Abstract
Background
Widespread uptake of COVID-19 vaccines will be essential to controlling the COVID-19 pandemic. Vaccines have been developed in unprecedented time and quantifying levels of hesitancy towards vaccination among the general population is of importance.
Methods
Systematic review and meta-analysis of studies using large nationally representative samples (n ≥ 1000) to examine the percentage of the population intending to vaccinate, unsure, or intending to refuse a COVID-19 vaccine when available. Generic inverse meta-analysis and meta-regression were used to pool estimates and examine time trends. PubMed, Scopus and pre-printer servers were searched from January-November 2020. Registered on PROSPERO (CRD42020223132).
Findings
Twenty-eight nationally representative samples (n = 58,656) from 13 countries indicate that as the pandemic has progressed, the percentage of people intending to vaccinate decreased and the percentage of people intending to refuse vaccination increased. Pooled data from surveys conducted during June-October suggest that 60% (95% CI: 49% to 69%) intend to vaccinate and 20% (95% CI: 13% to 29%) intend to refuse vaccination, although intentions vary substantially between samples and countries (I2 > 90%). Being female, younger, of lower income or education level and belonging to an ethnic minority group were consistently associated with being less likely to intend to vaccinate. Findings were consistent across higher vs. lower quality studies.
Interpretation
Intentions to be vaccinated when a COVID-19 vaccine becomes available have been declining across countries and there is an urgent need to address social inequalities in vaccine hesitancy and promote widespread uptake of vaccines as they become available.
Funding
N/A.
Keywords: COVID-19, Coronavirus, Vaccine, Intentions, Acceptance, Attitudes
1. Introduction
The COVID-19 pandemic has resulted in more than 1 million deaths worldwide from March to October 2020 [1] and is likely to continue to have far reaching impacts on healthcare systems [2]. The development of vaccines against COVID-19 has been occurring at unprecedented speed, and as of November 2020, there are multiple candidate vaccines in the final stages of testing [3]. The success of any vaccination programme is dependent on the proportion of the population willing to be vaccinated and based on recent estimates it is likely that up to three quarters of the population may require vaccination to bring an end to the pandemic [4], [5].
Early in the pandemic a small number of studies surveyed adults to gauge public willingness to be vaccinated against COVID-19 and although a number of studies were reliant on non-representative convenience samples, the majority of the populations sampled intended to vaccinate [6], [7], [8], [9]. For example, a cross country survey found a relatively high average level of intended vaccination (72%), although sample sizes of individual countries were low (N = ~600–800) and may not provide accurate nationally representative coverage [7].
However, as the pandemic has evolved there have been reports of widespread misinformation about COVID-19 [10], distrust in government [11] and public concerns about the safety of COVID-19 vaccines given their rapid development [12], all of which may have affected intended vaccine uptake. It is also unclear whether vaccine acceptability will be socio-demographically patterned. Disadvantaged minority groups have previously been shown to be less likely to intend to be vaccinated for influenza [13], [14]. However, a systematic review concluded that patterning for other demographic groups during previous influenza vaccination programmes is inconsistent [15]. Given that current evidence on socio-demographic patterning of COVID-19 vaccination intentions is lacking, it will be important to understand how vaccination intentions differ within and across countries to inform measures to improve public acceptability and uptake of vaccination programmes.
We conducted a rapid systematic review and meta-analysis of large nationally representative study samples to address the current lack of consensus on; i) the proportion of the population willing to be vaccinated against COVID-19 when a vaccine become available and how this differs across countries, ii) whether vaccination intentions have declined as the pandemic has progressed, iii) socio-demographic inequalities in intended vaccine uptake.
2. Method
To inform mass COVID-19 vaccination programmes, we used rapid systematic review methodology [16]. Rapid reviews provide timely evidence synthesises whilst maintaining the rigour of traditional systematic reviews [17] by using expedited review processes [17], [18], such as limiting databases searched or number of reviewers (e.g. cross-checking of a proportion of extraction as opposed to independent extraction by a second author).
Eligibility criteria. We included studies that measured intentions to be vaccinated against coronavirus in nationally representative samples of the general public. Published journal articles and pre-prints were eligible for inclusion. News articles reporting on opinion polls (with no corresponding scientific report including methodology used) were not eligible. The review is registered on PROSPERO (CRD42020223132).
Populations and study design: To be eligible studies were required to have used a sampling approach designed to be nationally representative on key population demographics of the country (e.g. gender, education level), such as quota sampling or random probability sampling. Sampling error is problematic when examining prevalence estimates in small sample sizes [19]. Because sampling error tends to be minimal at sample sizes of ≥ 1000 [19], as is considered common practice for nationally representative surveys [20], we limited eligibility to studies with a sample of n ≥ 1000 from the same country. Studies that collected non-general public samples (e.g. healthcare professionals, parents, students) were not eligible. Studies that used non-representative sampling (e.g. convenience sampling, snowball sampling) were not eligible and studies that lacked sufficient methodological information to determine sampling used were ineligible.
Interest (measure): To be eligible studies were required to include a question that measured intentions/willingness to use a vaccine for COVID-19 when one becomes available (e.g. ‘I would use a vaccine for COVID-19 when it becomes available’). Studies that exposed participants to information designed to alter vaccine intentions (e.g. experiments comparing how public health messages may improve vaccine intentions) were not eligible. Studies that compared willingness to take different types of vaccine (e.g. varying hypothetical effectiveness/price) were ineligible, unless they also included a questionnaire item measuring general willingness to take a COVID-19 vaccine.
Outcome: Studies were required to report the proportion of participants responding to the different response options for the vaccine intention question (e.g. Yes vs. No, Willing vs. Unsure Vs. Unwilling, Likely vs. Undecided vs. Unlikely).
Article identification strategy. During November 2020, we searched PUBMED and Scopus (2020 onwards) for published articles in peer reviewed journals. We used the following search terms: (COVID OR coronavirus OR SARS-COV-2) AND (Vaccine OR Vaccination) AND (Inten* OR willing* OR attitud* OR hypothetical). We also searched three pre-print servers; Open Science Framework (which hosts 30 other preprint archives, including PsychArxiv), MedrXiv and the Social Science Research Network (SSRN). One author conducted the initial title and abstract screening to exclude unrelated articles and a second author checked 25% of this (there were no discrepancies). A single author conducted full-text screening to determine eligibility and a second author cross-checked all eligibility decisions. For all eligible articles identified through searches, we used forward citation tracking (Google Scholar) and our knowledge of existing research to identify any further articles.
Data extraction. For each study one author extracted information, all extraction was cross-checked by a second author and disagreements were resolved through discussion. We extracted the following information; bibliographic information, country, sampling procedure (e.g. quota vs. probability), sample size, month of survey, measure of vaccination intentions and results. We extracted results based on response options, resulting in % choosing response options indicative of definitely or probably yes, % definitive or probable no, % unsure (if measure allowed for the latter). If studies had multiple waves of data collection, we extracted results from the most recent wave. We also extracted information on whether studies reported results of analyses examining demographic predictors of vaccination intentions that were commonly examined (i.e. at least 5 studies) in studies. To be eligible for extraction, demographic predictors were required to be examined adjusting for other demographics (i.e. zero-order correlations were not eligible) in order to be confident of independent effects. We prioritised extraction of results of analyses that examined vaccine intentions (i.e. reference of category of yes vs. other (unsure/no combined). If this analysis was not available, in order of priority we favoured extracting demographic results for analyses examining yes vs. no, then yes vs. unsure.
Risk of bias indicators. We considered methodological factors of studies that may impact on results by increasing risk of bias. Quota-based samples were rated as being higher in risk of bias than probability-based samples, as the latter tends to be a more accurate/representative sampling approach [21], [22]. We also reasoned that studies not having yet undergone formal peer review (unpublished pre-prints) may increase risk of bias and coded for this. For studies examining demographic predictors, we considered relatively smaller sample sizes (n < 2500) as higher in risk of bias due to concerns over small numbers of cases in analyses when examining sub-groups. For example, with n ≥ 2500, for minority sub-groups (e.g. ~ 2% of the population, such as Black people in the UK) there would be expected to be a minimum of 50 cases in analyses, as opposed to only 20 cases with n = 1000. Studies of demographic predictors that adjusted for attitudinal predictors (e.g. attitudes towards vaccination) of vaccine intentions were considered higher in risk of bias, as inclusion may mask associations between demographics and vaccination intentions. For example, if demographic patterning of vaccination intentions is caused in part by attitudinal differences, including attitudinal measures as predictor variables of intentions (alongside demographic factors) would reduce and ‘mask’ an association between a demographic factor and vaccination intentions.
Synthesis of evidence: We meta-analysed proportions of the samples ((Total sample N / 100) * % reporting) reporting: i) intending to vaccinate, ii) unsure, and iii) not intending to vaccinate. Analysis was performed using the ‘metafor’ package in R. We used a logit transformation on raw proportions in random effects, generic inverse variance meta-analyses with a restricted maximum-likelihood estimator. Transformations were conducted using the ‘escalc’ function in the metafor package. Back-transformed (inverse) logit values are presented in the text, whilst raw proportion data is presented in forest and funnel plots to aid interpretation. Heterogeneity was assessed with the I2 statistic. We conducted meta-regressions to examine the relationship between outcomes and month of study data collection (treating month as a continuous variable, e.g. March = 1, April = 2). We conducted leave-one-out analysis to examine the stability of the pooled estimates and identify any influential samples in the main analyses and meta-regressions. We reasoned that studies which did not use an ‘unsure’ or ‘undecided’ response option (e.g. responses grouped into yes vs. no, as opposed to yes vs. unsure vs. no) may affect vaccine acceptance and rejection estimates, so we examined if pooled estimates of intended vaccination and intended refusal differed between these two types of study. Furthermore, in sensitivity analyses we examined whether the inclusion of an ‘unsure/undecided’ response option moderated any effects of month of study data collection on intention estimates. We also examined the effect of month of study data collection separately in studies with vs. without an ‘unsure/undecided’ response option.
To address risk of bias, we conducted sub-group analyses to examine whether results differed between studies using quota vs. probability sampling and pre-prints vs. journal articles. We limited these analyses to studies which collected data early in the pandemic (March-May) to account for the majority of probability samples (3/4) and journal article sample (12/12) studies being conducted in this period. Sub-group analysis compared studies that did vs. did not allow for an ‘unsure/undecided’ response option. We also examined potential publication bias and small study effects (see online supplementary materials). Demographic predictors were measured and/or analysed differently across studies, so for each demographic we reported the proportion of studies finding evidence vs. no evidence of significant (p < .05) relationships with vaccination intentions, and whether results were similar when limited to studies that had larger sample sizes and did not adjust for attitudinal predictors in analyses (i.e. higher quality studies).
3. Results
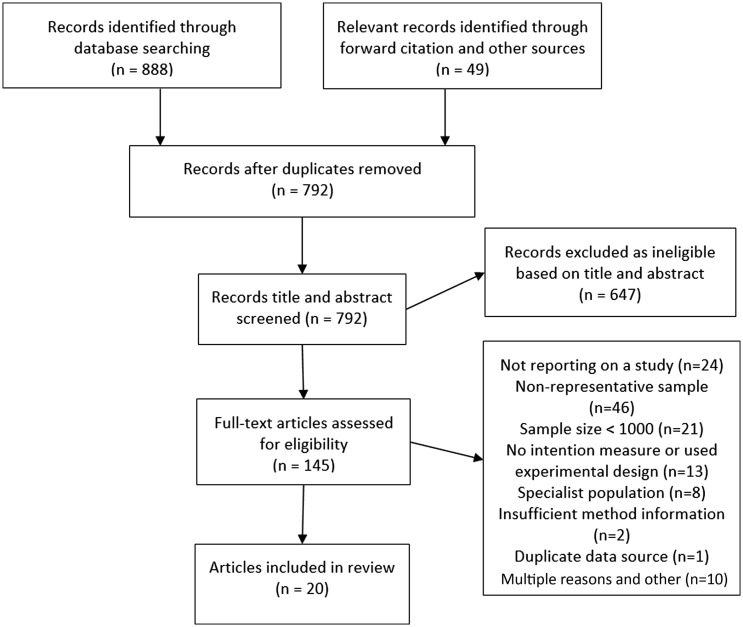
Study selection. A total of 792 unique articles were found through database searches and other sources. Of the 145 articles full-text screened, 20 articles reporting on 28 samples were eligible for inclusion. See Fig. 1 . For bibliographic information of all included articles, see online supplementary material.
Fig. 1.
Study selection flowchart.
Overview. Of the 28 samples included, the majority were from the UK and North America (7 = US, 2 = Canada, 7 = UK) as well as samples from France (n = 3), Australia, China, Denmark, Germany, Italy, Ireland Netherlands, Poland and Portugal. Full study information is reported in Table 1 . Sample sizes ranged from 1000 to 7547 (median = 1198). Samples were collected in the early phases of the pandemic (March-May, n = 18) and later (June onwards, n = 10). Twenty-four samples used quota-based sampling and four used probability sampling. Twelve articles were in peer-reviewed journals and 16 were pre-prints (at the time of identification). Sixteen studies included an ‘unsure/undecided’ response option for vaccination intentions and 12 studies did not include this response option.
Table 1.
Study and sample information of eligible articles included in review.
| Author | Country | Journal article or pre-print | Probability vs. quota sampling1 | N | Month | Measure2 | Intention results3 |
|---|---|---|---|---|---|---|---|
| Edwards | Australia | Pre-print | Quota | 3061 | August | If a safe and effective vaccine for COVID-19 is developed, would you… Would definitely [Y], probably [Y], probably not [N], definitely not [N] | Yes: 87% No: 13% |
| Leigh | Canada | Pre-print | Quota | 1996 | April-May | I will get vaccinated for the virus when it is developed… Strongly disagree [N], disagree [N], somewhat disagree [N], undecided [U], somewhat agree [Y], agree [Y], strongly agree [Y] |
Yes: 76% Unsure: 13% No: 11% |
| Taylor | Canada | Journal article | Quota | 1902 | May | If a vaccine for COVID-19 was available, would you get vaccinated? Yes [Y], no [N] | Yes: 80% No: 20% |
| Wang | China | Journal article | Quota | 2058 | March | Accept vaccination if the COVID-19 vaccine is successfully developed and approved for listing in the future? Yes [Y], no [N] | Yes: 91% No: 9% |
| Neumann-Böhme | Denmark | Journal article | Quota | 1000 | April | Would you be willing to get vaccinated against the novel coronavirus? Yes [Y], no [N], not sure [U] |
Yes: 80% Unsure: 12% No: 8% |
| Neumann-Böhme | France | Journal article | Quota | 1000 | April | Would you be willing to get vaccinated against the novel coronavirus? Yes [Y], no [N], not sure [U] |
Yes: 62% Unsure: 28% No: 10% |
| Ward | France | Pre-print | Quota | 5018 | April | Respondents were asked whether they would agree to get vaccinated if a vaccine against the COVID-19 was available: Certainly [Y], probably [Y], probably not [N], certainly not [N] |
Yes: 76% No: 24% |
| Hacquin | France | Pre-print | Quota | 1003 | September | Respondents were asked whether they would agree to get vaccinated if a vaccine against the COVID-19 were available Certainly [Y], probably [Y], probably not [N], certainly not [N] |
Yes: 52% No: 48% |
| Neumann-Böhme | Germany | Journal article | Quota | 1002 | April | Would you be willing to get vaccinated against the novel coronavirus? Yes [Y], no [N], not sure [U] |
Yes: 70% Unsure: 20% No:10% |
| Murphy | Ireland | Pre-print | Quota | 1041 | March-April | If a new vaccine were to be developed that could prevent COVID-19, would you accept it for yourself? Yes [Y], maybe [Y], no [N] |
Yes: 91% No: 10% |
| Graffigna | Italy | Journal article | Probability | 1004 | May | Willingness to vaccinate against COVID-19 whenever the vaccine is available Absolutely likely [Y], very likely [Y], neither likely or unlikely [U], unlikely [N], not likely at all [N] |
Yes: 59% Unsure: 26% No: 15% |
| Neumann-Böhme | Netherlands | Journal article | Quota | 1012 | April | Would you be willing to get vaccinated against the novel coronavirus? Yes [Y], no [N], not sure [U] |
Yes: 73% Unsure: 19% No: 8% |
| Feleszko | Poland | Pre-print | Quota | 1066 | June | If a vaccine against COVID-19 is available do you plan to vaccinate? Yes [Y], no [N], I do not know/it is difficult to answer [U] |
Yes: 37% Unsure 34% No: 28% |
| Neumann-Böhme | Portugal | Journal article | Quota | 1064 | April | Would you be willing to get vaccinated against the novel coronavirus? Yes [Y], no [N], not sure [U] |
Yes: 75% Unsure: 21% No: 5% |
| Murphy | UK | Pre-print | Quota | 2025 | March | If a new vaccine were to be developed that could prevent COVID-19, would you accept it for yourself? Yes [Y], maybe [Y], no [N] |
Yes: 94% No: 6% |
| Neumann-Böhme | UK | Journal article | Quota | 1009 | April | Would you be willing to get vaccinated against the novel coronavirus? Yes [Y], no [N], not sure [U] |
Yes: 79% Unsure: 15% No: 6% |
| Freeman | UK (England) | Journal article | Quota | 2501 | May | How likely it was that you would accept a vaccination for Coronavirus? Definitely [Y], probably [Y], possibly [Y], probably not [N], definitely not [N] |
Yes: 88% No: 12% |
| Roozenbeek |
UK | Journal article | Quota | 1150 | May | Participants were asked whether they would get vaccinated against COVID-19 if a vaccine were to become available Yes [Y], no [N] | Yes: 79% No: 21% |
| McAndrew | UK | Pre-print | Quota | 1663 | June | When a Coronavirus (COVID-19) vaccine becomes available, do you think you will or will not get vaccinated? Definitely will get vaccinated [Y], probably will get vaccinated [Y], probably will not get vaccinated [N], definitely will not get vaccinated[N], unsure [U] |
Yes: 69% Unsure:15% No:16% |
| Sherman | UK | Pre-print | Quota | 1504 | July | How likely would you be to have a COVID-19 vaccination when a coronavirus vaccination becomes available? Eleven-point scale from extremely unlikely to extremely likely. 0–2 [N], 3–7 [U], 8–10 [Y] | Yes: 64% Unsure: 27% No: 9% |
| Loomba | UK | Pre-print | Quota | 4001 | September | If a new coronavirus (COVID-19) vaccine became available, would you accept the vaccine for yourself? Yes [Y], definitely [Y], unsure but leaning towards yes [U], unsure but leaning towards no [U], no definitely not [N] | Yes: 54% Unsure: 40% No:6% |
| Romer | US |
Journal article | Probability | 1050 | March | If there were a vaccine that protected you from getting the coronavirus, how likely, if at all, would you be to decide to be vaccinated? Not at all likely [N], not too likely [N], somewhat likely [Y], very likely [Y] |
Yes: 86% No: 15% |
| Taylor | US | Journal article | Quota | 1772 | May | If a vaccine for COVID-19 was available, would you get vaccinated? Yes [Y], no [N] | Yes: 75% No: 25% |
| Carpiano | US | Pre-print | Probability | 1000 | May | If a vaccine against the coronavirus becomes available, do you plan to get vaccinated, or not? Yes [Y], no [N], not sure [U] |
Yes: 50% Unsure: 30% No: 20% |
| Callaghan | US | Pre-print | Quota | 5009 | May-June | Scientists around the world are working on developing a vaccine to protect individuals against the coronavirus. If a Vaccine is developed, would you pursue getting vaccinated for the coronavirus? Yes [Y], no [N] | Yes: 69% No: 31% |
| McAndrew | US |
Pre-print | Quota | 1198 | June | When a Coronavirus (COVID-19) vaccine becomes available, do you think you will or will not get vaccinated? Definitely will get vaccinated [Y], probably will get vaccinated [Y], probably will not get vaccinated [N], definitely will not get vaccinated [N], unsure [U] | Yes: 59% Unsure: 15% No: 25% |
| Loomba | US |
Pre-print | Quota | 4000 | September | If a new coronavirus (COVID-19) vaccine became available, would you accept the vaccine for yourself? Yes definitely [Y], unsure but leaning towards yes [U], unsure but leaning towards no [U], no definitely not [N] |
Yes: 44% Unsure: 41% No:15% |
| Daly | US | Pre-print | Probability | 7547 | October | How likely are you to get vaccinated for coronavirus when a vaccine becomes available Unsure [U], somewhat unlikely [N], very unlikely [N], somewhat likely [Y], very likely [Y] |
Yes: 54% Unsure: 14% No: 32% |
If sampling method was unclear from the description in the study method, we confirmed use of quota vs. probability by searching for the data source online (e.g. panel provider website).
[Y], [N] and [U] indicate extracted response options representing yes, no and unsure in the present meta-analysis.
Values may not equal 100 due to rounding of reported values in study manuscripts.
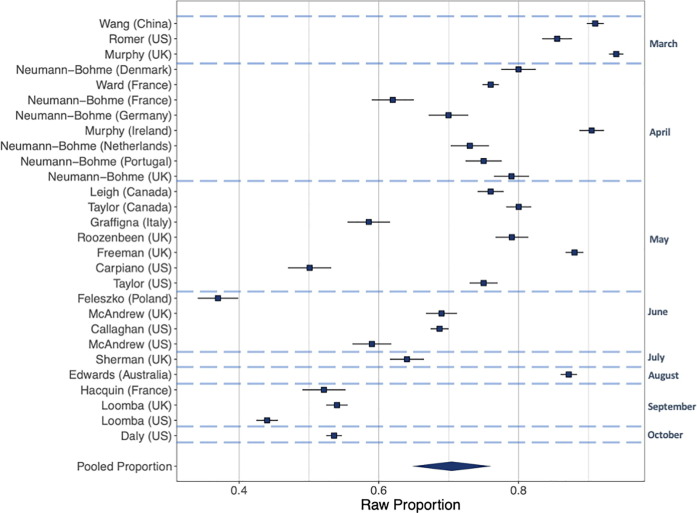
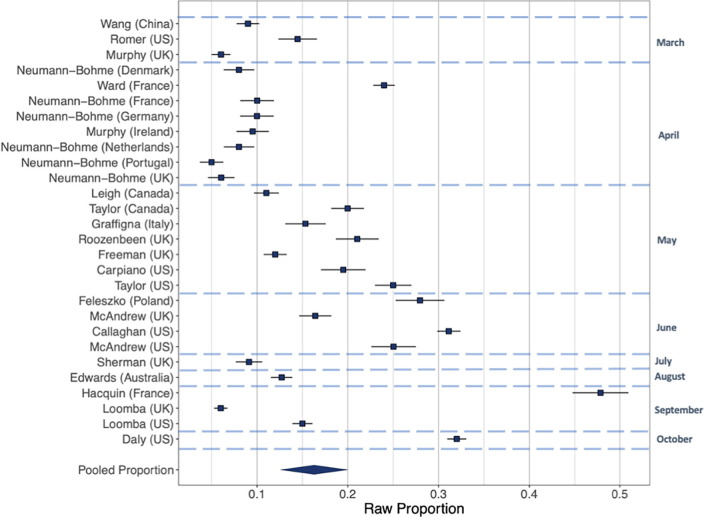
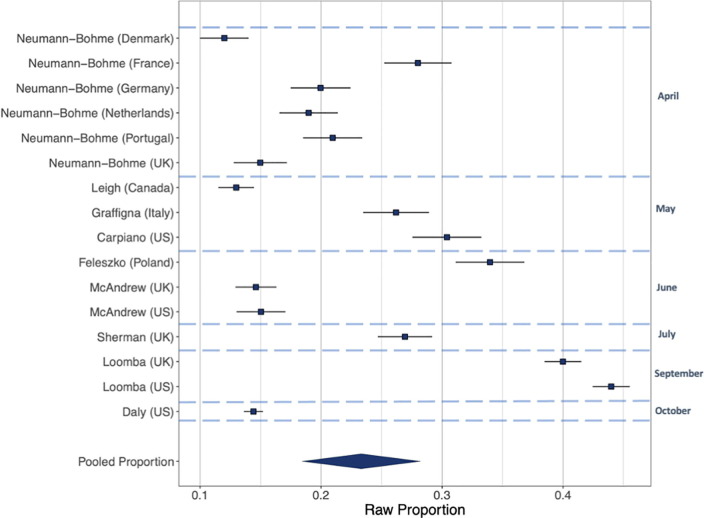
Intentions to vaccinate (Fig. 2, Fig. 3, Fig. 4). Including all 28 samples collected from March-October, the pooled proportion reporting intending to vaccinate was 0.729 [n = 28, 95% CI: 0.666 to 0.784: I2 = 99.6%], the proportion reporting they would refuse a vaccine was 0.143 [n = 28, 95% CI: 0.114 to 0.179: I2 = 99.3%] and the proportion reporting being unsure was 0.221 [n = 16, 95% CI: 0.178 to 0.271: I2 = 99.0%]. Values do not equal 100 as not all studies included an unsure response option. See online supplementary materials document for results in full.
Fig. 2.
Raw proportions of intentions to vaccinate across the 28 samples.
Fig. 3.
Raw proportions of individuals reporting intending not to vaccinate across 16 samples.
Fig. 4.
Raw proportions of individuals reporting unsure of vaccination across 28 studies.
Presence vs. absence of ‘unsure’ response option in survey. There was a significant difference in the proportion of participants intending to vaccinate when an ‘unsure/undecided’ response option was used vs. when there was no ‘unsure/undecided’ response option (X2(1) = 16.82, p < .001). When there was no unsure response option the proportion was 0.828 [95% CI: 0.759 to 0.880: I2 = 99.4%], and when unsure was a response option the proportion was 0.635 [95% CI: 0.569 to 0.670: I2 = 99.2%]. There was not a significant difference in the proportion intending to refuse a vaccine between samples with [0.124: 95% CI: 0.092 to 0.164: I2 = 99.0%] vs. without [0.172: 95% CI: 0.120 to 0.240: I2 = 99.4%] an ‘unsure/undecided’ response option (X2(1) = 2.12, p = .146).
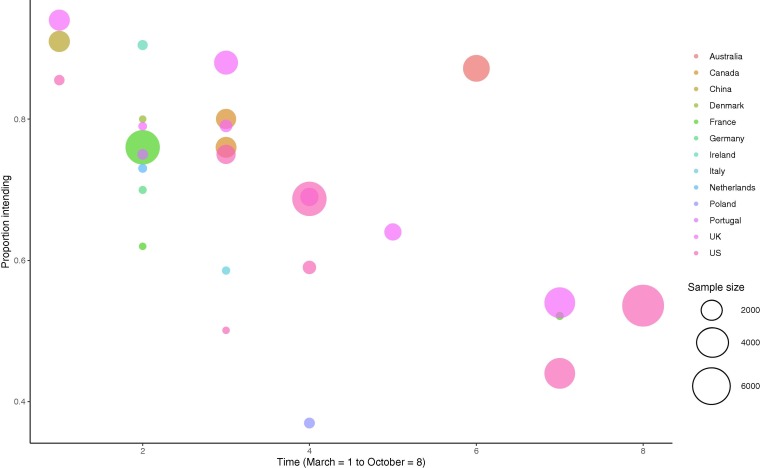
Time trends. There was a significant association between proportion of individuals intending to vaccinate and month study was conducted (coefficient = -0.25 [95% CI: -0.37 to -0.12], z = 3.83, p < .001), see Fig. 5 . There was also a significant association between intentions not to vaccinate and month of study (coefficient = 0.16 [95% CI: 0.03 to 0.28], z = 2.48, p = .013). Over time intentions to vaccinate decreased, and intentions not to vaccinate increased. For example, in studies (n = 18) that collected data during the early phase of the pandemic (March-May), the proportion intending to vaccinate was 79% and not intending to was 12%, compared with 60% and 20% June-October studies (n = 10). There was no association between proportion of individuals being unsure about vaccination and when the study was conducted (coefficient = 0.10 [95% CI: −0.03 to 0.24], z = 1.54, p = .124]. Because samples varied by country, to confirm time trend findings were not explained by different countries being sampled earlier vs. later in the pandemic, we replicated the meta-regressions for the two countries with multiple samples collected during different months. Among UK samples (n = 7, coefficient = −0.39 [95% CI: −0.57 to −0.22], z = 4.34, p < .001) and US samples (n = 7, coefficient = −0.22 [95% CI: −0.38 to −0.05], z = 2.53, p = .011)] the same negative associations were observed as in the main analyses. There was no evidence that the relationship between month of survey data collection and intending to be vaccinated was moderated by whether a study did vs. did not have an ‘unsure/undecided’ response options. See online supplementary materials.
Fig. 5.
Proportion of populations intending to vaccinate by country and time.
Publication and risk of bias analyses. There was minimal evidence of publication bias and leave out one analyses indicated limited variation in estimates (online supplementary materials document). We found no evidence that results differed between samples reported in journal articles vs. pre-prints. There was minimal evidence of differences in findings between studies using probability vs. quota sampling, with the exception of ‘unsure’ responses being lower in quota samples (see online supplementary materials).
Demographic predictors. Fourteen studies examined demographic predictors. See Table 2 . In 12/14 studies older adults were significantly more likely to report intending to vaccinate than younger, one study found no effect of age and in one study young adults (<25 years) were more likely than middle aged adults, but not older adults. In 9/14 studies males were more likely to intend to vaccinate than females (no significant association in five studies). Higher education level was associated with intending to vaccinate in 7/14 studies (no association in seven). White ethnic groups were more likely to vaccinate in 7/11 studies (no association in four). Higher income was associated with being more likely to intend to vaccinate in 8/9 studies and in one study there was no association. Presence of a health condition was examined in five studies. Presence of a health condition was a non-significant predictor in n = 4 and not having a health condition was associated with being more likely to intend to be vaccinated in one study. When analyses were limited to the five higher quality studies (large sample size, no inclusion of attitudinal predictors) the role of demographic factors was more consistent; 5/5 studies found that older adults, males and higher education levels were associated with increased likelihood of intending to vaccinate. Similarly, 3/4 studies found that being white and 4/4 found that those on higher income were more likely to intend to vaccinate. Presence of a health condition was non-significant in n = 2.
Table 2.
Studies examining demographic predictors of vaccination intentions.
| Author & Country | N1 | Comparison information | Age | Gender | Education | Ethnicity | Physical health condition | Income |
|---|---|---|---|---|---|---|---|---|
| Edwards (Australia)* |
3061 | Intention to vaccinate vs. other responses (do not intend and unsure) | Older adults more likely to vaccinate (55yrs + ) | Males more likely to vaccinate | Higher education qualification more likely to vaccinate | Non-significant | Non-significant | Higher income more likely to vaccinate |
| Hacquin (France)* |
4027 | Intention to vaccinate vs. do not intend | Older adults more likely to vaccinate (64yrs + ) | Males more likely to vaccinate | Higher than high school qualification more likely to vaccinate | – | – | – |
| Ward (France) |
5018 | Intention to vaccinate vs. do not intend (Model included attitudinal measures) | Older adults more likely to vaccinate (64yrs + ) | Males more likely to vaccinate | Non-significant | – | – | Higher income more likely to vaccinate |
| Murphy (Ireland) |
1041 | Intention to vaccinate vs. do not intend | Older adults (64yrs + ) more likely to vaccinate than 35–44 yr olds, but no other groups | Non-significant | Non-significant | Irish ethnicity more likely to vaccinate than non-Irish | No health condition more likely to vaccinate | Higher income more likely to vaccinate |
| Roozenbeek (UK) |
1150 | Intention to vaccinate vs. do not intend (Model included attitudinal measures) | Older adults more likely to vaccinate | Non-significant | Non-significant | – | – | – |
| Loomba (UK)* |
4001 | Intention to vaccinate vs. do not intend | Older adults (55yrs + ) more likely to vaccinate than younger (<25 yrs) | Males more likely to vaccinate | Higher education qualification more likely to vaccinate | Whites more likely to vaccinate than Black and Asian (and other) | – | Higher income more likely to vaccinate |
| McAndrew (UK) |
1663 | Intention to vaccinate vs. other responses (do not intend and unsure) | Older adults more likely to vaccinate | Non-significant | Non-significant | Non-significant | – | – |
| Murphy (UK) |
2025 | Intention to vaccinate vs. do not intend | Older adults (65yrs + ) more likely to vaccinate | Non-significant | Non-significant | Non-significant | Non-significant | Higher income more likely to vaccinate |
| Sherman (UK) |
1488 | Likelihood of being vaccinated, continuous measure | Older adults more likely to vaccinate | Non-significant | Non-significant | Non-significant | Non-significant | – |
| Daly (US)* |
7547 | Intention to vaccinate vs. unsure | Older adults more likely to vaccinate (65yrs + ) | Males more likely to vaccinate | Degree level education and above more likely to vaccinate | Whites more likely to vaccinate than African Americans (Black) | Non-significant | Higher income more likely to vaccinate |
| Carpiano (US) |
1000 | Intention to vaccinate vs. unsure |
Older adults (60yrs + ) more likely to vaccinate than 30-59yrs | Males more likely to vaccinate | College level education and above more likely to vaccinate | Whites more likely to vaccinate than African Americans, Hispanic and other | – | Non-significant |
| Callaghan (US) |
5009 | Intention to vaccinate vs. unsure (Model included attitudinal measures) | Older adults more likely to vaccinate | Males more likely to vaccinate | Non-significant | Whites more likely to vaccinate than African Americans | – | Higher income more likely to vaccinate |
| Loomba (US)* |
4000 | Intention to vaccinate vs. do not intend |
Young adults (<25yrs) more likely to vaccinate than other 25–54 | Males more likely to vaccinate | Higher educational qualification more likely to vaccinate | Whites more likely to vaccinate than African Americans (and other) | – | Higher income more likely to vaccinate |
| McAndrew (US) |
1198 | Intention to vaccinate vs. other responses (do not intend and unsure) | Non-significant | Males more likely to vaccinate | College level education and above more likely to vaccinate | Whites more likely to vaccinate than African Americans | – | – |
N may differ to Table 1 (e.g. Hacquin et al. examined demographic predictors across several waves of data collection, as opposed to only the final wave of data collection, as reported in Table 1).
indicates highest quality studies (N > 2500, no inclusion of COVID attitudinal or previous vaccination behaviour variables in analyses).
4. Discussion
Results of this systematic review and meta-analysis of 58,656 participants drawn from 28 large nationally representative study samples across 13 countries indicates that the percentage of the population intending to be vaccinated when a COVID-19 vaccine becomes available has declined markedly across countries as the pandemic has progressed. Numbers reporting that they will refuse a vaccine have increased over time and a substantial proportion of adults now intend to refuse a vaccine, when available (June-October estimate = 20%). There is also consistent socio-demographic patterning of vaccination intentions; being female, younger, of lower income or education level and belonging to an ethnic minority group are associated with a reduced likelihood of intending to be vaccinated when a vaccine become available.
Some of the present findings are in line with a recently published narrative review of studies examining vaccine intentions by Lin et al [23]. The authors concluded that COVID-19 vaccination intentions have declined over time and there was socioeconomic and demographic patterning of vaccine intentions. Lin et al.[23] included a number of small non-representative samples and results from news reports in their review. These methodological differences may explain why in the present research of large nationally representative studies we found more consistent evidence of gender differences.
Emerging evidence suggests that both exposure to misinformation about COVID-19 [10], [24] and public concerns over the safety of vaccines [25] may be contributing to the observed declines in intentions to be vaccinated, and this highlights the need for measures to address public acceptability, trust and concern over the safety and benefit of approved vaccines. As well as observing declines in intentions to vaccinate over time in our main analyses, we also note intentions differed markedly by country. When sampled during a similar period early on in the pandemic (March-April), 91% of adults in China reported intending to be vaccinated, compared to 76% of adults in France. However, the most recent estimate (September-October) in France is now 52% and similar to the US (54%).
Across studies males were more likely to report intending to vaccinate than females and this is consistent with previous research examining influenza vaccine hesitancy and some studies identifying higher uptake among males [26]. However, there is also recent evidence of greater influenza vaccine uptake among females in both the US and Hong Kong [27], [28]. A range of social and contextual factors may explain gender patterning of vaccination intentions (e.g. current or planned pregnancy). It will therefore be important to understand why females show lower intentions to vaccinate against COVID-19 and whether uptake shows a similar gender patterning. There was also very consistent socioeconomic patterning of vaccination intentions: lower income or education and ethnic minorities were less likely to intend to vaccinate. There was no evidence in any studies reviewed that presence of a chronic health condition was associated with increased vaccination intentions, even though these groups are at increased risk of dying from COVID-19. Measures are required to maximise vaccine uptake in vulnerable and disadvantaged groups who have already been disproportionately affected by the pandemic, such as those from lower income and ethnic minority groups [29], [30].
5. Strengths and limitations
Strengths of the present research are that we limited evidence synthesis to study designs that allow for accurate estimates of population level intentions with minimal sampling error, as small studies of non-representative samples are likely to provide biased estimates. A large proportion of the included studies used quota (as opposed to probability-based sampling) and were pre-prints yet to be peer reviewed (as opposed to published journal articles). However, the type of sampling method used (quota vs. probability) had minimal impact on intentions estimates and that studies reported in pre-prints produced similar effect estimates as peer-reviewed journals. Analyses also accounted for studies using different response formats and findings were similar. However, studies that included an ‘unsure/undecided’ response option had lower estimates of the proportion of the sampled population intending to vaccinate compared to studies with only two response options (e.g. ‘yes’ vs. no’), but there was no difference for the proportion of sampled populations indicating they would not vaccinate. These findings indicate that studies which do not include an ‘unsure’ response option overestimate vaccination intentions and that ‘unsure’ response options may be primarily driven by participants who are unsure but more likely to intend to be vaccinated than not get vaccinated.
We used a rapid systematic review approach and this resulted in us surveying only two online database for published articles and rather than two authors independently assessing article eligibility and extraction, one author was responsible with cross-checking by a second author. However, we retained a number of features of best practice for systematic review and meta-analysis (e.g. assessing risk of bias, publication bias and thorough searching of grey literature). There were fewer studies later (as opposed to earlier) in the pandemic and this may affect the reliability of the time trends analyses. However, we found evidence of declining vaccination intentions when data was analysed using meta-regression (continuous month-of-year variable), when comparing study estimates from early in the pandemic (March-May) to later (June-October) and when examining time trends within countries (UK, US). Furthermore, results of the time trends analyses were consistent irrespective of whether studies used an ‘unsure/undecided’ response option or not. Two included individual studies (US, France) also reported vaccination intentions over time in the same population and results were consistent [25], [31]. As included studies examined nationally representative samples we do not know whether a similar pattern of results would be expected among other population groups (e.g. healthcare workers) and research is required to address these questions [32], [33]. Findings of the present research are limited to studies that met eligibility criteria for having used large and nationally representative sampling and this tended to be developed western countries.
6. Conclusions
Intentions to vaccinate when a COVID-19 vaccine becomes available have been declining across countries and there is an urgent need to address social inequalities in vaccine hesitancy and promote widespread uptake of vaccines as they become available.
Data sharing
Study data files and analysis code are openly available on the Open Science Framework; https://osf.io/hj4ds/.
Contributors
The study was designed by ER. Data was collected by ER, AJ, IL and MD. AJ and ER analysed the data. The first draft was written by ER. All authors critically revised the manuscript and agree to be accountable for all aspects of the work.
7. Role of the funding source
There was no funding source for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
ER’s time was part-funded by the European Research Council and their support is gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.02.005.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.WHO. WHO COVID-19 Weekly Operational Update. 2020. Accessed 30/11/20 from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201005-weekly-epi-update-8.pdf.
- 2.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO, 2020. Accessed 30/11/20: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines.
- 4.Bartsch S.M., O'Shea K.J., Ferguson M.C., et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59(4):493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iboi E.A., Ngonghala C.N., Gumel A.B. Will an imperfect vaccine curtail the COVID-19 pandemic in the U.S.? Infectious Disease Modelling. 2020;5:510–524. doi: 10.1016/j.idm.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38(42):6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazarus J.V., Ratzan S.C., Palayew A., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020 doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams L., Gallant A.J., Rasmussen S., et al. Towards intervention development to increase the uptake of COVID-19 vaccination among those at high risk: outlining evidence-based and theoretically informed future intervention content. Br J Health Psychol. 2020;25(4):1039–1054. doi: 10.1111/bjhp.12468. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Jing R., Lai X., et al. Acceptance of covid-19 vaccination during the covid-19 pandemic in china. Vaccines. 2020;8(3):1–14. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roozenbeek J., Schneider C.R., Dryhurst S., et al. Susceptibility to misinformation about COVID-19 around the world. R Soc Open Sci. 2020;7(10) doi: 10.1098/rsos.201199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fancourt D., Steptoe A., Wright L. The Cummings effect: politics, trust, and behaviours during the COVID-19 pandemic. The Lancet. 2020;396(10249):464–465. doi: 10.1016/S0140-6736(20)31690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S adults. Ann Int Med. 2020 doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers L.B., Goodwin R. Determinants of adults' intention to vaccinate against pandemic swine flu. BMC Public Health. 2011;11(1):15. doi: 10.1186/1471-2458-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galarce E.M., Minsky S., Viswanath K. Socioeconomic status, demographics, beliefs and A(H1N1) vaccine uptake in the United States. Vaccine. 2011;29(32):5284–5289. doi: 10.1016/j.vaccine.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Schmid P., Rauber D., Betsch C., Lidolt G., Denker M.-L. Barriers of influenza vaccination intention and behavior – a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tricco AC, Langlois E, Straus SE, Organization WH. Rapid reviews to strengthen health policy and systems: a practical guide: World Health Organization; 2017.
- 17.Haby M.M., Chapman E., Clark R., Barreto J., Reveiz L., Lavis J.N. What are the best methodologies for rapid reviews of the research evidence for evidence-informed decision making in health policy and practice: a rapid review. Health Res Pol Syst. 2016;14(1):83. doi: 10.1186/s12961-016-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varker T., Forbes D., Dell L., et al. Rapid evidence assessment: increasing the transparency of an emerging methodology. J Eval Clin Pract. 2015;21(6):1199–1204. doi: 10.1111/jep.12405. [DOI] [PubMed] [Google Scholar]
- 19.American Association for Public Opinion Research. Margin of Sampling Error/Credibility Interval. Acessed 30/11/20 from: https://www.aapor.org/Education-Resources/Election-Polling-Resources/Margin-of-Sampling-Error-Credibility-Interval.aspx. 2020.
- 20.Gallup. How polls are conducted. Accessed 30/11/20 from: http://www.janda.org/c10/Lectures/topic05/GallupFAQ.htm. 1997.
- 21.Yeager D.S., Krosnick J.A., Chang L., et al. Comparing the accuracy of RDD telephone surveys and internet surveys conducted with probability and non-probability samples. Public Opinion Quarterly. 2011;75(4):709–747. [Google Scholar]
- 22.Malhotra N, Krosnick JA. The effect of survey mode and sampling on inferences about political attitudes and behavior: Comparing the 2000 and 2004 ANES to Internet surveys with nonprobability samples. Political Analysis 2007: 286-323.
- 23.Lin C., Tu P., Beitsch L.M. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines. 2021;9(1):16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul E, Steptoe A, Fancourt D. Anti-vaccine attitudes and risk factors for not agreeing to vaccination against COVID-19 amongst 32,361 UK adults: Implications for public health communications. medRxiv 2020: 2020.10.21.20216218.
- 25.Daly M, Robinson R. Willingness to vaccinate against COVID-19 in the US: Longitudinal evidence from a nationally representative sample of adults from April–October 2020. 2020. medRxiv. https://www.medrxiv.org/content/10.1101/2020.11.27.20239970v1. [DOI] [PMC free article] [PubMed]
- 26.Morgan R, Klein SL. The intersection of sex and gender in the treatment of influenza. Curr Opin Virol. 2019 Apr;35:35-41. doi: 10.1016/j.coviro.2019.02.009. Epub 2019 Mar 19. PMID: 30901632; PMCID: PMC6556398. [DOI] [PMC free article] [PubMed]
- 27.Applewhite A, Stancampiano FF, Harris DM, Manaois A, Dimuna J, Glenn J, Heckman MG, Brushaber DE, Sher T, Valery JR. A retrospective analysis of gender-based difference in adherence to influenza vaccination during the 2018-2019 season. Journal of Primary Care & Community Health. 2020 Sep;11:2150132720958532. [DOI] [PMC free article] [PubMed]
- 28.Chan D.P., Wong N.S., Wong E.L., Cheung A.W., Lee S.S. Household characteristics and influenza vaccination uptake in the community-dwelling elderly: a cross-sectional study. Prevent Med Rep. 2015;1(2):803–808. doi: 10.1016/j.pmedr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. The Lancet Respiratory Medicine. 2020;8(6):547–548. doi: 10.1016/S2213-2600(20)30228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacquin A, Altay S, de Araujo E, Chevallier C, Mercier H. Sharp rise in vaccine hesitancy in a large and representative sample of the French population: reasons for vaccine hesitancy. Psyarxiv.
- 32.Bell S., Clarke R., Mounier-Jack S., Walker J.L., Paterson P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. 2020;38(49):7789–7798. doi: 10.1016/j.vaccine.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman R.D., Yan T.D., Seiler M., et al. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine. 2020;38(48):7668–7673. doi: 10.1016/j.vaccine.2020.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.