Abstract
The capillary bed constitutes the obligatory pathway for almost all oxygen (O2) and substrate molecules as they pass from blood to individual cells. As the largest organ, by mass, skeletal muscle contains a prodigious surface area of capillaries that have a critical role in metabolic homeostasis and must support energetic requirements that increase as much as 100-fold from rest to maximal exercise. In 1919 Krogh’s 3 papers, published in the Journal of Physiology, brilliantly conflated measurements of muscle capillary function at rest and during contractions with Agner K. Erlang’s mathematical model of O2 diffusion. These papers single-handedly changed the perception of capillaries from passive vessels serving at the mercy of their upstream arterioles into actively contracting vessels that were recruited during exercise to elevate blood-myocyte O2 flux. Although seminal features of Krogh’s model have not withstood the test of time and subsequent technological developments, Krogh is credited with helping found the field of muscle microcirculation and appreciating the role of the capillary bed and muscle O2 diffusing capacity in facilitating blood-myocyte O2 flux. Today, thanks in large part to Krogh, it is recognized that comprehending the role of the microcirculation, as it supports perfusive and diffusive O2 conductances, is fundamental to understanding skeletal muscle plasticity with exercise training and resolving the mechanistic bases by which major pathologies including heart failure and diabetes cripple exercise tolerance and cerebrovascular dysfunction predicates impaired executive function.
Keywords: Exercise, capillary hemodynamics, red blood cell flux, O2 uptake kinetics
Introduction
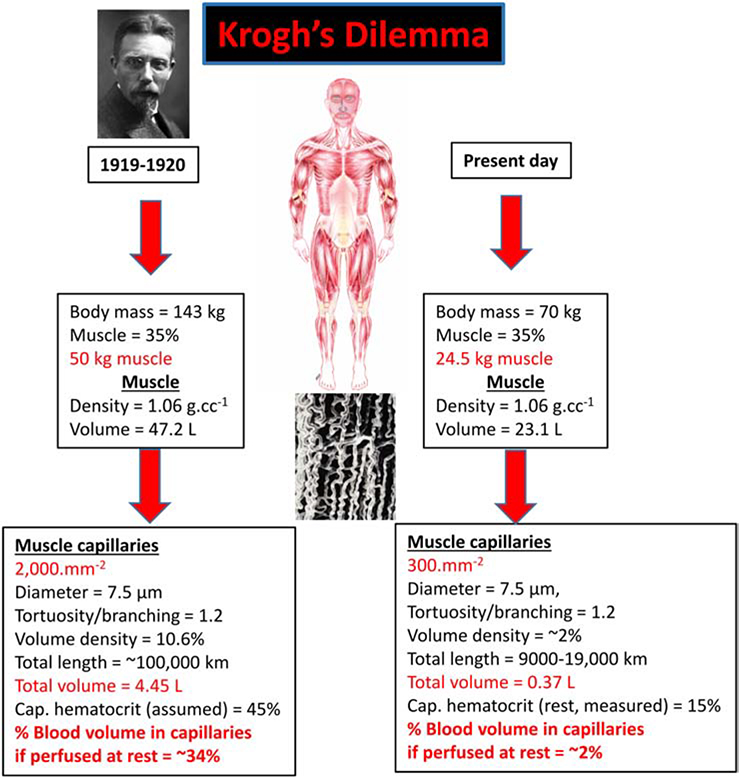
Shack August Steenberg Krogh (1874–1949) was a superb experimentalist who could conceive and fabricate state-of-the art purpose-built research instruments. These capabilities empowered his great intellect and unstoppable curiosity enabling him to address some of the most pressing scientific questions of the day, in physiology and beyond. One of his personal beliefs was that “Questions worthy of attack, show their worth by fighting back” a saying from the Danish polymath, Piet Hein, (27) and his measurements, as depicted in Fig. 1 (left side), emphasized the foundational dilemma regarding capillary function that he solved with his “capillary recruitment” theory. For his estimates of the total length of capillaries in an adult human, 100,000 km (!) (17), there simply was not sufficient blood volume to allow continuous perfusion of this vast network of vessels with a combined blood volume of nearly 5 L! The individual Krogh considered had 50 kg of muscle and for muscle to constitute ~35% of this body mass, this was an extraordinarily large person (143 kg, 315 lb). Their total blood volume at 8% body mass would have been ~12 L and skeletal muscle would have required 34% of that volume - which he quite rightly considered untenable.
Figure 1. Krogh’s dilemma that helped formulate the capillary recruitment hypothesis.
Left side: When Krogh calculated the total length of capillaries in skeletal muscle (17, page 10) he arrived at a total length of 100,000 km which captured the attention of scientists and school children the world over by being sufficient to circumscribe the Earth nearly three times at the equator! His calculation used a capillary density of 2,000.mm−2, a value several-fold higher than human muscle (24) and also considered an individual with 50 kg of skeletal muscle (which is possible, but extraordinary). This yielded a prodigious total capillary volume and Krogh, without evidence to the contrary, considered that capillaries contained the same hematocrit as systemic blood (i.e., ~45%). Krogh’s model of capillary recruitment was his solution to the hemodynamic infeasibility of devoting a third of total blood volume to the skeletal muscle capillary bed at rest. Right side: Using contemporary values for quadriceps muscle capillary density and a more reasonable estimate of skeletal muscle mass together with the substantially lower capillary hematocrit (~15%) measured in rodent muscles at rest (5,24,26), the blood volume devoted to the almost fully-perfused capillary bed is only ~2% of total blood volume.
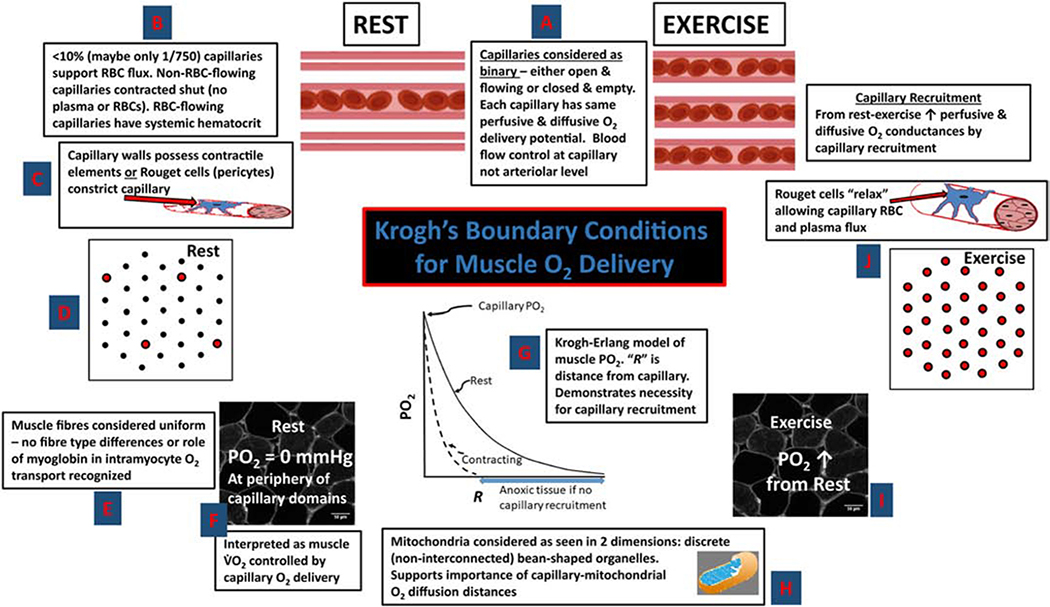
From his intravital microscopy observations in the tongue of deeply anesthetized frogs and other muscles and species, as well as India ink (carbon) infusions in muscles at rest, during contractions and post-mortem, Krogh spied a potential solution (18–20). What if, at least at rest, most capillaries were shut and did not support red blood cell (RBC) or plasma flux (Fig. 2)? During muscle contractions/exercise, these capillaries would then open and receive blood flow (capillary recruitment). For this schema to work, Krogh needed a mechanism for closing off capillaries to blood flow and so he considered that the capillaries themselves, or later, following Bjovulf J. Vimtrup’s experiments in Krogh’s laboratory (28), that Rouget’s cells (later termed pericytes) spontaneously constricted the capillary (rev. 24). At rest Krogh considered that a cyclical dilation and flow occurred among capillaries when some constrictor substance, which Krogh called “pituitin” was metabolized and temporarily released the impediment to flow through that particular capillary (17).
Figure 2. Central tenets to Krogh’s muscle capillary recruitment theory.
(anti-clockwise from top centre) (18–20): A. Capillaries have a binary function, either open and flowing or closed (no RBCs or plasma). All open capillaries (at rest or during contractions) are considered to have the same O2 delivery. B. At rest (non-contracting) most capillaries not flowing (i.e., closed’). C. Capillaries initially thought to be contractile, later Rouget cells (pericytes) considered to constrict the capillary. D. Cross section of resting muscle showing most capillaries closed. E. Discrete fibre types and role of myoglobin to transport O2 intracellularly not recognized. F. Erroneous O2 delivery limitation of at rest interpreted as intramyocyte PO2 of 0 mmHg. G. Krogh-Erlang model hypothesizes progressive fall in PO2 with distance “R” from capillary. H. Mitochondria considered independent “bean-shaped” structures reinforces notion of limiting “diffusion distances.” I. Exercise and capillary recruitment considered to elevate intramyocyte PO2. J. All capillaries recruited and flowing during contractions via relaxation of Rouget cells which increases muscle O2 diffusing capacity and facilitates elevated . Adapted from (24).
During exercise, essentially all capillaries were recruited and, in this fashion, intracapillary diffusion distances were decreased and overall muscle O2 diffusing capacity was increased to facilitate a ten-fold or so increase in metabolic rate (i.e., O2 upta e, ). A central piece of this puzzle was the work of Norwegian-born Torbjorn Gaarder in fish, performed in Krogh’s laboratory of Zoophysiology in Copenhagen, which supported the notion that muscle , even at rest, was O2 supply dependent (rev. 24). From this Krogh concluded that the PO2 was either zero or close to zero at the extremities from open capillaries. Moreover, Krogh considered that each capillary had the same RBC flux and O2 delivery potential that was unchanged between rest and exercise (Fig. 2). Thus, the only mechanism by which blood-muscle O2 flux could increase by 10-fold or so was by capillary recruitment (20).
To this point Krogh had made measurements of the muscle O2 diffusion constant, “D”, under hyperoxic conditions, that were remarkably close to those measured many decades later – a superb achievement (rev. 24). These measurements combined with his capillary counts, formed the basis for his 3rd Nobel prizewinning paper (20) and the one that he considered “most interesting” (24,27). Thus, by conflating his measurements of muscle capillaries and recruitment theory with a “D” that could only change by means of capillary recruitment, he and Erlang calculated the PO2 profiles in Fig. 2 (see central graph, refs. 18–20). With so few capillaries supporting RBC flux at rest, capillary recruitment was requisite for preventing massive muscle anoxia during exercise. In fact, the Krogh-Erlang equation predicted that intramyocyte PO2 increased during exercise. Further, the Krogh-Erlang model held that capillaries represented pinpoint sources of O2 delivery such that the tissue volume supplied by a capillary increased with distance from that capillary: In itself a most inefficient system (6).
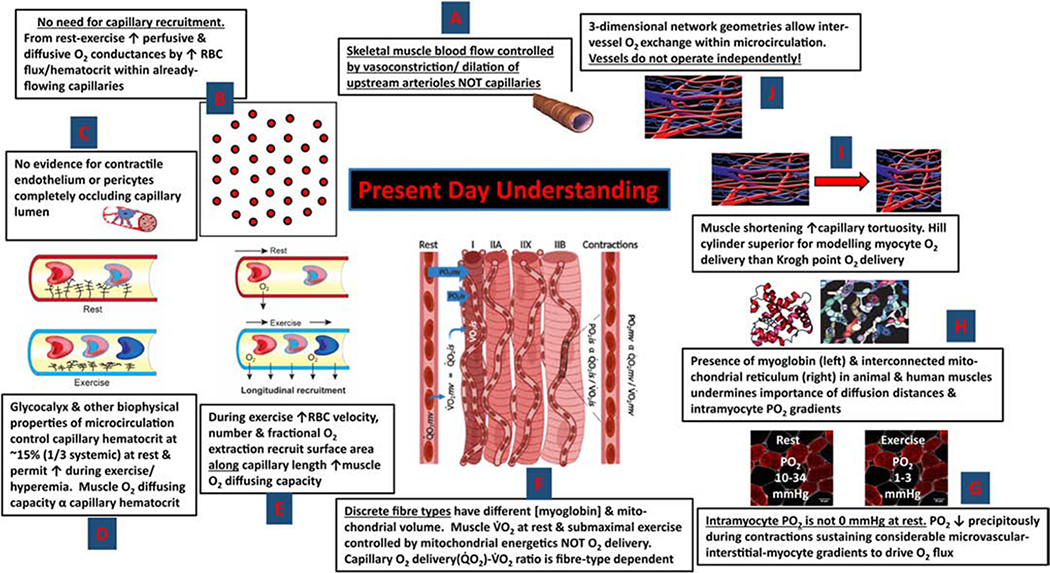
Figure 3 updates many of the seminal features of Krogh’s model with discoveries made over the intervening century and paints a very different picture of skeletal muscle O2 delivery at rest and during exercise. Firstly, note that, as before Krogh the principal site of muscle blood flow control has returned to the arterioles upstream of the capillaries (rev. 18). From Fig. 1 (right side) capillary density measurements from human quadriceps demonstrate that 300.mm−2 is more reasonable than 2,000.mm−2 (rev. 24) as is a muscle mass of ~23 kg (not 50 kg). Also, the observation of Damon & Duling (4) and others (5,26, rev 24) that capillary hematocrit may only be ~15% (due, in part, to the presence of the glycocalyx (4,5)) reduces the maximum blood volume necessary to fill the capillary bed to less than 1/10th that considered by Krogh (17), at least at rest (see Fig. 1, right side). Despite intensive investigation and sporadic reports of endothelial cells or pericytes narrowing the capillary lumen (9) no compelling evidence has been presented that either process can either fully constrict or dilate a capillary (rev. 24), and certainly not within the very few seconds necessary to explain the rapid dynamics required to support in vivo muscle kinetics (2,8,14,26, rev 24). Not only have many laboratories demonstrated the RBC flux in most skeletal muscle capillaries at rest under well-controlled physiological conditions (negating the presence of, or necessity for, capillary recruitment), but it is now recognized that capillary RBC velocity and flux as well as hematocrit vary hugely among capillaries – even those supplied by a common arteriole (4,15, rev 24). This evidence, combined with the realization that it is the number of RBCs within flowing capillaries adjacent to the contracting muscle fibers that determines tissue O2 diffusing capacity (7), challenges Krogh’s notion of capillaries each contributing equally to O2 delivery and having a solely binary function (i.e., either open or closed, ref 1).
Figure 3. Contemporary understanding of muscle capillary function and O2 delivery and myocyte PO2 at rest and during exercise.
(anti-clockwise from top centre): A. Capillary perfusion is under upstream arteriolar control (21). B. As most capillaries support flow at rest there is no mandate for de novo capillary recruitment (24,26). Increased O2 delivery occurs predominantly by elevated RBC velocity, flux and hematocrit in already-flowing capillaries (24). C. There is no compelling evidence that the skeletal muscle capillary endothelium is contractile or that pericytes close off capillaries at rest and allow rapid opening during exercise (24). D. The endothelial surface layer or glycocalyx helps reduce capillary hematocrit below systemic (4,5,24,26). E. Increased blood-myocyte O2 flux may occur by better utilizing capillary surface along the length of already flowing capillaries (i.e., longitudinal recruitment) (24). F. Skeletal muscles are comprised of discrete fibre types that vary in mitochondrial volume and oxidative potential with slow-twitch muscles regulating their microvascular PO2 higher at rest and during contractions than their fast-twitch counterparts (24). G. Intramyocyte PO2 at rest is between 10–34 mmHg and may fall as low as 1–3 mmHg during exercise without detectable gradients (13,24,25). H. Myoglobin (especially deoxymyoglobin) and the interconnected mitochondrial reticulum may facilitate intramyocyte O2 and possibly proton transport (3,24). I. Capillary tortuosity and branching, especially in shortened/contracted muscles, support the Hill cylinder geometry for modelling myocyte O2 delivery versus the Krogh-Erlang “point” delivery model (6). The Hill model is more efficient as the tissue volume supplied decreases, rather than increases, with distance from the capillaries. J. Capillaries are not each independent (or uniform) units of O2 supply and there are important interactions among capillaries as well as arterioles and venules (24).
Figure 3 also demonstrates that different fibre types, not recognized in Krogh’s day, have different mitochondrial volume densities and myoglobin content. That mitochondria form an interconnected network that may span the distance between sarcolemma and fibre center combined with the ability of (particularly) deoxygenated myoglobin (Mb) to transport O2 within the myocyte might negate the concept of intramuscular diffusion distances altogether (3): As indeed the physiological evidence supports (10). Direct measurements of microvascular and interstitial space PO2s in rats (Fig. 4A and E) and intramyocyte PO2s in dogs (13) and humans (25) demonstrate very different PO2 profiles from capillary to myocyte than hypothesized by Krogh (compare Krogh-Erlang graph in Fig. 2G with graph in Fig. 4E). In addition, it is now known that the 3-dimensional network of blood vessels (arterioles, capillaries and venules) facilitates significant intervessel O2 exchange (15) and, especially for contracted muscles with tortuous and branched capillaries (Fig 3i and j), the Hill cylinder geometry, where tissue volume supplied by a given capillary decreases with distance from that capillary, is more appropriate and efficient for O2 delivery (6, rev 24).
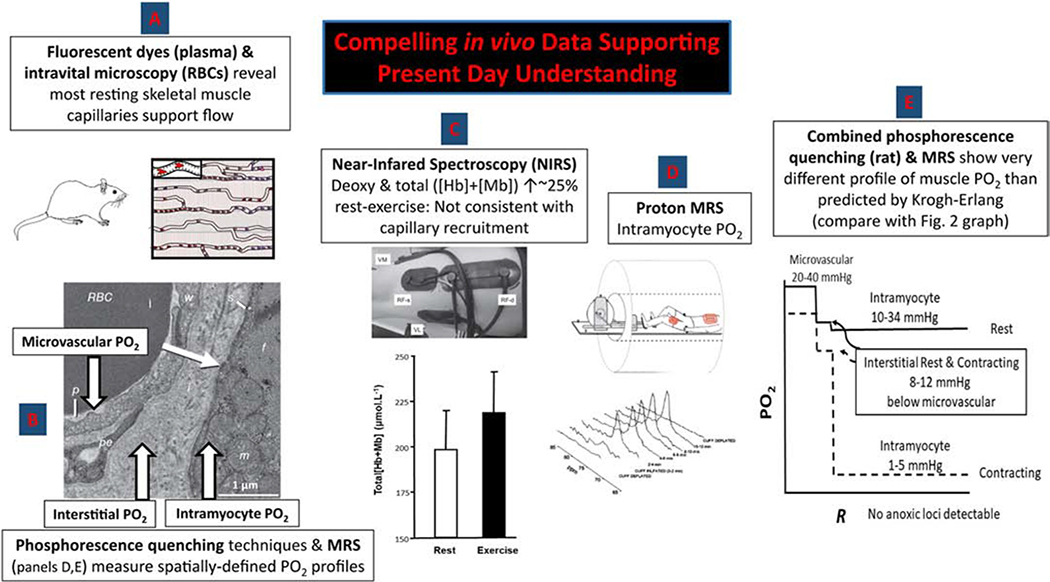
Figure 4. in vivo techniques and data supporting contemporary model of capillary function and muscle oxygenation.
A. Fluorescent dyes (plasma-borne) and intravital microscopy reveal the presence of plasma and/or RBC flow in the vast majority of capillaries in resting rodent muscles (24). B. Phosphorescence quenching techniques permit high-fidelity measurements of microvascular and interstitial PO2 in resting and contracting rodent muscles. RBC, red blood cell in capillary lumen; p, plasma in capillary; pe, pericyte; w, endothelial cell forming capillary wall; s, myocyte sarcolemma; f, myofibrils; m, mitochondrial reticulum (11,12). C. Time-resolved near-infrared spectroscopy (NIRS) facilitates measurement of absolute concentrations of deoxyhemoglobin (Hb)+myoglobin (Mb) and total [Hb+Mb] in resting and contracting human muscles demonstrating only a modest increase in [Hb] from rest to exercise (16). An observation inconsistent with substantial capillary recruitment. D. Proton magnetic resonance spectroscopy (MRS) permits measurement of intramyocyte PO2 at rest and during exercise (25). E. In contrast to the Krogh-Erlang’s progressive decrease of PO2 with distance from the capillary (“R”) note the substantial step decreases in that short physical space between the microvascular and interstitial spaces and on into the myocyte where the PO2 is extremely low and the profile very flat (24). Intramyocyte PO2s are thus far higher at rest and far lower during exercise than hypothesized by Krogh. Rather than simply recruiting more capillaries, the contemporary interpretation places great emphasis on dynamic events within the capillaries (i.e., changing RBC flux, velocity, hematocrit) for regulating perfusive and diffusive O2 delivery and the intramyocyte space for regulating diffusive O2 delivery.
Key to furthering our knowledge of microcirculatory function are the techniques presented in Figure 4 that constitute a level of interrogation far beyond that available to Krogh. Several of these are pertinent here including phosphorescence-quenching determination of microvascular and interstitial PO2’s (11,12) and MRS for resolution of intramuscular PO2 (25). But it is Near–Infrared Spectroscopy (NIRS) that is, perhaps, most widely used in humans. A veritable plethora of NIRS investigations have provided support for the concept that little capillary recruitment actually occurs in human muscle from rest to exercise (e.g., 16, Figure 4C). Specifically, supposing, as did Krogh, that at least 90% of capillaries were closed in resting muscle (no plasma, no RBCs) and these were then recruited during exercise such that they contained RBCs. The muscle hemoglobin+myoglobin concentration ([Hb+Mb]), which NIRS measures, would be expected to increase enormously (i.e., reflecting at least a 10-fold increase in [Hb]). However, actual measurements demonstrate typically a 20–30% elevation (14,24) and this can be achieved, at least in theory, by elevating the capillary hematocrit as has been demonstrated by contractions and other hyperemic states (4,5,26 rev 24)).
Why is it so important that we understand capillary function and the mechanistic bases for blood-myocyte O2 flux?
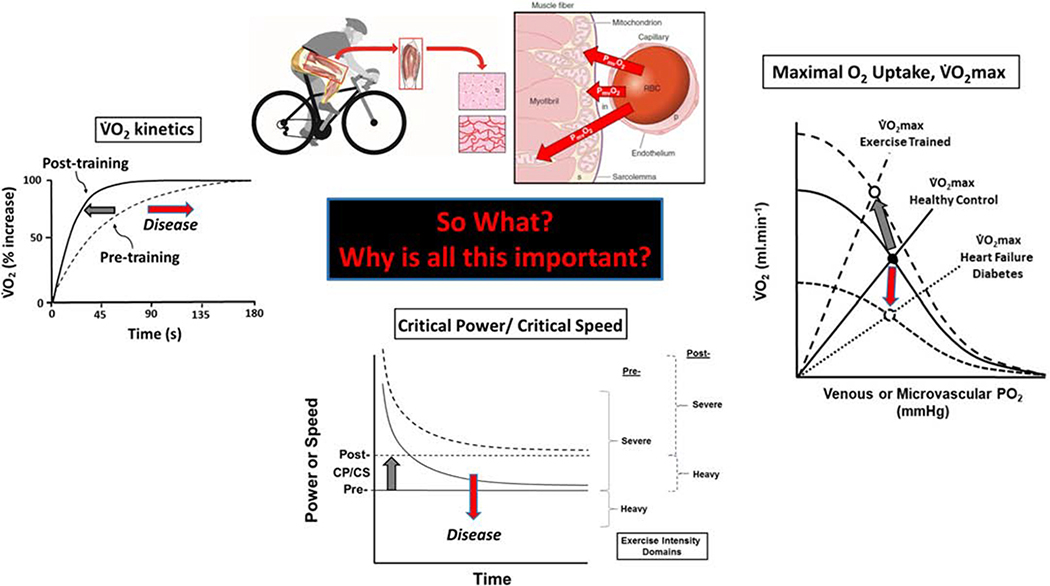
The skeletal muscle capillary endothelium presents a truly vast surface area – far bigger than a tennis court - for blood-myocyte interchange of O2, substrates such as glucose, amino acids and free-fatty acids as well as a host of metabolites and signaling molecules. If it is not recognized that most of that surface is available in health, then predations of diseases such as heart failure (12,26) and Type II diabetes (22), that reduce that surface area and therefore exchange capacity remain silent. Although it is now believed that Krogh’s capillary recruitment mechanism, by which contracted-shut capillaries opened to flow at the onset of exercise, is untenable he taught us to consider the function of the capillary bed as a whole. Moreover, his work helped us to consider that blood-muscle O2 flux is symbiosis of perfusive and diffusive O2 fluxes – although the former is now recognized to be controlled principally at the arteriolar level (21) and the latter by capillary hematocrit and longitudinal capillary recruitment (i.e., by increasing O2 exchange along the length of already-flowing capillaries (24, Fig. 3E)). Figure 5 presents three seminal parameters of aerobic function (23) inetics (rate of increase following exercise onset), critical power/critical speed (CP/CS, the highest metabolic rate, that can be sustained for a very long time’) and max (the highest achieved during large muscle mass exercise) (23). These parameters define exercise performance and each is dependent on achieving a rapid and substantial increase in blood-muscle O2 flux directed into the contracting myocytes. s exercise training improves exercise tolerance by means of speeding inetics and increasing P and max, so organic diseases (e.g., HF, ype II diabetes) and chronic inactivity that slow kinetics and decrease CP/CS and max in so doing impair exercise tolerance (23). Having an accurate model of capillary function is key to understanding the control of these parameters and their plasticity in health and disease - function and dysfunction - and designing effective therapeutic countermeasures to improve patients’ exercise tolerance, quality of life and reduce morbidity and mortality.
Figure 5. Three seminal parameters of aerobic function that are dependent on blood-myocyte O2 flux.
Left: he speed of the kinetics following the onset of dynamic exercise determines the size of the O2 deficit and thus the reliance on substrate-level phosphorylation and muscle high-energy phosphate perturbation (8). low kinetics in diseases such as heart failure (HF) and Type II diabetes (T2D) increases the O2 deficit following exercise onset and sows the seeds for exercise intolerance whereas fast kinetics are emblematic of elite distance runners and cyclists and predicate tight metabolic control and exceptional athletic performance. Center: The hyperbolic relationship between decreasing power or speed and increasing time-to-exhaustion yields two parameters: An asymptote (Critical Power, CP) and curvature constant W’ which represents a constant amount of work that can be performed above CP. The curve is typically established by having the individual cycle or run at a fixed power output (P; or speed) that exhausts them in 2–15 min. Judicious selection of 4 or 5 such exercise bouts on separate days defines P and W’ such that the limit of tolerance (tlim) = W’ (P-CP). Increased CP (notionally the highest power, or, more correctly, that can be sustained for a very long time) occurs with exercise training whereas CP decreases with diseases such as HF and T2D. Right: The eponymous Wagner diagram conflates perfusive (curved lines from Y-axis) and diffusive (straight lines from origin) O2 conductances that conflate to yield the maximal O2 upta e ( max; circles). Notice the substantial increase in both perfusive and diffusive O2 conductances that elevate the max with exercise training (large dashed lines) and their decrease with disease (small dashed lines) that conspire to reduce max. Gray arrows, exercise training; red arrows, diseases such as HF or T2D, for example. Redrawn from (23). It is crucial to understand the dominant role for capillaries in facilitating blood-myocyte O2 flux – as Krogh recognized – for that process dictates each of these parameters of aerobic function. By so doing the mechanistic role of capillary functional impairments in major diseases can be determined and more effective therapeutic countermeasures designed: Be they behavioral (e.g. physical activity, exercise training), pharmacological, or nutritional.
Highlights:
Krogh’s 1919 Nobel prizewinning papers conflated muscle capillary function at rest and during contractions with measurements of oxygen (O2) diffusion to produce a novel theory of muscle O2 delivery.
Essential components of Krogh’s theory included most capillaries being contracted shut at rest and being recruited during muscle contractions to increase O2 delivery and decrease O2 diffusion distances.
Discoveries in the intervening 100 years have moved the site of capillary blood flow upstream to the arterioles: There is no compelling evidence for capillary contractility or active closure.
Most capillaries support flow at rest and increased O2 delivery during contractions occurs via elevated blood flow and velocity within already flowing capillaries and recruitment of exchange surface along the capillaries (longitudinal recruitment).
Today it is recognized that capillaries do not have a simple binary function (i.e., open or closed) but changes in capillary hemodynamics increase perfusive and diffusive O2 conductances during contractions.
The impact of intramuscular and intramyocyte diffusion distances is mitigated by elevated intracapillary (increased hematocrit) and intramyocyte (myoglobin deoxygenation, mitochondrial interconnectivity) O2 diffusing capacity.
Footnotes
Conflict of interest
The authors of our manuscript titled “August Krogh: Muscle capillary function and oxygen delivery” have no conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angleys H & Østergaard L (2020). Krogh’s capillary recruitment hypothesis, 100 years on: Is the opening of previously closed capillaries necessary to ensure muscle oxygenation during exercise? Am J Physiol Heart Circ Physiol. 318, H425–H447. [DOI] [PubMed] [Google Scholar]
- 2.Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI & Poole DC (2002). Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol 133, 229–39. [DOI] [PubMed] [Google Scholar]
- 3.Clanton TL (2019). Managing the power grid: how myoglobin can regulate PO2 and energy distribution in skeletal muscle. J Appl Physiol (1985). 126, 787–790. [DOI] [PubMed] [Google Scholar]
- 4.Damon DH & Duling BR (1985). Evidence that capillary perfusion heterogeneity is not controlled in striated muscle. Am J Physiol 249, H386–392. [DOI] [PubMed] [Google Scholar]
- 5.Desjardins C & Duling BR (1990). Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol 258, H647–6454. [DOI] [PubMed] [Google Scholar]
- 6.Ellis CG, Potter RF & Groom AC (1983). The Krogh cylinder geometry is not appropriate for modelling O2 transport in contracted skeletal muscle. Adv Exp Med Biol 159, 253–268. [DOI] [PubMed] [Google Scholar]
- 7.Federspiel WJ, Popel AS (1986). A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res 32, 164–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi B, Hogan MC, & Gladden LB (2020). Microvascular O2 delivery and O2 utilization during metabolic transitions in skeletal muscle. One-hundred years after the pioneering work by August Krogh. Comparative Biochemistry and Physiology, Part A, In Press. [DOI] [PubMed] [Google Scholar]
- 9.Grubb S, Lauritzen M, & Aalkjaer C (2020). Brain capillary pericytes and neurovascular coupling. Comparative Biochemistry and Physiology, Part A, In Press. [DOI] [PubMed] [Google Scholar]
- 10.Hepple RT, Hogan MC, Stary C, Bebout DE, Mathieu-Costello O & Wagner PD (2000). Structural basis of muscle O2 diffusing capacity: evidence from muscle function in situ. J Appl Physiol 88, 560–566. [DOI] [PubMed] [Google Scholar]
- 11.Hirai DM, Colburn TD, Craig JC, Hotta K, Kano Y, Musch TI & Poole DC (2019). Skeletal muscle interstitial O2 pressures: bridging the gap between the capillary and myocyte. Microcirculation. 26, e12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai DM, Musch TI, Poole DC (2015). Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol. 309, H1419–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honig CR, Gayeski TE & Groebe K (1997). Myoglobin and oxygen gradients In: Crystal RG, West JB, Weibel ER & Barnes PJ (Eds.) The Lung: Scientific Foundations. New York, Raven Press. [Google Scholar]
- 14.Kindig CA, Richardson TE & Poole DC (2002). Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol 92, 2513–2520. [DOI] [PubMed] [Google Scholar]
- 15.Kissane RWP, Al-Shammari AA, & Egginton SE (2020). The importance of capillary distribution in supporting muscle function, building on Krogh’s seminal ideas. Comparative Biochemistry and Physiology, Part A, In Press. [DOI] [PubMed] [Google Scholar]
- 16.Koga S, Barstow TJ, Okushima D, Rossiter HB, Kondo N, Ohmae E, & Poole DC (2015). Validation of a high-power, time-resolved, near-infrared spectroscopy system for measurement of superficial and deep muscle deoxygenation during exercise. J Appl Physiol (1985). 118, 1435–1442. [DOI] [PubMed] [Google Scholar]
- 17.Krogh A (1922). The Anatomy and Physiology of Capillaries New Haven: Yale University Press. [Google Scholar]
- 18.Krogh A (1919a). The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol 52, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krogh A (1919b). The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol 52, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krogh A (1919c). The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 52, 457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ (2012). Peripheral circulation. Compr Physiol 2, 321–447. [DOI] [PubMed] [Google Scholar]
- 22.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI & Poole DC (2006). Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol. 291, H2439–2444. [DOI] [PubMed] [Google Scholar]
- 23.Poole DC, Behnke BJ & Musch TI (2020a). The role of vascular function on exercise capacity in health and disease [published online ahead of print, 2020 Jan 24]. J Physiol. 2020;10.1113/JP278931. doi: 10.1113/JP278931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole DC, Pittman RN, Musch TI & Østergaard L (2020b). ugust Krogh’s theory of muscle microvascular control and oxygen delivery: A paradigm shift based on new data. J. Physiol. In press, July 2020. [DOI] [PubMed] [Google Scholar]
- 25.Richardson RS, Duteil S, Wary C, Wray DW, Hoff J & Carlier PG (2006). Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson TE, Kindig CA, Musch TI & Poole DC (2003). Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol (1985). 95, 1055–1062. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Nielsen B (1995). August and Marie Krogh: Lives in Science. American Physiological Society, Oxford. [Google Scholar]
- 28.Vimtrup B (1922). Beiträge zur Anatomie der Capillaren. I. Über contraktile Elemente in der Gefasswand der Blutkapillaren. Zeitschrift für Anatomie und Entwicklungsgeschichte 65, 150–182. [Google Scholar]