Abstract
Mitral regurgitation remains the most common form of valve disease worldwide and given an aging population with a significant proportion of secondary mitral regurgitation, a transcatheter approach to mitral valve replacement has become a major goal of the transcatheter therapeutics field. Mitral regurgitation can be caused by disease of the leaflets (primary) or by diseases of the left atrium or left ventricle (LV) (secondary or functional), and may involve overlap of the two (mixed disease). The location of the mitral valve (and large size), the approach to anchoring a valve replacement, and concerns about left ventricular outflow tract (LVOT) obstruction are all issues that have made the transcatheter delivery of a valve replacement challenging. Despite these challenges, both transapical and transseptal devices are currently being developed, with several in early feasibility trials and several entering pivotal trials. As the field of transcatheter mitral valve replacement (TMVR) improves and develops, a critical part of evaluating patients with mitral valve disease will be utilizing the heart team approach to identify and individualize the most appropriate treatment for each patient.
Keywords: Transcatheter valve therapies, mitral valve surgery, transcatheter mitral valve replacement (TMVR), mitral valve replacement
Introduction
Advances in transcatheter technologies have revolutionized the treatment of aortic valve disease and have led to the approval of transcatheter aortic valve replacement (TAVR) for all categories of risk (1-6). The most common form of valve disease in the developed world, however, is mitral regurgitation (7). The prevalence of mitral regurgitation increases with age, with a relatively low prevalence in young adults of 0.5% (aged 18 to 44 years), and a substantial increase to nearly 10% in older adults (aged 75 years and older) (8). As advanced heart failure treatments have improved over time, the incidence of ischemic heart disease has increased, which has subsequently led to an increase in functional mitral regurgitation (9). This, coupled with increases in life expectancy in the developed world, has led to an aging population with mitral valve disease who may not be ideal candidates for a standard surgical therapy due to anatomic constraints or elevated surgical risk. Consequently, a transcatheter approach to mitral valve replacement has rapidly become a goal of both physician investigators and the transcatheter therapeutics industry. Although a substantial amount of thought and investigation, paired with extensive industry research and development, has been invested into the transcatheter mitral space, transcatheter mitral valve replacement (TMVR) has not achieved the same success (or rapid development and implementation) as TAVR. In this review, we will discuss the burden of mitral valve disease, the rationale for transcatheter treatment, the challenges for treatment, several of the current devices under investigation, and our thoughts on the future directions of the field. As we evaluate these new technologies and consider patients for treatment, it is most important to utilize a comprehensive heart team approach.
Etiologies and classification of mitral regurgitation
Unlike aortic stenosis, which has a relatively limited number of mechanisms for valve deterioration, mitral regurgitation can be caused by a variety of failures of both the mitral valve apparatus and the left ventricle (LV). Mitral regurgitation can be the consequence of dysfunction in the mitral valve annulus, the anterior and posterior mitral valve leaflets, the chordae tendineae, the anterolateral and posteromedial papillary muscles, and the left ventricular myocardium. Mitral regurgitation is generally classified as either primary (also referred to as degenerative or organic disease) or secondary (functional).
Primary mitral regurgitation
The culprit lesion in primary mitral regurgitation is typically the mitral valve leaflets. Diseases that result in primary mitral regurgitation include myxomatous degeneration, leaflet flail, mitral valve prolapse, rheumatic valve disease and connective tissue disorders.
Secondary mitral regurgitation
Secondary mitral regurgitation is generally caused by diseases of the left atrium and LV, and usually spares the mitral valve leaflets. However, several aspects of the mitral valve apparatus can be compromised in secondary mitral regurgitation. Left ventricular cardiomyopathies (either ischemic or non-ischemic) can result in tethering of the mitral valve leaflets (related to regional wall motion abnormalities or left ventricular remodeling), annular dilatation (which can be caused by either left atrial or left ventricular dilatation), and dysfunctional mitral valve closing (related either to reduced left ventricular contractility or dyssynchrony). Although the leaflets are thought to be spared in secondary mitral regurgitation, compensatory changes in the leaflets can occur, including leaflet thickening and increased leaflet area, thus leading to mixed disease that demonstrates elements of both primary and secondary mitral regurgitation (10).
Surgical classification of mitral regurgitation
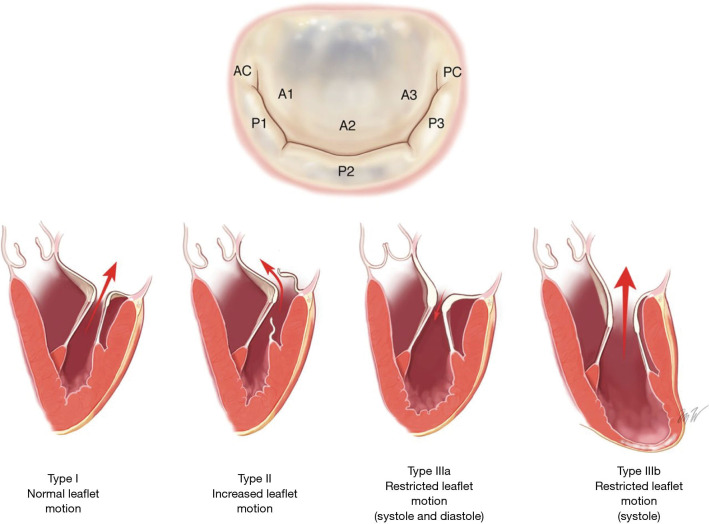
For surgical intervention of mitral valve disease, the Carpentier Classification has commonly been used (Figure 1). Carpentier type I disease is characterized by normal leaflet motion. Mitral regurgitation in this phenotype is typically related to a dilated mitral valve annulus which is most frequently related to posterior annular tethering (12), but may also be related to atrial enlargement (a phenomenon known as “atrial functional mitral regurgitation”). In atrial functional mitral regurgitation, the regurgitation is related to significant atrial enlargement, often in the setting of atrial fibrillation, and can be reversed when the underlying arrhythmia is corrected (13). Carpentier type II disease is distinguished by increased leaflet mobility. Leaflet and/or chordae pathologies are most commonly responsible. Primary leaflet abnormalities due to degenerative disease generally falls into this category. In Carpentier type III disease, the lesion is decreased leaflet mobility. This is further classified into type IIIa and type IIIb disease. In type IIIa disease, the leaflet mobility is restricted in both systole and diastole. Common causes are radiation therapy and rheumatic heart disease. In type IIIb, the mobility is limited during systole. This is caused by myocardial ischemia/dysfunction and ventricular remodeling, and the leaflets are preserved (14).
Figure 1.
Mitral valve anatomy and Carpentier Classification of mitral regurgitation. Reproduced with permission from the Journal of the American College of Cardiology (11).
Natural history of mitral regurgitation
The majority of patients with severe, chronic mitral regurgitation are able to live without symptoms for many years due to the ability of the LV to remodel. Eventually, if left untreated, patients will develop symptoms of heart failure and shortness of breath. The compensatory mechanisms of left ventricular remodeling lead to left ventricular dilatation and finally systolic dysfunction. Atrial fibrillation may also develop as the left atrium dilates. In patients with primary mitral regurgitation, the progression to symptoms is associated with increased morbidity and mortality (15,16). In secondary mitral regurgitation, however, the presence of at least moderate mitral regurgitation is associated with increased mortality (17). This concept was first demonstrated in patients with ischemic disease (18,19), but a more recent study of 45,900 patients with secondary mitral regurgitation demonstrated an increase in all-cause mortality in patients with at least mild mitral regurgitation, regardless of whether the etiology is ischemic or non-ischemic (20). The association of mortality with severity of mitral regurgitation has also been observed regardless of whether the disease has progressed to a symptomatic state (21).
Current guideline recommendations
Primary mitral regurgitation
According to the current American College of Cardiology/American Heart Association guidelines, mitral valve surgery is recommended for symptomatic patients with primary mitral regurgitation and ejection fraction above 30% (Class I, LOE B). Surgery is also recommended for asymptomatic primary mitral regurgitation patients with severe, chronic disease and left ventricular dysfunction with ejection fraction of 30% to 60%, or end-systolic diameter of at least 40 mm (Class I, LOE B). In addition, surgery may be considered for asymptomatic patients with normal left ventricular function if the likelihood of successful repair is considered high by the treating surgeon (22).
Secondary mitral regurgitation
The current guidelines recommend that, for patients with secondary mitral regurgitation, mitral valve surgery may be considered when undergoing concomitant coronary artery bypass grafting (CABG) or aortic valve replacement (AVR) (Class IIA, LOE C), when severe, chronic mitral regurgitation is present. Lastly, for patients with severe symptoms with chronic, severe mitral regurgitation (despite optimized goal-directed medical therapy), mitral valve surgery may be considered (Class IIB, LOE B) (22).
Surgical treatment of mitral regurgitation
Primary mitral regurgitation
The gold standard of care for patients with primary mitral regurgitation who meet criteria for intervention is surgical mitral valve repair (23,24). The rationale for surgical repair involves the restoration of physiologic leaflet motion (when the disease is primarily of the leaflets) through several techniques (e.g., leaflet resection, chordal transposition, annuloplasty repair, and implantation of artificial chordae). These techniques can be technically challenging to perform, however, in high-volume, expert mitral valve surgical centers, greater than 95% of primary mitral regurgitation cases can be successfully repaired (25,26). For primary mitral regurgitation patients at low surgical risk, a strategy of mitral valve repair at an expert center should be considered the optimal strategy, then subsequently surgical mitral valve replacement when repair is not possible.
Secondary mitral regurgitation
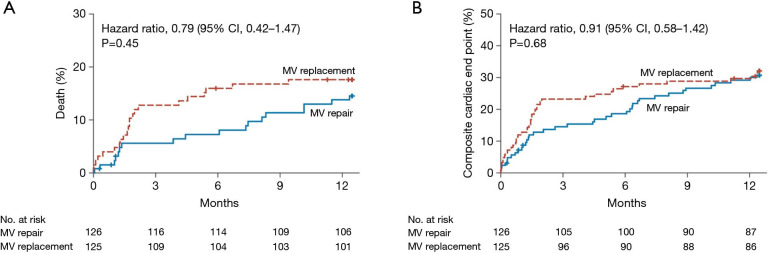
Whereas surgical mitral valve repair is preferred in the treatment of primary mitral regurgitation, the optimal strategy for surgical approach in secondary mitral regurgitation is not as clear. There is significant variation nationally in regards to surgical approach (repair versus replacement) in secondary mitral regurgitation (27). Previous studies have demonstrated a lower peri-operative risk of mortality in patients receiving a repair strategy in secondary mitral regurgitation (28-30), however, the opposing argument to this approach is that mitral valve replacement provides a more durable long-term result with a decreased risk of recurrent mitral regurgitation and perhaps improved long-term outcomes. The Cardiothoracic Surgical Trials Network (CTSN) conducted a large, multicenter randomized trial to address this issue (Figure 2). This trial compared outcomes of mitral valve repair and replacement in secondary mitral regurgitation (31). A total of 251 patients were randomized to either mitral valve repair or chordal-sparing replacement, and at 1 year, there was no difference in mortality. Recurrent moderate or severe mitral regurgitation was significantly lower in the replacement group (2.3%) versus the repair group (32.6%), however, there was no difference in the rate of a composite end point of major adverse cardiac or cerebrovascular events, quality of life and functional status (31). Given these findings, both mitral valve repair and mitral valve replacement are reasonable surgical options for patients with secondary mitral regurgitation with acceptable surgical risk.
Figure 2.
Rate of death and the composite cardiac end point in surgical mitral valve repair versus replacement. The composite end point of the rate of major adverse cardiac or cerebrovascular events included death, stroke, subsequent mitral-valve (MV) surgery, hospitalization for heart failure, and an increase in the New York Heart Association class of 1 or more. Crosses indicate that patients’ data were censored at that point. From: reference (31). Copyright 2014. Massachusetts Medical Society. Reprinted with permission.
Challenges of traditional mitral valve surgery
Mortality rates of up to 5% are associated with mitral valve surgery (32), and associated morbidity rates can range from 10% to 20% (32,33). The previously discussed aging population of mitral valve patients is also important to note, as the risk of traditional mitral valve surgery increases with age (ranging as high as 17% in octogenarians) (34). In addition, patients with secondary mitral regurgitation typically have reduced left ventricular function, which is also associated with an increased risk of traditional open surgery (34). A mitral valve repair approach has not been demonstrated to improve mortality when performed either for mitral regurgitation in the setting of reduced LV function or when performed at the time of concomitant CABG (35,36).
Rationale and technical challenges for TMVR
Rationale for transcatheter therapies for mitral valve disease
The higher risk nature of secondary mitral regurgitation patients (given advancing age, comorbidities and reduced LV function) combined with the lack of demonstrated impact of surgery on hard outcomes likely explains why most symptomatic severe mitral regurgitation patients are not referred for traditional surgery (37). Although there may not be a mortality benefit of treating severe secondary mitral regurgitation, there is likely a symptomatic benefit. In this case, it is reasonable to consider a transcatheter approach for treatment of mitral valve regurgitation. Given the less invasive nature of the transcatheter procedure, the traditional risks of surgery can be avoided, thus allowing for symptomatic improvement for the patient without the higher early peri-operative risk. Many lessons have been learned in TAVR which may be applicable to TMVR. In TAVR, the transcatheter approach is associated with lower risks of acute kidney injury, infection, atrial fibrillation, stroke and faster patient recoveries (2,3). Additionally, cardiopulmonary bypass is not required for most percutaneous approaches, thus avoiding its associated morbidity and mortality.
Technical challenges for TMVR
The technical challenges of implementing a fully percutaneous AVR were rapidly overcome by a combination of operator experience and technical device iteration. The mitral valve, given its location and valve complexity, makes the task of achieving a fully percutaneous valve replacement more formidable. Given the variety of etiologies of mitral regurgitation, multiple transcatheter repair techniques have been developed, but these generally target only one anatomic aspect of the mitral valve apparatus.
MitraClip edge-to-edge repair is the only approved transcatheter therapy in the US. Although successful for many patients with primary mitral regurgitation and high/prohibitive surgical risk as well as patients with secondary mitral regurgitation, it has limitations. These include anatomies that are not suitable for MitraClip repair, such as calcified leaflets, immobile leaflets due to prior radiation therapy or rheumatic disease, healed endocarditis, clefts, and multiple jets (Barlow’s disease). In addition, a significant proportion of patients may be left with moderate or more residual mitral regurgitation, which is associated with subsequent mortality and may account for some of the residual poor outcomes even in treated patients in the COAPT trial. One argument for TMVR over repair techniques is that a valve replacement may offer more durable results in mitral regurgitation reduction while allowing for the treatment of the variety of different anatomic pathologies.
The first technical challenge for TMVR is the location of the valve. To access the valve percutaneously, a transseptal puncture must be performed from the transfemoral approach. To deliver a valve from this approach, the delivery system must be equipped with a high degree of flexion to be able to navigate the transseptal puncture and then be appropriately oriented within the native mitral valve. The mitral valve is larger than the aortic valve, and thus a large delivery system must be able to pass through the transseptal puncture. Due to the challenges of the transseptal approach, several TMVR systems have been developed on the transapical platform. Although the Tendyne transapical platform has had success, the transapical approach, as demonstrated with the TAVR experience, is generally associated with poorer outcomes as compared to a transfemoral approach (1,2,5).
The second major technical challenge for TMVR is the ability to anchor the device. In TAVR, the aortic valve is calcified and rigid, and this lends itself to relatively easy anchoring of a bioprosthesis to the native annulus. Regurgitant mitral valves are generally not significantly calcified. In addition, the shape of the mitral valve annulus is typically D-shaped, and is dynamic throughout the cardiac cycle.
A third technical challenge for TMVR is the potential for compromising the left ventricular outflow tract (LVOT). The LVOT is known to be reduced after mitral valve surgery (38), and this has also been noted after mitral valve-in-ring and valve-in-MAC procedures (39,40) (and serves as a limiting factor for these procedures). The degree of left ventricular size and septal hypertrophy, as well as the aortomitral annular angle are all important factors to consider when planning for TMVR. Nonetheless, the early experience with TMVR devices (primarily in valve-in-valve and valve-in-ring procedures) has yielded several lessons which have helped to improve the prediction of LVOT obstruction (41).
TMVR devices under investigation
The initial clinical experience with TMVR has involved the placement of balloon-expandable TAVR bioprostheses within either annuloplasty rings (42,43) or degenerated surgical valves (44,45). This approach has also been utilized in patients with significant mitral annular calcification (without a prior surgical bioprosthesis), but high mortality, LVOT obstruction and reports of valve embolization have limited the utilization of this procedure (39,46). The transcatheter mitral valve-in-valve procedure with a balloon-expandable valve has yielded acceptable results (47), and consequently, has become the procedure of choice for high surgical risk patients with degenerated surgical mitral valve bioprostheses.
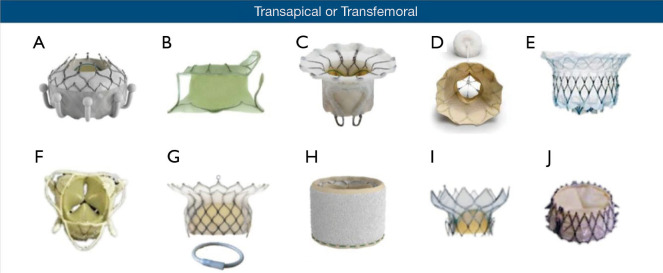
Currently, over 30 TMVR devices are in development (48,49), and the field is continuing to expand. The vast majority of these devices are in early safety and feasibility trials. We will focus this discussion on devices that are currently under clinical evaluation in the United States (Figure 3). For the purposes of this discussion, we will first discuss the transapical devices, and we will subsequently discuss the transfemoral/transseptal devices. As noted below, several of the transapical systems are being developed to transition to a transfemoral/transseptal approach.
Figure 3.
Current TMVR devices under development and clinical evaluation. (A) CardiAQ/EVOQUE (Edwards Lifesciences Inc.); (B) Tiara (Neovasc Inc.); (C) FORTIS (Edwards Lifesciences Inc.); (D) Tendyne (Abbott Inc.); (E) Intrepid (Medtronic Inc.); (F) Caisson (LivaNova); (G) HighLife Bioprosthesis and Subannular Implant (HighLife SAS); (H) SAPIEN M3 (Edwards Lifesciences Inc.); (I) Cardiovalve (Cardiovalve); (J) NaviGate (NaviGate Cardiac Structures Inc.). Reproduced with permission from the Journal of the American Heart Association (50). TMVR, transcatheter mitral valve replacement.
Transapical devices
Tendyne MV system
The Tendyne MV system (Abbott Structural, Santa Clara, CA, USA) is a self-expanding trileaflet porcine pericardial valve mounted on a nitinol frame. The valve is implanted via the transapical approach, and can be repositioned and retrieved. The nitinol stent has a D-shape to conform to the native mitral valve, and the device has an atrial sealing cuff to reduce paravalvular leak. The valve is anchored to the LV apex via a polyethylene tether. The first 30 patients treated in the early feasibility trial have been published (51), followed by publication of the first 100 patients treated (52). The trial enrolled patients considered high or prohibitive risk for traditional surgery with grade 3 or 4 mitral regurgitation. The majority of treated patients had secondary mitral regurgitation (89%). Technical success was achieved in 97% of patients, and no periprocedural deaths were observed. The death rate at 30 days was 6%, and the degree of mitral regurgitation at 30 days was either none or trivial in 98.8% of patients, with this persisting at 98.4% at 12 months. At 1 year, overall survival was 72.4%. These results led to the first CE Mark approval of a TMVR device in January, 2020.
The results of the early feasibility trial have led to the formation of the pivotal trial for the Tendyne system, the SUMMIT trial (clinical trial to evaluate the safety and effectiveness of using the Tendyne mitral valve system for the treatment of symptomatic mitral regurgitation; clinicaltrials.gov, NCT03433274), which will randomize patients to either the Tendyne system or MitraClip. In addition, this trial will involve an arm of patients treated with Tendyne implantation into severe mitral annular calcification. The early results of the first nine patients treated with Tendyne-in-MAC have recently been published (53). The device was successfully implanted in all 9 patients. One patient required alcohol septal ablation for LVOT obstruction (due to valve malrotation) and in follow up (mean 12 months), one cardiac death and one non-cardiac death occurred (53).
Neovasc tiara mitral valve
The Tiara transcatheter mitral valve (Neovasc Inc., Richmond, Canada) is a self-expanding trileaflet bovine pericardial valve that is mounted on a nitinol frame. The valve is implanted via the transapical approach. The Tiara has a large atrial skirt to help seat the device and to minimize paravalvular leak, and it utilizes ventricular anchors into the LV myocardium. The first implant of the Tiara device was performed in Vancouver in 2014, and the early feasibility trial is ongoing (54). A transfemoral/transseptal system Tiara system is currently under development.
Intrepid mitral valve
The Intrepid valve system (Medtronic Inc., Redwood City, CA, USA), is a self-expanding trileaflet bovine pericardial valve that is mounted on a nitinol stent frame. The Intrepid system operates via the transapical approach. The atrial portion of the valve is large and is designed to appropriately seat the device and seal it to the native annulus. The first implant was performed in 2014, and results from the first 50 implanted patients have been published (55). In this initial 50 patient experience, successful device placement was achieved in 96% of patients, and the mortality rate at 30 days was 14%. All patients surviving to 30 days had none to mild mitral regurgitation. These early results have led to the formation of the US pivotal trial, APOLLO (TMVR with the Medtronic intrepid TMVR system in patients with severe symptomatic mitral regurgitation; clinicaltrials.gov, NCT 03242642). There are two arms to the trial design. In the first arm, patients with symptomatic, severe mitral regurgitation will be randomized to either the Intrepid TMVR system or traditional surgery. Patients who are considered ineligible for surgery will be enrolled in the second arm and treated with the Intrepid system. The primary trial endpoint is a composite endpoint of all-cause mortality, stroke, re-operation (or re-intervention), and cardiovascular hospitalization at 1 year. Secondary endpoints include safety endpoints (AKI, disabling stroke, prolonged ventilation, deep wound infection, and major bleeding) and efficacy endpoints (improvement in mitral regurgitation, NYHA class, and quality of life). Recent changes have been proposed in the APOLLO trial design, including the use of MitraClip in the control arm, incorporation of a MAC registry, the addition of international sites, and the incorporation of a transseptal new valve design platform.
Of note, the FORTIS valve (Edwards Lifesciences, Irvine, CA, USA), is a self-expanding bovine pericardial valve mounted on a nitinol stent frame that was first implanted in 2014, however, further study of this device has been discontinued as elevated rates of valve thrombosis were noted in the early experience.
Transfemoral/transseptal devices
CardiAQ/EVOQUE transcatheter mitral valve
The initial CardiAQ valve (CardiAQ Valve Technologies, Irvine, CA, USA), was developed for both transapical and transfemoral/transseptal access (56). The CardiAQ valve was a self-expanding trileaflet bovine pericardial valve mounted on a nitinol stent frame. The valve was designed with a polyester fabric skirt and band, and polyurethane foam-covered anchors to grasp the leaflets and chords (for anchoring). The lessons learned from the initial CardiAQ experience have informed the formation of the US early feasibility study of the EVOQUE valve (Edwards Lifesciences, Irvine, CA, USA). The EVOQUE mitral valve replacement system is similar to the CardiAQ system with unique features. It consists of a trileaflet bovine pericardial valve mounted on a nitinol frame and has an intra-annular skirt to minimize paravalvular leak. The atrial and ventricular profiles are smaller than the initial CardiAQ valve. The EVOQUE valve is available in two sizes (44 and 48 mm), and is delivered via a transfemoral/ transseptal approach with a delivery system that includes multiple planes of flexion and independent depth control. The early feasibility and Canadian compassionate use studies are currently enrolling. The results of the first 14 patients treated with the EVOQUE valve were recently presented (57). Procedural success was achieved in 13/14 patients (93%), with one conversion to open surgery 1/14 (7%). One patient developed LVOT obstruction. At 30 days, 13/14 (93%) of patients were alive. Mitral regurgitation was eliminated in 80% of patients, and the remaining 20% had 1+ mitral regurgitation.
Caisson TMVR system
The Caisson TMVR system (LivaNova, Maple Grove, MN, USA), is a self-expanding, trileaflet porcine valve that is mounted on a nitinol stent frame. The Caisson system consists of an anchor which engages underneath the mitral valve annulus with ventricular feet as well as atrial holding features to anchor to the atrial surface of the valve. The valve is designed to then sit within the anchor. The anchor is D-shaped to conform to the mitral valve annulus. This valve has been recently discontinued.
Sapien M3
The Sapien M3 (Edwards Lifesciences, Irvine, CA, USA) system is a combination of a modified Sapien 3 TAVR valve and a coiling nitinol docking system. The M3 valve is a balloon-expandable bovine pericardial valve mounted on a cobalt-chromium stent frame. The nitinol coiling system is placed around the mitral valve leaflets and the M3 valve is placed within it. The system is delivered via the transfemoral, transseptal approach. The initial results of the first ten treated patients were recently published (58). A total of ten patients with severe mitral regurgitation were treated with the Sapien M3 system, with nine valves successfully implanted. All patients who received the valve had a reduction in mitral regurgitation to trivial or none. At 30 days, there were no significant adverse events (including stroke, rehospitalization, LVOT obstruction, device migration or conversion to open surgery). Recently, the results of the first 35 treated patients were presented (59). Procedural success was achieved in 31/35 (88.6%), and the all-cause mortality rate at 30 days was 2.9% (n=1). There was also one disabling stroke at 30 days.
In addition to the valves described above, other technologies are under development and remain in the early stages with only anecdotal cases described at this time. Other devices in development include the HighLife valve (HighLife SAS, France), the Cardiovalve (Cardiovalve, Israel), the Cephea Valve (Cephea Valve Technologies), the AltaValve (4C Medical Technologies Inc.), the NSCI NaviGate valve (NaviGate Cardiac Structures Inc.), and the MValve (MValve Ltd., Israel).
Conclusions & future directions
While the field of TAVR has rapidly developed and has transformed the paradigm of the treatment of aortic valve disease, the field of TMVR has experienced a slower rate of progress. This progress has largely been limited by both the location, size, and the complexity of the mitral valve. Advancing the development of TMVR is critical, as mitral regurgitation is the most common valvular lesion in the world, and substantial advances in treatment of heart failure has led to an aging population with mitral regurgitation who may be at either high or prohibitive risk for open surgery. Currently, many devices are under development, with the majority of platforms involving an expandable stent frame with a mounted trileaflet bioprosthesis. Despite the early success of one transapical platform, technological improvements that reduce the device delivery profile (which presents a challenge given the large size of the mitral valve as compared to the aortic valve) and delivery systems (including significant ability for flexion and turning) to facilitate transseptal transvenous delivery will continue and likely make a transseptal approach both feasible and preferred.
As these devices are developed and studied, it will be critical to rigorously study them as compared to traditional surgery, which is a lesson learned well from the TAVR experience. In that regard, the APOLLO and SUMMIT trials will be important to follow in terms of trial design and results. The complexity of both the mitral valve as well as the transcatheter treatment approaches necessitate complex decision-making and each patient should be evaluated on an individual basis with the heart team approach.
Video.

Transcatheter mitral valve replacement: latest advances and future directions.
Acknowledgments
Funding: None.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: HCH reports institutional research grants from Abbott Vascular, Ancora, Boston Scientific, Edwards Lifesciences, Medtronic, and WL Gore; consultant fees from Abbott Vascular, Edwards Lifesciences, and Medtronic; equity from Microinterventional Devices. WYS reports institutional research grants from Edwards Lifesciences, Medtronic; consultant fees from Edwards Lifesciences, Medtronic, Cardiac Dimensions and Microinterventional Devices. The other author has no conflicts of interest to declare.
References
- 1.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609-20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 3.Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705. 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 4.Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706-15. 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 5.Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;371:967-8. 10.1056/NEJMc1408396 [DOI] [PubMed] [Google Scholar]
- 6.Reardon MJ, Van Mieghem NM, Popma JJ. Surgical or transcatheter aortic-valve replacement. N Engl J Med 2017; 377:197-8. [DOI] [PubMed] [Google Scholar]
- 7.Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart 2016;102:75-85. 10.1136/heartjnl-2014-307020 [DOI] [PubMed] [Google Scholar]
- 8.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. 10.1016/S0140-6736(06)69208-8 [DOI] [PubMed] [Google Scholar]
- 9.Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014;63:185-6. 10.1016/j.jacc.2013.08.723 [DOI] [PubMed] [Google Scholar]
- 10.Chaput M, Handschumacher MD, Tournoux F, et al. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008;118:845-52. 10.1161/CIRCULATIONAHA.107.749440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone GW, Vahanian AS, Adams DH, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 1: clinical trial design principles: a consensus document from the mitral valve academic research consortium. J Am Coll Cardiol 2015;66:278-307. 10.1016/j.jacc.2015.05.046 [DOI] [PubMed] [Google Scholar]
- 12.Maslow A. Mitral valve repair: an echocardiographic review: part 1. J Cardiothorac Vasc Anesth 2015;29:156-77. 10.1053/j.jvca.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 13.Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol 2011;58:1474-81. 10.1016/j.jacc.2011.06.032 [DOI] [PubMed] [Google Scholar]
- 14.Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295-301. 10.1161/01.CIR.0000151097.30779.04 [DOI] [PubMed] [Google Scholar]
- 15.Magida MG, Streitfeld FH. The natural history of rheumatic heart disease in the third, fourth, and fifth decades of life. II. Prognosis with special reference to morbidity. Circulation 1957;16:713-22. 10.1161/01.CIR.16.5.713 [DOI] [PubMed] [Google Scholar]
- 16.Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996;335:1417-23. 10.1056/NEJM199611073351902 [DOI] [PubMed] [Google Scholar]
- 17.Trichon BH, Felker GM, Shaw LK, et al. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003;91:538-43. 10.1016/S0002-9149(02)03301-5 [DOI] [PubMed] [Google Scholar]
- 18.Lamas GA, Mitchell GF, Flaker GC, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation 1997;96:827-33. 10.1161/01.CIR.96.3.827 [DOI] [PubMed] [Google Scholar]
- 19.Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759-64. 10.1161/01.CIR.103.13.1759 [DOI] [PubMed] [Google Scholar]
- 20.Sannino A, Smith RL, Schiattarella GG, et al. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a systematic review and meta-analysis. JAMA Cardiol 2017; 2:1130-9. 10.1001/jamacardio.2017.2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. 10.1056/NEJMoa041451 [DOI] [PubMed] [Google Scholar]
- 22.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 23.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. 10.1093/ejcts/ezs455 [DOI] [PubMed] [Google Scholar]
- 24.Yun KL, Miller DC. Mitral valve repair versus replacement. Cardiol Clin 1991;9:315-27. 10.1016/S0733-8651(18)30304-7 [DOI] [PubMed] [Google Scholar]
- 25.Castillo JG, Anyanwu AC, Fuster V, et al. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg 2012;144:308-12. 10.1016/j.jtcvs.2011.12.054 [DOI] [PubMed] [Google Scholar]
- 26.Gillinov AM, Blackstone EH, Nowicki ER, et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg 2008;135:885-93, 893.e1-2. [DOI] [PubMed]
- 27.Di Salvo TG, Acker MA, Dec GW, et al. Mitral valve surgery in advanced heart failure. J Am Coll Cardiol 2010;55:271-82. 10.1016/j.jacc.2009.08.059 [DOI] [PubMed] [Google Scholar]
- 28.Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg 2001;122:1125-41. 10.1067/mtc.2001.116557 [DOI] [PubMed] [Google Scholar]
- 29.Reece TB, Tribble CG, Ellman PI, et al. Mitral repair is superior to replacement when associated with coronary artery disease. Ann Surg 2004;239:671-5; discussion 675-7. 10.1097/01.sla.0000124297.40815.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Radi OO, Austin PC, Tu JV, et al. Mitral repair versus replacement for ischemic mitral regurgitation. Ann Thorac Surg 2005;79:1260-7; discussion 1260-7. 10.1016/j.athoracsur.2004.09.044 [DOI] [PubMed] [Google Scholar]
- 31.Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. 10.1056/NEJMoa1312808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gammie JS, O'Brien SM, Griffith BP, et al. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation 2007;115:881-7. 10.1161/CIRCULATIONAHA.106.634436 [DOI] [PubMed] [Google Scholar]
- 33.Goodney PP, Stukel TA, Lucas FL, et al. Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg 2003;238:161-7. 10.1097/01.SLA.0000081094.66659.c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta RH, Eagle KA, Coombs LP, et al. Influence of age on outcomes in patients undergoing mitral valve replacement. Ann Thorac Surg 2002;74:1459-67. 10.1016/S0003-4975(02)03928-0 [DOI] [PubMed] [Google Scholar]
- 35.Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol 2007;49:2191-201. 10.1016/j.jacc.2007.02.043 [DOI] [PubMed] [Google Scholar]
- 36.Wu AH, Aaronson KD, Bolling SF, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005;45:381-7. 10.1016/j.jacc.2004.09.073 [DOI] [PubMed] [Google Scholar]
- 37.Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. 10.1093/eurheartj/ehm001 [DOI] [PubMed] [Google Scholar]
- 38.Rosendal C, Hien MD, Bruckner T, et al. Left ventricular outflow tract: intraoperative measurement and changes caused by mitral valve surgery. J Am Soc Echocardiogr 2012;25:166-72. 10.1016/j.echo.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 39.Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the First Multicenter Global Registry. JACC Cardiovasc Interv 2016;9:1361-71. 10.1016/j.jcin.2016.04.022 [DOI] [PubMed] [Google Scholar]
- 40.Paradis JM, Del Trigo M, Puri R, et al. Transcatheter valve-in-valve and valve-in-ring for treating aortic and mitral surgical prosthetic dysfunction. J Am Coll Cardiol 2015;66:2019-37. 10.1016/j.jacc.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 41.Bapat V, Pirone F, Kapetanakis S, et al. Factors influencing left ventricular outflow tract obstruction following a mitral valve-in-valve or valve-in-ring procedure, part 1. Catheter Cardiovasc Interv 2015;86:747-60. 10.1002/ccd.25928 [DOI] [PubMed] [Google Scholar]
- 42.Himbert D, Brochet E, Radu C, et al. Transseptal implantation of a transcatheter heart valve in a mitral annuloplasty ring to treat mitral repair failure. Circ Cardiovasc Interv 2011;4:396-8. 10.1161/CIRCINTERVENTIONS.111.962332 [DOI] [PubMed] [Google Scholar]
- 43.Descoutures F, Himbert D, Maisano F, et al. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg 2013;44:e8-15. 10.1093/ejcts/ezt155 [DOI] [PubMed] [Google Scholar]
- 44.Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv 2010;76:608-15. 10.1002/ccd.22618 [DOI] [PubMed] [Google Scholar]
- 45.Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010;121:1848-57. 10.1161/CIRCULATIONAHA.109.924613 [DOI] [PubMed] [Google Scholar]
- 46.Guerrero M, Urena M, Pursnani A, et al. Balloon expandable transcatheter heart valves for native mitral valve disease with severe mitral annular calcification. J Cardiovasc Surg (Torino) 2016;57:401-9. [PubMed] [Google Scholar]
- 47.Yoon SH, Whisenant BK, Bleiziffer S, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J 2019;40:441-51. 10.1093/eurheartj/ehy590 [DOI] [PubMed] [Google Scholar]
- 48.Regueiro A, Granada JF, Dagenais F, et al. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol 2017;69:2175-92. 10.1016/j.jacc.2017.02.045 [DOI] [PubMed] [Google Scholar]
- 49.De Backer O, Piazza N, Banai S, et al. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv 2014;7:400-9. 10.1161/CIRCINTERVENTIONS.114.001607 [DOI] [PubMed] [Google Scholar]
- 50.Testa L, Popolo Rubbio A, Casenghi M, et al. Transcatheter mitral valve replacement in the transcatheter aortic valve replacement era. J Am Heart Assoc 2019;8:e013352. 10.1161/JAHA.119.013352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller DWM, Farivar RS, Jansz P, et al. Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J Am Coll Cardiol 2017;69:381-91. 10.1016/j.jacc.2016.10.068 [DOI] [PubMed] [Google Scholar]
- 52.Sorajja P, Moat N, Badhwar V, et al. Initial feasibility study of a new transcatheter mitral prosthesis: the first 100 patients. J Am Coll Cardiol 2019;73:1250-60. 10.1016/j.jacc.2018.12.066 [DOI] [PubMed] [Google Scholar]
- 53.Sorajja P, Gössl M, Babaliaros V, et al. Novel transcatheter mitral valve prosthesis for patients with severe mitral annular calcification. J Am Coll Cardiol 2019;74:1431-40. 10.1016/j.jacc.2019.07.069 [DOI] [PubMed] [Google Scholar]
- 54.Cheung A. Early experience of TIARA transcatheter mitral valve replacement system. Ann Cardiothorac Surg 2018;7:787-91. 10.21037/acs.2018.09.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bapat V, Rajagopal V, Meduri C, et al. Early experience with new transcatheter mitral valve replacement. J Am Coll Cardiol 2018;71:12-21. 10.1016/j.jacc.2017.10.061 [DOI] [PubMed] [Google Scholar]
- 56.Sondergaard L, Ussia GP, Dumonteil N, et al. The CardiAQ transcatheter mitral valve implantation system. EuroIntervention 2015;11 Suppl W:W76-7. [DOI] [PubMed]
- 57.Webb J, Hensey M, Fam N, et al. Early experience with the EVOQUE Mitral Valve Replacement System. J Am Coll Cardiol 2020. doi: . 10.1016/S0735-1097(20)31741-1 [DOI] [Google Scholar]
- 58.Webb JG, Murdoch DJ, Boone RH, et al. Percutaneous transcatheter mitral valve replacement: first-in-human experience with a new transseptal system. J Am Coll Cardiol 2019;73:1239-46. 10.1016/j.jacc.2018.12.065 [DOI] [PubMed] [Google Scholar]
- 59.Makkar R, O'Neill W, Whisenant B, et al. TCT-8 updated 30-day outcomes for the U.S. early feasibility study of the SAPIEN M3 Transcatheter Mitral Valve Replacement System. J Am Coll Cardiol 2019. doi: . 10.1016/j.jacc.2019.08.030 [DOI] [Google Scholar]