Abstract
Oral administration of the adenosine receptor (ADOR) antagonist, 7-methylxanthine (7-MX), reduces both form-deprivation and lens-induced myopia in mammalian animal models. We investigated whether topically instilled caffeine, another non-selective ADOR antagonist, retards vision-induced axial elongation in monkeys. Beginning at 24 days of age, a 1.4% caffeine solution was instilled in both eyes of 14 rhesus monkeys twice each day until the age of 135 days. Concurrent with the caffeine regimen, the monkeys were fitted with helmets that held either −3 D (−3D/pl caffeine, n = 8) or +3 D spectacle lenses (+3D/pl caffeine, n = 6) in front of their lens-treated eyes and zero-powered lenses in front of their fellow-control eyes. Refractive errors and ocular dimensions were measured at baseline and periodically throughout the lens-rearing period. Control data were obtained from 8 vehicle-treated animals also reared with monocular −3 D spectacles (−3D/pl vehicle). In addition, historical comparison data were available for otherwise untreated lens-reared controls (−3D/pl controls, n = 20; +3D/pl controls, n = 9) and 41 normal monkeys. The vehicle controls and the untreated lens-reared controls consistently developed compensating axial anisometropias (−3D/pl vehicle = −1.44 ± 1.04 D; −3D/pl controls = −1.85 ± 1.20 D; +3D/pl controls = +1.92 ± 0.56 D). The caffeine regime did not interfere with hyperopic compensation in response to +3 D of anisometropia (+1.93 ± 0.82 D), however, it reduced the likelihood that animals would compensate for −3 D of anisometropia (+0.58 ± 1.82 D). The caffeine regimen also promoted hyperopic shifts in both the lens-treated and fellow-control eyes; 26 of the 28 caffeine-treated eyes became more hyperopic than the median normal monkey (mean (±SD) relative hyperopia = +2.27 ± 1.65 D; range = +0.31 to +6.37 D). The effects of topical caffeine on refractive development, which were qualitatively similar to those produced by oral administration of 7-MX, indicate that ADOR antagonists have potential in treatment strategies for preventing and/or reducing myopia progression.
Keywords: myopia, hyperopia, emmetropization, caffeine, adenosine receptors, axial length
1. Introduction
Ocular growth and emmetropization are regulated by visual feedback associated with the eye’s refractive state. The vision-dependent cascade that regulates ocular growth and emmetropization begins in the retina where the sign of optical defocus is encoded (i.e., the direction of growth required to eliminate an existing refractive error). Direction specific signals are communicated from the neural retina through the retinal pigment epithelium (RPE), to the choroid, and finally to the sclera, where alterations in the biochemistry and tissue biomechanics produce changes in the eye’s axial elongation rate that normally reduce or eliminate refractive errors (see Troilo et al., 2019 (Troilo, Smith, Nickla, Ashby, Tkatchenko, Ostrin, Gawne, Pardue, Summers, Kee, Schroedl, Wahl & Jones, 2019) for a recent review). It is likely that behavioral and environmental factors that influence the operation of this cascade are responsible for common refractive errors, such as myopia, in children.
Although there are still many gaps in our knowledge concerning the constituent components and the operating characteristics of this vision-dependent cascade, multiple potential cellular, biochemical, and molecular components have been identified in each of the major ocular structures involved in the cascade (Troilo et al., 2019). Identifying and understanding the role of cascade components is valuable because it may be possible to design treatment regimens that control and/or manipulate a given component in a manner that would prevent or eliminate common refractive errors. In this respect, there is strong evidence that cholinergic muscarinic receptors (McBrien, Moghaddam & Reeder, 1993, Raviola & Wiesel, 1985, Stone, Lin & Laties, 1991, Tigges, Iuvone, Fernandes, Sugrue, Mallorga, Laties & Stone, 1999) are a key component in this cascade and that therapeutic treatment strategies employing cholinergic muscarinic receptor antagonists, like atropine (Bedrossian, 1971, Chia, Chua, Wen, Fong, Goon & Tan, 2014, Shih, Chen, Chou, Ho, Lin & Hung, 1999, Wildsoet, Chia, Cho, Guggenheim, Polling, Read, Sankaridurg, Saw, Trier, Walline, Wu & Wolffsohn, 2019) or pirenzepine (Siatkowski, Cotter, Crockett, Miller, Novack, Zadnik & Group, 2008), are effective in reducing the rate of myopia progression in children.
There is growing evidence that adenosine receptors (ADORs) are also elements within the emmetropization cascade or, at the least, can indirectly influence the operating characteristics of the cascade. In primates, all of the known ADOR subtypes have been localized in the neural retina, RPE, choroid and sclera (Beach, Hung, Arumugam, Smith & Ostrin, 2018, Brass, Zarbin & Snyder, 1987, Cui, Trier, Chen, Zeng, Yang, Hu & Ge, 2008, Dong, An, Ren, Yan, Zhou, Lu, Hu, Chen & Qu, 2007, Wan, Cui, Yang, Hu, Li, Hu, Trier & Zeng, 2011). Although it is likely that ADORs are involved in a variety of cellular functions, ADORs have been shown to have physiological effects that are relevant to the emmetropization cascade. For example, ADORs influence the release of retinal neurotransmitters known to be involved in regulating ocular growth (e.g., dopamine and acetylcholine) (Cunha, 2001, Dong et al., 2007, Oliveira & Correia-de-SA, 2005, Salmi, Chergui & Fredholm, 2005, Sohni & Hartwick, 2014). ADORs alter choroidal blood flow (Polska, Ehrlich, Luksch, Fuchsjager-Mayrl & Schmetterer, 2003) and regulate fluid transport across the RPE (Kawahara, Hikichi, Kitaya, Takahashi, Mori & Yoshida, 2005) (i.e., factors that may be involved in vision-dependent changes in choroidal thickness (Zhang & Wildsoet, 2015)), and collagen synthesis by scleral fibroblasts (Cui, Trier, Zeng, Wu, Yu, Hu, Chen & Ge, 2011, Nie, Huo, Yang, Gao, Zeng, Trier & Cui, 2012, Trier, Olsen, Kobayashi & Ribel-Madsen, 1999, Zhou, Huang, An, Lu, Qin, Jiang, Li, Wang, Chen & Qu, 2010). Moreover, visual manipulations that produce axial myopia alter the pattern of ADOR expression in the major ocular tissues involved in the emmetropization cascade (Cui, Trier, Zeng, Wu, Yu & Ge, 2010), and genetic deletion of the A2A receptor has been shown to produce axial myopia in mice (Zhou et al., 2010).
More importantly, at least with respect to potential treatment strategies for myopia, the oral administration of the non-selective ADOR antagonist, 7-methylxanthine (7-MX), has been shown to reduce the axial myopic changes produced by either form deprivation or hyperopic defocus in a variety of mammalian animal models (rabbits (Nie et al., 2012), guinea pigs (Cui et al., 2011), and monkeys (Hung, Arumugam, Ostrin, Patel, Trier, Jong & Smith III, 2018)) and to impede the course of myopia in children, particularly those who, prior to treatment, exhibited relatively moderate rates of myopic progression (Trier, Ribel-Madsen, Cui & Christensen, 2008). 7-MX, which is a metabolite of caffeine, was initially studied as a potential treatment strategy for myopia because it does not readily cross the blood-brain barrier and, consequently, does not have the arousal effects of other adenosine antagonists.
The purpose of this study was to determine if topically instilled caffeine is also effective in retarding axial elongation in infant rhesus monkeys. Caffeine, like 7-MX, is a non-selective ADOR antagonist and a common, well-tolerated dietary element. We chose to investigate caffeine eye drops because caffeine is a more potent adenosine receptor antagonist than 7-MX (Daly, Butts-Lamb & Padgett, 1983), topically applied caffeine readily penetrates the eye (Kronschlager, Forsman, Yu, Talebizadeh, Lofgren, Meyer, Bergquist & Soderberg, 2014, Varma, Kovtun & Hegde, 2010, Yoon & Danesh-Meyer, 2019), and high concentrations of caffeine can be delivered to the eye topically without resulting in blood serum levels that have central nervous system stimulating effects (Kronschlager et al., 2014, Varma et al., 2010), thus reducing potential systemic side effects associated with oral administration.
2. Methods
2.1. Subjects and Rearing Procedures
Fourteen infant rhesus monkeys (Macaca mulatta) that were obtained at 2–3 weeks of age were the primary subjects. Throughout the study, the monkeys were housed in our non-human primate nursery that was illuminated by broadband “white” fluorescent lights (Philips TL735, correlated color temperature = 3500 K; Philips Lighting, Sommerset, NJ, USA) on a 12-hour light/12-hour dark cycle. During the lights-on cycle, which began at 7:00 AM, the illuminances at different locations within the caging area ranged from 342 to 688 lux. At 24 ± 3 days of age, the monkeys were fitted with light-weight helmets that held either −3 D (−3D/pl caffeine monkeys, n = 8) or +3 D spectacle lenses (+3D/pl caffeine monkeys, n = 6) in front of their lens-treated eyes and zero-powered lenses (plano or pl lenses) in front of their fellow-control eyes. Except for the brief daily periods when the lenses were cleaned and the few extra seconds that it took to administer the caffeine eye drops, the helmets were worn continuously until 135 ± 3 days of age. Previous studies have demonstrated that normal infant monkeys consistently demonstrate compensating refractive-error changes in response to these degrees of imposed optical defocus (Hung et al., 2018, Hung, Arumugam, She, Ostrin & Smith III, 2018, Hung, Crawford & Smith III, 1995, Smith III & Hung, 1999, Smith III, Hung, Arumugam & Huang, 2013). Our husbandry and lens-rearing regimens have been described in detail previously (Hung et al., 2018, Smith III & Hung, 1999, Smith III et al., 2013).
Concurrent with the onset of lens wear, one drop of a 1.4% caffeine solution (Greenpark Compounding Pharmacy, Houston, TX, USA) was instilled in both eyes of these lens-reared monkeys twice a day (BID), once at the start of the daily lights-on cycle and once at about 3:30 pm. The treatment drops consisted primarily of caffeine citrate (2.8% caffeine citrate, 1.4% caffeine base), hydroxypropyl methylcellulose, and sterile water. The concentration of the caffeine solution that we employed was slightly higher than that employed in most previous human studies (e.g., 1.4% vs 1.0%) (Chandra, Gaur & Varma, 2011). The solution was buffered, and preservatives were excluded to ensure that the drops were well tolerated by the infant monkeys. Nevertheless, because the ocular fissures of infant monkeys are small, a portion of each drop was blinked out of the eye shortly after installation. Consequently, there is some uncertainty about the exact amount of caffeine that effectively remained in contact with the eye after a given application.
Control data for the eye drop regimen were obtained from an additional 8 infant monkeys that were treated binocularly with the vehicle solution on the same BID schedule as the caffeine-treated monkeys. These vehicle controls also wore −3 D lenses in front of one eye and plano lenses in front of their fellow eyes (−3D/pl vehicle group). Control data for normal emmetropization, lens-induced myopia (LIM) and lens-induced hyperopia (LIH), most of which were reported in previous publications, were available for 41 infant monkeys reared with unrestricted vision (i.e., normal controls; two of these normal controls were studied during the course of this investigation) (Hung et al., 2018, Hung et al., 2018, Hung, Ramamirtham, Huang, Qiao-Grider & Smith III, 2008, Qiao-Grider, Hung, Kee, Ramamirtham & Smith III, 2007, Smith 3rd, Hung, Arumugam, Holden, Neitz & Neitz, 2015, Smith III & Hung, 1999, Smith III, Hung & Huang, 2012) and otherwise untreated infant monkeys reared with either monocular −3 D (n = 20; −3D/pl control group; two of these animals were studied concurrently with the caffeine-treated monkeys) or +3 D treatment lenses (n = 9; +3D/pl control group) (Hung et al., 1995, Smith III & Hung, 1999, Smith III et al., 2013, Smith III, Hung, Huang, Blasdel, Humbird & Bockhorst, 2010, Smith III, Hung, Kee, Qiao-Grider & Ramamirtham, 2003). The rearing procedures, husbandry protocols, measurement methods for the −3D/pl vehicle group, the normal controls, and lens-reared controls were similar in every respect to those used with the caffeine-treated monkeys. The only significant change has been an upgrade to our A-scan ultrasound system. We now use a slightly higher frequency probe (13 MHz vs 7 MHz), but we still employed the same contact measurement methods. New technologies have also been added to the measurement protocol in recent years (e.g., a Spectralis SD-OCT system for measuring choroidal thickness). In these instances, the comparison databases are much smaller, as noted in the text.
2.2. Ocular Biometry
The refractive errors, corneal powers, and axial dimensions were measured for both eyes at ages corresponding to the start of lens wear and subsequently at approximately two-week intervals throughout the lens-rearing period. To make these measurements, the monkeys were anesthetized (intramuscular injection: ketamine hydrochloride, 15–20 mg/kg, and acepromazine maleate, 0.15–0.2 mg/kg; topical: one drop 0.5% tetracaine hydrochloride), and cycloplegia was induced by the topical instillation of 1 drop of 1% tropicamide OU, 25 and 20 minutes prior to making the biometric measures. Each eye’s refractive state along the pupillary axis was measured by retinoscopy independently by two experienced investigators. The results were averaged using matrix notation (Harris, 1988), and the eye’s ametropia (i.e., refractive error) was defined as the spherical-equivalent, spectacle-plane refractive correction (95% limits of agreement = ±0.60 D) (Kee, Hung, Qiao-Grider, Roorda & Smith III, 2004). A hand-held keratometer (Alcon Auto-keratometer: Alcon, Inc., St. Louis, MO, USA) was employed to measure central corneal curvature along the pupillary axis. Three readings were averaged and the mean spherical-equivalent corneal power was calculated using an assumed refractive index of 1.3375 (95% limits of agreement = +0.49 to −0.37 D) (Kee, Hung, Qiao, Habib & Smith III, 2002). In a few instances, the corneas of very young infants had curvatures that were too steep to be measured using the keratometer. In those cases, corneal power was determined using a corneal video topographer (EyeSys 2000; EyeSys Vision, Inc. Houston, TX, USA). The eyes’ axial dimensions, in particular, anterior chamber depth, lens thickness and vitreous chamber depth, were measured by A-scan ultrasonography using a 13-MHz transducer (OTI Scan 1000; Ultra Medical, Saarbruecken-Gersweiler, Germany). Ten separate measurements were averaged (95% limits of agreement for vitreous chamber depth = ±0.05 mm) (Smith III et al., 2012).
Subfoveal choroidal thickness was measured at the onset of lens wear and at several additional time points during the treatment period using spectral-domain optical coherence tomography (SD-OCT; Spectralis, Heidelberg, Germany). The 95% limits of agreement for the average central choroidal thickness were −10.4 to +5.9 μm (for details see Hung et al. (Hung et al., 2018)). Choroidal thickness data were obtained from all of the caffeine-treated monkeys, the vehicle-control monkeys, and 7 normal control animals (some of the normal control data were reported previously in Hung et al. (Hung et al., 2018)).
Intraocular pressure (IOP) was measured at the end of the treatment period in both eyes of 13 caffeine-treated monkeys, the 8 vehicle controls, and 8 control monkeys using either a Tono-pen (Reichert Inc., of Buffalo, NY) or a TonoVet (iCare, Revenio Group Corporation, Finland). Pupil diameters were measured from photographs obtained under typical laboratory lighting during the last month of treatment. The photographs were taken approximately 1 hour after the second daily installation of caffeine or the vehicle solution. Also near the end of the lens-rearing period, blood samples were obtained via femoral vein puncture near the middle of the daily light cycle from 2 normal controls and 6 caffeine-treated monkeys. Assays were performed for serum levels of caffeine and two major caffeine metabolites in monkeys, theophylline and theobromine. We did not assay for paraxanthine, a relatively minor metabolite of caffeine in monkeys (Stavric, R. & Gilbert, 1984).
All rearing and experimental procedures were reviewed and approved by the University of Houston’s Institutional Animal Care and Use Committee and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3. Statistical Methods
Statistical analyses were performed using Minitab (Release 16.2.4, Minitab Inc, State College, PA) and Super ANOVA software (Abacus Concepts, Inc, Berkeley, CA). One-way ANOVAs were also used to examine between-group differences in ocular parameters at ages corresponding to the start and end of lens wear. Because the distribution of refractive errors in normal monkeys at ages corresponding to the end of the treatment period is leptokurtic, nonparametric Mann-Whitney tests were used to compare end-of-treatment refractive errors between normal-control and treated-subject groups. Mixed-design ANOVAs with Greenhouse-Geisser (G-G) corrections for multiple testing were used to compare the longitudinal changes in anisometropia and choroidal thickness. Some cross-sectional between-group analyses were conducted using Student T-tests. Paired-student T-tests were employed to examine the interocular differences in ocular parameters within a given subject group. The relationship between refractive error and the ratio between vitreous chamber depth and anterior corneal radius (both expressed in mm) were characterized using linear regression analysis.
3. Results
3.1. General Observations
The caffeine-treated infants, like the control animals, were observed at approximately two-hour intervals throughout the daily lights-on cycle. At these times, the animals were fed infant formula by bottle until weaning, and the animals were inspected to ensure that the helmets were properly fitted and that the lenses were clean. No differences in behavior or activity levels between caffeine-treated and control animals were apparent on casual observation at any time during the treatment period. There were also no differences in weight gains (normal controls vs caffeine, 0.66 kg vs 0.64 kg; T =0.48, P = 0.64); and at the end of the treatment period, there were no differences in IOP between fellow-control eyes of caffeine monkeys (10.2 ± 2.3 mmHg) and the left eyes of normal controls (11.2 ± 1.8 mmHg; T = 1.17, P = 0.26). In addition, the pupil diameters in the caffeine monkeys (3.54 ± 0.34 mm) were similar to those in the vehicle (3.59 ± 0.48 mm, T = 0.25, P = 0.81) and normal controls (3.79 ± 0.49 mm, T = 1.24, P = 0.24). No alterations in the anterior segment of the caffeine treated eyes were noted on slit-lamp examination, and visual inspection of the OCT images revealed normal retinal organization.
If all of the caffeine in the eye drops had entered the systems of the infant monkeys, the total daily dose for individual animals would have been about 2.8 mg of caffeine (i.e., 4.7 mg/kg at baseline). To determine the levels of caffeine absorbed systemically from the topically instilled drops, blood samples, which were obtained at the midpoint of the lights-on cycle from 6 caffeine-treated infants near the end of the treatment period (mean (±SD) weight = 1.31 ± 0.18 kg; a maximum daily caffeine dose of 2.2 mg/kg) and 2 age-matched controls, were analyzed for methylxanthines. The detection limit for the serum methylxanthine screen was 50 parts per billion (50 ng/ml). No traces of caffeine or the caffeine metabolites, theobromine and theophylline, were detected in either of the two control monkeys. In the caffeine-treated monkeys, the average (±SD) serum levels of caffeine and theophylline were 203 ± 58 ng/ml (range = 154 to 249 ng/ml) and 159 ± 60 (range = 91 to 249 ng/ml), respectively. Trace amounts of theobromine were detected in all six caffeine-treated infants. To put these serum caffeine levels into perspective, a single cup of coffee can provide an effective caffeine dose of 0.4 to 2.5 mg/kg in adult humans and result in an average plasma concentration maximum of 250 to 2000 ng/ml 15 to 120 minutes after consumption (Fredholm, Battig, Holmen, Nehlig & Zvartau, 1999, Gelal, Guven, Balkan, Artok & Benowitz, 2003).
3.2. Refractive Development
At the start of the lens-rearing period, there were no differences in refractive error, corneal power, anterior chamber depth, lens thickness or vitreous chamber depth between any of the subject groups (F = 0.69 to 2.04; P = 0.08 to 0.63). All of the infants in the vehicle- and caffeine-treated groups exhibited the moderate degrees of hyperopia that are typically observed in normal macaques (Qiao-Grider et al., 2007). The average refractive errors for the right eyes of the −3D/pl (+3.92 ± 1.24 D) and +3D/pl caffeine monkeys (+3.94 ± 1.35 D) were comparable to those of the monkeys in the vehicle (+3.45 ± 1.17 D, T = 0.79 and 0.72, P = 0.44 and 0.49) and normal control groups (+3.89 ± 1.79 D, T = 0.18 and 0.18, P = 0.86 and 0.86). The two eyes of individual vehicle- and caffeine-treated animals were also well-matched (see Table 1). At baseline, there were no significant interocular differences in refractive error, corneal power, anterior chamber depth, lens thickness, vitreous chamber depth, or choroidal thickness in either the vehicle controls (P = 0.25 to 0.75) or the −3D/pl (P = 0.33 to 0.84) and the +3D/pl caffeine treatment groups (P = 0.15 to 0.53).
Table 1.
Average (±SD) Baseline and End-of-Treatment Refractive Errors and Biometric Measures
| Group | Refractive Error (D) | Corneal Power (D) | Vitreous Chamber Depth (mm) | Vitreous Chamber / Corneal Radius Ratio | Choroidal Thickness (μm) |
|---|---|---|---|---|---|
| Baseline | |||||
| Vehicle −3D/pl | |||||
| Treated eye | +3.45 ± 1.17 | 63.17 ± 1.26 | 8.32 ± 0.30 | 1.557 ± 0.044 | 124.0 ± 22.0 |
| Fellow eye | +3.48 ± 1.14 | 63.27 ± 1.21 | 8.30 ± 0.32 | 1.554 ± 0.043 | 125.9 ± 20.6 |
| Caffeine −3D/pl | |||||
| Treated eye | +3.92 ± 1.24 | 61.40 ± 1.62 | 8.49 ± 0.25 | 1.543 ± 0.037 | 132.4 ± 15.9 |
| Fellow eye | +3.93 ± 1.17 | 61.44 ± 1.33 | 8.51 ± 0.23 | 1.559 ± 0.025 | 131.7 ± 15.5 |
| Caffeine +3D/pl | |||||
| Treated eye | +3.94 ± 1.35 | 62.01 ± 2.25 | 8.52 ± 0.43 | 1.563 ± 0.033 | 134.3 ± 20.8 |
| Fellow eye | +3.84 ± 1.27 | 61.85 ± 2.44 | 8.50 ± 0.45 | 1.555 ± 0.040 | 131.1 ± 26.1 |
| Normal Controls | |||||
| Right Eye | +3.83 ± 1.77 | 61.52 ± 2.03 | 8.62 ± 0.30 | 1.572 ± 0.056 | 121.9 ± 19.3 |
| Left Eye | +3.80 ± 1.70 | 61.59 ± 1.90 | 8.62 ± 0.30 | 1.573 ± 0.053 | 119.8 ± 15.9 |
| End of Treatment | |||||
| Vehicle −3D/pl | # | # | # | ||
| Treated eye | +1.92 ± 2.02 | 57.51 ± 1.24 | 9.63 ± 0.45 | 1.640 ± 0.069 | 159.7 ± 34.3 |
| Fellow eye | +3.36 ±1.15 | 57.69 ± 1.49 | 9.35 ± 0.36 | 1.598 ± 0.052 | 167.5 ± 34.2 |
| Caffeine −3D/pl | |||||
| Treated eye | +4.60 ± 1.96 | 56.28 ± 1.60 | 9.49 ± 0.28 | 1.582 ± 0.060 | 164.8 ± 15.9 |
| Fellow eye | +4.02 ± 1.03 | 56.45 ± 1.64 | 9.50 ± 0.34 | 1.587 ± 0.037 | 157.5 ± 14.5 |
| Caffeine +3D/pl | # | # | # | # | |
| Treated eye | +5.68 ± 1.74 | 55.82 ± 2.05 | 9.31 ± 0.53 | 1.537 ± 0.052 | 176.1 ± 19.6 |
| Fellow eye | +3.75 ± 2.38 | 55.99 ± 2.11 | 9.67 ± 0.62 | 1.602 ± 0.079 | 156.0 ± 24.9 |
| Normal Controls | |||||
| Right Eye | +2.42 ± 0.91 | 55.89 ± 1.59 | 9.76 ± 0.29 | 1.618 ± 0.046 | 143.6 ± 27.4 |
| Left Eye | +2.35 ± 0.97 | 55.97 ± 1.64 | 9.75 ± 0.31 | 1.619 ± 0.049 | 142.4 ± 21.8 |
Significant interocular difference (P < 0.05)
As reported previously, the optically imposed anisometropias consistently produced compensating interocular differences in refractive error in the lens-reared controls (see Hung et al. (Hung et al., 2018) for examples of compensating refractive-error changes for individual lens-reared control animals as well as end-of-treatment summary data). Seventeen of the 20 infants in the −3D/pl control group developed relative myopic refractive errors in their treated eyes that were at least −0.50 D in magnitude. All 9 of the +3D/pl controls exhibited relative hyperopic refractive errors of at least +0.50 D in their treated eyes that reduced the degree of imposed anisometropia.
The topical vehicle regimen did not prevent the development of lens-induced myopic anisometropia. In Figure 1, the spherical-equivalent refractive errors are plotted as a function of age for the treated (filled symbols) and fellow eyes (open symbols) of individual −3D/pl vehicle-control monkeys. For reference, the thin solid lines in each panel show the longitudinal refractive errors for individual normal control monkeys. Although two vehicle controls (Fig 1A and B) manifested only small degrees of relative myopia in their treated eyes at the end of the treatment period (>−0.5 D), the other six vehicle controls exhibited obvious and consistent compensating myopic changes in their treated eyes that were similar to those typically observed in the negative-lens-reared controls.
Figure 1.

Spherical-equivalent, spectacle-plane refractive corrections plotted as a function of age for vehicle control monkeys reared with −3 D lenses in front of their treated eyes (filled symbols) and zero-powered lenses in front of their fellow eyes (open symbols). The first and last symbols in each individual plot represent the start and end of the treatment period, respectively. The thin solid lines in each function represent data for the right eyes of the 41 normal control monkeys. The panels are arranged from A to H according to the degree of relative myopic anisometropia observed at the end of the lens-rearing period. Comparable plots showing longitudinal refractive-error data for individual −3D/pl lens-reared control animals can be found in Figure 1 of Hung et al.(Hung et al., 2018)
In contrast, as shown in Figure 2, which shows the longitudinal spherical-equivalent refractive errors for the treated (filled symbols) and fellow eyes (open symbols) of individual −3D/pl caffeine monkeys, the topical caffeine regimen greatly reduced the likelihood that the animals reared with −3 D of imposed monocular hyperopic defocus developed compensating myopic ametropias in their treated eyes. Only two of the eight −3D/pl caffeine monkeys developed compensating myopic anisometropias (panels 2G and 2H). In contrast to the consistent pattern of refractive compensation exhibited by the −3D/pl vehicle and lens-reared controls, there were no systematic interocular differences in the refractive errors of three of the −3D/pl caffeine monkeys (panels 2D–2F) and three of the animals in this group exhibited increasing relative hyperopic ametropias in their lens-treated eyes (panels 2A–2C). These relative hyperopic changes are particularly significant because the resulting anisometropias in these −3D/pl caffeine monkeys increased the degree of hyperopic defocus that the lens-treated eyes experienced.
Figure 2.
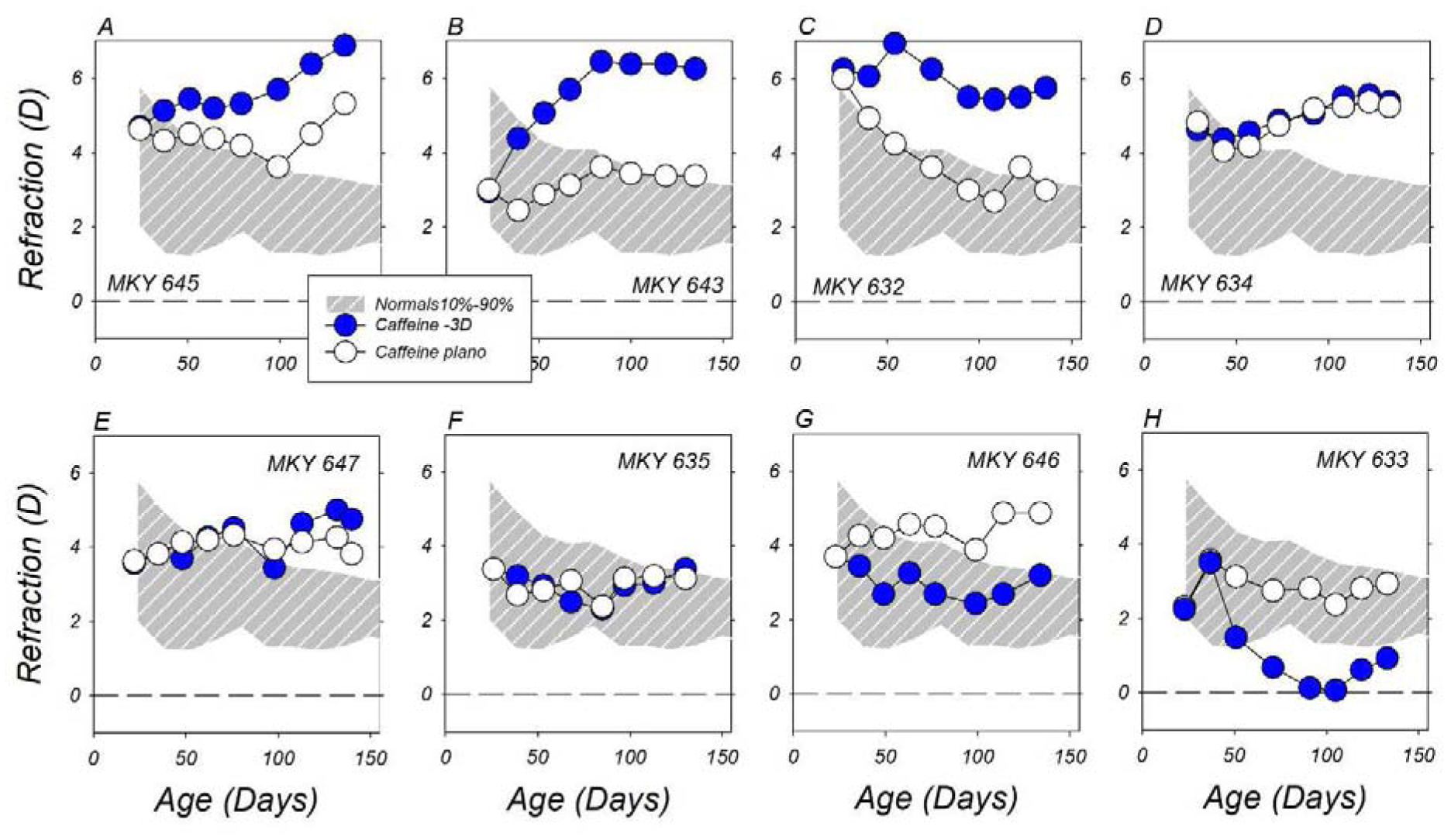
Spherical-equivalent, spectacle-plane refractive corrections plotted as a function of age for caffeine-treated monkeys reared with −3 D lenses in front of their treated eyes (filled symbols) and zero-powered lenses in front of their fellow eyes (open symbols). The first and last symbols in each individual plot represent the start and end of the treatment period, respectively. The thin solid lines in each function represent data for the right eyes of the 41 normal control monkeys. The panels are arranged from A to H according to the degree of relative myopic anisometropia observed at the end of the lens-rearing period.
On the other hand, the topical caffeine regimen did not prevent compensating refractive changes to monocular positive-powered lenses (Figure 3). All six +3D/pl caffeine-treated monkeys developed relative hyperopic refractive errors in their lens-treated eyes. These hyperopic anisometropias, like those observed in the lens-reared controls, emerged early in the treatment period and were relatively stable over the last half of the observation period. The smallest hyperopic anisometropia was observed in the animal (MKY F691, panel 3F) that showed large hyperopic refractive shifts in both eyes.
Figure 3.
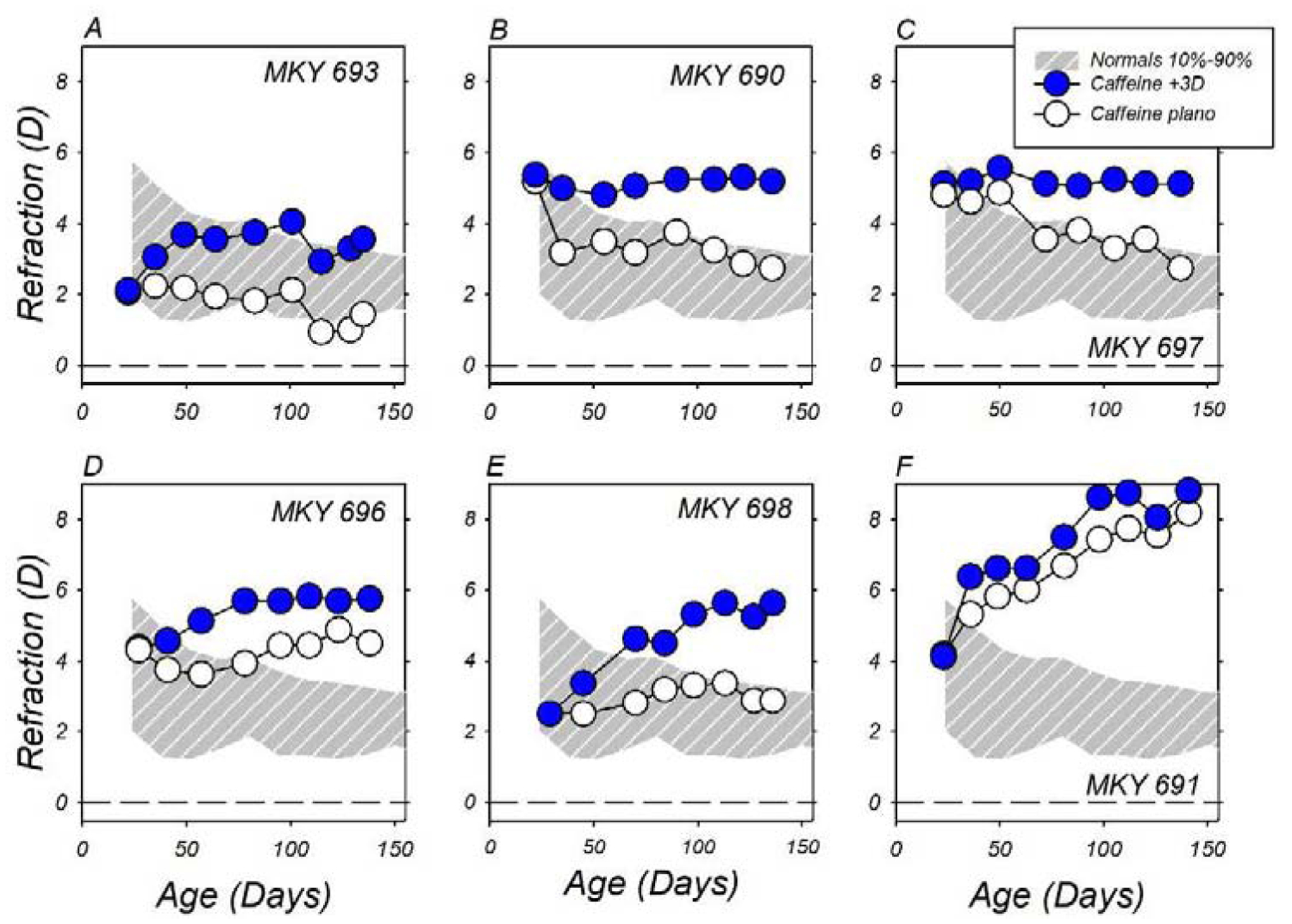
Spherical-equivalent, spectacle-plane refractive corrections plotted as a function of age for caffeine-treated monkeys reared with +3 D lenses in front of their lens-treated eyes (filled symbols) and zero-powered (plano) lenses in front of their fellow eyes (open symbols). The first and last symbols in each individual function represent the start and end of the treatment period, respectively. The thin solid lines in each plot represent data for the right eyes of the 39 normal control monkeys. The panels are arranged from A to F according to the degree of hyperopia observed in the lens-treated eyes at the end of the lens-rearing period.
Figure 4 summarizes the effects of the caffeine regimen on the interocular differences in refractive error that are normally produced by the lens-rearing procedures. The magnitude of anisometropia (lens-treated eye ametropia – fellow eye ametropia) is plotted as a function of age for individual −3D/pl vehicle controls (Fig 4A) and the −3D/pl (Fig 4B) and +3D/pl caffeine-treated monkeys (Fig 4 C). The large symbols with error bars to the right in each plot illustrate the mean (±SD) anisometropias at the end of the treatment period for the vehicle controls (cyan fill), the lens-reared controls (red fill) and the caffeine-treated monkeys (blue fill). Qualitatively and quantitatively the longitudinal pattern of anisometropias in the −3D/pl vehicle controls was similar to that observed in the −3D/pl lens-reared controls (F = 0.33, P = 0.80). Like the lens-reared controls the majority of vehicle controls (6 of 8 versus 17 of 20) exhibited anisometropias that were more than 2 SDs more myopic than the mean anisometropia for normal monkeys. At the end of the lens-rearing period, the average anisometropias for the −3D/pl vehicle controls and the −3D/pl lens-reared controls were −1.44 ± 1.04 D and −1.85 ± 1.20 D, respectively (T = 0.90, P = 0.38).
Figure 4.

Interocular differences in refractive error (lens-treated eye – fellow eye) plotted as a function of age for individual −3D/pl vehicle controls (A) and −3D/pl (B) and +3D/pl caffeine monkeys (C). The first and last symbols in each individual function represent the start and end of the treatment period, respectively. The shaded area in each plot represents ±2 SDs of the mean anisometropia for the 41 normal control monkeys. The symbols with errors bars on the right in each plot show the average end-of-treatment anisometropias for the vehicle controls (cyan fill), caffeine-treated monkeys (blue fill) and the lens-reared controls (red fill). See Figure 3 in Hung et al. (Hung et al., 2018) for similar longitudinal plots of anisometropia for the lens-reared control monkeys.
As shown in Figure 4B, the direction and magnitude of anisometropia varied greatly between animals in the −3D/pl caffeine group and the development pattern of anisometropia was very different from that observed in the −3D/pl vehicle (F = 4.61, P = 0.02) and lens-reared control monkeys (F = 7.79, P = 0.0006). While two of the −3D/pl caffeine monkeys exhibited myopic anisometropias that were comparable to those typically observed in the −3D/pl vehicle and lens-reared controls, three of the −3D/pl caffeine monkeys developed hyperopic anisometropias and the other three −3D/pl caffeine monkeys, like normal control monkeys, maintained similar refractive errors in both eyes throughout the treatment period. On the other hand, in the vehicle and lens-reared control animals, the −3D/pl lens-rearing strategy consistently produced compensating myopic anisometropias that were outside the 95% confidence limits for anisometropias in normal monkeys (shaded areas). At the end of the treatment period, the mean anisometropia for the −3D/pl caffeine monkeys (+0.58 ± 1.82 D) was significantly more hyperopic than that exhibited by either the −3D/pl vehicle (T = 2.72, P = 0.02) or lens-reared controls (T = 3.48, P = 0.007).
In contrast, as illustrated in Figure 4C, all of the +3D/pl caffeine monkeys consistently developed compensating hyperopic anisometropias that were outside the 95% confidence limits for normal monkeys. Similarly, all 9 of the +3D/pl control animals developed hyperopic anisometropias that were above the range of anisometropias in age-matched normal monkeys. A mixed-design ANOVA showed that the +3/pl control monkeys and +3D/pl caffeine monkeys had similar developmental patterns of anisometropia (F = 0.38, P = 0.75). At ages corresponding to the end of the treatment period, the mean (±SD) anisometropias for the +3D/pl controls (+1.87 ± 0.55 D) and +3D/pl caffeine-treated monkeys (+1.93 ± 0.82 D) were nearly identical (T = −0.16, P = 0.88).
In comparison to normal control monkeys, the caffeine and lens-rearing regimens in combination altered the course of emmetropization in both the lens-treated and fellow-control eyes, and consequently, the final refractive errors observed at the end of the treatment period. Figures 5A, 5B and 5C compare the changes in refractive error from baseline that took place during the observation period between the right eyes of normal control monkeys and the lens-treated eyes of −3D/pl vehicle controls and the −3D/pl and +3D/pl caffeine-treated monkeys, respectively. As illustrated by the red lines, the median normal control monkey typically became less hyperopic/more myopic during the observation period, i.e., they exhibited emmetropization from their initial hyperopic ametropias. In contrast, the majority of the caffeine-treated eyes showed relatively smaller myopic shifts or they became more hyperopic. At the end of the treatment period, only one treated eye in the −3D/pl caffeine group exhibited refractive-error changes that were at or below the median normal control animal. Over the course of the treatment period, the average changes in refractive error from baseline for the treated eyes of the −3D/pl (+0.67 ± 1.56D, T = 3.22, P = 0.008) and +3D/pl caffeine-treated monkeys (+1.57 ± 1.66 D, T = 3.94, P = 0.006) were significantly more hyperopic than that for the normal controls (mean change = −1.36 ± 1.82 D). Similarly, the lens-treated eyes of the −3D/pl caffeine monkeys exhibited more hyperopic/less myopic changes in refractive error than the lens-treated eyes of the vehicle controls (mean change = −1.52 ± 1.91, T = −2.52, P = 0.03).
Figure 5.

Relative changes in refractive error for the lens-treated eyes of individual vehicle controls (A) and caffeine monkeys reared with −3D (B) and +3D of imposed anisometropia (C) plotted as a function of age. The data were normalized to the refractive corrections at the start of the treatment period. The first and last symbols in each individual function represent the start and end of the treatment period, respectively. The red line in each plot shows the median refractive-error changes observed in normal control animals. (D) Ametropias obtained at ages corresponding to the end of the lens-rearing period for individual animals. The right and left eyes of the normal monkeys are represented by open symbols. For the lens-reared animals, the filled and open symbols represent the lens-treated and control eyes, respectively
Figure 5D illustrates the spherical-equivalent refractive errors for both eyes of individual animals at the end of the observation period. In comparison to normal monkeys (right eye median = +2.44 D), both the lens-treated (+5.19 D, P = 0.002) and fellow-control eyes of the −3D/pl caffeine monkeys (+3.81 D, P = 0.0003) were significantly more hyperopic. On average, the lens-treated eyes of the −3D/pl caffeine monkeys (+4.59 ± 1.96) were also significantly more hyperopic than those of the −3D/pl vehicle (+1.92 ± 2.02, T = 2.69, P = 0.02) and lens-reared controls (+0.97 ± 2.25, T = 4.24, P = 0.001). In addition, the fellow eyes of the −3D/pl caffeine monkeys were significantly more hyperopic than the fellow eyes of −3D/pl lens-reared controls (+4.02 ± 1.03 D vs +2.82 ± 1.60 D, T = 2.34, P = 0.03). Although the fellow eyes of the −3D/pl caffeine monkeys were also more hyperopic than those of the −3D/pl vehicle controls, these differences were not statistically significant (+3.36 ± 1.15 D, T = 1.24, P = 0.25). There were no between-group differences in the final refractive errors for treated and fellow eyes of the −3D/pl vehicle controls and the −3D/pl lens-reared controls (P > 0.05).
At the end of the rearing period, the lens-treated eyes of the +3D/pl caffeine monkeys (+5.41 D, P = 0.0002) were significantly more hyperopic than the eyes of normal monkeys. Although 5 of the 6 fellow eyes of the +3D/pl caffeine monkeys were more hyperopic than the median normal monkey, the ametropias for these fellow eyes were not significantly different from those of normal monkeys (median = +2.81 D, P = 0.06). On average, the final refractive errors for the lens-treated and fellow control eyes of the +3D/pl caffeine monkeys were also statistically similar to those of the +3D/pl lens-reared controls (treated eyes: +5.68 ± 1.71 D vs +4.79 ± 0.92 D, T = 1.15, P = 0.29; fellow eyes: +3.75 ± 2.38 D vs +2.92 ± 0.99 D, T = 0.81, P = 0.45).
3.3. Ocular Components
At the end of the treatment period, the dimensions of the anterior segments of both eyes of the caffeine-treated monkeys were similar to those of the age-matched normal control animals. In particular, there were no between-group differences in the average corneal power (T = −0.03 to 0.80, P = 0.44 to 0.98) or lens thickness (T = 0.79 to 1.98, P = 0.09 to 0.44). The anterior chamber depths of both eyes of the −3D/pl caffeine monkeys and the fellow control eyes of the +3D/pl caffeine monkeys were also not significantly different from those in normal control monkeys (T = −1.96 to −0.40, P = 0.08 to 0.70). However, the anterior chamber depths in lens-treated eyes of +3D/pl caffeine monkeys were marginally shallower than that observed in normal monkeys (2.89 mm vs 3.08 mm; T = −2.39, P = 0.03). This difference may reflect the fact that at the start of the treatment period the anterior chamber depths of the +3D/pl caffeine monkeys were on average shallower, although not statistically, than those of the animals in the other subject groups (see Table 1).
The anisometropias observed in the −3D/pl vehicle controls and +3D/pl caffeine monkeys were associated with alterations in the thickness of the treated eye’s choroid that were similar in nature to the defocus-induced choroidal thickness changes observed in many species (Troilo et al., 2019). As illustrated in Figure 6, which compares the average (±SEM) longitudinal changes in sub-foveal choroidal thickness between subject groups, the normal controls (gray symbols) exhibited age-dependent increases in choroidal thickness. The choroidal thickness in the two eyes of the controls were well matched throughout the observation period and at the end of the observation period, the mean (±SEM) increases in choroidal thickness were 22.9 ± 10.1 μm and 22.5 ± 8.2 μm for the right and left eyes of the normal controls, respectively (T = 0.03, P = 0.98). At the start of the treatment period the choroidal thickness in the two eyes of the vehicle controls were similar (124.1 ± 7.8 μm vs 125.9 ± 7.3 μm, T = −0.67, P = 0.53), however, as illustrated in panel A, the imposed hyperopic defocus produced a rapid and significant relative decrease in choroidal thickness in the lens-treated eyes (cyan symbols). During the first 30 days of the treatment period, the changes in choroidal thickness in the fellow eyes of the vehicle controls (white symbols) were comparable to those in the normal controls, however, the increases in choroidal thickness in the lens-reared eyes were smaller than the fellow-eye changes in 7 of the 8 vehicle controls. As a result the choroidal thickness in the lens-reared eyes at 42 (mean ±SEM = 120.6 ± 9.3 μm vs 142.3 ± 9.03 μm, T = −4.38, P = 0.003) and 56 days of age (128.4 ± 9.6 μm vs 142.2 ± 12.2 μm, T = −3.35, P = 0.01), were significantly thinner, and the interocular differences were larger than any of the mean interocular differences observed in the normal control monkeys (right eye – left eye range = −5.4 to +3.6 μm). As the −3D/pl vehicle controls compensated for the imposed anisometropia, the degree of hyperopic defocus experienced by the lens-treated eye and the interocular differences in choroidal thickness decreased.
Figure 6.
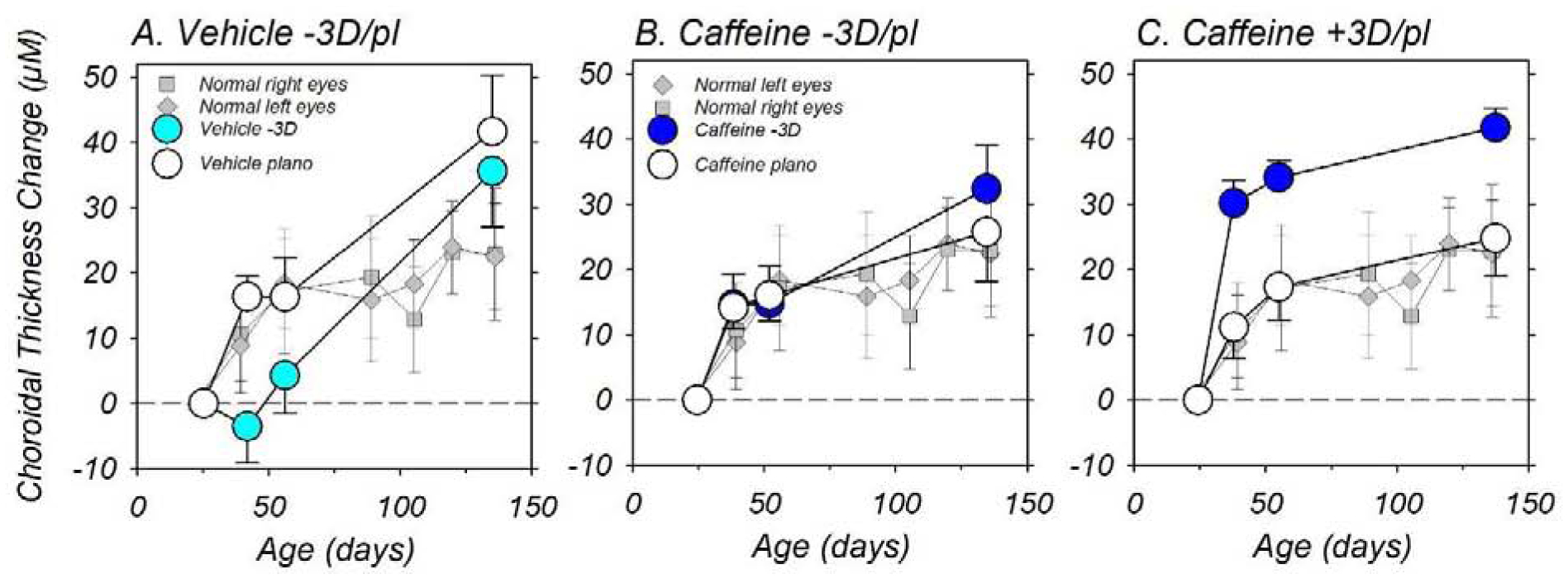
The average (±SEM) relative changes in choroidal thickness for the lens-treated (filled circles) and fellow control eyes (open circles) of the −3D/pl vehicle controls (A) and caffeine-treated monkeys reared with −3 D (B) and +3 D of imposed anisometropia (C). The solid grey squares represent data from control monkeys. The first and last symbols in each individual function represent the start and end of the treatment period, respectively.
The caffeine regimen did not alter the normal age-dependent increases in choroidal thickness. As illustrated in Figures 6B and C, the fellow control eyes of both the +3D/pl (F = 0.27, P = 0.80) and the −3D/pl caffeine treated monkeys (F = 0.57, P = 0.59) exhibited the same pattern of age-dependent increases in choroidal thickness as the normal control monkeys, and at the end of the treatment period the average increases in choroidal thickness were similar to those of the normal control monkeys (−3D/pl caffeine = 25.8 ± 7.6 μm, T = −0.23, P = 0.82; +3D/pl caffeine = 24.9 ± 5.9 μm, T = −0.17, P = 0.87).
As in the other subject groups, there were also no interocular differences in choroidal thickness in the +3D/pl caffeine monkeys at the start of the treatment period (134.3 ± 8.5 μm vs 131.1 ± 10.6 μm, T = 1.25, P = 0.27). However, as shown in Figure 6C, the optically imposed myopic defocus produced a rapid and significant relative increase in choroidal thickness in the lens-reared eyes that was apparent at the first measurement session following the onset of treatment (164.5 ± 8.47 μm vs 142.35 ± 9.72 μm, T = 8.81, P = 0.0001). This relative increase in choroidal thickness was sustained throughout the treatment period despite the fact that these animals compensated for much of the optically imposed defocus.
Interestingly, the caffeine regimen prevented the expected relative reduction in choroidal thickness in the lens-reared eyes of the −3D/pl caffeine monkeys (Fig 6B). Instead, throughout the observation period, the average increases in choroidal thickness in the lens-treated and the fellow-control eyes of the −3D/pl caffeine monkeys were well-matched and very similar to those exhibited by the normal control monkeys.
The axial nature of the refractive errors in the caffeine monkeys is demonstrated in Figure 7. In panel A, the interocular differences in vitreous chamber depth obtained at the end of the treatment period are shown for individual animals in each subject group. As expected, the average vitreous chamber depth in the two eyes of the normal controls were well-matched (open diamonds; 9.76 mm vs 9.75 mm; T = 0.76, P = 0.45). In comparison, both lens-reared control groups (red symbols) showed significant compensating interocular differences in vitreous chamber depth. Specifically, the −3D/pl lens-reared controls exhibited deeper vitreous chambers in their relatively myopic lens-treated eyes (10.33 ± 0.56 mm vs 9.96 ± 0.49 mm; T = 5.69, P < 0.001), whereas the +3D/pl lens-reared controls exhibited shallower vitreous chambers in their relatively hyperopic lens-treated eyes (9.51 ± 0.31 mm vs 9.83 ± 0.31 mm; T = −11.52, P < 0.001). Similarly, the vitreous chambers in the relatively myopic treated eyes of −3D/pl vehicle controls (cyan symbols) were significantly deeper than those for their fellow control eyes (9.63 ± 0.45 mm vs 9.35 ± 0.36; T = 4.54, P = 0.003). The interocular vitreous chamber differences in all three lens-reared control groups were significantly larger than those found in normal monkeys (P < 0.001). For the caffeine-treated monkeys (blue symbols), the animals reared with +3D treatment lenses also had relatively shallower vitreous chambers in their relatively hyperopic lens-treated eyes (9.31 mm vs 9.67 mm; T = −6.54, P = 0.001). The average interocular difference in vitreous chamber depth in the +3D/pl caffeine monkeys was similar in magnitude to that in the +3D/pl lens-reared controls (−0.37 mm vs −0.32 mm; T = 0.77, P = 0.47), but significantly different from the interocular vitreous chamber differences in the normal monkeys (T = 6.51, P = 0.001). On the other hand, the interocular differences in vitreous chamber depth were highly variable within the −3D/pl caffeine monkeys. The average interocular difference for this group was significantly smaller than that found in the −3D/pl vehicle controls (−0.01 ± 0.29 mm vs +0.28 ± 0.17 mm; T = −2.41, P = 0.04) and similar to that for the normal monkeys (T = 0.20, P = 0.85). The high degree of variability in the interocular differences in vitreous chamber depths within this group reflected the inter-subject differences in anisometropia (see Figure 4). For example, in the two −3D/pl caffeine monkeys that developed myopic anisometropias, the vitreous chamber depths were deeper in their lens-treated eyes. On the other hand, in the three −3D/pl caffeine monkeys that developed the large hyperopic anisometropias, the vitreous chamber depths were shallower in their lens-treated eyes.
Figure 7.
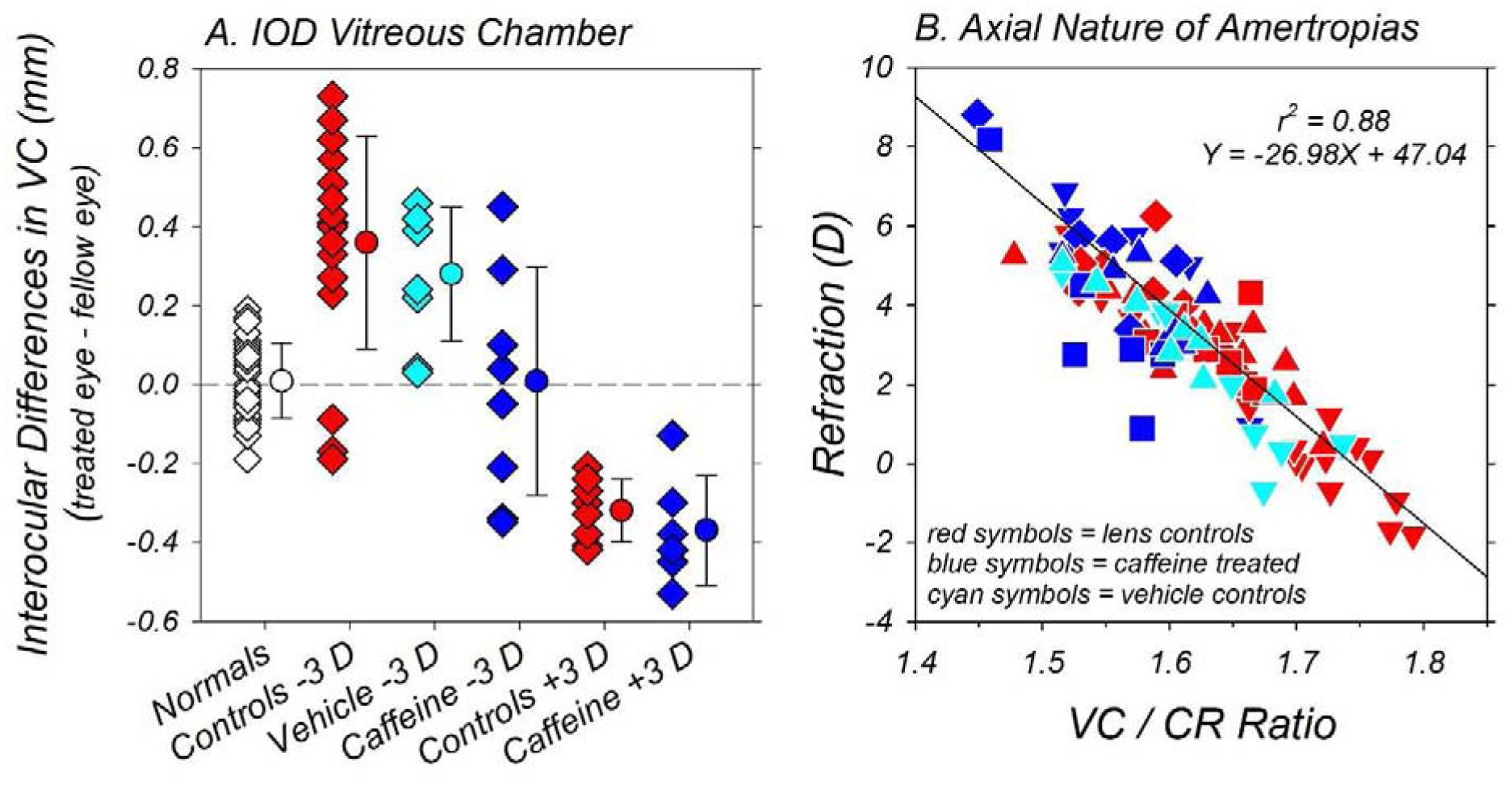
(A) Interocular differences in vitreous chamber depth (right or lens-treated eye – left or fellow eye) obtained at the end of the observation period for individual animals in each subject group. The data for the normal controls, the lens-reared controls, the vehicle controls, and caffeine-treated monkeys are represented by the white, red, cyan and blue filled symbols, respectively. The symbols with errors bars on the right in each plot show the average (±SD) end-of-treatment anisometropias. (B) End-of-treatment ametropias plotted as function of the ratio of the vitreous chamber depth and anterior corneal radius for individual vehicle controls (cyan symbols), lens-reared controls (red symbols) and caffeine-treated monkeys (blue symbols). The lens-treated and fellow eyes of the animals reared with −3D of imposed anisometropia are represented by  and
and  symbols, respectively. The lens-treated and fellow eyes of animals reared with +3D of imposed anisometropia are represented by
symbols, respectively. The lens-treated and fellow eyes of animals reared with +3D of imposed anisometropia are represented by  and
and  symbols, respectively. The solid line represents the best fitting linear regression for all of the data included in the plot.
symbols, respectively. The solid line represents the best fitting linear regression for all of the data included in the plot.
The end-of-treatment refractive errors were correlated with vitreous chamber depth. In Figure 7B, refractive error is plotted as a function of the ratio of vitreous chamber depth and anterior corneal radius (VC/CR) for both eyes of individual caffeine monkeys (blue symbols), vehicle controls (cyan symbols), and lens-reared controls (red symbols). Because neither the lens-rearing procedures nor the caffeine regimen altered corneal power, plotting the VC/CR ratio reduces the effects of the normal inter-subject differences in corneal power on axial growth during emmetropization and provides a clearer indication of how treatment-induced changes in vitreous chamber elongation contributed to an eye’s final refractive state. Linear regression analysis that included all the data in panel B indicated that refractive error was significantly correlated with the VC/CR ratio (r2 = 0.88; P < 0.0001) in the lens-reared monkeys. The more hyperopic eyes typically had lower VC/CR ratios (i.e., relatively shorter vitreous chambers for given corneal power) than the generally less hyperopic/more myopic eyes. The overall pattern of results was similar for all the subjects and the data for each of the subject groups appeared to be adequately described by the regression line.
4. Discussion
The key findings of this study were that topically instilled caffeine, a non-selective adenosine receptor antagonist, eliminated the consistent myopic compensation normally observed in young monkeys reared with imposed hyperopic anisometropia, but did not interfere with the hyperopic compensation in response to imposed myopic anisometropia. In addition, the caffeine regimen promoted absolute hyperopic shifts in the majority of both the lens-treated and fellow-control eyes of the caffeine monkeys. The changes in vision-dependent emmetropization observed in the caffeine-treated monkeys were associated with alterations in choroidal thickness and vitreous chamber elongation rate.
4.1. Topical Caffeine versus Oral 7-Methylxanthine in Monkeys
In infant monkeys, the effects of topical caffeine on vision-dependent emmetropization were qualitatively and quantitatively similar to the effects produced by oral 7-MX. Figure 8 compares the mean (±SEM) refractive errors obtained at the end of the treatment period for normal monkeys, lens-reared controls, vehicle controls, and lens-reared monkeys treated with either topical caffeine or oral 7-MX (Hung et al., 2018). During the treatment period, the 7-MX monkeys received 100 mg/kg of 7-MX by mouth twice per day at the start and end of the daily lights-on cycle. The biometric methods and the lens-rearing procedures were virtually identical for the 7-MX- and caffeine-treated animals. Figure 8 reveals that both ADOR antagonists prevented myopic compensation to imposed hyperopic defocus, and both ADOR antagonists appeared to facilitate the compensating hyperopic shifts produced by imposed myopic defocus. In addition, the average refractive errors in the lens-treated (+4.59 ± 0.69 D) and fellow eyes of the −3D/pl caffeine monkeys (+4.02 ± 0.36 D) were comparable to those in the lens-treated (+4.89 ± 0.96 D; T = −0.25, P = 0.81) and fellow eyes of the 7-MX monkeys reared with −3D of imposed anisometropia (+4.54 ± 0.67 D; T = −0.69, P = 0.50). Similarly there were no significant differences between the average refractive errors of the lens-treated (+5.68 ± 0.70 D vs +6.10 ± 0.70 D; T = −0.43, P = 0.68) and fellow eyes of the caffeine and 7-MX monkeys reared with +3D of imposed anisometropia (+3.75 ± 0.97 D vs +4.31 ± 0.80 D; T = −0.47, P = 0.67). In addition, the average refractive errors for the lens-treated and fellow eyes of both the caffeine- and 7-MX-treated monkeys were more hyperopic than normal control animals and, with the exception of the refractive errors for the fellow eyes of the +3D/pl caffeine monkeys (T = 1.29, P = 0.13), all of these differences were significant (T = 5.07 to 2.23, P = 0.002 to 0.04).
Figure 8.
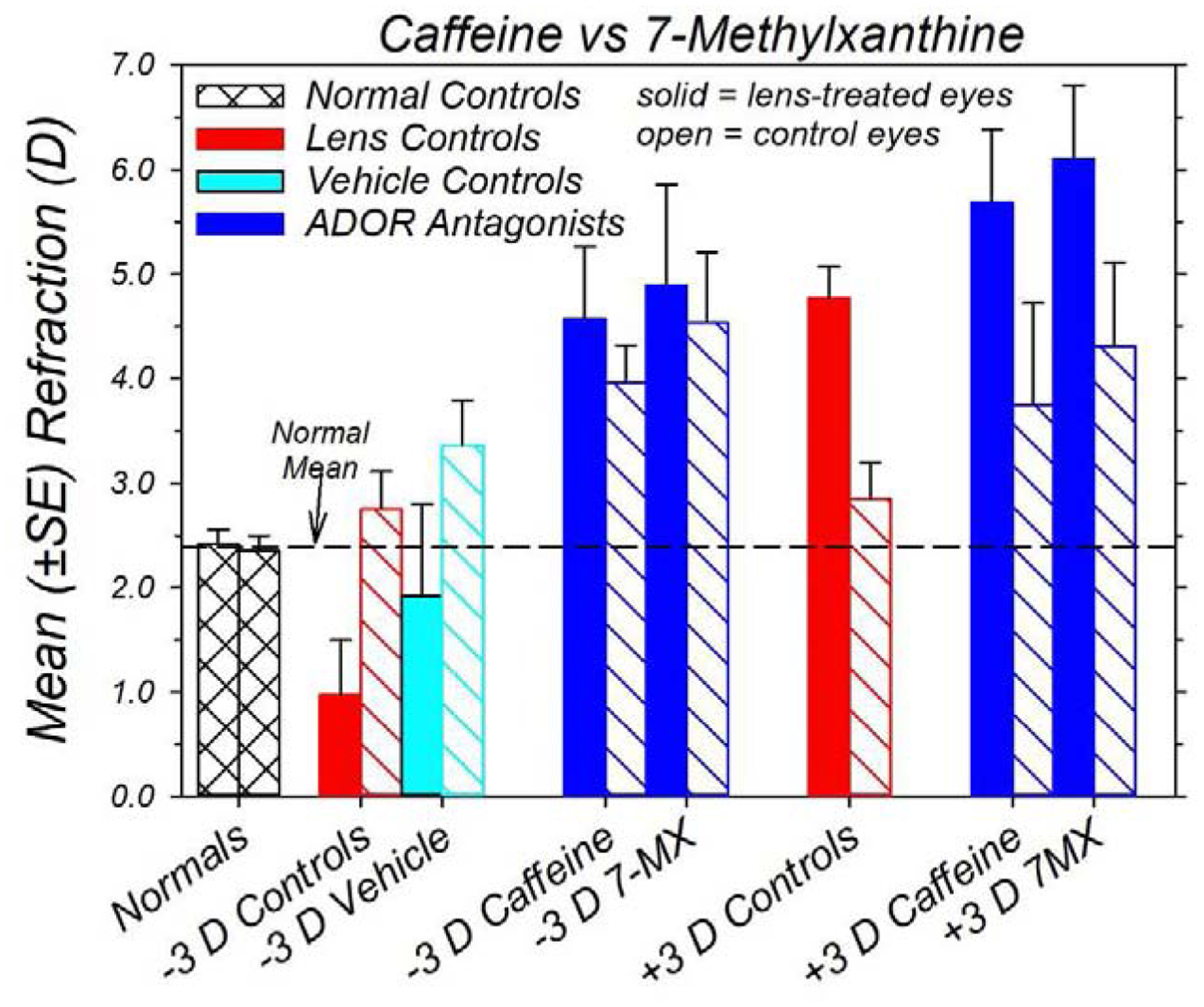
Mean (±SEM) ametropias for both eyes of normal monkeys (n = 41) and the lens-treated (filled bars) and fellow-controls eyes (open bars) for the −3D/pl vehicle controls (cyan fill and outline), lens-reared control monkeys (red fill and outline; −3D/pl, n = 20; +3D/pl, n = 9) and lens-reared monkeys treated with either oral 7-MX (−3D/pl, n = 10; +3D/pl, n = 6) or caffeine (blue fill and outline). The data were obtained at ages corresponding to the end of the lens-rearing period. The 7-MX data are from Hung et al. (Hung et al., 2018). During the treatment period, the 7-MX monkeys received 100 mg/kg of 7-MX by mouth twice per day at the start and end of the daily lights-on cycle.
The ocular component changes responsible for the ametropias in the caffeine and 7-MX monkeys were also similar. Neither the caffeine nor the 7-MX treatments produced systematic alterations in corneal power, anterior chamber depth or lens thickness. On the other hand, the anisometropias and absolute refractive errors observed in both the 7-MX (Hung et al., 2018) and caffeine monkeys were significantly correlated with vitreous chamber depth, and both treatment regimens interfered with the decrease in choroidal thickness normally produced by imposed hyperopic defocus. There was one apparent difference in the effects of oral 7-MX and topical caffeine. Whereas, the caffeine regimen did not alter the age-related increases in choroidal thickness in the fellow control eyes, we previously reported a sustained increase in choroidal thickness in the fellow control eyes of 7-MX monkeys relative to normal control eyes. The absolute age-dependent increases in fellow-eye choroidal thickness in 7-MX and caffeine-treated monkeys were very similar. For instance, the average end-of-treatment increases in fellow-eye choroids in the −3D/pl caffeine and −3D/pl 7-MX monkeys were 24.9 ± 5.9 and 24.4 ± 4.0 μm, respectively. In this study our normal monkey data set was more extensive and the age-dependent increases in choroidal thickness were greater than those observed in our 7-MX study.
Comparisons across studies involving different animal species suggest that the protective effects of 7-MX on vision-induced myopia may be dose dependent (Hung et al., 2018). In this respect, it is interesting that there was good quantitative agreement between the results for the 7-MX and caffeine treatment regimens in monkeys. It seems unlikely that the two different administration regimens would coincidently result in similar dosages of ADOR antagonists. However, it is possible that both treatment regimens resulted in saturated dosage levels.
4.2. ADOR Antagonists: Interspecies Comparisons
In addition to the results from this study, oral administration of the ADOR antagonist 7-MX has previously been shown to reduce vision-induced myopia in guinea pigs (Cui et al., 2011), rabbits (Nie et al., 2012), and monkeys (Hung et al., 2018). The effects of 7-MX have been less consistent in chickens. Oral 7-MX (30 mg/kg) has been reported to produce a small, but significant, reduction in the degree of negative-lens-induced myopia in chickens. However, the same dosage did not reduce the degree of form-deprivation myopia in chickens (Wang K, et al. IOVS 2014; 55:ARVO E-Abstract 3040). More recently, Liu et al (2019) (Liu, Schaeffel, Trier & Feldkaemper, 2019) confirmed that neither oral 7-MX (100 mg/kg, BID) at doses equivalent to those shown to be effective in preventing lens-induced myopia in monkeys nor intravitreal injections of 7-MX altered the course of form-deprivation myopia in chickens. The absence of robust protective effects of ADOR antagonists against myopia in chickens may reflect the structural differences in the scleras of chickens and commonly used mammalian models. Like humans, the scleras of guinea pigs, rabbits, and monkeys are composed of only a fibrous layer. In contrast, while the chicken sclera also has a fibrous layer, it has a much thicker cartilaginous layer (Troilo et al., 2019). As outlined below, there is evidence that suggests that the primary site of the anti-myopia action of ADOR antagonists is on the biochemistry and biomechanics of the fibrous sclera. If that is the case, the failure of 7-MX to suppress form-deprivation myopia in chickens may be due to the dominant cartilaginous component in the chick sclera (Liu et al., 2019).
4.3. Site and Mechanisms of Actions of Caffeine
It is possible that caffeine influences the operational properties of the vision-dependent cascade that normally mediates emmetropization. The fact that the ocular component changes that are responsible for the refractive changes in the caffeine-treated monkeys were identical to those responsible for the compensating ametropias produced by imposed defocus in lens-reared control monkeys (Hung, Wallman & Smith III, 2000, Qiao-Grider, Hung, Kee, Ramamirtham & Smith III, 2010) is in agreement with this hypothesis. The idea that caffeine influences the emmetropization cascade is feasible because all four ADORs subtypes have been identified in the neural retina, the retinal pigment epithelium, the choroid and the sclera (Beach et al., 2018, Brass et al., 1987, Cui et al., 2010), the major ocular components involved in the cascade (Troilo et al., 2019). Moreover, there is evidence that ADORs are directly involved in the emmetropization process. For example, visual manipulations that produce myopia alter ADOR expression in cascade components (Cui et al., 2010) and genetic deletion of selective ADOR subtypes produces axial myopia in mice (Zhou et al., 2010). In addition, there are a number of ways in which ADORs and their antagonists could indirectly influence key cascade components (e.g., by modulating dopamine or acetylcholine transmission (Cunha, 2001, Moo-Puc, Gongora-Alfaro, Alvarez-Cervera, Pineda, Arankowshy-Sandoval & Heredia-Lopez, 2003, Oliveira & Correia-de-SA, 2005, Salmi et al., 2005) or altering ocular circadian rhythms (Sohni & Hartwick, 2014)).
Because 7-MX does not readily penetrate the blood-brain barrier (Shi & Daly, 1999), and presumably not the blood-retina barrier, we previously speculated that the site of action of 7-MX was unlikely to be in the retina, but more likely to be in the RPE, choroid or sclera (Hung et al., 2018). Many of the early studies of the effects of oral 7-MX on refractive development were motivated by the hypothesis that ADOR antagonists directly altered the biochemistry and biomechanics of the fibrous layers of the sclera, making it more resistant to stretch and axial elongation, even in animals reared with unrestricted vision (Trier et al., 1999). In this respect, all four ADOR subtypes have been identified on human scleral fibroblasts (Cui et al., 2008), and animals studies confirmed that 7-MX appeared to strengthen the collagen components of the fibrous sclera, at least in mammalian species (Cui et al., 2011, Nie et al., 2012, Trier et al., 1999). In contrast to 7-MX, topical caffeine readily penetrates the globe (Kronschlager et al., 2014) in concentrations that are sufficient to reduce cataract formation (Kronschlager, Lofgren, Yu, Talebizadeh, Varma & Soderberg, 2013, Varma & Hegde, 2010, Varma et al., 2010), which raises the possibility that retinal components were involved in the refractive changes observed in our caffeine-treated monkeys. However, the similarities between the refractive changes produced by oral 7-MX and topical caffeine suggest that the site and mechanisms of action are likely the same for both treatment regimens. Assuming that oral 7-MX does not reach the retina, it is reasonable to suppose that the effects of topical caffeine were due to actions in the RPE, choroid and/or sclera.
How does topical caffeine reach these potential target sites? There are several potential routes. Caffeine could be absorbed by conjunctival blood vessels and indirectly reach the posterior globe via the vascular system, most likely at a very dilute levels. However, caffeine readily penetrates the cornea and enters the aqueous humor (Kronschlager et al., 2013, Varma et al., 2010, Varma, Kovtun & Hegde, 2011). Once in the eye, given the relatively rapid turnover rate for aqueous in the anterior chamber (Varma et al., 2010), it is unlikely that substantial amounts of caffeine would diffuse directly to the retina. On the other hand, substantial amounts of caffeine could reach the RPE, choroid, and sclera via unconventional, non-trabecular pathways, both the uveoscleral and uveovortex outflow pathways (see Johnson et al. for a review (Johnson, McLaren & Overby, 2017)). Although the largest amount of aqueous leaves the eye via the conventional trabecular meshwork pathway and enters the blood stream, in non-human primates a significant percentage of the aqueous (mean = 37.4% ± 4% in 3 to 23 year-old rhesus monkeys) leaves the eye via these non-trabecular routes (Gabelt, Gottanka, Lutjen-Drecoll & Kaufman, 2003, Johnson et al., 2017), which would deliver topically applied caffeine in a relatively direct manner to the likely target tissues in the posterior globe. A potential advantage of these non-trabecular delivery pathways, particularly the uveoscleral pathway, is that the caffeine would not be rapidly swept away by circulating blood, and thus would be available to the potential target sites for a longer period of time.
Although it seems reasonable to hypothesize that the effects of caffeine and 7-MX are mediated via the vision-dependent mechanisms that normally regulate emmetropization, there are numerous potential ways in which topically instilled caffeine could influence ocular growth and refractive development. For example, it is possible that both 7-MX and caffeine influence refractive develop through non-adenosine receptors (Sanderson, Dartt, Trinkaus-Randall, Pintor, M.M., Delamere, Fletcher, Salt, Grosche & Mitchell, 2014).
4.4. Changes in Choroidal Thickness
In young animals, hyperopic and myopic defocus consistently produce decreases and increases in choroidal thickness, respectively (Hung et al., 2000, Troilo, Nickla & Wildsoet, 2000, Wildsoet & Wallman, 1995). However, in the caffeine-treated monkeys, the hyperopic fellow-control eyes and the negative-lens-treated eyes exhibited normal age-dependent increases in choroidal thickness despite experiencing significant amounts of hyperopic defocus. It appears that the caffeine regimen overrode the normal choroidal response to hyperopic defocus. On the other hand, interocular comparisons in the +3D/pl caffeine monkeys indicated that the caffeine treatment did not prevent the increase in choroidal thickness normally produced by the imposed myopic anisometropia. These choroidal results, along with the differences in anisometropic compensation in the two caffeine-treated groups, suggest that the caffeine regimen selectively impacted mechanisms that normally increase the rate of axial elongation. There is growing evidence that vision-guided ocular growth is mediated by bidirectional mechanisms. For instance, it has recently been shown that imposed hyperopic and myopic defocus affect different sets of retinal genes in monkeys (Tkatchenko, Troilo, Benavente-Perez & Tkatchenko, 2018). Although the site of action of caffeine is unknown, its effects appear to be selective for the direction of axial growth.
Although the changes in choroidal thickness observed in our vehicle controls and the +3D/pl caffeine monkeys were in the appropriate direction, the magnitude of these changes was too small to directly explain the associated refractive-error changes. For example, based on calculations using a schematic eye model for 3-week-old monkeys, (Qiao-Grider et al., 2007) a 22 μm increase in relative choroidal thickness (i.e., the average interocular difference in choroidal thickness observed in the +3D/pl caffeine monkeys at the end of the treatment period) would be expected to produce a hyperopic shift of less than 0.25 D. The dioptric effect of a similar increase in choroidal thickness would be smaller in the longer eyes of monkeys at the end of the treatment period. As evidenced by the interocular differences in vitreous chamber depth in the +3D/pl caffeine monkeys, which were almost 10 times larger than the increases in choroidal thickness, the relative and absolute hyperopic shifts observed in the caffeine monkeys were due to a reduction in the elongation rate of the posterior globe.
In addition to its role in emmetropization, the choroid plays a key role in maintaining the health of the retina. Because variations in choroidal thickness, both increases and decreases, have been associated with retinal disease (Dhoot, Huo, Yuan, Xu, Srivistava, Ehlers, Traboulsi & Kaiser, 2013, Kim, Lee, Joe, Kim & Yoon, 2013, Kim, Oh, Kwon, Yoo & Huh, 2011), there is increasing interest in identifying and understanding factors that influence choroidal thickness. In particular, a number of previous studies have reported that a single oral administration of caffeine or the consumption of coffee, the most common dietary source of caffeine, reduced choroidal thickness in healthy adults. These transient decreases in choroidal thickness in humans were observed minutes after caffeine administration, persisted for several hours, and returned to baseline values within 24 hours (Altinkaynak, Ceylan, Kartal, Keles, Ekinci & Olcaysu, 2016, Vural, Kara, Sayin, Pirhan & Ersan, 2014, Zengin, Cinar, Karahan, Tuncer & Kucukerdonmez, 2014). In contrast the fellow control eyes of the caffeine-treated monkeys exhibited normal age-dependent increases in choroidal thickness.
Given the similarities between the eyes of humans and macaques, it seems unlikely that the different choroidal responses reflect a between-species difference. There are a number of methodological differences between this study and the previous human studies. Whereas we employed infant monkeys, all the previous human studies involved adults. This is potentially important because whereas choroidal thickness increases with age in children (Read, Alonso-Caneiro, Vincent & Collins, 2015, Read, Collins, Vincent & Alonso-Caneiro, 2013), it decreases with age in human adults (Altinkaynak et al., 2016, Ikuno, Kawaguchi, Nouchi & Yasuno, 2010, Margolis & Spaide, 2009, Vural et al., 2014). Thus, it is possible that age may influence the degree of change produced by caffeine (Altinkaynak et al., 2016, Vural et al., 2014). The routes of caffeine administration were also different between our study and the previous human studies. However, since oral 7-MX produced changes in choroidal thickness in monkeys that were comparable to those produced by topical caffeine (Hung et al., 2018), it seems unlikely that different choroidal responses can be attributed to the route of administration. These differences are also unlikely to represent differences in the amount of adenosine antagonist administered. At least with respect to serum levels, our topical caffeine regimen resulted in relatively low methylxanthine levels. On the other hand, the oral dosage of 7-MX in our previous study were much higher than those used in these previous human studies involving caffeine. Thus, the methylxanthine dosages in our two studies probably bracketed the expected blood levels of methylxanthines in the previous human caffeine studies (White, Padowski, Zhong, Chen, Luo, Lazarus, Layton & McPherson, 2016). The duration of treatment is one of the most obvious procedural differences between these studies, single administration in the human studies, which produced transient thinning, versus twice a day for months in our monkeys, which did not interfere with normal maturational increases in choroidal thickness. Unfortunately, our measurement schedule did not allow us to determine if there were transient duration-dependent differences in the direction of the choroidal changes produced by caffeine in our monkeys.
4.5. Clinical Implications
The results of this study, together with those from our previous investigation of the effects of oral 7-MX on vision-dependent ocular growth (Hung et al., 2018), indicate that ADOR antagonists have potential in treatment strategies for preventing and/or reducing the progression of myopia in children. In particular, both topical caffeine and oral 7-MX (Hung et al., 2018) decrease the axial elongation normally produced by hyperopic defocus, one of the primary risk factors believed to be involved in the development and progression of myopia in children (Gifford, Richdale, Kang, Aller, Lam, Liu, Michaud, Mulder, Orr, Rose, Sanders, Seidel, Tideman & Sankaridurg, 2019). However, neither of these treatment strategies interfere with the ability of imposed myopic defocus to slow axial elongation, the most common optical treatment strategy employed to reduce myopia progression in children (Wildsoet et al., 2019). In this respect, ADOR antagonists and optical treatment strategies could possibly work in an additive or synergistic manner resulting in more therapeutic benefit than either treatment strategy alone.
Although relatively little is known about the possible pathophysiological effects of topical caffeine on the eye (Yoon & Danesh-Meyer, 2019), the available data suggest that topical caffeine is potentially a safe therapeutic agent for managing myopia in children. First, caffeine is a common dietary constituent that is considered generally safe for human consumption. In fact, regular intake of caffeine, most commonly from coffee and other beverages, has been reported to be associated with some significant health benefits in adults (Poole, Kennedy, Roderick, Fallowfield, Hayes & Parkes, 2017). Caffeine eye drops do not appear to have toxic effects on the eyes of monkeys or rats (Kronschlager et al., 2014), but have been reported to protect the rat eye from several types of cataract (Kronschlager et al., 2013, Varma et al., 2010, Varma et al., 2011). Topical and oral caffeine have been used in studies of IOP in adult humans without any reported adverse events (Chandra et al., 2011). Moreover, neither oral nor topical caffeine altered IOP in normal adult humans (Adams & Brubaker, 1990, Chandra et al., 2011). An advantage of topical administration of caffeine is that high concentrations of the drug may be administered to the eye while reducing the likelihood of significant systemic absorption. This is particularly important with caffeine because of its known psychoactive effects.
There are, however, as outline above, uncertainties about whether the observed anti-myopia effects are local or due to systemically absorbed caffeine. It will be important to determine how topical caffeine reaches the targets responsible for its anti-myopia effects in order to understand the potential use of topical caffeine for myopia management in humans.
4.6. Study Limitations
Because monkeys are a scarce resource, we employed relatively small numbers of subjects, which given the variability in the −3D/pl subject group, is a concern. However, each monkey was studied longitudinally. Consequently, extensive amounts of data were collected from a given animal, and the data collected were very reliable. In this respect, we believe it is unlikely that the between subject variability in this group represents measurement artifacts. However, the observed high inter-animal variability could reflect several subject factors related to the effectiveness of the caffeine treatments, including differences in the amount of caffeine from each treatment drop that remained in contact with eye (some animals may have blinked a larger proportion of the treatment drops out of the eye). In addition, in monkeys, as in humans, there are substantial inter-subject differences in the non-trabecular outflow rates (Gabelt et al., 2003) and the half-life of serum levels of caffeine, possibly due to genetic variations in the activity of enzymes that degrade caffeine (Uno, Uehara, Murayama & Yamazaki, 2011). The blood levels of theophylline, a metabolic byproduct of caffeine metabolism, in our caffeine-treated monkeys varied from 91 to 249 ng/ml, suggesting that some animals may have metabolized caffeine more efficiently than others. Thus, individual differences in effective caffeine dosages and pharmacokinetics could have contributed to the observed variability in the −3D/pl caffeine group. Unfortunately we did not analyze blood caffeine levels in all of our treated monkeys, precluding a reasonable examination of this possibility.
With respect to extrapolating the results from this study to treatment strategies for myopia, a limitation of this study is that the amount of caffeine that was administered was not precisely controlled, primarily because much of a given caffeine drop that was instilled into an infant monkey eye was blinked out of the palpebral fissure. As a consequence, we cannot with confidence specify the amount of caffeine that was available for absorption by the eye. Because of the rapid growth of the anterior segment of the infant monkey eye, it is also likely that the amount of caffeine available from a given drop of topical caffeine probably increased with age as the palpebral fissure enlarged. This trend was probably counterbalanced in part by the fact that in humans, and probably monkeys, the caffeine half-life is much longer in neonates, not reaching adult clearing times until about 6 months of age in human infants (Fredholm et al., 1999). The exact maturation rate of the enzyme system that degrades caffeine in infant monkeys is not known. Thus, it is problematic extrapolating the caffeine dosage regimen that we employed in this study for potential use in treatment strategies for managing myopia progression in children.
In several respects the caffeine concentrations that we measured in our treated animals were lower than expected. For example, in anesthetized rats binocular instillation of a caffeine solution that was similar in concentration to that used in this study resulted in a blood concentration of caffeine of 14,000 ng/ml two hours after treatment. Taking into account that our monkeys were about 6.5–7.0 times larger than an adult rat at the time the blood samples were taken, one might expect to see peak blood concentrations of between about 2000 ng/ml to 2150 ng/ml. The lower concentrations levels measured in our monkeys are no doubt due, in part, to the delay between treatment and measurement. The half-life for caffeine in adult monkeys is about 3–5 hours. However, our measured concentrations were still lower than one might expect. As mentioned above much of the apparent discrepancy is probably related to fact that large portions of the caffeine eye drops were lost due to reflex blinking follow instillation of the caffeine drops.
While much is to be learned about the protective effects of caffeine eye drops on vision-induced myopia, the results of this study justify further investigations of the potential benefits of ADOR antagonists in the management of myopia.
Highlights.
Topical caffeine prevented myopic compensation for imposed hyperopic defocus
Topical caffeine did not interfere with hyperopic shifts produced by myopic defocus
Caffeine treatment slowed vitreous chamber elongation in infant monkeys
Caffeine and oral 7-methylzanthine have similar effects on refractive development
Adenosine receptor antagonists have potential in the management of myopia
Acknowledgments
This work was supported by National Institutes of Health Grants EY-03611 and EY-07551 and funds from the Brien Holden Vision Institute and the UH Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests
E.L. Smith III is an author on patents related to optical and pharmaceutical treatment strategies for myopia and is a consultant to Nevakar Inc, SightGlass Vision, Treehouse Eyes, Acucela Inc, and Essilor of America. The other authors have no conflicts of interest relevant to the research described in this manuscript.
References
- Adams BA, & Brubaker RF (1990). Caffeine has no clinically significant effect on aqueous humor flow in the normal human eye. Ophthalmology, 97, 1030–1031. [DOI] [PubMed] [Google Scholar]
- Altinkaynak H, Ceylan E, Kartal B, Keles S, Ekinci M, & Olcaysu OO (2016). Measurement of choroidal thickness following caffeine intake in healthy subjects. Current Eye Research, 41, 708–704. [DOI] [PubMed] [Google Scholar]
- Beach KM, Hung L-F, Arumugam B, Smith ELI, & Ostrin L (2018). Adenosine receptor distribution in Rhesus monkey ocular tissue. Experimental Eye Research, 174, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrossian RH (1971). The effect of atropine on myopia. Annals of Ophthalmology, 3, 891–897. [PubMed] [Google Scholar]
- Brass KM, Zarbin MA, & Snyder SH (1987). Endogenous adenosine and adenosine receptors localized to ganglion cells of the retina. Proceedings of the National Academy of Science USA, 84, 3906–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P, Gaur A, & Varma P (2011). Effects of caffeine on the intraocular pressure in patients with primary open angle glaucoma. Clinical Ophthalmology, 5, 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia A, Chua W-H, Wen L, Fong A, Goon YY, & Tan D (2014). Atropine for the treatment of childhood myopia: Changes after stopping atropine 0.01%, 0.1% and 0.5%. American Journal of Ophthalmology, 157, 451–457. [DOI] [PubMed] [Google Scholar]
- Cui D, Trier K, Chen X, Zeng J, Yang X, Hu J, & Ge J (2008). Distribution of adenosine receptors in human sclera fibroblasts. Molecular Vision, 14, 523–529. [PMC free article] [PubMed] [Google Scholar]
- Cui D, Trier K, Zeng J, Wu K, Yu M, & Ge J (2010). Adenosine receptor protein changes in guinea pigs with form deprivation myopia. Acta Ophthalmologica, 88, 759–765. [DOI] [PubMed] [Google Scholar]
- Cui D, Trier K, Zeng J, Wu K, Yu M, Hu J, Chen X, & Ge J (2011). Effects of 7-methylxanthine on sclera in form deprivation myopia in guinea pigs. Acta Ophthalmologica, 89, 328–334. [DOI] [PubMed] [Google Scholar]
- Cunha RA (2001). Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochemistry International, 38, 107–125. [DOI] [PubMed] [Google Scholar]
- Daly JW, Butts-Lamb P, & Padgett W (1983). Subclasses of adenosine receptors in the central nervous system: Interaction with caffeine and related methylxathines. Cellular and Molecular Neurobiology, 3, 69–80. [DOI] [PubMed] [Google Scholar]
- Dhoot DS, Huo S, Yuan A, Xu D, Srivistava S, Ehlers JP, Traboulsi E, & Kaiser PK (2013). Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. British Journal of Ophthalmology, 97, 66–69. [DOI] [PubMed] [Google Scholar]
- Dong F, An J. h., Ren Y. p., Yan D. s., Zhou X. t., Lu F, Hu D. n., Chen J. f., & Qu J (2007). Expression of dopamine receptor D2 and adenosine receptor A2A in human retinal pigment epithelium. Chinese Journal of Ophthalmology, 43, 1110–1113. [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, & Zvartau EE (1999). Actions of caffeine in the brain with special reerence to factors that contribute to its widespread use. Pharmacological Review, 51, 83–133. [PubMed] [Google Scholar]
- Gabelt BT, Gottanka J, Lutjen-Drecoll E, & Kaufman PL (2003). Aqueous humor dynamices and trabecular meshwork and anterior ciliary muscle morphologic changes with age in rhesus monkeys. Investigative Ophthalmology & Visual Science, 44, 2118–2125. [DOI] [PubMed] [Google Scholar]
- Gelal R, Guven H, Balkan D, Artok L, & Benowitz NL (2003). Influence of menthol on caffeine disposition and pharmacodynamics in healthy normal volunteers. European Journal of Clinical Pharmacology, 59, 417–422. [DOI] [PubMed] [Google Scholar]
- Gifford KL, Richdale K, Kang P, Aller TA, Lam CS, Liu YM, Michaud L, Mulder J, Orr JB, Rose KA, Sanders KJ, Seidel D, Tideman JWI, & Sankaridurg P (2019). IMI - Clinical Management Guidelines Reprot. Investigative Ophthalmology & Visual Science, 60, 184–203. [DOI] [PubMed] [Google Scholar]
- Harris WF (1988). Algebra of sphero-cylinders and refractive errors, and their means, variance, and standard deviation. American Journal of Optometry and Physiological Optics, 65, 794–902. [DOI] [PubMed] [Google Scholar]
- Hung L-F, Arumugam B, Ostrin L, Patel N, Trier K, Jong M, & Smith III EL (2018). The adenosine receptor antagonist, 7-methylxanthine, alters emmetropizing responses in infant monkeys. Investigative Ophthalmology & Visual Science, 59, 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Arumugam B, She Z, Ostrin L, & Smith III EL (2018). Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Experimental Eye Research , (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Crawford MLJ, & Smith III EL (1995). Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine, 1, 761–765. [DOI] [PubMed] [Google Scholar]
- Hung L-F, Ramamirtham R, Huang J, Qiao-Grider Y, & Smith EL III (2008). Peripheral refraction in normal infant rhesus monkeys. Investigative Ophthalmology & Visual Science, 49, 3747–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Wallman J, & Smith III EL (2000). Vision dependent changes in the choroidal thickness of macaque monkeys. Investigative Ophthalmology & Visual Science, 41, 1259–1269. [PubMed] [Google Scholar]
- Ikuno Y, Kawaguchi K, Nouchi T, & Yasuno Y (2010). Choroidal thickness in healthy Japanese subjects. Investigative Ophthalmology & Visual Science, 51, 2173–2176. [DOI] [PubMed] [Google Scholar]
- Johnson M, McLaren JW, & Overby DR (2017). Unconventional aqueous humor outflow: A review. Experimental Eye Research, 158, 94–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Hikichi T, Kitaya N, Takahashi J, Mori F, & Yoshida A (2005). Adenosine agonist regulation of outward active transport of fluorescein across retinal pigment epithelium in rabbits. Experimental Eye Research, 80, 493–499. [DOI] [PubMed] [Google Scholar]
- Kee C. s., Hung L-F, Qiao-Grider Y, Roorda A, & Smith III EL (2004). Effects of optically imposed astigmatism on emmetropization in infant monkeys. Investigative Ophthalmology & Visual Science, 45, 1647–1659. [DOI] [PubMed] [Google Scholar]
- Kee C. s., Hung L-F, Qiao Y, Habib A, & Smith III EL (2002). Prevalence of astigmatism in infant monkeys. Vision Research, 42, 1349–1359. [DOI] [PubMed] [Google Scholar]
- Kim JT, Lee DH, Joe SG, Kim JG, & Yoon YH (2013). Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Investigative Ophthalmology & Visual Science, 54, 3378–3387. [DOI] [PubMed] [Google Scholar]
- Kim S-W, Oh JS, Kwon S-S, Yoo J, & Huh K (2011). Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-relatead macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina, The journal of retinal and vitreous diseases, 31, 1904–1911. [DOI] [PubMed] [Google Scholar]
- Kronschlager M, Forsman E, Yu Z, Talebizadeh N, Lofgren S, Meyer LM, Bergquist J, & Soderberg P (2014). Pharmacokinetics for topically applied caffeine in the rat. Experimental Eye Research, 122, 94–101. [DOI] [PubMed] [Google Scholar]
- Kronschlager M, Lofgren S, Yu Z, Talebizadeh N, Varma SD, & Soderberg P (2013). Caffeine eye drops protect against UV-B cataract. Experimental Eye Research, 113, 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Schaeffel F, Trier K, & Feldkaemper MP (2019). Effects of 7-methylxanthine on deprivation myopia and retinal dopamine release. Ophthalmic Research, 63, 347–357. [DOI] [PubMed] [Google Scholar]
- Margolis R, & Spaide RF (2009). A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. American Journal of Ophthalmology, 147, 811–815. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, & Reeder AP (1993). Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Investigative Ophthalmology & Vison Science, 34, 205–215. [PubMed] [Google Scholar]
- Moo-Puc RE, Gongora-Alfaro JL, Alvarez-Cervera FJ, Pineda JC, Arankowshy-Sandoval G, & Heredia-Lopez F (2003). Caffeine and muscarinic antagonists act in synergy to inhibit haloperidol-induced catalepsy. Neuropharmacology, 45 (493–503) [DOI] [PubMed] [Google Scholar]
- Nie H-H, Huo L-J, Yang X, Gao Z-Y, Zeng JW, Trier K, & Cui D-M (2012). Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. International Journal of Ophthalmology, 5, 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, & Correia-de-SA P (2005). Protein kinase A and Ca(v)I (L-type) channels are common targets to facilitatory adenosine A2A and muscarinic M1 receptors on rat motoneurons. Neurosignals, 14, 262–272. [DOI] [PubMed] [Google Scholar]
- Polska E, Ehrlich P, Luksch A, Fuchsjager-Mayrl G, & Schmetterer L (2003). Effects of adenosine on intraocular pressure, optic neruve head blood flow, and choroidal blood flow in healthy humans. Investigative Ophthalmology & Visual Science, 44, 3110–3114. [DOI] [PubMed] [Google Scholar]
- Poole R, Kennedy O, Roderick P, Fallowfield JA, Hayes PC, & Parkes J (2017). Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. British Medical Journal, 359, j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung L-F, Kee C. s., Ramamirtham R, & Smith III E (2007). Normal ocular development in young rhesus monkeys (Macaca mulatta). Vision Research, 47, 1424–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung L-F, Kee C. s., Ramamirtham R, & Smith III EL (2010). Nature of the refractive errors in rhesus monkeys (Macaca mulatta) with experimentally induced ametropias. Vision Research, 50, 1867–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, & Wiesel TN (1985). An animal model of myopia. New England Journal of Medicine, 312, 1609–1615. [DOI] [PubMed] [Google Scholar]
- Read SA, Alonso-Caneiro D, Vincent SJ, & Collins MJ (2015). Longitudinal changes in choroidal thickness and eye growth in childhood. Investigative Ophthalmology & Visual Science, 56, 3103–3112. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Vincent SJ, & Alonso-Caneiro D (2013). Choroidal thickness in childhood. Investigative Ophthalmology & Visual Science, 54, 3586–3593. [DOI] [PubMed] [Google Scholar]
- Salmi P, Chergui K, & Fredholm BB (2005). Adenosine-dopamine interactions revealed in knockout mice. Journal of Molecular Neuroscience, 26, 239–244. [DOI] [PubMed] [Google Scholar]
- Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, M.M. C, Delamere NA, Fletcher E, Salt TE, Grosche A, & Mitchell CH (2014). Purines in the eye: Recent evidence for the physiological and pathological roles of purines in the RPE, retinal neurons, astrocytes, Mueller cells, lens, trabecular mechwork, cornea and lacrimal gland. Experimental Eye Research, 127, 207–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, & Daly JW (1999). Chronic effects of xanthines on levels of central receptors in mice. Cellular and Molecular Neurobiology, 19, 719–732. [DOI] [PubMed] [Google Scholar]
- Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, & Hung PT (1999). Effects of different concentrations of atropine on controlling myopia in myopic children. Journal of Ocular Pharmacology and Therapeutics, 15, 85–90. [DOI] [PubMed] [Google Scholar]
- Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K, & Group, U.S.P.S. (2008). Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% prenzepine ophthalmic gel in children with myopia. Journal of American Association of Pediatric Ophthalmogy and Strabismus, 12, 332–339. [DOI] [PubMed] [Google Scholar]
- Smith EL 3rd, Hung L-F, Arumugam B, Holden BA, Neitz M, & Neitz J (2015). Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Investigative Ophthalmology & Visual Science, 56, 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith III EL, & Hung L-F (1999). The role of optical defocus in regulating refractive development in infant monkeys. Vision Research, 39, 1415–1435. [DOI] [PubMed] [Google Scholar]
- Smith III EL, Hung L-F, Arumugam B, & Huang J (2013). Negative lens-induced myopia in infant monkeys: Effects of high ambient lighting. Investigative Ophthalmology & Visual Science, 54, 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith III EL, Hung L-F, & Huang J (2012). Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology & Visual Science, 53, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith III EL, Hung L-F, Huang J, Blasdel T, Humbird T, & Bockhorst K (2010). Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Investigative Ophthalmology & Visual Science, 51, 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith III EL, Hung L-F, Kee C. s., Qiao-Grider Y, & Ramamirtham R (2003). Continuous ambient lighting and lens compensation in infant monkeys. Optometry and Vision Science, 80, 374–382. [DOI] [PubMed] [Google Scholar]
- Sohni P, & Hartwick ATE (2014). Adenosine modulates light response of rat retinal ganglion cell photoreceptors through a cAMP-mediated pathway. Journal of Physiology, 592, 4201–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavric B, R. K, & Gilbert SG (1984). Automated high-performance liquid chromatographic assay for monitoring caffeine and its metabolites in biological fluids of monkeys consumng caffeine. Journal of Chromatography, 310, 107–118. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, & Laties AM (1991). Muscarinic antagonist effects on experimental chick myopia. Experimental Eye Research, 52, 755–758. [DOI] [PubMed] [Google Scholar]
- Tigges M, Iuvone M, Fernandes A, Sugrue MF, Mallorga P, Laties AM, & Stone RA (1999). Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optometry and Vision Science, 76, 397–407. [DOI] [PubMed] [Google Scholar]
- Tkatchenko TV, Troilo D, Benavente-Perez A, & Tkatchenko AV (2018). Gene expression in response to optical defocus of opposite signs reveals bidirectional mechanisms of visually guided eye growth. PLOS Biology, 16, e2006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier K, Olsen EB, Kobayashi T, & Ribel-Madsen SM (1999). Biochemical and ultrastructural changes in rabbit sclera after treatment with 7-methylxanthine, theobromine, acetazolamide, or L-ornithine. British Journal of Ophthalmology, 83, 1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier K, Ribel-Madsen SM, Cui D, & Christensen SB (2008). Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. Journal of Ocular Biology Diseases and Informatics, 1, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Nickla D, & Wildsoet C (2000). Choroid thickness changes during altered eye growth and refractive state in the common marmoset (Callithrix jaccbus). Investigative Ophthalmology & Visual Science, 41, 1249–1258. [PubMed] [Google Scholar]
- Troilo D, Smith ELI, Nickla DL, Ashby R, Tkatchenko AV, Ostrin L, Gawne TJ, Pardue MT, Summers JA, Kee C. s., Schroedl F, Wahl S, & Jones L (2019). IMI - Report on Experimental Models of Emmetropization and Myopia. Investigative Ophthalmology & Visual Science, 60 (Special Issue No. 3), 31–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Uehara S, Murayama N, & Yamazaki H (2011). CYP1D1, pseudogenized in human, is expressed and encodes a functional drug-metabolizing enzyme in cynomolgus monkey. Biochemical Pharmacology, 81, 442–450. [DOI] [PubMed] [Google Scholar]
- Varma SD, & Hegde KR (2010). Prevention of oxidative damage to lens by caffeine. Journal of Ocular Pharmacology and Therapeutics, 26, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma SD, Kovtun S, & Hegde K (2010). Effectiveness of topical caffeine in cataract prevention: studies with galactose cataract. Molecular Vision, 16, 2626–2633. [PMC free article] [PubMed] [Google Scholar]
- Varma SD, Kovtun S, & Hegde KR (2011). Role of UV irradiation and oxidative stress in cataract formation. Medical prevention by nutritional antioxidants and metabolic agonists. Eye Contact Lens, 37, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural AD, Kara N, Sayin N, Pirhan D, & Ersan HBA (2014). Choroidal thickness changes after a single administration of coffee in healthy subjects. Retina, The Journal of Retinal and Choroidal Diseases, 34, 1223–1228. [DOI] [PubMed] [Google Scholar]
- Wan W. j., Cui D. m., Yang X, Hu J. m., Li C. x., Hu S. l., Trier K, & Zeng J. w. (2011). Expression of adenosine receptors in human retinal pitment epithelium cells in vitro. Chinese Medical Journal, 124, 1139–1144. [PubMed] [Google Scholar]
- White JR, Padowski JM, Zhong Y, Chen G, Luo S, Lazarus P, Layton ME, & McPherson S (2016). Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowy in coffee chilled or hot versu chilled energy drink in health young adults. Clinical Toxicology, 54, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildsoet C, & Wallman J (1995). Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Research, 35, 1175–1194. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Chia A, Cho P, Guggenheim JA, Polling JR, Read SA, Sankaridurg P, Saw SM, Trier K, Walline JJ, Wu PC, & Wolffsohn JS (2019). IMI - Interventions for controling myopia onset and progression report. Investigative Ophthalmology & Visual Science, 60, M106–M131. [DOI] [PubMed] [Google Scholar]
- Yoon JJ, & Danesh-Meyer HV (2019). Caffeine and the eye. Survey of Ophthalmology, 64, 334–344. [DOI] [PubMed] [Google Scholar]
- Zengin MO, Cinar E, Karahan E, Tuncer I, & Kucukerdonmez C (2014). The effects of caffeine on choroidal thickness in young healthy subjects. Cutaneous and Ocular Toxicology, 34, 112–116. [DOI] [PubMed] [Google Scholar]
- Zhang Y, & Wildsoet CF (2015). RPE and choroid mechanisms underlying ocular growth and myopia. Progress in Molecular Biology and Translational Science, 134, 221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Huang Q, An J, Lu R, Qin X, Jiang L, Li Y, Wang J, Chen J, & Qu J (2010). Genetic deletion of the Adensoine A2A receptor confers postnatal developmet of relative myopia in mice. Investigative Ophthalmology & Visual Science, 51, 4362–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]


