD-L1 inhibitor Olaparib: PARP inhibitor |
DCR | 74 |
| NCT03110328 | II | Second-line (iCCA, eCCA) | Pembrolizumab | Pembrolizumab: PD-1 antibody | PFS OS Best overall response |
33 | |
| NCT04211168 | II | Second-line (iCCA, eCCA, GBC) | Toripalimab plus lenvatinib | Toripalimab: PD-1 antibody Lenvatinib: TKI |
ORR AEs |
44 | |
| NCT03797326 | II | Second-line (AVC, iCCA, eCCA, GBC) | Pembrolizumab plus lenvatinib | Pembrolizumab: PD-1 antibody Lenvatinib: TKI |
ORR AEs |
600 | |
| NCT04010071 | II | Second-line (AVC, iCCA, eCCA, GBC) | Toripalimab plus axitinib | Toripalimab: PD-1 antibody Axitinib: TKI |
ORR PFS |
60 | |
|
NCT03704480 (IMMUNO-BIL) |
II | Second-line (iCCA, eCCA, GBC) | Durvalumab plus tremelimumab | Durvalumab plus tremelimumab plus paclitaxel | Durvalumab: PD-L1 inhibitor Tremelimumab: anti-CTLA-4 agent |
PFS | 102 |
| NCT03999658 | II | Second- or later-line (AVC, iCCA, eCCA, GBC) | STI-3031 | STI-3031: PD-L1 inhibitor | ORR | 220 | |
| NCT03475953 | I/II | Second- or later-line (AVC, iCCA, eCCA, GBC) | Avelumab plus regorafenib | Avelumab: PD-L1 inhibitor Regorafenib: TKI |
RP2D | 362 | |
| NCT03801083 | II | Second- or later-line (AVC, iCCA, eCCA, GBC) | TILs | TILs: Tumor-Infiltrating Lymphocytes | ORR | 59 | |
| NCT04057365 | II | Second- or later-line (iCCA, eCCA, GBC) | Nivolumab plus DKN-01 | Nivolumab: PD-1 antibody DKN-01: humanized monoclonal antibody against the DKK1 protein |
ORR | 30 | |
| NCT04298008 | II | Third-line (AVC, iCCA, eCCA, GBC) | AZD6738 (ceralasertib) plus durvalumab | AZD6738: ATR and ATM inhibitor Durvalumab: PD-L1 inhibitor |
DCR | 26 |
This table includes ongoing clinical trials assessing immunotherapy as first-, second-, or later-line treatment. 5-FU: 5-fluorouracil; AEs, adverse events; ATM, ataxia-telangiectasia mutation; AVC, ampulla of Vater cancer; BTC, biliary tract cancer; CisGem, cisplatin plus gemcitabine combination; CTLA-4, cytotoxic T-lymphocyte antigen 4; DCR: disease control rate; DLTs, dose-limiting toxicities; eCCA: extrahepatic cholangiocarcinoma; FGFR, fibroblast growth factor receptor; GBC, gallbladder cancer; GEMOX, gemcitabine plus oxaliplatin; iCCA, intrahepatic cholangiocarcinoma; ORR, overall response rate; OS, overall survival; PARP, poly ADP ribose polymerase; PDGFR, platelet-derived growth factor receptor; PD-1, programmed death 1, PFS, progression-free survival; RP2D, recommended phase II dose; S-1: tegafur/gimeracil/oteracil; TILs: tumor infiltrating lymphocytes; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor.
Herein, we provide an overview on the current knowledge regarding the predictive biomarkers of the response to ICIs in advanced BTCs, especially focusing on the role of programmed death-ligand 1 (PD-L1) expression, tumor mutational burden (TMB), mismatch repair deficiency (dMMR), high microsatellite instability (MSI-H), and DNA damage repair (DDR) gene mutations in this setting.
We performed research using PubMed/Medline, Cochrane Library, and Scopus with the keywords “biliary tract cancer” OR “cholangiocarcinoma” OR “intrahepatic cholangiocarcinoma” OR “extrahepatic cholangiocarcinoma” OR “gallbladder cancer” AND “immunotherapy” OR “immune checkpoint inhibitors” AND “PD-L1” OR “tumor mutational burden” OR “TMB” OR “MSI” OR “DDR” OR “DNA damage repair” OR “tumor microenvironment.” We selected pivotal registration studies. We also selected the most relevant and pertinent studies considering the quality of the studies in terms of their applicability, how they were conducted, statistical analysis, number of patients enrolled, and outcomes. For ongoing clinical trials, we searched in the clinicaltrials.gov database for currently recruiting and active trials, not simply recruiting trials, using the following keywords: “biliary tract cancer” OR “cholangiocarcinoma” OR “intrahepatic cholangiocarcinoma” OR “extrahepatic cholangiocarcinoma” OR “gallbladder cancer” AND “immunotherapy” OR “immune checkpoint inhibitors.” We restricted our research to phase I, II, or III trials focused on the metastatic/advanced setting.
2. PD-L1 Expression
The expression of PD-L1 assessed by immunohistochemistry has been shown to correlate with response to ICIs in several tumor types, including non-small cell lung cancer (NSCLC), gastric cancer, and urothelial carcinoma [27,28,29]. However, few data are available in BTC patients treated with ICIs so far. According to previous reports, PD-L1 expression has been reported to range from approximately 45% to 65% of immune cells within the tumor microenvironment and from 10% to 70% of tumor specimens [30,31]; in addition, PD-L1 expression by both intra-tumoral inflammatory or neoplastic cells has been related to tumor aggressiveness and worse survival [32,33]. First, Gani and colleagues evaluated the association between clinical outcomes and PD-L1 expression in 54 iCCA tumor samples, with PD-L1 assessed within the tumor front (TF) and in tumor-associated macrophages (TAMs) [34]. Of note, iCCAs expressing PD-L1 in the TF had a 60% reduced survival compared to PD-L1-negative patients [34]. Similar results were mirrored in more recent studies on other BTC subtypes, including pCCA and dCCA [30,31,32,33].
Regarding the predictive value of PD-L1 in this setting, interesting data may be extracted by the subgroup analyses of clinical trials assessing ICIs in advanced BTCs [35]. Among these, the KEYNOTE-028 phase Ib trial (Table 2) exclusively enrolled PD-L1-positive patients, with at least a 1% modified proportion score or interface pattern, which were treated with 10 mg/kg of pembrolizumab every two weeks [36]. According to the results of this study, after a median follow-up of 7.5 months, the overall response rate (ORR) was 13.0% in 23 previously treated BTC patients, with a median progression-free survival (PFS) and an overall survival (OS) of 1.8 and 5.7 months, respectively [36]. Similarly, the KEYNOTE-158 phase II trial investigated the role of 200 mg of pembrolizumab every three weeks in pretreated BTC patients with advanced disease [36]. At a median follow-up of 5.7 months in the overall population, a disappointing ORR of 5.8% was detected, with median PFS and OS of 2.0 and 7.4 months, respectively [36]. In a subgroup analysis especially focused on PD-L1-positive (n = 61) and PD-L1-negative (n = 34) BTC patients, the ORR was 6.6% in the first group and 2.9% in PD-L1-nonexpressers [36].
Table 2.
Reported outcomes of single-agent immune checkpoint inhibitors in advanced biliary tract cancer (BTC).
| Phase | Setting, (Type of BTC) | ICI | Agents Description | Number of Patients | Outcomes |
|---|---|---|---|---|---|
| Ib [36] | Second- or later-line (iCCA, eCCA, GBC) | Pembrolizumab | Pembrolizumab: PD-1 inhibitor | 24 (all patients had PD-L1 ≥1%) | mPFS 1.8 months |
| mOS 5.7 months | |||||
| ORR 13% | |||||
| SD rate 17% | |||||
| II [36] | Second- or later-line (iCCA, eCCA, GBC) | Pembrolizumab | Pembrolizumab: PD-1 inhibitor | 104 (61 patients had PD-L1 ≥1%) | mPFS 2.0 months |
| mOS 7.4 months | |||||
| ORR 5.8% (6.6% in PD-L1+; 2.9% in PD-L1-) | |||||
| II [40] | Second- or later-line (iCCA, eCCA, GBC) | Nivolumab | Nivolumab: PD-1 inhibitor | 30 | mPFS 1.4 months |
| mOS 5.2 months | |||||
| PR rate 3% | |||||
| II [37] | Second- or later-line (iCCA, eCCA, GBC) | Nivolumab | Nivolumab: PD-1 inhibitor | 54 | mPFS 3.7 months |
| mOS 14.2 months | |||||
| ORR 22% | |||||
| DCR 50% | |||||
| II [41] | Second- or later-line (iCCA, eCCA, GBC) | Durvalumab | Durvalumab: PD-L1 inhibitor | 42 | mPFS 1.5 months |
| mOS 8.1 months | |||||
| PR rate 4.8% | |||||
| I [42] | Second- or later-line (iCCA, eCCA, GBC) | M7824 | M7824: PD-L1 inhibitor | 30 | mOS 12.7 months |
| ORR 20% |
AVC, ampulla of Vater cancer; DCR, disease control rate; eCCA, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; iCCA, intrahepatic cholangiocarcinoma; m, median; ORR, overall response rate; OS, overall survival; PD-1, programmed death 1; PFS, progression-free survival; PR, partial response; SD, stable disease.
Another PD-1 inhibitor, nivolumab, was evaluated as a second-line treatment in 54 BTC patients with advanced disease in a recently published phase II trial [37]. In this study conducted by Kim and colleagues, the median PFS and OS in the overall population were 3.7 and 14.2 months, respectively, with an ORR of 22% and disease control rate (DCR) of 59% (Table 2). In 18 PD-L1-positive patients (≥1% of tumor cells expressing PD-L1 as a cutoff), a statistically significantly superior median PFS was observed compared to PD-L1-negative BTCs (10.4 months versus 2.3 months; HR, 0.23; 95% CI, 0.10–0.51; p < 0.001) [37]. In addition, a clinically meaningful superior median OS was observed in PD-L1-positive patients, despite not reaching statistical significance (not reached versus 10.8 months) [37].
Overall, the role of PD-L1 expression in predicting the response to ICIs in BTC is still to be defined. In addition, several methodological issues must be taken into account when discussing this topic in BTC, as well as in other tumor types [38,39]. Among these, the use of different PD-L1 assays, the lack of guidelines, the differences in scoring systems, and the discrepancy between metastatic and primary lesions have been suggested to be implied in reporting discordant results.
3. TMB
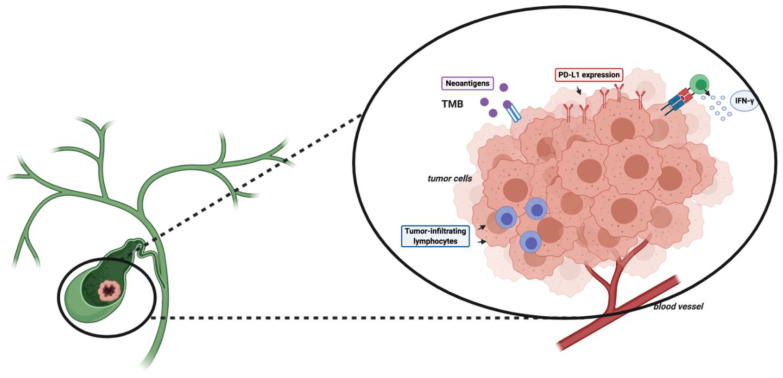
Besides PD-L1 expression, TMB has been associated with responses to ICIs in several tumor types, despite this biomarker not having been prospectively validated yet [43]. TMB is commonly defined as the overall number of somatic nonsynonymous mutations per megabase (Mut/Mb), including frame-shift mutations, insertions, point mutations, and deletions [44,45]. The onset of these mutations is involved in the synthesis of abnormal proteins, which can act as neoantigens, activating antitumor responses (Figure 1) [46].
Figure 1.
Schematic figure reporting some potential biomarkers of the response to immune checkpoint inhibitors (ICIs). Abbreviations: TMB, tumor mutational burden; PD-1, programmed death 1; PD-L1, programmed death-ligand 1.
As in the case of PD-L1, TMB assessment is widely influenced by the kits and methods used that have been suggested to report different values in the same sample, and consequently, great attention and caution should be paid when comparing TMB values between studies using different methods [47,48]. In a genomic study by Weinberg and colleagues on 1502 BTCs, TMB was investigated in 352 tumor samples [49]. Based on a cutoff of 17 Mut/Mb, the authors observed that 4% of samples (14/352) had high TMB (TMB-H) [49]; of note, the proportion of TMB-H tumors was different in distinct BTC subgroups, with 5.8% (6/104), 3.5% (7/198), and 2% (1/50) of GBCs, iCCAs, and eCCAs, defined as TMB-H in this genomic report [49].
In terms of clinical responses to ICIs, data on TMB in BTC are sparse and anecdotal. A recently published study by Zhang and colleagues reported three BTC cases (one iCCA and two dCCAs) with TMB-H, which were treated with ICIs [50]; of note, all of these patients achieved response to immunotherapy, with two partial responses (PRs) and one case of complete response (CR) [50]. However, recent phase I and II clinical trials evaluating ICIs in advanced BTC did not report data in terms of TMB. Further studies are needed to understand the putative role of TMB in predicting the response to ICIs in BTC patients [50].
4. dMMR/MSI-H
In addition to PD-L1 and TMB, MSI is considered a potentially meaningful predictive biomarker of the response to ICIs, and has been associated with dMMR [51]. More specifically, MSI results in the accumulation of mutations, leading to the formation of neoantigens and the activation of antitumor immune responses [52,53].
Of note, the proportion of MSI-H status among BTC patients is controversial, as suggested by recent studies on this topic reporting conflicting results [54]. In fact, a landmark whole exome-sequencing report conducted by Nakamura and colleagues highlighted concurrent dMMR or MSI-H status in 36% of 260 BTC patients [55]. This proportion has been revised downward in a systematic review by Silva, estimating a proportion of dMMR and/or MSI-H of 10%, 5%–13%, and 5% in iCCA, eCCA, and GBC, respectively [56]. In addition, two reports by Weinberg and Winkelmann reported even lower proportions, with the former observing only 1% of dMMR by immunohistochemistry in 102 BTC specimens; conversely, the latter highlighted only seven cases of MSI-H/dMMR in a cohort of 352 BTCs (2%) [49,57].
As regards the predictive value of dMMR/MSI-H, few data are available so far. However, it is worth noting that in the previously discussed phase II trial on nivolumab monotherapy conducted by Kim and colleagues, all responders were microsatellite stable (MSS) [37]. Similarly, in the report by Zhang, the three BTC patients who achieved PR or CR with ICIs were all MSS [50]. In addition, the previously discussed KEYNOTE-158 and KEYNOTE-028 trials exploring the role of pembrolizumab in pretreated patients with advanced BTC reported an interesting finding: in fact, all responders to the ICI were microsatellite stable, adding further confusion on the putative role of MSI [36]. Of note, 95.2% (99/104) of the BTC patients enrolled in the KEYNOTE-158 were MSS, and pembrolizumab reported a disappointing response rate of 5.8%, as previously reported [36]. Conversely, only one MSI-H patient was included in the KEYNOTE-028 study, where no data on MSI were available in 37.5% of the enrolled BTCs [36].
Although the scarcity of data precludes from making a strong statement regarding the effective role of dMMR/MSI-H, available evidence seems to suggest an overall modest value of these biomarkers. Conversely, the evaluation of these biomarkers in concert with other potentially meaningful predictors could provide more useful information, as indicated by recently published studies on DDR, TMB, and MSI-H in this setting (Table 3).
Table 3.
Table reporting some possible advantages and disadvantages of the frequently used biomarkers for predicting the response to immunotherapy.
| Pros/Cons | PD-L1 | MSI-H/dMMR | TMB |
|---|---|---|---|
| Advantages |
|
|
|
| Disadvantages |
|
|
|
dMMR, mismatch repair deficiency; IHC, immunohistochemistry; MSI-H, high microsatellite instability; PCR, polymerase chain reaction; PD-L1, programmed death-ligand 1; TMB, tumor mutational burden.
5. DDR
Among the most promising predictive biomarkers of the response to immunotherapy, recent years have witnessed growing attention toward DDR gene alterations, based on preclinical and early phase clinical trials supporting this biological rationale [58,59]. Of note, DDR gene aberrations impair DNA damage repair processes, with subsequent accumulation of DNA damage [60]; in physiological conditions, genes such as poly (ADP-ribose) polymerase 1 and 2 (PARP1 and PARP2) play a key role in maintaining genomic stability and in avoiding the accumulation of these mutations, with the inhibition of these genes representing a timely topic in medical oncology [61].
Based on these premises, mutations in DDR genes have been recently studied in BTC, reporting interesting data on their possible role and their impact on modifying the responses to ICIs [62]. First, the proportion of DDR gene mutations in BTC has been reported to occur in approximately 30% of patients, while Breast Related Cancer Antigens (BRCA) mutations seem to fluctuate between 1% and 7%, according to previous reports [63,64,65]. A recently published study by Spizzo and colleagues analyzed tumor samples from 1292 BTC patients (iCCA, n = 746; eCCA, n = 189; GBC, n = 353) using next-generation sequencing [66]. Of note, BRCA mutations were observed in 3.6% of tumor samples, without showing significant differences according to tumor site [66]; in addition, an important finding of this report is the association between BRCA mutations, MSI/dMMR, and TMB-H, something that supports the evaluation of ICIs in a specific subgroup of BTC patients, with DDR gene mutations potentially representing biomarkers predictive of the response to immunotherapy [66,67].
Nonetheless, there is currently no consensus on the methods for testing DDR gene alterations and few data are available on the effective role of DDR gene mutations in BTC. Lastly, none of the recent studies investigating ICIs as a monotherapy or in combination with other anticancer agents in metastatic BTC have reported the number of patients harboring DDR aberrations; further studies are warranted in this direction to shed light on this promising—and still barely known—landscape.
6. TME
The tumor microenvironment (TME) represents another promising biomarker whose role as a predictor of the response to ICIs is under evaluation in several tumor types, with preclinical studies suggesting that TME could modify and modulate the host immune response against tumors [68,69,70]. As regards BTC, recent reports have highlighted that these hepatobiliary tumors are desmoplastic malignancies with the TME showing immunosuppressive innate immune cells, including tumor-associated macrophages and myeloid-derived suppressor cells [71,72,73]. In addition, the existence of distinct subgroups of tumors has been suggested, with immunologically “hot” and “cold” BTCs [74]. As regards the former, enhanced immune molecular expression, higher CD8+ T cell density, and a superior response rate to immunotherapy have been observed; in addition, this BTC subgroup seems to report increased PD-1 and PD-L1 expression, together with higher CD8+ T cell infiltration and enhanced granzyme B activity [75]. Conversely, the immune “cold” subgroup—which seems to represent the majority of these malignancies on the basis of the response rate observed in clinical trials assessing ICIs—presents a prevalence of immunosuppressive cells (e.g., tumor-associated macrophages and tolerogenic dendritic cells) and a non-T cell-infiltrated TME [75]. However, these results are still preliminary and offer an overall limited level of evidence.
7. Conclusions
ICIs are being assessed in advanced BTC, as a monotherapy or in combination with other anticancer agents, reporting controversial results so far; of note, most patients show disappointing clinical outcomes. Responses seem limited to a small percentage of BTCs. Unfortunately, the available data on the predictors of the response to ICIs in BTC are conflicting, and no single biomarker may select patients likely to benefit from this therapeutic approach. Moreover, we are aware that discussing potentially meaningful predictors could appear preliminary in a setting where immunotherapy is still trying to “find its way.” However, the identification of reliable predictors of the response to immunotherapy represents a compelling and urgent need in this aggressive malignancy with limited treatment options.
Author Contributions
Conceptualization, A.R., A.D.R. and G.B.; methodology, A.R. and A.D.R.; software, A.R. and A.D.R.; validation, G.B.; formal analysis, A.R. and A.D.R.; investigation, A.R. and A.D.R.; resources, A.R. and A.D.R.; data curation, A.R. and A.D.R.; writing—original draft preparation, A.R. and A.D.R.; writing—review and editing, A.R. and A.D.R.; visualization, A.R., A.D.R. and G.B.; supervision, G.B.; project administration, A.R. and A.D.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Razumilava N., Gores G.J. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013;11:e13–e14. doi: 10.1016/j.cgh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzo A., Brandi G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: Reflections on a standard of care. Expert Rev. Gastroenterol. Hepatol. 2020:1–3. doi: 10.1080/17474124.2021.1864325. [DOI] [PubMed] [Google Scholar]
- 3.Banales J.M., Cardinale V., Carpino G., Marzioni M., Andersen J.B., Invernizzi P., Lind G.E., Folseraas T., Forbes S.J., Fouassier L., et al. Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat. Rev. Gastroenterol. Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi S., Gores G.J. Emerging molecular therapeutic targets for cholangiocarcinoma. J. Hepatol. 2017;67:632–644. doi: 10.1016/j.jhep.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liau J.Y., Tsai J.H., Yuan R.H., Chang C.N., Lee H.J., Jeng Y.M. Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Mod. Pathol. 2014;27:1163–1173. doi: 10.1038/modpathol.2013.241. [DOI] [PubMed] [Google Scholar]
- 6.Tella S.H., Kommalapati A., Borad M.J., Mahipal A. Second-line therapies in advanced biliary tract cancers. Lancet Oncol. 2020;21:e29–e41. doi: 10.1016/S1470-2045(19)30733-8. [DOI] [PubMed] [Google Scholar]
- 7.Bridgewater J., Galle P.R., Khan S.A., Llovet J.M., Park J.W., Patel T., Pawlik T.M., Gores G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Tariq N.U., McNamara M.G., Valle J.W. Biliary tract cancers: Current knowledge, clinical candidates and future challenges. Cancer Manag. Res. 2019;11:2623–2642. doi: 10.2147/CMAR.S157092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandi G., Rizzo A., Dall’Olio F.G., Felicani C., Ercolani G., Cescon M., Frega G., Tavolari S., Palloni A., De Lorenzo S., et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: A retrospective single-center experience. Int. J. Hyperth. 2020;37:479–485. doi: 10.1080/02656736.2020.1763484. [DOI] [PubMed] [Google Scholar]
- 10.Valle J., Wasan H., Palmer D.H., Cunningham D., Anthoney A., Maraveyas A., Madhusudan S., Iveson T., Hughes S., Pereira S.P., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 11.Valle J.W., Furuse J., Jitlal M., Beare S., Mizuno N., Wasan H., Bridgewater J., Okusaka T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2014;25:391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 12.Valle J.W., Lamarca A., Goyal L., Barriuso J., Zhu A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7:943–962. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamarca A., Barriuso J., McNamara M.G., Valle J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020;73:170–185. doi: 10.1016/j.jhep.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Massa A., Varamo C., Vita F., Tavolari S., Peraldo-Neia C., Brandi G., Rizzo A., Cavalloni G., Aglietta M. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers. 2020;12:2308. doi: 10.3390/cancers12082308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou S., Li J., Zhou H., Frech C., Jiang X., Chu J.S., Zhao X., Li Y., Li Q., Wang H., et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014;5:5696. doi: 10.1038/ncomms6696. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Alfa G.K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., Paulson A.S., Borad M.J., Gallinson D., Murphy A.G., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abou-Alfa G.K., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., Cleary J.M., Catenacci D.V., Borad M.J., Bridgewater J., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzo A., Ricci A.D., Brandi G. Futibatinib, an investigational agent for the treatment of intrahepatic cholangiocarcinoma: Evidence to date and future perspectives. Expert Opin. Investig. Drugs. 2020:1–8. doi: 10.1080/13543784.2021.1837774. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferro V., El-Rayes B.F., Droz dit Busset M., Cotsoglou C., Harris W.P., Damjanov N., Masi G., Rimassa L., Personeni N., Braiteh F., et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzo A., Ricci A.D., Tavolari S., Brandi G. Circulating Tumor DNA in Biliary Tract Cancer: Current Evidence and Future Perspectives. Cancer Genom. Proteom. 2020;17:441–452. doi: 10.21873/cgp.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma B., Meng H., Tian Y., Wang Y., Song T., Zhang T., Wu Q., Cui Y., Li H., Zhang W., et al. Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer. 2020;20:318. doi: 10.1186/s12885-020-06804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churi C.R., Shro_ R., Wang Y., Rashid A., Kang H.C., Weatherly J., Zuo M., Zinner R., Hong D., Meric-Bernstam F., et al. Mutation Profiling in Cholangiocarcinoma: Prognostic and Therapeutic Implications. PLoS ONE. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakrabarti S., Kamgar M., Mahipal A. Targeted Therapies in Advanced Biliary Tract Cancer: An Evolving Paradigm. Cancers. 2020;12:2039. doi: 10.3390/cancers12082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzo A., Ricci A.D., Brandi G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2020;20:1–10. doi: 10.1080/17474124.2021.1864325. [DOI] [PubMed] [Google Scholar]
- 25.Morizane C., Ueno M., Ikeda M., Okusaka T., Ishii H., Furuse J. New developments in systemic therapy for advanced biliary tract cancer. Jpn. J. Clin. Oncol. 2018;48:703–711. doi: 10.1093/jjco/hyy082. [DOI] [PubMed] [Google Scholar]
- 26.Vogel A., Bathon M., Saborowski A. Immunotherapies in clinical development for biliary tract cancer. Expert Opin. Investig. Drugs. 2020:1–13. doi: 10.1080/13543784.2021.1868437. [DOI] [PubMed] [Google Scholar]
- 27.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 28.Rizzo A., Mollica V., Ricci A.D., Maggio I., Massucci M., Rojas Limpe F.L., Fabio F.D., Ardizzoni A. Third- and later-line treatment in advanced or metastatic gastric cancer: A systematic review and meta-analysis. Future Oncol. 2020;16:4409–4418. doi: 10.2217/fon-2019-0429. [DOI] [PubMed] [Google Scholar]
- 29.Powles T., Walker J., Andrew Williams J., Bellmunt J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat. Rev. 2020;82:101925. doi: 10.1016/j.ctrv.2019.101925. [DOI] [PubMed] [Google Scholar]
- 30.Sabbatino F., Villani V., Yearley J.H., Deshpande V., Cai L., Konstantinidis I.T., Moon C., Nota S., Wang Y., Al-Sukaini A., et al. PD-L1 and HLA Class I Antigen Expression and Clinical Course of the Disease in Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2016;22:470–478. doi: 10.1158/1078-0432.CCR-15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye Y., Zhou L., Xie X., Jiang G., Xie H., Zheng S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J. Surg. Oncol. 2009;100:500–504. doi: 10.1002/jso.21376. [DOI] [PubMed] [Google Scholar]
- 32.Fontugne J., Augustin J., Pujals A., Compagnon P., Rousseau B., Luciani A., Tournigand C., Cherqui D., Azoulay D., Pawlotsky J.M., et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:24644–24651. doi: 10.18632/oncotarget.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter D., Herrmann E., Schnitzbauer A.A., Zeuzem S., Hansmann M.L., Peveling-Oberhag J., Hartmann S. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71:383–392. doi: 10.1111/his.13238. [DOI] [PubMed] [Google Scholar]
- 34.Gani F., Nagarajan N., Kim Y., Zhu Q., Luan L., Bhaijjee F., Anders R.A., Pawlik T.M. Program Death 1 Immune Checkpoint and Tumor Microenvironment: Implications for Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2016;23:2610–2617. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]
- 35.Ricci A.D., Rizzo A., Brandi G. Immunotherapy in Biliary Tract Cancer: Worthy of a Second Look. Cancer Control. 2020;27:1073274820948047. doi: 10.1177/1073274820948047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piha-Paul S.A., Oh D.Y., Ueno M., Malka D., Chung H.C., Nagrial A., Kelley R.K., Ros W., Italiano A., Nakagawa K., et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer. 2020;147:2190–2198. doi: 10.1002/ijc.33013. [DOI] [PubMed] [Google Scholar]
- 37.Kim R.D., Chung V., Alese O.B., El-Rayes B.F., Li D., Al-Toubah T.E., Schell M.J., Zhou J.M., Mahipal A., Kim B.H., et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020;6:888–894. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ancevski Hunter K., Socinski M.A., Villaruz L.C. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol. Diagn. Ther. 2018;22:1–10. doi: 10.1007/s40291-017-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemery S., Keegan P., Pazdur R. First FDA Approval Agnostic of Cancer Site—When a Biomarker Defines the Indication. N. Engl. J. Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 40.Ueno M., Ikeda M., Morizane C., Kobayashi S., Ohno I., Kondo S., Okano N., Kimura K., Asada S., Namba Y., et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019;4:611–621. doi: 10.1016/S2468-1253(19)30086-X. [DOI] [PubMed] [Google Scholar]
- 41.Ioka T., Ueno M., Oh D.Y., Fujiwara Y., Chen J.S., Doki Y., Mizuno N., Park K., Asagi A., Hayama M., et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC) J. Clin. Oncol. 2019;37:387. doi: 10.1200/JCO.2019.37.4_suppl.387. [DOI] [Google Scholar]
- 42.Yoo C., Oh D.Y., Choi H.J., Kudo M., Ueno M., Kondo S., Chen L.T., Osada M., Helwig C., Dussault I., et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer. 2020;8:e000564. doi: 10.1136/jitc-2020-000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritterhouse L.L. Tumor mutational burden. Cancer Cytopathol. 2019;127:735–736. doi: 10.1002/cncy.22174. [DOI] [PubMed] [Google Scholar]
- 44.McNamara M.G., Jacobs T., Lamarca A., Hubner R.A., Valle J.W., Amir E. Impact of high tumor mutational burden in solid tumors and challenges for biomarker application. Cancer Treat. Rev. 2020;89 doi: 10.1016/j.ctrv.2020.102084. [DOI] [PubMed] [Google Scholar]
- 45.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z., Duan J., Cai S., Han M., Dong H., Zhao J., Zhu B., Wang S., Zhuo M., Sun J., et al. Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients with Non-Small Cell Lung Cancer With Use of a Next-Generation Sequencing Cancer Gene Panel. JAMA Oncol. 2019;5:696–702. doi: 10.1001/jamaoncol.2018.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chalmers Z.R., Connelly C.F., Fabrizio D., Gay L., Ali S.M., Ennis R., Schrock A., Campbell B., Shlien A., Chmielecki J., et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg B.A., Xiu J., Lindberg M.R., Shields A.F., Hwang J.J., Poorman K., Salem M.E., Pishvaian M.J., Holcombe R.F., Marshall J.L., et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 2019;10:652–662. doi: 10.21037/jgo.2018.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W., Shi J., Wang Y., Zhou H., Zhang Z., Han Z., Li G., Yang B., Cao G., Ke Y., et al. Next-generation sequencing-guided molecular-targeted therapy and immunotherapy for biliary tract cancers. Cancer Immunol. Immunother. 2020 doi: 10.1007/s00262-020-02745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudley J.C., Lin M.T., Le D.T., Eshleman J.R. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer Res. 2016;22:813–820. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 52.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang L., Chang M., Chang H.M., Chang F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl. Immunohistochem. Mol. Morphol. 2018;26:e15–e21. doi: 10.1097/PAI.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 54.Verlingue L., Malka D., Allorant A., Massard C., Ferte C., Lacroix L., Rouleau E., Auger N., Ngo M., Nicotra C., et al. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur. J. Cancer. 2017;87:122–130. doi: 10.1016/j.ejca.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura H., Arai Y., Totoki Y., Shirota T., Elzawahry A., Kato M., Hama N., Hosoda F., Urushidate T., Ohashi S., et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 56.Silva V.W., Askan G., Daniel T.D., Lowery M., Klimstra D.S., Abou-Alfa G.K., Shia J. Biliary carcinomas: Pathology and the role of DNA mismatch repair deficiency. Chin. Clin. Oncol. 2016;5:62. doi: 10.21037/cco.2016.10.04. [DOI] [PubMed] [Google Scholar]
- 57.Winkelmann R., Schneider M., Hartmann S., Schnitzbauer A.A., Zeuzem S., Peveling-Oberhag J., Hansmann M.L., Walter D. Microsatellite Instability Occurs Rarely in Patients with Cholangiocarcinoma: A Retrospective Study from a German Tertiary Care Hospital. Int. J. Mol. Sci. 2018;19:1421. doi: 10.3390/ijms19051421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown J.S., O’Carrigan B., Jackson S.P., Yap T.A. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2017;7:20–37. doi: 10.1158/2159-8290.CD-16-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yap T.A., Plummer R., Azad N.S., Helleday T. The DNA Damaging Revolution: PARP Inhibitors and Beyond. Am. Soc. Clin. Oncol. Educ. Book. 2019;39:185–195. doi: 10.1200/EDBK_238473. [DOI] [PubMed] [Google Scholar]
- 60.Rizzo A., Mollica V., Cimadamore A., Santoni M., Scarpelli M., Giunchi F., Cheng L., Lopez-Beltran A., Fiorentino M., Montironi R., et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells. 2020;9:2051. doi: 10.3390/cells9092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pilié P.G., Tang C., Mills G.B., Yap T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019;16:81–104. doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reisländer T., Groelly F.J., Tarsounas M. DNA Damage and Cancer Immunotherapy: A STING in the Tale. Mol. Cell. 2020;80:21–28. doi: 10.1016/j.molcel.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 63.Lamarca A., Barriuso J., McNamara M.G., Valle J.W. Biliary Tract Cancer: State of the Art and potential role of DNA Damage Repair. Cancer Treat. Rev. 2018;70:168–177. doi: 10.1016/j.ctrv.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Golan T., Raitses-Gurevich M., Kelley R.K., Bocobo A.G., Borgida A., Shroff R.T., Holter S., Gallinger S., Ahn D.H., Aderka D., et al. Overall Survival and Clinical Characteristics of BRCA-Associated Cholangiocarcinoma: A Multicenter Retrospective Study. Oncologist. 2017;22:804–810. doi: 10.1634/theoncologist.2016-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdel-Wahab R., Yap T.A., Madison R., Pant S., Cooke M., Wang K., Zhao H., Bekaii-Saab T., Karatas E., Kwong L.N., et al. Genomic profiling reveals high frequency of DNA repair genetic aberrations in gallbladder cancer. Sci. Rep. 2020;10:22087. doi: 10.1038/s41598-020-77939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spizzo G., Puccini A., Xiu J., Goldberg R.M., Grothey A., Shields A.F., Arora S.P., Khushmann M., Salem M.E., Battaglin F., et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open. 2020;5:e000682. doi: 10.1136/esmoopen-2020-000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ricci A.D., Rizzo A., Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): A new Pandora’s box? ESMO Open. 2020;5:e001042. doi: 10.1136/esmoopen-2020-001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu T., Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 69.Hui L., Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 70.Frankel T., Lanfranca M.P., Zou W. The Role of Tumor Microenvironment in Cancer Immunotherapy. Adv. Exp. Med. Biol. 2017;1036:51–64. doi: 10.1007/978-3-319-67577-0_4. [DOI] [PubMed] [Google Scholar]
- 71.Mertens J.C., Rizvi S., Gores G.J. Targeting cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1454–1460. doi: 10.1016/j.bbadis.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabris L., Sato K., Alpini G., Strazzabosco M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology. 2020 doi: 10.1002/hep.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romano M., De Francesco F., Gringeri E., Giordano A., Ferraro G.A., Di Domenico M., Cillo U. Tumor Microenvironment Versus Cancer Stem Cells in Cholangiocarcinoma: Synergistic Effects? J. Cell. Physiol. 2016;231:768–776. doi: 10.1002/jcp.25190. [DOI] [PubMed] [Google Scholar]
- 74.Wu H.J., Chu P.Y. Role of Cancer Stem Cells in Cholangiocarcinoma and Therapeutic Implications. Int. J. Mol. Sci. 2019;20:4154. doi: 10.3390/ijms20174154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loeuillard E., Conboy C.B., Gores G.J., Rizvi S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019;1:297–311. doi: 10.1016/j.jhepr.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.