Abstract
Background
Considering the heavy economic burden of osteoporotic fractures, the limits of healthcare resources, and the recent availability of new anti-osteoporosis drugs, there is continuing interest in economic evaluation studies of osteoporosis management strategies.
Objectives
This study aims to (1) systematically review recent economic evaluations of drugs for osteoporosis and (2) to apply an osteoporosis-specific guideline to critically appraise them.
Methods
A literature search was undertaken using PubMed, EMBASE, National Health Service Economic Evaluation database, and the Cost-Effectiveness Analysis Registry to identify original articles containing economic evaluations of anti-osteoporosis drugs, published between 1 July, 2013 and 31 December, 2019. A recent European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases-International Osteoporosis Foundation (ESCEO-IOF) guideline for the conduct and reporting of economic evaluations in osteoporosis was used to assess the quality of included articles.
Results
The database search retrieved 3860 records, of which 27 studies fulfilled the inclusion criteria. These studies were conducted in 15 countries; 12 active drugs were assessed, including various traditional pharmacological treatments such as bisphosphonates, raloxifene, strontium ranelate, denosumab, and teriparatide, and new agents such as abaloparatide, romosozumab, and gastro-resistant risedronate. Eight out of 12 studies that compared traditional oral bisphosphonates to other active interventions (denosumab, zoledronic acid, gastro-resistant risedronate, and teriparatide) suggested that the other active agents were generally cost-effective or dominant. Additionally, the cost-effectiveness of sequential therapy has recently been assessed and indications are that it can lead to extra health benefits (larger gains in quality-adjusted life-year). The key drivers of cost effectiveness included baseline fracture risk, drug effect on the risk of fractures, drug cost, and medication adherence/persistence. The current average score for quality assessment was 17 out of 25 (range 2–15); room for improvement was observed for most studies, which could potentially be explained by the fact that most studies were published prior to the osteoporosis-specific guideline. Greater adherence to guideline recommendations was expected for future studies. The quality of reporting was also suboptimal, especially with regard to treatment side effects, treatment effect after discontinuation, and medication adherence.
Conclusions
This updated review provides an overview of recently published cost-effectiveness analyses. In comparison with a previous review, recent economic evaluations of anti-osteoporosis drugs were conducted in more countries and included more active drugs and sequential therapy as interventions/comparators. The updated economic evidence could help decision makers prioritize health interventions and the unmet/unreported quality issues indicated by the osteoporosis-specific guideline could be useful in improving the transparency, quality, and comparability of future economic evaluations in osteoporosis.
Electronic supplementary material
The online version of this article (10.1007/s40273-020-00965-9) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| In comparison with oral bisphosphonates (including generic forms), other active interventions (such as denosumab, zoledronic acid, gastro-resistant risedronate, or teriparatide) were generally cost effective or dominant |
| Sequential therapy has the potential to generate extra health benefits and to be cost effective in comparison with monotherapy, although more clinical and economic data are needed |
| Although several studies partially followed the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases-International Osteoporosis Foundation guideline, quality was largely insufficient for most articles. Our study highlighted that insufficiently implemented and/or reported recommendations should be included in future studies; this could be useful in improving the transparency, quality, and comparability of economic evaluations in osteoporosis |
Introduction
Osteoporosis is a skeletal disease associated with a significant health and economic burden, which has become an increasing global health problem considering the aging population characterized by multi-morbidity. The morbidity and mortality imposed by osteoporotic fractures along with the negative impact on patients’ quality of life are important clinical considerations [1]. Worldwide, osteoporosis causes more than 8.9 million fractures annually, resulting in an osteoporotic fracture every 3 s [2]. In the European Union, 22 million women and 5.5 million men had osteoporosis in 2010 [3]. As a result of changes in population demography, the annual number of fragility fractures was expected to rise from 3.5 million in 2010 to 4.5 million in 2025, corresponding to an increase of 28% [4]. In the USA, over 1.5 million fractures per year were attributable to osteoporosis, resulting in direct healthcare costs of 12–18 billion US dollars [5]. Improving osteoporosis care and reducing spiraling fracture-related costs pose worldwide challenges.
Health economic evaluations have become increasingly important to support the setting of priorities in healthcare and to help decision makers allocate healthcare resources efficiently in the context of limited healthcare resources, the ongoing aging of the population, and the heavy economic burden of osteoporotic fractures, as well as the recent availability of new agents for osteoporosis management (e.g., abaloparatide, romosozumab, gastro-resistant risedronate). In 2015, a study systematically reviewed all economic evaluations of anti-osteoporosis drugs published up to 31 June, 2013 and suggested that anti-osteoporosis drugs were generally cost effective in comparison with no treatment in postmenopausal women aged over 60–65 years with low bone mass, especially those with prior vertebral fractures. However, given the heterogeneity of fracture risk, comparators, country setting, model structure, and incorporation of medication adherence, as well as the lack of head-to-head comparisons, it remained challenging to make comparisons between studies [6]. In addition, the quality of reporting was largely insufficient for most studies, despite the fact that guidelines for conducting health economic evaluations have been widely available for many years.
Recently, a guideline for the conduct and reporting of economic evaluations in the field of osteoporosis has been designed by a working group convened by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation (IOF) [7]. Although several disease-specific recommendations for economic evaluations have been developed, this guideline is the first that provides a list of recommendations and minimum requirements for the design, conduct, and reporting of an osteoporosis-specific economic evaluation. Osteoporosis-specific recommendations in this guideline, which supplement general and national guidelines, could guide researchers in designing appropriate and high-quality economic evaluations and help decision makers and reviewers to assess the quality of these studies, and further to improve the transparency and comparability of these studies and maintain methodologic standards [7]. Therefore, assessing how recent studies adhere to the osteoporosis-specific guideline is important in identifying the main limitations of these studies, and further to indicate some of the most important recommendations that should be taken into account in future studies.
An overview of currently available studies regarding cost-effectiveness analyses of drugs for osteoporosis would thus be useful to guide researchers in designing and conducting high-quality economic evaluations, in identifying gaps in current evidence, and to help administrators make decisions based on high-quality evidence. We therefore updated and undertook this review to (1) systematically identify and review economic evaluations published between 2013 and 2019 on drugs for osteoporosis and (2) to critically appraise their quality using the recent osteoporosis-specific guideline, and also to provide insight into key drivers of cost-effectiveness ratios.
Methods
Literature Search
A systematic literature search was undertaken to identify recent cost-effectiveness analyses of drugs for osteoporosis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [8]. The search was conducted using several databases including PubMed, EMBASE (Ovid), National Health Service Economic Evaluation Database (NHS EED) [the database ceased to be updated after March 2015] and the Cost-Effectiveness Analysis (CEA) Registry (the database can serve as an archive only until 2018). We restricted our analysis to articles published between 1 July, 2013 and 31 December, 2019, as prior articles were covered in the previous review [6]. An initial search was conducted in PubMed and EMBASE using a search strategy (see Appendix 1 in the Electronic Supplementary Material [ESM]) designed according to the Population, Intervention, Comparator, Outcome (PICO) criteria with the help of an expert library specialist. The key word ‘osteoporosis’ was used in the NHS EED and the CEA Registry database.
Study Selection
First, duplicates were identified and removed. Second, two reviewers (NL, DC) independently applied inclusion and exclusion criteria to screen titles and abstracts of the remaining articles. Third, full-text versions of eligible articles were screened in-depth by two independent reviewers (NL and DC, LS, DP, SS, or RB). A consensus meeting with a third reviewer (MH) was used to resolve discrepancies. Finally, reference lists and citations of eligible articles were checked manually for additional relevant studies.
Studies were included if they were published in English between July 2013 and December 2019 and contained a full economic evaluation (the comparative analysis of alternative interventions in terms of both costs and consequences) of anti-osteoporosis drugs. Non-original articles (e.g., editorials, reviews, conference proceedings), partial economic evaluations, and non-specific drug studies (e.g., only use vitamin D and/or calcium as interventions, studies regarding screening strategies, intervention thresholds, medication adherence, nutrition, model of care, fracture liaison services, and lifestyle) were excluded.
Data Extraction and Quality Assessment
A standardized data-extraction form was developed to collect data from eligible studies. Study characteristics regarding publication (author, year of publication, journal), study design (country, population, perspective, model type, outcome measure, time horizon, comparators, intervention duration, cost type, discount rates, year of valuation), study outcomes (results and sensitivity analysis), and funding source were extracted by one reviewer (NL) and checked by another reviewer (DC, LS, DP, SS, RB, or IK). Incremental cost-effectiveness ratios (ICERs) were reported as provided in the articles. Afterwards, for comparability reasons, all ICERs were converted into 2019 US dollars using the Organisation for Economic Cooperation and Development exchange rate and inflation rate [9]. We then synthetized and analyzed ICERs of active agents compared to traditional oral bisphosphonates (first-line treatments in most countries), and of sequential therapies (e.g., abaloparatide/teriparatide followed by alendronate) by using US$100,000 per quality-adjusted life-year (QALY) gained as the willingness-to-pay (WTP) threshold. Other information such as country, treatment duration, and annual drug cost was also extracted. In addition, we checked included studies, especially one-way sensitivity analyses, to identify key drivers of cost effectiveness; these were eventually chosen through team discussion.
The conduct and reporting quality of included articles were then appraised using the ESCEO-IOF guideline for economic evaluations in osteoporosis by two independent reviewers (NL with DC, LS, DP, SS, RB, or IK). The whole assessment consisted of two parts. Part one included recommendations for the design and conduct of an economic evaluation in osteoporosis; 29 recommendations were addressed in nine categories (type of economic evaluation, method for the conduct of economic evaluation, modeling technique, base-case analysis and population, mortality, fracture costs and utility, treatment characteristics, sensitivity analyses, and outcomes). Part two was an osteoporosis-specific checklist with nine recommendations for reporting, including the reporting and justification of key modeling aspects (choice of model, transition probabilities, effect of fracture on costs, mortality, and utility) and key treatment characteristics (the effect of treatment per fracture site, the effect of treatment after discontinuation, the inclusion and approach used to model medication adherence, therapy costs, and side effects) [7].
Each recommendation of these two parts was scored using ‘Yes’ (fulfilled the requirement of reporting), ‘No’ (did not fulfill the requirement), ‘Part’ (partially fulfilled the requirement), or ‘Not Applicable’ according to the operationalization of the guideline (Appendix 2 in the ESM). To estimate a score for reporting, we assigned a score of 1 for ‘Yes’, 0.5 for ‘Part’, and 0 for ‘No’. Discrepancies in rating were resolved by consensus and consultation with a third reviewer (MH). It is worth noting that in the scoring system we excluded recommendations that were not directly connected to the quality level of studies (i.e., ‘use ICUROS data’, ‘use FRAX® or GARVAN® tools’, ‘consider sequential therapy as intervention’, and ‘in the absence of hip/wrist specific efficacy data, use non-vertebral or clinical fracture efficacy data as replacement’).
Results
Results of Study Selection
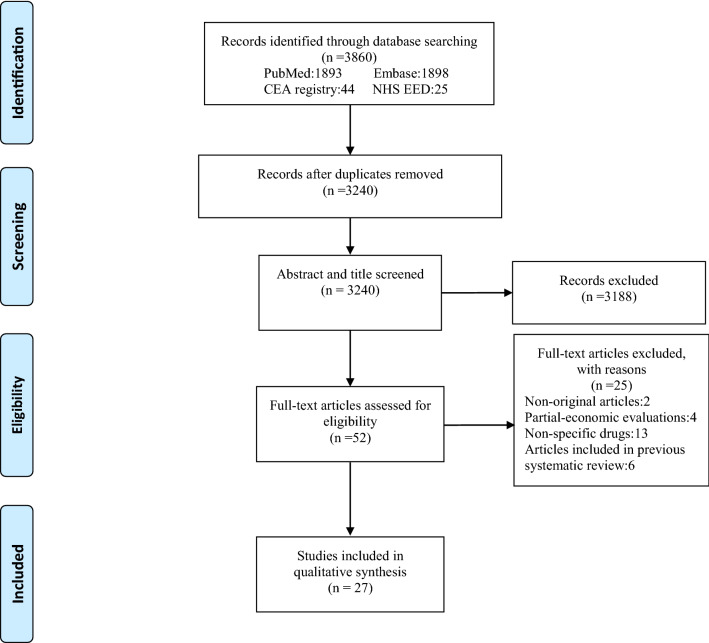
Figure 1 shows the PRISMA flowchart for the identification of studies. The database search retrieved 3860 records, of which 620 were found to be duplicates and removed. We reviewed all titles and abstracts of the remaining 3240 studies and subsequently excluded 3188 articles that did not meet our inclusion criteria. Upon review of the full text of the remaining 52 studies, 25 articles were excluded for reasons such as being non-original articles (n = 2), partial-economic evaluations (n = 4), reporting on non-specific drugs (n = 13), and studies included in previous review (n = 6). A total of 27 articles were included in our study for data extraction and quality assessment.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of study selection. CEA cost-effectiveness analysis, NHS EED National Health Service Economic Evaluation database
Overview of Included Studies
The characteristics of included studies are reported in Table 1. These studies were conducted in 15 different countries. The USA accounted for the largest number (n = 7); 12 studies were conducted in Asia, i.e., three each in Japan (n = 3), China (n = 3), and Iran (n = 3). Five studies were performed in five different European countries. Twelve of the 27 studies were published in osteoporosis journals, particularly in Osteoporosis International (n = 5).
Table 1.
Characteristics of published articles assessing the cost effectiveness of drugs for osteoporosis
| References | Journal | Country | Study perspectivea | Model type | Outcome measure | Time horizon | Cost type | Discount rates (costs, QALY) | Funding source |
|---|---|---|---|---|---|---|---|---|---|
| Active drug(s) vs no treatment/calcium + vitamin D | |||||||||
| Golmohamdi et al. [12] | Electronic Physician | Iran | Healthcare | No | BMD | 12 and 36 months | Direct costs | NR | NR |
| Kwon et al. [32] | Journal of Bone Metabolism | Korea | Societal | Markov cohort model | QALY | Lifetime | Direct costs | 5%, 5% | Takeda Pharmaceutical Company Limited, Korea |
| Ito et al. [36] | BMJ Open | USA | Healthcare | Markov cohort model | QALY | Lifetime | Direct costs | 3%, 3% | NR |
| Mohd-Tahir et al. [17] | International Journal of Rheumatic Diseases | Malaysia | Healthcare | No | Fracture events | 0–15 years | Direct costs | NR | NR |
| Cui et al. [34] | Osteoporosis International | China | Healthcare | Markov microsimulation model | QALY | Lifetime | Direct costs | 3%, 3% | China Postdoctoral Science Foundation Grant, NSFC, Beijing Natural Science Foundation |
| Taheri et al. [33] | Iranian Journal of Pharmaceutical Research | Iran | Healthcare | Markov cohort model | QALY | Lifetime | Direct costs | 7.2%, 5% | CinnaGen Corporation |
| Active drug(s) vs active drug(s) | |||||||||
| Parthan et al. [21] | Bone | Sweden | Healthcare | Markov cohort model | QALY | Lifetime | Direct costs | 3%, 3% | NR |
| Venice et al. [10] | Journal of Clinical Densitometry | Mexico | Healthcare | No | All costs are compared | 12 months | Direct costs | NR | NR |
| Waure et al. [26] | BioMed Research International | Italy | Healthcare | Markov cohort model | QALY | Lifetime | Direct costs | 3%, 3% | Amgen Inc |
| Darbà et al. [25] | ClinicoEconomics and Outcomes Research | Spain | Healthcare | Markov cohort model | QALY | Lifetime | Direct costs | 3%, 3% | Amgen SA Barcelona, Spain, and GSK |
| Miraci et al. [11] | International Journal of Pharmacy and Pharmaceutical Sciences | Albania | Healthcare | No | Average percentage of change in BMD | 12 months | Direct costs | NR | NR |
| Silverman et al. [22] | Journal of Osteoporosis | USA | Healthcare | Markov cohort model | QALY | Lifetime | Direct costs | 3%, 3% | Amgen Inc |
| Chen et al. [31] | Patient Preference and Adherence | China | Healthcare | Markov microsimulation model | QALY | Lifetime | Direct costs | 3%, 3% | NSFC and Jiangsu Research on Philosophy and Social Science in University of Education Department of Jiangsu Province |
| Karnon et al. [14] | Cost Effectiveness and Resource Allocation | Australia | Healthcare | Markov cohort model | QALY | 10 years | Direct costs | 5%, 5% | No funding |
| Azar et al. [13] | Medical journal of the Islamic Republic of Iran | Iran | Healthcare | Decision tree model | QALY | 2 years | Direct costs | 3%, 3% | NR |
| Mori et al. [18] | Osteoporosis International | Japan | Healthcare, societal and governmental | Markov microsimulation model | QALY | Lifetime | Direct and indirect costs | 3%, 3% | NR |
| Mori et al. [19] | Osteoporosis International | USA | Societal | Markov microsimulation model | QALY | Lifetime | Direct costs | 3%, 3% |
Veterans Affairs Special Fellowship in Advanced Geriatrics |
| Moriwaki et al. [30] | Osteoporosis International | Japan | Healthcare | Markov microsimulation model | QALY | Lifetime | Direct costs | 2%, 2% | Asahi Kasei Pharma Corporation |
| O’Hanlon et al. [35] | Clinical Therapeutics | USA | Healthcare | Markov cohort model | QALY | 1.5 years; Lifetime | Direct costs | 3%, 3% | NR |
| Yoshizawa et al. [29] | Archives of Osteoporosis | Japan | Societal | Markov cohort model | QALY | Lifetime | Direct costs | 3%, 3% | NR |
| Chokchalermwong et al. [23] | Journal of the Medical Association of Thailand | Thailand | Societal | Markov microsimulation model | QALY | Lifetime | Direct costs | 3%, 3% | NR |
| Coyle et al. [24] | Medical Decision Making Policy & Practice | Canada | Healthcare | Markov cohort model | QALY | Lifetime | Direct costs | 1.5%, 1.5% | NR |
| Hiligsmann et al. [27] | Osteoporosis International | France | Healthcare | Markov microsimulation model | QALY | Lifetime | Direct costs | 3%, 3% | Teva and Theramex |
| Li et al. [15] | The Journal of the North American Menopause Society | China | Healthcare | Markov cohort model | QALY | 40 years | Direct and indirect costs | 3%, 3% |
NSFC, Science and Technology Department of Fujian Province of China |
| Sequential therapies | |||||||||
| Hiligsmann et al. [28] | Seminars in Arthritis and Rheumatism | USA | Healthcare | Markov microsimulation model | QALY | Lifetime | Direct costs | 3%, 3% | Radius Health, Inc., Waltham MA |
| Le et al. [16] | Annals of Pharmacotherapy | USA | Healthcare | Discrete-event simulation model | QALY | 10 years | Direct costs | 3%, 3% | Radius Health, Inc |
| Mori et al. [20] | JBMR PLUS | USA | Healthcare and societal | Markov microsimulation model | QALY | Lifetime | Direct and indirect costs | 3%, 3% | JMDC Inc., SMS CO. and LTD |
BMD bone mineral density, NR not reported, NSFC National Natural Science Foundation of China, QALY quality-adjusted life-year
aAs stated by the authors
Most studies used the healthcare perspective (n = 21), some with a societal perspective (n = 4), while one study used both societal and healthcare perspectives, and another study reported societal, healthcare, and governmental perspectives. All studies included direct costs and only three also considered indirect costs [15, 18, 20]. However, we found that some studies including both direct and indirect costs were not defined as having a societal perspective, although this was the original information stated by authors reported in Table 1; no adjustment and correction were made for this. Nineteen studies applied a lifetime horizon while others considered truncated time horizons [10–15]. A Markov model was used in 21 studies, consisting of a Markov cohort model (n = 12) or a Markov microsimulation model (n = 9). One study applied a discrete-event simulation model [16], another a decision-tree model [13]. Quality-adjusted life-years (QALYs) were used as the outcome in these 23 studies with a model. The remaining four studies used no model [10–12, 17]. One out of the four conducted a cost-minimization analysis [10], in which costs were compared. Another two studies [11, 12] used bone mineral density (BMD) as the final outcome and ICER was calculated based on the differences of costs and BMD of different interventions. Furthermore, the number of fracture events was regarded as the outcome in the fourth study [17], ICER was calculated based on the differences of average annual costs divided by the difference of numbers of hip fractures prevented between bisphosphonates and the combination of calcium and vitamin D. Fourteen studies were funded by pharmaceutical companies or national public funds, while 13 studies did not mention the source of funding or had no funding.
Table 2 presents characteristics of the studied population, the active intervention and comparator, year of costing valuation, sensitivity analysis, and the main results of the articles. Study populations differed between studies in BMD T-score, mean age, history of fracture, or even tolerance of oral bisphosphonates. Some studies included patients stratified for age and two studies included only a male population [21, 22].
Table 2.
Results of cost-effectiveness analyses of drugs for osteoporosis
| References | Population (base case) | Comparators | Year of costing valuation | Sensitivity analysis | Results |
|---|---|---|---|---|---|
| Active drug(s) vs no treatment/calcium + vitamin D | |||||
| Golmohamdi et al. [12] | Postmenopausal women with osteoporosis | Zoledronic acid vs placebo | 2013 | No | One percent increase of BMD on femoral neck, hip trochanter, total hip, and lumbar spine requires further cost of US$386, US$264, US$388, and US$347, respectively. Zoledronic acid is a cheaper and better approach and can be considered as a dominant approach |
| Kwon et al. [32] | Postmenopausal women aged 55, 60, or 65 years with BMD T-scores from − 2.0 to − 2.4 at the femoral neck, and without previous osteoporotic fractures | Calcium/ vitamin D vs (raloxifene + calcium/ vitamin D) or (risedronate + calcium/vitamin D) | 2014 | One-way | In comparison with calcium/vitamin D supplements, drug therapy (raloxifene or risedronate + calcium/vitamin D) had an ICER of US$16,472 and US$6741 per QALY gained for treatment started at the age of 55 and 60 years, respectively. Given the WTP threshold (US$25,700), pharmaceutical treatment was cost effective. For older women starting medication at 65 years of age, pharmaceutical intervention was a dominant strategy |
| Ito et al. [36] | Women aged 85 years with BMD T-score ≤ − 2.0 at the spine, hip, or radius who resided in nursing homes | (Zoledronic acid + calcium/vitamin D) vs usual care (calcium/vitamin D) | 2017 | One-way probabilistic | In comparison with usual care, zoledronic acid had an ICER of US$207,400 per QALY gained. Given the WTP threshold (US$100,000), zoledronic acid was not cost effective |
| Mohd-Tahir et al. [17] | Adult patients diagnosed with osteoporosis related to glucocorticoid drugs use | Oral bisphosphonates (alendronate, risedronate or ibandronate) vs calcium/vitamin D | 2014 | No | Overall, in comparison with calcium/vitamin D, the use of bisphosphonates could not be considered cost effective for treatment of all patients with GIO. Bisphosphonates were considered cost effective if started in patients more than 60 years old. However, bisphosphonates were not cost effective in patients with GIOP secondary osteoporosis. The ICERs of bisphosphonates in patients with previous fracture or with rheumatoid arthritis were estimated at MYR 108,603 and MYR 25,699 per QALY gained, respectively. Given the WTP threshold (MYR 26,317), bisphosphonates were cost effective in patients with rheumatoid arthritis |
| Cui et al. [34] | Postmenopausal osteoporotic women | Zoledronic acid vs no treatment | 2019 | One-way | In comparison with no treatment, zoledronic acid had ICERs of US$26,637, US$22,129, US$20,338, US$19,285, US$18,181, US$16,680, US$15,047, and US$14,447 per QALY at FRAX threshold 0.02, 0.06, 0.07,0.08, 0.09, 0.1, 0.5, and 1, respectively. Zoledronic acid was cost effective when the 10-year probability of major osteoporotic fracture based on FRAX was above 7% |
| Taheri et al. [33] | Women aged 70 years, BMD T-score − 2.5 with previous fracture or BMD T-score -3.0 without prior fracture | Teriparatide vs no treatment | 2018 | One-way probabilistic | In comparison with no treatment, teriparatide was indicated to be more costly and associated with fewer fractures, more life-years, and more QALYs, with an ICER of IRR 254,750,619 per QALY gained. It could be considered as a cost-effective treatment in severe PMO at the WHO recommended threshold |
| Active drug(s) versus active drug(s) | |||||
| Parthan et al. [21] | Men aged 75 years and older with osteoporosis | Denosumab vs (generic alendronate, strontium ranelate, zoledronic acid, generic risedronate, ibandronate, and teriparatide) | 2012 | One-way probabilistic | Total lifetime costs for denosumab, generic alendronate, strontium ranelate, zoledronic acid, generic risedronate, ibandronate, and teriparatide were €31,004, €33,731, €34,788, €34,796, €34,826, €35,983, and €37,461, respectively. Total QALYs were 5.23, 5.15, 5.15, 5.17, 5.13, 5.12, and 5.22, respectively In comparison with other treatments, denosumab had the lowest costs and highest QALYs. Denosumab dominated all comparators |
| Venice et al. [10] | Postmenopausal women aged 45–79 years with low BMD in the lumbar spine and/or right hip, a Karnofsky index of 90–100 | Alendronate vs zoledronic acid | 2010 | No | Compared with oral alendronate, zoledronic acid provided an annual savings of 15% of the direct costs for 1 year. Zoledronic acid infusion is also linked to a higher increase in BMD and compliance |
| Waure et al. [26] | Postmenopausal women aged 65 years with a BMD T-score < − 4 SD | Denosumab vs (risedronate, generic and branded alendronate, ibandronate, and strontium ranelate) | 2009 | Probabilistic | The ICERs for denosumab, in comparison with risedronate, generic alendronate, branded alendronate, ibandronate, and strontium ranelate were estimated at €10,302, €18,047, €17,133, €2158, and €69 per QALY gained, respectively. Given the WTP threshold (€30,000), denosumab is cost effective |
| Darbà et al. [25] | Postmenopausal women age 65 years with BMD T-score ≤ 2.5 or less | Denosumab vs (alendronate, ibandronate, risedronate, strontium ranelate, and no treatment) | 2013 | Probabilistic, multivariate, univariate | The ICER for denosumab compared with no treatment, alendronate, risedronate, and ibandronate were estimated at €6823, €16,294, €4895, and €2205 per QALY gained, respectively. Given the WTP threshold (€20,000), denosumab is cost effective. Denosumab is a dominant treatment option in comparison with strontium ranelate |
| Miraci et al. [11] | Menopausal or postmenopausal women aged 50 years with T-score − 1 to -6, diagnosed for the first time | Ibandronate vs alendronate | NR | No | The cost/efficacy ratio (1% change of BMD) was 13.434 units for ibandronate and 31.677 units for alendronate type A1. Ibandronate is more effective and cost effective than alendronate in the treatment of osteoporosis |
| Silverman et al. [22] | Men with a mean age of 78 years with BMD T-score of − 2.12 and a vertebral fracture prevalence of 23% | Denosumab vs (generic alendronate, risedronate, ibandronate, teriparatide, and zoledronic acid) | 2013 | One-way probabilistic | Compared with generic alendronate, denosumab had an ICER of US $16,888 per QALY gained. Given the WTP threshold (US $100,000), denosumab is cost effective. Compared with risedronate, ibandronate, teriparatide, and zoledronic acid, denosumab is a dominant option |
| Chen et al. [31] | Postmenopausal women aged 65 years with BMD T-score ≤ -2.5 at the femoral neck and without previous fractures (initial population) | Raloxifene vs conventional treatment (calcitonin or alendronate or calcium/vitamin D) | 2015 | One-way probabilistic | Compared with conventional treatment, treatment with raloxifene had an ICER of US$36,891 per QALY gained. Given the WTP threshold of US$20,000), raloxifene was not cost effective |
| Karnon et al. [14] | Women with a mean age of 72 years (range 60–90 years) with a mean BMD T-score at the femoral neck of − 2.15, and with 24% of women having experienced a previous fracture | Patented denosumab vs generic alendronate | 2012 | Deterministic probabilistic | Compared with alendronate, denosumab had an ICER of AUD$246,749 per QALY gained. Given the WTP threshold (AUD$100,000), denosumab is not cost effective |
| Azar et al. [13] | Postmenopausal women aged over 60 years with BMD T-score ≤ 2.5 and with at least a previous spine, wrist, or hip fracture caused by osteoporosis | Risedronate vs (alendronate and teriparatide) | 2014 | One-way | Compared with risedronate, alendronate and teriparatide had ICERs of US$-2178 and US$483,783 per QALY gained. Given the WTP threshold (US$14,010), alendronate is the dominant and cost-effective treatment option. The treatment strategy of teriparatide is more expensive than risedronate and alendronate and is associated with very little increase in QALYs |
| Mori et al. [18] | Postmenopausal osteoporotic women without previous hip or vertebral fractures at various ages of therapy initiation (65, 70, 75, and 80 years) | Alendronate vs denosumab vs no treatment | 2016 | One-way probabilistic | For patients aged 75 and 80 years, denosumab was cost saving from any of the three perspectives, in comparison with alendronate For patients aged 65 and 70 years, denosumab had an ICER of US$25,700 and US$5000 per QALY gained, from a societal perspective, and did not exceed a WTP threshold (US$50,000). Therefore, denosumab was a cost-effective option |
| Mori et al. [19] | Community-dwelling non-Hispanic white women at different starting ages (65, 70, 75, and 80 years) and without previous hip, vertebral, or wrist fractures |
Oral bisphosphonate vs no treatment, combined strategy (bisphosphonate + falls prevention exercise) and (falls prevention exercise only) |
2014 | One-way probabilistic | Compared with an oral bisphosphonate alone, the combined strategy had ICERs of US$202,020, US$118,460, US$46,870, and US$17,640 per QALY for patients aged 65, 70, 75, and 80 years, respectively. Given the WTP threshold (US $100,000), the combined strategy for patients ages 75 and 80 years was cost effective. The combined strategy provided better health at lower cost than the falls prevention exercise alone at ages 70, 75, and 80 years |
| Moriwaki et al. [30] | Women of 70 years with BMD T-score of − 2.5 and a previous vertebral fracture | Alendronate + basic treatment (placebo + calcium + vitamin D) vs basic treatment; (zoledronic acid + basic treatment) vs (alendronate + basic treatment) | 2016 |
Deterministic probabilistic |
For patients 70 years of age, zoledronic acid was dominated by alendronate. However, the incremental QALY is quite small in extent. Considering the advantage of annual zoledronic acid treatment in compliance and persistence, zoledronic acid may be a cost-effective treatment option compared to alendronate |
| O'Hanlon et al. [35] | Women of 70 years with BMD T-scores < − 2.5 and a previous vertebral fracture | Alternative bone-forming agent profiles vs the teriparatide reference case; alternative bone-forming agent profiles vs (sequential teriparatide + denosumab) | 2016 | No | In comparison with teriparatide, alternative bone-forming agent profiles produced a net monetary benefit of US$17,000,000 per 10,000 treated patients during the 1.5 years and US$80,000,000 over a lifetime horizon that included 3.5 years of maintenance treatment with denosumab |
| Yoshizawa et al. [29] | Women aged 75 years with BMD T-score − 2.87 and with a previous vertebral body fracture | Denosumab vs alendronate; alendronate vs no treatment | 2016 | Deterministic probabilistic | Compared with alendronate, denosumab had an ICER of US$40,241 per QALY gained. Assuming a WTP threshold (US$50,000), denosumab was cost effective |
| Chokchalermwong et al. [23] | Postmenopausal women aged ≥ 50 years with osteoporosis and without previous fractures | (Oral bisphosphonates, raloxifene, strontium ranelate, and denosumab) vs no treatment | 2015 | One-way probabilistic | In comparison with no treatment, none of the alternative drugs were cost effective at baseline case. For women from the age of 65 years, with a BMD T-score ≤ -2.5, oral bisphosphonates were the only drugs cost effective (the ICER was THB130,049), followed by denosumab and raloxifene, respectively. Strontium ranelate was dominated by no treatment |
| Coyle et al. [24] | Women aged 70–74 years with osteoporosis and without previous fractures who are able to tolerate oral bisphosphonates | Alendronate vs etidronate vs risedronate vs zoledronic acid vs denosumab vs no treatment | 2017 | Probabilistic | For patients who can tolerate oral bisphosphonates, in comparison with no treatment, alendronate, risedronate, zoledronic acid, and denosumab had ICERs of CAN$3751, CAN$85,557, CAN$83,503, and CAN$238,523 per QALY gained, respectively. Given the WTP threshold (CAN$50,000), alendronate was the only cost-effective drug. In comparison with alendronate, risedronate and etidronate were dominated, and zoledronic acid and denosumab were associated with a high ICER. For patients who are unable to tolerate oral bisphosphonates, dependent on age and fracture history, compared with no treatment, the ICER for zoledronic acid had a range from CAN$17,770 to CAN$94,365 per QALY. Denosumab was dominated by zoledronate or had an ICER greater than CAN$3.0 million |
| Hiligsmann et al. [27] | Postmenopausal women aged 60–80 years with BMD T-score ≤ -2.5 and/or prevalent vertebral fractures | Gastro-resistant risedronate vs (generic risedronate, alendronate and no treatment) | 2017 | One-way probabilistic | The ICER for GR risedronate, compared with alendronate, generic risedronate and no treatment, ranged from €2037 to €21,875 per QALY gained. Given the WTP threshold (€60,000), GR risedronate was cost effective In women aged 75 years and older, GR risedronate was even shown to be dominant |
| Li et al. [15] | Women aged over 60 years with BMD T-score ≤ 2.5 in the lumbar spine or femoral neck and without previous fractures | (Alendronate, zoledronic acid, raloxifene and teriparatide) vs calcium/vitamin D; (alendronate, raloxifene and teriparatide) vs zoledronic acid | 2018 | Univariate probabilistic | Compared with calcium/vitamin D, zoledronic acid had an ICER of US$7864 per QALY gained. Given the WTP threshold (US$28,624), zoledronic acid was cost-effective. The ICER of teriparatide versus zoledronic acid was US$470,797 per QALY gained, which exceeded the threshold. Alendronate and raloxifene were dominated by zoledronic acid |
| Sequential therapies | |||||
| Hiligsmann et al. [28] | Women aged 50–80 years with a BMD T-score ≤ -3.5 and without fracture history or with a BMD T-score − 2.5 to − 3.5 and a history of at least one osteoporotic fracture | (Sequential abaloparatide + alendronate) vs (sequential teriparatide + alendronate) and no treatment | 2017 | One-way probabilistic | In comparison with sequential TPTD/ALN therapy, sequential ABL/ALN therapy was dominant in all simulated populations. Compared with no treatment, in women with BMD T-score ≤ 3.5 (age over 70 years), the sequential ABL/ALN therapy was cost saving; and in women with a BMD T-score -2.5 to -3.5 and history of one osteoporotic fracture, the ICER was estimated at US$125,493, US$91,394, US$81,865, US$51,906, US$38,763, US$31,390, and US$28,086 for patients aged 50 years, 55 years, 60 years, 65 years, 70 years, 75 years, and 80 years, respectively. Given the WTP threshold (US$100,000), sequential ABL/ALN therapy was cost effective for patients aged over 55 years |
| Le et al. [16] | Postmenopausal women aged 68.8 years with osteoporosis | (Sequential abaloparatide + alendronate) vs (sequential teriparatide + alendronate) and (placebo + alendronate) | 2017 | One-way probabilistic | Compared with TPTD/ALN over a 10-year time horizon, ABL/ALN was dominant. In comparison with PBO/ALN, ABL/ALN had an ICER of US$333,266 per QALY gained. In high-risk women, ABL/ALN was also cost effective in comparison with TPTD/ALN, and had an ICER of US$188,891 per QALY gained relative to PBO/ALN |
| Mori et al. [20] | Community-dwelling white osteoporotic women aged 65, 70, 75, and 80 years with previous vertebral fracture | (Sequential teriparatide + alendronate) vs alendronate alone | 2018 | One-way probabilistic | Compared with alendronate alone from the societal perspective, sequential teriparatide/alendronate had an ICER of US$434,400, US$330,000, US$280,100, and US$290,800 per QALY for women aged 65, 70, 75, and 80 years, respectively. From a healthcare perspective, the ICERs were US$441,700, US$336,700, US$288,200, and US$299,100 per QALY, respectively, Given the WTP threshold (US$150,000), sequential teriparatide/alendronate was not cost effective unless the costs of generic/biosimilar teriparatide were heavily discounted with respect to the current brand cost |
ABL abaloparatide, ALN alendronate, AUD Australian Dollar, BMD bone mineral density, GIOP glucocorticoid-induced osteoporosis, GR gastro-resistant, ICER incremental cost-effectiveness ratio, IRR Iranian Rial, NR not reported, PBO placebo, PMO postmenopausal osteoporosis, PTH parathyroid hormone, QALY quality-adjusted life-year, MYR Malaysian Ringgit, THB Thai Baht, TPTD teriparatide, WTP willingness to pay, WHO World Health Organization
Twelve active drugs were assessed in the studies, including various pharmacological treatments such as bisphosphonates (alendronate, etidronate, risedronate, ibandronate, and zoledronic acid), raloxifene, strontium ranelate, denosumab, and teriparatide, and including new agents such as abaloparatide, romosozumab, and gastro-resistant risedronate. Twelve studies included two or more active drugs in their analysis [13, 15–17, 21–28]. Oral bisphosphonates were included in 11 studies [13–15, 18, 21, 22, 24–26, 29, 30] and compared with other active interventions. There were three studies [16, 20, 28] considering sequential therapies as comparators, while six studies [15, 17, 30–32, 36] made the comparison between active osteoporotic drugs and calcium/vitamin D3 and ten studies [12, 18, 19, 23–25, 27–29, 33, 34] included no treatment as the comparator. Treatment duration in most studies was similar to randomized controlled trials, indications, or guidelines (e.g., 3 or 5 years for anti-resoptive agents, 12–24 months for anabolic agents). Both a deterministic sensitivity analysis (e.g., one-way, multivariate) and a probabilistic sensitivity analysis were conducted in 17 studies. Two studies [24, 26] applied only a probabilistic sensitivity analysis and three studies applied only a one-way sensitivity analysis [13, 32, 34]. Sensitivity analysis was not conducted in five studies [10–12, 17, 35]. We presented the WTP threshold in Table 2 as stated by the authors and no adjustment was made. The WTP threshold was shown to be different even through studies had been conducted in the context of the same country.
Table 3 summarizes the results of the cost-effectiveness analysis between traditional oral bisphosphonates and other active drugs in 2019 US dollars. Annual drug costs for branded oral bisphosphonates had a range from US$123 to US$1874; the cost for generic oral bisphosphonates was much lower, from US$7 to US$458. The annual cost of denosumab differed steeply between countries, from US$608 to US$1811. Several studies made comparisons between denosumab and oral bisphosphonates.
Table 3.
Cost-effective analyses between oral bisphosphonates and other active drugs for osteoporosis
| References | Country | Intervention and comparator | Treatment duration (years) | Annual drug costs (intervention/comparator) |
ICER |
|---|---|---|---|---|---|
| Coyle et al. [24] | Canada | Denosumab vs alendronate | 2 | US$663/US$123 | US$2,376,812 |
| Darbà et al. [25] | Spain | Denosumab vs alendronate | 5 | US$608/US$237 | US$23,746 |
| Waure et al. [26] | Italy | Denosumab vs generic alendronate | 4 | US$842/US$458 | US$29,980 |
| Denosumab vs branded alendronate | 4 | US$842/US$502–528 | US$28,462 | ||
| Karnon et al. [14] | Australia | Patented denosumab vs generic alendronate | 5 | US$624/US$230 | US$284,397 |
| Mori et al. [18] | Japan | Denosumab vs alendronate (SP, 65 years) | 5 | US$799/US$246 | US$27,375 |
| Denosumab vs alendronate (SP, 70 years) | US$5326 | ||||
| Denosumab vs alendronate (HP, 65 years) | US$32,061 | ||||
| Denosumab vs alendronate (HP, 70 years) | US$7137 | ||||
| Denosumab vs alendronate (GP, 65 years) | US$28,546 | ||||
| Denosumab vs alendronate (GP, 70 years) | US$6178 | ||||
| Parthan et al. [21] | Sweden | Denosumab vs generic alendronate | 5 | US$733/US$49 | Dominant |
| Silverman et al. [22] | USA | Denosumab vs generic alendronate | 5 | US$1811/US$33 | US$18,532 |
| Yoshizawa et al. [29] | Japan | Denosumab vs alendronate | 5 | US$743/US$289 | US$40,969 |
| Darbà et al. [25] | Spain | Denosumab vs risedronate | 5 | US$608/US$414 | US$7134 |
| Waure et al. [26] | Italy | Denosumab vs risedronate | 4 | US$842/US$455 | US$17,114 |
| Parthan et al. [21] | Sweden | Denosumab vs generic risedronate | 5 | US$733/US$64 | Dominant |
| Silverman et al. [22] | USA | Denosumab vs risedronate | 5 | US$1811/US$1874 | Dominant |
| Darbà et al. [25] | Spain | Denosumab vs ibandronate | 5 | US$608/US$227 | US$3213 |
| Waure et al. [26] | Italy | Denosumab vs ibandronate | 4 | US$842/US$819 | US$3585 |
| Parthan et al. [21] | Sweden | Denosumab vs ibandronate | 5 | US$733/US$544 | Dominant |
| Silverman et al. [22] | USA | Denosumab vs ibandronate | 5 | US$1811/US$1462 | Dominant |
| Coyle et al. [24] | Canada | Zoledronic acid vs alendronate | 2 | US$298/US$123 | US$535,359 |
| Li et al. [15] | China | Zoledronic acid vs alendronate | 3/5 | US$536/US$555 | Dominant |
| Moriwaki et al. [30] | Japan | Zoledronic acid vs alendronate | 3 | US$350/US$273 | Dominant |
| Hiligsmann et al. [27] | France | GR risedronate vs alendronate | 3 | US$58/US$55 | US$2401 |
| Azar et al. [13] | Iran | Teriparatide vs generic risedronate | 2 | US$1757/US$7 | US$522,424 |
| Hiligsmann et al. [27] | France | GR risedronate vs generic risedronate | 3 | US$58/US$37 | US$2759 |
GP governmental perspective, GR gastro-resistant, HP healthcare perspective, ICER incremental cost-effectiveness ratio, SP societal perspective
Specifically, eight studies [14, 18, 21, 22, 24–26, 29] made comparisons between denosumab and oral alendronate, of which five studies [18, 22, 25, 26, 29] demonstrated that denosumab was cost effective, and one study [21] showed that denosumab was a dominant option if we applied US$100,000 per QALY gained as the WTP threshold. In addition, when compared with risedronate and ibandronate, denosumab was also shown to be cost effective [25, 26] or dominant [21, 22]. However, two studies [14, 24] showed that denosumab was not cost effective with large ICERs when compared with alendronate; this was caused by minimal incremental QALYs. In addition, comparisons between oral and non-oral bisphosphonates were performed in some studies. Three studies [15, 20, 24] were conducted between zoledronic acid and oral alendronate, with one study indicating that zoledronic acid was dominant [15]; in the other two studies, zoledronic acid was not cost effective or was dominated by alendronate [20, 24]. As a new formulation of bisphosphonates, gastro-resistant risedronate was cost effective in comparison with alendronate and risedronate in one study [27]. Furthermore, another study compared teriparatide with risedronate, showing that teriparatide was not cost effective. Overall, 67% studies (eight of a total 12 studies) or 82% of comparisons (23 of a total 28 studies) suggested that active interventions (denosumab, zoledronic acid, gastro-resistant risedronate, or teriparatide) were cost effective when compared with traditional oral bisphosphonates. Additionally, comparisons between active interventions were also made in some studies; two studies showed that denosumab was cost effective [26] or dominant [21] when compared with strontium ranelate. Zoledronate acid and teriparatide were dominated by denosumab in another two studies [21, 22].
Table 4 presents three studies [16, 20, 28] that estimated the cost effectiveness of sequential therapies from the US perspective. Hiligsmann et al. [28] analyzed populations with different BMD T-scores at baseline, and the study of Mori et al. [20] assessed women at different ages and from both healthcare and societal perspectives. Hiligsmann et al. [28] and Le et al. [16] assessed sequential therapies starting with 1.5 years of abaloparatide or teriparatide, followed by 5 years of alendronate as the treatment duration. In the study of Mori et al. [20], 2 years of initial treatment with teriparatide was followed by 10 years of alendronate. The monthly drug costs for abaloparatide were similar between studies, at approximately US$1700; the cost of teriparatide was from US$1711 to US$3722 per month. Abaloparatide followed by alendronate was shown to be dominant when compared with teriparatide followed by alendronate in two studies [16, 28]. In addition, when compared with a placebo or no treatment, Hiligsmann et al. [28] showed that abaloparatide followed by alendronate was cost saving or cost effective in different populations. In the study of Le et al. abaloparatide or teriparatide followed by alendronate was not cost effective when compared with a placebo followed by alendronate [16]. Furthermore, Mori et al. [20] compared sequential therapy (teriparatide followed by alendronate) with alendronate alone at different ages and economic perspectives, indicating that sequential therapy was not cost effective. The high drug costs of abaloparatide and teriparatide largely affected ICERs when compared with no treatment, a placebo, and with alendronate alone.
Table 4.
Cost-effective analyses of sequential therapy
| References | Country | Population | Comparator (treatment duration) | Monthly drug costs | ICER |
|---|---|---|---|---|---|
| Hiligsmann et al. [28] | USA | BMD T-score ≤ − 3.5, age 70 or − 3.5 ≤ BMD T-score ≤ − 2.5 and history of one osteoporotic fracture, age 70 y | Sequential ABL(1.5y)/ALN(5y) vs sequential TPTD(1.5)/ALN(5y) |
ABL US$1695 TPTD US$3387 ALN US$10 |
Dominant |
| Le et al. [16] | USA | Aged ≥ 65 y with a prior vertebral fracture | Sequential ABL(1.5y)/ALN(5y) vs sequential TPTD(1.5)/ALN(5y) |
ABL US$1795 TPTD US$3722 ALN US$10 |
Dominant |
| Hiligsmann et al. [28] | USA | BMD T-score ≤ − 3.5, age 70 y | Sequential ABL(1.5y)/ALN(5y) vs no treatment |
ABL US$1695 ALN US$10 |
Cost saving |
| − 3.5 ≤ BMD T-score ≤ − 2.5 and history of one osteoporotic fracture, age 70 y | US$40,428 | ||||
| Le et al. [16] | USA | Aged ≥ 65 y with a prior vertebral fracture | Sequential ABL(1.5y)/ALN(5y) vs PBO/ALN(5y) |
ABL US$1795 ALN US$10 |
US$347,577 |
| Sequential TPTD(1.5y)/ALN(5y) vs PBO/ALN(5y) | TPTD US$3722 | US$991,854 | |||
| Mori et al. [20] | USA | Age 65 y (SP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) |
TPTD US$1711 ALN US$17 |
US$442,263 |
| Age 70 y (SP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) | US$335,973 | |||
| Age 75 y (SP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) | US$285,170 | |||
| Age 80 y (SP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) | US$296,063 | |||
| Age 65 y (HP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) | US$449,695 | |||
| Age 70 y (HP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) | US$342,794 | |||
| Age 75 y (HP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) | US$293,416 | |||
| Age 80 y (HP) | Sequential TPTD(2y)/ALN(10y) vs ALN(10y) | US$304,514 |
ABL abaloparatide, ALN alendronate, BMD bone mineral density, HP healthcare perspective, ICER incremental cost-effectiveness ratio, SP societal perspective, TPTD teriparatide, y years
Critical Appraisal
Table 5 presents the results of the quality assessment of the design and conduct of economic evaluations in osteoporosis using the ESCEO-IOF guideline. Substantial differences were observed between studies with an average score of 17 out of 25 (range 2–25). Although some studies followed several recommendations of the guideline, room for improvement was observed for most studies.
Table 5.
Quality appraisal of cost-effectiveness analyses of drugs for osteoporosis using the ESCEO-IOF guideline
| Item | Recommendation | Article references | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [12] | [32] | [36] | [17] | [34] | [33] | [21] | [10] | [26] | [25] | [11] | [22] | [31] | ||
| Type of economic evaluation | Cost-utility analysis using QALY as the outcome | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Method for the conduct of an economic evaluation | A model-based economic evaluation | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Modeling technique | Lifetime horizon | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Markov model is appropriate (6-month/1-year cycle length) | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | |
| Avoid hierarchy of fractures and restrictions after fracture events | NA | Part | Part | No | Yes | Yes | Part | NA | Part | No | No | Part | Yes | |
| Hip, clinical vertebral, and non-vertebral non-hip fracture | Yes | Yes | Yes | Yes | Yes | Part | Yes | Part | Yes | Yes | No | Yes | Yes | |
| Base-case analysis and population | Multiple scenarios: age range, BMD, and fracture risk scenarios | No | Yes | Part | Part | Part | No | No | No | No | Yes | No | No | Part |
| The FRAX® or GARVAN® tools can be used to model fracture | ||||||||||||||
| Increased risk after fracture events within the model | No | Yes | Yes | No | Yes | No | No | No | No | Yes | No | No | Yes | |
| Mortality | Excess mortality after hip fractures and clinical vertebral fractures | No | Yes | Yes | No | No | Part | Yes | No | No | Yes | No | Yes | Yes |
| Proportion attributed to the fracture (e.g., 25–30%) mortality that is attributable to the fracture event | No | No | No | No | No | Yes | Yes | No | No | No | No | No | No | |
| Fracture costs and utility | Societal and/or healthcare payer perspective | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Acute fracture costs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | |
| Long-term costs after hip fracture (attributable to the fracture) | No | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | |
| First year and subsequent years’ effects of fractures on disutility | No | Part | Yes | No | Yes | Part | Yes | No | Yes | Yes | No | Yes | Yes | |
| National ICUROS data if available | ||||||||||||||
| An additional effect (on costs and/or utility) after multiple fractures | No | No | No | No | No | No | No | No | No | Yes | No | No | No | |
| Treatment characteristics | Treatment duration similar to guidelines or RCTs | Yes | Yes | Yes | No | Yes | NA | Yes | No | Yes | Yes | No | Yes | Yes |
| Comparators: no treatment and relevant active osteoporotic agent(s) | Yes | No | Yes | No | Yes | No | No | No | No | Yes | No | No | No | |
| Sequential therapy may be considered as intervention/comparators | ||||||||||||||
| Efficacy data from RCTs, (network) meta-analysis | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | |
| In the absence of hip/wrist specific efficacy data, use of non-vertebral or clinical fracture efficacy data | ||||||||||||||
| Treatment effects after discontinuation depending on treatment | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | |
| Medication adherence as base case or sensitivity | No | Yes | No | No | Yes | No | No | No | No | No | No | No | Yes | |
| Drug costs and administration/monitoring costs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Adverse events | No | Yes | Yes | No | No | No | No | Yes | Yes | No | No | No | No | |
| Sensitivity analyses | One-way sensitivity analyses | No | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes |
| Probabilistic sensitivity analyses | No | No | Yes | No | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | |
| Outcomes | Presentation of disaggregated outcomes, incremental costs, and outcomes for each intervention and incremental cost-effectiveness ratios | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Scoring | 7 | 20 | 21 | 5.5 | 19.5 | 16.5 | 18.5 | 4.5 | 15.5 | 21 | 2 | 17.5 | 20.5 | |
| Item | Recommendation | Article references | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [14] | [13] | [18] | [19] | [30] | [35] | [29] | [23] | [24] | [27] | [15] | [28] | [16] | [20] | ||
| Type of economic evaluation | Cost-utility analysis using QALY as outcome | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Method for the conduct of economic evaluation | A model-based economic evaluation | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Modeling technique | Lifetime horizon | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Markov model is appropriate (6-month/1-year cycle length) | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | |
| Avoid hierarchy of fractures and restrictions after fracture events | Yes | No | Part | Part | Yes | Part | Part | Part | Part | Yes | Part | Yes | Part | Part | |
| Hip, clinical vertebral, and non-vertebral non-hip fracture | Yes | Yes | Part | Yes | Yes | Yes | Part | Yes | Yes | Yes | Part | Yes | Yes | Yes | |
| Base-case analysis and population | Multiple scenarios: age range, BMD, and fracture risk scenarios | Part | No | Part | Part | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Part | Part |
| The FRAX® or GARVAN® tools can be used to model fracture | |||||||||||||||
| Increased risk after fracture events within the model | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | |
| Mortality | Excess mortality after hip fractures and clinical vertebral fractures | Part | No | Part | Part | Part | Yes | Part | No | Yes | Yes | Yes | Yes | Part | Part |
| Proportion attributed to the fracture (e.g., 25–30%) mortality that is attributable to the fracture event | No | No | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | No | Yes | |
| Fracture costs and utility | Societal and/or healthcare payer perspective | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Acute fracture costs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Long-term costs after hip fracture (attributable to the fracture) | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| First year and subsequent years’ effects of fractures on disutility | Yes | Yes | Yes | Yes | Yes | Part | Yes | Yes | Part | Yes | Yes | Yes | Yes | Part | |
| National ICUROS data if available | |||||||||||||||
| An additional effect (on costs and/or utility) after multiple fractures | Yes | No | No | No | No | No | No | Yes | No | Yes | No | Yes | No | No | |
| Treatment characteristics | Treatment duration similar to guidelines or RCTs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Comparators: no treatment and relevant active osteoporotic agent(s) | No | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | |
| Sequential therapy may be considered as intervention/comparators | |||||||||||||||
| Efficacy data from RCTs, (network) meta-analysis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| In the absence of hip/wrist specific efficacy data, use of non-vertebral or clinical fracture efficacy data | |||||||||||||||
| Treatment effects after discontinuation depending on treatment | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Part | Yes | Yes | Yes | |
| Medication adherence as base case or sensitivity | Yes | No | Yes | Yes | Yes | No | Yes | No | No | Yes | No | Yes | Yes | Yes | |
| Drug costs and administration/monitoring costs | Yes | Yes | Yes | Yes | Yes | Part | Yes | Yes | Yes | Yes | Yes | Yes | Part | Yes | |
| Adverse events | No | No | No | No | No | No | No | Yes | No | No | No | Yes | Yes | No | |
| Sensitivity analyses | One-way sensitivity analyses | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Probabilistic sensitivity analyses | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Outcomes | Presentation of disaggregated outcomes, incremental costs, and outcomes for each intervention and incremental cost-effectiveness ratios | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Scoring | 20 | 11 | 21 | 21.5 | 20.5 | 15.5 | 20.5 | 19.5 | 18 | 24 | 16.5 | 25 | 20 | 20 | |
Scoring: ‘use ICUROS data’, ‘use FRAX® or GARVAN® tools’, ‘consider sequential therapy as intervention’, and ‘in the absence of hip/wrist specific efficacy data, use of non-vertebral or clinical fracture efficacy data as replacement’ was not included in the scoring system
BMD bone mineral density, NA not applicable, QALY quality-adjusted life-year, RCTs randomized controlled trials
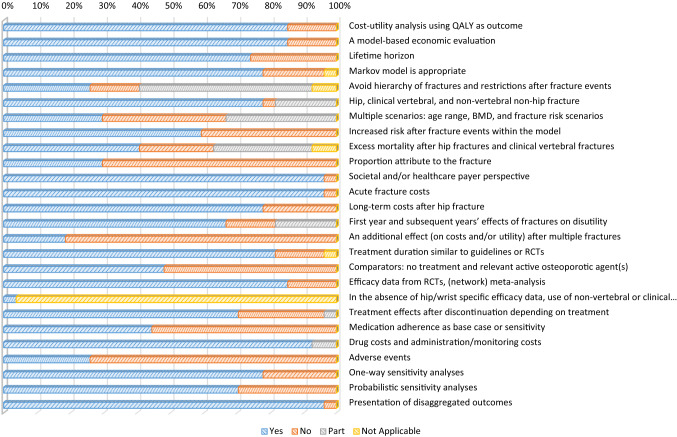
Figure 2 shows the percentage of studies that fully, partially, or did not report the individual recommendations in the guideline. The most frequently unreported recommendations were ‘an additional effect after multiple fractures’ (i.e., an additional effect on costs and/or utility should be modeled), ‘adverse events’ (i.e., important side effects that have an impact on costs and/or utility need to be included), and ‘proportion attributed to the fracture’ (i.e., a proportion of excess mortality attributed to the fracture should be included). In addition, some recommendations such as ‘avoid hierarchy of fractures and restrictions after fracture events’ (e.g., the absence of a non-hip fracture after a previous hip fracture or a limit to the number of fracture events) and ‘multiple scenarios’ (i.e., include age range and fracture risk levels) were frequently partially reported.
Fig. 2.
Proportion of studies meeting individual items recommended in ESCEO-IOF guideline (total studies: 27). BMD bone mineral density, QALY quality-adjusted life-year, RCTs randomized controlled trials
The results of reporting quality assessment are presented in Table 6; most recommendations were well reported with an average score of 6.8 out of 9 (range 0.5–9). The quality of reporting was suboptimal for ‘treatment side effects’ (i.e., describing the approaches and data sources used for costs and utilities effects of adverse events). Furthermore, ‘medication adherence’ (i.e., describing approaches and data sources used for modeling medication adherence) was poorly reported in some articles [10–13, 15, 17, 23, 26, 33], as well as ‘treatment effect after discontinuation’ in six articles (i.e., these studies did not assume a linear decrease of the effect after discontinuation for a period similar to the duration of treatment) [10–13, 23, 24].
Table 6.
Quality of reporting of published articles assessing the cost effectiveness of drugs for osteoporosis using the ESCEO-IOF osteoporosis specific checklist
| Item | Recommendation | Article references | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [12] | [32] | [36] | [17] | [34] | [33] | [21] | [10] | [26] | [25] | [11] | [22] | [31] | ||
| Transition probabilities | Report the transition probabilities and how they were estimated (including increased fracture risk) | No | Yes | Yes | No | Yes | Yes | Yes | NA | Yes | Yes | No | Yes | Yes |
| Excess mortality after fractures | Describe approaches and data sources used for the excess mortality after fractures | No | Yes | Yes | No | No | Yes | Yes | NA | No | Yes | No | Yes | Yes |
| Fractures costs | Describe approaches and data sources used for fractures costs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Fractures effects on utility | Describe approaches and data sources used for the effects of fractures on utility | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Treatment effect during treatment | Describe fully the methods used for the identification, selection, and synthesis of clinical effectiveness data (per fracture site) | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Treatment effect after discontinuation | Describe fully the methods used for the treatment effect after discontinuation | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Medication adherence | Describe approaches and data sources used for modeling medication adherence | No | Yes | Yes | No | Yes | No | Yes | No | No | Yes | No | Yes | Yes |
| Treatment costs | Describe approaches and data sources used for therapy costs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Part | Yes | Yes | Yes | Yes | Yes |
| Treatment side effects | Describe approaches and data sources used for costs and utilities effects of adverse events | No | Yes | Yes | No | No | No | No | No | Yes | No | No | No | No |
| Scoring | 2 | 9 | 9 | 3 | 7 | 7 | 8 | 0.5 | 7 | 8 | 1 | 8 | 8 | |
| Item | Recommendation | Article references | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [14] | [13] | [18] | [19] | [30] | [35] | [29] | [23] | [24] | [27] | [15] | [28] | [16] | [20] | ||
| Transition probabilities | Report the transition probabilities and how they were estimated (including increased fracture risk) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Excess mortality after fractures | Describe approaches and data sources used for the excess mortality after fractures | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Fracture costs | Describe approaches and data sources used for fracture costs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fracture effects on utility | Describe approaches and data sources used for the effects of fractures on utility | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Treatment effect during treatment | Describe fully the methods used for the identification, selection, and synthesis of clinical effectiveness data (per fracture site) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Treatment effect after discontinuation | Describe fully the methods used for the treatment effect after discontinuation | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes |
| Medication adherence | Describe approaches and data sources used for modeling medication adherence | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| Treatment costs | Describe approaches and data sources used for therapy costs | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Treatment side effects | Describe approaches and data sources used for costs and utilities effects of adverse events | No | No | No | No | No | No | No | Yes | No | No | No | Yes | Part | No |
| Scoring | 8 | 5 | 8 | 8 | 8 | 8 | 8 | 6 | 7 | 8 | 6 | 9 | 8.5 | 8 | |
NA not applicable
Key Drivers of Cost Effectiveness
Several drivers of cost effectiveness were identified, including baseline fracture risk, drug effect on the risk of fractures, drug cost, and medication adherence/persistence.
Baseline Fracture Risk
Most studies indicated that the increase of baseline fracture risk and the age of patients were associated with favorable results of cost-effectiveness analyses of osteoporotic drugs. For instance, Moriwaki et al. [30] indicated that the incremental costs and incremental QALYs of zoledronic acid compared with alendronate tended to be small, with an increase of T-scores. Moreover, Chokchalermwong et al. [23] reported that, compared to no treatment, the ICER of bisphosphonates was 130,049 THB per QALY when starting the drug from the age of 65 years, with a BMD T-score ≤ − 2.5. However, denosumab was cost effective from the age of 80 years and over.
Drug Effect on the Risk of Fractures
Twelve studies [15, 18, 20–23, 25, 29–32, 36] reported that the cost effectiveness result of osteoporotic drugs is most sensitive to changes in the effect of osteoporotic drugs on the risk of fractures. Silverman et al. [22] indicated that when the relative risk of hip fracture with denosumab is lowered from 0.38 (baseline) to 0.18, denosumab still dominates the generic alendronate. However, when this relative risk is increased to 0.78, denosumab is no longer a cost-effective option. This finding is similar to the study of Parthan et al. [21] and Yoshizawa et al. [29]. In addition, Moriwaki et al. [30] reported that the relative risk of hip fracture with zoledronic acid had a relatively strong effect on the estimated incremental net monetary benefit; compared to alendronate, zoledronic acid could be a cost-effective option if the relative risk was equal to 0.34 (lower limit).
Drug Cost
Variation in drug costs could lead to different cost-effectiveness results of anti-osteoporosis drugs. The strong effect of drug cost was reported in several studies [13, 14, 20, 23, 27–30, 33, 34]. Mori et al. [20] compared sequential therapy (teriparatide followed by alendronate) to alendronate alone and reported that results were most sensitive to the changes in the estimated cost of teriparatide. If the cost of a generic/biosimilar was estimated to be 15% of the brand (i.e., 85% less), the annual cost of teriparatide would be $6490 for a 65-year-old cohort; or if the cost of a generic/biosimilar was estimated to be 35% of the brand (i.e., 65% less), the annual cost of teriparatide would be $11,461 for a 75-year-old cohort; the ICERs of sequential teriparatide/alendronate were below the WTP threshold of $150,000/QALY. Moriwaki et al. [30] also reported that if the cost of zoledronic acid was lowered by 30%, zoledronic acid could be a cost-effective option compared with alendronate. Additionally, Karnon et al. [14] indicated that there is a near-zero probability that denosumab is cost effective at a threshold of $100,000/QALY compared with alendronate at the current price; however, if the price of denosumab was reduced by 50%, the incremental cost per QALY gained falls to $50,068.
Medication Adherence/Persistence
Anti-osteoporosis medications have shown to be effective in reducing fracture risk; however, as a chronic disease, non-adherence to pharmacological treatment in osteoporosis is a well-recognized problem, which would result not only in deteriorating clinical outcomes, but also in decreased cost effectiveness of pharmacotherapy. Several studies [18, 20, 27, 29, 31, 34] reported that the persistence and adherence rates of osteoporosis medications have marked effects on the cost-effectiveness ratios. For instance, Mori et al. [18] indicated that denosumab was cost effective or even cost saving in comparison with weekly oral alendronate, mainly driven by the higher persistence rate of denosumab leading to higher efficacy. In addition, Hiligsmann et al. [27] also reported that the ICERs of gastro-resistant risedronate were markedly affected by the incremental difference in persistence between gastro-resistant risedronate and the active comparator treatment. Moreover, the study of Chen et al. [31] demonstrated that medication persistence and adherence had a great impact on clinical and cost effectiveness, high raloxifene persistence and adherence improved clinical effectiveness, but the costs were also higher. Raloxifene treatment became cost effective compared with a conventional treatment strategy if raloxifene persistence and adherence decreased by 30–50%.
Discussion
This updated review identified 27 economic evaluations of drugs for osteoporosis published between July 2013 and 2019. Twelve active drugs were assessed in the studies, including bisphosphonates (alendronate, etidronate, risedronate, gastro-resistant risedronate, ibandronate, and zoledronic acid), romosozumab, raloxifene, strontium ranelate, denosumab, teriparatide, and abaloparatide. When compared with traditional oral bisphosphonates, 67% of the studies (eight of the total 12 studies) or 82% of the comparisons (23 of the total 28 comparisons) showed that the alternative drugs (denosumab, zoledronic acid, gastro-resistant risedronate, and teriparatide) were cost effective or dominant at the WTP threshold of US$100,000 per QALY gained. In particular, most studies suggested that denosumab was a cost-effective or dominant option compared with oral bisphosphonates. It should however be noted that recent studies have shown a rapid decrease of BMD and an increased risk of vertebral fractures after discontinuation of denosumab [37, 38] and that these effects have not been included in economic evaluations; accordingly, the cost effectiveness of denosumab could be over-estimated.
Additionally, within the total 27 studies, the source of funding and the role of the funder were fully reported in only 14 studies. It is further interesting to note that three [22, 25, 26] out of eight studies conducted comparing denosumab with oral bisphosphonates, showing that denosumab was cost effective or dominant, were funded by industry. For the remaining five studies that did not mention funding or had no funding, only three (60%) indicated that denosumab was cost effective or dominant. The potential bias in industry-sponsored studies may therefore exist; however, given the limited studies, it is difficult to draw a clear conclusion. Previously, another study [39] comparing economic evaluations of bisphosphonates for the treatment of osteoporosis suggested that the funding source (industry vs non-industry) did not seem to significantly affect the reporting of ICERs below the US$20,000 and US$50,000 thresholds.
Furthermore, some new formulations of bisphosphonates also led to a higher health benefit than traditional oral tablet bisphosphonates. One of the included studies showed that gastro-resistant risedronate was cost effective when compared with traditional oral alendronate [27]. In addition, some recent studies also indicated that new effervescent formulation of alendronate could be an intriguing option in reducing the occurrence of adverse gastrointestinal events in anti-osteoporosis treatment, thus increasing adherence to therapy and anti-fracture efficacy [40]. More research is needed to investigate both the clinical and economic benefits of these new formulations of oral bisphosphonates.
With emerging evidence about the value of sequential therapy [41, 42], sequential therapy was included in three studies [16, 20, 28]. When mutually comparing anabolic agents, sequential treatment starting with abaloparatide followed by alendronate was shown to be dominant compared with sequential therapy starting with teriparatide followed by alendronate. These three studies also compared the cost effectiveness of sequential therapy with no treatment, placebo, or alendronate alone, indicating mixed results. Incremental cost-effectiveness ratios were strongly affected by the extremely high drug costs of anabolic agents. One study [20] demonstrated that their results were sensitive to the cost of teriparatide, reporting that the cost of a generic/biosimilar product needed to be 65–85% lower than the brand for sequential teriparatide/alendronate to be cost effective.
After our search period, another study suggested that sequential treatment starting with abaloparatide followed by alendronate was cost effective in comparison to generic alendronate monotherapy for US postmenopausal women aged ≥ 60 years at an increased risk of fractures. This also dominated sequential treatment starting with alendronate followed by abaloparatide and then again by alendronate [43].
This review updates a previous systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis [6]. The previous review identified 39 economic evaluations of drugs in postmenopausal osteoporosis published in the period 2008–13, an average of 6.5 studies per year. In our review, 4.5 studies per year were identified. Given that new osteoporosis medications continue to emerge on the market, the previous review does not include some medications that were not available that time, but are currently frequently used. The cost effectiveness of some medications was not conclusive because of the limited number of studies in the previous review, but the evidence became clearer in our updated review. In addition, with newer evidence being available after the publication of the previous review, the comparator in the economic evaluation might also changed. For example, vitamin D and calcium (or no treatment) were common comparators in previous studies. However, most studies (74%) in our review made comparisons between active osteoporotic interventions and traditional oral bisphosphonates, as well as mutual comparisons between different alternatives.
Moreover, in comparison with the previous review, where evaluations were mainly conducted in Europe, many evaluations in the updated review were conducted outside of Europe and especially in Asia, where osteoporosis is an increasing burden [44]. Thirty-three percent of the studies in our review applied the Markov microsimulation model in comparison with 21% in the previous review, indicating the increasing use of Markov microsimulation model in recent years, which supports the suggestion that the Markov microsimulation model is an evolution of a health economic model used in osteoporosis. The Markov individual state-transition model overcomes the memory-less nature of the Markov cohort model and is preferred to capture all the interactions between events and the changing risks of future fractures and mortality [45].
There are several extra findings identified in our review in comparison with the previous review. However, a comparison between the two studies remains difficult owing to the large heterogeneity in country setting, model structure, fracture risk, drug costs, and incorporation of medication adherence. In addition, the use of FRAX® or GARVAN® tools [46] indicates a slight increase (5%) in comparison with studies included in the previous review, but it is still inadequate (22%).
To assess the quality of included studies, unlike the general checklist applied in the previous review, we used an osteoporosis-specific guideline [7] to critically appraise the studies included in this review. In comparison with the general quality assessment tools relied on in the previous review, the osteoporosis-specific guideline serves as a minimum standard for all economic analyses in osteoporosis; the guideline’s specificity enables better identification of unmet quality issues within recent studies and indicates some highly important criteria that should be met and improved in future studies, and further helps to reduce inter-study heterogeneity, thereby facilitating inter-study comparisons. Although a few studies followed several of the guideline’s recommendations, given that most of the studies were published prior to the osteoporosis-specific guideline, the guide was not available to assist researchers in designing appropriate and high-quality economic evaluations, which may be why most studies did not adhere to several recommendations/criteria of the guideline and scored poorly for some criteria. Room for improvement was observed.
With regard to osteoporosis-specific recommendations, the frequently unmet/unreported recommendations such as ‘an additional effect after multiple fractures on cost and/or utility’, ‘important adverse events’, and ‘a proportion of excess mortality attributed to the fracture’ should be modeled/included in future studies. As for osteoporosis-specific checklist for reporting, considering several partially or not reported recommendations including ‘treatment side effects’, ‘medication adherence’, and ‘treatment effect after discontinuation’ would limit transparency, comparability, and use by decision maker; these missing or partially reported recommendations should receive more attention and be modeled/included in future studies. Therefore, the osteoporosis-specific guideline, which supplements the generally accepted methodologic standards, can be useful in improving the transparency, quality, and comparability of economic evaluations in osteoporosis, thus increasing its potential for use by decision makers and leading to a more effective allocation of resources [7].
Moreover, it is important that researchers should be aware of and use the guideline. Interestingly, since the publication of the ESCEO-IOF guideline (between October 2018 and August 2020), nine economic evaluations have used and referenced the guideline. Specifically, these nine studies all reported that the conduct of the economic evaluation adheres to this recent published osteoporosis-specific guideline. However, only four studies [20, 28, 34, 47] clearly showed how their studies followed the recommendations of the guideline. Therefore, to successfully implement this guideline, we recommend that future studies include a table in the main text or appendix stating clearly how the criteria were met, and/or the reasons for non-adherence (if appropriate), which would lead to improved study transparency.
Additionally, some key drivers of cost effectiveness were identified in this review, including baseline fracture risk, drug effect on the risk of fractures, drug cost, and medication adherence/persistence. These key drivers were frequently reported to be the most influential factors in the cost-effectiveness ratio, and should therefore be incorporated in future economic evaluations.
Although the present study followed recommendations for conducting reviews of economic evaluations [48], there may have been some potential limitations to our study. First, because of limited space in Table 2 and for clarity, only base-case results were included in our results analysis. Second, reviewers involved in the quality assessment proposed different opinions in scoring for some recommendations; discrepancies in rating were resolved by a third reviewer (MH) and reached a consensus with the first author (NL). In addition, differentiating between partially or fully reported was difficult for some recommendations; the final interpretation/assessment was performed by the first author in agreement with a third reviewer (MH), who assessed all papers. Third, although the osteoporosis-specific guideline aimed to complement and align with most general guidelines for economic evaluations, some differences can be observed. For instance, the ESCEO-IOF guideline treats one-way and probabilistic sensitivity analyses equally in scoring, while other guidelines may treat them separately. Fourth, some key drivers of cost effectiveness were identified during the review of the articles. We did not perform a systematic quantitative assessment to identify key drivers of cost effectiveness.
Conclusions
In comparison with evaluations listed in a previous review, recent economic evaluations were conducted in more countries, and included more active drugs and sequential therapy as comparators. A comparison between studies remains difficult. In total, this updated review included 27 studies on the cost effectiveness of drugs for osteoporosis, suggesting that some active interventions (denosumab, zoledronic acid, gastro-resistant risedronate, or teriparatide) were cost effective or dominant when compared with oral bisphosphates. However, given the limited number of studies on the cost-effectiveness of sequential therapy that have been conducted so far, further research would be needed to investigate adequate evidence of the beneficial effect of this new form of intervention over single anti-osteoporosis interventions alone. In addition, the results of a quality appraisal indicate that greater adherence to the osteoporosis-specific guideline is expected to improve the transparency, quality, and comparability of future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Declarations
Funding
No funding was received for the conduct of the study or the preparation of this article.
Conflicts of Interest/Competing Interests
Nannan Li is funded by the China Scholarship Council (grant number 201909110080). Lei Si is funded by a National Health and Medical Research Council Early Career Fellowship (grant number GNT1139826). Mickaël Hiligsmann has received research grants through institutions from Amgen, Bayer, Radius Health, and ViiV-Healthcare GSK. Anneleis Boonen received a research grant from Abbvie and Celgene, and honorariums from UCF, Lilly, and Galapagos. Jean-Yves Reginster has served on paid advisory boards, and received consulting fees, lecture fees, and/or grant support from IBSA-Genevrier, Mylan, Radius Health, Pierre Fabre, Echolight, Teva, Cniel, and the Dairy Research Council.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data analyzed as part of this study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Authors’ Contributions
NL: study rationale and design, literature search, literature selection, quality assessment of studies, interpretation and reflection, writing the manuscript. DC: literature selection, data extraction, quality assessment, reviewing the manuscript. LS: study rationale and design, literature selection, data extraction, quality assessment, reviewing the manuscript. DP: literature selection, data extraction, quality assessment, reviewing the manuscript. SS: literature selection, data extraction, quality assessment, reviewing the manuscript. RB: literature selection, data extraction, quality assessment, reviewing the manuscript. IK: data extraction, quality assessment, reviewing the manuscript. SB: literature selection, reviewing the manuscript. AB: study rationale and design, interpretation and reflection, reviewing the manuscript. SE: study rationale and design, interpretation and reflection, reviewing the manuscript. JP: study rationale and design, interpretation and reflection, reviewing the manuscript. JYR: reviewing the manuscript. MH: study rationale and design, interpretation and reflection, reviewing the manuscript, guarantor of the study.
References
- 1.Morizio P, Burkhart JI, Ozawa S. Denosumab: a unique perspective on adherence and cost-effectiveness compared with oral bisphosphonates in osteoporosis patients. Ann Pharmacother. 2018;52(10):1031–1041. doi: 10.1177/1060028018768808. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, Cooper C, Rizzoli R, Reginster JY. Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif Tissue Int. 2019;104(3):235–238. doi: 10.1007/s00223-018-00512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy. 2013;11(5):485–497. doi: 10.1007/s40258-013-0047-8. [DOI] [PubMed] [Google Scholar]
- 6.Hiligsmann M, Evers SM, Sedrine WB, Kanis JA, Ramaekers B, Reginster JY, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205–224. doi: 10.1007/s40273-014-0231-1. [DOI] [PubMed] [Google Scholar]
- 7.Hiligsmann M, Reginster JY, Tosteson AN, Bukata SV, Saag KG, Gold DT, et al. Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos Int. 2019;30(1):45–57. doi: 10.1007/s00198-018-4744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.OECD. Purchasing power parities (PPPs), data and methodology. https://www.oecd.org/std/purchasingpowerparitiespppsdata.htm#eurodenominatedseries. Accessed 25 Sep 2020.
- 10.Chávez-Valencia V, Arce-Salinas CA, Espinosa-Ortega F. Cost-minimization study comparing annual infusion of zoledronic acid or weekly oral alendronate in women with low bone mineral density. J Clin Densitom. 2014;17(4):484–489. doi: 10.1016/j.jocd.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 11.MiraÇi M, Demeti A, Ylli Z, KelliÇi S, Tarifa D. The cost-effectiveness of ibandronate and alendronate for the treatment of osteoporosis in a specialized clinic in Tirana. Int J Pharm Pharm Sci. 2015;7(10):207–211. [Google Scholar]
- 12.Golmohamdi FR, Abbasi M, Karyani AK, Sari AA. Cost-effectiveness of zoledronic acid to prevent and treat postmenopausal osteoporosis in comparison with routine medical treatment. Electron Physician. 2016;8(12):3434. doi: 10.19082/3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azar AA, Rezapour A, Alipour V, Sarabi-Asiabar A, Gray S, Mobinizadeh M, et al. Cost-effectiveness of teriparatide compared with alendronate and risedronate for the treatment of postmenopausal osteoporosis patients in Iran. Med J Islam Repub Iran. 2017;31:39. doi: 10.14196/mjiri.31.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnon J, Shafie AS, Orji N, Usman SK. What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia. Cost Effect Resour Alloc. 2016;14(1):11. doi: 10.1186/s12962-016-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Zheng B, Liu M, Zhou H, Zhao L, Cai H, Huang J. Cost-effectiveness of antiosteoporosis strategies for postmenopausal women with osteoporosis in China. Menopause. 2019;26(8):906–914. doi: 10.1097/GME.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 16.Le QA, Hay JW, Becker R, Wang Y. Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann Pharmacother. 2019;53(2):134–143. doi: 10.1177/1060028018798034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohd-Tahir NA, Thomas P, Mohamed-Said MS, Makmor-Bakry M, Li SC. Cost-effectiveness of bisphosphonates for prevention of fracture related to glucocorticoid-induced osteoporosis in Malaysia. Int J Rheum Dis. 2018;21(3):647–655. doi: 10.1111/1756-185X.13206. [DOI] [PubMed] [Google Scholar]
- 18.Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos Int. 2017;28(5):1733–1744. doi: 10.1007/s00198-017-3940-4. [DOI] [PubMed] [Google Scholar]
- 19.Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of combined oral bisphosphonate therapy and falls prevention exercise for fracture prevention in the USA. Osteoporos Int. 2017;28(2):585–595. doi: 10.1007/s00198-016-3772-7. [DOI] [PubMed] [Google Scholar]
- 20.Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of sequential teriparatide/alendronate versus alendronate-alone strategies in high-risk osteoporotic women in the US: analyzing the impact of generic/biosimilar teriparatide. JBMR Plus. 2019;3(11):e10233. doi: 10.1002/jbm4.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parthan A, Kruse M, Agodoa I, Silverman S, Orwoll E. Denosumab: a cost-effective alternative for older men with osteoporosis from a Swedish payer perspective. Bone. 2014;1(59):105–113. doi: 10.1016/j.bone.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Silverman S, Agodoa I, Kruse M, Parthan A, Orwoll E. Denosumab for elderly men with osteoporosis: a cost-effectiveness analysis from the US payer perspective. J Osteoporos. 2015;1(2015):627631. doi: 10.1155/2015/627631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chokchalermwong S, Lochid-Amnuay S, Kapol N, Wiroonpochid E, Ongphiphadhanakul B. Economic evaluation of drug treatment for fracture prevention in Thai postmenopausal women with osteoporosis without fracture history. J Med Assoc Thailand. 2019;102(1):62–70. [Google Scholar]
- 24.Coyle D. Cost-effectiveness of pharmacological treatments for osteoporosis consistent with the revised economic evaluation guidelines for Canada. MDM Policy Pract. 2019;4(1):2381468318818843. doi: 10.1177/2381468318818843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darbà J, Kaskens L, Vilela FS, Lothgren M. Cost-utility of denosumab for the treatment of postmenopausal osteoporosis in Spain. Clinicoecon Outcomes Res. 2015;7:105. doi: 10.2147/CEOR.S78349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Waure C, Specchia ML, Cadeddu C, Capizzi S, Capri S, Di Pietro ML, et al. The prevention of postmenopausal osteoporotic fractures: results of the health technology assessment of a new antiosteoporotic drug. BioMed Res Int. 2014;2014:975927. doi: 10.1155/2014/975927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiligsmann M, Reginster JY. Cost-effectiveness of gastro-resistant risedronate tablets for the treatment of postmenopausal women with osteoporosis in France. Osteoporos Int. 2019;30(3):649–658. doi: 10.1007/s00198-018-04821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide vs teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum. 2019;49(2):184–196. doi: 10.1016/j.semarthrit.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Yoshizawa T, Nishino T, Okubo I, Yamazaki M. Cost-effectiveness analysis of drugs for osteoporosis treatment in elderly Japanese women at high risk of fragility fractures: comparison of denosumab and weekly alendronate. Arch Osteoporos. 2018;13(1):94. doi: 10.1007/s11657-018-0509-6. [DOI] [PubMed] [Google Scholar]
- 30.Moriwaki K, Mouri M, Hagino H. Cost-effectiveness analysis of once-yearly injection of zoledronic acid for the treatment of osteoporosis in Japan. Osteoporos Int. 2017;28(6):1939–1950. doi: 10.1007/s00198-017-3973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Si L, Winzenberg TM, Gu J, Jiang Q, Palmer AJ. Cost-effectiveness of raloxifene in the treatment of osteoporosis in Chinese postmenopausal women: impact of medication persistence and adherence. Patient Prefer Adherence. 2016;10:415. doi: 10.2147/PPA.S100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon JW, Park HY, Kim YJ, Moon SH, Kang HY. Cost-effectiveness of pharmaceutical interventions to prevent osteoporotic fractures in postmenopausal women with osteopenia. J Bone Metab. 2016;23(2):63–77. doi: 10.11005/jbm.2016.23.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taheri S, Fashami FM, Peiravian F, Yousefi P. Teriparatide in the treatment of severe postmenopausal osteoporosis: a cost-utility analysis. Iran J Pharm Res. 2019;18(2):1073. doi: 10.22037/ijpr.2019.1100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui L, He T, Jiang Y, Li M, Wang O, Jiajue R, et al. Predicting the intervention threshold for initiating osteoporosis treatment among postmenopausal women in China: a cost-effectiveness analysis based on real-world data. Osteoporos Int. 2020;31(2):307–316. doi: 10.1007/s00198-019-05173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Hanlon CE, Parthan A, Kruse M, Cartier S, Stollenwerk B, Jiang Y, et al. A model for assessing the clinical and economic benefits of bone-forming agents for reducing fractures in postmenopausal women at high, near-term risk of osteoporotic fracture. Clin Ther. 2017;39(7):1276–1290. doi: 10.1016/j.clinthera.2017.05.348. [DOI] [PubMed] [Google Scholar]
- 36.Ito K. Cost-effectiveness of single-dose zoledronic acid for nursing home residents with osteoporosis in the USA. BMJ Open. 2018;8(9):e022585. doi: 10.1136/bmjopen-2018-022585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 2017;32(6):1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- 38.Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JE, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 39.Fleurence RL, Spackman DE, Hollenbeak CS. Does the funding source influence the results in economic evaluations? A case study in bisphosphonates for the treatment of osteoporosis. Pharmacoeconomics. 2010;28(4):295–306. doi: 10.2165/11530530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Invernizzi M, Cisari C, Carda S. The potential impact of new effervescent alendronate formulation on compliance and persistence in osteoporosis treatment. Aging Clin Exp Res. 2015;27(2):107–113. doi: 10.1007/s40520-014-0256-5. [DOI] [PubMed] [Google Scholar]
- 41.Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32(2):198–202. doi: 10.1002/jbmr.3051. [DOI] [PubMed] [Google Scholar]
- 42.Kanis JA, Cooper C, Rizzoli R, Reginster JY. Review of the guideline of the American College of Physicians on the treatment of osteoporosis. Osteoporos Int. 2018;29(7):1505–1510. doi: 10.1007/s00198-018-4504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide followed by alendronate vs. alendronate monotherapy in women at increased risk of fracture: a US payer perspective. Semin Arthritis Rheum. 2020;50(3):394–400. doi: 10.1016/j.semarthrit.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Xia W, Cooper C, Li M, Xu L, Rizzoli R, Zhu M, et al. East meets West: current practices and policies in the management of musculoskeletal aging. Aging Clin Exp Res. 2019;31(10):1351–1373. doi: 10.1007/s40520-019-01282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Si L, Winzenberg TM, Palmer AJ. A systematic review of models used in cost-effectiveness analyses of preventing osteoporotic fractures. Osteoporos Int. 2014;25(1):51–60. doi: 10.1007/s00198-013-2551-y. [DOI] [PubMed] [Google Scholar]
- 46.Kanis JA, Harvey NC, Johansson H, Liu E, Vandenput L, Lorentzon M, et al. A decade of FRAX: how has it changed the management of osteoporosis? Aging Clin Exp Res. 2020;32(2):187–196. doi: 10.1007/s40520-019-01432-y. [DOI] [PubMed] [Google Scholar]
- 47.Peng K, Yang M, Tian M, Chen M, Zhang J, Wu X, et al. Cost-effectiveness of a multidisciplinary co-management program for the older hip fracture patients in Beijing. Osteoporos Int. 2020;31(8):1545–1553. doi: 10.1007/s00198-020-05393-1. [DOI] [PubMed] [Google Scholar]
- 48.Jefferson T, Demicheli V, Vale L. Quality of systematic reviews of economic evaluations in health care. JAMA. 2002;287(21):2809–2812. doi: 10.1001/jama.287.21.2809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.