Abstract
BACKGROUND
Depression is closely related to coronary artery disease (CAD). However, the association of depression before angiography with major adverse cardiovascular event (MACE) is still unknown.
METHODS
In a prospective cohort study, 410 patients underwent angiography for the first time between 2016 and 2017 in Dr. Heshmat Hospital, Rasht, Iran. Demographic and medical information were collected and depressive symptoms were assessed using Beck Depression Inventory-II (BDI-II). The patients were followed for one year after angiography. Chi-square test and analysis of variance (ANOVA) were performed to compare demographic and clinical characteristics of patients between different levels of depressive symptoms. Multiple Cox regression analysis was performed to assess the association between depression symptoms before angiography and MACE rate controlled for the effect of confounders.
RESULTS
Of 410 patients, follow-up data were available for 380 (95%) patients. the MACE occurred in 134 (35%) patients. Depressive symptoms were observed in 42% of patients. Based on multivariable Cox regression analysis, adjusted for CAD severity, the risk of one-year MACE occurrence in patients with mild, moderate, and severe depressive symptoms was 1.96 [95% confidence interval (CI) for hazard ratio (HR): 1.30-2.94], 1.88 (95% CI for HR: 1.15-3.09), and 2.81 (95% CI for HR: 1.56-5.06) times that of patients without depressive symptoms, respectively. Depression in patients before angiography increased the risk of MACE up to 2.045 times.
CONCLUSION
The results showed that MACE in patients with depression was more than patients without depression. MACE in different levels of depression (mild, moderate, severe) was not significantly different.
Keywords: Depression, Angiography, Coronary Artery Disease
Introduction
Depression is common among patients with coronary artery disease (CAD), with a prevalence of 25% up to 50%.1,2 Several recent studies have found that depression is also associated with an increased risk of mortality and adverse cardiovascular events among patients with CAD or heart failure (HF), which was independent of the severity of the coronary lesions, ventricular function, and other factors of prognostic significance.3-5
The studies have also shown that psychological factors have a similar role as common risk factors of cardiovascular diseases (CVDs) in the mortality of patients suffering from CVDs.6,7 Depression leads to the incidence of CVDs, exacerbation of symptoms, poor prognosis of the disease, poor results of treatment, and also increased risk of mortality caused by re-infarction after myocardial infarction (MI).8-10
A meta-analysis revealed a pooled odds ratio (OR) of 2.25 [95% confidence interval (CI): 1.73-2.93] for all-cause mortality, 2.71 (95% CI: 1.68-4.36) for cardiac mortality, and 1.59 (95% CI: 1.37-1.85) for cardiac events, associated with depressive symptoms or disorders among post-MI patients.3 Furthermore, depressive symptoms following MI have been associated with increased hospital readmissions.11,12
Some studies have reported a relationship between depression and revascularization.13,14 In patients with CAD, depression diagnosis at the time of angiography significantly predicted coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) procedure rates over the next 5 years.13
Depression at the time of angiography also increases the risk of major adverse cardiovascular event (MACE) during the next one year by up to two times.15
MACEs are the important causes of morbidity and mortality in patients with CAD. The detection and treatment of the risk factors for MACE are critical to improve the patients’ health and longevity.16
Depression is the most common psychiatric disorders among patients with CAD.1 To assess whether depression symptoms increase the risk of mortality and MACE among patients with CAD, we hypothesized that patients with depression symptoms would have an increased risk of all-cause and MACE, compared with patients with no depression.
Materials and Methods
This research was a prospective cohort study. 410 patients with inclusion criteria who underwent angiography for the first time between September 2016 and November 2017 in Dr. Heshmat Hospital, Rasht, Iran, entered into the study.
The patients over 80 years old, the patients with the history of MI, angiography, or CABG on month prior to the sampling, and the patients with known malignancies were excluded from the study. Demographic information and also the data regarding medications, cigarette smoking, diabetes, hypertension (HTN), dyslipidemia, and history of heart disease (HD) were collected through a sociodemographic and medical interview. History of HD was determined by patients’ self-report of a previous admission, MI, PCI, or CABG. This study was based on the research proposal, approved by the Deputy of Research and Technology of Guilan University of Medical Sciences, Rasht, with Ethics Committee code of IR.GUMS.REC.1395.168.
For assessing depressive symptoms and their severity, Beck Depression Inventory-II (BDI-II) self-reporting questionnaire was used.17 Beck et al. measured its internal consistency to be between 0.73 to 0.92.18 It was completed by a psychologist before angiography. Since some of the patients could have been suffering from the lack of enough education to complete the questionnaire, the psychologist read and completed each question for the subjects in order to prevent any bias in the report. The data were collected in post angiography unit. The questionnaire consisted of 21 questions scoring from 0 to 3 (0 = no sign, 3 = the highest degree of signage) and the score range was from 0 to 63. Depression assessment was done based on total score: normal (0 to 13), mild depression (14 to 19), moderate depression (20 to 28), and severe depression (≥ 29).19 BDI has been standardized for the population of Iran and its Persian version’s reliability and validity have been confirmed.20
All patients were followed for at least one year after their first angiography. A year after the procedure, each participant was telephoned and MACE items were reviewed. MACEs in this study are defined as re-MI, cardiac mortality, revascularization procedures [percutaneous transluminal coronary angioplasty (PTCA), CABG, and re-angiography], or readmission due to HD and cerebrovascular events (e.g., stroke).
The participants were also observed in terms of undergoing treatment using antidepressant medication during the one year of the study, and the ones given medical depression treatment were excluded from the study. The deceased participants’ cause and time of death were collected through an interview with one of the immediate relatives.
Mean ± standard deviation (SD) and frequency (percentage) were used to describe the quantitative and qualitative patients’ characteristics, respectively. Chi-square test was performed to compare the categorical patients’ characteristics between different levels of depressive symptoms. Analysis of variance (ANOVA) was used to compare mean age and total cholesterol (TC) level among the different depressive symptoms categories. MACE-free survival was defined as survival without any MACE including death, CABG, re-hospitalization, stroke, re-angiography, PCI, MI, and re-vascularization and the first time of MACE occurrence was considered for MACE-free survival time. In the univariable analyses, Kaplan-Meier method was used to estimate MACE-free survival and log-rank test was performed to compare survival between patient's groups according to demographic variables or extent and severity of CAD. Multiple Cox regression analyses were carried out to assess the association between depression symptoms before angiography and MACE rate controlled for the effect of confounders. A forward stepwise procedure with likelihood ratio method was used to enter the covariates into the model and hazard ratios (HRs) and 95% CIs were calculated. As the extent and severity of CAD was highly correlated (r = 0.834, P < 0.001), because of not having collinearity problem, we assessed them in the separate regression models and did not entered in the models simultaneously. All statistical tests were two-sided and P < 0.050 was considered statistically significant. All analyses were performed using SPSS software (version 21.0, IBM Corporation, Armonk, NY, USA).
Results
Of 410 patients who were recruited and assessed in the baseline, follow-up data were available for 380 (95%) patients. According to the baseline data, there was no significant difference between patients with and without follow-up data (details are not shown). Demographic and clinical characteristics of patients entered into the analyses are summarized in table 1. 86 (23%), 50 (13%), and 23 (6%) patients had mild, moderate, and severe depressive symptoms, respectively. At the time of angiography, depressive symptoms were not observed in 221 (58%) patients.
Table 1.
Distribution of blood pressure (BP) indices in study population on admission date
| Factor | Total (n = 380) | Depression status* |
P | |||
|---|---|---|---|---|---|---|
| None [221 (58)] | Mild [86 (23)] | Moderate [50 (13)] | Severe [23 (6)] | |||
| Sex | < 0.001 | |||||
| Male | 204 (54) | 146 (71) | 38 (19) | 12 (6) | 8 (4) | |
| Female | 176 (46) | 75 (42) | 48 (27) | 38 (22) | 15 (9) | |
| Age (year) | 58.00 ± 9.24 | 58.00 ± 9.49 | 59.00 ± 9.78 | 59.00 ± 6.95 | 58.00 ± 9.50 | 0.880 |
| Education | < 0.001 | |||||
| Illiterate | 155 (41) | 76 (49) | 42 (27) | 24 (16) | 13 (8) | |
| Less than diploma | 163 (43) | 98 (60) | 32 (20) | 23 (14) | 10 (6) | |
| Diploma and higher | 62 (16) | 47 (76) | 12 (19) | 3 (5) | 0 (0) | |
| Smoker | 0.229 | |||||
| Yes | 82 (22) | 53 (65) | 13 (15) | 9 (11) | 7 (9) | |
| No | 298 (78) | 168 (56) | 73 (25) | 41 (14) | 16 (5) | |
| Residency | 0.020 | |||||
| Urban | 208 (55) | 135 (65) | 38 (18) | 26 (13) | 9 (4) | |
| Rural | 172 (45) | 89 (50) | 48 (28) | 24 (14) | 14 (8) | |
| Hx-HTN | 0.051 | |||||
| Yes | 177 (47) | 90 (51) | 48 (27) | 28 (16) | 11 (6) | |
| No | 203 (53) | 131 (65) | 38 (19) | 22 (11) | 12 (5) | |
| Hx-DM | 0.340 | |||||
| Yes | 152 (40) | 81 (53) | 36 (24) | 25 (16) | 10 (7) | |
| No | 228 (60) | 140 (61) | 50 (22) | 25 (11) | 13 (6) | |
| Hx-HD | 0.090 | |||||
| Yes | 177 (47) | 92 (52) | 43 (24) | 30 (17) | 12 (7) | |
| No | 203 (53) | 129 (64) | 43 (21) | 20 (10) | 11 (5) | |
| TC (mg/dl) | 157.00 ± 42.88 | 154.00 ± 39.31 | 163.00 ± 51.64 | 156.00 ± 37.18 | 173.00 ± 40.80 | 0.120 |
| Extent of CAD | 0.887 | |||||
| None | 123 (32) | 70 (57) | 22 (18) | 22 (18) | 9 (7) | |
| 1-vessel CAD | 74 (20) | 48 (65) | 19 (26) | 4 (5) | 3 (4) | |
| 2-vessel CAD | 79 (21) | 45 (57) | 20 (25) | 10 (13) | 4 (5) | |
| 3 or more-vessel CAD | 104 (27) | 58 (56) | 25 (24) | 14 (14) | 7 (6) | |
| Severity of CAD | 0.355 | |||||
| Normal | 123 (32) | 70 (57) | 22 (18) | 22 (18) | 9 (7) | |
| Mild | 14 (4) | 8 (58) | 3 (21) | 2 (14) | 1 (7) | |
| Moderate | 25 (7) | 19 (76) | 4 (16) | 2 (8) | 0 (0) | |
| Severe | 218 (57) | 124 (57) | 57 (26) | 24 (11) | 13 (6) | |
Data are presented as mean ± standard deviation (SD) or frequency and percentage
Percent in rows was calculated in total of patients in each level
Hx-HTN: History of hypertension; Hx-DM: History of diabetes; Hx-HD: History of heart disease; TC: Total cholesterol; CAD: Coronary artery disease
During the follow-up time, overall, at least one of the MACEs occurred in 134 (35%) patients. On the other hand, frequency of MACE occurrence was 4 deaths, 49 CABGs, 59 re-hospitalizations, 1 stroke, 17 re-angiographies, 40 PCIs, and 11 MIs. Table 2 illustrates incidence of at least one of the MACEs according to the patients’ depressive symptoms status.
Table 2.
Major adverse cardiovascular events (MACEs) according to the patients’ depressive symptoms
| Depression status | Total (n = 380) | MACE* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Re-hospitalization | PCI | Re-angiography | CABG | MI | Stroke | Mortality | ||
| None | 221 (58.0) | 163 (74.0) | 23 (10.0) | 19 (9.0) | 8 (4.0) | 23 (10.0) | 6 (3.0) | 1 (0.5) | 0 (0) |
| Mild | 86 (23.0) | 46 (54.0) | 16 (19.0) | 10 (12.0) | 4 (5.0) | 15 (17.0) | 1 (1.0) | 0 (0) | 3 (4.0) |
| Moderate | 50 (13.0) | 28 (56.0) | 13 (26.0) | 7 (14.0) | 4 (8.0) | 7 (14.0) | 3 (6.0) | 0 (0) | 0 (0) |
| Severe | 23 (6.0) | 9 (39.0) | 7 (30.0) | 4 (17.0) | 1 (4.0) | 4 (17.0) | 1 (4.0) | 0 (0) | 1 (4.0) |
| P | < 0.001 | 0.004 | 0.428 | 0.606** | 0.352 | 0.418** | - | 0.017** | |
Data are presented as frequency and percentage
Percent was calculated in total of patients of each depressive symptoms level. Some patients experienced more than one event;
P-values reported with caution of low sample size
MACE: Major adverse cardiovascular event; PCI: Percutaneous coronary intervention; CABG: Coronary artery bypass grafting; MI: Myocardial infarction
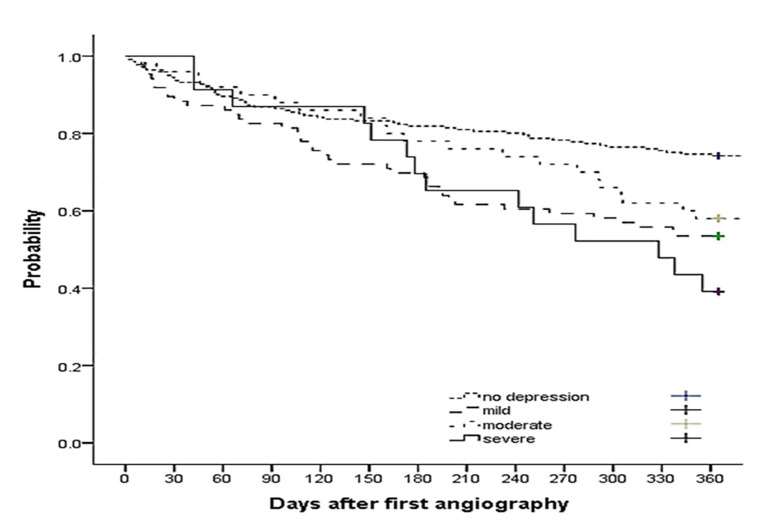
In overall, the one-year MACE-free survival was 65% (95% CI: 61%-69%). As shown in table 2, patients with depression experienced a higher occurrence of MACE. The one-year MACE-free survival was lower in patients with higher severity of depression than in those without depression (54% with 95% CI: 61.1%-68.9%, 58% with 95% CI: 44%-72%, and 39% with 95% CI: 20%-59% for patients with mild, moderate, and severe depressive symptoms compared to 74% with 95% CI: 68%-80% for patients without depressive symptoms, P < 0.001) (Figure 1).
Figure 1.
Major adverse cardiovascular event (MACE)-free survival probability during the first year after angiography procedure according to depressive symptoms status at baseline. The one-year MACE-free survival was lower in patients with higher severity of depression than those without depression (P < 0.001).
Female sex (P < 0.001), lower education (P < 0.001), living in rural area (P = 0.020), and having history of HTN (P = 0.050) were related to higher level of depressive symptoms (Table 1); however, none of them was related to the MACE-free survival (P > 0.050 for all analyses) (Table 3) and of the factors that were studied, only severity and frequency of vessel involvement CAD were significantly related to MACE-free survival. Patients with less severity and frequency of vessel involvement CAD had significantly higher MACE-free survival (P < 0.010 for both) (Table 3). One-year MACE-free survival was 84% (with 95% CI: 78%-90%), 64% (with 95% CI: 39%-89%), 88% (with 95% CI: 74%-100%), and 52% (with 95% CI: 46%-58%) for patients with normal, mild, moderate, and severe CAD, respectively. Furthermore, One-year MACE-free survival was 84% (with 95% CI: 78%-90%), 60% (with 95% CI: 48%-72%), 56% (with 95% CI: 42%-70%), and 55% (with 95% CI: 45%-65%) for patients with normal, 1, 2, and 3 or more vessel involvement CAD, respectively.
Table 3.
Univariable Cox regression analysis of demographic and clinical characteristics of patients related to major adverse cardiovascular events (MACEs) after first angiography
| Factor | HR | 95% CI | P |
|---|---|---|---|
| Sex (male) | 0.985 | 0.70-1.39 | 0.933 |
| Age | 1.002 | 0.98-1.02 | 0.855 |
| Education | 0.568* | ||
| Illiterate | 1.114 | 0.68-1.83 | 0.672 |
| Less than diploma | 0.911 | 0.55-1.51 | 0.717 |
| Diploma and higher | 1.000 | ||
| Smoker (yes) | 0.816 | 0.53-1.26 | 0.360 |
| Residency (urban) | 1.015 | 0.72-1.43 | 0.933 |
| Hx-HTN (yes) | 1.012 | 0.72-1.42 | 0.947 |
| Hx-DM (yes) | 1.227 | 0.87-1.73 | 0.241 |
| Hx-HD (yes) | 1.201 | 0.85-1.69 | 0.292 |
| TC | 1.002 | 1.00-1.01 | 0.358 |
| Extent of CAD | < 0.001* | ||
| None | 1.000 | ||
| 1-vessel CAD | 2.870 | 1.63-5.05 | < 0.001 |
| 2-vessel CAD | 3.352 | 1.93-5.81 | < 0.001 |
| 3 or more-vessel CAD | 3.365 | 1.99-5.68 | < 0.001 |
| Severity of CAD | < 0.001* | ||
| Normal | 1.000 | ||
| Mild | 2.449 | 0.92-6.53 | 0.073 |
| Moderate | 0.740 | 0.22-2.49 | 0.627 |
| Severe | 3.621 | 2.24-5.85 | < 0.001 |
| Depression status | < 0.001* | ||
| None | 1.000 | ||
| Mild | 2.091 | 1.39-3.13 | < 0.001 |
| Moderate | 1.729 | 1.05-2.83 | 0.030 |
| Severe | 2.654 | 1.48-4.77 | 0.001 |
P-values demonstrated that there was a significant relation between categorical variables as a whole and MACEs after first angiography.
HR: Hazard ratio; CI: Confidence interval; Hx-HTN: History of hypertension; Hx-DM: History of diabetes; Hx-HD: History of heart disease; CAD: Coronary artery disease; MACEs: Major adverse cardiovascular events
The Cox regression analysis demonstrated that, adjusted for CAD extent, the risk of MACE in patients with mild, moderate, and severe depressive symptoms was 2.10 (95% CI: 1.38-3.11, P < 0.001), 1.93 (95% CI: 1.17-3.18, P = 0.010), and 3.06 (95% CI: 1.70-5.51, P < 0.001) times that of patients without depressive symptoms (Table 4, Model 1). Similarly in the second regression model, adjusted for CAD severity, the risk of one-year MACE occurrence in patients with mild, moderate, and severe depressive symptoms was 1.96 (95% CI: 1.30-2.94, P = 0.001), 1.88 (95% CI: 1.15-3.09, P = 0.012), and 2.81 (95% CI: 1.56-5.06, P = 0.001) times that of patients without depressive symptoms (Table 4, Model 2).
Table 4.
Multivariable Cox regression analyses of factors related to major adverse cardiovascular events (MACEs) after first angiography
| Factors | Model 1* |
P | Model 2* |
P | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Depression status | < 0.001** | < 0.001** | ||||
| None | 1.000 | 1.000 | ||||
| Mild | 2.075 | 1.383-3.111 | < 0.001 | 1.956 | 1.303-2.935 | 0.001 |
| Moderate | 1.932 | 1.172-3.184 | 0.010 | 1.882 | 1.146-3.091 | 0.012 |
| Severe | 3.057 | 1.697-5.506 | < 0.001 | 2.810 | 1.560-5.062 | 0.001 |
| Extent of CAD | < 0.001 | |||||
| None | 1.000 | |||||
| 1-vessel CAD | 3.119 | 1.760-5.530 | < 0.001 | |||
| 2-vessel CAD | 3.538 | 2.039-6.142 | < 0.001 | |||
| 3 or more-vessel CAD | 3.517 | 2.080-5.948 | < 0.001 | |||
| Severity of CAD | < 0.001 | |||||
| Normal | 1.000 | |||||
| Mild | 2.490 | 0.934-6.635 | 0.068 | |||
| Moderate | 0.911 | 0.269-3.082 | 0.881 | |||
| Severe | 3.765 | 2.327-6.094 | < 0.001 | |||
Cox regression models indicated significant hazard ratios (HRs) of baseline depressive symptoms status and major adverse cardiovascular events (MACEs), respectively adjusted for coronary artery disease (CAD) extent (model 1) and CAD severity (model 2);
P-values demonstrated that there was a significant relation between depression status as a whole and MACE after first angiography.
HR: Hazard ratio; CI: Confidence interval; CAD: Coronary artery disease
Discussion
This study investigates the relationship between depressive symptoms and MACE. The results showed that MACE in patients with depression was more than patients without depression. MACE in different levels of depression was not significantly different. Depression in patients before angiography increased the risk of MACE up to 2.045 times.
MACE is an important factor of mortality and morbidity in patients with CAD.13 In one of the first studies, it was shown that mortality in patients with and without depression was, respectively, 17% and 3% which was significantly different.21 The study of Tsai et al. during a 32-month follow-up showed that HTN, having triple-vessel disease, stent implantation, and uric acid were associated with MACE in this study.16
Li et al. in a 1-year follow-up showed that the incidence of MACE in patients with depression was significantly higher than patients without depression in both PCI and CABG patients.22
The study of Frasure-Smith et al. indicated that although scores more than 14 (≥ 14) in BDI among men increased the risk of MACE, this was lower among female patients.23 Meyer et al. indicated that depression could independently predict the occurrence of mortality and MACE; furthermore, it could be associated with better survival during the fallow-up.24 The bidirectional link between depression and revascularization has been reported in several studies.25,26
In a 1-year follow-up, we have indicated that the depression status was associated with the increase in MACE risk, mortality, and readmission and also MACE was higher in patients with depression than patients without depression.
The study of Sullivan et al.13 was aligned with our study. In their study, 198 patients suffering from stable coronary disease underwent selective angiography during a 5-year follow-up. The results indicated that depression status was associated with revascularization and higher healthcare costs. While in the study of Sullivan et al.,13 Hamilton Rating Scale for Depression (HRSD) was used to investigate the patients, we used BDI in our study. In a prospective study in 2015, 2390 patients were followed for 8.8 years. The patients with depression were at increased risks of all-cause mortality [relative risk (RR) = 2.84, 95% CI: 1.25-6.49] compared with patients without depression. When patients were stratified according to CAD status, depression increased the risk of mortality among patients with no CAD but not among patients with CAD.27
Despite the difference in the subjects and depression measurement methods in these studies, undesirable effects of depression on MACE were very similar.
Several explanations might be suggested for the higher risk of cardiovascular events in patients with depression:
Endothelial dysfunction, platelet abnormalities, autonomic nervous system (ANS) dysfunction, and inflammation as evidenced by elevated C-reactive protein (CRP) and cytokines may link depression with adverse cardiac outcomes.28
Patients with depression are less likely to engage in health-promoting behaviors including maintenance of a healthy diet, regular exercise, smoking cessation, adherence to medications, stress reduction, and completion of cardiac rehabilitation programs.28
Decreasing secondary preventive behaviors in patients with depression can aggravate HD and increase the risk of mortality, which can explain the prevalence of MACE in these patients.
This study had several limitations. In this research, the emphasis was on depressive symptoms as self-reported by the patients and without clinical interview. As in self-report, the patient may report his feelings incorrectly, therefore, it can adversely affect the findings of the study.
The presence of a psychologist to investigate the symptoms of depression and heart specialists in the research team, accuracy in diagnosis of CAD severity, arterial involvement based on angiography, and the detailed data collected from the patients’ cardiac medical history were the merits of this study. Additionally, this study was done at a referral hospital in north of Iran which covers a large number of patients in this area.
Our study showed that the higher frequency or severity of vessel involvement CAD along with depressive symptoms at the time of angiography could predict the occurrence of MACE during the year post angiography.
Conclusion
The prevalence of depression in patients undergoing angiography and its relationship with the increase of MACE in patients with and without depression indicates the importance of assessing the psychological status of these patients. Since depression causes increasing financial burden on health system, screening patients with depression may allow physicians to provide more appropriate and cost-effective care for them.
Acknowledgments
The authors wish to thank Dr. Heshmat Hospital staff for their cooperation and also Vice Chancellor for Research of Guilan University of Medical Sciences for their financial support.
This study was based on the research proposal, approved by the Deputy of Research and Technology of Guilan University of Medical Sciences with Ethics Committee code of IR.GUMS.REC.1395.168.
This study was financially supported by the Deputy of Research and Technology of Guilan University of Medical Sciences, Rasht, Iran (Grant No. 95052311).
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Connerney I, Sloan RP, Shapiro PA, Bagiella E, Seckman C. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosom Med. 2010;72(9):874–81. doi: 10.1097/PSY.0b013e3181f65fc1. [DOI] [PubMed] [Google Scholar]
- 2.Damen NL, Versteeg H, Boersma E, Serruys PW, van Geuns RJ, Denollet J, et al. Depression is independently associated with 7-year mortality in patients treated with percutaneous coronary intervention: Results from the RESEARCH registry. Int J Cardiol. 2013;167(6):2496–501. doi: 10.1016/j.ijcard.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: A meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–16. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Zuidersma M, Conradi HJ, van Melle JP, Ormel J, de Jonge P. Self-reported depressive symptoms, diagnosed clinical depression and cardiac morbidity and mortality after myocardial infarction. Int J Cardiol. 2013;167(6):2775–80. doi: 10.1016/j.ijcard.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Murphy B, Rogerson M, Worcester M, Elliott P, Higgins R, Le Grande M, et al. Predicting mortality 12 years after an acute cardiac event: Comparison between inhospital and 2-month assessment of depressive symptoms in women. J Cardiopulm Rehabil Prev. 2013;33(3):160–7. doi: 10.1097/HCR.0b013e318283927f. [DOI] [PubMed] [Google Scholar]
- 6.Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):511–23. doi: 10.1016/j.pcad.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, et al. Depression as a risk factor for coronary artery disease: Evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305–15. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 9.Carney RM, Freedland KE, Steinmeyer B, Blumenthal JA, de JP, Davidson KW, et al. History of depression and survival after acute myocardial infarction. Psychosom Med. 2009;71(3):253–9. doi: 10.1097/PSY.0b013e31819b69e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parakh K, Thombs BD, Fauerbach JA, Bush DE, Ziegelstein RC. Effect of depression on late (8 years) mortality after myocardial infarction. Am J Cardiol. 2008;101(5):602–6. doi: 10.1016/j.amjcard.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Myers V, Gerber Y, Benyamini Y, Goldbourt U, Drory Y. Post-myocardial infarction depression: Increased hospital admissions and reduced adoption of secondary prevention measures-a longitudinal study. J Psychosom Res. 2012;72(1):5–10. doi: 10.1016/j.jpsychores.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Reese RL, Freedland KE, Steinmeyer BC, Rich MW, Rackley JW, Carney RM. Depression and rehospitalization following acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(6):626–33. doi: 10.1161/CIRCOUTCOMES.111.961896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan MD, LaCroix AZ, Spertus JA, Hecht J, Russo J. Depression predicts revascularization procedures for 5 years after coronary angiography. Psychosom Med. 2003;65(2):229–36. doi: 10.1097/01.psy.0000058370.50240.aa. [DOI] [PubMed] [Google Scholar]
- 14.Park MW, Kim JH, Her SH, Cho JS, Choi MS, Gweon TG, et al. Effects of percutaneous coronary intervention on depressive symptoms in chronic stable angina patients. Psychiatry Investig. 2012;9(3):252–6. doi: 10.4306/pi.2012.9.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hance M, Carney RM, Freedland KE, Skala J. Depression in patients with coronary heart disease. A 12-month follow-up. Gen Hosp Psychiatry. 1996;18(1):61–5. doi: 10.1016/0163-8343(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 16.Tsai IT, Wang CP, Lu YC, Hung WC, Wu CC, Lu LF, et al. The burden of major adverse cardiac events in patients with coronary artery disease. BMC Cardiovasc Disord. 2017;17(1):1. doi: 10.1186/s12872-016-0436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 18.Beck At, Ward Ch, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZJ, Guo M, Si TM, Jiang MM, Liu SM, Liu YY, et al. Association of depression with adverse cardiovascular events after percutaneous coronary intervention. Coron Artery Dis. 2013;24(7):589–95. doi: 10.1097/MCA.0b013e3283650234. [DOI] [PubMed] [Google Scholar]
- 20.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory-Second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21(4):185–92. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 21.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270(15):1819–25. [PubMed] [Google Scholar]
- 22.Li XM, Li TT, Cong HL, Guo ZG, Song JH, Zhao R, et al. Impact of depression on prognosis of patients with coronary heart disease undergoing revascularization. Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40(2):99–103. [PubMed] [Google Scholar]
- 23.Frasure-Smith N, Lesperance F, Irwin MR, Sauve C, Lesperance J, Theroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62(4):302–8. doi: 10.1016/j.biopsych.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Meyer T, Hussein S, Lange HW, Herrmann-Lingen C. Anxiety is associated with a reduction in both mortality and major adverse cardiovascular events five years after coronary stenting. Eur J Prev Cardiol. 2015;22(1):75–82. doi: 10.1177/2047487313505244. [DOI] [PubMed] [Google Scholar]
- 25.Rothenbacher D, Hahmann H, Wusten B, Koenig W, Brenner H. Symptoms of anxiety and depression in patients with stable coronary heart disease: Prognostic value and consideration of pathogenetic links. Eur J Cardiovasc Prev Rehabil. 2007;14(4):547–54. doi: 10.1097/HJR.0b013e3280142a02. [DOI] [PubMed] [Google Scholar]
- 26.Shibeshi WA, Young-Xu Y, Blatt CM. Anxiety worsens prognosis in patients with coronary artery disease. J Am Coll Cardiol. 2007;49(20):2021–7. doi: 10.1016/j.jacc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier R, Bacon SL, Arsenault A, Dupuis J, Laurin C, Blais L, et al. Relative associations between depression and anxiety on adverse cardiovascular events: Does a history of coronary artery disease matter? A prospective observational study. BMJ Open. 2015;5(12):e006582. doi: 10.1136/bmjopen-2014-006582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: Epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925. [DOI] [PMC free article] [PubMed] [Google Scholar]