Abstract
Impaired exercise following Fontan is a surrogate of morbidity. Single-center longitudinal data exist, but there is a lack of contemporary multi-center data. Ramp cycle ergometry was re-performed in consented participants who had originally participated in the Pediatric Heart Network’s Fontan cross-sectional study. Annualized change was evaluated at maximal and submaximal exercise. Associations between these outcomes and patient characteristics were analyzed. There were 336 participants in Fontan 3, mean age 23.2 years. Paired measurements of peak oxygen consumption (peak VO2) were available for 95; peak exercise data at Fontan 3 were available for 275. Percent-predicted peak VO2 declined by 0.8 ± 1.7% per year (p < 0.001). At Fontan 3, the lowest performing peak VO2 tertile had the highest rate of overweight and obesity (p < 0.001). Female gender was more prevalent in the highest performing tertile (p = 0.004). Paired data at the ventilatory anaerobic threshold (VO2 at VAT) were available for 196; VAT data at Fontan 3 were available for 311. Percent-predicted VO2 at VAT decreased by 0.8 ± 2.6% per year (p < 0.001). At Fontan 3, VO2 at VAT was better preserved than peak VO2 across all tertiles, with higher rates of overweight and obesity in the lower performing group (p = 0.001). Female gender (p < 0.001) and left ventricular morphology (p = 0.03) were associated with better performance. Submaximal exercise is better preserved than maximal in the Fontan population, but declined at the same rate over the study period. The overall longitudinal rate of decline in exercise performance is slower than what has been described previously.
Keywords: Fontan, Exercise physiology, Congenital heart disease
Introduction
The Fontan procedure has allowed for the survival of generations of children born with heterogeneous forms of critical congenital heart disease characterized by a single effective pumping chamber. The original procedure has undergone a number of modifications since the first description, and now typically involves the creation of a total cavopulmonary connection (TCPC), using either an extracardiac tube graft or an intra-atrial lateral tunnel [1–8]. While Fontan palliation allows for survival through the first few decades of life, there is a progressive decline in the efficiency of the circulation over time, which correlates with a progressive increase in morbidity and mortality [9–14].
Diminished exercise capacity is associated with increased morbidity and mortality in many forms of congenital and acquired heart disease, and is a useful marker of overall circulatory health [15–23]. In 2008, the Pediatric Heart Network (PHN) reported cross-sectional exercise data for a large cohort of patients who had undergone the Fontan operation (Fontan I). Those data demonstrated impaired exercise capacity among all age groups and suggested that exercise impairment was more pronounced in older participants [24, 25]. Although exercise capacity was diminished at both peak exercise (65% predicted) and at the ventilatory anaerobic threshold (78% predicted), the impairment was more pronounced at peak exercise, a discordance in exercise measures that is different relative to those with a normal biventricular circulation. The few published longitudinal studies evaluating exercise performance after Fontan confirmed the progressive decline in exercise capacity, estimated to diminish by 1.3–2.6% predicted per year, but are limited by the small size of the cohorts, usually drawn from a single site, and primarily comprised patients who received their Fontan procedure before the widespread adoption of the TCPC [26–28].
Given the importance of exercise performance as a surrogate of circulatory wellness and the absence of robust longitudinal exercise data from those who have undergone the modern TCPC, the PHN undertook a follow-up for participants from the original Fontan cross-sectional study [29]. For this study, we sought to understand factors associated with impaired exercise capacity after Fontan, to determine the slope of the decline in exercise performance over time, and to evaluate performance at peak versus sub-maximal exercise. We hypothesized that while exercise capacity would likely be impaired, the degree of impairment would be worse at peak exercise and the rate of change in impairment in the modern era would be diminished relative to previous reports.
Methods
All patients who participated in the original PHN Fontan Cross-Sectional Study (Fontan 1) were screened for enrollment in the Fontan follow-up study (Fontan 3). A survey-based follow-up (Fontan 2) was completed in the intervening period [30]. Those who had been converted to a two-ventricle circulation, had undergone heart transplant, or who had died since enrollment in Fontan 1 were not eligible for participation in Fontan 3. Eligible participants were approached for consent/assent by the institution at which original consent was obtained. All enrolled subjects were then offered the opportunity to participate in cardiopulmonary exercise testing, and those who successfully completed testing comprised the study cohort.
Following consent/assent, maximal exercise testing was performed using a standardized ramp protocol on an electronically braked cycle ergometer as previously described in the Fontan 1 study [24, 25]. Equipment was calibrated to manufacturers’ specifications, and testing was performed with standard protocols previously used in children, adolescents, and adult subjects with Fontan physiology [24, 25]. Participants pedaled in an unloaded state for three minutes and workload was then increased continuously with a slope chosen to achieve each participant’s predicted maximal work rate after 10 to 12 min of cycling. Metabolic measurements were assessed on a breath-by-breath basis throughout exercise. Maximal effort was defined as achieving a respiratory exchange ratio (RER) of equal to or greater than 1.10 [25]. Analysis of peak oxygen consumption (peak VO2) was limited to those participants who reached the threshold for maximal effort, but submaximal data were included, when interpretable, regardless of the value of the RER achieved during the exercise test.
Demographic and patient-specific anatomic and clinical characteristics were summarized using standard descriptive statistics. Normally distributed variables were reported as mean and standard deviation. Comparisons were made between Fontan 1 and Fontan 3 exercise measurements using the paired student t test. Annualized rate of change was calculated by dividing the differences between the two time points by the time gap. Regression analysis was performed to evaluate factors associated with change in exercise outcomes. Exercise performance at Fontan 3 was then evaluated in a cross-sectional manner. To facilitate comparison between the high and lower performers, the cross-sectional cohort was divided into tertiles based on percent predicted peak VO2 and minute oxygen consumption at the anaerobic threshold (VO2 at VAT). The differences among the characteristics of the subjects in these tertiles were evaluated. Regression analysis was performed on both the maximal and sub-maximal cross-sectional data to evaluate potential risk factors for poor performance at Fontan 3.
Results
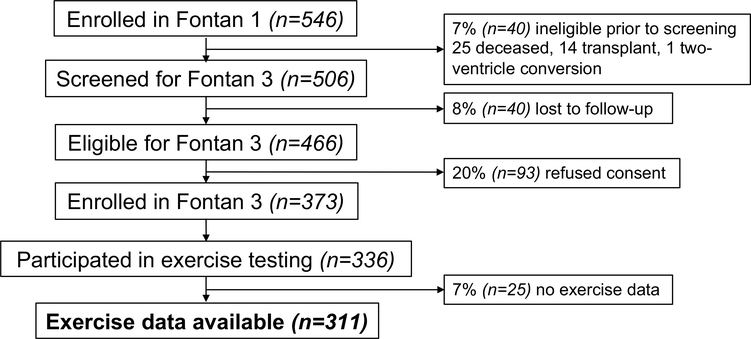
We identified 506 participants from Fontan 1 who were thought to be alive with an intact Fontan circulation. Of these, 40 were deemed lost to follow-up leaving 466 potential participants, of which 373 enrolled in Fontan 3. From this group, at least partial exercise data were available for 311 participants (Fig. 1). For the 311 participants with exercise data, the mean age at time of original Fontan procedure was 3.2 ± 1.6 years (Table 1), while the mean age at time of exercise testing for Fontan 3 was 21.1 ± 3.3 years. The majority of the participants were male (61%), and ventricular morphology was nearly evenly split between single left ventricular morphology (54%) and either single right or undetermined ventricular morphology (46%). Most participants had undergone a form of TCPC, 55% intra-atrial lateral tunnel vs 30% extracardiac conduit; 15% had undergone either an older version of the Fontan or a different modification. A pacemaker was present in 5% of participants.
Fig. 1.
Fontan 3 enrollment
Table 1.
Participant characteristics at Fontan 3
| Characteristic | N | Mean ± SD or n (%) |
|---|---|---|
| Age at Fontan, years | 311 | 3.2 ± 1.6 |
| Age at exercise testing, years | 311 | 21.1 ± 3.3 |
| Height, cm | 311 | 168.7 ± 9.3 |
| Weight, kg | 311 | 67.0 ± 15.7 |
| Body surface area, m2 | 281 | 1.8 ± 0.2 |
| BMI categories | 311 | |
| Normal | 221 (71%) | |
| Overweight | 57 (18%) | |
| Obese | 33 (11%) | |
| Sex | 311 | |
| Female | 120 (39%) | |
| Male | 191 (61%) | |
| Race | 310 | |
| Other | 60 (19%) | |
| White | 250 (81%) | |
| Ventricular morphology | 311 | |
| Left | 167 (54%) | |
| Right | 89 (29%) | |
| Mixed | 55 (18%) | |
| Type of Fontan | 311 | |
| Atriopulmonary connection | 39 (13%) | |
| TCPC lateral tunnel | 172 (55%) | |
| TCPC extracardiac conduit | 92 (30%) | |
| Other | 8 (3%) | |
| Pacemaker present | 311 | |
| No | 295 (95%) | |
| Yes | 16 (5%) |
BMI body mass index, TCPC total cavopulmonary connection
Of the 311 participants in Fontan 3, 95 achieved an RER > 1.10 at both Fontan 1 and Fontan 3 and therefore had paired data at peak exercise (Table 2, Fig. 2a). Statistically significant declines over time in resting oxygen saturation, oxygen saturation at peak exercise, peak work rate, percent predicted peak work rate, peak VO2, and percent predicted peak VO2 were noted in this group. The annualized rate of decline of percent predicted peak VO2 was 0.8 percentage points per year. There were no statistically significant associations between patient characteristics and change in percent predicted peak VO2 (data not presented).
Table 2.
Change in peak exercise performance from Fontan I to Fontan III
| Characteristic | N | Fontan I | Fontan III | Annualized change | p value* |
|---|---|---|---|---|---|
| Age at exercise testing | 95 | 13.7 ± 2.8 | 23.1 ± 2.9 | <0.001 | |
| Peak VO2, mL/kg/min | 95 | 28.1 ± 6.2 | 26.6 ± 7.5 | −0.2 ± 0.7 | 0.03 |
| % predicted VO2 | 95 | 68.5 ± 14.3 | 61.4 ± 15.9 | −0.8 ± 1.7 | <0.001 |
| Peak work rate, W | 95 | 101.1 ± 40.2 | 128.2 ± 39.2 | 2.9 ± 4.6 | <0.001 |
| % predicted peak work rate | 95 | 68.7 ± 15.4 | 55.6 ± 15.9 | −1.4 ± 1.6 | <0.001 |
| Maximum respiratory rate | 95 | 52.6 ± 12.3 | 45.9 ± 10.7 | −0.7 ± 1.2 | <0.001 |
| Peak heart rate | 95 | 161.0 ± 20.5 | 153.3 ± 23.7 | −0.8 ± 2.1 | <0.001 |
| % predicted peak heart rate | 95 | 78.0 ± 9.8 | 77.8 ± 11.9 | −0.0 ± 1.0 | 0.85 |
| Resting O2 saturation | 94 | 94.1 ± 4.2 | 93.2 ± 4.4 | −0.1 ± 0.4 | 0.03 |
| Peak O2 saturation | 89 | 91.4 ± 5.0 | 90.7 ± 4.4 | −0.1 ± 0.5 | 0.13 |
VO2 oxygen consumption, W Watts, O2 oxygen
Paired Student’s t test
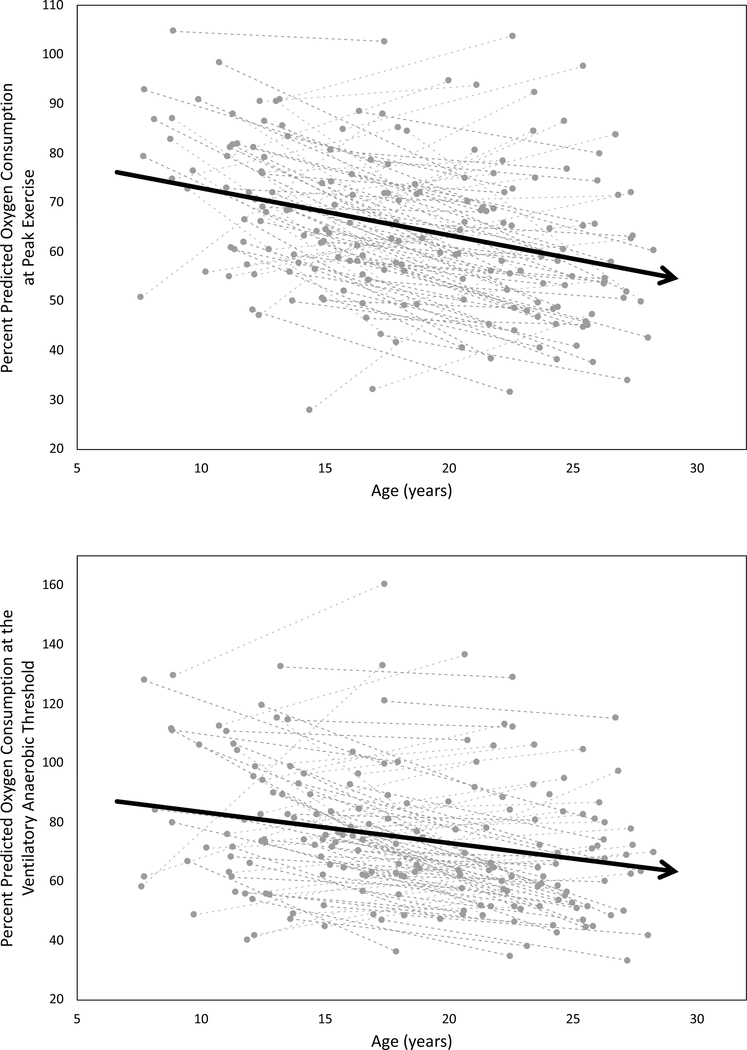
Fig. 2.
Longitudinal percent-predicted exercise data for individual subjects with a paired maximal tests (VO2) and b paired data at the ventilatory anaerobic threshold (VAT). The dashed lines represent each participant, while the solid lines with arrows represents the trend line for the cohort. Both plots demonstrate a decline in exercise capacity from Fontan 1 to Fontan 3
Paired data at sub-maximal exercise were available in 196 to 262 subjects, depending on the measurement (Table 3, Fig. 2b). These data likewise demonstrated a decline in raw measures of exercise performance as well as in percent predicted values. Although there was an increase in percent predicted forced vital capacity, there was a decrease of the FEV1/FVC ratio consistent with a mild progression of obstructive airway disease. Despite this finding, the VE/VCO2 ratio at the ventilatory anaerobic threshold improved, consistent with an improvement in ventilatory efficiency. There were again no statistically significant associations between patient characteristics and change in percent predicted VO2 at VAT (data not presented).
Table 3.
Change in exercise performance at the ventilatory anaerobic threshold from Fontan I to Fontan III
| Characteristic | N | Fontan 1 | Fontan 3 | Annualized change | p value* |
|---|---|---|---|---|---|
| Age at exercise testing | 270 | 12.2 ± 3.2 | 21.6 ± 3.3 | <0.001 | |
| VO2 at VAT, mL/kg/min | 196 | 19.4 ± 6.5 | 17.0 ± 5.7 | −0.3 ± 0.6 | <0.001 |
| % predicted VO2 at VAT | 196 | 80.0 ± 24.7 | 72.1 ± 24.8 | −0.8 ± 2.6 | <0.001 |
| % predicted FVC | 251 | 76.1 ± 14.9 | 81.6 ± 16.7 | 0.6 ± 1.5 | <0.001 |
| % predicted FEV1 | 247 | 76.7 ± 15.2 | 81.1 ± 16.3 | 0.5 ± 1.6 | <0.001 |
| FEV1/FVC at rest | 248 | 88.8 ± 8.0 | 85.7 ± 7.8 | −0.3 ± 1.0 | <0.001 |
| VE/VCO2 at VAT | 194 | 43.4 ± 10.7 | 33.4 ± 4.6 | −1.1 ± 1.1 | <0.001 |
| Resting O2 saturation | 262 | 94.2 ± 4.0 | 93.4 ± 4.6 | −0.1 ± 0.5 | 0.001 |
Paired Student’s t test
VO2 oxygen consumption, VAT ventilatory anaerobic threshold, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, VE/VCO2 ratio of minute ventilation to carbon dioxide production, O2 oxygen
For the group that was able to reach maximal effort at Fontan 3 (n = 275), the mean percent predicted peak VO2 was 59% (Table 4, Fig. 3a). When divided into tertiles based on percent predicted peak VO2, the highest performing tertile was associated with younger age (p = 0.007), lower weight (p < 0.001), normal (not elevated) body mass index (BMI) (p < 0.001), and female gender (p = 0.004). A potential trend was noted toward lower peak VO2 in participants with an atriopulmonary Fontan (p = 0.11), but there was no difference based on ventricular morphology (p = 0.61).
Table 4.
Exercise performance at Fontan III divided into tertiles by percent predicted peak oxygen consumption
| Characteristic | Total |
Tertile 1 |
Tertile 2 |
Tertile 3 |
p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | ||
| Peak VO2, mL/kg/min | 275 | 26.1 ± 7.3 | 91 | 19.5 ± 4.0 | 92 | 26.1 ± 4.8 | 92 | 32.6 ± 6.1 | <0.001* |
| Percent predicted peak VO2 (%) | 275 | 60 ± 16 | 91 | 43 ± 7 | 92 | 59 ± 4 | 92 | 78 ± 11 | <0.001* |
| Age at exercise testing, years | 275 | 21.1 ± 3.3 | 91 | 21.4 ± 3.6 | 92 | 21.7 ± 3.2 | 92 | 20.2 ± 3.0 | 0.007* |
| Height, cm | 275 | 168.9 ± 9.3 | 91 | 169.2 ± 9.7 | 92 | 169.0 ± 9.2 | 92 | 168.6 ± 9.0 | 0.89* |
| Weight, kg | 275 | 67.0 ± 15.7 | 91 | 72.3 ± 20.0 | 92 | 66.6 ± 13.4 | 92 | 62.1 ± 10.9 | <0.001* |
| BMI categories | 275 | 91 | 92 | 92 | <0.001† | ||||
| Normal | 196 (71%) | 52 (57%) | 64 (70%) | 80 (87%) | |||||
| Overweight | 51 (19%) | 20 (22%) | 19 (21%) | 12 (13%) | |||||
| Obese | 28 (10%) | 19 (21%) | 9 (10%) | 0 (0%) | |||||
| Gender | 275 | 91 | 92 | 92 | 0.004† | ||||
| Female | 102 (37%) | 24 (26%) | 32 (35%) | 46 (50%) | |||||
| Male | 173 (63%) | 67 (74%) | 60 (65%) | 46 (50%) | |||||
| Race | 274 | 90 | 92 | 92 | 0.31† | ||||
| White | 224 (82%) | 69 (77%) | 78 (85%) | 77 (84%) | |||||
| Other | 50 (18%) | 21 (23%) | 14 (15%) | 15 (16%) | |||||
| Ventricular morphology | 275 | 91 | 92 | 92 | 0.61† | ||||
| Left | 146 (53%) | 48 (53%) | 44 (48%) | 54 (59%) | |||||
| Right | 80 (29%) | 28 (31%) | 28 (30%) | 24 (26%) | |||||
| Mixed | 49 (18%) | 15 (16%) | 20 (22%) | 14 (15%) | |||||
| Type of Fontan | 275 | 91 | 92 | 92 | 0.11† | ||||
| TCPC lateral tunnel | 157 (57%) | 48 (53%) | 60 (65%) | 49 (53%) | |||||
| TCPC extracardiac conduit | 79 (29%) | 26 (29%) | 19 (21%) | 34 (37%) | |||||
| Atriopulmonary connection | 32 (12%) | 13 (14%) | 10 (11%) | 9 (10%) | |||||
| Other | 7 (3%) | 4 (4%) | 3 (3%) | 0 (0%) | |||||
| Pacemaker present | 275 | 91 | 92 | 92 | 0.22† | ||||
| No | 260 (95%) | 83 (91%) | 88 (96%) | 89 (97%) | |||||
| Yes | 15 (5%) | 8 (9%) | 4 (4%) | 3 (3%) | |||||
One-way ANOVA
Fisher’s Exact test
VO2 oxygen consumption, BMI body mass index, TCPC total cavopulmonary connection
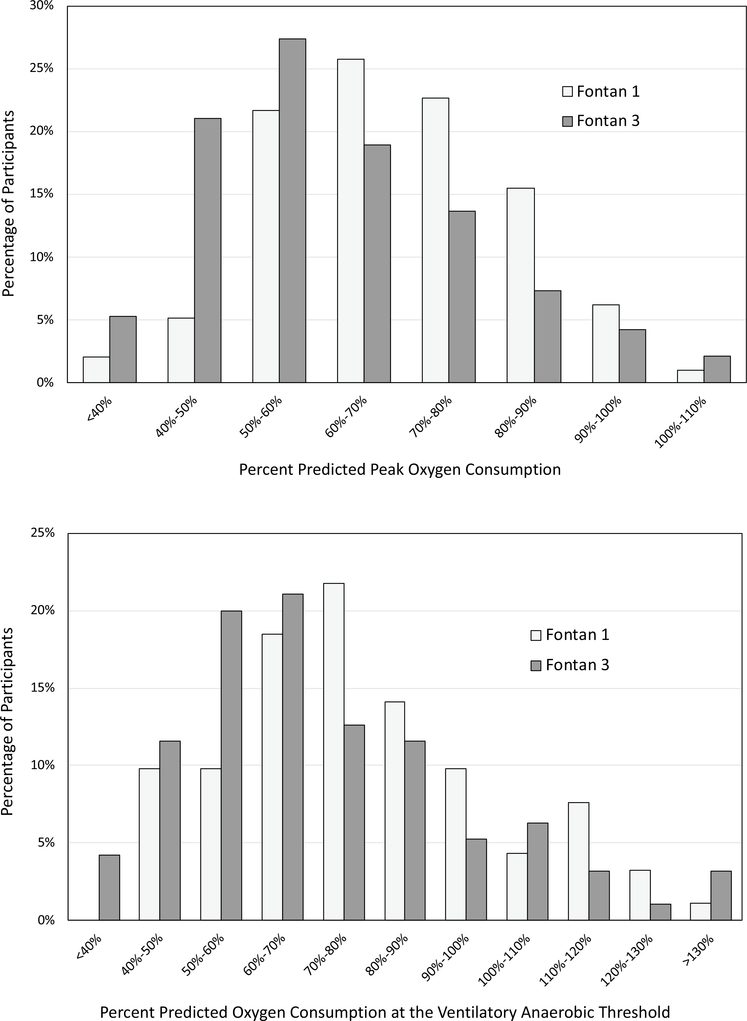
Fig. 3.
Histogram of exercise performance oxygen consumption at a peak exercise and b oxygen consumption at the anaerobic threshold. The y-axis is percentage of patients and the x axis is percent predicted for age and gender. The light bars represent data from Fontan 1 while the dark bars represent data from Fontan 3. Both histograms demonstrate a shift toward lower performance from Fontan 1 to Fontan 3
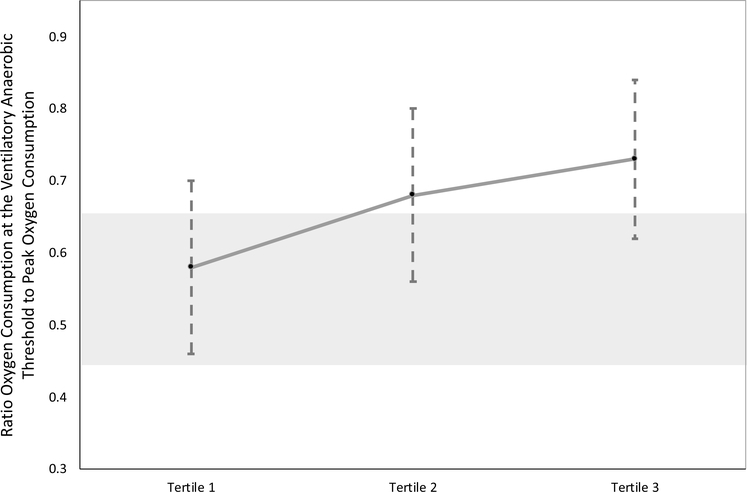
For participants with data at the ventilatory anaerobic threshold at Fontan 3 (n = 311), the percent predicted VO2 at VAT was 73% (Table 5, Fig. 3b). When divided into tertiles based on percent predicted VO2 at VAT, younger age (p = 0.006), lower weight (p < 0.001), lower BMI (p = 0.001), and female gender (p < 0.001) were again associated with inclusion in the highest performing tertile. We additionally found that single left ventricular morphology was more common in the highest performing tertile (p = 0.03). The presence of a pacemaker was most common in the lowest performing tertile (p = 0.02). When we evaluated the ratio of VO2 at VAT to peak VO2, we found an increase in the ratio for those in the higher performing tertile of VO2 at VAT (Fig. 4).
Table 5.
Exercise performance at Fontan III divided into tertiles by oxygen consumption at the ventilatory anaerobic threshold
| Characteristic | Total |
Tertile 1 |
Tertile 2 |
Tertile 3 |
p value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | ||
| VO2 at VAT, mL/kg/min | 311 | 17.2 ± 5.6 | 103 | 12.4 ± 2.6 | 104 | 16.6 ± 2.9 | 104 | 22.7 ± 5.0 | <0.001* |
| Percent predicted VO2 at VAT (%) | 311 | 73 ± 25 | 103 | 50 ± 9 | 104 | 69 ± 5 | 104 | 101 ± 20 | <0.001* |
| Peak VO2, mL/kg/min | 271 | 26.2 ± 7.3 | 93 | 21.9 ± 5.4 | 89 | 25.1 ± 5.9 | 89 | 31.8 ± 6.8 | <0.001* |
| Ratio of VO2 at VAT to Peak VO2 | 271 | 0.66 ± 0.13 | 93 | 0.58 ± 0.12 | 89 | 0.68 ± 0.12 | 89 | 0.73 ± 0.11 | <0.001* |
| Age at exercise testing, years | 311 | 21.1 ± 3.3 | 103 | 21.8 ± 3.3 | 104 | 21.1 ± 3.5 | 104 | 20.3 ± 3.1 | 0.006* |
| Height, cm | 311 | 168.7 ± 9.3 | 103 | 169.6 ± 10.0 | 104 | 169.1 ± 9.1 | 104 | 167.3 ± 8.8 | 0.17* |
| Weight, kg | 311 | 67.0 ± 15.7 | 103 | 71.9 ± 18.0 | 104 | 66.9 ± 15.4 | 104 | 62.3 ± 11.8 | <0.001* |
| BMI categories | 311 | 103 | 104 | 104 | 0.001† | ||||
| Normal | 221 (71%) | 64 (62%) | 71 (68%) | 86 (83%) | |||||
| Overweight | 57 (18%) | 19 (18%) | 24 (23%) | 14 (13%) | |||||
| Obese | 33 (11%) | 20 (19%) | 9 (9%) | 4 (4%) | |||||
| Gender | 311 | 103 | 104 | 104 | <0.001† | ||||
| Female | 120 (39%) | 25 (24%) | 39 (38%) | 56 (54%) | |||||
| Male | 191 (61%) | 78 (76%) | 65 (63%) | 48 (46%) | |||||
| Race | 310 | 102 | 104 | 104 | 0.82† | ||||
| White | 250 (81%) | 84 (82%) | 82 (79%) | 84 (81%) | |||||
| Other | 60 (19%) | 18 (18%) | 22 (21%) | 20 (19%) | |||||
| Ventricular morphology | 311 | 103 | 104 | 104 | 0.03† | ||||
| Left | 167 (54%) | 53 (51%) | 46 (44%) | 68 (65%) | |||||
| Right | 89 (29%) | 33 (32%) | 34 (33%) | 22 (21%) | |||||
| Mixed | 55 (18%) | 17 (17%) | 24 (23%) | 14 (13%) | |||||
| Type of Fontan | 311 | 103 | 104 | 104 | 0.13† | ||||
| TCPC lateral tunnel | 172 (55%) | 64 (62%) | 62 (60%) | 46 (44%) | |||||
| TCPC extracardiac conduit | 92 (30%) | 23 (22%) | 30 (29%) | 39 (38%) | |||||
| Atriopulmonary connection | 39 (13%) | 14 (14%) | 9 (9%) | 16 (15%) | |||||
| Other | 8 (3%) | 2 (2%) | 3 (3%) | 3 (3%) | |||||
| Pacemaker present | 311 | 103 | 104 | 104 | 0.02† | ||||
| No | 295 (95%) | 93 (90%) | 103 (99%) | 99 (95%) | |||||
| Yes | 16 (5%) | 10 (10%) | 1 (1%) | 5 (5%) | |||||
VO2 oxygen consumption, VAT ventilatory anaerobic threshold, BMI body mass index, TCPC total cavopulmonary connection
One-way ANOVA
Fisher’s Exact test
Fig. 4.
Ratio of VO2 at VAT to peak VO2 across Fontan 3 tertiles for those who had both sub-maximal and maximal data. The shaded area represents the normal range for those with a biventricular circulation. The hashed lines represent the standard deviation for each tertile
Discussion
This study is the largest longitudinal evaluation of exercise performance in children born with single ventricle physiology and palliated with the Fontan procedure. The majority of participants underwent staged palliation, culminating in a TCPC, which remains the primary form of surgical palliation. The results confirm previous findings that exercise capacity declines as children grow into adolescents and young adults, although the rate of decline was substantially lower than previously reported, perhaps related to the increased prevalence of the TCPC in this more contemporary cohort [25–28]. Factors associated with worse performance at the time of Fontan 3 included older age, heavier weight, increased BMI, and male gender. The presence of a single left ventricle was more common in those in the highest performing tertile at the ventilatory anaerobic threshold. While an improvement was noted in the calculated ventilatory efficiency from Fontan 1 to Fontan 3, this is likely related to the known increase in the ratio of tidal volume to dead space that occurs as a natural consequence of growth [31].
Although all measures of exercise performance in Fontan 3 were substantially below population norms, oxygen consumption during sub-maximal exercise was better preserved relative to peak exercise, consistent with the cross-sectional findings of Fontan 1. While the reason for better performance during sub-maximal exercise was not delineated in the present study, it is likely related to inherent limitations of the Fontan circulation. In the absence of a subpulmonary pump, preload is maintained by the pressure gradient across the pulmonary vascular bed; the difference between central venous pressure and atrial pressure [32–34]. As exercise intensity increases, central venous pressure must also rise to allow for increased transpulmonary blood flow. This increase in central venous pressure, along with an increase in heart rate, is what allows cardiac output to keep pace with metabolic demand. However, at higher levels of exertion, the increase in pressure required to provide adequate ventricular preload begins to exceed the physiologic limit of an individual’s maximal central venous pressure. This physiologic limit, or ceiling, is that point at which exercise is limited by an inability to raise central venous pressure any further.
Given the importance of exercise as a surrogate of overall cardiovascular health across a variety of cardiovascular disease states [15–23], it is important to understand the relationship between VO2 at VAT and peak VO2 after Fontan. In circulations with two ventricles, the ratio between VO2 at VAT and peak VO2 is relatively fixed at 45–65%, regardless of the fitness of the individual patient [21]. However, that ratio is altered for those who have undergone Fontan palliation, particularly at higher levels of performance, suggesting that an improvement in exercise capability may be preferentially expressed by an improvement in VO2 at VAT [24, 25, 29]. While VO2 at VAT is more technically difficult to measure than peak VO2, it is similarly associated with important morbidities and mortality [22, 23, 35]. In light of the physiologic limitations after Fontan, this population may be one in which VO2 at VAT is a more useful measure.
In this study, although both maximal and sub-maximal exercise capacities were below population norms for the study cohort as a whole, the participants who comprised the best performing tertile at the ventilatory anaerobic threshold were able to perform right at the expected level for age and sex during sub-maximal exercise. This cohort also had the highest ratio of VO2 at VAT to peak VO2, again demonstrating the discontinuity in these exercise measures at higher levels of activity. Importantly, the relative preservation of VO2 at VAT in this tertile suggests that there may be a group of patients for whom the Fontan physiology is more stable and resilient over time. While more work needs to be done to identify why some patients are able to perform sub-maximal exercise at near-normal levels, the implication of this finding is that a significant subset of Fontan patients can be expected to perform activities of daily living and many recreational physical activities at levels nearly equal to their healthy peers well into early adulthood. Although maximal exercise may be limited by the physiologic ceiling, sub-maximal activity is likely substantially more important and common in day-to-day life.
The most significant demographic differences between the upper and lower tertiles in this study were BMI and the rates of overweight and obesity. Only 18% of the upper tertile were either overweight or obese versus 39% for the lower tertile. Recent studies of exercise performance in the Fontan population suggest that sustained regular physical activity is associated with superior exercise performance [36, 37]. This appears to be specifically related to increases in lean lower extremity mass, bone density, and lower body mass index [36–38]. Augmentation of cardiac output during exercise by both lower extremity and respiratory muscle groups appears to be at least in part associated with improved exercise performance. The findings of our study support this concept.
The findings in the present study have important implications for the long-term health of those with Fontan physiology. We know from previous studies that there is a threshold of exercise capacity below which patients are more likely to demonstrate increased morbidities associated with heart failure [15–17]. Taking the cross-sectional data from Fontan 1 and applying the slope of decline from previous longitudinal studies suggest that patients with Fontan physiology would cross the threshold for symptomatic heart failure early in their third decade. The data from this study, while still demonstrating a decline, suggest that the period of relative wellness in patients who undergo a TCPC may be longer than would have been anticipated using the older data in patients with older versions of the Fontan procedure.
Limitations
This study was limited to the select group of survivors from Fontan 1 who chose to participate in Fontan 3 and who were able to participate in exercise testing. This creates an inherent survivor bias and eliminates those whose physical impairment made exercise participation impossible. As a consequence, the results of this study likely represent a more positive view of exercise performance across the cohort than what is actually true among patients with single ventricle heart disease more generally. Further, the data from Fontan 1 and Fontan 3 represent two points separated by nearly a decade. By necessity, we assumed a linear rate of decline for this analysis, but this assumption may not be accurate. It is plausible that the rate of decline is slow until a threshold is reached, at which point the slope of the decline accelerates, and it is equally plausible that the slope of the decline is simply non-linear.
Conclusion
Exercise capacity among those with Fontan physiology is diminished relative to age- and gender-matched normative values. While indices of exercise performance decrease over time, the rate in this cohort is slower than has been previously described. Sub-maximal aerobic exercise capacity is better preserved than maximal aerobic capacity in the Fontan population, but declines at the same rate during the second and third decades of life. Non-obese body composition is associated with better maximal and sub-maximal exercise, while female gender and left ventricular morphology are associated with improved exercise performance at the ventilatory anaerobic threshold. Within the larger group, there exists a subgroup with preserved VO2 at VAT. Future work should focus on understanding the characteristics of this subgroup and on determining whether medical or interventional alterations in management can improve the performance of the entire cohort of those who have undergone the Fontan procedure.
Acknowledgments
Funding Funding for this project was provided by the National Heart, Lung, and Blood Institute (NHLBI U01 HL068270, HL109741, HL109781, HL109816, HL109818, HL109777, HL109778, HL109673, HL109743, HL109737, HL068270). The views expressed are those of the authors, and do not represent official positions of NHLBI or NIH.
Abbreviations
- TCPC
Total cavopulmonary connection
- PHN
Pediatric Heart Network
- RER
Respiratory exchange ratio
- Peak VO2
Peak oxygen consumption
- VO2 at VAT
Oxygen consumption at the ventilator anaerobic threshold
- BMI
Body mass index
Footnotes
Data Availability The data for this study is housed at the Healthcore, which served as the data coordinating center.
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical Approval This study was approved by the institutional review board at each participating institution and complied with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate All participants and/or their guardian provided informed consent and / or assent, as indicated.
Consent for Publication Disclosure of plan for publication was included in the informed consent, if required by individual participating institutions.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bridges ND, Lock JE, Castaneda AR (1990) Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 82:1681–1689 [DOI] [PubMed] [Google Scholar]
- 2.de Leval MR, Kilner P, Gewillig M, Bull C (1988) Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thoracic Cardiovasc Surg 96:682–695 [PubMed] [Google Scholar]
- 3.DeLeon SY, Idriss FS, Ilbawi MN, Muster AJ, Paul MH, Cole RB, Riggs TW, Berry TE (1983) The role of the Glenn shunt in patients undergoing the Fontan operation. J Thoracic Cardiovasc Surg 85:669–677 [PubMed] [Google Scholar]
- 4.Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP (1973) An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg 66:613–621 [PubMed] [Google Scholar]
- 6.Zellers TM, Driscoll DJ, Humes RA, Feldt RH, Puga FJ, Danielson GK (1989) Glenn shunt: effect on pleural drainage after modified Fontan operation. J Thorac Cardiovasc Surg 98:725–729 [PubMed] [Google Scholar]
- 7.Norwood WI, Jacobs ML (1993) Fontan’s procedure in two stages. Am J Surg 166:548–551 [DOI] [PubMed] [Google Scholar]
- 8.Lemler MS, Scott WA, Leonard SR, Stromberg D, Ramaciotti C (2002) Fenestration improves clinical outcome of the Fontan procedure: a prospective, randomized study. Circulation 105:207–212 [DOI] [PubMed] [Google Scholar]
- 9.Hirsch JC, Goldberg C, Bove EL, Salehian S, Lee T, Ohye RG, Devaney EJ (2008) Fontan operation in the current era: a 15-year single institution experience. Ann Surg 248:402–410 [DOI] [PubMed] [Google Scholar]
- 10.Khairy P, Fernandes SM, Mayer JE Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ (2008) Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 117:85–92 [DOI] [PubMed] [Google Scholar]
- 11.Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, Rush CH, Gaynor JW, Goldberg DJ (2012) 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol 60:1018–1025 [DOI] [PubMed] [Google Scholar]
- 12.d’Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, Bullock A, Justo RN, Grigg LE, Sholler GF, Hope S, Radford DJ, Gentles TL, Celermajer DS, Winlaw DS (2014) Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 130:S32–S38 [DOI] [PubMed] [Google Scholar]
- 13.Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O’Leary PW, Driscoll DJ, Cetta F (2015) 40-year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol 66:1700–1710 [DOI] [PubMed] [Google Scholar]
- 14.Downing TE, Allen KY, Glatz AC, Rogers LS, Ravishankar C, Rychik J, Faerber JA, Fuller S, Montenegro LM, Steven JM, Spray TL, Nicolson SC, Gaynor JW, Goldberg DJ (2017) Long-term survival after the Fontan operation: Twenty years of experience at a single center. J Thorac Cardiovasc Surg 154(243–253):e2. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham JW, Nathan AS, Rhodes J, Shafer K, Landzberg MJ, Opotowsky AR (2017) Decline in peak oxygen consumption over time predicts death or transplantation in adults with a Fontan circulation. Am Heart J 189:184–192 [DOI] [PubMed] [Google Scholar]
- 16.Inuzuka R, Diller GP, Borgia F, Benson L, Tay EL, Alonso-Gonzalez R, Silva M, Charalambides M, Swan L, Dimopoulos K, Gatzoulis MA (2012) Comprehensive use of cardiopulmonary exercise testing identifies adults with congenital heart disease at increased mortality risk in the medium term. Circulation 125:250–259 [DOI] [PubMed] [Google Scholar]
- 17.Fernandes SM, Alexander ME, Graham DA, Khairy P, Clair M, Rodriguez E, Pearson DD, Landzberg MJ, Rhodes J (2011) Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis 6:294–303 [DOI] [PubMed] [Google Scholar]
- 18.Diller GP, Giardini A, Dimopoulos K, Gargiulo G, Muller J, Derrick G, Giannakoulas G, Khambadkone S, Lammers AE, Picchio FM, Gatzoulis MA, Hager A (2010) Predictors of morbidity and mortality in contemporary Fontan patients: results from a multi-center study including cardiopulmonary exercise testing in 321 patients. Eur Heart J 31:3073–3083 [DOI] [PubMed] [Google Scholar]
- 19.Giardini A, Specchia S, Tacy TA, Coutsoumbas G, Gargiulo G, Donti A, Formigari R, Bonvicini M, Picchio FM (2007) Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol 99:1462–1467 [DOI] [PubMed] [Google Scholar]
- 20.Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, Johansson B, Bouzas B, Mullen MJ, Poole-Wilson PA, Francis DP, Gatzoulis MA (2005) Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 112:828–835 [DOI] [PubMed] [Google Scholar]
- 21.Franco V (2011) Cardiopulmonary exercise test in chronic heart failure: beyond peak oxygen consumption. Curr Heart Fail Rep 8:45–50 [DOI] [PubMed] [Google Scholar]
- 22.Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, Schneider S, Schwarz A, Senges J (2002) Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 106:3079–3084 [DOI] [PubMed] [Google Scholar]
- 23.Tsai HY, Tsai WJ, Kuo LY, Lin YS, Chen BY, Lin WH, Shen SL, Huang HY (2018) Oxygen consumption at anaerobic threshold predicts cardiac events after heart transplantation. Transplant Proc 50:2742–2746 [DOI] [PubMed] [Google Scholar]
- 24.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ, Pediatric Heart Network I (2008) Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 52:85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J, Pediatric Heart Network I (2008) A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 52:99–107 [DOI] [PubMed] [Google Scholar]
- 26.Fernandes SM, McElhinney DB, Khairy P, Graham DA, Landzberg MJ, Rhodes J (2010) Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatr Cardiol 31:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giardini A, Hager A, Pace Napoleone C, Picchio FM (2008) Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg 85:818–821 [DOI] [PubMed] [Google Scholar]
- 28.Jenkins PC, Chinnock RE, Jenkins KJ, Mahle WT, Mulla N, Sharkey AM, Flanagan MF (2008) Decreased exercise performance with age in children with hypoplastic left heart syndrome. J Pediatr 152:507–512 [DOI] [PubMed] [Google Scholar]
- 29.Atz AM, Zak V, Mahony L, Uzark K, D’Agincourt N, Goldberg DJ, Williams RV, Breitbart RE, Colan SD, Burns KM, Margossian R, Henderson HT, Korsin R, Marino BS, Daniels K, McCrindle BW, Pediatric Heart Network I (2017) Longitudinal outcomes of patients with single ventricle after the Fontan procedure. J Am Coll Cardiol. 69:2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atz AM, Zak V, Mahony L, Uzark K, Shrader P, Gallagher D, Paridon SM, Williams RV, Breitbart RE, Colan SD, Kaltman JR, Margossian R, Pasquali SK, Allen K, Lai WW, Korsin R, Marino BS, Mirarchi N, McCrindle BW, Pediatric Heart Network I (2015) Survival data and predictors of functional outcome an average of 15 years after the Fontan procedure: the pediatric heart network Fontan cohort. Congenit Heart Dis 10:E30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giardini A, Odendaal D, Khambadkone S, Derrick G (2011) Physiologic decrease of ventilatory response to exercise in the second decade of life in healthy children. Am Heart J 161:1214–1219 [DOI] [PubMed] [Google Scholar]
- 32.Navaratnam D, Fitzsimmons S, Grocott M, Rossiter HB, Emmanuel Y, Diller GP, Gordon-Walker T, Jack S, Sheron N, Pappachan J, Pratap JN, Vettukattil JJ, Veldtman G (2016) Exercise-induced systemic venous hypertension in the Fontan circulation. Am J Cardiol 117:1667–1671 [DOI] [PubMed] [Google Scholar]
- 33.Gewillig M, Goldberg DJ (2014) Failure of the Fontan circulation. Heart Fail Clin 10:105–116 [DOI] [PubMed] [Google Scholar]
- 34.Goldberg DJ, Avitabile CM, McBride MG, Paridon SM (2013) Exercise capacity in the Fontan circulation. Cardiol Young 23:824–830 [DOI] [PubMed] [Google Scholar]
- 35.Malhotra R, Bakken K, D’Elia E, Lewis GD (2016) Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 4:607–616 [DOI] [PubMed] [Google Scholar]
- 36.Cordina R, Celermajer DS, d’Udekem Y (2018) Lower limb exercise generates pulsatile flow into the pulmonary vascular bed in the setting of the Fontan circulation. Cardiol Young 28:732–733 [DOI] [PubMed] [Google Scholar]
- 37.O’Byrne ML, Desai S, Lane M, McBride M, Paridon S, Goldmuntz E (2017) Relationship between habitual exercise and performance on cardiopulmonary exercise testing differs between children with single and biventricular circulations. Pediatr Cardiol 38:472–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avitabile CM, Leonard MB, Zemel BS, Brodsky JL, Lee D, Dodds K, Hayden-Rush C, Whitehead KK, Goldmuntz E, Paridon SM, Rychik J, Goldberg DJ (2014) Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart 100:1702–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]