Abstract
Objective:
Treatment guidelines for Tourette’s Disorder (TD) are based on patients’ degree of tic severity and impairment. However, clear benchmarks for determining tic severity and impairment have not been established. This study examined benchmarks of tic severity and tic impairment using the Yale Global Tic Severity Scale (YGTSS) and the Clinical Global Impression of Severity (CGI-S).
Method:
Individuals with TD or another Tic Disorder (N = 519) recruited across nine sites were administered a diagnostic interview, the YGTSS, and the CGI-S. Correlations and trend analyses contrasted YGTSS scores across CGI-S ratings. A logistic regression model examined predictive benchmarks for tic severity, tic impairment, and global severity. Model classifications were compared against CGI-S ratings, and agreement was examined using kappa.
Results:
Spearman correlations between the CGI-S and YGTSS scores ranged from 0.54 to 0.63 (p < 0.001). Greater CGI-S ratings were associated with a linear stepwise increase in YGTSS Total Tic scores, Impairment scores, and Global Severity scores. Despite moderate-to-strong associations (ρ = 0.45–0.56, p < 0.001) between the CGI-S and predictive logistical regression models, only fair agreement was achieved when applying classification benchmarks (κ = 0.21–0.32, p < 0.001).
Conclusions:
CGI-S ratings are useful to characterize benchmarks for tic severity, tic impairment, and global severity on the YGTSS. Logistic regression model benchmarks had only fair agreement with the CGI-S and underscore the heterogeneity of TD symptoms. Collectively, findings offer guidance on the delineation of tic severity categorizations to apply evidence-based treatment recommendations.
Keywords: Tourette Disorder, YGTSS, tic severity, impairment, clinical severity, assessment
Tourette’s Disorder and persistent tic disorders (collectively referred to as TD) are neuropsychiatric conditions with a childhood onset (Bloch and Leckman, 2009), which affect about 1% of the pediatric population (Knight et al., 2012; Scahill et al., 2014; Scharf et al., 2015). Individuals with TD exhibit a wide range of symptoms that include simple motor tics (e.g., repetitive eye-blinking, quick head jerk), complex motor tics (e.g., combination of movements, writing tics, whole body tics), simple vocal tics (e.g., coughing, throat clearing), and complex vocal tics (e.g., words, short phrases). Tics tend to emerge in early school-aged years and peak in severity during early adolescence (Bloch and Leckman, 2009). Although tic severity often declines in early adulthood, some individuals with TD exhibit tics throughout adulthood (Bloch and Leckman, 2009). In addition to tics, the clinical picture of TD is often complicated by co-occurring psychiatric conditions, including anxiety disorders, attention-deficit hyperactivity disorders (ADHD), and obsessive-compulsive disorder (OCD) (Freeman et al., 2000). Tics and co-occurring conditions are associated with functional impairment (Conelea et al., 2013; Storch et al., 2007a) and contribute to decreased quality of life (Eddy et al., 2011; Storch et al., 2007b).
There are two empirically supported treatment options for individuals with TD: pharmacotherapy (e.g., antipsychotic and alpha-2 agonist medications) (Weisman et al., 2013) and behavior therapy (e.g., habit reversal training, exposure with response prevention, comprehensive behavioral intervention for tics) (McGuire et al., 2014; McGuire et al., 2015c; Piacentini et al., 2010; Wilhelm et al., 2012). Decisions to pursue treatment for TD should be made collaboratively with patients and families, and incorporate multiple factors (e.g., tic severity, tic impairment, patient preference, and accessibility of treatments). However, patients and families often rely upon clinicians for guidance and in turn, clinicians rely on professional treatment guidelines. These treatment guidelines universally recommend incorporating tic severity and tic impairment in treatment planning (Murphy et al., 2013; Pringsheim et al., 2019, 2012; Roessner et al., 2011; Steeves et al., 2012; Verdellen et al., 2011). For tics that are “mild” in severity, psychoeducation is often the recommended intervention (Murphy et al., 2013). Behavior therapy is typically recommended for tics of “moderate” severity or greater; and pharmacotherapy recommended when tics are “severe” (Murphy et al., 2013). While nuanced differences exist from guideline to guideline (Murphy et al., 2013; Pringsheim et al., 2019, 2012; Roessner et al., 2011; Steeves et al., 2012; Verdellen et al., 2011), the commonality across all guidelines is that increasing levels of severity require increasing levels of clinical intervention. Unfortunately, the delineation of these descriptive severity categories is unclear and likely influenced by a clinician’s level of experience with TD. This can result in differences in tic severity classification among clinicians and consequently different treatment recommendations. A clear consensus on criteria that constitute “mild”, “moderate”, and “severe” severity in TD can guide clinicians (and allied care providers) to recommend appropriate levels of clinical intervention.
Tic severity and tic impairment are commonly measured using the Yale Global Tic Severity Scale (YGTSS) (Leckman et al., 1989; McGuire et al., 2018). Although the YGTSS is the gold standard for assessing tic severity and impairment (McGuire et al., 2012), there are no established benchmarks for distinguishing mild, moderate, or severe levels of illness. The Clinical Global Impression-Severity (CGI-S) scale is a general measure of disorder severity often used in research studies (Guy, 1976). This clinician-rated scale has been applied to measure global severity in TD, and incorporates tic severity and tic impairment into a single ordinal rating (e.g., mild, moderate, severe) (Guy, 1976; Leckman et al., 1989). Because the CGI-S considers tic severity and tic impairment into a single rating, it represents an ideal index to determine YGTSS benchmarks that correspond with descriptive severity categories used in treatment guidelines. To date, only one study has examined the relationship benchmarks between the CGI-S and the YGTSS (Leckman et al., 1989). Leckman and colleagues (1989) found a stepwise increase in the Global Severity score for each increasingly severe CGI-S category (Leckman et al., 1989). Although informative, this report only examined YGTSS Global Severity score (Total Tic score + Impairment score) and was limited to a single recruitment site that included only 90 youth and adults (Leckman et al., 1989). Given that the YGTSS Total Tic score is the primary outcome in most clinical trials (Murphy et al., 2017; Piacentini et al., 2010; Scahill et al., 2001; Wilhelm et al., 2012), benchmarks for this scale can inform the application of treatment guidelines and recommendations. Here, we examined benchmarks of tic severity, tic impairment, and global severity using the YGTSS and CGI-S in a large multi-site sample of well-characterized patients with TD.
Methods
Participants
Participants were 519 individuals with TD (465 Tourette Disorder, 41 Chronic Motor Tic Disorder, 5 Chronic Vocal Tic Disorder, and 8 Transient Tic Disorder) who were recruited across nine U.S. academic Tourette and Obsessive-Compulsive Disorder specialty clinics. These participants were ascertained as part of multiple independent studies either focusing on phenomenological research or clinical trials (University of California Los Angeles, n = 170; University of South Florida, n = 99; University of Wisconsin-Milwaukee, n = 76; Massachusetts General Hospital and Harvard Medical School, n = 47; Johns Hopkins University, n = 41; University of Texas Health Science Center at San Antonio, n = 40; Yale Child Study Center, n = 35; University of Utah, n = 8; Weill Cornell Medical College, n = 3) (Bennett et al., 2020; Himle et al., 2012; Johnco et al., 2016; McCracken et al., 2008; McGuire et al., 2015a; McGuire et al., 2016; Piacentini et al., 2010; Ricketts et al., 2016; Storch et al., 2017; Wilhelm et al., 2012).
Measures
Psychiatric Diagnoses.
In most cases, psychiatric diagnoses were determined using an age-appropriate structured diagnostic interview administered by a trained clinician [i.e., Anxiety Disorder Interview Schedule-Parent and Child Version (ADIS-C/P; Silverman et al., 2001; Silverman and Albano, 1996), n = 175; the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002), n = 122; Kiddie-Schedule for Affective Disorders (KSADS), n = 98; Mini International Neuropsychiatric Interview-KID (MINI-KID; Sheehan et al., 2010), n = 25]. In 99 patients, psychiatric diagnoses were based on a clinical interview conducted by a board certified child psychiatrist or psychologist followed by a best estimate procedure conducted by two doctoral-level clinicians using all available information (Leckman et al., 1982).
Yale Global Tic Severity Scale (YGTSS) (Leckman et al., 1989).
The YGTSS is a clinician-rated interview that measures current tic severity and impairment. It has a stable factor structure (Storch et al., 2007c), as well as excellent reliability and validity (Leckman et al., 1989; McGuire et al., 2018; Storch et al., 2005). Motor and phonic tics are rated separately across five dimensions. Ratings are summed to produce a Total Tic score (range: 0–50), which serves as the gold standard measure of tic severity. The Impairment score is separate from tic severity and reflects overall tic impairment (range: 0–50). The Total Tic score and Impairment score are summed to produce a Global Severity score (range: 0–100).
Clinical Global Impression-Severity (CGI-S) Scale (Guy, 1976).
The CGI-S is a 7-point clinician-rating designed to measure overall illness severity in TD. Although co-occurring conditions are often present in TD, clinicians were trained to focus on TD symptom severity when rating the CGI-S using tic-specific anchors. Scores on the CGI-S range from: (1) “Normal presentation/no illness,” (2) “Borderline illness severity,” (3) “Mild illness severity,” (4) “Moderate illness severity,” (5) “Marked illness severity,” (6) “Severe illness severity,” and (7) “Extreme illness severity.”
Procedures
Local institutional review boards approved all study procedures for each individual research protocol. Study data were collected after adult participants provided consent, parents provided permission for minors, and minors provided assent. Prior to the administration of the YGTSS and CGI-S, raters were trained to reliability. Supervision on assessments varied slightly across individual protocols. However, all raters received regular supervision on assessments either via regular meetings with local study investigators who had extensive experience with TD assessments, or monthly teleconference calls led by study investigators with extensive TD assessment experience. De-identified data were aggregated across the studies for the secondary analyses here.
Analytic Plan
First, sample characteristics and distribution of YGTSS and CGI-S scores were examined (see Table 1). T-tests and Mann-Whitney U tests compared differences in tic severity (YGTSS, CGI-S) and impairment between youth (7–17 years of age) and adults (18+ years of age). Spearman correlations (ρ) examined the association between CGI-S and the YGTSS Total Tic score, YGTSS Impairment score, and YGTSS Global Severity score. Second, YGTSS Total Tic scores, YGTSS Impairment scores, YGTSS Global Severity scores, and counts of co-occurring psychiatric diagnoses were indexed by CGI-S categories (see Table 2 and Figure 1). Third, to examine the influence of co-occurring psychiatric conditions on CGI-S ratings, chi-square tests examined differences in the counts of anxiety disorders, ADHD, OCD, and depressive disorders between CGI-S categories. Fourth, a trend analysis compared the YGTSS Total Tic score, YGTSS Impairment score, and YGTSS Global Severity score across CGI-S ratings using an analysis of variance (ANOVA). Polynomial contrasts and Bonferroni post-hoc tests determined significant trends. Finally, a logistic regression model was applied using the PLUM function in SPSS 25.0 to determine tic severity, tic impairment, and global severity benchmarks on the YGTSS using CGI-S ratings. Spearman correlations (ρ) examined the relationship between the logistic regression model classifications and CGI-S classifications. Mutual classification of proposed tic severity, tic impairment, and global severity benchmarks were compared with the original CGI-S rating using Cohen’s kappa (κ): ≤ 0 as no agreement, 0.01–0.20 as none to slight agreement, 0.21–0.40 as fair agreement, 0.41– 0.60 as moderate agreement, 0.61–0.80 as substantial agreement, and 0.81–1.00 as almost perfect agreement (Landis and Koch, 1977).
Table 1.
Demographic and Clinical Characteristics of the Sample (N = 519)
| Gender | N (%) |
|---|---|
| Male | 371 (71.5%) |
| Race & Ethnicity | |
| Caucasian | 402 (77.5%) |
| Hispanic | 63 (12.1%) |
| Asian American/Pacific Islander | 22 (4.2%) |
| African American | 8 (1.5%) |
| Other Race (e.g., biracial) | 13 (2.5%) |
| Unknown Race/Ethnicity | 11 (2.1%) |
| Medication Status | |
| Antipsychotic | 80 (15.4%) |
| Alpha-2 agonist | 105 (20.2%) |
| Any tic medication | 165 (31.8%) |
| Co-Occurring Psychiatric Diagnoses | |
| Any attention deficit hyperactivity disorder (ADHD) | 162 (31.2%) |
| Any obsessive-compulsive disorder (OCD) | 120 (23.1%) |
| Any anxiety disordersa | 118 (36.2%) |
| Any depressive disordersb | 25 (4.8%) |
| Participants with one or more co-occurring psychiatric diagnosis | 314 (60.5%) |
| Mean (SD) [Range] | |
| Age | 15.92 (11.13) [5–69] |
| YGTSS Total Tic Score | 24.03 (7.19) [7–46] |
| YGTSS Impairment Score | 24.37 (9.30) [0–50] |
| YGTSS Global Severity Score | 48.40 (14.25) [7–96] |
Note: YGTSS = Yale Global Tic Severity Scale
Anxiety disorders included separation anxiety, social phobia, generalized anxiety, specific phobia, panic disorder, agoraphobia, and anxiety disorders not otherwise specified.
Depressive disorders included major depressive disorder, dysthymia, or depressive disorder not otherwise specified.
Table 2.
Tic Symptom Severity, Tic Impairment, and Current Co-occurring Psychiatric Conditions for CGI-Severity scores (N = 519)
| CGI-Severity | YGTSS Total Tic Score | YGTSS Impairment Score | YGTSS Global Severity Score | ADHD | OCD | Anxiety Disordersa | Depressive Disordersb | |
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Mean (SD) | Mean (SD) | N (%) | N (%) | N (%) | N (%) | |
| Borderline Illness | 12 | 12.42 (4.52) | 7.08 (6.90) | 19.50 (8.40) | 6 (50%) | 4 (33%) | 5 (42%) | 2 (17%) |
| Mild Illness | 58 | 19.26 (6.54) | 16.90 (6.70) | 36.16 (10.32) | 16 (28%) | 11 (19%) | 21 (36%) | 4 (7%) |
| Moderate Illness | 259 | 22.28 (5.94) | 22.57 (7.66) | 44.85(10.89) | 66 (25%) | 45 (17%) | 83 (32%) | 8 (3%) |
| Marked Illness | 165 | 27.76 (5.73) | 29.78 (7.97) | 57.54 (10.84) | 66 (67%) | 52 (32%) | 69 (42%) | 9 (5%) |
| Severe Illness | 25 | 34.12 (6.62) | 33.04 (8.63) | 67.16 (12.84) | 8 (47%) | 8 (47%) | 10 (40%) | 2 (8%) |
| Extreme Illness | 0 | --- | --- | --- | --- | --- | --- | --- |
Note: YGTSS = Yale Global Tic Severity Scale, ADHD = Attention-Deficit Hyperactivity Disorder, OCD = Obsessive-Compulsive Disorder.
Anxiety disorders included separation anxiety, social phobia, generalized anxiety, specific phobia, panic disorder, agoraphobia, and anxiety disorders not otherwise specified.
Depressive disorders included major depressive disorder, dysthymia, or depressive disorder not otherwise specified.
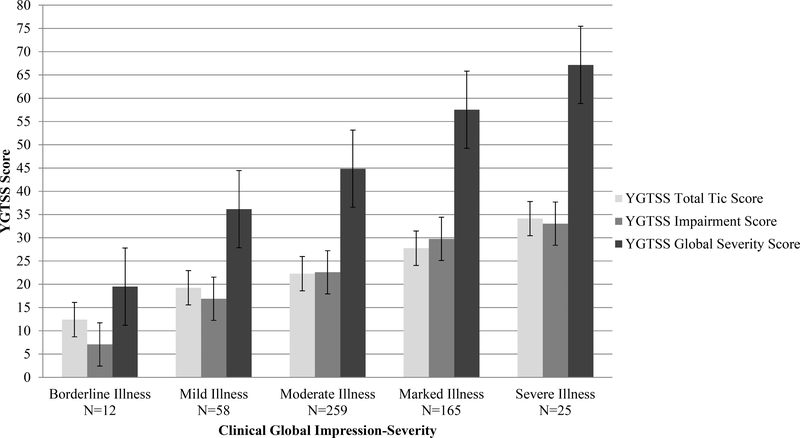
Figure 1.
The distribution of tic symptom severity, tic impairment, and global severity on the Yale Global Tic Severity Scale (YGTSS) for each Clinical Global Impression of Severity (CGI-Severity) category from the original ratings (N = 519).
Results
Participant Characteristics.
Table 1 presents demographic and clinical characteristics of the 418 youth and 101 adults in the combined sample. The average YGTSS Total Tic score, Impairment score, and Global Severity score were 24.03 + 7.19, 24.37 + 9.30, and 48.40 + 14.25, respectively (see Table 1). There were no differences by age group on Total Tic score (t517 = 1.82, p = 0.07, d = 0.20), Impairment score (t517 = 0.00, p = 0.99, d = 0.00), and Global Severity Score (t517 = 0.91, p = 0.36, d = 0.10). The distribution of CGI-S ratings are presented in Table 2. Mann-Whitney U tests found no significant difference between the mean rank of youth and adults on the CGI-S (Z = −0.17, p = 0.86). Given these small, non-significant differences in YGTSS and CGI-S scores, youth and adults were examined together. Across all participants, positive associations were observed between CGI-S ratings and YGTSS Total Tic score (ρ = 0.54, p < 0.001), YGTSS Impairment score (ρ = 0.54, p < 0.001), and YGTSS Global Severity score (ρ = 0.63, p < 0.001).
The Influence of Concomitant Psychiatric Disorders on CGI-S Ratings.
The frequencies of anxiety (χ2 = 4.50, p = 0.34, V = 0.09) and depressive disorders (χ2 = 6.61, p = 0.16, V = 0.11) were not significantly different across CGI-S categories. However, there were differences in the frequency of ADHD (χ2 = 12.23, p = 0.02, V = 0.15) and OCD (χ2 = 13.73, p = 0.008, V = 0.16) across CGI-S categories. Participants with Marked severity had a higher co-occurrence of ADHD (χ2 = 9.91, p = 0.002, V = 0.15) and OCD (χ2 = 11.42, p < 0.001, V = 0.16) compared to those with Moderate severity. No other significant difference between CGI-S categories was present for either ADHD (χ2 = 0.11–3.53, p = 0.06–0.74, V = 0.02–0.17) or OCD (χ2 = 0.01–3.33, p = 0.07–0.96, V = 0.01–0.14). Taken together, these results suggest that concomitant psychiatric disorders did not have a large effect on CGI-S ratings.
Tic Severity, Impairment, and Global Severity Across CGI-S Ratings.
Scores on the CGI-S ranged from Borderline to Severe, with no participants classified as Extreme. The average and standard error for YGTSS Total Tic scores, Impairment scores, and Global Severity scores across CGI-S ratings are presented in Figure 1. There were clear stepwise increases in the mean YGTSS Total Tic scores, Impairment scores, and Global Severity scores for each incremental CGI-S rating (see Figure 1). This visual impression was supported by an ANOVA contrasting YGTSS Total Tic scores (F4,514 = 60.55, p < 0.001), Impairment scores (F4,514 = 60.67, p < 0.001), and Global Severity score (F4,514 = 94.44, p < 0.001) across CGI-S categories (see Table 2). Polynomial contrasts revealed a significant linear trend in YGTSS Total Tic scores across CGI-S categories (p < 0.001), but no significant quadratic (p = 0.11) or cubic trends (p = 0.24). Post-hoc tests showed significantly lower YGTSS Total Tic scores for each incremental CGI-S category. Borderline severity had lower YGTSS Total Tic Scores than Mild severity (d = 1.09, p < 0.003), Mild lower than Moderate (d = 0.50, p < 0.005), Moderate lower than Marked (d = 0.94, p < 0.001), and Marked lower than Severe (d = 1.09, p < 0.001). The average stepwise difference for each CGI-S category ranged from 3 to 7 points. The largest stepwise increases in YGTSS Total Tic scores were between the Borderline to Mild group and Marked to Severe group (7-point increases). Comparatively, the increases from Mild to Moderate to Marked groups was smaller and relatively consistent across categorical steps (4 to 5 points, see Table 2 and Figure 1).
A similar pattern of greater impairment on the YGTSS Impairment Scale was observed across increasing CGI-S ratings. Polynomial contrasts found a significant linear trend in YGTSS Impairment scores (p < 0.001) across CGI-S categories, but no significant quadratic (p = 0.15) or cubic trends (p = 0.52). Post-hoc tests confirmed that Borderline severity had significantly lower impairment than Mild (d = 1.46, p < 0.001), Mild less than Moderate (d = 0.76, p < 0.001), and Moderate less than Marked (d = 0.93, p < 0.001). However, no significant difference in YGTSS Impairment scores was found between Marked and Severe ratings on the CGI-S (d = 0.40, p = 0.49). There was an average change of 3 to 10 points on the YGTSS Impairment score for each increase in CGI-S category. The largest stepwise increase was between the Borderline to Mild group (9-point increase), with the increase between Mild to Moderate to Marked being smaller (6 to 7 points, see Table 2 and Figure 1). The smallest increase was between Marked and Severe categories on the CGI-S (4 points).
Finally, the YGTSS Global Severity score also displayed greater severity across increasing CGI-S ratings. Polynomial contrasts found a significant linear trend in YGTSS Global Severity scores (p < 0.001) across CGI-S categories, but no significant quadratic (p = 0.88) or cubic trends (p = 0.85). Post-hoc tests confirmed that Borderline severity had lower YGTSS Global Severity scores than Mild severity (d = 1.66, p < 0.001), Mild lower than Moderate (d = 0.81, p < 0.001), Moderate lower than Marked (d = 1.17, p < 0.001), and Marked lower than Severe (d = 0.87, p < 0.001). This resulted in an average difference of 8 to 17 points on the YGTSS Global Severity score for each increase in CGI-S category. The largest stepwise increase was between the Borderline and Mild groups (16 points, see Table 2 and Figure 1), followed by the increase between the Moderate to Marked groups (13 points). The distinction between other stepwise categories (Mild to Moderate, and Marked to Severe) was smaller (8 to 10 points).
Determining Clinical Severity Based on YGTSS.
Logistic regression models for tic severity, tic impairment, and global severity are displayed in Table 3. There was a moderate association between CGI-S and tic severity benchmarks identified by the logistic regression model (ρ = 0.45, p < 0.001), which mutually classified 296 cases with the CGI-S (57%) and had fair agreement (κ = 0.24, 95% CI: 0.18, 0.31, p < 0.001). For tic impairment, there was also a moderate association between the CGI-S and the benchmarks predicted by the logistic regression model (ρ = 0.43, p < 0.001), which mutually classified 279 cases with the CGI-S (54%) and exhibited fair agreement (κ = 0.21, 95% CI: 0.15, 0.27, p < 0.001). Finally for global severity, there was a strong association between the CGI-S and the benchmarks identified by the logistic regression model (ρ = 0.56, p < 0.001), which mutually classified 312 cases with the CGI-S (60%) and displayed fair agreement (κ = 0.32, 95% CI: 0.25, 0.38, p < 0.001). Although the predictive logistic regression models and the CGI-S classified similar counts of participants for tic severity, tic impairment, and global severity, agreement on the participants placed in those categories was generally unimpressive (kappas ranging from .21 to .32). Given the level of observed agreement, we looked at a model that considered YGTSS Impairment scores > 10 as having Moderate severity on the CGI-S (in line with YGTSS guidance). However, it did not substantially improve accuracy of predictive classification.
Table 3.
Potential Models for Benchmarks of Tic Severity, Impairment, and Global Severity on the Yale Global Tic Severity Scale (YGTSS)
| Logistic Regression Model | |||
|---|---|---|---|
| CGI-Severity | Total Tic Scorea n (%) | Impairment Scoreb n (%) | Global Severity Scorec n (%) |
| Normal | 0 (0%) | 0 (0%) | 0 (0%) |
| Borderline Illness | 0 (%) | 0 (0%) | 2 (0.4%) |
| Mild Illness | 14 (2.7%) | 10 (1.9%) | 30 (5.8%) |
| Moderate Illness | 346 (66.7%) | 295 (56.8%) | 330 (63.6%) |
| Marked Illness | 156 (30.1%) | 210 (40.5%) | 151 (29.1%) |
| Severe Illness | 3 (0.6%) | 4 (0.8%) | 6 (1.2%) |
| Extreme Illness | 0 (%) | 0 (0%) | 0 (0%) |
Note:
YGTSS Total Tic Score: 0 = normal (inferred), 1–6 = borderline (inferred), 7–10 = mild, 11–27 = moderate, 28–43 = marked, 43–46 = severe, 47–50 = extreme (inferred).
YGTSS Impairment Score: not included = normal and borderline, 0–7 = mild, 8–29 = moderate, 30–42 = marked, 43–50 = severe, not included = extreme.
YGTSS Global Severity Score: 0–9 = borderline, 10–25 = mild, 26–55 = moderate, 56–81 = marked, 82–96 = severe, not included = extreme.
Discussion
This study used a large, well-characterized, treatment-seeking sample to develop objective benchmarks of tic severity, tic impairment, and global severity classifications using the YGTSS and CGI-S. Positive associations were observed between CGI-S ratings and the YGTSS scores with minimal influence of co-occurring psychiatric conditions on CGI-S categories. These findings suggest that CGI-S ratings were primarily driven by tic severity and tic impairment in this sample and not affected by the presence of common psychiatric comorbidities.
Trend analyses found a linear progression of increasing YGTSS scores across CGI-S ratings. Indeed, the average tic severity (3 to 7 points), tic impairment (3 to 10 points), and global severity (8 to 17 points) scores on the YGTSS increased for each severity level on the CGI-S. The global severity scores across CGI-S ratings were comparable to those reported by Leckman and colleagues (Leckman et al., 1989), and the differentiation of tic severity and tic impairment scores across CGI-S ratings delineated the contribution of each component to overall severity (see Figure 1). The confluence of YGTSS scores and CGI-S categories underscores the importance of balancing both tic severity and an individuals’ level of tic impairment in an evidence-based clinical assessment (McGuire et al., 2012).
While the current sample provides descriptive benchmarks and ranges to differentiate between severity categories using the YGTSS (see Figure 1), the logistic regression model encountered challenges when seeking to establish optimal categorical cut-points. Although the predicted benchmarks had moderate-to-strong associations with up to 60% mutual case classification with the CGI-S, agreement on the specific participants placed into the same severity categories was only fair. The limited overall agreement between the logistic regression model categorizations and CGI-S categorizations raises concerns about the generalizability and precision of benchmarks identified for tic severity, tic impairment, and global severity. Although predictive regression models have identified severity benchmarks in OCD (Lewin et al., 2014; Storch et al., 2015), it may be that tic severity and impairment alone are not the only determining factors in severity as measured by the CGI-S. Consider a child who has a frequent and intense “eye poking tic” but few other tics. This child might have a low YGTSS Total Tic score, but this tic could be associated with considerable social and medical consequences and contribute to a Marked CGI-S rating. In comparison, another child with several frequent simple tics of mild intensity that go unnoticed by peers may contribute to a moderate YGTSS Total Tic score, a low YGTSS Impairment score and culminate in a Mild severity rating on the CGI-S. Thus, the degree to which specific tics are perceived as bothersome and contribute to disability across functional domains may be an important consideration in rating overall severity on the CGI-S that is not fully captured by YGTSS ratings. The modified Hopkins Motor/Vocal Tic Scale that measures the severity of most bothersome tics may be a useful complement to the YGTSS in clinical research (McGuire et al., 2015b; Walkup et al., 1992).
Several study limitations warrant consideration. First, this was a sample of outpatient treatment-seeking youth and adults. There were no cases in the Extreme CGI-S category and few in the Borderline (n = 12) and Severe categories (n = 25). The few cases on either end of the severity spectrum may have influenced the regression models’ accuracy in determining optimal benchmarks. Second, although consistent with clinical practice, the ratings of the YGTSS and the CGI-S were conferred by the same clinician. Third, assessments were conducted by trained clinicians, but we did not evaluate inter-rater reliability across sites and raters. The item anchors on the YGTSS dimensions offer clinicians guidance on scoring the measure. However, the YGTSS is not a self-report scale. Eliciting the information needed to rate the motor and vocal tic dimensions on the YGTSS requires training and experience. Finally, although the YGTSS is the gold-standard measure of tic severity, its limitations have been described and the revised version of the scale offers some, albeit modest, improvements (McGuire et al., 2018). Nonetheless, the YGTSS is the most commonly used measure in research and clinical practice. These YGTSS benchmarks are offered to help clinicians characterize TD severity and apply treatment guidelines.
Although definitive benchmarks remain elusive, the current findings have implications for research and clinical practice. In research, these results can assist with the classification of overall severity across TD treatment trials to permit greater comparability. In clinical practice, findings may help translate results from clinical trials that use the YGTSS and promote appropriate implementation of evidence-based treatment guidelines Consider the case of a child with a YGTSS Total Tic score of 15 and Impairment score of 20 consistent with Mild rating on the CGI-S. Although concerned parents may advocate for immediate intervention, the clinician could appropriately offer reassurance and psychoeducation about the severity of tics. If tic severity later increased by 5 points on the YGTSS Total Tic score, then an appropriate level of intervention would be recommended (in collaboration with the patient/family) due to moderate severity of TD. In response to a more dramatic and sustained increase in tic severity and tic impairment (e.g. an increase of 10 points on YGTSS Total Tic score and 20 points on YGTSS Impairment score), a greater level of intervention would warrant consideration due to a presentation consistent with a Marked rating on the CGI-S. While the CGI-S balances tic severity and tic impairment into a single rating, it is influenced by the clinician’s overall experience with TD. Expert clinicians may intuitively apply these guidelines due to years of clinical experience. However, the development of benchmarks can help treatment recommendations be consistently applied across treatment centers, even among clinicians who are less experienced with TD populations. When doing so, individual patient treatment recommendations properly consider patient and family preference, tic severity, related distress and impairment, and access to treatment.
In summary, treatment recommendations for TD should properly consider tic severity and tic impairment. In the current study, a stepwise increase was observed on YGTSS Total Tic score, Impairment score, and Global Severity score across CGI-S ratings. Although predictive models using YGTSS scores produced only moderate agreement with CGI-S ratings, our findings provide general guidance for YGTSS scores that characterize overall severity and are not influenced by common psychiatric comorbidities. In doing so, this report offers direction for determining severity categorizations that underlie tic treatment algorithms developed by leading experts. While clinicians with considerable experience with TD may intuitively rely on the CGI-S to guide treatment, the YGTSS is less influenced by clinician experience due to its standardized administration and anchor points that guide severity ratings. Thus, the YGTSS scoring framework presented here can help guide clinicians less experienced with TD to appropriately interpret treatment algorithms in real-world clinical practice.
Acknowledgements:
The authors would like to express their appreciation to the children and parents who participated in these research projects. This work was supported in part by grants to Dr. McGuire (Tourette Association of America, American Academy of Neurology, American Psychological Foundation), Dr. Essoe (Tourette Association of America), Dr. Scahill (R01MH069874, Tourette Association of America), Dr. Wilhelm (R01MH069877), Dr. Peterson (RO1MH069875), Dr. Woods (Tourette Association of America), Dr. McCracken (T32MH073517), and Dr. Piacentini (T32MH073517, R01MH070802, Tourette Association of America). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, NIH, or other grant organizations.
Author disclosures: Dr. McGuire reports receiving research support from the Tourette Association of American (TAA), the American Academy of Neurology (AAN). He has served as a consultant to Bracket Global, Syneos Health, and Luminopia, and also received royalties from Elsevier. Dr. Piacentini has received grant or research support from the National Institute of Health (NIH), PCORI, Pfizer Pharmaceuticals, and the TLC Foundation for BFRBs. He has received book royalties from Guilford Press and Oxford University Press. He has received speaking honoraria and travel support from the Tourette Association of America and the International Obsessive Compulsive Disorder Foundation (IOCDF) and consultant fees from Luminopia. Dr. Storch has received research support from the NIH, Red Cross, ReBuild TX, Texas Higher Education Coordinating Board, IOCDF, and Greater Houston Community Foundation. He reports receiving royalties from Elsevier Publications, Springer, American Psychological Association, John Wiley & Sons Inc, Jessica Kingsley, Oxford, and Lawrence Erlbaum. He has been a consultant for Levo Therapeutics, and serves on the speaker’s bureau and scientific advisory board for the IOCDF. He also reported receiving research support from the McIngvale Endowed Chair. Dr. Ricketts has received research support from the TAA and NIMH. Dr. Woods has received speaker’s honoraria from the TAA and royalties from Guilford Press and Oxford University Press. Dr. Peterson has received research support and speaker’s honoraria from the NIH, TAA, and receives royalties from Oxford University Press. Dr. Walkup has received research support from Patient-Centered Outcomes Research Institute. He is an unpaid advisor to the Anxiety and Depression Association of America (ADAA) and the Trichotillomania Learning Center. He is an unpaid Director on the Board of Directors of the Tourette Association of America. He has received royalties for books from Guilford Press and Oxford University Press and educational materials from Wolters Kluwer. He has served as a paid speaker the American Academy of Child and Adolescent Psychiatry, the American Psychiatric Association and the American Academy of Pediatrics. Dr. Wilhelm is a presenter for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies; she has received royalties from Elsevier Publications, Guilford Publications, New Harbinger Publications, Springer, and Oxford University Press. Dr. Wilhelm has also received speaking honoraria from various academic institutions and foundations, including the International Obsessive Compulsive Disorder Foundation, Tourette Association of America, and Brattleboro Retreat. In addition, she received payment from the Association for Behavioral and Cognitive Therapies for her role as Associate Editor for the Behavior Therapy journal, as well as from John Wiley & Sons, Inc. for her role as Associate Editor on the journal Depression & Anxiety. Dr. Wilhelm has also received honorarium from One-Mind for her role in PsyberGuide Scientific Advisory Board. Dr. Wilhelm has received salary support from Novartis and Telefonica Alpha, Inc. Dr. Ramsey has received travel award support from the TAA. Dr. Essoe has received research support from the TAA. Dr. Himle has received speaker’s honoraria and travel support from the TAA, including through their program partnership with the Centers for Disease Control and Prevention, and has received research support from the NIH and TAA. Dr. Lewin reported receiving research support from the All Children’s Hospital Research Foundation, Centers for Disease Control and Prevention, and IOCDF; reported serving on the speaker’s bureau for the TAA and IOCDF; reported receiving travel support from the TAA, American Psychological Association, ADAA, NIMH, and Rogers Memorial Hospital; reported receiving consulting fees from Bracket and Prophase Inc; reported receiving book royalties from Springer; reported receiving honoraria from Oxford Press, Children’s Tumor Foundation, and University of Central Oklahoma; and reported being on the scientific and clinical advisory board for the IOCDF and the board of directors for the Society for Clinical Child and Adolescent Psychology and American Board of Clinical Child and Adolescent Psychology. Dr. Chang has received research support from the NIMH. Dr. Murphy has received research funding from Auspex Pharmaceuticals, NIMH, Shire Pharmaceuticals, Pfizer, F. Hoffmann–La Roche Ltd, AstraZeneca Pharmaceuticals, Centers for Disease Control and Prevention, Massachusetts General Hospital, Sunovion Pharmaceuticals, Neurocrine Biosciences, PANDAS Network, and Psyadon Pharmaceuticals. Dr. McCracken has received grant or research support from NIH, Seaside Therapeutics, Roche, and Otsuka. He has served as a consultant to BioMarin and PharmaNet. Dr. Scahill has research support from NIH and US Department of Defense. He has served as a consultant for pharmaceutical companies including Roche, Janssen, Yamo, Teva. He has received book royalties from Oxford, Guilford and American Psychological Association. He has also received support from the Marcus Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph F. McGuire, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine; Semel Institute of Neuroscience and Human Behavior, University of California Los Angeles.
John Piacentini, Semel Institute of Neuroscience and Human Behavior, University of California Los Angeles.
Eric A. Storch, Department of Psychiatry, Baylor College of Medicine.
Emily J. Ricketts, Semel Institute of Neuroscience and Human Behavior, University of California Los Angeles.
Douglas W. Woods, Marquette University.
Alan L. Peterson, Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center at San Antonio; Research and Development Service, South Texas Veterans Health Care System; Department of Psychology, University of Texas at San Antonio.
John T. Walkup, Ann and Robert H. Lurie Children’s Hospital of Chicago.
Sabine Wilhelm, Massachusetts General Hospital and Harvard Medical School.
Kesley Ramsey, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine.
Joey K.-Y. Essoe, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine.
Michael B. Himle, Department of Psychology, University of Utah.
Adam B. Lewin, Departments of Pediatrics, Psychiatry and Behavioral Neuroscience, University of South Florida.
Susanna Chang, Semel Institute of Neuroscience and Human Behavior, University of California Los Angeles.
Tanya K. Murphy, Departments of Pediatrics, Psychiatry and Behavioral Neuroscience, University of South Florida; All Children’s Hospital, Johns Hopkins Medicine.
James T. McCracken, Semel Institute of Neuroscience and Human Behavior, University of California Los Angeles.
Lawrence Scahill, Marcus Autism Center, Emory University School of Medicine.
References
- Bennett SM, Capriotti M, Bauer C, Chang S, Keller AE, Walkup J, Woods D, Piacentini J, 2020. Development and Open Trial of a Psychosocial Intervention for Young Children With Chronic Tics: The CBIT-JR Study. Behav Ther, 51, 659–669. 10.1016/j.beth.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, 2009. Clinical course of Tourette syndrome. J Psychosom Res, 67, 497–501. 10.1016/j.jpsychores.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conelea CA, Woods DW, Zinner SH, Budman CL, Murphy TK, Scahill LD, Compton SN, Walkup JT, 2013. The Impact of Tourette Syndrome in Adults: Results from the Tourette Syndrome Impact Survey. Community Ment Health J, 49, 110–120. 10.1007/s10597-011-9465-y [DOI] [PubMed] [Google Scholar]
- Eddy CM, Rizzo R, Gulisano M, Agodi A, Barchitta M, Calì P, Robertson MM, Cavanna AE, 2011. Quality of life in young people with Tourette syndrome: a controlled study. J Neurol, 258, 291–301. 10.1007/s00415-010-5754-6 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. SCID-I/P; New York, NY. [Google Scholar]
- Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P, 2000. An international perspective on Tourette syndrome: selected findings from 3500 individuals in 22 countries. Dev Med Child Neurol, 42, 436–447. 10.1111/j.1469-8749.2000.tb00346.x [DOI] [PubMed] [Google Scholar]
- Guy W, 1976. ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service. [Google Scholar]
- Himle MB, Freitag M, Walther M, Franklin SA, Ely L, Woods DW, 2012. A randomized pilot trial comparing videoconference versus face-to-face delivery of behavior therapy for childhood tic disorders. Behav Res Ther, 50, 565–570. doi: 10.1016/j.brat.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Johnco C, McGuire JF, McBride NM, Murphy TK, Lewin AB, Storch EA, 2016. Suicidal ideation in youth with tic disorders. J Affect Disord, 200, 204–211. 10.1016/j.jad.2016.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T, 2012. Prevalence of Tic Disorders: A Systematic Review and Meta-Analysis. Pediatr Neurol, 47, 77–90. 10.1016/j.pediatrneurol.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG, 1977. The measurement of observer agreement for categorical data. Biometrics, 159–174. [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ, 1989. The Yale Global Tic Severity Scale: Initial Testing of a Clinician-Rated Scale of Tic Severity. J Am Acad Child Adolesc Psychiatry, 28, 566–573. 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson D, Belanger A, Weissman MM, 1982. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry, 39, 879–883. doi: 10.1001/archpsyc.1982.04290080001001 [DOI] [PubMed] [Google Scholar]
- Lewin AB, Piacentini J, De Nadai AS, Jones AM, Peris TS, Geffken GR, Geller DA, Nadeau JM, Murphy TK, Storch EA, 2014. Defining clinical severity in pediatric obsessive-compulsive disorder. Psychol Assess, 26, 679–684. 10.1037/a0035174 [DOI] [PubMed] [Google Scholar]
- McCracken JT, Suddath R, Chang S, Thakur S, Piacentini J, 2008. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette syndrome. J Child Adolesc Psychopharmacol, 18, 501–508. doi: 10.1089/cap.2007.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Arnold E, Park JM, Nadeau JM, Lewin AB, Murphy TK, Storch EA, 2015a. Living with tics: reduced impairment and improved quality of life for youth with chronic tic disorders. Psychiatry Res, 225, 571–579. doi: 10.1016/j.psychres.2014.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Kugler BB, Park JM, Horng B, Lewin AB, Murphy TK, Storch EA, 2012. Evidence-based assessment of compulsive skin picking, chronic tic disorders and trichotillomania in children. Child Psychiatry Hum Dev, 43, 855–883. doi: 10.1007/s10578-012-0300-7 [DOI] [PubMed] [Google Scholar]
- McGuire JF, McBride N, Piacentini J, Johnco C, Lewin AB, Murphy TK, Storch EA, 2016. The premonitory urge revisited: An individualized premonitory urge for tics scale. J Psychiatric Res, 83, 176–183. 10.1016/j.jpsychires.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Brennan EA, Lewin AB, Murphy TK, Small BJ, Storch EA, 2014. A meta-analysis of behavior therapy for Tourette Syndrome. J Psychiatric Res, 50, 106–112. 10.1016/j.jpsychires.2013.12.009 [DOI] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Scahill L, Woods DW, Villarreal R, Wilhelm S, Walkup JT, Peterson AL, 2015b. Bothersome tics in patients with chronic tic disorders: Characteristics and individualized treatment response to behavior therapy. Behav Res Ther, 70, 56–63. 10.1016/j.brat.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Storch EA, Murphy TK, Ricketts EJ, Woods DW, Walkup JW, Peterson AL, Wilhelm S, Lewin AB, McCracken JT, Leckman JF, Scahill L, 2018. A multicenter examination and strategic revisions of the Yale Global Tic Severity Scale. Neurology 90, e1711–e1719. 10.1212/WNL.0000000000005474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Ricketts EJ, Piacentini J, Murphy TK, Storch EA, Lewin AB, 2015c. Behavior Therapy for Tic Disorders: an Evidenced-Based Review and New Directions for Treatment Research. Curr Dev Disord Rep, 2, 309–317. 10.1007/s40474-015-0063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Fernandez TV, Coffey BJ, Rahman O, Gavaletz A, Hanks CE, Tillberg CS, Gomez LI, Sukhodolsky DG, Katsovich L, Scahill L, 2017. Extended-Release Guanfacine Does Not Show a Large Effect on Tic Severity in Children with Chronic Tic Disorders. J Child Adolesc Psychopharmacol, 27, 762–770. 10.1089/cap.2017.0024 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Lewin AB, Storch EA, Stock S, 2013. Practice Parameter for the Assessment and Treatment of Children and Adolescents With Tic Disorders. J Am Acad Child Adolesc Psychiatry, 52, 1341–1359. 10.1016/j.jaac.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, Walkup JT, 2010. Behavior Therapy for Children With Tourette Disorder: A Randomized Controlled Trial. JAMA, 303, 1929–1937. 10.1001/jama.2010.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, Carroll A, Dion Y, Luscombe S, Steeves T, Sandor P, 2012. Canadian Guidelines for the Evidence-Based Treatment of Tic Disorders: Pharmacotherapy. Can J Psychiatry, 57, 133–143. 10.1177/070674371205700302 [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Holler-Managan Y, Okun MS, Jankovic J, Piacentini J, Cavanna AE, Martino D, Müller-Vahl K, Woods DW, Robinson M, Jarvie E, Roessner V, Oskoui M, 2019. Comprehensive systematic review summary: Treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology, 92, 907–915. 10.1212/WNL.0000000000007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts EJ, Goetz AR, Capriotti MR, Bauer CC, Brei NG, Himle MB, Espil FM, Snorrason Í, Ran D, Woods DW, 2016. A randomized waitlist-controlled pilot trial of voice over Internet protocol-delivered behavior therapy for youth with chronic tic disorders. J Telemed Telecare, 22, 153–162. doi: 10.1177/1357633X15593192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, Strand G, Stern JS, Termine C, Hoekstra PJ, the ESSTS Guidelines Group, 2011. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry, 20, 173–196. 10.1007/s00787-011-0163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AFT, Cohen DJ, Leckman JF, 2001. A Placebo-Controlled Study of Guanfacine in the Treatment of Children With Tic Disorders and Attention Deficit Hyperactivity Disorder. Am J Psychiatry, 158, 1067–1074. 10.1176/appi.ajp.158.7.1067 [DOI] [PubMed] [Google Scholar]
- Scahill L, Specht M, Page C, 2014. The prevalence of tic disorders and clinical characteristics in children. J Obsessive Compuls Relat Disord, 3, 394–400. 10.1016/j.jocrd.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y, 2015. Population prevalence of Tourette syndrome: A systematic review and meta-analysis. Mov Disord, 30, 221–228. 10.1002/mds.26089 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B, 2010. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). Journal Clin Psychiatry, 71, 313–326. doi: 10.4088/JCP.09m05305whi [DOI] [PubMed] [Google Scholar]
- Silverman WK, Saavedra LM, Pina AA, 2001. Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. J Am Acad Child Adolesc Psychiatry, 40, 937–944. doi: 10.1097/00004583-200108000-00016 [DOI] [PubMed] [Google Scholar]
- Silvermnan WK, Albano AM, 1996. The anxiety disorders interview schedule for DSM-IV: child and parent versions. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Steeves T, McKinlay BD, Gorman D, Billinghurst L, Day L, Carroll A, Dion Y, Doja A, Luscombe S, Sandor P, Pringsheim T, 2012. Canadian Guidelines for the Evidence-Based Treatment of Tic Disorders: Behavioural Therapy, Deep Brain Stimulation, and Transcranial Magnetic Stimulation. Can J Psychiatry, 57, 144–151. 10.1177/070674371205700303 [DOI] [PubMed] [Google Scholar]
- Storch EA, De Nadai AS, Do Rosário MC, Shavitt RG, Torres AR, Ferrão YA, Miguel EC, Lewin AB, Fontenelle LF, 2015. Defining clinical severity in adults with obsessive–compulsive disorder. Compr Psychiatry, 63, 30–35. doi: 10.1016/j.comppsych.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Johnco C, McGuire JF, Wu MS, McBride NM, Lewin AB, Murphy TK, 2017. An initial study of family accommodation in children and adolescents with chronic tic disorders. Eur Child Adolesc Psychiatry, 26, 99–109. doi: 10.1007/s00787-016-0879-5. [DOI] [PubMed] [Google Scholar]
- Storch EA, Lack CW, Simons LE, Goodman WK, Murphy TK, Geffken GR, 2007a. A Measure of Functional Impairment in Youth with Tourette’s Syndrome. J Pediatr Psychol, 32, 950–959. 10.1093/jpepsy/jsm034 [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Lack C, Milsom VA, Geffken GR, Goodman WK, Murphy TK, 2007b. Quality of Life in Youth With Tourette’s Syndrome and Chronic Tic Disorder. J Clin Child Adolesc Psychol, 36, 217–227. 10.1080/15374410701279545 [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Fernandez M, Krishnan M, Geffken GR, Kellgren AR, Goodman WK, 2007c. Factor-analytic study of the Yale Global Tic Severity Scale. Psychiatry Res, 149, 231–237. 10.1016/j.psychres.2006.03.017 [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Sajid M, Allen P, Roberti JW, Goodman WK, 2005. Reliability and validity of the Yale Global Tic Severity Scale. Psychol Assess, 17, 486–491. 10.1037/1040-3590.17.4.486 [DOI] [PubMed] [Google Scholar]
- Verdellen C, van de Griendt J, Hartmann A, Murphy T, the ESSTS Guidelines Group, 2011. European clinical guidelines for Tourette Syndrome and other tic disorders. Part III: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry, 20, 197–207. 10.1007/s00787-011-0167-3 [DOI] [PubMed] [Google Scholar]
- Walkup J, Rosenberg LA, Brown J, Singer HS, 1992. The validity of instruments measuring tic severity in Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry, 31, 472–477. 10.1097/00004583-199205000-00013 [DOI] [PubMed] [Google Scholar]
- Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH, 2013. Systematic review: Pharmacological treatment of tic disorders – Efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev, 37, 1162–1171. 10.1016/j.neubiorev.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, Chang S, Liu H, Dziura J, Walkup JT, Scahill L, 2012. Randomized Trial of Behavior Therapy for Adults With Tourette Syndrome. Arch Gen Psychiatry, 69, 795–803. 10.1001/archgenpsychiatry.2011.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]