Abstract
目的
明确人参皂苷20(S)-Rg3促进卵巢癌细胞抑癌基因von Hippel-Lindau(VHL)表达的机制。
方法
Real-time PCR和Western blotting检测20(S)-Rg3处理前后卵巢癌细胞SKOV3中的VHL、DNA甲基转移酶DNMT1、DNMT3A和DNMT3B的mRNA和蛋白水平。Real-time PCR检测甲基转移酶抑制剂5-氮杂-2-脱氧胞苷(5-Aza-CdR)处理卵巢癌细胞SKOV3前后VHL的mRNA水平变化。甲基化特异性PCR(MSP)检测20(S)-Rg3单纯处理组和20(S)-Rg3处理且过表达DNMT3A组的VHL基因启动子区的甲基化水平,并检测VHL的mRNA和蛋白表达水平。免疫组化检测课题组前期获得的20(S)-Rg3处理组及对照组的裸鼠皮下移植瘤组织中VHL和DNMT3A的蛋白表达。
结果
20(S)-Rg3处理后,卵巢癌细胞SKOV3中VHL的mRNA水平升高到阴性对照细胞的2倍以上,蛋白水平亦上调(P < 0.05);DNMT3A的mRNA水平和蛋白水平均下降(P < 0.05),DNMT3B的mRNA水平略有升高但蛋白水平无明显变化(P>0.05),DNMT1的mRNA和蛋白水平均无变化(P>0.05)。2 μmol/L和5 μmol/L的5-Aza-CdR处理后,SKOV3细胞的VHL mRNA水平升高到阴性对照细胞的3倍以上(P < 0.05)。20(S)-Rg3使VHL基因启动子区的甲基化水平降低(P < 0.05),在20(S)-Rg3处理的同时过表达DNMT3A,则VHL基因启动子甲基化水平再次升高,同时VHL mRNA和蛋白水平均降低(P < 0.05)。免疫组织显示,相对于对照组,20(S)-Rg处理组的裸鼠皮下移植瘤组织中VHL的表达上调、DNMT3A的表达下调。
结论
20(S)-Rg3通过抑制DNMT3A介导的启动子甲基化而促进卵巢癌细胞中VHL的表达。
Keywords: 卵巢癌, 人参皂苷, VHL, 甲基化
Abstract
Objective
To explore the mechanism by which ginsenoside 20(S)-Rg3 upregulates the expression of tumor suppressor von Hippel-Lindau (VHL) gene in ovarian cancer cells.
Methods
Ovarian cancer cell line SKOV3 treated with 20(S)-Rg3 were examined for mRNA and protein levels of VHL, DNMT1, DNMT3A and DNMT3B by real-time PCR and Western blotting, respectively. The changes in VHL mRNA expression in SKOV3 cells in response to treatment with 5-Aza-CdR, a DNA methyltransferase inhibitor, were detected using real-time PCR. VHL gene promoter methylation was examined with methylation-specific PCR and VHL expression levels were determined with real-time PCR and Western blotting in non-treated or 20(S)-Rg3-treated SKOV3 cells and in 20(S)-Rg3-treated DNMT3A-overexpressing SKOV3 cells. VHL and DNMT3A protein levels were detected by immunohistochemistry in subcutaneous SKOV3 cell xenografts in nude mice.
Results
Treatment of SKOV3 cells with 20(S)-Rg3 significantly upregulated VHL and downregulated DNMT3A expressions at both the mRNA and protein levels (P < 0.05) and upregulated DNMT3B expression only at the mRNA level, but did not cause significant changes in either the mRNA or protein level of DNMT1. Treatment of the cells with 2 and 5 μmol/L 5-Aza-CdR obviously increased VHL mRNA expression by by over 3 folds (P < 0.05). 20(S)-Rg3 significantly decreased the methylation level in the promoter region of VHL gene, and this effect was abrogated by DNMT3A overexpression in the cells (P < 0.05). Immunohistochemisty showed a significantly increased VHL expression but a lowered DNMT3A expression in subcutaneous SKOV3 cell xenografts in 20 (S)-Rg3-treated nude mice.
Conclusion
Ginsenoside 20(S)-Rg3 upregulates VHL expression in ovarian cancer cells by suppressing DNMT3A-mediated DNA methylation.
Keywords: ovarian cancer, ginsenoside, von Hippel-Lindau, methylation
卵巢癌是死亡率最高的妇科恶性肿瘤[1]。因卵巢位于盆腔深部、卵巢癌早期无特异性的症状和体征,加之缺乏有效的早期筛查方法和特异的诊断指标,导致卵巢癌难以被早期发现,约三分之二的卵巢癌患者于中晚期才被确诊[2]。目前,晚期卵巢癌患者的5年生存率仅为15%~45%[3]。因此,改善晚期卵巢癌患者的预后是亟待解决的医学问题。筛选获得靶向关键分子的卵巢癌治疗新药将是改善卵巢癌患者治疗效果的一个重要途径。
人参皂苷是人参具有药物活性的精华成分[4]。目前已分离获得70种以上的人参皂苷单体,包括Ra1-3、Rb1-3、Rg1、Rg3、Rh1等[5]。其中Rg3的抗肿瘤活性吸引了众多注目,已报道Rg3在胶质瘤[6]、结肠癌[7]、肺癌[8]、前列腺癌[9]、乳腺癌[10]、宫颈癌[11]、卵巢癌[12]等多种肿瘤中具有抗肿瘤作用。Rg3根据第20位碳原子(C20)上羟基的空间位置而分为20(S)-Rg3和20(R)-Rg3两个同型异构体[13]。我们课题组前期研究发现,20(S)-Rg3通过上调卵巢癌细胞中的von Hippel-Lindau(VHL)的表达而减弱卵巢癌细胞的侵袭转移能力[12]。VHL的一个重要功能是介导缺氧诱导因子-1α(HIF-1α)经泛素-蛋白酶体途径降解[14]。常氧下,HIF-1α的关键脯氨酸残基由脯氨酸羟化酶区域蛋白羟基化,使得HIF-1α被VHL/E3泛素连接酶复合物识别,进而被多泛素化并被26S蛋白酶体降解[15]。VHL基因失活则使得HIF-1α蓄积,进而促进VEGF、TGF-α等细胞因子表达,加快细胞能量代谢、血管生长等过程,促进肿瘤的发生发展[16]。VHL基因的失活机制主要包括基因突变、杂合性缺失和基因启动子区的甲基化[17-18]。但是,20(S)-Rg3上调抑癌基因VHL的机制尚不明确。
本研究从DNA甲基化这一转录前水平的调控机制入手,发现20(S)-Rg3通过下调DNA甲基转移酶DNMT3A而削弱了其介导的VHL基因启动子区甲基化,使得VHL表达水平升高,为20(S)-Rg3能成为治疗卵巢癌的新型药物提供理论依据。
1. 材料和方法
1.1. 实验材料
人卵巢癌细胞株SKOV3(中国科学院细胞库);人参皂苷20(S)-Rg3(天士力制药集团股份有限公司);1640培养基(GIBCO);5-Aza-CdR(Sigma);DNMT3A过表达载体(Addgene)[19]。质粒转染试剂XtremeGENE HP(Roche);基因组DNA提取试剂盒(天根生化科技);EZ-DNA Methylation-Direct试剂盒(Zymo Research);RNA提取试剂盒RNA fast 200(上海飞捷生物);反转录试剂盒RevertAid first strand cDNA synthesis Kit(Thermo Fisher);甲基化PCR试剂盒Epi Taq HS和real-time PCR试剂SYBR Green Master Mix(TaKaRa);小鼠抗人β-actin多克隆抗体、兔抗人VHL多克隆抗体、兔抗人DNMT3A单克隆抗体(Cell Signaling Technology);HRP-羊抗兔/鼠IHC二抗试剂盒(福州迈新生物技术)。
1.2. 细胞培养与20(S)-Rg3处理
SKOV3细胞用含10%胎牛血清的RPMI 1640培养基在37 ℃、5%CO2、100%湿度的条件下培养。种板后24 h使细胞密度达到50%,更换新的完全培养基,并加入终浓度为80 μg/mL的20(S)-Rg3,继续培养24 h后提取总DNA、RNA或48h后提取总蛋白备用。
1.3. 使用5-Aza-CdR处理细胞
3×105个细胞/孔接种于6孔板中,培养24 h后细胞汇合度达到40%~50%时加5-Aza-CdR储存液(10 mmol/L)使其终浓度分别达到1、2或5 μmol/L。放入37 ℃、5% CO2和饱和湿度的培养箱继续培养48 h提取总RNA。
1.4. 质粒转染
4×105个细胞/孔接种于6孔板中,约24 h后细胞汇合度达70%时按照X-treme GENE HP DNA转染试剂说明书进行瞬时转染。每孔使用转染试剂6 μL,质粒2 μg。转染后继续培养48 h提取总RNA,72 h提取总蛋白。
1.5. DNA提取、重亚硫酸盐处理和甲基化特异性PCR(MSP)
利用MethPrimer(<a href="http://www.urogene.org/methprimer/" target="_blank">http://www.urogene.org/methprimer/</a>)获取VHL基因启动子区的CpG岛信息,并设计MSP引物(序列信息见<xref ref-type="table" rid="Table1">表 1</xref>)。提取基因组DNA后,用EZ DAN Methylation-Direct Kit进行DNA重亚硫酸盐处理及纯化回收。按下列体系进行MSP反应:Epi Taq HS 0.1 μL,10×Epi Taq PCR Buffer 2 μL,dNTP mixture 2.4 μL,MgCl<sub>2</sub>(25 mmol/L)2.4 μL,上、下游引物各0.8 μL,硫化的DNA < 40 ng,灭菌水补足至25 μL;反应条件:94℃ 5 min,之后94 ℃ 30 s,49 ℃ 30 s,72 ℃ 30 s,32个循环,之后72 ℃10 min。PCR产物进行3%琼脂糖凝胶电泳。
1.6. 细胞总RNA提取、反转录与real-time PCR
按照RNAFast 200试剂盒说明书提取细胞总RNA。核酸蛋白浓度分析仪检测RNA浓度和A260/280比值。采用反转录试剂盒RevertAid first strand cDNA synthesis Kit进行RNA反转录:总RNA 2 μg,random hexamer primer 1 μL,用nuclease-free water补足体积到12 μL,在PCR仪中65 ℃ 5 min,然后冰上急冷5 min;再加入5 × Reaction Buffer 4 μL,dNTP Mixture 2 μL,RNase Inhibitor 1 μL,M-MuLV Reverse Transcriptase 1 μL,将PCR管放入预热的PCR仪,设置反应条件25 ℃5 min,42 ℃ 60 min,70 ℃ 5 min。将得到的cDNA保存于-20℃备用。按照SYBR Green Master Mix说明书进行realtime PCR反应:2×SYBR Premix Ex Taq 10 μL,10 μmol/L上、下游引物各0.4 μL,cDNA 1 μL,灭菌水7.4 μL,realtime PCR反应条件为预变性95 ℃ 30 s,然后95 ℃ 5 s、60 ℃ 30 s,重复40个循环,之后接熔解曲线条件。采用CFX-96 real-time PCR system(Bio Rad)软件通过2-ΔΔCt方法计算基因的相对表达量。引物序列见表 1。
1.
序列信息
Primer sequences
| Genes | Sequences (5'-3') |
| Real-time PCR Primer | |
| VHL | F: GCAGGCGTCGAAGAGTACG |
| R:CGGACTGCGATTGCAGAAGA | |
| DNMT3A | F: TATTGATGAGCGCACAAGAGAGC |
| R:GGGTGTTCCAGGGTAACATTGAG | |
| DNMT3B | F: GACTCGAAGACGCACAGCTG |
| R:CTCGGTCTTTGCCGTTGTTATA | |
| DNMT1 | F: GTTCTTCCTCCTGGAGAATGTC |
| R: GGGCCACGCCGTACTGTTGCAG | |
| β-actin | F: TCCCTGGAGAAGAGCTACGA |
| R: AGCACTGTGTTGGCGTACAG | |
| MSPPrimer | |
| VHL methylated(M) | F: GGAGGATTATTTGAATTTAGGAGTTC |
| R: TAAAACAAAATCTCACTCTATCGC | |
| VHL unmethylated(U) | F: GGATTATTTGAATTTAGGAGTTTGA |
| R: TAAAACAAAATCTCACTCTATCACC |
1.7. Western blotting
提取总蛋白,BCA法定量,蛋白变性后按照每孔30 μg进行10%SDS-PAGE电泳,320 mA转膜90 min,5%脱脂奶粉封闭,一抗4℃孵育过夜(均为1:1000),二抗室温孵育1 h(1:2000),化学发光。
1.8. 20(S)-Rg3治疗的裸鼠皮下移植瘤组织以及对照组织切片的制备
课题组前期进行了20(S)-Rg3治疗裸鼠皮下移植瘤的实验[12],在裸鼠左前肢背侧皮下接种SKOV3细胞悬液0.1 mL(2×106个),10 d后肿瘤体积大于10 mm3时随机将裸鼠分成对照组和20(S)-Rg3干预组,后续30 d中干预组隔天尾静脉注射5 mg/kg的20(S)-Rg3,对照组则尾静脉注射相同体积的PBS。观察结束时颈椎脱臼处死裸鼠,剥离肿瘤,4%多聚甲醛固定,石蜡包埋并切片。
1.9. 免疫组织化学
石蜡脱蜡、水化,高压抗原修复,3%过氧化氢阻断内源性过氧化物酶,一抗4 ℃孵育过夜,二抗室温孵育15 min,DAB显色,苏木素复染,盐酸酒精分化,氨水返蓝,梯度酒精脱水,二甲苯透明,中性树胶封片,显微镜下观察并拍照。结果判定标准:VHL和DNMT3A以细胞质内有棕黄色颗粒为阳性。
1.10. 统计分析
所有数据以均数±标准差表示,用统计学软件SPSS 21.0进行统计分析。两组之间差异比较采用独立样本双侧t检验。甲基化特异PCR结果采用χ2检验。P < 0.05表示差异有统计学意义。
2. 结果
2.1. 20(S)-Rg3上调卵巢癌细胞SKOV3的VHL表达
Real-time PCR结果显示,相对于对照细胞,终浓度80 μg/mL的20(S)-Rg3处理24 h后卵巢癌细胞SKOV3的VHL mRNA水平显著升高(图 1A)。与mRNA水平的变化一致,Western blotting结果显示,20(S)-Rg3令SKOV3细胞的VHL蛋白表达水平显著增强(图 1B)。
1.
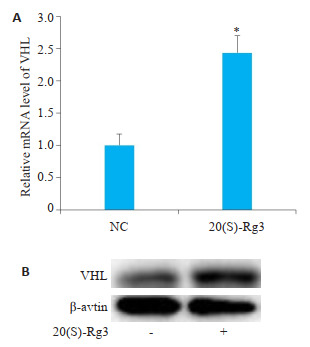
20(S)-Rg3上调卵巢癌细胞SKOV3的VHL
20(S)-Rg3 upregulates VHL in ovarian cancer cell line SKOV3. A: Real-time PCR shows elevatated VHL mRNA level in 20(S)-Rg3-treated cells; B: Western blotting shows increased VHL protein level in 20(S)-Rg3-treated cells. *P < 0.05.
2.2. 20(S)-Rg3降低卵巢癌细胞中VHL基因启动子区的甲基化水平
Real-time PCR结果显示,卵巢癌细胞SKOV3中VHL的mRNA水平随着DNA甲基转移酶抑制剂5-氮杂-2-脱氧胞苷(5-Aza-CdR)处理浓度的增加而升高(图 2A)。调取VHL基因序列上游3000个碱基作为其启动子区域,采用MethPrimer在线分析发现这一区域存在CpG岛(图 2B)。甲基化特异性PCR(MSP)结果显示,20(S)-Rg3处理后卵巢癌细胞SKOV3的VHL启动子区甲基化水平下降(图 2C)。
2.
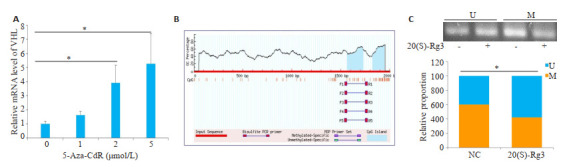
20(S)-Rg3降低VHL基因启动子区的甲基化水平
20(S)-Rg3 reduces methylation level in VHL gene promoter.A: Real-time PCR shows increased VHL mRNAlevel in 5-Aza-CdRtreated SKOV3 cells; B: The promoter region of VHL gene is predicted to contain CpG islands; C: MSP results show significantly decreased methylation level in the promoter region of VHL gene by 20(S)-Rg3. U=Unmethylated. M=Methylated. *P < 0.05.
2.3. 20(S)-Rg3通过下调卵巢癌细胞中甲基转移酶DNMT3A表达而增强VHL的表达
Real-time PCR结果显示,在用20(S)-Rg3处理卵巢癌细胞SKOV3后,DNMT3A的RNA水平下降、DNMT3B的RNA水平略有升高,但DNMT1的RNA水平无显著变化(图 3A)。Western blotting结果显示DNMT3A的蛋白水平明显降低,而DNMT3B和DNMT1蛋白水平无明显变化(图 3B)。在卵巢癌细胞SKOV3中过表达DNMT3A以及过表达DNMT3A同时用20(S)-Rg3处理,real-time PCR和Western blotting检测发现,与对照组相比,单纯过表达DNMT3A后VHL的mRNA和蛋白水平均较对照组下降,DNMT3A过表达拮抗了20(S)-Rg3上调VHL表达的作用(图 3C、D)。MSP结果显示单纯过表达DNMT3A后,VHL基因启动子区的甲基化水平升高(图 3E)。
3.

20(S)-Rg3通过抑制DNMT3A介导的DNA甲基化而上调VHL
20(S)-Rg3 upregulates VHL via suppressing DNMT3A-mediated methylation in the promoter region of VHL gene. A: Real-time PCR results show that 20(S)-Rg3 treatment decreases DNMT3A mRNA level and increases DNMT3B mRNA level without affecting DNMT1 mRNA in the cells; B: Western blotting results show that 20(S)-Rg3 treatment decreases DNMT3A and increases DNMT3B protein expressions without affecting DNMT1 expression in the cells; C: Real-time PCR shows reduced VHL mRNA level in 20(S)-Rg3-treated cells overexpressing DNMT3A; D: Western blotting results show lowered VHL protein expression in 20(S)-Rg3-treated cells overexpressing DNMT3A; E: MSP results show increased methylation level in the promoter region of VHL gene in 20(S)-Rg3-treated cells overexpressing DNMT3A. U=Unmethylated. M= Methylated. Ns : non-significant; *P < 0.05.
2.4. 免疫组化检测
采用免疫组织化学检测组织中VHL和DNMT3A的表达结果显示,相对于对照组,20(S)-Rg3治疗过的移植瘤组织中VHL表达升高而DNMT3A表达降低(图 4)。
4.
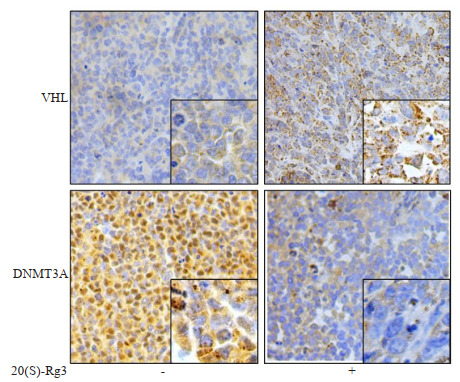
20(S)-Rg3治疗的SKOV3细胞裸鼠皮下移植瘤组织中VHL表达增强而DNMT3A表达减弱
Immunohistochemical staining showing increased VHL and lowered DNMT3A expressions in subcutaneous tumors derived SKOV3 cells in 20(S)-Rg3-treated nude mice (Original magnification, ×200; insets, ×400).
3. 讨论
本研究证实20(S)-Rg3下调卵巢癌细胞中DNMT3A的蛋白水平,从而抑制其介导的VHL基因启动子甲基化,促进VHL的表达。
20(S)-Rg3抑制卵巢癌细胞恶性表型的重要机制之一为上调抑癌基因VHL基因,但相关分子机制不明[12, 20]。在卵巢癌细胞中,20(S)-Rg3在mRNA水平即上调VHL,提示VHL mRNA转录增多或降解减少,相关调控可能发生在转录前、转录或转录后水平,如启动子CpG岛甲基化、非编码RNA等表观遗传调控或转录因子调控等。5-Aza-CdR升高VHL mRNA水平的现象反映出VHL受到DNA甲基转移酶的负调控,而当使用20(S)-Rg3处理后,哺乳动物体内3种主要的DNA甲基转移酶中,仅催化从头甲基化的DNMT3A表达下降,而维持DNA甲基化的DNMT1和催化从头甲基化的DNMT3B表达无变化,提示20(S)-Rg3导致的人卵巢癌细胞VHL表达上调可能与DNMT3A有关。实验结果证实了20(S)-Rg3下调DNMT3A蛋白水平、抑制其介导的DNA甲基化这一表观遗传调控机制,最终促进了VHL表达。这与研究报道的肿瘤中VHL的低表达与启动子区异常高甲基化相关的结果相符[21-22]。DNMT3A在多种肿瘤中发挥促癌作用,其在卵巢癌组织中异常高表达,参与促进卵巢癌细胞的增殖、迁移和侵袭等[23]。下调DNMT3A蛋白水平是20(S)-Rg3抑制卵巢癌细胞恶性表型的另一重要机制。20(S)-Rg3不影响DNMT1和DNMT3B的表达,仅下调DNMT3A,显示20(S)-Rg3对DNMT3A的抑制作用具有一定的特异性。文献报道DNMT3A的蛋白表达水平受到microRNA的负调控[24-26],同时其蛋白稳定性受到泛素-蛋白酶体途径的调控[27-28]。我们已获得了20(S)-Rg3影响的卵巢癌细胞microRNA表达谱,但尚未鉴定到靶向抑制DNMT3A的microRNA[29]。有关20(S)-Rg3降低DNMT3A蛋白水平的机制有待探讨。此外,我们前期发现,在卵巢癌细胞中DNMT3A通过介导microRNA前体基因启动子区甲基化而抑制部分具有抑癌作用的microRNA包括miR-532-3p、miR-603等的表达,从而促进了癌细胞的增殖、迁移和侵袭[29-32],本研究获得的DNMT3A抑制VHL表达的证据在此基础上进一步丰富了DNMT3A促癌作用的分子机制。
综上,人参皂苷20(S)-Rg3通过削弱DNMT3A介导的启动子甲基化而促进卵巢癌细胞中VHL的表达,这一结果拓展了对20(S)-Rg3抗卵巢癌分子机制的认识,为20(S)-Rg3成为临床治疗卵巢癌的候选药物提供了一定的理论依据。
Biography
王莉洁,硕士,住院医师,E-mail: wlj0061@163.com
Funding Statement
国家自然科学基金(81702576);陕西省自然科学基础研究计划项目(2020JM-376)
Supported by National Natural Science Foundation of China (81702576)
Contributor Information
王 莉洁 (Lijie WANG), Email: wlj0061@163.com.
李 旭 (Xu LI), Email: lixu56@mail.xjtu.edu.cn.
赵 乐 (Le ZHAO), Email: zhaole2@mail.xjtu.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018, 68(1): 7-30.] [DOI] [PubMed] [Google Scholar]
- 2.Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann Oncol. 2019;30(5):672–705. doi: 10.1093/annonc/mdz062. [Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger[J].Ann Oncol, 2019, 30(5): 672-705.] [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. doi: 10.1016/S0140-6736(17)33326-3. [Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J]. Lancet, 2018, 391(10125): 1023-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CZ, Anderson S, Du W, et al. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14(1):7–16. doi: 10.3724/SP.J.1009.2016.00007. [Wang CZ, Anderson S, Du W, et al. Red ginseng and cancer treatment [J]. Chin J Nat Med, 2016, 14(1): 7-16.] [DOI] [PubMed] [Google Scholar]
- 5.Zhou QL, Zhu DN, Yang XW, et al. Development and validation of a UFLC-MS/MS method for simultaneous quantification of sixty-six saponins and their six aglycones: Application to comparative analysis of red ginseng and white ginseng. J Pharm Biomed Anal. 2018;159:153–65. doi: 10.1016/j.jpba.2018.06.048. [Zhou QL, Zhu DN, Yang XW, et al. Development and validation of a UFLC-MS/MS method for simultaneous quantification of sixty-six saponins and their six aglycones: Application to comparative analysis of red ginseng and white ginseng[J]. J Pharm Biomed Anal, 2018, 159: 153-65.] [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Wei X, Shen L, et al. 20(S)-ginsenoside-Rg3 reverses temozolomide resistance and restrains epithelial-mesenchymal transition progression in glioblastoma. Cancer Sci. 2019;110(1):389–400. doi: 10.1111/cas.13881. [Chen Z, Wei X, Shen L, et al. 20(S)-ginsenoside-Rg3 reverses temozolomide resistance and restrains epithelial-mesenchymal transition progression in glioblastoma[J]. Cancer Sci, 2019, 110(1): 389-400.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu R, Qian F, Wang X, et al. Targeted delivery of 20(S)-ginsenoside Rg3-based polypeptide nanoparticles to treat colon cancer. Biomed Microdevices. 2019;21(1):18. doi: 10.1007/s10544-019-0374-0. [Qiu R, Qian F, Wang X, et al. Targeted delivery of 20(S)-ginsenoside Rg3-based polypeptide nanoparticles to treat colon cancer[J]. Biomed Microdevices, 2019, 21(1): 18.] [DOI] [PubMed] [Google Scholar]
- 8.Wang XJ, Zhou RJ, Zhang N, et al. 20(S)-ginsenoside Rg3 sensitizes human non-small cell lung cancer cells to icotinib through inhibition of autophagy. Eur J Pharmacol. 2019;850:141–9. doi: 10.1016/j.ejphar.2019.02.023. [Wang XJ, Zhou RJ, Zhang N, et al. 20(S)-ginsenoside Rg3 sensitizes human non-small cell lung cancer cells to icotinib through inhibition of autophagy[J]. Eur J Pharmacol, 2019, 850: 141-9.] [DOI] [PubMed] [Google Scholar]
- 9.Peng Y, Zhang R, Yang X, et al. Ginsenoside Rg3 suppresses the proliferation of prostate cancer cell line PC3 through ROS-induced cell cycle arrest. Oncol Lett. 2019;17(1):1139–45. doi: 10.3892/ol.2018.9691. [Peng Y, Zhang R, Yang X, et al. Ginsenoside Rg3 suppresses the proliferation of prostate cancer cell line PC3 through ROS-induced cell cycle arrest[J]. Oncol Lett, 2019, 17(1): 1139-45.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo YH, Kuruganti R, Gao Y. Recent advances in ginsenosides as potential therapeutics against breast cancer. Curr Top Med Chem. 2019;19(25):2334–47. doi: 10.2174/1568026619666191018100848. [Guo YH, Kuruganti R, Gao Y. Recent advances in ginsenosides as potential therapeutics against breast cancer[J]. Curr Top Med Chem, 2019, 19(25): 2334-47.] [DOI] [PubMed] [Google Scholar]
- 11.Bian S, Zhao Y, Li F, et al. 20(S)-ginsenoside Rg3 promotes HeLa cell apoptosis by regulating autophagy. Molecules. 2019;24(20):3655. doi: 10.3390/molecules24203655. [Bian S, Zhao Y, Li F, et al. 20(S)-ginsenoside Rg3 promotes HeLa cell apoptosis by regulating autophagy[J]. Molecules, 2019, 24(20): 3655.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Zhao L, Zhang Y, et al. Ginsenoside 20(S)-Rg3 targets HIF-1alpha to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One. 2014;9(9):e103887. doi: 10.1371/journal.pone.0103887. [Liu T, Zhao L, Zhang Y, et al. Ginsenoside 20(S)-Rg3 targets HIF-1alpha to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells[J]. PLoS One, 2014, 9(9): e103887.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Xu X, Wei X, et al. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol Res. 2019;148(104409) doi: 10.1016/j.phrs.2019.104409. [Luo C, Xu X, Wei X, et al. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms[J]. Pharmacol Res, 2019, 148(104409).] [DOI] [PubMed] [Google Scholar]
- 14.Tarade D, Ohh M. The HIF and other quandaries in VHL disease. Oncogene. 2018;37(2):139–47. doi: 10.1038/onc.2017.338. [Tarade D, Ohh M. The HIF and other quandaries in VHL disease[J]. Oncogene, 2018, 37(2): 139-47.] [DOI] [PubMed] [Google Scholar]
- 15.Aki D, Li Q, Li H, et al. Immune regulation by protein ubiquitination: roles of the E3 ligases VHL and Itch. Protein Cell. 2019;10(6):395–404. doi: 10.1007/s13238-018-0586-8. [Aki D, Li Q, Li H, et al. Immune regulation by protein ubiquitination: roles of the E3 ligases VHL and Itch[J]. Protein Cell, 2019, 10(6): 395-404.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrotta S, Roberti D, Bencivenga D, et al. Effects of germline VHL deficiency on growth, Metabolism, and mitochondria. N Engl J Med. 2020;382(9):835–44. doi: 10.1056/NEJMoa1907362. [Perrotta S, Roberti D, Bencivenga D, et al. Effects of germline VHL deficiency on growth, Metabolism, and mitochondria[J]. N Engl J Med, 2020, 382(9): 835-44.] [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Kim JH, Jang HJ, et al. Clinicopathologic significance of VHL gene alteration in clear-cell renal cell carcinoma: An updated meta-analysis and review. Int J Mol Sci. 2018;19(9):2529. doi: 10.3390/ijms19092529. [Kim HS, Kim JH, Jang HJ, et al. Clinicopathologic significance of VHL gene alteration in clear-cell renal cell carcinoma: An updated meta-analysis and review[J]. Int J Mol Sci, 2018, 19(9): 2529.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrot-Zhang J, Chambwe N, Damrauer JS, et al. Comprehensive analysis of genetic ancestry and Its molecular correlates in cancer. Cancer Cell. 2020;37(5):639–54 e6. doi: 10.1016/j.ccell.2020.04.012. [Carrot-Zhang J, Chambwe N, Damrauer JS, et al. Comprehensive analysis of genetic ancestry and Its molecular correlates in cancer [J]. Cancer Cell, 2020, 37(5): 639-54 e6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZX, Mann JR, Hsieh CL, et al. Physical and functional interactions between the human DNMT3L protein and members of the de novo methyltransferase family. J Cell Biochem. 2005;95(5):902–17. doi: 10.1002/jcb.20447. [Chen ZX, Mann JR, Hsieh CL, et al. Physical and functional interactions between the human DNMT3L protein and members of the de novo methyltransferase family[J]. J Cell Biochem, 2005, 95 (5): 902-17.] [DOI] [PubMed] [Google Scholar]
- 20.Ricketts CJ, De Cubas AA, Fan H, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018;23(12):3698. doi: 10.1016/j.celrep.2018.06.032. [Ricketts CJ, De Cubas AA, Fan H, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma [J]. Cell Rep, 2018, 23(12): 3698.] [DOI] [PubMed] [Google Scholar]
- 21.Ganeshan D, Menias CO, Pickhardt PJ, et al. Tumors in von hippellindau syndrome: From head to toe-comprehensive state-of-the-Art review. Radiographics. 2018;38(3):849–66. doi: 10.1148/rg.2018170156. [Ganeshan D, Menias CO, Pickhardt PJ, et al. Tumors in von hippellindau syndrome: From head to toe-comprehensive state-of-the-Art review[J]. Radiographics, 2018, 38(3): 849-66.] [DOI] [PubMed] [Google Scholar]
- 22.Dizman N, Philip EJ, Pal SK. Genomic profiling in renal cell carcinoma. Nat Rev Nephrol. 2020;16(8):435–51. doi: 10.1038/s41581-020-0301-x. [Dizman N, Philip EJ, Pal SK. Genomic profiling in renal cell carcinoma[J]. Nat Rev Nephrol, 2020, 16(8): 435-51.] [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Pei M, Li Z, et al. Double-negative feedback interaction between DNA methyltransferase 3A and microRNA-145 in the Warburg effect of ovarian cancer cells. Cancer Sci. 2018;109(9):2734–45. doi: 10.1111/cas.13734. [Zhang S, Pei M, Li Z, et al. Double-negative feedback interaction between DNA methyltransferase 3A and microRNA-145 in the Warburg effect of ovarian cancer cells[J]. Cancer Sci, 2018, 109(9): 2734-45.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen BF, Chan WY. The de novo DNA methyltransferase DNMT3A in development and cancer. Epigenetics. 2014;9(5):669–77. doi: 10.4161/epi.28324. [Chen BF, Chan WY. The de novo DNA methyltransferase DNMT3A in development and cancer[J]. Epigenetics, 2014, 9(5): 669-77.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018;19(2):81–92. doi: 10.1038/nrg.2017.80. [Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation[J]. Nat Rev Genet, 2018, 19(2): 81-92.] [DOI] [PubMed] [Google Scholar]
- 26.Shi W, Tang T, Li X, et al. Methylation-mediated silencing of miR-133a-3p promotes breast cancer cell migration and stemness via miR-133a-3p/MAML1/DNMT3A positive feedback loop. J Exp Clin Cancer Res. 2019;38(1):429. doi: 10.1186/s13046-019-1400-z. [Shi W, Tang T, Li X, et al. Methylation-mediated silencing of miR-133a-3p promotes breast cancer cell migration and stemness via miR-133a-3p/MAML1/DNMT3A positive feedback loop[J]. J Exp Clin Cancer Res, 2019, 38(1): 429.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen ZG, Wang YJ, Chen RS, et al. Ube2b-dependent degradation of DNMT3a relieves a transcriptional brake on opiate-induced synaptic and behavioral plasticity. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0533-y. [Chen ZG, Wang YJ, Chen RS, et al. Ube2b-dependent degradation of DNMT3a relieves a transcriptional brake on opiate-induced synaptic and behavioral plasticity[J]. Mol Psychiatry, 2019.] [DOI] [PubMed] [Google Scholar]
- 28.Artemov AV, Zhigalova N, Zhenilo S, et al. VHL inactivation without hypoxia is sufficient to achieve genome hypermethylation. Sci Rep. 2018;8(1):10667. doi: 10.1038/s41598-018-28795-y. [Artemov AV, Zhigalova N, Zhenilo S, et al. VHL inactivation without hypoxia is sufficient to achieve genome hypermethylation[J]. Sci Rep, 2018, 8(1): 10667.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Zheng X, Lu J, et al. Ginsenoside 20(S)-Rg3 inhibits the warburg effect via modulating DNMT3A/miR-532-3p/HK2 pathway in ovarian cancer cells. Cell Physiol Biochem. 2018;45(6):2548–59. doi: 10.1159/000488273. [Zhou Y, Zheng X, Lu J, et al. Ginsenoside 20(S)-Rg3 inhibits the warburg effect via modulating DNMT3A/miR-532-3p/HK2 pathway in ovarian cancer cells[J]. Cell Physiol Biochem, 2018, 45(6): 2548-59.] [DOI] [PubMed] [Google Scholar]
- 30.Zheng X, Zhou Y, Chen W, et al. Ginsenoside 20(S)-Rg3 prevents PKM2-targeting miR-324-5p from H19 sponging to antagonize the warburg effect in ovarian cancer cells. Cell Physiol Biochem. 2018;51(3):1340–53. doi: 10.1159/000495552. [Zheng X, Zhou Y, Chen W, et al. Ginsenoside 20(S)-Rg3 prevents PKM2-targeting miR-324-5p from H19 sponging to antagonize the warburg effect in ovarian cancer cells[J]. Cell Physiol Biochem, 2018, 51(3): 1340-53.] [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Chen H, He F, et al. Ginsenoside 20(S)-Rg3 upregulates HIF-1alpha-targeting miR-519a-5p to inhibit the Warburg effect in ovarian cancer cells. Clin Exp Pharmacol Physiol. 2020;47(8):1455–63. doi: 10.1111/1440-1681.13321. [Lu J, Chen H, He F, et al. Ginsenoside 20(S)-Rg3 upregulates HIF-1alpha-targeting miR-519a-5p to inhibit the Warburg effect in ovarian cancer cells[J]. Clin Exp Pharmacol Physiol, 2020, 47(8): 1455-63.] [DOI] [PubMed] [Google Scholar]
- 32.Lu J, Wang L, Chen W, et al. miR-603 targeted hexokinase-2 to inhibit the malignancy of ovarian cancer cells. Arch Biochem Biophys. 2019;661:1–9. doi: 10.1016/j.abb.2018.10.014. [Lu J, Wang L, Chen W, et al. miR-603 targeted hexokinase-2 to inhibit the malignancy of ovarian cancer cells[J]. Arch Biochem Biophys, 2019, 661:1-9.] [DOI] [PubMed] [Google Scholar]


