Abstract
Purpose:
The NIH Undiagnosed Diseases Network (UDN) evaluates participants with disorders that have defied diagnosis, applying personalized clinical and genomic evaluations and innovative research. The clinical sites of the UDN are essential to advancing the UDN mission; this study assesses their contributions relative to standard clinical practices.
Methods:
We analyzed retrospective data from four UDN clinical sites, from July 2015-September 2019, for diagnoses, new disease gene discoveries and the underlying investigative methods.
Results:
Of 791 evaluated individuals, 231 received 240 diagnoses and 17 new disease-gene associations were recognized. Straightforward diagnoses on UDN exome and genome sequencing occurred in 35% (84/240). We considered these tractable in standard clinical practice, although genome sequencing is not yet widely available clinically. The majority (156/240, 65%) required additional UDN-driven investigations, including 90 diagnoses that occurred after prior non-diagnostic exome sequencing and 45 diagnoses (19%) that were non-genetic. The UDN-driven investigations included complementary/supplementary phenotyping, innovative analyses of genomic variants and collaborative science for functional assays and animal modeling.
Conclusion:
Investigations driven by the clinical sites identified diagnostic and research paradigms that surpass standard diagnostic processes. The new diagnoses, disease gene discoveries and delineation of novel disorders represent a model for genomic medicine and science.
Keywords: Exome sequencing, Genome sequencing, Phenotyping, Ultra-rare diseases, Undiagnosed diseases
INTRODUCTION
The Undiagnosed Diseases Network (UDN) is a research consortium supported by the NIH Common Fund to diagnose participants with refractory medical conditions and conduct collaborative research on the etiology and pathophysiology of undiagnosed diseases.1–4 Accepted participants undergo personalized clinical and research evaluations at one of 12 clinical sites. Since 70–80% of undiagnosed diseases are due to rare and ultra-rare genetic disorders (affecting less than 200,000 and 2,000 in the US, respectively),5,6 sequencing plays a central role in the UDN, with exome sequencing (ES) and genome sequencing (GS) performed through collaborations among the clinical sites and the sequencing core laboratories. Emerging laboratory methods, such as RNA sequencing (RNAseq) analyses for resolving the significance of genetic variants occur at the clinical sites. Additional network resources including model organism screening centers (MOSC), a metabolomics core laboratory, a coordinating center and a biorepository enhance the network’s research mission. The UDN diagnostic rate is ~30%,3 noteworthy in view of the extensive prior diagnostic failures, including non-diagnostic ES. This enhanced diagnostic yield in these extremely difficult cases is due to a combination of methods, including customized clinical evaluations with detailed phenotyping, innovative genomic analysis and, importantly, close collaborations within the network and outside, including data sharing.3 These activities allow the UDN to diagnose refractory cases, overcoming existing constraints of clinical practice and of disease-, technology-, or body-system-limited clinical research programs.
ES is increasingly available through other research studies and in clinical practice, with a diagnostic yield of 25–40% in ES naïve probands.7–10 When third-party or public payers cover costs, general genetics and exome clinics can utilize ES in their diagnostic protocol.11–15 However, several factors complicate the clinical application of ES.16–19 First, most participants who undergo ES obtain a non-diagnostic result, either with no variants, or variants of uncertain significance (VUS) that cannot be resolved further through clinical means. Pursuit of candidate genes often requires further research-level verification. Case matching resources such as GeneMatcher20 are powerful, but often do not produce successful collaborations. Variants of uncertain significance in known disease genes can remain unresolved, despite clinical follow-up studies in >50%.10,21 Subsequent reanalysis of non-diagnostic ES data by clinical laboratories can yield diagnoses in up to 15% of cases.22–27 However, clinicians have few further options if this reanalysis is also non-diagnostic. Second, in >10% of cases, differences in the interpretation of ES between the ordering clinician and testing laboratory occur, and may be difficult to resolve.21 Third, clinical laboratories may not report variants in known disease-associated genes if there is phenotypic discordance, or variants in genes not currently associated with disease, leading to missed opportunities for clinicians to solve cases.28 Finally, time and reimbursement are significant barriers to pursuing all the above activities. Clinical ES utilization is rising,29–31 but pre- and post-ES activities are labor intensive, requiring time outside the reimbursable face-to-face clinical encounters.29,32,33
The UDN has pioneered and optimized diagnostic and research strategies to overcome these constraints. Geographic and financial barriers are minimized, with participants being accepted solely on the clinical manifestations, thereby providing equitable enrollment and evaluations. It is unique among genomic research networks in adopting the N-of-1 paradigm, rather than a cohort approach, enabling personalized in-person phenotyping and genomic evaluations beyond what is available in a clinical setting, or in studies that gather historical records and perform genomic evaluations. Collaborative science within the network facilitates disease gene discovery.
Genomic data analyses in the UDN have distinctive elements. 1) Dual analysis of UDN-generated sequencing data is available at the sequencing core laboratory and the individual clinical sites. 2) Reanalysis of raw data from pre-UDN non-diagnostic ES and GS occurs at the clinical sites. The UDN clinical sites’ research analytical pipelines and other adjunct methods can identify variants that a commercial laboratory might not report, resulting in a resolution rate of 40% in the ES non-diagnostic cases, as previously reported.28 3) New and innovative analysis techniques are periodically applied to unsolved cases, since no such case is considered “closed.” Despite the inherent clinical and etiological heterogeneity of the participants, the network has been successful in making diagnoses, discovering disease-causing genes and expanding rare disease phenotypes using data sharing and collaborative efforts.3,28 The UDN paradigm goes well beyond what is currently possible in a busy and time-constrained clinical diagnostic setting. We illustrate the value of this with an in-depth analysis of data from four UDN clinical sites.
MATERIALS AND METHODS
Ethics Statement:
This retrospective study was conducted under the UDN protocol approved by the central institutional review board at the National Human Genome Research Institute, with referral, review, and evaluation processes previously described.3 Informed consent was obtained from all subjects.
Invitations for participation were extended to the seven clinical sites that had been part of the UDN since its inception in 2014. Four clinical sites participated, including Duke/Columbia University Medical Centers, the NIH Undiagnosed Diseases Program, Stanford Medicine, and Vanderbilt University Medical Center. Application and evaluation processes performed at these four sites are representative of all clinical sites, described in the UDN manual of operations and published previously3. Informed consent was obtained from all subjects. The data collection period was July 2015 through September 2019. The full cohort included all applicants to these clinical sites and, among these, a smaller cohort of individuals who were accepted, evaluated, and received a diagnosis. Demographic information as well as details of the diagnoses, internal collaborations and geographic location of the participants, were downloaded from the UDN Gateway.34 The clinical sites provided details on UDN-driven investigations for all diagnoses. A subset of the diagnoses and disease genes (September 2015-May 2017) was published previously.3 Management changes due to diagnoses have been reported4 and are not included in this paper. Quantitative analyses were conducted using SPSS 26.0.
UDN-driven investigations:
The clinical sites collaboratively initiated and completed several research processes to increase the rate of diagnoses. All applicants underwent a medical record review which identified steps for a personalized evaluation, including phenotyping, sequencing or other laboratory tests as follows.
Complementation/supplementation of prior clinical data: Phenotypic gaps were filled and new diagnostic clues were sought through temporally concentrated specialty evaluations, re-examination of prior studies (including imaging, pathology slides, etc.), and phenotype-driven imaging, procedures, and genetic/non-genetic laboratory studies. If UDN genomic results were already available at the time of the clinical evaluation, the phenotyping was further customized, as indicated.
Innovative analysis of genomic data and collaborative investigations to advance genomic science: When feasible, pre-existing raw sequence data (FASTQ/BAM) from prior non-diagnostic ESs were reanalyzed through research pipelines at the clinical sites.28,35 A non-diagnostic ES was operationalized as one in which there were no variants, or a single heterozygous variant was found in genes for autosomal recessive conditions, or VUSs in novel candidate or known disease-associated genes that could not be resolved further. For others, new ES or GS was performed at the UDN sequencing core laboratory, with dual analysis of the data by the core and the clinical sites. Emerging variants were manually curated at the clinical sites (see Supplementary materials). Functional assays such as RT-PCR were performed when indicated. Variants of interest were further evaluated using internal data sharing, case matching, animal modeling and RNASeq. External data sharing for case matching and collaborations occurred through PhenomeCentral, GeneMatcher,24 myGene2,36 and, through individualized UDN participant webpages.36
Classification of UDN diagnoses:
In accordance with the UDN diagnosis-coding tool,3 the diagnoses made after UDN evaluation were classified as: 1) clinical diagnoses, 2) diagnoses due to phenotype-directed testing, 3) diagnoses stemming from ES/GS, or 4) diagnoses on non-sequencing, genome-wide diagnostic assay (chromosomal microarray (CMA) (footnote in Table 2 defines the diagnosis classification). New disease gene discoveries were included in these diagnoses.
Table 2.
Classification of diagnoses (n=240) and case examples describing UDN-driven investigations leading to diagnoses.
| Classification of diagnosis | Example Case Description | Diagnosis | UDN-driven investigations to establish diagnosis | |
|---|---|---|---|---|
| Clinical diagnosesa (n=16, 7%) | Case 1: 22y male with episodic thrombocytopenia, lymphopenia, neutropenia, recurrent fevers | Autoimmune thrombocytopenic purpura |
Complementation/supplementation of prior clinical data (aggregate evidence led to diagnosis): B cell flow cytometry and Fas mediated apoptosis normal. Intermittent anti-platelet antibodies. Recurrent severe episodes of thrombocytopenia, (neutropenia possibly secondary to treatment), responded to anti-CD20 therapy but complicated by serum sickness, ultimately stabilized after bone marrow transplant on NIH protocol treating refractory autoimmune disease. This is a non-genetic diagnosis. |
|
| Case 2: 60y male with periodic fevers, monthly episodes lasting 7–10 days | Waldenstrom macroglobulinemia |
Complementation/supplementation of prior clinical data (aggregate evidence led to diagnosis): IgM elevation mild but bone marrow aspirate flow cytometry showed 1% clonal population of kappa-restricted plasmacytoid CD38/CD138+ cells. This is a non-genetic diagnosis. |
||
| Diagnoses due to phenotype-directed testingb (n=29, 12%) | Non-genetic laboratory testing (n=12) | Case 3: 26y female with progressive limb girdle weakness | Anti-HMGCR myopathy |
Complementation/supplementation of prior clinical data (neurology evaluation) and subsequent targeted testing: Intermittent weakness was consistent with autoimmune myopathy: anti-HMGCR and anti-MDA5 antibodies identified on myositis panel (not included in myositis panel prior to UDN). This is a non-genetic diagnosis. |
| Case 4: 29y male with acquired transfusion-dependent dyserythropoietic anemia. | Autoimmune dyserythropoietic anemia |
Complementation/supplementation of prior clinical data (bone marrow aspirate) and subsequent targeted testing: Bone marrow aspirate pathology led to molecular study: increased population of gamma-delta T cells likely mediating cytotoxic immune response against erythroid precursors. Responded to cyclosporine. This is a non-genetic diagnosis. |
||
| Single gene testing (n=17) | Case 5: 4y female with neurodevelopmental regression and non-diagnostic pre-UDN ES | PLA2G6-associated Neurodegeneration with brain iron accumulation 2B (MIM#256600) |
Complementation/supplementation of prior clinical data (neurology evaluation) and subsequent targeted testing : Neurological phenotype consistent with NBIA: MLPA for PLA2G6 revealed novel homozygous deletion encompassing noncoding exon 1, with RT-PCR demonstrating absent PLA2G6 expression. |
|
| Case 6: 39y male with cognitive decline, personality changes, cerebral volume loss, and non-diagnostic pre-UDN ES | Frontotemporal dementia (MIM#105550) |
Complementation/supplementation of prior clinical data (consultation with neurology expert at another UDN clinical site) and subsequent targeted testing: Progressive phenotype was consistent with C9orf72 repeat expansion disease; testing showed >44 repeats, not detectable on ES |
||
| Diagnoses stemming from ES/GS and downstream analysesc (n=190, 79%) | Exome sequencing (n=116) | Case 7: 6m male with severe congenital hypotonia and fine tremor and non-diagnostic pre-UDN ES | Congenital myopathy with tremor (MIM# 618524), due to de novo variant in MYBPC1 NM 002465.3:c.776T>C (p.Leu259Pro) |
Innovative analysis of genomic data (reanalysis of pre-UDN non-diagnostic ES) and collaborations outside network (case-matching and functional assays): Detected on UDN reanalysis of raw ES data. Variant was not reported on pre-UDN ES due to perceived poor phenotypic fit. Three other similarly affected cases identified, leading to new disease association for MYBPC1. |
| Case 8: 20m male with infantile spasms, microcephaly, lamellar cataracts, developmental delays and non-diagnostic pre-UDN ES | Neurodevelopmental disorder with epilepsy, cataracts, feeding difficulties and delayed brain myelination (MIM#617393), due to de novo variant in NACC1 NM_052876.3:c.892C>T (p.Arg298Trp) |
Innovative analysis of genomic data (reanalysis of pre-UDN sequences) and collaborations within and outside network (case-matching): Reanalysis of pre-UDN ES data prioritized a de novo variant in NACC1. Additional individuals identified through GeneMatcher and the UDN patient webpage, leading to new disease gene identification. |
||
| Genome sequencing (n=74) | Case 9: 10y female with congenital neuropathy, muscle weakness, calf atrophy, and abnormal gait, and non-diagnostic pre-UDN ES | Charcot-Marie-Tooth disease, axonal, type 2S (MIM#616155), caused by biallelic variants in IGHMBP2 NM_002180.2:c.1730T>C (p.Leu577Pro) and c.1235+894C>A |
Innovative analysis of genomic data (dual analysis of UDN sequence data by clinical site) and functional assay at clinical site Maternal missense pathogenic variant reported on pre-UDN ES. Clinical site reanalysis of UDN GS data revealed a paternally inherited cryptic intronic splice site acceptor. Direct sequencing of IGHMBP2 cDNA with and without a nonsense mediated decay (NMD) inhibitor confirmed inclusion of a mini pseudoexon in mRNA. The abnormal transcript was subject to NMD |
|
| Case 10: 31y female with adult-onset, slowly progressive distal asymmetric myopathy with scapular winging, mild facial weakness and non-diagnostic pre-UDN ES | Multisystem proteinopathy 3 (MIM#615424), caused by a de novo heterozygous 500 base pair deletion encompassing exon 10 of HNRNPA1 NM_031157.3:c.1063+15_*5-68del |
Innovative analysis of genomic data (dual analysis of UDN sequence data by clinical site + RNASeq) and collaborations within network (between the clinical site and the sequencing core) Collaborative analysis of UDN GS data by the clinical site and sequencing core identified the deletion which was confirmed by qPCR. RNASeq on patient fibroblasts confirmed that the aberrant transcript escapes NMD and results in truncation of the M9 protein domain. |
||
| Case 11: 6y female with macrocephaly, developmental delay and dysmorphic features, and non-diagnostic ES | Kleefstra syndrome type 2, caused by a heterozygous 127 kb deletion of 7q36.1 involving exons 8-55 of KMT2C |
Innovative analysis of genomic data (dual analysis of UDN sequence data and manual curation by clinical site): Not reported as diagnostic due to size and presence of deletions in normal individuals; however, manual curation of KMT2C isoforms by the clinical site revealed that this distal deletion occurred in the isoform most expressed in brain tissue, whereas the deletions in healthy individuals in DGV were proximal and not in the brain expressed isoform. |
||
| Diagnoses on non-sequencing, genome-wide diagnostic assay: Chromosome microarray (CMA)d (n=5, 2%) | Case 12: 10y female with severe developmental delay, absent speech, joint laxity, tethered cord, and non-diagnostic pre-UDN ES | Wieacker-Wolff syndrome (MIM#314580), due to a de novo heterozygous 95 kb deletion involving exon 1 of ZC4H2 |
Innovative analysis of genomic data (CMA performed on more advanced platform): Deletion was detected on a high density CMA. Pre-UDN SNP-based CMA did not detect the deletion, likely due to being below the level of resolution. Reason for nondetection in UDN GS unclear. |
|
Clinical diagnoses: Defined as diagnosis made on clinical grounds, including aggregate assessment of non-specific test results: also conferred when clinical diagnostic criteria were met, or pathognomonic signs were present
Diagnoses due to phenotype-directed testing: When the clinical manifestations were suggestive of a disorder or group of disorders, further targeted genetic or non-genetic laboratory tests were performed or reviewed and led to diagnoses. These were further classified into “non-genetic laboratory testing” and “single gene testing”
Diagnoses stemming from ES or GS and downstream analyses: These included diagnoses with first-pass ES or GS, or with further innovative genomics and network and outside collaborations involving animal modeling and functional assays.
Diagnoses on non-sequencing, genome-wide diagnostic assay: Chromosome microarray (CMA): CMA performed with more sensitive platform, or when it had not been obtained prior to the UDN
HMGCR = 3-hydroxy-3-methylglutaryl-coenzyme A reductase; MDA5= melanoma differentiation-associated gene 5; ES=exome sequencing; GS= genome sequencing; NBIA= Neurodegeneration with brain iron accumulation; MLPA= Multiplex ligation-dependent probe amplification; DGV= Database of Genomic Variants
Comparisons to clinical genetics practice:
To assess if ES in the UDN alone with phenotype integration was enough to achieve a diagnosis (analogous to clinical practice), the clinical sites were asked to categorize each diagnosis as (1) straightforward or (2) requiring additional UDN-driven investigations detailed above, and to describe those. Straightforward diagnoses on ES were defined as those in which the UDN ES or GS detected compelling variant(s) that matched the phenotype, even if that phenotype was obtained by the UDN-driven deep complementary phenotyping. These diagnoses were considered achievable in current standard clinical practice. Straightforward diagnoses on GS were also included as not requiring additional UDN investigations, because GS will soon become increasingly available in clinical practice.
For further comparisons to clinical practice, the genetics clinics at Duke, Stanford and Vanderbilt provided information on distances traveled by non-UDN patients to their general genetics clinics and results from these genetics clinics’ ES reanalysis through commercial laboratories, when ES had been non-diagnostic. The NIH clinical site does not have a comparable clinical genetics practice and so was not included in these comparisons.
RESULTS
Demographics:
Across the four clinical sites, 2490 applications were received and 964 individuals (39%) were accepted; clinical and genomic evaluations were complete on 791 participants (remainder 173 are undergoing evaluations). Of the 791 evaluated, 231 (29%) received 240 diagnoses; seven received two diagnoses and one received three. The median time to diagnosis (time from start of in-person clinical evaluation to results disclosure) was 185 days (0–1399). Of the 231 diagnosed individuals, 90 had a pre-UDN non-diagnostic ES (Table 1). Details of each diagnosis are in Table S1.
Table 1.
Demographics, presenting clinical manifestations and pre-UDN ES status of the 231 diagnosed individuals (who had a total of 240 diagnoses).
| Variable | Value (%) | |
|---|---|---|
| Median age | Pediatric (n=155) | 6 years |
| Adult (n=76) | 34.5 years | |
| Gender | Female | 131 (57%) |
| Male | 100 (43%) | |
| Race | White | 179 (78%) |
| Asian | 18 (8%) | |
| Black | 13 (5%) | |
| Other | 21 (9%) | |
| Ethnicity | Hispanic | 26 (11%) |
| Non-Hispanic | 168 (73%) | |
| Unknown | 37 (16%) | |
| Presenting symptoms categorya | Neurologic | 130 (56%) |
| Multiple congenital anomalies | 18 (8%) | |
| Musculoskeletal | 17 (7%) | |
| Other (18 systems) | 66 (29%) | |
| Pre-UDN ES | Prior ES performed was non-diagnosticb | 90 (39%) |
| No prior ES | 141 (61%) | |
Reported by clinical site at application review
Prior non-diagnostic ES defined as one with either: no variants, heterozygous variants in autosomal recessive disease genes, variants of uncertain significance in a known disease gene or in a novel candidate gene, which could not be resolved further
UDN-driven investigations:
Complementation and supplementation of prior clinical data: Detailed and iterative phenotyping led to 16 clinical diagnoses, mainly non-genetic (Case examples 1 and 2 in Table 2, Figure 1, and Table S1.) Phenotyping also identified differential diagnostic possibilities that were confirmed by subsequent specific testing, producing a genetic or non-genetic diagnosis in 29 individuals (Case examples 3–6 in Table 2, Figure 1, and Table S1). Cumulatively, the phenotyping led to 45 of 240 (19%) diagnoses.
Innovative analysis of genomic data, non-sequencing genomic approaches and collaborative investigations to advance genomic science: 190 of 240 diagnoses (79%) stemmed from reanalysis of prior non-diagnostic ES and new ES or GS performed in the UDN (Tables 2 and 3, Table S1). Broadly, ES led to 116 diagnoses and GS to 74 diagnoses. The UDN-driven genomic analyses were as follows:
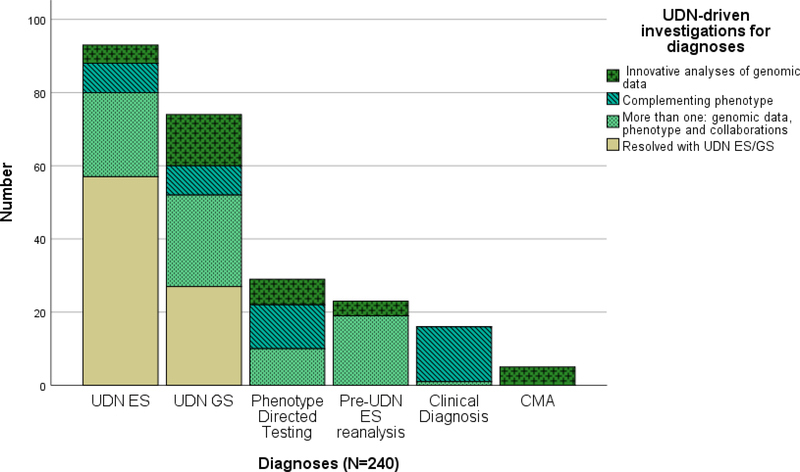
Figure 1. Details of the 240 diagnoses.
The beige portions of the bars indicate diagnoses that were made in a straightforward manner from ES/GS that was performed by the UDN sequencing core with integration of the phenotype by the UDN clinical sites. The 57 diagnoses (24%) that were due to UDN ES and 27 diagnoses (11%) that were due to UDN GS are similar to what could be accomplished in a regular genetics clinic. The green portions indicate diagnoses that were made with additional UDN-driven investigations that are difficult to accomplish in regular clinical settings.. In aggregate the majority of diagnoses (n= 156 of 240, 65%) occurred due to the additional and most often multiple UDN-driven investigations, initiated at the clinical sites.
Table 3.
Diagnoses made by ES or GS (190 of 240 diagnoses)
| Sequencing and other efforts | Diagnoses on ES= 116 | Diagnoses on GS= 74 | Totals | |||
|---|---|---|---|---|---|---|
| Pre-UDN ES data reanalysis at clinical sites | ES through UDN sequencing core | Clinical site dual analysis of UDN ES data | GS through UDN sequencing core | Clinical site dual analysis of UDN GS data | ||
| Straightforward ES/GS diagnoses | 0 | 57a | n/a b | 27a | n/a b | 84 (44%) |
| UDN investigations beyond ES/GS required for diagnosisc | 23d | 29 | 7d | 38 | 9d | 106 (56%) |
| Totals | 23 | 86 | 7 | 65 | 9 | 190 |
Diagnoses that were made on ES or GS through the UDN in a straightforward manner, by reconciling the ES or GS results with the phenotype, similar to a clinical genetics setting. The remainder (n=106) needed UDN-driven investigations.
When the diagnosis was identified by both the UDN sequencing core laboratory and on clinical site analysis, attribution was given to the UDN sequencing core.
Details of additional investigations may be found in Table S1.
Diagnoses (n= 39, 21%) solely attributed to UDN clinical sites’ innovative research analyses of the ES and GS data.
Reanalysis of pre-UDN non-diagnostic ES data:
Remarkably, of the 231 diagnosed individuals, 90 (39%) had a non-diagnostic ES prior to UDN enrollment. Reanalysis of pre-UDN sequence data was completed for 53 individuals and resulted in 23 diagnoses, with a 43% diagnostic rate with reanalysis (Table 3, Case examples 7 and 8 in Table 2).
Other methods to resolve prior ES non-diagnostic cases:
Among the remaining 67 (of 90) prior non-diagnostic ES, GS resolved 41 (45%) and repeat ES in the UDN (due to prior outdated ES) resolved another eight (8%). The remainder of the ES non-diagnostic cases were resolved without genomic sequencing, i.e., via clinical diagnosis in four (4%), diagnosis on phenotype-directed testing in 11 (12%), and diagnosis on CMA in three (3%).
C omparison of the types of diagnoses in the ES non-diagnostic cases (n=90) to the 150 diagnoses in the ES naïve cases, showed no significant difference in the types of diagnoses (clinical diagnoses on phenotype-directed tests, genomic and CMA diagnoses) (Figure S1). T Regardless of whether ES had been performed previously, a personalized approach resulted in similar distribution of types of diagnoses, and in many of these reflexive GS following non-diagnostic ES would not have solved them.
Dual analysis of UDN generated ES/GS data:
Concurrent dual analysis of sequence data through the sequencing core pipeline and the clinical sites’ research pipelines resulted in a larger number of candidate variants, as expected. These were manually curated at the clinical sites and reiteratively integrated with the phenotype, for prioritization as disease-associated or as novel genes (Table 3 and Supplementary Material). These clinical site investigations resulted in seven additional ES diagnoses and nine additional GS diagnoses beyond those issued in the report by the UDN sequencing core laboratory (Case examples 9–11 in Table 2, Table 3). Overall, 39 of the 190 (21%) genomic diagnoses were due to the clinical sites’ analyses of pre-UDN and UDN-generated sequence data (Table 3). It is to be noted that straightforward ES (n=57) and GS (n=27) diagnoses were attributed to the sequencing core laboratory, even when the dual clinical site analysis detected these. The details of these straightforward diagnoses are below in the section on Comparisons of the UDN to genetics clinics.
CMA diagnoses:
Five diagnoses resulted from CMA studies, either because a prior CMA had never been performed or because a higher-resolution CMA was employed using the N-of-1 approach. These diagnoses demonstrate the utility of CMA in specific instances, e.g., case example 12 in Table 2.
RNASeq:
Collaborations among UDN sites generated RNASeq data for 45 individuals in the diagnosed cohort and these contributed to diagnoses in seven of 45 (16%). Example findings included allelic imbalance, evidence of functional impact of non-coding variants such as splicing, and evidence of nonsense-mediated decay (Case examples 9 and 10 in Table 2).
New disease gene identification with collaborative science:
Seventeen new gene-disease associations were included among the 190 sequencing diagnoses (Table S2). These discoveries arose through multiple avenues (Case example 8 in Table 2, Table S1). Collaborations with the UDN MOSC contributed to eight of the 17 novel disease-gene discoveries. Other internal collaborations included partnerships with the UDN Metabolomics Core and disease or technological experts at UDN sites (Table 2). In aggregate, internal data sharing and collaboration occurred for 131/240 (55%) diagnoses. External collaborations occurred for 64/240 (27%) diagnoses, with 34/64 (53%) contributing to the diagnosis.
Comparison of the UDN to genetics clinics:
Table 4 describes UDN processes relative to standard clinical practices. Straightforward diagnoses on UDN ES and GS provided 84 diagnoses (35% of all 240), due to compelling sequence variation and congruence with the presenting phenotype (Figure 1, Table 3). The remaining 156 diagnoses (65%) that were not straightforward required additional UDN-driven investigations that are difficult in clinical practice, with many requiring more than one such investigation (Figure 1, Table 2, and Table S1).
Table 4.
Comparison of UDN-driven investigations at the clinical sites, to standard clinical genetics practice
| Characteristics/Investigations | UDN | Clinical Practice |
|---|---|---|
| Participant characteristics | Refractory to multiple prior clinical and laboratory evaluations, and often ES negative | More likely to not have ES, may or may not have failed prior clinical evaluations |
| Time spent on pre-. post-, and face-to-face activities | Face-to-face time represents a minority of time required for clinical and research activities (record review, literature review, phenotyping, bioinformatics, variant curation, RNASeq, collaborative science, integration of all data) | Limited by clinical demands and financial constraints to a few hours for all activities |
|
Equity in access: • Geographic access • Financial considerations |
Accessible to all in USA and internationallya All eligible irrespective of finances |
Regional access more likelya Financial considerations likely factor |
| Complementation/Supplementation of prior clinical data | Personalized, temporally concentrated, comprehensive N-of-1 clinical consultations/laboratory tests/imaging/procedures • Fills in phenotypic gaps and generates additional clinical information • Leads to clinical diagnoses, diagnoses on targeted testing and contributes to genomic diagnoses |
Variable, less likely to be temporally concentrated and comprehensive Time and financially constrained in filling in gaps and obtaining new information |
| Innovative analyses of genomic data | Straightforward diagnoses on UDN sequencing • ES/GS (35% diagnostic yield) Research reanalysis of pre-UDN raw data from non-diagnostic ES (diagnostic yield of 43%) • Multiple other approaches to resolving prior ES negatives Dual analysis of UDN generated genomic data by UDN core lab and clinical sites • Clinical site analysis led to additional genomic diagnoses (8%) Manual curation of research variants generated by clinical site and core lab genomic analysis RNASeq: Internal collaborations led to generation and analyses of RNASeq (contributed to diagnoses in 15%) New disease gene identification • 8% of genomic diagnoses were novel disease gene associations • Can be pursued with internal collaborations |
Straightforward diagnoses on clinical ES (diagnostic yield 25–30% in literature). GS less widely available Standard reanalysis of negative ES with same pipeline (diagnosis yield of 6.5% at Duke, Stanford and Vanderbilt), 10–15% in literature • Limited further options to resolve ES negatives Dual analysis unavailable due to lack of bioinformatics in clinics Clinicians do not receive research variants from clinical labs for curation Limited availability of RNASeq, with the clinical laboratory determining access New disease gene identification • Time and resource constrained |
See Figure S2 for detailed travel data
The 84 straightforward ES (n=57) and GS (n=27) diagnoses could be comparable to what could occur in standard clinical practice. However, four of the 57 straightforward ES diagnoses had been missed with a pre-UDN ES, due to interim new disease gene reports (n=1), and analytical pipeline differences (n=3). Of the 27 straightforward GS diagnoses, 12 occurred in ES naïve cases, since the clinical sites opted to perform GS without ES on these; our review determined that 11 of these 12 diagnoses could have occurred with clinical ES. Among the remaining 15 straightforward GS diagnoses that had prior non-diagnostic ES, we estimate that only four could not have occurred with ES, since these were due to variants not easily detected on exomes, such as structural and non-coding variants. For the other 11, recent improvements in capture kits, analytic pipelines, and interim disease-gene associations could have resulted in these diagnoses on a current ES. Thus, only nine of the 84 straightforward diagnoses could not have been readily achievable in clinical practice with ES alone.
The clinical genetics practices at Duke, Stanford and Vanderbilt request reanalysis of non-diagnostic exomes by the commercial laboratories that had performed the ES originally. This standard clinical reanalysis yielded diagnoses in 2/28 (7%) at Duke over four years, 6/83 (7%) at Stanford over four years and 0/10 at Vanderbilt over one year (the Vanderbilt genetics clinics started requesting reanalysis only in the last year). In contrast, the UDN diagnosis rate with reanalysis of non-diagnostic ES over four years (n= 23/53, 43%) was significantly higher (Fisher’s exact test, p<0.001).
Data from Duke, Stanford, and Vanderbilt indicated that the travel distances to the UDN were qualitatively much greater than to their general genetics clinics (Figure S2). This indicates that the UDN serves a geographically broader constituency than the general genetics clinics at the participating sites.
DISCUSSION
The mission of the UDN is to evaluate individuals with mystery illnesses and to provide innovative insights into rare and undiagnosed diseases. Applications are reviewed and acceptance is determined using the same inclusion criteria across clinical sites within the network. If indicated on medical record review, recommendations for additional clinically available testing are provided to the referring physician prior to acceptance, maximizing network resources for more challenging patients. UDN applicants accepted for participation are clinically and etiologically heterogeneous, with the underlying disorders including various rare and ultra-rare genetic diseases as well as non-genetic disorders. The UDN clinical sites have developed a systematic, innovative, comprehensive, and reiterative diagnostic paradigm, outlined in the UDN manual of operations, and the evaluation is personalized to each participant. Critical components include the reconsideration of prior clinical and genomic data, filling-in phenotyping gaps, generating and analyzing new clinical and genomic data, and working interactively with researchers inside and outside the UDN. The network interface allows clinical hypotheses to be rapidly moved to exploration and the infrastructure allows for facile data sharing for case-matching and functional assays (in vitro molecular studies and animal modeling). It is noteworthy that most of these UDN-driven investigations entail research activities that are difficult to achieve in standard clinical practice, with multiple avenues being necessary for diagnostic resolution in the majority of participants (Figure 1). Therefore, the UDN is unique in complementing its N-of-1 patient-facing activities with cutting-edge research, providing a model for precision and translational medicine.
The N-of-1 model adopted by the UDN is not the traditional method utilized for disease gene discovery, but the network has been successful in identifying and pursuing candidate genes for ultra-rare disorders. Internal and external collaborative science initiated at the four studied clinical sites resulted in 17 disease gene discoveries through case-matching, animal modeling and other molecular studies. Indeed, 7% of the 231 diagnosed individuals were found to have a novel disease. Several of the new disease gene associations have resulted in establishment of patient foundations for advocacy and further research into pathophysiology and therapeutics.
The UDN diagnoses both genetic and non-genetic diseases (e.g., anti-HMGCR myopathy, Table 2 and Schnitzler syndrome, Table S1) through its participant-centric deep phenotyping. Of the 240 diagnoses, 45 (19%) were due to clinical synthesis of data or due to phenotype-directed testing. Although some of these diagnoses were tractable in a clinical setting, they had been missed previously by clinical diagnostic services, sometimes by multiple institutions: it is often difficult to know prior to evaluating a patient if a diagnosis could have occurred in standard clinical setting.. The keys to these diagnoses were: 1) a personalized planning of evaluations to fill phenotypic gaps and obtain additional information; 2) a temporally concentrated suite of specialty consultations, imaging, and laboratory tests, often within a week; and 3) synthesis of the emerging data by the specialists and the primary UDN investigators (medical geneticists, other clinicians, bioinformaticians, research genetic counselors, etc.). This N-of-1 precision medicine model of the UDN provides diagnostic power beyond what is available in clinical settings or in other research studies focused on cohorts. Furthermore, UDN clinical sites keep unsolved cases open indefinitely; diagnoses can occur years after individuals complete their evaluation,28 due to many reasons, such as reanalysis of data and adopting new technologies such as RNASeq. Thus, more than the present 231 out of 791 individuals in this cohort may be diagnosed with time.
For every UDN participant suspected to have a genetic disease, the capabilities and limitations of prior genomic testing are considered. For example, early ES had lower coverage compared with current ES, and clinical laboratories are less likely to report novel candidate gene variants or variants in disease genes that are inconsistent with the phenotype.28,37 The decision regarding further genomic testing is dependent on such considerations. For example, CMA was utilized if a prior CMA was not done or was on an outdated platform and provided five diagnoses that were due to large CNVs (95 Kb- 3 Mb), which are most optimally detected by CMA, rather than next-generation sequencing such as GS. The UDN sequencing core provides clinical reports consistent with ACMG/AMP reporting guidelines and, additional research variants that may warrant further investigation but do not meet the core’s criteria for clinical reporting; these include coding and non-coding variants, structural variants and trinucleotide repeats. Supplementing this report are the labor-intensive and evolving research bioinformatics output at the clinical sites, a unique UDN-driven investigation. Variants forthcoming from these dual analyses are pursued at the clinical sites, by extensive manual curation, functional assays, etc. contributing to increased diagnostic sensitivity. In addition, the clinical sites’ research reanalysis of pre-UDN non-diagnostic ES data results in previously missed diagnoses and novel candidate gene identification (23 of 53 reanalyses, 43%), at rates that are much higher than reported in the literature.22–24 Prior non-diagnostic ESs are also solved with non-genomic approaches, rather than always moving on to GS for all such cases (Figure S1). Successful resolution of these ES non-diagnostics allows conservation of UDN sequencing resources for other refractory cases.
The UDN diagnosis rate of ~30% might seem similar to that achieved with ES in standard genetics or exome clinics, but it is to be emphasized that these diagnoses occur in the context of high frequencies of prior non-diagnostic ES and non-diagnostic clinical evaluations, often at multiple tertiary centers. As hubs for undiagnosed disease data generation and integration in the UDN, the clinical sites can perform many investigations beyond those of a general genetics clinic. The personalized, compressed, iterative, comprehensive, and multi-disciplinary UDN evaluations are difficult to achieve in standard clinics. We recognize that most of the straightforward diagnoses (35%, 84 of 240) made by the UDN ES and GS could be achievable in standard genetics clinics. Although GS is not yet standard of care in clinical practice, we elected to include the GS diagnoses in this calculation to provide a more conservative estimate, since many of the straightforward diagnoses made on GS could have occurred with ES, and it is likely that GS will become more broadly available to clinicians in the near future.
However, the remainder (65%, 156 of 240) of diagnoses required multiple UDN-driven investigations initiated at the clinical sites, such as the research reanalysis of raw data from prior non-diagnostic ES and the dual analysis of genomic data that are unique to the UDN and unavailable in genetics clinics, beyond reanalysis that may use the same standard clinical pipeline.22,24 Furthermore, clinicians are unlikely to receive a list of research variants from laboratories that perform clinical ES for curation; the laboratories may include VUS in known disease genes or candidate genes, but only if there is some evidence for an association. Indeed, 39 of 190 (21%) genomic diagnoses in this study occurred directly due to the genomic sequence analysis at the clinical sites. Notably, the UDN has a mandate to accept individuals without regard to their insurance or financial status; therefore geographic and financial barriers are minimized beyond what can be achieved in clinical practice, to improve equity in access to those with the most diagnostically intractable diseases. As a result, individuals who have no access to clinical ES or are denied ES coverage by insurance continue to be accepted by the network,38 leading to some diagnoses that could be tractable in a clinical setting. This number is declining as clinical reimbursement for ES increases, although unlikely to ever become zero. However, we do anticipate increasingly more participants will enroll in the UDN with a prior non-diagnostic ES (current rate is ~75%, unpublished data). Hence, the proportion of straightforward diagnoses is likely to decrease, with more diagnoses requiring UDN-driven investigations. Unlike genetics clinics, the UDN also provides resolution to non-genetic diseases and can explore new methods such as long-read sequencing and optical mapping of the genome in refractory phenotypes, techniques that are still at the frontier of genomic research. Finally, unlike in most clinical settings, the UDN also standardizes its data, generating Human Phenotype Ontology terms39 for the phenotype and sharing genomic data internally and externally, for further larger-scale cohort-type research studies.
Looking toward sustainability of this network, various models have been proposed, including adding more sites to decrease travel burden/costs, creating a tiered system to identify cases requiring additional research resources, scaling down the evaluations to only the most pertinent, greater use of telemedicine to decrease travel costs, and exploration of alternative funding sources. However, each of these have inherent limitations, and network discussions are ongoing.
Certain elements of the clinical sites’ investigations could potentially be implemented into clinical practice. Clinicians could ask laboratories for a list of candidate genes and for variants in disease genes that were not reported due to poor phenotypic fit when ES reports are non-diagnostic. If there is discordance between a laboratory report and the clinician’s interpretation, further discussions with the laboratory for possible reasons (such as alternative transcripts)40 may result in resolution. Clinicians could periodically curate VUS through population and disease databases, apart from waiting for ES reanalysis by the original testing laboratory. Candidate gene follow-up is feasible in clinical settings, when successful case-matching and collaborations occur without extended efforts. However, time and reimbursement constraints and lack of network expertise and resources are barriers to clinicians performing the extensive activities of the UDN clinical sites.
Our study has limitations. The retrospective design could have resulted in recall bias in the clinical sites’ assessment of the UDN-driven activities that contribute to diagnoses, and it is possible that for some diagnoses the level of assertion may change over time, with additional data. Lack of objective data from the general genetics clinics at the UDN sites for many variables (such as data on other research collaborations that the genetics clinics may have established for non-diagnostic ES), precluded more direct comparisons. We also do not have the time interval between the original ES and the reanalysis for the non-diagnostic ES (both from the UDN cohort and the corresponding genetics clinics), thus tempering this comparison, since longer time intervals could result in higher diagnostic rates. We do not describe the role of internal collaborators such as the MOSC in detail (publications listed in Table S3), since the focus was on the clinical sites.
In conclusion, the UDN is a unique research network that directly benefits patients and simultaneously conducts rigorous research. The UDN clinical sites are integral elements of the network, incorporating critical research into the clinical evaluations to obtain incremental diagnoses and pursuing novel candidate genes to causality through collaborative science. Our analysis indicated that 65% of the UDN diagnoses would not be achieved in typical clinical settings. UDN-driven investigations inform the science and practice of genomic medicine. Even as both the clinical and research milieus are rapidly evolving with emerging technological advances, the network has the expertise and infrastructure to be on the frontier of new diagnostic paradigms for rare and ultra-rare diseases.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all of the individuals and their families for their participation in this study. Research reported in this manuscript was supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director under Award Number(s) U01HG007672 (Duke University), U01HG007708 (Stanford Medicine), U01HG007674 (Vanderbilt University Medical Center), and U01HG007530 (Coordinating Center at Harvard Medical School). The NIH clinical site (UDP) was supported by the National Human Genome Research Institute (NHGRI) Intramural Research Program under Award Number HG000215-17. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
Pengfei Liu is an employee of Baylor College of Medicine and derives support through a professional services agreement with Baylor Genetics, which performs clinical genetic testing services.
Matthew T Wheeler is a stockholder of Personalis Inc.
David B. Goldstein is a founder of and holds equity in Q State Biosciences and Praxis Therapeutics; holds equity in Apostle Inc., and serves as a consultant to AstraZeneca, Gilead Sciences and GoldFinch Bio, outside the submitted work.
Undiagnosed Diseases Network Member List:
Maria T. Acosta, Margaret Adam, David R. Adams, Pankaj B. Agrawal, Mercedes E. Alejandro, Justin Alvey, Laura Amendola, Ashley Andrews, Euan A. Ashley, Mahshid S. Azamian, Carlos A. Bacino, Guney Bademci, Eva Baker, Ashok Balasubramanyam, Dustin Baldridge, Jim Bale, Michael Bamshad, Deborah Barbouth, Pinar Bayrak-Toydemir, Anita Beck, Alan H. Beggs, Edward Behrens, Gill Bejerano, Jimmy Bennet, Beverly Berg-Rood, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, Stephanie Bivona, Elizabeth Blue, John Bohnsack, Carsten Bonnenmann, Devon Bonner, Lorenzo Botto, Brenna Boyd, Lauren C. Briere, Elly Brokamp, Gabrielle Brown, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, Peter Byers, William E. Byrd, John Carey, Olveen Carrasquillo, Ta Chen Peter Chang, Sirisak Chanprasert, Hsiao-Tuan Chao, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, Matthew Coggins, F. Sessions Cole, Heather A. Colley, Cynthia M. Cooper, Heidi Cope, William J. Craigen, Andrew B. Crouse, Michael Cunningham, Precilla D’Souza, Hongzheng Dai, Surendra Dasari, Mariska Davids, Jyoti G. Dayal, Matthew Deardorff, Esteban C. Dell’Angelica, Shweta U. Dhar, Katrina Dipple, Daniel Doherty, Naghmeh Dorrani, Emilie D. Douine, David D. Draper, Laura Duncan, Dawn Earl, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Cecilia Esteves, Tyra Estwick, Marni Falk, Liliana Fernandez, Carlos Ferreira, Elizabeth L. Fieg, Laurie C. Findley, Paul G. Fisher, Brent L. Fogel, Irman Forghani, Laure Fresard, William A. Gahl, Ian Glass, Rena A. Godfrey, Katie Golden-Grant, Alica M. Goldman, David B. Goldstein, Alana Grajewski, Catherine A. Groden, Andrea L. Gropman, Irma Gutierrez, Sihoun Hahn, Rizwan Hamid, Neil A. Hanchard, Kelly Hassey, Nichole Hayes, Frances High, Anne Hing, Fuki M. Hisama, Ingrid A. Holm, Jason Hom, Martha Horike-Pyne, Alden Huang, Yong Huang, Rosario Isasi, Fariha Jamal, Gail P. Jarvik, Jeffrey Jarvik, Suman Jayadev, Jean M. Johnston, Lefkothea Karaviti, Emily G. Kelley, Jennifer Kennedy, Dana Kiley, Isaac S. Kohane, Jennefer N. Kohler, Deborah Krakow, Donna M. Krasnewich, Elijah Kravets, Susan Korrick, Mary Koziura, Joel B. Krier, Seema R. Lalani, Byron Lam, Christina Lam, Brendan C. Lanpher, Ian R. Lanza, C. Christopher Lau, Kimberly LeBlanc, Brendan H. Lee, Hane Lee, Roy Levitt, Richard A. Lewis, Sharyn A. Lincoln, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo, Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, Marta M. Majcherska, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Kenneth Maravilla, Thomas C. Markello, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Jacob McCauley, Allyn McConkie-Rosell, Colleen E. McCormack, Alexa T. McCray, Elisabeth McGee, Heather Mefford, J. Lawrence Merritt, Matthew Might, Ghayda Mirzaa, Eva Morava, Paolo M. Moretti, Marie Morimoto, John J. Mulvihill, David R. Murdock, Mariko Nakano-Okuno, Avi Nath, Stan F. Nelson, John H. Newman, Sarah K. Nicholas, Deborah Nickerson, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina GS. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips III, Jennifer E. Posey, Lorraine Potocki, Barbara N. Pusey, Aaron Quinlan, Wendy Raskind, Archana N. Raja, Deepak A. Rao, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Natalie Rosenwasser, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Susan L. Samson, Mario Saporta, C. Ron Scott, Judy Schaechter, Timothy Schedl, Kelly Schoch, Daryl A. Scott, Prashant Sharma, Vandana Shashi, Jimann Shin, Rebecca Signer, Catherine H. Sillari, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Edward C. Smith, Kevin S. Smith, Emily Solem, Lilianna Solnica-Krezel, Rebecca C. Spillmann, Joan M. Stoler, Nicholas Stong, Jennifer A. Sullivan, Kathleen Sullivan, Angela Sun, Shirley Sutton, David A. Sweetser, Virginia Sybert, Holly K. Tabor, Cecelia P. Tamburro, Queenie K.-G. Tan, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Brianna M. Tucker, Tiina K. Urv, Adeline Vanderver, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Stephanie Wallace, Nicole M. Walley, Chris A. Walsh, Melissa Walker, Jennifer Wambach, Jijun Wan, Lee-kai Wang, Michael F. Wangler, Patricia A. Ward, Daniel Wegner, Mark Wener, Tara Wenger, Katherine Wesseling Perry, Monte Westerfield, Matthew T. Wheeler, Jordan Whitlock, Lynne A. Wolfe, Jeremy D. Woods, Shinya Yamamoto, John Yang, Guoyun Yu, Diane B. Zastrow, Chunli Zhao, Stephan Zuchner
REFERENCES
- 1.Gahl WA, Wise AL, Ashley EA. The Undiagnosed Diseases Network of the National Institutes of Health: A National Extension. JAMA. 2015;314(17):1797–1798. [DOI] [PubMed] [Google Scholar]
- 2.Fund NC, 2017. https://undiagnosed.hms.harvard.edu/ 2020. [Google Scholar]
- 3.Splinter K, Adams DR, Bacino CA, et al. Effect of Genetic Diagnosis on Patients with Previously Undiagnosed Disease. N Engl J Med. 2018;379(22):2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gahl WA, Tifft CJ. The NIH Undiagnosed Diseases Program: lessons learned. JAMA. 2011;305(18):1904–1905. [DOI] [PubMed] [Google Scholar]
- 5.https://globalgenes.org. Rare Disease Statistics. 2015.
- 6.Nguengang Wakap S, Lambert DM, Olry A, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Need AC, Shashi V, Hitomi Y, et al. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet. 2012;49(6):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Deignan JL, Dorrani N, et al. Clinical exome sequencing for genetic identification of rare mendelian disorders. JAMA. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Muzny DM, Xia F, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dustin Baldridge M, Heeley Jennifer, Vineyard Marisa,, Linda Manwaring M, Toler Tomi L., Fassi Emily, Fiala Elise,, Sarah Brown P, Goss Charles W., Willing Marcia, Grange Dorothy K.,, Beth A. Kozel M, and Shinawi Marwan. The Exome Clinic and the role of medical genetics expertise in the interpretation of exome sequencing results. Genetics in Medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowdin S, Gilbert A, Bedoukian E, et al. Recommendations for the integration of genomics into clinical practice. Genet Med. 2016. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaridis KN, McAllister TM, Babovic-Vuksanovic D, et al. Implementing individualized medicine into the medical practice. American journal of medical genetics Part C, Seminars in medical genetics. 2014;166c(1):15–23. [DOI] [PubMed] [Google Scholar]
- 13.Lazaridis KN, Schahl KA, Cousin MA, et al. Outcome of Whole Exome Sequencing for Diagnostic Odyssey Cases of an Individualized Medicine Clinic: The Mayo Clinic Experience. Mayo Clin Proc. 2016;91(3):297–307. [DOI] [PubMed] [Google Scholar]
- 14.Shashi V The utility of the traditional medical genetics diagnostic evaluation in the context of next-generation sequencing for undiagnosed genetic disorders. J Intellect Disabil Res. 2014;16(2):176–182. [DOI] [PubMed] [Google Scholar]
- 15.Williams MS, Buchanan AH, Davis FD, et al. Patient-Centered Precision Health In A Learning Health Care System: Geisinger’s Genomic Medicine Experience. Health Aff (Millwood). 2018;37(5):757–764. [DOI] [PubMed] [Google Scholar]
- 16.Bertier G, Senecal K, Borry P, Vears DF. Unsolved challenges in pediatric whole-exome sequencing: A literature analysis. Crit Rev Clin Lab Sci. 2017;54(2):134–142. [DOI] [PubMed] [Google Scholar]
- 17.Brittain HK, Scott R, Thomas E. The rise of the genome and personalised medicine. Clin Med (Lond). 2017;17(6):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volk A, Conboy E, Wical B, Patterson M, Kirmani S. Whole-Exome Sequencing in the Clinic: Lessons from Six Consecutive Cases from the Clinician’s Perspective. Molecular syndromology. 2015;6(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thevenon J, Duffourd Y, Masurel-Paulet A, et al. Diagnostic odyssey in severe neurodevelopmental disorders: toward clinical whole-exome sequencing as a first-line diagnostic test. Clin Genet. 2016;89(6):700–707. [DOI] [PubMed] [Google Scholar]
- 20.Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36(10):928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shashi V, McConkie-Rosell A, Schoch K, et al. Practical considerations in the clinical application of whole-exome sequencing. Clin Genet. 2015. [DOI] [PubMed] [Google Scholar]
- 22.Wenger AM, Guturu H, Bernstein JA, Bejerano G. Systematic reanalysis of clinical exome data yields additional diagnoses: implications for providers. Genet Med. 2017;19(2):209–214. [DOI] [PubMed] [Google Scholar]
- 23.Eldomery MK, Coban-Akdemir Z, Harel T, et al. Lessons learned from additional research analyses of unsolved clinical exome cases. Genome medicine. 2017;9(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiatt SM, Amaral MD, Bowling KM, et al. Systematic reanalysis of genomic data improves quality of variant interpretation. Clin Genet. 2018;94(1):174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, Meng L, Normand EA, et al. Reanalysis of Clinical Exome Sequencing Data. N Engl J Med. 2019;380(25):2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewans LJ, Schofield D, Shrestha R, et al. Whole-exome sequencing reanalysis at 12 months boosts diagnosis and is cost-effective when applied early in Mendelian disorders. Genet Med. 2018;20(12):1564–1574. [DOI] [PubMed] [Google Scholar]
- 27.Wright CF, McRae JF, Clayton S, et al. Making new genetic diagnoses with old data: iterative reanalysis and reporting from genome-wide data in 1,133 families with developmental disorders. Genet Med. 2018;20(10):1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shashi V, Schoch K, Spillmann R, et al. A comprehensive iterative approach is highly effective in diagnosing individuals who are exome negative. Genet Med. 2019;21(1):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fennell AP, Hunter MF, Corboy GP. The changing face of clinical genetics service delivery in the era of genomics: a framework for monitoring service delivery and data from a comprehensive metropolitan general genetics service. Genet Med. 2020;22(1):210–218. [DOI] [PubMed] [Google Scholar]
- 30.Williams JL, Faucett WA, Smith-Packard B, Wagner M, Williams MS. An assessment of time involved in pre-test case review and counseling for a whole genome sequencing clinical research program. J Genet Couns. 2014;23(4):516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukenik-Halevy R, Ludman MD, Ben-Shachar S, Raas-Rothschild A. The time-consuming demands of the practice of medical genetics in the era of advanced genomic testing. Genet Med. 2016;18(4):372–377. [DOI] [PubMed] [Google Scholar]
- 32.Maiese DR, Keehn A, Lyon M, Flannery D, Watson M. Current conditions in medical genetics practice. Genet Med. 2019;21(8):1874–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attard CA, Carmany EP, Trepanier AM. Genetic counselor workflow study: The times are they a-changin’? J Genet Couns. 2019;28(1):130–140. [DOI] [PubMed] [Google Scholar]
- 34.Undiagnosed Diseases Network, 2014. Accessed 25 Sep 2017. [Google Scholar]
- 35.Zastrow DB, Kohler JN, Bonner D, et al. A toolkit for genetics providers in follow-up of patients with non-diagnostic exome sequencing. J Genet Couns. 2019;28(2):213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MyGene2, 2020. http://www.mygene2.org.
- 37.Shashi V, Geist J, Lee Y, et al. Heterozygous variants in MYBPC1 are associated with an expanded neuromuscular phenotype beyond arthrogryposis. Hum Mutat. 2019;40(8):1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuter CM, Kohler JN, Bonner D, et al. Yield of whole exome sequencing in undiagnosed patients facing insurance coverage barriers to genetic testing. J Genet Couns. 2019;28(6):1107–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köhler S, Doelken SC, Mungall CJ, et al. The Human Phenotype Ontology project: linking molecular biology and disease through phenotype data. Nucleic Acids Res. 2014;42(D1):D966–D974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoch K, Tan QK, Stong N, et al. Alternative transcripts in variant interpretation: the potential for missed diagnoses and misdiagnoses. Genet Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.