Abstract
BACKGROUND
Although the antioxidant properties of ginger have been revealed, there is little available information on the effectiveness of ginger on inflammatory disorders such as atherosclerosis. This study was carried out to examine the effect of ginger on improving the complication of atherosclerosis.
METHODS
This study was a double-blind, placebo-controlled, randomized clinical trial conducted on patients with atherosclerosis. Participants in the ginger and control groups received 1600 mg of powdered ginger or placebo (wheat flour) in capsules daily for 8 weeks. Weight, body mass index (BMI), fasting blood sugar (FBS), cholesterol, triglyceride (TG), low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL), high-density lipoprotein (HDL), lipoprotein (a) [Lp(a)], high-sensitivity C-reactive protein (hs-CRP), and total anti-oxidant capacity (TAC) were assessed before and after the intervention.
RESULTS
Ginger consumption in the intervention group significantly reduced serum Lp(a) level (27.25 ± 1.30 ng/ml vs. 23.57 ± 0.97 ng/ml) (P = 0.040) and also the level of hs-CRP in the intervention group was 1.90 ± 0.33 µg/ml and 1.24 ± 0.15 µg/ml (P = 0.010) before and after intervention, respectively, but the levels of Lp(a) and hs-CRP were not decreased significantly in the placebo group. The level of TAC in the ginger group was 0.71 ± 0.05 mM and after the trial was 0.57 ± 0.04 mM (P = 0.050); no significant differences were seen in TAC when ginger was administered at 1600 mg/daily for 60 days. Also the level of Lp(a) and hs-CRP but not TAC reduced significantly in ginger group compared to placebo group after intervention.
CONCLUSION
This study showed that ginger had anti-atherosclerosis and anti-glycemic properties associated through a significant decreased Lp(a) and FBS in patients with atherosclerosis supplemented with ginger.
Keywords: Atherosclerosis, Ginger, Lipoprotein (a), C-reactive Protein, Oxygen Radical Absorbance Capacity
Introduction
Cardiovascular disease (CVD) is one of the most important causes of death worldwide and accounts for 10% of all causes of disease in the world.1 Atherosclerosis and thrombosis have been identified as the major causes of CVD. Inflammatory processes in the endothelial cells of the vessel wall initiate atherosclerosis, which is associated with retained low-density lipoprotein (LDL).2 Macrophage is transformed into foam cell upon LDL uptake and the gradual accumulation of foam cells and proliferation of smooth muscle cells (SMCs) in the inner lining of the arteries result in fiber-fat plaque (atheroma). Depending on the size and the degree of luminal involvement, the propensity of a plaque to rupture is determined which causes acute coronary syndrome (ACS).3
Measuring the levels of lipids and inflammatory markers is used to evaluate the risk of future coronary heart disease (CHD). In particular, recent studies revealed that plasma levels of lipoprotein (a) [Lp(a)] may be an important risk marker for atherosclerosis.4 Lp(a) is an LDL-like particle, rich in about 45% cholesterol and also contains apo Lp(a) [apo(a)]. Diet, physical exercise, and other environmental influences seem not to change the serum level of Lp(a).5 However, Lp(a) is a strong risk factor for CVD but the lack of clinical trial data has resulted in Lp(a) being mostly ignored by clinical guidelines assessing the prohibition of CVD.6
C-reactive protein (CRP) is an acute-phase protein produced exclusively in the liver, involved in the immunological process, and mainly used in screening and prediction of cardiovascular problems.7 Inflammatory markers, specifically high-sensitivity CRP (hs-CRP) as a novel biomarker, help identify those patients who would benefit more from pharmacological intervention.8
Reactive oxygen species (ROS) are very active small molecules derived from oxygen that have long been known to be components of the killing response of immune cells to microbial invasion. Recent evidence has shown that ROS play a key role as a messenger in normal cell signal transduction and cell cycling. Their ability to modify LDL, react with endothelial-derived nitric oxide (NO) subsequently forming proxynitrite, and raise the expression of various genes important for leukocyte recruitment within the arterial wall is the basis of the oxidant injury theory of atherosclerosis.9
Today, there are many medicinal herbs around the world used to treat diseases. Ginger (Zingiber officinale Roscoe) is a known spice used in medicinal purpose and cooking since ancient time. Several studies that most of them were animal and in vitro-based demonstrated the antioxidant and anti-inflammatory properties of ginger.10
Ginger also was found to prevent arachidonic acid metabolism which leads to reducing prostaglandin synthesis and improving inflammation as non-steroidal anti-inflammatory drugs (NSAIDs).11 Since ginger has particular fewer side effects than conventional inflammation-reducing drugs, it is a preferred method to slow down the process of developing atherosclerotic plaques.12
Given that the plasma Lp(a) is a factor that increases in CVD and ginger as an anti-inflammatory and antioxidant drug can reduce the incidence of atherosclerosis, it is expected that consumption of ginger may reduce serum Lp(a) and other inflammatory factors. Several studies demonstrated the effectiveness of ginger on the reduced inflammatory mediators but the anti-atherosclerotic potential of ginger is not well documented. In the present randomized controlled trial (RCT), the efficacy of ginger supplement in the Lp(a), hs-CRP, total antioxidant capacity (TAC), Lipid profile, and fasting blood sugar (FBS) levels was evaluated in patients with atherosclerosis.
Materials and Methods
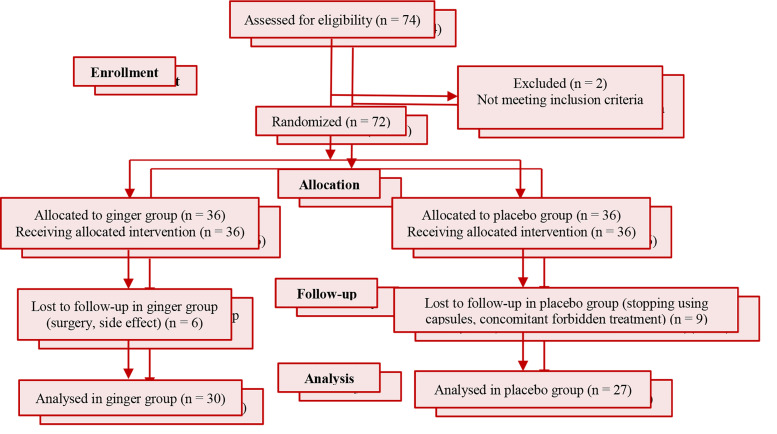
We undertook this randomized, double-blind, active and placebo-controlled trial at Imam Khomeini and Golestan Hospitals in Ahvaz, Iran. The study took place between October and December 2015. A total of 74 male patients were evaluated for eligibility at first. Two of them were excluded from the study because they did not meet full criteria for inclusion. The remaining (72) were divided randomly to ginger (n = 36) and placebo (n = 36) groups with permuted block randomization. Coded packages containing treatment components were provided to participants by clinical staff unaware of treatment allocation. Six patients in ginger group and also nine patients in placebo group were lost to follow-up because of some side effects, need for surgery, stopping using capsules, and etc. Finally, this study was completed and analyzed based on data from 57 patients (30 in the ginger group and 27 in the placebo group). Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Because some laboratory data are affected by estrogen and progesterone hormones, women were excluded from this study. The male patients aged 40 to 70 years with involvement of at least one coronary artery in angiography which were undergoing medical treatment not revascularization met our inclusion criteria. Major exclusion criteria were as follows: supplement intake to less than 80 percent of the total, changes in routine treatment received before the study (change in the type and amount of medication during intervention), drug side effects, taking a vitamin supplement or warfarin, and unwillingness to continue cooperation in the study. The concomitant use of drug treatment for atherosclerosis was permitted but patients were instructed to maintain their diet, exercise, and pharmacological therapy during the study period. After giving a full explanation of the study to the patients and obtaining informed consent from them, patients were interviewed and filled a questionnaire about their demographic characteristics including age, history of other diseases, type and amount of medication, and quality of life. Then participants received 1600 mg of ginger rhizome powder or placebo per day (1 capsule of 800 mg after lunch and 1 capsule of 800 mg after dinner) for two months.
Figure 1.
Flowchart describing the progress of the patients through the trial
Weight was measured without shoes or socks while standing erect with weight scale (Seca Colorata 760, Germany) and height was measured from head to foot. The body mass index (BMI) [body weight (kg)/height squared (m2)] was also calculated.
Blood samples were taken after 12 hours fasting, either before or after the intervention. Serum was separated and stored at -70 °C until assaying serum markers [Lp(a), hs-CRP, TAC, cholesterol, high-density lipoprotein (HDL), LDL, very-low-density lipoprotein (VLDL), triglyceride (TG), FBS] and BMI level.
Lp(a) concentrations were determined using enzyme-linked immunosorbent assay (ELISA) kit (a quantitative sandwich enzyme immunoassay) (Catalog #: KA1041, Abnova Corporation, Taiwan). Measurement of serum hs-CRP was done by sandwich ELISA technique using kit supplied by IBL (IBL, Hamburg, Germany, Cat. No.: IB59126). Serum TAC was measured by oxidation reduction colorimetric method at a wavelength of 490 nm, using manufacturer´s protocol (Zellbio® kit, Cat. No.: ZB-TAC96, Netherlands). FBS was determined by the colorimetric enzymatic method (Pars Azmoon kit, Iran). Lipid profile was measured by enzymatic photometric method, using Pars Azmoon test kits.
The independent Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, approved this study with number: IR.AJUMS.REC.1394.294. All study participants provided written informed consent prior to involvement. This trial is registered with Iranian Registry of Clinical Trials (IRCT) information (number: IRCT2015091524025N1).
Data were reported as mean ± standard deviation (SD) and absolute number (percentage) for continuous and categorical variables, respectively. Kolmogorov-Smirnov test (K-S test) was used for checking normality assumption and according to its result, appropriate (parametric or non-parametric) tests were used. Also chi-squared test evaluated the frequency differences of qualitative variables between two groups. Independent t-test was used to compare means between case and control groups, also paired sample t-test was used for quantitative variables before and after the trial. Multiple line are regression evaluated the association between groups and risk factors by adjusting baseline variables. All statistical analyses were performed using SPSS software (version 20, IBM Corporation, Armonk, NY, USA) and a P-value less than 0.050 was considered as significant.
Results
72 male patients were registered for treatment and randomly devided in two groups. Fifteen patients were dropped out from the study owing to the concomitant use of forbidden treatment, stopping using capsules, and losing to follow up. Finally, 57 patients completed the trial (30 in the ginger group and 27 in the placebo group).
Both study groups were well matched for baseline characteristics of the age, artery involvement, and risk factors containing hypertension (HTN), smoking, and diabetes (Table 1). After random allocation, both groups were analyzed for risk factors such as smoking, HTN, and diabetes and no significant difference was found between ginger group and placebo group. The average of individuals age in the ginger group was 56.40 ± 7.70 years and in the placebo group was 56.35 ± 7.74 years; this difference was not statistically significant (P = 0.916). The individuals with HTN in the placebo group were 11 (40.7%) and in the trial group were 15 persons (50.0%) that was also not statistically significant (P = 0.483). The number of smokers in the placebo group was 4 (14.8%) and in the trial group was 4 persons (13.3%); this was statistically meaningless. The diabetics in the placebo group were 12 (44.4%) and in the trial group were 13 (43.3%) persons; this difference was not statistically significant.
Table 1.
Demographic, lifestyle, and laboratory variables for participants
| Variables | Placebo group* (n = 27) | Ginger group (n = 30) | P | |
|---|---|---|---|---|
| Demographic | Age (year) | 56.35 ± 7.74 | 56.40 ± 7.70 | 0.916# |
| Lifestyle | Weight (kg) | 76.35 ± 2.80 | 77.07 ± 2.10 | 0.824# |
| BMI (kg/m2) | 26.02 ± 0.88 | 27.81 ± 0.76 | 0.123# | |
| HTN | 11 (40.7) | 15 (50.0) | 0.483$ | |
| Diabetes | 12 (44.4) | 13 (43.3) | 0.933$ | |
| Smoking | 4 (14.8) | 4 (13.3) | 0.872£ | |
| Artery involvement | One coronary artery | 13 (48.1) | 21 (70.0) | 0.243$ |
| Two coronary arteries | 8 (29.6) | 5 (16.7) | ||
| Three coronary arteries | 6 (22.2) | 4 (13.3) | ||
| Laboratory | FBS (mg/dl) | 148.73 ± 15.90 | 134.57 ± 10.90 | 0.848# |
| Cholesterol (mg/dl) | 152.46 ± 4.60 | 163.27 ± 7.20 | 0.158# | |
| HDL (mg/dl) | 38.41 ± 0.86 | 39.67 ± 1.43 | 0.465# | |
| LDL (mg/dl) | 89.42 ± 4.40 | 93.29 ± 5.30 | 0.707# | |
| VLDL (mg/dl) | 26.88 ± 3.50 | 35.55 ± 2.90 | 0.007# | |
| TG (mg/dl) | 138.12 ± 16.20 | 184.50 ± 15.50 | 0.044# | |
| Lp(a) (ng/ml) | 27.63 ± 2.60 | 27.26 ± 6.70 | 0.515# | |
| CRP (µg/ml) | 1.53 ± 0.18 | 1.91 ± 0.33 | 0.991# | |
| TAC (mM) | 0.77 ± 0.53 | 0.71 ± 0.05 | 0.197# |
Continues and categorical variables are reported as mean ± standard deviation (SD) and absolute number (percentage), respectively
Independent sample t-test
Chi-square test
Fisher’s exact test
BMI: Body mass index; HTN: Hypertension, FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; VLDL: Very-low-density lipoprotein; TG: Triglyceride; Lp(a): Lipoprotein (a); CRP: C-reactive protein; TAC: Total antioxidant capacity
We showed that there were no significant differences between BMI, FBS, weight, cholesterol, LDL, HDL, Lp(a), CRP, and TAC before the intervention in table 1.
The final analysis was done in two ways after intervention. Table 2 and figure 2 compare the changes from baseline to post-treatment in each group (ginger and placebo groups) independently.
Table 2.
Comparison of measured indices in placebo and ginger groups before and after medical intervention
| Variables | Placebo |
P | Ginger |
P | ||
|---|---|---|---|---|---|---|
| After | Before | After | Before | |||
| Weight (kg) | 76.99 ± 2.70 | 77.74 ± 2.70 | 0.005 | 77.06 ± 2.10 | 74.73 ± 2.00 | < 0.001* |
| BMI (kg/m2) | 26.02 ± 0.85 | 26.52 ± 0.86 | 0.005 | 27.81 ± 0.76 | 26.98 ± 0.72 | < 0.001* |
| FBS (mg/dl) | 145.74 ± 15.60 | 148.07 ± 15.50 | 0.760 | 134.56 ± 10.40 | 117.70 ± 9.80 | 0.003* |
| Cholesterol (mg/dl) | 150.88 ± 4.70 | 151.81 ± 4.80 | 0.821 | 163.26 ± 7.20 | 161.83 ± 6.40 | 0.841 |
| HDL (mg/dl) | 38.41 ± 0.86 | 39.82 ± 0.66 | 0.070 | 39.66 ± 1.40 | 41.60 ± 1.00 | 0.100 |
| LDL (mg/dl) | 89.81 ± 4.30 | 86.48 ± 4.70 | 0.460 | 93.28 ± 5.30 | 87.60 ± 5.30 | 0.302 |
| VLDL (mg/dl) | 26.55 ± 3.30 | 27.85 ± 2.70 | 0.461 | 36.14 ± 2.90 | 31.82 ± 3.30 | 0.281 |
| TG (mg/dl) | 138.12 ± 16.20 | 138.62 ± 12.80 | 0.970 | 184.50 ± 15.50 | 154.96 ± 17.10 | 0.120 |
Values are expressed as mean ± standard deviation (SD) (paired sample t-test was used)
A significant difference between the indices before and after in placebo and ginger groups
BMI: Body mass index; FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; VLDL: Very-low-density lipoprotein; TG: Triglyceride
Figure 2.
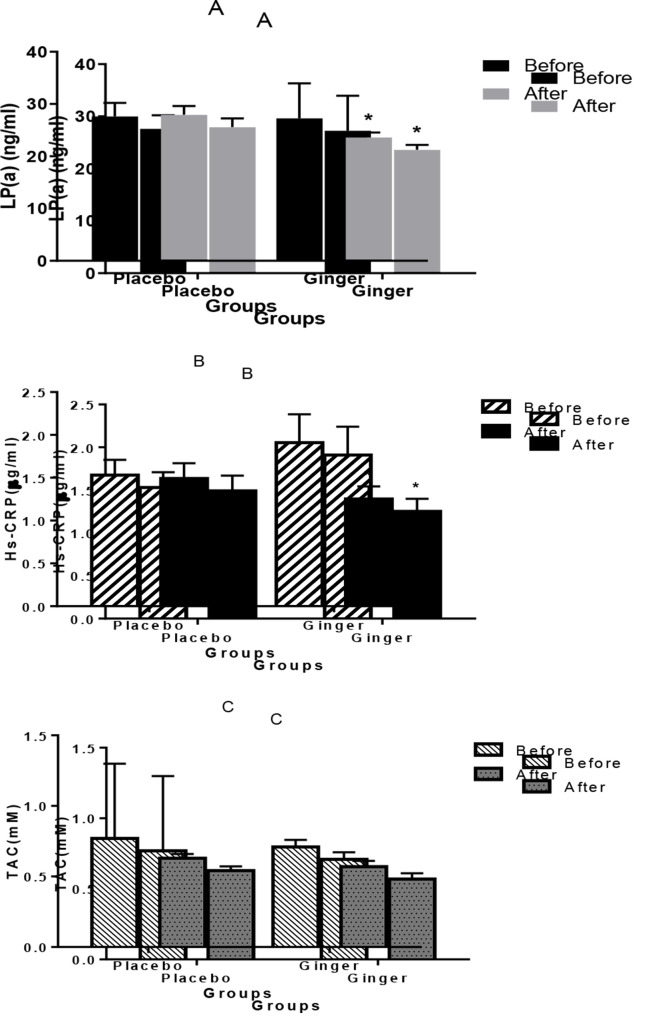
Patients with atherosclerosis in the ginger and control groups received 1600 mg of powdered ginger or placebo (wheat flour) in capsules daily for 8 weeks. Serum levels of lipoprotein (a) [Lp(a)] (A), high-sensitivity C-reactive protein (hs-CRP) (B), and total antioxidant capacity (TAC) (C) have been shown in ginger and placebo groups before and after the intervention. Statistical significances are indicated as follows: * P < 0.050. Data are presented as mean ± standard error of the mean (SEM).
Table 3 evaluates the association between groups and risk factors when adjusted for confounder factors (such as age, smoking, HTN, and diabetes) and baseline variables.
Table 3.
Outcome variables of ginger and placebo groups with control of baseline and other variables*
| Variables | B | 95% CI | P |
|---|---|---|---|
| Weight | -3.75 | (-5.20, -2.30) | < 0.001 |
| BMI | -1.25 | (-1.77, -0.72) | < 0.001 |
| FBS | -20.47 | (-38.19, -2.75) | 0.024 |
| Cholesterol | 5.32 | (-8.55, 19.19) | 0.445 |
| HDL | 0.07 | (-2.60, 2.75) | 0.957 |
| LDL | -0.60 | (-13.36, 12.16) | 0.925 |
| VLDL | 0.94 | (-7.72, 9.59) | 0.829 |
| TG | 1.44 | (-43.62, 46.51) | 0.949 |
| Lp(a) | -4.21 | (-8.16, -0.25) | 0.038 |
| CRP | -0.39 | (-0.79, 0.01) | 0.053 |
| TAC | -0.06 | (-0.18, 0.06) | 0.255 |
The model includes age, smoking, hypertension (HTN), and diabetes (Multiple linear regression test)
BMI: Body mass index; FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; VLDL: Very-low-density lipoprotein; TG: Triglyceride; Lp(a): Lipoprotein (a); CRP: C-reactive protein; TAC: Total antioxidant capacity; CI: Confidence interval
Table 2 shows the effect of taking ginger and placebo capsules on the level of BMI, FBS, and lipid profiles at the beginning and end of the trial. The analysis of data shows that the amount of weight, BMI, and FBS in the ginger group was decreased, and this was statistically significant (P < 0.050) but not in the placebo group. The levels of cholesterol, TG, LDL, and VLDL were declined in the ginger group but these were not statistically significant. In addition, the level of HDL had no significant increase in the ginger group (P = 0.100).
Figure 2 shows the level of Lp(a), CRP, and TAC in ginger and placebo groups at the beginning and end of the intervention. The mean ± SEM Lp(a) in the ginger group was 27.25 ± 1.30 ng/ml and after the trial was 23.57 ± 0.97 ng/ml (P = 0.040). The level of CRP in the ginger group was 1.90 ± 0.33 µg/ml and after the trial was 1.24 ± 0.15 µg/ml (P = 0.010). In addition, the level of TAC in the ginger group was 0.71 ± 0.05 mM and after the trial was 0.57 ± 0.04 mM (P = 0.050). Within the data analysis, the level of Lp(a) and CRP significantly decreased in the ginger group (P < 0.050) but not in the placebo group. No differences were seen in TAC when ginger was administered at 1600 mg/daily for 60 days (P = 0.050).
According to the results in table 3, weight (P < 0.001), BMI (P < 0.001), FBS (P = 0.024), and Lp(a) (P = 0.038) significantly decreased in the ginger group compared to the placebo group. There was a borderline decrease in CRP, but this was not statistically significant (P = 0.053). No significant differences were seen in TAC, cholesterol, and lipid profiles after trial.
Discussion
The main underlying cause of CVD is a process known as atherosclerosis.13 Inflammation, oxidative stress, and dyslipidemia are playing important roles in the progression of atherosclerosis.14 According to previous studies, medicinal plants have numerous health benefits.15 Medicinal herb is a plant that is able to change the physiological and pathological processes and can be used to prevent or treat diseases.16 The purpose of this study was to investigate the effect of ginger supplementation on Lp(a), TAC, hs-CRP, and lipid profile in the patients with therosclerosis.
Based on previous animal and human studies, some herbal medicine can reduce body weight.17,18 Our results showed that ginger supplementation was able to reduce weight in patients with CVD. Studies on using ginger supplementation as a weight loss component were controversial. Although some studies reported that ginger produced a significant reduction in body weight, there is little evidence to support its effectiveness as a weight loss supplement.19,20
Dyslipidemia is the most important contributing factor to the pathogenesis of atherosclerosis. LDL, VLDL remnants, chylomicron remnants, small dense LDL (sdLDL), Lp(a), and oxidized LDL are pro-atherogenic and HDLs are anti-atherogenic Lps.21 Although there is some scientific evidence for the positive impact of ginger supplementation on dyslipidemia, these supportive data are mostly based upon animal and in vitro experiments.22 Based on the results of our study, HDL, LDL, and TG are statistically the same following 8-week ginger supplementation. In a human trial, it was demonstrated that 4 g of ginger given daily for 4 months to patients with coronary artery disease (CAD) failed to affect significantly blood lipids.23 These controversies surrounding animal and human studies may be because of the interaction of other lipid-lowering drugs. In the other words, the level of cholesterol, LDL, and TG had been already controlled by these drugs.
Natural antioxidants, in particular tannins, flavonoids, and C and E vitamins have the ability to maintain β-cells performance and decrease glucose levels in the blood.24 The blood sugar-lowering impact of ginger supplementation has been discussed in a large number of studies. It is well known that ginger can act as a hypoglycemic agent in patients with type 2 diabetes.18,25 Based on our findings, there was a 12.5% decrease in FBS in patients who consumed ginger supplements for 8 weeks compared to control subjects.
The evidence from literature-based meta-analyses of prospective studies suggests the potential importance of Lp(a) in CVD.26,27 Lp(a) is independently associated with CVD risk.5 Elevated Lp(a) levels may develop atherosclerosis via Lp(a)-derived cholesterol entrapment in the intima, inflammatory cell recruitment, and/or the binding of pro-inflammatory oxidized phospholipids (OxPLs). The pro-thrombotic antifibrinolytic actions of apo(a) are expressed, on the one hand, as inhibition of fibrinolysis with enhancement of clot stabilization and on the other hand, as enhanced coagulation via the inhibition of tissue factor pathway inhibitor (TFPI).4
Based on the recent evidence on the role of Lp(a) as contributing factor to the development of CVD, decreased Lp(a) level may reduce the risk of atherosclerosis.28 Since lifestyle in general (diet, exercise, etc.) has no remarkable impact on Lp(a), only pharmacologic interventions or extracorporeal Lp apheresis may be suitable to lower Lp(a) level in patients.6 Previous studies confirm that Lp(a) is lowered by estrogens, niacin, and Lp apheresis.29 There is little randomized intervention showing that any kind of herbal medicine reduces Lp(a). Since many studies are conducted on the effect of chemical drugs on the level of Lp(a), finding an herbal drug with fewer side effects can be helpful in this process.
The mechanism of the effect of Lp(a)-lowering drugs is unclear but may be related to regulation of LDL receptor expression and modifications of Lp(a) catabolism.30 Our study showed that ginger was a potent medicinal herb that reduced Lp(a) levels in patients with atherosclerosis.
The CRP is synthesized by the liver and used mainly as a marker of subclinical chronic vascular inflammation, and it has a predictive value of future cardiovascular events.31 It is well known that some herbal medicine showed significant anti-inflammatory properties. The herbal properties of ginger are similar to NSAIDs; therefore, it can regulate biochemical pathways which are activated with chronic inflammation.18
Based on the results of our study, CRP, as a pro-inflammatory cytokine and marker of atherosclerosis, decreased significantly following consumption of ginger powder for 8 weeks. Our result is similar to the result of the other human studies. In a double-blinded, placebo-controlled clinical trial, 12 weeks of ginger supplementation reduced the level of CRP in patients with type 2 diabetes.32 Another study by Shidfar et al. showed that 3 months supplementation of ginger improved CRP levels.25 This reduces inflammation and its complications in patients with CVD.
Oxidative stress is one of the contributory factors that have long been recognized as being involved in the development of CVD. Thus, plasma antioxidants that show antioxidant/anti-inflammatory effects have been linked to a lower risk of CVD.9 Antioxidant compounds, rich in some planet and herbal medicine, are able to protect cell membranes against damage.7,33
Although ginger contains polyphenol compounds (6-gingerol and its derivatives) which have a high antioxidant activity,34 our results could not show significant changes in TAC. The results of this study are in contrast with other studies which used a higher dose and/or longer intervention.18,25 It seems that to assess the merits of the antioxidant properties of ginger, the patient should consume a higher dose (at least 3 g per day). Further studies are being undertaken to fully explain the effective dose of ginger to show its antioxidant characteristics. Our previous study showed that 6-gingerol decreased proteoglycan in vascular SMCs (VSMCs) stimulated by transforming growth factor beta (TGF-β).35
Conclusion
This study demonstrated that daily consumption of 1600 mg of ginger in capsules for 8 weeks by patients with CVD led to lowering FBS as well as variation in Lp(a) and a decrease of weight and BMI. Moreover, it seems effective in decreasing CRP but the intergroup changes was borderline and further study is needed. These data show that ginger supplement has anti-inflammatory anti-glycemic properties and main effect on reducing Lp(a).
Acknowledgments
This paper was obtained from an MSc thesis approved and financially supported by Ahvaz Jundishapur University of Medical Sciences (Grant no. CMRC 9411).
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Mackay J, Mensah GA. The atlas of heart disease and stroke. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 3.Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol. 2001;88(7B):3J–6J. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur Heart J. 2010;31(23):2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Blom D, Seidah NG, et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: An analysis of 10 clinical trials and the LDL receptor's role. J Lipid Res. 2016;57(6):1086–96. doi: 10.1194/jlr.P065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassner U, Schlabs T, Rosada A, Steinhagen-Thiessen E. Lipoprotein(a)--An independent causal risk factor for cardiovascular disease and current therapeutic options. Atheroscler Suppl. 2015;18:263–7. doi: 10.1016/j.atherosclerosissup.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 7.Kooti W, Mansouri E, Ghasemiboroon M, Harizi M, Ashtary-Larky D, Afrisham R. The Effects of Hydroalcoholic Extract of Apium graveolens Leaf on the Number of Sexual Cells and Testicular Structure in Rat. Jundishapur J Nat Pharm Prod. 2014;9(4):e17532. doi: 10.17795/jjnpp-17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra B, Panja M. High sensitive C-reactive protein: A novel biochemical markers and its role in coronary artery disease. J Assoc Physicians India. 2005;53:25–32. [PubMed] [Google Scholar]
- 9.Wang Y, Chun OK, Song WO. Plasma and dietary antioxidant status as cardiovascular disease risk factors: A review of human studies. Nutrients. 2013;5(8):2969–3004. doi: 10.3390/nu5082969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werneke U, Earl J, Seydel C, Horn O, Crichton P, Fannon D. Potential health risks of complementary alternative medicines in cancer patients. Br J Cancer. 2004;90(2):408–13. doi: 10.1038/sj.bjc.6601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grzanna R, Lindmark L, Frondoza CG. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–32. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Blair EY, McLachlan AJ. Investigation of the effects of herbal medicines on warfarin response in healthy subjects: A population pharmacokinetic-pharmacodynamic modeling approach. J Clin Pharmacol. 2006;46(11):1370–8. doi: 10.1177/0091270006292124. [DOI] [PubMed] [Google Scholar]
- 13.Frostegard J. SLE, atherosclerosis and cardiovascular disease. J Intern Med. 2005;257(6):485–95. doi: 10.1111/j.1365-2796.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 14.Tietge UJ. Hyperlipidemia and cardiovascular disease: Inflammation, dyslipidemia, and atherosclerosis. Curr Opin Lipidol. 2014;25(1):94–5. doi: 10.1097/MOL.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 15.Shirali S, Shokri Mashhadi N, Ashtary-Larky D, Safania T, Barari A. Effects of silymarin supplementation on leptin, adiponectin and paraoxanase levels and body composition during exercise: A randomized double-blind placebo controlled clinical trial. Jundishapur J Nat Pharm Prod. 2017;11(4) [Google Scholar]
- 16.Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin J Nat Med. 2016;14(10):732–45. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- 17.Goyal RK, Kadnur SV. Beneficial effects of Zingiber officinale on goldthioglucose induced obesity. Fitoterapia. 2006;77(3):160–3. doi: 10.1016/j.fitote.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffari-Khosravi H, Talaei B, Jalali BA, Najarzadeh A, Mozayan MR. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2014;22(1):9–16. doi: 10.1016/j.ctim.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol. 2008;46(2):409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe PA, Granner ML, Conway JM, Ainsworth BE, Dobre M. Availability of weight-loss supplements: Results of an audit of retail outlets in a southeastern city. J Am Diet Assoc. 2006;106(12):2045–51. doi: 10.1016/j.jada.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Koba S, Hirano T. Dyslipidemia and atherosclerosis. Nihon Rinsho. 2011;69(1):138–43. [PubMed] [Google Scholar]
- 22.Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): A hot remedy for cardiovascular disease? Int J Cardiol. 2009;131(3):408–9. doi: 10.1016/j.ijcard.2007.07.107. [DOI] [PubMed] [Google Scholar]
- 23.Bordia A, Verma SK, Srivastava KC. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1997;56(5):379–84. doi: 10.1016/s0952-3278(97)90587-1. [DOI] [PubMed] [Google Scholar]
- 24.Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron Physician. 2016;8(1):1832–42. doi: 10.19082/1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shidfar F, Rajab A, Rahideh T, Khandouzi N, Hosseini S, Shidfar S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J Complement Integr Med. 2015;12(2):165–70. doi: 10.1515/jcim-2014-0021. [DOI] [PubMed] [Google Scholar]
- 26.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102(10):1082–5. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 27.Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, et al. Lipoprotein(a) levels and risk of future coronary heart disease: Large-scale prospective data. Arch Intern Med. 2008;168(6):598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 28.Tsimikas S. Lipoprotein(a): Novel target and emergence of novel therapies to lower cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):157–64. doi: 10.1097/MED.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bos S, Yayha R, van Lennep JE. Latest developments in the treatment of lipoprotein (a). Curr Opin Lipidol. 2014;25(6):452–60. doi: 10.1097/MOL.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 30.Norata GD, Ballantyne CM, Catapano AL. New therapeutic principles in dyslipidaemia: Focus on LDL and Lp(a) lowering drugs. Eur Heart J. 2013;34(24):1783–9. doi: 10.1093/eurheartj/eht088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghafourian M, Ashtary-Larky D, Chinipardaz R, Eskandary N, Mehavaran M. Inflammatory biomarkers' response to two different intensities of a single bout exercise among soccer players. Iran Red Crescent Med J. 2016;18(2):e21498. doi: 10.5812/ircmj.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014;65(4):515–20. doi: 10.3109/09637486.2014.880671. [DOI] [PubMed] [Google Scholar]
- 33.Mansouri E, Ghasemiboroon M, Asadi Samani M, Alamiri F, Ashtary-Larky D, Kafash Farkhad N, et al. The effect of hydro-alcoholic extract of apium graveolens L. leaf on delivery rate in female rats, weight and gender ratio of infants. Jundishapur J Natur Pharm Products. 2017;12(1):e28802. [Google Scholar]
- 34.Stoilova I, Krastanov A, Stoyanova A, Denev P, Gargova S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007;102(3):764–70. [Google Scholar]
- 35.Kamato D, Babaahmadi Rezaei H, Getachew R, Thach L, Guidone D, Osman N, et al. (S)-[6]-Gingerol inhibits TGF-beta-stimulated biglycan synthesis but not glycosaminoglycan hyperelongation in human vascular smooth muscle cells. J Pharm Pharmacol. 2013;65(7):1026–36. doi: 10.1111/jphp.12060. [DOI] [PubMed] [Google Scholar]