Abstract
目的
基于网络药理学方法探讨白藜芦醇(RES)治疗阿尔茨海默病(AD)的作用机制。
方法
利用PubChem、BATMANTCM、Genecards、AD、TTD、String 11.0、AlzData、SwissTargetPrediction、Metascape等数据库,获得RES所有作用靶点和人类AD相关的靶点,使用Venny 2.1工具取交集,获得RES治疗AD的靶点。构建靶点蛋白质相互作用网络,基因本体论(GO)与KEGG通路富集分析,使用Cytoscape 3.7.1构建RES治疗AD的“靶点-信号通路”网络图。使用SwissDock(<a href="http://www.swissdock.ch/docking" target="_blank">http://www.swissdock.ch/ docking</a>)服务器进行分子对接验证。通过蛋白免疫印迹检测RES处理AD细胞模型(293Tau细胞)的pS396、pS199、Tau5、CDK5、糖原合酶激酶3β(GSK3β)和p-GSK3β的蛋白水平。
结果
分析得到RES作用靶点182个,AD相关靶点525个,RES治疗AD的靶点36个,其中34.6%的靶点为蛋白质修饰酶、27.7%的靶点为代谢物相互转化酶、13.8%的靶点为基因特异性转录调节因子、10.3%的靶点为转运蛋白。RES治疗AD的核心关键靶点为INS、APP、ESR1、MMP9、IGF1R、CACNA1C、MAPT(即微管相关蛋白Tau)、MMP2、TGFB1与GSK3B。GO生物学过程富集分析显示,RES治疗AD主要涉及对β-淀粉样蛋白的反应、转移酶活性的正调节、跨膜受体蛋白酪氨酸激酶信号通路、行为、学习或记忆、老化、跨膜转运的调控等多个生物功能。KEGG通路富集分析表明富集最显著的信号通路为阿尔茨海默病通路、PI3K-AKT信号通路、cGMP-PKG信号通路、MAPK信号通路等。分子对接结果显示,RES与ESR1、GSK3B、MMP9、IGF1R、APP与INS结合较好。蛋白免疫印迹结果显示,50 μmol/L RES处理12 h可以通过调控CDK5、核心靶点GSK3β活性显著降低293Tau细胞pS396和pS199水平(P < 0.001)。
结论
RES可以通过Aβ、Tau病理过程治疗AD,可通过调控CDK5与GSK3β改善AD病理改变。
Keywords: 网络药理学, 白藜芦醇, 阿尔茨海默病, 作用机制
Abstract
Objective
To investigate the therapeutic mechanism of resveratrol (RES) for Alzheimer's disease (AD) in light of network pharmacology.
Methods
We searched PubChem, BATMAN-TCM, Genecards, AD, TTD, String 11.0, AlzData, SwissTargetPrediction, Metascape and other databases for the therapeutic targets of RES and human AD-related targets. The intersection was determined using Venny 2.1 to obtain the therapeutic targets of RES for AD. The protein-protein interaction (PPI) network was constructed, the gene ontology (GO) was enriched and the Kyoto Encyclopedia of Genes and Genomes pathway (KEGG pathway) were analyzed. Cytoscape 3.7.1 software was used to construct a target-signaling pathway network of RES in the treatment of AD. Molecular docking verification was carried out on SwissDock (<a href="http://www.swissdock.ch/docking" target="_blank">http://www.swissdock.ch/docking</a>). We examined a 293Tau cell model of AD for changes in protein levels of pS396, pS199, Tau5, CDK5, glycogen synthase kinase 3β (GSK3β) and p-GSK3β in response to RES treatment using Western blotting.
Results
We obtained 182 targets of RES, 525 targets related to AD, and 36 targets of RES for AD treatment, among which 34.6% of the targets were protein-modifying enzymes, 27.7% were metabolite invertase, 13.8% were gene-specific transcriptional regulators, and 10.3% were transporters. The core key targets of RES in the treatment of AD included INS, APP, ESR1, MMP9, IGF1R, CACNA1C, MAPT (microtubule- associated protein Tau), MMP2, TGFB1 and GSK3B. Enrichment analysis of GO biological process suggested that the biological function of RES in AD treatment mainly involved the response to β-amyloid protein, positive regulation of transferase activity, the transmembrane receptor protein tyrosine kinase signaling pathway, regulation of behavior, learning or memory, aging, and transmembrane transport. KEGG pathway enrichment analysis showed that the most significantly enriched signaling pathways were AD pathway, PI3K-AKT signaling pathway, cGMP-PKG signaling pathway, and MAPK signaling pathway. Molecular docking results showed that RES had strong binding with ESR1, GSK3B, MMP9, IGF1R, APP and INS. In the cell model of AD, treatment with 50 μmol/L RES for 12 h significantly reduced the levels of pS396 and pS199 by regulating CDK5 and GSK3β activity (P < 0.001).
Conclusion
RES produces therapeutic effects on AD by acting on multiple targets and affecting multiple signaling pathways and improves AD-associated pathologies via a direct action on Aβ and Tau pathological processes.
Keywords: network pharmacology, resveratrol, Alzheimer's disease, mechanism
阿尔茨海默病(AD)是最为常见的一种神经退行性疾病,表现为不可逆的神经元死亡和进展性的认知功能障碍,约有60%~80%的痴呆病例与AD有关[1-2]。AD的发病机制至今仍不清楚,美国FDA批准了5种药物用于AD的治疗:多奈哌齐、利凡斯的明、加兰他敏、美金刚和Namzaric®(多奈哌齐和美金刚缓释剂的组合)[3],其中多奈哌齐、利凡斯的明、加兰他敏为乙酰胆碱酯酶抑制剂,美金刚为NMDA受体拮抗剂。上述AD治疗药物均为对症治疗且疗效有限,因此寻找能直接针对AD病理改变的候选药物并阐明其作用机制具有重要意义。
白藜芦醇(RES)是从多种水果中提取的非黄酮类多酚化合物[4-5]。由于RES具有抗氧化、抗炎和抗微生物等特性[6],其治疗潜力已被广泛研究。据报道,RES在AD、癫痫、帕金森病、亨廷顿病、肌萎缩侧索硬化症和神经损伤的体外模型中均显示出明显的治疗效果[6-7]。虽然RES在不同的行为测试中可改善认知功能[8-9],但RES治疗AD仍缺乏体内与临床研究证据。因此,有必要系统深入的对RES治疗AD的药理作用进行研究。网络药理学可系统、便捷的揭示药物活性成分与疾病靶点之间潜在的复杂相互作用,进而检测这种相互作用对系统功能和行为的影响[10]。本文基于网络药理学筛选出RES治疗AD的靶点,并对靶蛋白进行GO生物学过程及KEGG通路分析,明确RES治疗AD的核心靶点与潜在分子机制,在AD细胞模型中进行了初步验证,为进一步实验及临床应用提供理论依据。
1. 资料和方法
1.1. 收集RES作用靶点
使用PubChem数据库<sup>[<xref ref-type="bibr" rid="b11">11</xref>]</sup>(<a href="https://pubchem.ncbi.nlm.nih.gov/" target="_blank">https://pubchem.ncbi.nlm.nih.gov/</a>)获取RES的Canonical SMILES编号,将所得编号输入SwissTargetPrediction数据库<sup>[<xref ref-type="bibr" rid="b12">12</xref>]</sup>(<a href="http://www.swisstargetprediction.ch/" target="_blank">http://www.swisstargetprediction.ch/</a>)筛选RES作用靶点。此外,进一步使用BATMAN-TCM数据库<sup>[<xref ref-type="bibr" rid="b13">13</xref>]</sup>(<a href="http://bionet.ncpsb.org/batman-tcm/" target="_blank">http://bionet.ncpsb.org/batman-tcm/</a>)查询RES潜在靶点,合并两数据库获得靶点使用Excel表格去重后得到RES所有作用靶点。
1.2. AD靶点的获取
以“Alzheimer's disease”为关键词在Genecards数据库(<a href="https://www.genecards.org/" target="_blank">https://www.genecards.org/</a>)、AD数据库<sup>[<xref ref-type="bibr" rid="b14">14</xref>]</sup>(<a href="https: //www.cbligand.org/AD/login.php" target="_blank">https: //www.cbligand.org/AD/login.php</a>)以及TTD数据库<sup>[<xref ref-type="bibr" rid="b15">15</xref>]</sup> (<a href="http://bidd.nus.edu.sg/group/cjttd/" target="_blank">http://bidd.nus.edu.sg/group/cjttd/</a>)查询人类AD靶点。对1.1中RES作用靶点与AD靶点使用Venny 2.1 (<a href="https://bioinfogp.cnb.csic.es/tools/venny/index.html" target="_blank">https://bioinfogp.cnb.csic.es/tools/venny/index.html</a>)工具取交集,获得RES治疗AD的靶点。进一步使用Panther数据库(<a href="http://www.pantherdb.org/" target="_blank">http://www.pantherdb.org/</a>)对RES治疗AD靶点进行功能分类。
1.3. 构建靶点蛋白质-蛋白质相互作用(PPI)网络
将RES与AD共同靶点上传至String 11.0数据库(<a href="https://string-db.org/" target="_blank">https://string-db.org/</a>),选择物种为“Homo sapiens”,设定置信度大于0.4,获得靶点PPI网络。网络中的节点(nodes)代表靶蛋白,边(edges)代表靶蛋白与靶蛋白之间存在相互作用。度值(degree)为网络图中一个节点的与其他节点的连接数量,度值越高该节点在网络中越重要。将PPI网络导出为tsv格式文件,导入Cytoscape 3.7.1软件<sup>[<xref ref-type="bibr" rid="b16">16</xref>]</sup>(<a href="https://cytoscape.org/" target="_blank">https://cytoscape.org/</a>)使用Cytohubba插件根据度值筛选核心靶点。
1.4. 基因本体论(GO)与KEGG通路富集分析
Metascape<sup>[<xref ref-type="bibr" rid="b17">17</xref>]</sup>(<a href="https://metascape.org/gp" target="_blank">https://metascape.org/gp</a>)是一个功能强大的基因功能注释分析工具,使用Metascape对RES治疗AD靶蛋白进行GO生物学过程与KEGG信号通路富集分析。分析阈值设置为最小富集因子Enrichment为1.5、最小重叠靶点为3以及<italic>P</italic> < 0.01。使用微生信在线绘图网站(<a href="http://www.bioinformatics.com.cn" target="_blank">www.bioinformatics.com.cn</a>)对GO生物学过程与KEGG通路富集结果绘制气泡图与KEGG信号通路图。
1.5. 构建“靶点-信号通路”网络图
为直观的显示RES治疗AD的靶点参与KEGG信号通路情况,使用Cytoscape 3.7.1构建“靶点-信号通路”网络图。
1.6. AlzData数据库验证AD信号通路靶点变化
AlzData数据库<sup>[<xref ref-type="bibr" rid="b18">18</xref>]</sup>(<a href="http://www.alzdata.org/" target="_blank">http://www.alzdata.org/</a>)对684例AD病人以及562例对照脑组织基因表达谱进行了整合分析,我们使用该数据库检索关键信号通路的靶点在AD病人中的变化情况。
1.7. 分子对接验证核心靶点
将PPI网络中度值排名最高的10个靶点视为核心靶点,使用对接网络服务器SwissDock(<a href="http://www.swissdock.ch/docking" target="_blank">http://www.swissdock.ch/docking</a>)对RES与核心靶点进行分子对接,以验证其与核心靶点的结合强度。使用PubChem数据库查找RES三维结构,保存为SDF格式,使用OpenBabel软件转换为Mol2格式,进一步使用UCSF Chimera软件添加氢原子。在RCSB PDB数据库(<a href="http://www.rcsb.org/" target="_blank">http://www.rcsb.org/</a>)查找核心靶点,使用UCSF Chimera软件进行蛋白质文件预处理。点击Tools->Structure Editing->Dock Prep,在弹出窗口中不选择Write Mol2 file。在Add Hydrogen for Dock Prep窗口保留默认设置,完成后保存为pdb格式文件。在SwissDock网站分别上传RES配体与靶蛋白进行分子对接。
1.8. 药品及试剂
RES(SRT501,MCE公司),CCK-8试剂盒(B34302) (Bimake生物科技有限公司)。二甲基亚砜(DMSO)、DMEM培养基(美国Sigma)。pS396(p-Tau at Ser396) (美国Biosource),Tau5(总Tau)(美国Millipore),Tau1 (Ser-198/199/202位点非磷酸化Tau)(美国Chemicon),pS199(p-Tau at Ser199)(美国Invitrogen),GSK3β(总GSK3β)和p-GSK3β(p-GSK3β at Ser9)(Cell Signaling technology),CDK5与p-CDK5(Tyr15)(Santa Cruz Biotechnology),GAPDH(英国Abcam)。
1.9. 细胞培养及处理
细胞模型是研究AD的有效方法之一,本研究选用过表达人Tau的AD细胞模型。人胚肾细胞293细胞(293WT)与稳转人野生型Tau的293细胞(293Tau)来自本实验室。细胞培养于添加10%胎牛血清的DMEM培养基,置于37 ℃、5%CO2培养箱中培养。RES使用DMSO配置成100 mmol/L母液待用。293Tau细胞种植于96孔板,密度为20×104,24 h后分别给与0、25、50、75、100 μmol/L RES处理6 h,由此确定RES处理293Tau细胞的最佳浓度。进一步使用最佳浓度处理0、6、12、24、36 h确定RES处理时间。293Tau细胞分为3组,分别为293Tau、293Tau + DMSO(溶剂对照)、293Tau+RES,观察RES对Tau磷酸化的影响。
1.10. CCK-8活性检测
严格根据CCK-8试剂盒使用说明书检测CCK-8活性,用BioTek酶标仪(BioTek Laboratory Instruments,美国)检测在450 nm处的吸光度值A450 nm。
1.11. 蛋白免疫印迹检测
1.11.1. 细胞样品
吸弃细胞培养基,用预冷的PBS溶液清洗残留培养基和死亡脱落细胞,根据细胞密度每孔加入100~200 µL细胞裂解液,将裂解液收集到EP管中。100 ℃沸水中煮沸10 min,然后在冰中超声5 s。离心15 min(4 ℃,转速14 000 g),吸取上清液并进行分装。测完蛋白浓度后按比例加入溴酚蓝(终浓度0.2%)和β-巯基乙醇(终浓度10%),-80 ℃分装保存备用。
1.11.2. Western blotting
根据测得的蛋白质浓度,计算出各样品的上样量。常规电泳和转膜后,用5%脱脂牛奶封闭NC膜,加抗p-S396-Tau,p-S199-Tau,Tau5,GSK3β,p-GSK3β等一抗4 ℃孵育过夜。次日用TBSTween-20洗涤NC膜后,二抗(IRDye@800CW,1:10 000)孵育1 h(室温避光)。再用TBS-Tween-20洗涤后,Odyssey红外扫描仪(Li-Cor biosciences,美国)扫描,用ImageJ统计软件(NIH, Bethesda, MD, USA)进行灰度值统计分析。
1.12. 统计学方法
采用SPSS16.0版软件录入数据并进行统计学处理,实验数据均以均数±标准差表示。3组间计量资料比较采用单因素方差分析,两两比较采用Tukey多重比较检验,P < 0.05为差异有统计学意义。
2. 结果
2.1. RES、AD靶点的获取
SwissTargetPrediction数据库与BATMAN-TCM数据库中获得RES作用靶点分别为87、100个,去重后得到182个作用靶点。以“Alzheimer's disease”为疾病关键词,在Genecards数据库、AD数据库以及TTD数据库分别检索到100、320、143个AD相关靶点,去重后得到525个AD靶点。
2.2. RES治疗AD PPI网络的构建与分析
182个RES作用靶点与525个AD相关靶点使用Venny 2.1求交集,获得RES治疗AD的靶点36个(图 1A)。RES治疗AD的36个靶点具体为:ADAM17、ADRA2C、ADRB2、AHR、APP、CACNA1C、CACNA1D、CDK1、CDK5、CHRNA7、ESR1、ESR2、FGFR2、GSK3A、GSK3B、IGF1R、INS、INSR、KIT、LCK、MAOA、MAPT、MME、MMP1、MMP2、MMP9、NOS1、NQO2、PDE4D、SLC6A4、TGFB1、TTR、UCHL1、VCP、VDR与WEE1(表 1)。使用Panther数据库将RES治疗AD 36个靶点分为8类,前4的功能为:蛋白质修饰酶(34.6%)、代谢物相互转化酶(27.7%)、基因特异性转录调节因子(13.8%)、转运蛋白(10.3%)(图 1B)。蛋白质修饰酶中CDK1、CDK5、GSK3A、GSK3B与WEE1为丝氨酸/苏氨酸蛋白激酶,MME、MMP1、MMP2、MMP9为金属蛋白酶。
1.

RES作用靶点与AD靶点交集及功能分类
ntersection and functional classification of RES and AD targets. A: The Venn diagram was applied to obtain the intersection of the RES and AD target. B: Panther classification categorized target proteins of RES against AD. The percentages of the proteins in their functional class are shown in the pie chart.
1.
RES治疗AD靶点
Target proteins of RES againstAD
| Number | Gene ID | Protein description | Gene symbol |
| 1 | 6868 | ADAM metallopeptidase domain 17 | ADAM17 |
| 2 | 152 | Adrenoceptor alpha 2C | ADRA2C |
| 3 | 154 | Adrenoceptor beta 2 | ADRB2 |
| 4 | 196 | Aryl hydrocarbon receptor | AHR |
| 5 | 351 | Amyloid beta precursor protein | APP |
| 6 | 775 | Calcium voltage-gated channel subunit alpha1 C | CACNA1C |
| 7 | 776 | Calcium voltage-gated channel subunit alpha1 D | CACNA1D |
| 8 | 983 | Cyclin dependent kinase 1 | CDK1 |
| 9 | 1020 | Cyclin dependent kinase 5 | CDK5 |
| 10 | 1139 | Cholinergic receptor nicotinic alpha 7 subunit | CHRNA7 |
| 11 | 2099 | Estrogen receptor 1 | ESR1 |
| 12 | 2100 | Estrogen receptor 2 | ESR2 |
| 13 | 2263 | Fibroblast growth factor receptor 2 | FGFR2 |
| 14 | 2931 | Glycogen synthase kinase 3 alpha | GSK3A |
| 15 | 2932 | Glycogen synthase kinase 3 beta | GSK3B |
| 16 | 3480 | Insulin like growth factor 1 receptor | IGF1R |
| 17 | 3630 | Insulin | INS |
| 18 | 3643 | Insulin receptor | INSR |
| 19 | 3815 | KIT proto-oncogene, Receptor tyrosine kinase | KIT |
| 20 | 3932 | LCK proto-oncogene, Src family tyrosine kinase | LCK |
| 21 | 4128 | Monoamine oxidase A | MAOA |
| 22 | 4137 | Microtubule associated protein tau | MAPT |
| 23 | 4311 | Membrane metalloendopeptidase | MME |
| 24 | 4312 | Matrix metallopeptidase 1 | MMP1 |
| 25 | 4313 | Matrix metallopeptidase 2 | MMP2 |
| 26 | 4318 | Matrix metallopeptidase 9 | MMP9 |
| 27 | 4842 | Nitric oxide synthase 1 | NOS1 |
| 28 | 4835 | N-ribosyldihydronicotinamide:quinone reductase 2 | NQO2 |
| 29 | 5144 | Phosphodiesterase 4D | PDE4D |
| 30 | 6532 | Solute carrier family 6 member 4 | SLC6A4 |
| 31 | 7040 | Transforming growth factor beta 1 | TGFB1 |
| 32 | 7276 | Transthyretin | TTR |
| 33 | 7345 | Ubiquitin C-terminal hydrolase L1 | UCHL1 |
| 34 | 7415 | Valosin containing protein | VCP |
| 35 | 7421 | Vitamin D receptor | VDR |
| 36 | 7465 | WEE1 G2 checkpoint kinase | WEE1 |
将RES治疗AD的36个靶蛋白导入String 11.0数据库进行蛋白质互作分析,得到RES治疗AD靶点PPI网络图(图 2)。该PPI网络共有36个节点,125条边,平均度值为6.94,其中NQO2未与其他靶点发生相互作用。将PPI网络图导入Cytoscape 3.7.1软件,使用Cytohubba插件进行网络拓扑分析,节点颜色越深度值越高,即与更多的节点发生相互作用。结果显示,RES治疗AD度值排名前10的依次为INS、APP、ESR1、MMP9、IGF1R、CACNA1C、MAPT、MMP2、TGFB1与GSK3B (图 3),其中INS度值高达27。
2.

RES治疗AD靶点PPI网络
PPI network of the targets of RES for treatment of AD.
3.
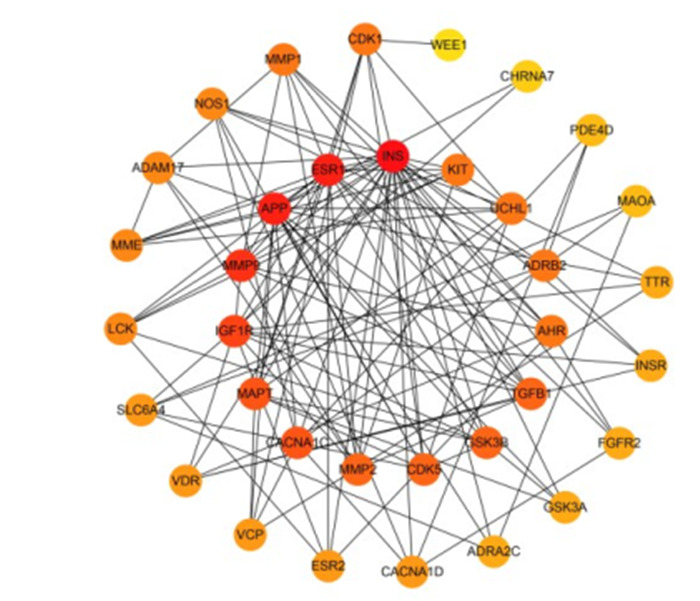
RES治疗AD靶点拓扑分析
Topological analyses of the targets of RES for treatment of AD. The nodes represent the target proteins and the edges represent the interaction among the targets. A redder color indicates a higher degree of interaction.
2.3. GO生物学过程与KEGG通路富集分析
通过Metascape数据库对RES治疗AD的36个靶点进行GO生物学过程与KEGG通路富集分析。GO生物学过程前20条富集信息(图 4),X轴代表富集因子,Y轴代表GO生物学过程名称,气泡大小代表GO生物学过程中的靶点数量,气泡颜色为-log10(p value)代表富集显著性。GO生物学过程主要涉及对β-淀粉样蛋白的反应、转移酶活性的正调节、跨膜受体蛋白酪氨酸激酶信号通路、行为、学习或记忆、老化、跨膜转运的调控等(图 4)。
4.
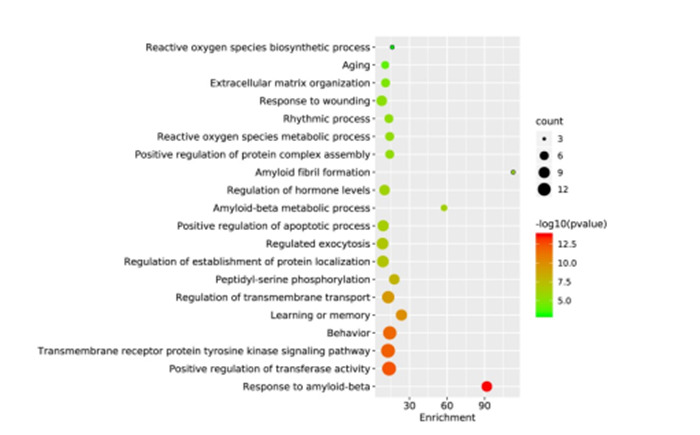
GO富集分析生物学过程Top20气泡图
Top 20 bubble chart of biological process of GO enrichment analysis. The X-axis represents the enrichment factor, the bubble size represents the count of targets enriched in terms and the color represents the P value.
KEGG通路富集主要涉及阿尔茨海默病、内吞作用、cGMP-PKG信号通路、5-羟色胺能突触、多巴胺能突触、钙信号通路、MAPK信号通路、GnRH信号通路、胆碱能突触、雌激素信号途径、PI3K-Akt信号通路、AMPK信号通路等(图 5)。KEGG富集分析信号通路详细信息见表 2。
5.
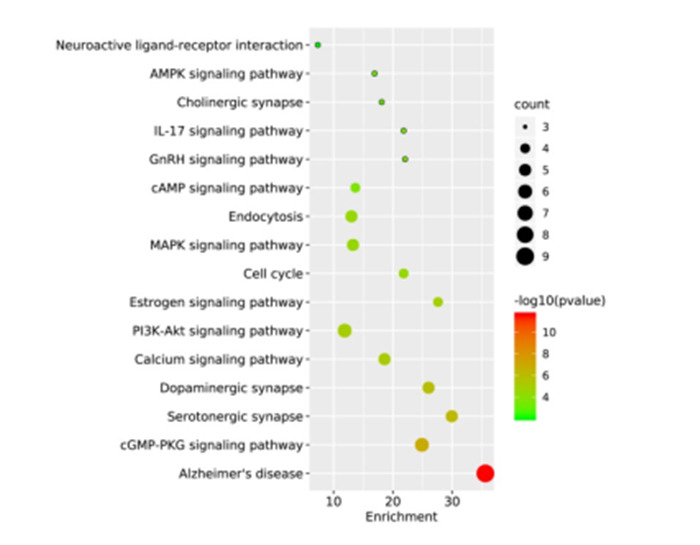
KEGG通路富集分析气泡图
Bubble chart of KEGG pathway enrichment analysis. The X-axis represents the enrichment factor, bubble size represents the count of targets enriched in terms and the color represents the P value.
2.
RES治疗AD KEGG通路富集分析
KEGG pathway enrichment analysis of RES againstAD
| Pathway | Enrichment | P | Count | Symbols |
| Alzheimer's disease | 35.62 | 2.67E-12 | 9 | APP, CACNA1C, CACNA1D, CDK5, GSK3B, MAPT, MME, NOS1, ADAM17 |
| cGMP-PKG signaling pathway | 24.92 | 1.35E-07 | 6 | ADRA2C, ADRB2, CACNA1C, CACNA1D, INS, INSR |
| Serotonergic synapse | 29.95 | 6.59E-07 | 5 | APP, CACNA1C, CACNA1D, MAOA, SLC6A4 |
| Dopaminergic synapse | 26.03 | 1.32E-06 | 5 | CACNA1C, CACNA1D, GSK3A, GSK3B, MAOA |
| Calcium signaling pathway | 18.60 | 6.87E-06 | 5 | ADRB2, CACNA1C, CACNA1D, CHRNA7, NOS1 |
| PI3K-Akt signaling pathway | 11.87 | 9.99E-06 | 6 | FGFR2, GSK3B, IGF1R, INS, INSR, KIT |
| Estrogen signaling pathway | 27.63 | 1.31E-05 | 4 | ESR1, ESR2, MMP2, MMP9 |
| Cell cycle | 21.83 | 3.32E-05 | 4 | CDK1, GSK3B, TGFB1, WEE1 |
| MAPK signaling pathway | 13.27 | 3.49E-05 | 5 | CACNA1C, CACNA1D, FGFR2, MAPT, TGFB1 |
| Endocytosis | 13.02 | 3.83E-05 | 5 | ADRB2, FGFR2, IGF1R, KIT, TGFB1 |
| cAMP signaling pathway | 13.67 | 2.03E-04 | 4 | ADRB2, CACNA1C, CACNA1D, PDE4D |
| GnRH signaling pathway | 22.07 | 3.40E-04 | 3 | CACNA1C, CACNA1D, MMP2 |
| IL-17 signaling pathway | 21.83 | 3.51E-04 | 3 | GSK3B, MMP1, MMP9 |
| Cholinergic synapse | 18.13 | 6.04E-04 | 3 | CACNA1C, CACNA1D, CHRNA7 |
| AMPK signaling pathway | 16.92 | 7.39E-04 | 3 | IGF1R, INS, INSR |
| Neuroactive ligand-receptor interaction | 7.33 | 7.87E-03 | 3 | ADRA2C, ADRB2, CHRNA7 |
为更直观的显示参与RES治疗AD涉及KEGG通路所涉及的靶点,进一步使用Cytoscape 3.7.1构建了“靶点-信号通路”网络图(图 6)。“靶点-信号通路”网络图中共有45个节点,73条边,平均度值为3.24。KEGG通路阿尔茨海默病通路度值最高为9,其次为PI3K-Akt信号通路、cGMP-PKG信号通路、MAPK信号通路等。
6.
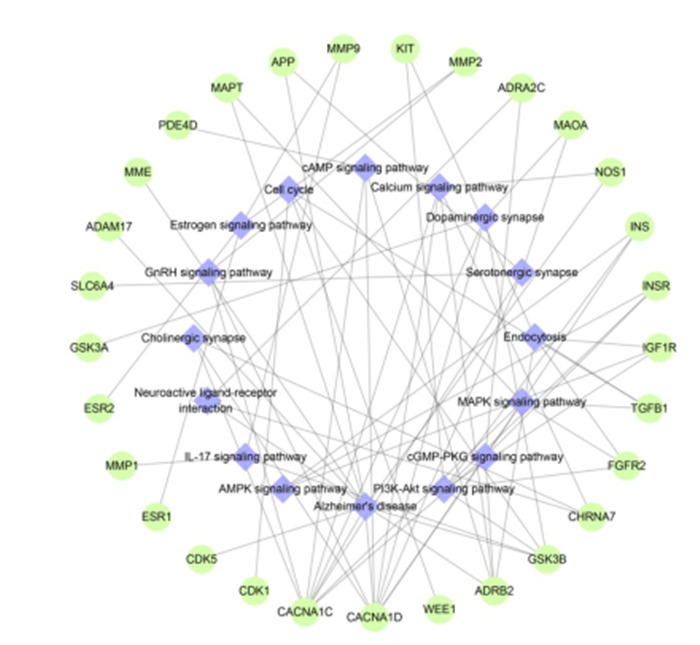
RES治疗AD“靶点-信号通路”网络图
Target-signaling pathway network diagram of RES for treatment of AD. Green nodes represent the target proteins and purple diamond nodes represent the enriched KEGG pathway.
进一步使用AlzData数据库检索富集最显著、度值最高的阿尔茨海默病(hsa05010)信号通路相关靶点在AD病人颞叶皮层中的变化情况,结果显示参与该通路的APP、CDK5与GSK3B在AD病人中均显著下调(图 7)。本研究绘制了参与阿尔茨海默病信号通路的9个靶点在KEGG通路图中的具体位置,参与靶点显示为红色(图 8)。
7.
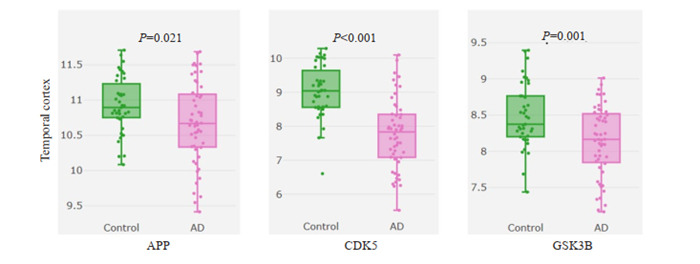
AD病人中参与阿尔茨海默病信号通路蛋白变化
Changes in proteins involved in Alzheimer's disease signaling pathway in AD patients. APP: Amyloid beta precursor protein; CDK5: Cyclin dependent kinase 5; GSK3B: Glycogen synthase kinase 3 beta.
8.
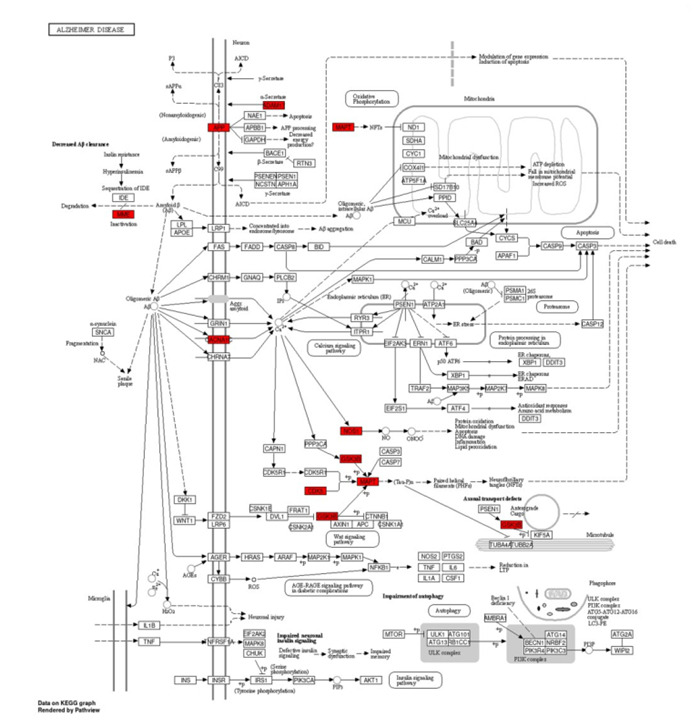
RES调控阿尔茨海默病信号通路靶点(红色标示)
Targets (red) regulated by RES inAlzheimer's disease signaling.
2.4. RES-核心靶点分子对接
使用SwissDock验证RES与核心靶点的结合,选择G最低的对接模型。Delta G数值负数为结合后可释放的自由能,其绝对值越大、结合越稳定。RES与ESR1、GSK3B、MMP9、IGF1R、APP与INS分子对接结果(表 3)。对接结果显示RES与核心靶点均有较强的相互作用,其中RES与ESR1的Delta G数值最低为-8.27 kcal/mol,RES与GSK3B的Delta G为-7.71 kcal/mol。
3.
RES-核心靶点分子对接
Molecular docking of the core target proteins with RES
| Target | PDB | deltaG (kcal/mol) | deltaGvdw | FullFitness (kcal/mol) | Energy (kcal/mol) |
| ESR1 | 2QE4 | -8.27 | -48.86 | -2062.72 | -7.36 |
| GSK3B | 2O5K | -7.71 | -44.97 | -1991.81 | -1.27 |
| MMP9 | 1ITV | -7.43 | -46.61 | -2522.84 | -8.21 |
| IGF1R | 1K3A | -7.32 | -41.57 | -1998.44 | 4.18 |
| APP | 2FK3 | -7.26 | -43.70 | -3359.63 | 1.83 |
| INS | 2QIU | -7.03 | -34.82 | -662.92 | 0.54 |
2.5. 不同RES浓度及时间对293Tau细胞的影响
本研究采用过表达人Tau的AD细胞模型(293Tau细胞),相比293Tau细胞293 WT不表达人Tau(图 9A)。为确定RES处理细胞最佳浓度,分别使用0、25、50、75、100 μmol/L RES处理293Tau细胞6 h。CCK-8结果显示,75与100 μmol/L RES会降低细胞存活率,即50 μmol/L是RES处理293Tau细胞的最佳浓度(图 9B)。进一步使用50 μmol/L RES处理293Tau细胞0、6、12、24、36 h,结果显示,RES处理293Tau细胞24、36 h可明显降低细胞存活率,因此50 μmol/L、12 h是RES处理293Tau细胞的最佳浓度和时间(图 9C)。
9.

不同RES浓度及时间对293Tau细胞的影响
Dose- and time-dependent effects of RES on 293Tau cells. A: Levels of Tau5 (total Tau) and Tau1 (nonphosphorylated Tau at Ser-198/199/202) measured by Western blotting; B: Relative cell viabilities of 293Tau cells after treatment with different concentration of RES for 6 h determined by cell counting kit-8 (CCK8) assay (n=6); C: Relative cell viabilities of 293Tau cells treated with 50 μmol/L RES for 0-36 h shown by CCK8 assay (n=3). Data are presented as Means±SD. *P < 0.05, ***P < 0.001 vs 0 μmol/L or 0 h.
2.6. RES通过调控GSK3β降低293Tau细胞Tau蛋白磷酸化
50 μmol/L RES处理12 h后检测各组Tau蛋白磷酸化水平、GSK3β活性。免疫印迹结果显示,RES处理可显著降低293Tau pS396和pS199水平,明显升高pGSK3β(非激活型GSK3β)水平。RES处理293Tau细胞并不影响各组间总的CDK5水平,但可以显著降低CDK5酪氨酸15位点磷酸化水平(图 10)。
10.

RES降低293Tau细胞Tau磷酸化水平
RES reduces tau phosphorylation level in 293Tau cells. A, B: Levels of Tau5 (total Tau), pS199 (p-Tau at Ser199) and pS396 (p-Tau at Ser396) measured by Western blotting and quantitative analysis (n=3); C, D: Levels of total GSK3β (t-GSK3β) and pGSK3β (p-GSK3β at Ser9) measured by Western blotting (n=3). E, F: Levels of CDK5 and p-CDK5 (p-CDK5 at Tyr15) measured by Western blotting (n=3). Data are presented as Mean±SD. *P < 0.05, ***P < 0.001.
3. 讨论
RES是一种神经保护特性的多酚化合物,具有抗氧化、抗炎、抗癌和抗淀粉样蛋白等作用[19-20],在体内和体外的实验中具有抗AD的作用[21-22]。本研究获得36个RES治疗AD的靶点,其中ADRB2、CDK5、GSK3A、GSK3B、LCK、MAPT(微管相关蛋白Tau)、MME、MMP2、TGFB1、VDR与AD Tau病理学变化显著相关,ADAM17、ADRB2、CACNA1C、CDK5、GSK3A、GSK3B、MMP2、PDE4D、TGFB1、UCHL1、VCP与Aβ病理学变化显著相关,其中ADRB2、CDK5、GSK3A、GSK3B、MMP2、TGFB1与Aβ、Tau病理过程均显著相关[18]。研究表明姜黄素可以通过阻碍大鼠CDK5的激活降低Aβ聚集与Tau过度磷酸化从而改善AD[23]。本研究在AD细胞模型(293Tau细胞)中发现RES处理可以抑制CDK5的激活。此外,蛋白功能分类表明MME、MMP1、MMP2、MMP9为金属蛋白酶,其中MMP2与MMP9可降解Aβ且在AD脑中的表达增加[24]。
GO富集分析结果表明,RES治疗AD的靶点富集最显著的生物学过程是β-淀粉样蛋白的反应,此外富集生物学过程还涉及转移酶活性的正调节、跨膜受体蛋白酪氨酸激酶信号通路、行为、学习或记忆、老化等。RES治疗AD靶蛋白KEGG通路主要富集在阿尔茨海默病、内吞作用、cGMP-PKG信号通路、5-羟色胺能突触、多巴胺能突触、钙信号通路、MAPK信号通路、GnRH信号通路、胆碱能突触、雌激素信号途径、PI3K-Akt信号通路、AMPK信号通路等。研究表明RES可抗氧化、抑制乙酰胆碱酯酶活力和抗凋亡,对老年性痴呆小鼠认知功能具有一定保护作用[8],同时有效地逆转Aβ25-35导致的学习记忆的损伤[25],减少了淀粉样蛋白前体蛋白(APP)的淀粉样蛋白裂解,增强了淀粉样β肽的清除率,抑制淀粉样蛋白生成途径并刺激自噬等过程降解Aβ聚集,补充RES有利于Tau蛋白的去磷酸化并抑制其聚集[26-27]。这说明RES可以通过影响AD病理改变的方式例如Aβ、Tau、胆碱能突触等治疗AD。
RES治疗AD的36个靶点可形成复杂的PPI网络,其中INS、APP、ESR1、MMP9、IGF1R、CACNA1C、MAPT、MMP2、TGFB1与GSK3B是RES治疗AD的核心靶点。淀粉样前体蛋白APP一种在全身细胞中广泛表达的蛋白质,其突变可导致早发性AD[28]。APP被剪切后可形成Aβ,Aβ在细胞外沉淀聚积后具有很强的神经毒性作用。GSK3B在脑内神经元高表达,是Tau蛋白磷酸化过程中关键激酶,不仅可以影响Tau蛋白磷酸化还可以影响Aβ的形成[29]。有研究报道miR-137调控CACNA1C参与了AD的发生[30]。丝氨酸/苏氨酸蛋白激酶的功能是使蛋白质上的丝氨酸和苏氨酸残基发生磷酸化,蛋白功能分类显示CDK1、CDK5、GSK3A、GSK3B、WEE1为丝氨酸/苏氨酸蛋白激酶。分子对接结果显示RES与GSK3B有较强的结合作用。本研究在AD细胞模型(293Tau细胞)中发现50 μmol/L RES处理12 h可显著降低Tau蛋白磷酸化水平,进一步检测Tau蛋白磷酸化过程中关键激酶GSK3β(GSK3B),发现RES处理可明显升高p-GSK3β水平。以上结果提示,RES可以通过调控GSK3β改善AD病理改变。
本研究基于网络药理学系统分析明确了RES治疗AD的核心靶点,通过GO富集分析与KEGG通路分析阐释其关键信号通路及分子机制,发现RES可以通过AD Aβ、Tau病理过程改善AD。使用分子对接技术对RES与核心靶点的结合进行了验证,并在AD细胞模型中验证了RES可通过调控CDK5与GSK3β改善AD病理改变。本研究可为RES治疗AD的临床应用提供理论和实验依据,为后续机制研究提供思路和方法。
Biography
方迎艳,博士,副教授,E-mail: fyingyan@hbpu.edu.cn
Funding Statement
国家自然科研基金(81902860);湖北理工学院校级人才引进科研项目(19XJK04R)
Supported by National Natural Science Foundation of China (81902860)
Contributor Information
方 迎艳 (Yingyan FANG), Email: fyingyan@hbpu.edu.cn.
曾 鹏 (Peng ZENG), Email: zengp@hust.edu.cn.
References
- 1.李 青峰, 邢 潇丹, 冯 前进. 基于耦合的卷积-图卷积神经网络的阿尔茨海默病的磁共振诊断方法. http://www.j-smu.com:81/CN/10.12122/j.issn.1673-4254.2020.04.13. 南方医科大学学报. 2020;40(4):531–7. doi: 10.12122/j.issn.1673-4254.2020.04.13. [李青峰, 邢潇丹, 冯前进.基于耦合的卷积-图卷积神经网络的阿尔茨海默病的磁共振诊断方法[J].南方医科大学学报, 2020, 40(4): 531-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonkusare SK, Kaul CL, Ramarao P. Dementia of Alzheimer's disease and other neurodegenerative disorders: memantine, a new hope. Pharmacol Res. 2005;51(1):1–17. doi: 10.1016/j.phrs.2004.05.005. [Sonkusare SK, Kaul CL, Ramarao P. Dementia of Alzheimer's disease and other neurodegenerative disorders: memantine, a new hope[J]. Pharmacol Res, 2005, 51(1): 1-17.] [DOI] [PubMed] [Google Scholar]
- 3.Melnikova I. Therapies for Alzheimer's disease. Nat Rev Drug Discov. 2007;6(5):341–2. doi: 10.1038/nrd2314. [Melnikova I. Therapies for Alzheimer's disease[J]. Nat Rev Drug Discov, 2007, 6(5): 341-2.] [DOI] [PubMed] [Google Scholar]
- 4.Medina-Bolivar F, Condori J, Rimando AM, et al. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry. 2007;68(14):1992–2003. doi: 10.1016/j.phytochem.2007.04.039. [Medina-Bolivar F, Condori J, Rimando AM, et al. Production and secretion of resveratrol in hairy root cultures of peanut[J]. Phytochemistry, 2007, 68(14): 1992-2003.] [DOI] [PubMed] [Google Scholar]
- 5.Rocha-González HI, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther. 2008;14(3):234–47. doi: 10.1111/j.1755-5949.2008.00045.x. [Rocha-González HI, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases[J]. CNS Neurosci Ther, 2008, 14(3): 234-47.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, et al. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed Pharmacother. 2020;127:110234–9. doi: 10.1016/j.biopha.2020.110234. [Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, et al. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway[J]. Biomed Pharmacother, 2020, 127: 110234-9.] [DOI] [PubMed] [Google Scholar]
- 7.Sarubbo F, Moranta D, Asensio VJ, et al. Effects of resveratrol and other polyphenols on the most common brain age-related diseases. Curr Med Chem. 2017;24(38):4245–66. doi: 10.2174/0929867324666170724102743. [Sarubbo F, Moranta D, Asensio VJ, et al. Effects of resveratrol and other polyphenols on the most common brain age-related diseases[J]. Curr Med Chem, 2017, 24(38): 4245-66.] [DOI] [PubMed] [Google Scholar]
- 8.罗 莉, 黄 忆明. 白藜芦醇对老年性痴呆小鼠认知功能的影响. 中南大学学报:医学版. 2006;25(4):566–9. doi: 10.3321/j.issn:1672-7347.2006.04.024. [罗莉, 黄忆明.白藜芦醇对老年性痴呆小鼠认知功能的影响[J].中南大学学报:医学版, 2006, 25(4): 566-9.] [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–79. doi: 10.1038/sj.emboj.7601758. [Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis[J]. EMBO J, 2007, 26(13): 3169-79.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YQ, Mao X, Guo QY, et al. Network pharmacology-based approaches capture essence of Chinese herbal medicines. Chin Herb Med. 2016;8(2):107–16. doi: 10.1016/S1674-6384(16)60018-7. [Zhang YQ, Mao X, Guo QY, et al. Network pharmacology-based approaches capture essence of Chinese herbal medicines[J]. Chin Herb Med, 2016, 8(2): 107-16.] [DOI] [Google Scholar]
- 11.Kim S, Thiessen PA, Cheng TJ, et al. Literature information in PubChem: associations between PubChem records and scientific articles. J Cheminform. 2016;8:32–40. doi: 10.1186/s13321-016-0142-6. [Kim S, Thiessen PA, Cheng TJ, et al. Literature information in PubChem: associations between PubChem records and scientific articles[J]. J Cheminform, 2016, 8: 32-40.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daina A, Michielin O, Zoete V. Swiss Target Prediction: updated data and new features for efficient prediction of protein targets of small molecules. NucleicAcids Res. 2019;47(W1):W357–64. doi: 10.1093/nar/gkz382. [Daina A, Michielin O, Zoete V. Swiss Target Prediction: updated data and new features for efficient prediction of protein targets of small molecules[J]. NucleicAcids Res, 2019, 47(W1): W357-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu ZY, Guo FF, Wang Y, et al. BATMAN-TCM: a bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine. Sci Rep. 2016;6:21146–55. doi: 10.1038/srep21146. [Liu ZY, Guo FF, Wang Y, et al. BATMAN-TCM: a bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine[J]. Sci Rep, 2016, 6: 21146-55.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu HB, Wang LR, Lv M, et al. AlzPlatform: an Alzheimer's disease domain-specific chemogenomics knowledgebase for polypharmacology and target identification research. J Chem Inf Model. 2014;54(4):1050–60. doi: 10.1021/ci500004h. [Liu HB, Wang LR, Lv M, et al. AlzPlatform: an Alzheimer's disease domain-specific chemogenomics knowledgebase for polypharmacology and target identification research[J]. J Chem Inf Model, 2014, 54(4): 1050-60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YX, Zhang S, Li FC, et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. NucleicAcids Res. 2019;60(12):981–9. doi: 10.1093/nar/gkz981. [Wang YX, Zhang S, Li FC, et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics[J]. NucleicAcids Res, 2019, 60(12): 981-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon P. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. doi: 10.1101/gr.1239303. [Shannon P. Cytoscape: a software environment for integrated models of biomolecular interaction networks[J]. Genome Res, 2003, 13(11): 2498-504.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou YY, Zhou B, Pache L, et al. Metascape provides a biologistoriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523–9. doi: 10.1038/s41467-019-09234-6. [Zhou YY, Zhou B, Pache L, et al. Metascape provides a biologistoriented resource for the analysis of systems-level datasets[J]. Nat Commun, 2019, 10: 1523-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu M, Zhang DF, Luo RC, et al. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in Alzheimer's disease. Alzheimer's Dement. 2018;14(2):215–29. doi: 10.1016/j.jalz.2017.08.012. [Xu M, Zhang DF, Luo RC, et al. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in Alzheimer's disease[J]. Alzheimer's Dement, 2018, 14(2): 215-29.] [DOI] [PubMed] [Google Scholar]
- 19.Lange KW, Li SM. Resveratrol, pterostilbene, and dementia. BioFactors. 2018;44(1):83–90. doi: 10.1002/biof.1396. [Lange KW, Li SM. Resveratrol, pterostilbene, and dementia[J]. BioFactors, 2018, 44(1): 83-90.] [DOI] [PubMed] [Google Scholar]
- 20.Jardim FR, de Rossi FT, Nascimento MX, et al. Resveratrol and brain Mitochondria: a review. Mol Neurobiol. 2018;55(3):2085–101. doi: 10.1007/s12035-017-0448-z. [Jardim FR, de Rossi FT, Nascimento MX, et al. Resveratrol and brain Mitochondria: a review[J]. Mol Neurobiol, 2018, 55(3): 2085-101.] [DOI] [PubMed] [Google Scholar]
- 21.Ahmed T, Javed S, Javed S, et al. Resveratrol and Alzheimer's disease: mechanistic insights. Mol Neurobiol. 2017;54(4):2622–35. doi: 10.1007/s12035-016-9839-9. [Ahmed T, Javed S, Javed S, et al. Resveratrol and Alzheimer's disease: mechanistic insights[J]. Mol Neurobiol, 2017, 54(4): 2622-35.] [DOI] [PubMed] [Google Scholar]
- 22.Drygalski K, Fereniec E, Koryciński K, et al. Resveratrol and Alzheimer's disease, from molecular pathophysiology to clinical trials. Exp Gerontol. 2018;113:36–47. doi: 10.1016/j.exger.2018.09.019. [Drygalski K, Fereniec E, Koryciński K, et al. Resveratrol and Alzheimer's disease, from molecular pathophysiology to clinical trials[J]. Exp Gerontol, 2018, 113: 36-47.] [DOI] [PubMed] [Google Scholar]
- 23.Das TK, Jana P, Chakrabarti SK, et al. Curcumin downregulates GSK3 and Cdk5 in scopolamine-induced Alzheimer's disease rats abrogating Aβ40/42 and tau hyperphosphorylation. J Alzheimer's Dis Rep. 2019;3(1):257–67. doi: 10.3233/ADR-190135. [Das TK, Jana P, Chakrabarti SK, et al. Curcumin downregulates GSK3 and Cdk5 in scopolamine-induced Alzheimer's disease rats abrogating Aβ40/42 and tau hyperphosphorylation[J]. J Alzheimer's Dis Rep, 2019, 3(1): 257-67.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim NKH, Villemagne VL, Soon CPW, et al. Investigation of matrix metalloproteinases, MMP-2 and MMP-9, in plasma reveals a decrease of MMP-2 in Alzheimer's disease. J Alzheimer's Dis. 2011;26(4):779–86. doi: 10.3233/JAD-2011-101974. [Lim NKH, Villemagne VL, Soon CPW, et al. Investigation of matrix metalloproteinases, MMP-2 and MMP-9, in plasma reveals a decrease of MMP-2 in Alzheimer's disease[J]. J Alzheimer's Dis, 2011, 26(4): 779-86.] [DOI] [PubMed] [Google Scholar]
- 25.沈 兆星, 肖 谦, 赵 宇星, et al. Ghrelin对糖尿病大鼠海马DKK-1表达和学习记忆功能的影响. http://www.j-smu.com:81/CN/Y2016/V36/I04/500. 南方医科大学学报. 2016;36(4):500–5. doi: 10.3969/j.issn.1673-4254.2016.04.010. [沈兆星, 肖谦, 赵宇星, 等. Ghrelin对糖尿病大鼠海马DKK-1表达和学习记忆功能的影响[J].南方医科大学学报, 2016, 36(4): 500-5.] [DOI] [PubMed] [Google Scholar]
- 26.Jia YM, Wang N, Liu XW. Resveratrol and amyloid-beta: mechanistic insights. Nutrients. 2017;9(10):1122–9. doi: 10.3390/nu9101122. [Jia YM, Wang N, Liu XW. Resveratrol and amyloid-beta: mechanistic insights[J]. Nutrients, 2017, 9(10): 1122-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashrafizadeh M, Zarrabi A, Najafi M, et al. Resveratrol targeting tau proteins, amyloid-beta aggregations, and their adverse effects: an updated review. Phytother Res. 2020;34(11):2867–88. doi: 10.1002/ptr.6732. [Ashrafizadeh M, Zarrabi A, Najafi M, et al. Resveratrol targeting tau proteins, amyloid-beta aggregations, and their adverse effects: an updated review[J]. Phytother Res, 2020, 34(11): 2867-88.] [DOI] [PubMed] [Google Scholar]
- 28.Defina PA, Moser RS, Glenn M, et al. Alzheimer's disease clinical and research update for health care practitioners. J Aging Res. 2013;52:207178–85. doi: 10.1155/2013/207178. [Defina PA, Moser RS, Glenn M, et al. Alzheimer's disease clinical and research update for health care practitioners[J]. J Aging Res, 2013, 52: 207178-85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.余 锋, 贾 芳芳. 运动调控GSK-3β介导阿尔茨海默症的机制探析. https://www.cnki.com.cn/Article/CJFDTOTAL-NTXZ202002008.htm. 南京体育学院学报. 2020;19(2):52–9. [余锋, 贾芳芳.运动调控GSK-3β介导阿尔茨海默症的机制探析[J].南京体育学院学报, 2020, 19(2): 52-9.] [Google Scholar]
- 30.姜扬.非编码RNAmiR-137调控tau蛋白磷酸化和L型钙离子通道CACNA1C参与阿尔茨海默病的发生[D].北京: 中国医科大学, 2018.


