Abstract
Background:
The disproportionate burden of more aggressive breast cancer subtypes among African American/Black women may stem from multilevel determinants. However, data are limited regarding the impacts of neighborhood social environmental characteristics among Black women.
Methods:
We evaluated the association between neighborhood-level socioeconomic status (nSES) and breast cancer subtypes in the Women’s Circle of Health and Women’s Circle of Health Follow-up Study, which included 1,220 Black women diagnosed from 2005 to 2017 with invasive breast cancer. nSES at diagnosis was measured using NCI’s census tract-level SES index. We used multilevel multinomial logistic regression models to estimate the association of nSES with breast cancer subtypes [triple-negative breast cancer (TNBC), HER2-positive vs. luminal A], adjusting for individual-level SES, body mass index, and reproductive factors. We tested for interactions by neighborhood racial composition.
Results:
Compared to census tracts characterized as high nSES, the relative risk ratios (RRR) for TNBC were 1.81 (95% CI: 1.20–2.71) and 1.95 (95% CI: 1.27–2.99) for women residing in areas with intermediate and low nSES, respectively (p-trend: .002). Neighborhood racial composition modified the association between nSES and TNBC; the highest relative risk of TNBC was among women residing in low nSES areas with low proportions of Black residents.
Conclusions:
Black women residing in socioeconomically disadvantaged neighborhoods may have an increased risk of TNBC, particularly among areas with lower proportions of Black residents.
Impact:
Places people live may influence breast tumor biology. A deeper understanding of multilevel pathways contributing to tumor biology is needed.
Keywords: Neighborhood socioeconomic status, Neighborhood racial composition, Triple-negative breast cancer, Black women
INTRODUCTION
In the United States, incidence rates of aggressive types of breast cancer, including estrogen receptor-negative (ER-) and triple-negative breast cancer (TNBC), are highest in African American/Black women (hereafter, ‘Black women’) (1). The risk of TNBC continues to be approximately twice as high among Black women as White women (1), which contributes to the large racial disparity in breast cancer mortality (1, 2). Although TNBC is associated with germline BRCA1 mutations, the incidence of BRCA1 mutations is lower among Black than White women (3), suggesting there are other mechanisms that promote more aggressive breast cancer phenotypes among Black women.
While earlier studies have focused on individual-level socioeconomic status (SES) as a major social determinant of breast cancer outcomes, neighborhood social environmental factors may impact breast cancer phenotypes beyond what can be attributed to individual-level SES (4). Black populations are more likely to reside in communities characterized by lower neighborhood-level socioeconomic status (nSES) (5, 6), which may result from intergenerational transmission of lower individual-level SES, housing and mortgage-lending discrimination and other forms of structural racism (6–8). Recent epigenetic research has shown that neighborhood social factors influence methylation patterns of genes that likely contribute to heterogeneity in tumor biology (9–11). Preclinical animal models also support the connection between adverse social environments and altered physiological processes that promote tumor growth in the mammary gland (12–14).
While a few prior studies suggested that Black women residing in socioeconomically deprived neighborhoods may be more likely to develop aggressive breast cancer subtypes (15–18), these studies, except for one (15), were unable to disentangle the effects of aggregated characteristics of individual residents (compositional effect) and the neighborhoods themselves (contextual effect). To our knowledge, no study has examined the association of nSES with TNBC independent of individual-level SES factors among Black women.
Residing in racially or ethnically homogeneous neighborhoods is posited to be associated with greater social support and lower exposure to discrimination (19), indicating a possibility that these neighborhoods may offer a buffering effect against the negative influences of disadvantaged nSES on some health outcomes. Furthermore, women with a higher level of education may be less susceptible to the influence of disadvantaged neighborhoods (20). However, studies are needed to understand if the association between nSES and breast cancer subtypes varies by racial/ethnic composition or individual-level education.
In the present population-based study of Black women with breast cancer, we aimed to evaluate the associations of nSES with breast cancer subtypes, including the relative risk of TNBC compared with luminal A, independent of individual-level sociodemographic, lifestyle, and reproductive factors. We also examined whether the associations were modified by proportion of Black residents and individual-level education.
MATERIALS AND METHODS
Study Population
We analyzed data from the Women’s Circle of Health Study (WCHS) (21) and the Women’s Circle of Health Follow-Up Study (WCHFS) (22), which is an ongoing cohort of Black breast cancer survivors built upon the infrastructure of WCHS. The present study used baseline data from cases recruited in both studies using the same methodology. In brief, eligible cases included English-speaking women aged 20 to 75 years who self-identified as Black, with a newly diagnosed histologically confirmed breast cancer, and who had no prior history of cancer except non-melanoma skin cancer. Cases were identified by rapid case ascertainment in 10 counties of New Jersey by the New Jersey State Cancer Registry (NJSCR). Detailed information on pre-diagnostic individual-level SES, reproductive, and lifestyle factors was collected during baseline in-person interviews, conducted approximately 9 months after diagnosis. The overall response rate was 43%, but 83% of those identified by NJSCR staff as potential participants completed the study, indicating excellent cooperation rate (22, 23). Recruiting minorities, particularly Black participants, for research has historically been challenging (24–26). However, our response rates are comparable to other cohorts of cancer survivors not focused on minorities (27, 28). Furthermore, we found that the distributions of tumor characteristics among participants in our study were very similar to the distributions among all eligible breast cancer cases in the target areas (22), suggesting the current study is representative of all Black women diagnosed with breast cancer in New Jersey.
For this study, we included 1,385 Black women with invasive breast cancer who completed the baseline interview through April 2018 (diagnosed between 2005 and 2017). Among them, ER and PR status were available for 1,369 (98.8%) and HER2 status was available for 1,330 (96.0%). After excluding 57 cases without ER, PR or HER2 status information, 66 cases without complete and valid residential addresses, 28 cases whose nSES scores were unknown, and 14 cases with missing data on covariates, a total of 1,220 cases were included in the analysis. We found no difference with respect to age, education, household poverty level, insurance status, parity, and BMI when comparing women included in the analysis with those who were not. Written informed consent was provided by all the study participants. The study was conducted in accordance with the U.S. Common Rule, and was approved by the Institutional Review Boards at all participating institutions.
Breast Cancer Subtypes
ER, PR and HER2 status were obtained from patients’ pathology reports and NJSCR. Subtypes were classified into three mutually exclusive, clinically-relevant subtypes as follows: luminal A (ER+ or PR+, and HER2-); HER2-positive (HER2+); and TNBC (ER-, PR-, and HER2-). Luminal A, which tends to have a more favorable prognosis than other subtypes, was the reference group in the analysis.
Neighborhood-Level SES (nSES)
We used the census tract-level SES index calculated and made available by the National Cancer Institute’s Surveillance Research Program, which is a time-dependent score constructed via factor analysis of seven variables measuring different aspects of census tract SES. Details of this index were published previously (29). In brief, the census tract-level SES measures were chosen based on Yost et al. (30) and included: education index (weighted school years) (31), percent unemployed, percent working class, median household income, percent below 150% of poverty line, median house value, and median rent. The time-dependent nSES scores based on the 2010 census definitions were generated for each year between 2006 and 2015 and for each SEER catchment area including New Jersey, using a series of American Community Survey five-year data. A greater score indicates a higher census tract-level SES.
Participants’ residential addresses at diagnosis were geocoded to latitude and longitude coordinates by the NJSCR and spatially joined to year 2010 census tracts. The nSES index for each participant was calculated by linking their census tract to the corresponding nSES index for their year of diagnosis, except for cases diagnosed before 2006 or after 2015 where the index for the year 2006 or 2015 respectively was used.
Statistical Analysis
We compared characteristics of study participants by breast cancer subtypes using ANOVA or chi-square test as appropriate. We used multilevel multinomial logistic regression models to estimate relative risk ratios (RRRs) and 95% CIs of HER2-positive and TNBC vs. Luminal A (reference) by levels of nSES. Specifically, we used a random-intercept model with level 1 and level 2 at the individual (n=1,220) and census tract level (n=547), respectively, to account for the geographic clustering of cases. Study participants were categorized into tertiles based on the distribution of nSES scores among luminal A cases with the third tertile (T3) representing the highest nSES and serving as the reference category. A crude model with just nSES was run first. The second model adjusted for the following individual-level covariates: age at diagnosis, education (≤high school, some college, ≥college), household poverty level (calculated based on total household income, number of people supported by that income, and the federal poverty line; <100% federal poverty line, ≥100% federal poverty line, unknown), type of health insurance (private, Medicaid, Medicare, uninsured, other/unknown), menopausal status (yes, no), parity/age at first birth/lactation (nulliparous, <25y never breastfed, <25y ever breastfed, ≥25y never breastfed, ≥25y ever breastfed), and BMI (calculated using self-reported weight and height one year prior to diagnosis). The third model additionally adjusted for census tract-level percentage of Black residents (quintile; from 2010 Census data). Other covariates considered were year of diagnosis, country of origin, marital status, age at menarche, oral contraceptive use, smoking status, physical activity level, alcohol consumption, and census tract-level percentage of Hispanic residents. These variables were not included because they did not change the effect estimates (RRR) by >5% using backward elimination and were not significantly associated with breast cancer subtypes in the multivariable-adjusted model. To test the robustness of the results, we adjusted for the census tract-level proportion of non-White residents instead of the proportion of Black residents in the sensitivity analysis.
Because neighborhood racial/ethnic composition and individual-level education may buffer against the influence of disadvantaged neighborhoods (19, 20), we developed a priori hypotheses to examine whether the associations were modified by percentage of Black residents and individual-level education via likelihood ratio tests with and without the product term between nSES and the stratification term. We further evaluated the joint effects of nSES (tertile) and percentage of Black residents (high vs. low defined by the median value of 52.12%) on breast cancer subtypes, with high nSES and high percentage of Black residents as the reference category. Similar joint-effects analysis was conducted for nSES and individual-level education. We also explored if the associations between nSES and breast cancer subtypes were modified by age, country of origin, household poverty level, menopausal status, parity and lactation. We used Stata version 16.0 (StataCorp LP, Texas) for all analyses.
RESULTS
Among the analytic sample, 710 (58.2%) women had luminal A subtype, 231 (18.9%) HER2-positive, and 279 (22.9%) TNBC. Compared to luminal A, women diagnosed with the other subtypes were younger, and more likely to be premenopausal and to have private insurance. TNBC cases were also less likely to be Caribbean-born and to have obtained a college degree (or above), and were more likely to be parous, to have their first birth at younger ages, and to live in census tracts with a higher proportion of Hispanic residents (Table 1).
Table 1.
Baseline Characteristics Among Black Women with Breast Cancer by Tumor Subtype in the WCHS and WCHFS (n = 1,220)
| Breast cancer subtype | |||||||
|---|---|---|---|---|---|---|---|
| Luminal A | HER2-positive | Triple-negative | p-value2 | ||||
| n = 710 (58.2%) | n = 231 (18.9%) | n = 279 (22.9%) | |||||
| n | (%)1 | n | (%)1 | n | (%)1 | ||
| Age (Mean±SD) | 54.8 | ±10.7 | 52.6 | ±11.0 | 52.2 | ±10.7 | <.001 |
| Year of diagnosis | .13 | ||||||
| ≤2010 | 173 | (24.4) | 57 | (24.7) | 85 | (30.5) | |
| >2010 | 537 | (75.6) | 174 | (75.3) | 194 | (69.5) | |
| Country of origin | .033 | ||||||
| U.S. born | 596 | (83.9) | 184 | (79.7) | 240 | (86.0) | |
| Caribbean born | 87 | (12.3) | 39 | (16.9) | 23 | (8.2) | |
| Other | 27 | (3.8) | 8 | (3.5) | 16 | (5.7) | |
| Education | .001 | ||||||
| ≤High school graduate | 295 | (41.5) | 82 | (35.5) | 117 | (41.9) | |
| Some college | 186 | (26.2) | 80 | (34.6) | 102 | (36.6) | |
| ≥College | 229 | (32.3) | 69 | (29.9) | 60 | (21.5) | |
| Household income poverty | .55 | ||||||
| <100% federal poverty line | 119 | (16.8) | 42 | (18.2) | 53 | (19.0) | |
| ≥100% federal poverty line | 545 | (76.8) | 177 | (76.6) | 215 | (77.1) | |
| Unknown | 46 | (6.5) | 12 | (5.2) | 11 | (3.9) | |
| Health insurance | .035 | ||||||
| Private | 370 | (52.1) | 143 | (61.9) | 159 | (57.0) | |
| Medicaid | 104 | (14.6) | 27 | (11.7) | 35 | (12.5) | |
| Medicare | 120 | (16.9) | 22 | (9.5) | 29 | (10.4) | |
| Uninsured | 79 | (11.1) | 26 | (11.3) | 39 | (14.0) | |
| Other/unknown | 37 | (5.2) | 13 | (5.6) | 17 | (6.1) | |
| Marital status | .21 | ||||||
| Married/living as married | 254 | (35.8) | 72 | (31.2) | 108 | (38.7) | |
| Single/other | 456 | (64.2) | 159 | (68.8) | 171 | (61.3) | |
| Post-menopausal | 456 | (64.2) | 128 | (55.4) | 154 | (55.2) | .007 |
| Age at menarche, y | .85 | ||||||
| <12 | 189 | (26.6) | 67 | (29.0) | 74 | (26.5) | |
| 12–13 | 354 | (49.9) | 107 | (46.3) | 133 | (47.7) | |
| >13 | 167 | (23.5) | 57 | (24.7) | 72 | (25.8) | |
| Parity/age at first birth | .017 | ||||||
| Nulliparous | 124 | (17.5) | 43 | (18.6) | 31 | (11.1) | |
| <25 y | 390 | (54.9) | 130 | (56.3) | 184 | (66.0) | |
| ≥25 y | 196 | (27.6) | 58 | (25.1) | 64 | (22.9) | |
| Ever breastfed among parous women | 239 | (40.8) | 86 | (45.7) | 90 | (36.3) | .14 |
| Ever oral contraceptive use | 499 | (70.4) | 161 | (69.7) | 194 | (69.5) | .96 |
| Smoking status | .85 | ||||||
| Never | 413 | (58.2) | 142 | (61.5) | 164 | (58.8) | |
| Former | 167 | (23.5) | 46 | (19.9) | 65 | (23.3) | |
| Current | 130 | (18.3) | 43 | (18.6) | 50 | (17.9) | |
| Any vigorous physical activity | 149 | (21.0) | 51 | (22.1) | 50 | (17.9) | .45 |
| Nondrinkers of alcohol | 406 | (57.2) | 135 | (58.4) | 165 | (59.1) | .86 |
| BMI 1 year before diagnosis (kg/m2, mean±SD) | 31.9 | ±7.0 | 30.6 | ±6.8 | 31.4 | ±7.0 | .16 |
| nSES score3 (mean±SD) | 9473 | ±872 | 9472 | ±901 | 9331 | ±847 | .033 |
| Census tract-level racial/ethnic density | |||||||
| % Black residents (mean±SD) | 50.0 | ±31.0 | 45.2 | ±30.4 | 47.1 | ±31.2 | .10 |
| % White residents (mean±SD) | 32.6 | ±26.1 | 34.4 | ±24.9 | 34.2 | ±25.8 | .32 |
| % Hispanic residents (mean±SD) | 19.2 | ±17.1 | 20.9 | ±18.2 | 23.2 | ±20.1 | .002 |
WCHS, Women’s Circle of Health Study; WCHFS, Women’s Circle of Health Follow-Up Study.
Values are n (%) unless otherwise noted.
ANOVA or chi-square test was used as appropriate
NCI’s census tract-level SES index is a time-dependent score constructed by factor analysis. See Methods for details.
Lower nSES was associated with a higher relative risk of TNBC (relative to luminal A) in crude and multivariable-adjusted models (Table 2). Compared to the crude model, adjusting for individual-level covariates did not materially alter the results while additionally controlling for the census tract-level proportion of Black residents strengthened the associations. Compared to census tracts characterized by high nSES (T3), the RRRs of TNBC subtype were 1.81 (95% CI: 1.20–2.71) and 1.95 (95% CI: 1.27–2.99) for participants residing in census tracts with intermediate (T2) and low nSES (T1) respectively (p-trend: .002). The association of lower nSES with higher relative risk of HER2-positive was marginally significant (RRRT1 vs. T3: 1.54, 95% CI: 0.98–2.40; p-trend: .054). The results were not essentially altered in the sensitivity analysis adjusting for census tract-level proportion of non-White residents. For example, compared to the high nSES (T3), the RRRs for TNBC were 1.81 (95% CI: 1.21–2.70) for T2 and 2.05 (95% CI: 1.31–3.19) for T1 (p-trend: .001).
Table 2.
Relative Risk Ratios (RRRs) and 95% Confidence Intervals (CIs) of Neighborhood Socioeconomic Status (nSES) with Breast Cancer Subtypes
| Tertile of nSES score | |||||
|---|---|---|---|---|---|
| T1 Lowest nSES | T2 Middle nSES | T3 Highest nSES | p-trend | ||
| Luminal A | n (%) | 237 (33.4) | 237 (33.4) | 236 (33.2) | |
| HER2-positive | n (%) | 79 (34.2) | 78 (33.8) | 74 (32.0) | |
| Model 11 | RRR [95% CI] | 1.06 [0.74–1.53] | 1.05 [0.73–1.51] | 1 [reference] | .73 |
| Model 22 | RRR [95% CI] | 1.19 [0.80–1.77] | 1.13 [0.77–1.65] | 1 [reference] | .39 |
| Model 33 | RRR [95% CI] | 1.54 [0.98–2.40] | 1.38 [0.91–2.09] | 1 [reference] | .054 |
| Triple-negative | n (%) | 110 (39.4) | 102 (36.6) | 67 (24.0) | |
| Model 11 | RRR [95% CI] | 1.63 [1.15–2.33] | 1.52 [1.06–2.17] | 1 [reference] | .005 |
| Model 22 | RRR [95% CI] | 1.51 [1.03–2.21] | 1.47 [1.02–2.14] | 1 [reference] | .028 |
| Model 33 | RRR [95% CI] | 1.95 [1.27–2.99] | 1.81 [1.20–2.71] | 1 [reference] | .002 |
Multilevel multinomial logistic regression models were used with luminal A as the reference group. Model 1 was a crude model.
Model 2 adjusted for individual-level covariates: age at diagnosis, education, household poverty level, health insurance, menopausal status, parity/age at first birth/lactation, and BMI 1 year prior to diagnosis.
Model 3 additionally adjusted for census tract-level proportion of Black residents.
Residing in neighborhoods with higher proportions of Black residents was associated with lower relative risks of HER2-positive and TNBC (RRRQ4-highest vs. Q1-lowest: 0.54, 95% CI: 0.34–0.86; p-trend: .003; Table 3). In stratified analysis, we found stronger associations of nSES with HER2-positive and TNBC subtypes among Black women residing in census tracts with lower proportions of Black residents, while no association was observed for those residing in areas with higher proportions of Black residents (p-for-interaction: .051; Supplementary Table 1). The relative risk of TNBC was highest among Black women residing in areas characterized by low nSES and lower proportions of Black residents (Fig. 1).
Table 3.
Relative Risk Ratios (RRRs) and 95% Confidence Intervals (CIs) of Census Tract-Level Proportion of Black Residents with Breast Cancer Subtypes1
| Census tract-level proportion of Black residents | ||||||
|---|---|---|---|---|---|---|
| Q1 (lowest) | Q2 | Q3 | Q4 (highest) | p-trend | ||
| Luminal A | n (%) | 178 (25.1) | 178 (25.1) | 174 (24.5) | 180 (25.4) | |
| HER2-positive | n (%) | 71 (30.7) | 58 (25.1) | 59 (25.5) | 43 (18.6) | |
| RRR [95% CI] | 1 [reference] | 0.74 [0.48–1.15] | 0.79 [0.50–1.23] | 0.50 [0.30–0.83] | .024 | |
| Triple-negative | n (%) | 72 (25.8) | 83 (29.8) | 64 (22.9) | 60 (21.5) | |
| RRR [95% CI] | 1 [reference] | 0.91 [0.60–1.37] | 0.68 [0.43–1.05] | 0.54 [0.34–0.86] | .003 | |
Multilevel multinomial logistic regression models were used with luminal A as the reference group. Model adjusted for age at diagnosis, education, household poverty level, health insurance, menopausal status, parity/age at first birth/lactation, BMI 1 year prior to diagnosis, and census tract-level nSES score.
Fig. 1. Relative Risk Ratios (RRRs) and 95% Confidence Intervals (CIs) for Triple-Negative Breast Cancer by Neighborhood Socioeconomic Status (nSES) and Census Tract-Level Proportion of Black Residents.
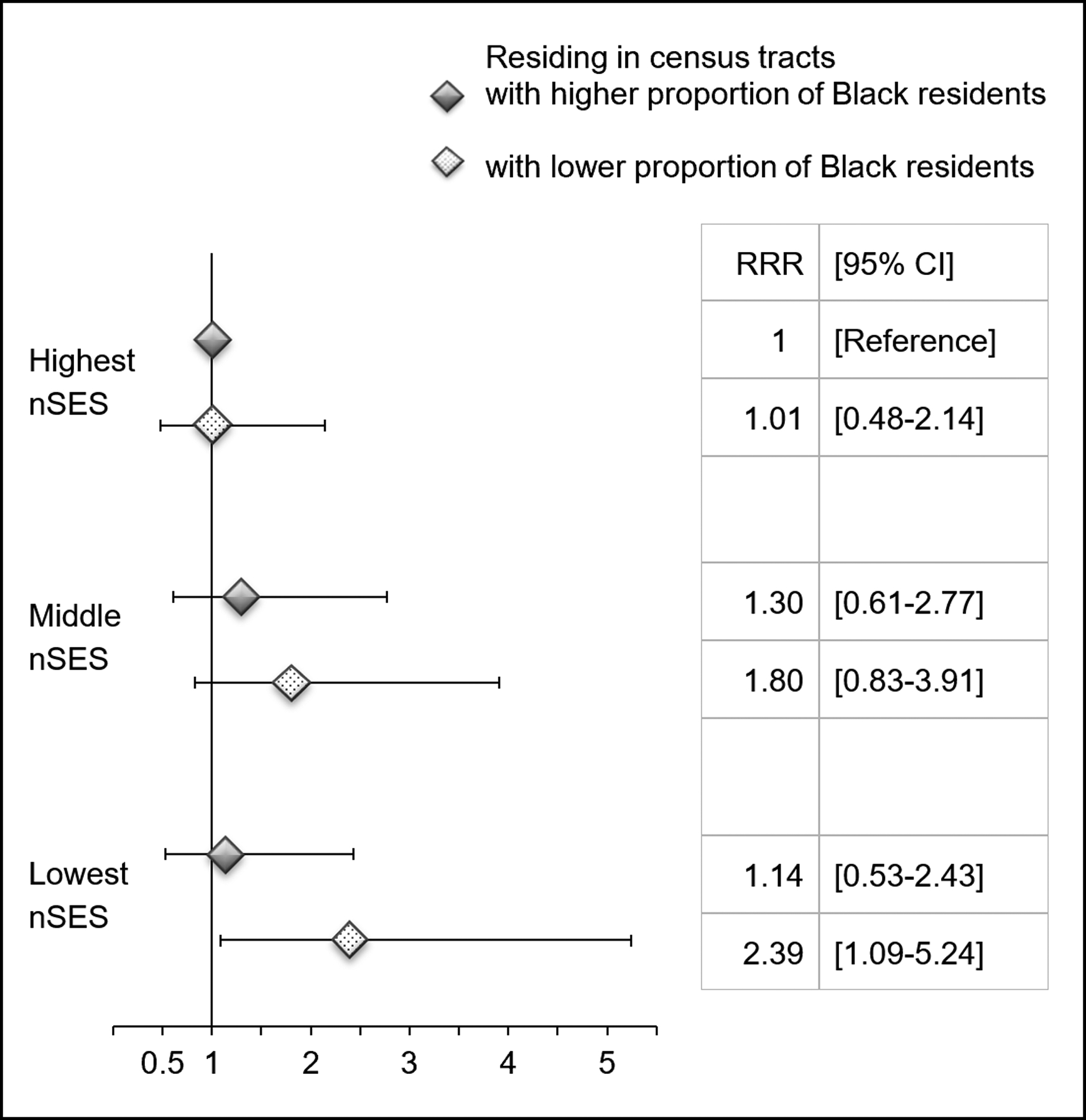
nSES scores were categorized into tertiles. Median value (52.12%) was used to define low vs. high proportion of Black residents. Model adjusted for age at diagnosis, education, household poverty level, health insurance, menopausal status, parity/age at first birth/lactation, and BMI 1 year prior to diagnosis.
In the multivariable-adjusted models including neighborhood-level factors, higher individual-level education (i.e., ≥ college) was not significantly associated with the relative risk of HER2-positive (RRR: 0.99, 95% CI: 0.64–1.54; p-trend: .88) or TNBC (RRR: 0.77, 95% CI: 0.50–1.17; p-trend: .40) compared with the education level of high school or less. Results remained materially unchanged in the model without adjusting for neighborhood-level factors. The interaction tests by education, as well as other individual-level factors (e.g., age, parity) were not significant. However, we found the association between nSES and TNBC was more pronounced among women who had relatively lower individual-level education, based on the stratified (Supplementary Table 2) and the joint-effects analysis (Supplementary Fig. 1). There was a suggestion that the association between nSES and TNBC was stronger among postmenopausal women, but we did not detect any significant interaction by menopausal status (Supplementary Table 3).
DISCUSSION
In this population-based study of Black women with breast cancer, we found that those residing in lower nSES areas had greater relative risk of TNBC. We observed this association only among women residing in lower Black density neighborhoods. Given that TNBC remains more common among Black than White women (1), and the causes are hypothesized to result from multilevel factors (4, 32), research to evaluate multilevel determinants of tumor biology is warranted. To our knowledge, this is the first study to evaluate neighborhood influences on breast cancer subtypes, including TNBC, independent of individual-level sociodemographic, lifestyle and reproductive factors.
Emerging research supports the negative influences of residing in disadvantaged neighborhoods on the risk of ER- or TNBC among Black women. In a population-based study in Chicago comparing Black and White women with breast cancer, the individual- and neighborhood-level SES together accounted for 26% of the racial disparity in ER-/PR- tumors that was not transmitted through reproductive factors (18). In this study, individual-level education, income, and census tract-level concentrated disadvantage and affluence were combined into a single measure. Although not statistically significant, findings using SEER-wide data suggested a decreased risk of TNBC among Black women residing in areas with the highest vs. lowest nSES (17). In addition, a study from the California Cancer Registry found that women in lower nSES areas were more likely to be diagnosed with TNBC (33). These two studies used the NCI’s census tract-level SES index as in our study, and the Yost index (30), respectively. However, since individual-level risk factors are not routinely collected by cancer registries, they could not control for the influence of individual-level SES, reproductive or other risk factors. Therefore, whether these associations were caused by neighborhood contextual characteristics or the aggregated characteristics of the individuals in those areas remained unknown.
The only other study that evaluated the association between nSES and breast cancer subtypes independent of individual-level risk factors was the Black Women’s Health Study (15). The nSES was measured as a score based on indicators of census block-level income and education. Although the authors did not evaluate TNBC, they found that the risk of ER- subtype was 24% lower in the highest quintile of nSES compared to the lowest, which supports our observation for TNBC.
Studies conducted in Chicago showed that residents in disadvantaged neighborhoods were more likely to report a higher level of social isolation, less social support and perceived lack of safety (34, 35), which may induce breast carcinogenesis via altering stress pathways (32, 36). In vivo animal studies showed that restraint stress decreased tumor suppressor p53 function and promoted tumorigenesis including breast cancer (13, 14), demonstrating from basic science studies that there is a link between social isolation and cancer outcomes. Epigenetic data has also demonstrated that methylation patterns vary by neighborhood-level attributes and contribute to stress pathways (9, 10). Given that several TNBC-specific methylated regions have been identified (11), more studies are needed to understand the mechanistic pathways involved in the association of disadvantaged neighborhoods, DNA methylation, and TNBC development. In addition to stress pathways, lack of safe neighborhoods may contribute to lower vitamin D synthesis owing to less time spent outdoors, which was associated with a higher risk of breast cancer including ER- and TNBC among Black women (3, 23).
Another novel finding from our study is that the association between nSES and TNBC risk was observed only among Black women residing in areas with lower proportions of Black residents, suggesting that higher density of the same race/ethnicity may buffer the potential impacts of socioeconomically disadvantaged neighborhoods. Our results also indicate that residing in areas with higher proportions of Black residents was associated with lower relative risk of TNBC, which is consistent with a 2020 report (37). We observed similar results using proportion of non-White residents in lieu of proportion of Black residents. In addition, our results remained unchanged when the percentage of Hispanic residents was additionally adjusted, supporting the robustness of our findings. The percentage of the population from one racial/ethnic group is a frequently used measure of racial/ethnic density, which has been hypothesized to affect cancer outcomes via psychosocial mechanisms (e.g., social support), cultural norms (e.g., health behaviors), and healthcare resources (19). Specific to breast cancer biology, less neighborhood racial/ethnic similarity may be associated with lower social support, greater social isolation, and higher exposure to discrimination (19), which supports our observation that Black women residing in areas with low nSES and lower proportions of Black residents have the highest relative risk of TNBC. Although cultural norms may be another pathway linking racial/ethnic density to cancer biology, our models have controlled for lifestyle and reproductive factors. Our findings suggest that social resources in neighborhoods with greater racial/ethnic similarity may buffer against the stresses associated with residing in disadvantaged neighborhoods (38).
Previous studies did not observe a significant association between individual-level education and breast cancer risk among Black women (15, 39), but none evaluated the association with TNBC when nSES and other individual-level factors were considered. Higher education was not significantly associated with the relative risk of TNBC in our study, which may be due to limited statistical power. There was a suggestion from our study that Black women with higher levels of education may be less susceptible to the negative impact of socioeconomically deprived neighborhoods, possibly because they have more resources to cope with neighborhood-related stressors and they are less influenced by their immediate surroundings.
We could only identify risk factors for more aggressive forms of breast cancer relative to luminal A, and a concern was that the higher relative risk of TNBC among women residing in socioeconomically disadvantaged neighborhoods was due to the lower risk of luminal A in these neighborhoods. A previous study demonstrated that low parity and late age at first birth are the primary factors mediating the positive association between nSES and the risk of ER+ breast cancer (15). We adjusted for these covariates in our analysis. Lifestyle factors including physical activity and alcohol intake may be in the potential common pathways for the neighborhood effects on both subtypes of breast cancer (40, 41). We found that these variables did not influence our results. More epidemiological studies with large samples of racial/ethnic minority women are needed to disentangle the relations between social environment and breast cancer subtypes. Future studies will need to integrate multilevel data (e.g., neighborhood characteristics, stressors, health behaviors, biomarker data) to better understand the pathways through which neighborhood features influence breast tumor biology.
The racial density under study was not able to capture the relative locations of racial/ethnic groups within the area (i.e., residential segregation). Future studies are needed to examine the association between racial residential segregation and TNBC risk. We used NCI’s nSES index to characterize the neighborhood socioeconomic environment, and the index is available only at the census tract level. However, census tracts are designed to be relatively homogenous with respect to population SES and living conditions. It has been shown that census tract-level socioeconomic measures, which performed comparably with census block group measures, could be used to monitor US socioeconomic inequalities in health (42). The NCI’s nSES index was found to be in high agreement with other commonly used nSES indices (e.g., Yost index) in New Jersey (29). Furthermore, we did not have data on residential history. We encourage future studies to examine the impact of early-life neighborhood environment (32). Moves prior to diagnosis were unlikely to influence our results since 70.8% of our participants reported that they had lived in their current neighborhoods for 5 years or more. Excluding participants who had recent moves did not materially alter our findings. Finally, residual confounding cannot be ruled out, as it is an intrinsic limitation of all observational studies.
One major strength of this study is that we were able to recruit a large population-based sample of Black women with breast cancer with relatively complete information on tumor characteristics, which allowed us to study aggressive phenotypes among this understudied group. We also collected detailed information on individual-level SES, reproductive and lifestyle factors. Therefore, we could control for these important breast cancer risk factors and assess the extent to which observed associations were attributable to neighborhood socioeconomic deprivation.
In conclusion, we found that lower nSES was associated with higher relative risk of TNBC among Black women, particularly if the neighborhoods had a lower density of Black residents. These findings imply that places people live may influence breast tumor biology. A deeper understanding of multilevel pathways contributing to tumor biology is needed (43), which may inform more effective strategies to address disparities in breast cancer outcomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Mandi Yu for sharing NCI’s census tract-level socioeconomic status index. We thank all the study participants for their contribution to this study.
Funding support: This work was supported by National Institute on Minority Health and Health Disparities and National Cancer Institute (K99MD013300 to B. Qin, R01CA185623 to E.V. Bandera, K. Demissie, and C.-C. Hong, R01CA100598, P01CA151135 and P30CA016056 to C.B. Ambrosone, K01CA193527 to A.A.M. Llanos, K07CA222158 to J.J. Plascak, P30CA072720 to Rutgers Cancer Institute of New Jersey); the Breast Cancer Research Foundation; a gift from the Philip L. Hubbell family; the New Jersey Alliance for Clinical and Translational Science which was supported by the NIH National Center for Advancing Translational Sciences (NCATS) under Award Number UL1TR0030117; and the New Jersey State Cancer Registry which was supported by the NCI SEER Program (HHSN261201300021I; N01-PC-2013-00021), the National Program of Cancer Registries (NU5U58DP006279-02-00), the New Jersey Department of Health and the Rutgers Cancer Institute of New Jersey. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations list:
- BMI
body mass index
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- NJSCR
New Jersey State Cancer Registry
- nSES
neighborhood-level socioeconomic status
- PR
progesterone receptor
- RRR
relative risk ratio
- TNBC
triple-negative breast cancer
- WCHS
Women’s Circle of Health Study
- WCHFS
Women’s Circle of Health Follow-Up Study
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
REFERENCES
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–51. [DOI] [PubMed] [Google Scholar]
- 2.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol. 2018;36:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, Satariano WA, Glaser SL. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. 2015;121:2314–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson RJ, Sharkey P. Neighborhood selection and the social reproduction of concentrated racial inequality. Demography. 2008;45:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothwell J, Massey DS. Geographic Effects on Intergenerational Income Mobility. Econ Geogr. 2015;91:83–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solon G Intergenerational income mobility in the United States. The American Economic Review. 1992;82:393–408. [Google Scholar]
- 8.Pager D, Shepherd H. The Sociology of Discrimination: Racial Discrimination in Employment, Housing, Credit, and Consumer Markets. Annu Rev Sociol. 2008;34:181–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman LA, Kaljee LM. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA Surg. 2017;152:485–93. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA, Zhao W, Wang X, Ratliff SM, Mukherjee B, Kardia SLR, Liu Y, Roux AVD, Needham BL. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: The Multi-Ethnic Study of Atherosclerosis. Epigenetics. 2017;12:662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stirzaker C, Zotenko E, Song JZ, Qu W, Nair SS, Locke WJ, Stone A, Armstong NJ, Robinson MD, Dobrovic A, Avery-Kiejda KA, Peters KM, French JD, Stein S, Korbie DJ, Trau M, Forbes JF, Scott RJ, Brown MA, Francis GD, Clark SJ. Methylome sequencing in triple-negative breast cancer reveals distinct methylation clusters with prognostic value. Nat Commun. 2015;6:5899. [DOI] [PubMed] [Google Scholar]
- 12.McClintock MK, Conzen SD, Gehlert S, Masi C, Olopade F. Mammary cancer and social interactions: identifying multiple environments that regulate gene expression throughout the life span. J Gerontol B Psychol Sci Soc Sci. 2005;60 Spec No 1:32–41. [DOI] [PubMed] [Google Scholar]
- 13.Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, McClintock MK. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci U S A. 2009;106:22393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, Zhao Y, Wang X, Levine AJ, Hu W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:7013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer JR, Boggs DA, Wise LA, Adams-Campbell LL, Rosenberg L. Individual and neighborhood socioeconomic status in relation to breast cancer incidence in African-American women. Am J Epidemiol. 2012;176:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andaya AA, Enewold L, Horner MJ, Jatoi I, Shriver CD, Zhu K. Socioeconomic disparities and breast cancer hormone receptor status. Cancer Causes Control. 2012;23:951–8. [DOI] [PubMed] [Google Scholar]
- 17.Akinyemiju TF, Pisu M, Waterbor JW, Altekruse SF. Socioeconomic status and incidence of breast cancer by hormone receptor subtype. Springerplus. 2015;4:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauscher GH, Campbell RT, Wiley EL, Hoskins K, Stolley MR, Warnecke RB. Mediation of Racial and Ethnic Disparities in Estrogen/Progesterone Receptor-Negative Breast Cancer by Socioeconomic Position and Reproductive Factors. Am J Epidemiol. 2016;183:884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang CY, Tseng M. Ethnic density and cancer: A review of the evidence. Cancer. 2018;124:1877–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shariff-Marco S, Yang J, John EM, Sangaramoorthy M, Hertz A, Koo J, Nelson DO, Schupp CW, Shema SJ, Cockburn M, Satariano WA, Yen IH, Ponce NA, Winkleby M, Keegan TH, Gomez SL. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2014;23:793–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G, Pawlish K, Godbold J, Furberg H, Fatone A, Valdimarsdottir H, Yao S, Li Y, Hwang H, Davis W, Roberts M, Sucheston L, Demissie K, Amend KL, Tartter P, Reilly J, Pace BW, Rohan T, Sparano J, Raptis G, Castaldi M, Estabrook A, Feldman S, Weltz C, Kemeny M. Conducting Molecular Epidemiological Research in the Age of HIPAA: A Multi-Institutional Case-Control Study of Breast Cancer in African-American and European-American Women. J Oncol. 2009;2009:871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandera EV, Demissie K, Qin B, Llanos AAM, Lin Y, Xu B, Pawlish K, Plascak JJ, Tsui J, Omilian AR, McCann W, Yao S, Ambrosone CB, Hong CC. The Women’s Circle of Health Follow-Up Study: a population-based longitudinal study of Black breast cancer survivors in New Jersey. J Cancer Surviv. 2020;14:331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin B, Xu B, Ji N, Yao S, Pawlish K, Llanos AAM, Lin Y, Demissie K, Ambrosone CB, Hong CC, Bandera EV. Intake of vitamin D and calcium, sun exposure, and risk of breast cancer subtypes among black women. Am J Clin Nutr. 2020;111:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shavers-Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2:31–45. [DOI] [PubMed] [Google Scholar]
- 25.Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. [DOI] [PubMed] [Google Scholar]
- 27.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States). Cancer Causes Control. 2005;16:545–56. [DOI] [PubMed] [Google Scholar]
- 28.Kwan ML, Ambrosone CB, Lee MM, Barlow J, Krathwohl SE, Ergas IJ, Ashley CH, Bittner JR, Darbinian J, Stronach K, Caan BJ, Davis W, Kutner SE, Quesenberry CP, Somkin CP, Sternfeld B, Wiencke JK, Zheng S, Kushi LH. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19:1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25:81–92. [DOI] [PubMed] [Google Scholar]
- 30.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–11. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Deapen D, Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States). Cancer Causes Control. 1998;9:369–80. [DOI] [PubMed] [Google Scholar]
- 32.Williams DR, Mohammed SA, Shields AE. Understanding and effectively addressing breast cancer in African American women: Unpacking the social context. Cancer. 2016;122:2138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. [DOI] [PubMed] [Google Scholar]
- 34.Rankin BH, Quane JM. Neighborhood Poverty and the Social Isolation of Inner-City African American Families. Social Forces. 2000;79:139–64. [Google Scholar]
- 35.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277:918–24. [DOI] [PubMed] [Google Scholar]
- 36.Antonova L, Aronson K, Mueller CR. Stress and breast cancer: from epidemiology to molecular biology. Breast Cancer Res. 2011;13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linnenbringer E, Geronimus AT, Davis KL, Bound J, Ellis L, Gomez SL. Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among Black and White women. Breast Cancer Res Treat. 2020;180:437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajrouch KJ, Reisine S, Lim S, Sohn W, Ismail A. Situational stressors among African-American women living in low-income urban areas: the role of social support. Women Health. 2010;50:159–75. [DOI] [PubMed] [Google Scholar]
- 39.Conroy SM, Shariff-Marco S, Koo J, Yang J, Keegan TH, Sangaramoorthy M, Hertz A, Nelson DO, Cockburn M, Satariano WA, Yen IH, Ponce NA, John EM, Gomez SL. Racial/Ethnic Differences in the Impact of Neighborhood Social and Built Environment on Breast Cancer Risk: The Neighborhoods and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2017;26:541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pizot C, Boniol M, Mullie P, Koechlin A, Boniol M, Boyle P, Autier P. Physical activity, hormone replacement therapy and breast cancer risk: A meta-analysis of prospective studies. Eur J Cancer. 2016;52:138–54. [DOI] [PubMed] [Google Scholar]
- 41.Williams LA, Olshan AF, Hong CC, Bandera EV, Rosenberg L, Cheng TD, Lunetta KL, McCann SE, Poole C, Kolonel LN, Palmer JR, Ambrosone CB, Troester MA. Alcohol Intake and Breast Cancer Risk in African American Women from the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2017;26:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–82. [DOI] [PubMed] [Google Scholar]
- 43.Alvidrez J, Castille D, Laude-Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and Health Disparities Research Framework. Am J Public Health. 2019;109:S16–s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


