Abstract
Background:
Limited studies have investigated racial/ethnic survival disparities for breast cancer (BC) defined by estrogen receptor (ER) and progesterone receptor (PR) status in a multiethnic population.
Methods:
Using multivariable Cox proportional hazards models, we assessed associations of race/ethnicity with ER/PR-specific BC mortality in 10,366 Californian women diagnosed with BC from 1993-2009. We evaluated joint associations of race/ethnicity, healthcare, sociodemographic, and lifestyle factors with mortality.
Results:
Among women with ER/PR+ BC, BC-specific mortality was similar among Hispanic and Asian American women, but higher among African American women (hazard ratio (HR) 1.31, 95% confidence interval 1.05-1.63) compared to non-Hispanic White (NHW) women. BC-specific mortality was modified by surgery type, hospital type, education, neighborhood socioeconomic status (SES), smoking history, and alcohol consumption. Among African American women, BC-specific mortality was higher among those treated at non-accredited hospitals (HR 1.57, CI 1.21-2.04) and those from lower SES neighborhoods (HR 1.48, CI 1.16-1.88) compared to NHW women without these characteristics. BC-specific mortality was higher among African American women with at least some college education (HR 1.42, CI 1.11-1.82) compared to NHW women with similar education. For ER−/PR− disease, BC-specific mortality did not differ by race/ethnicity and associations of race/ethnicity with BC-specific mortality varied only by neighborhood SES among African American women.
Conclusions:
Racial/ethnic survival disparities are more striking for ER/PR+ than ER−PR− BC. Social determinants and lifestyle factors may explain some of the survival disparities for ER/PR+ BC.
Impact:
Addressing these factors may help reduce the higher mortality of African American women with ER/PR+ BC.
Introduction
Breast cancer (BC) mortality rates in the United States (U.S.) have declined by 40% since 1989, but disparities persist (1,2) and are widening between African American and non-Hispanic White (NHW) women (3). Compared to NHW women, African American women have worse BC survival, Hispanic women have worse or similar survival, and Asian American women have similar or better survival (4–6). Tumor biology, treatment, healthcare, patient characteristics, medical history, behavioral factors, and social determinants have been shown to affect BC survival (7–10), but questions remain about the drivers of the observed survival disparities (6,11–13). Survival is lower for estrogen receptor (ER) negative and progesterone receptor (PR) negative (ER−/PR−) BC than ER or PR positive (ER/PR+) BC (14–18). ER−/PR− BC accounts for about 20% of new BC diagnoses, and is more frequently diagnosed among African American and Hispanic women (19). Studies that examined racial/ethnic survival disparities for BC defined by ER, PR, human epidermal growth factor receptor 2 (HER2), or other tumor markers (14,20–26) and underlying factors are largely limited to comparisons of African American and NHW women; only one study has examined subtype-specific survival in a more diverse sample of BC patients (11). Less is known about the factors contributing to the generally better BC survival of Hispanic and Asian American women compared to NHW and African American women. A better understanding of the contributing factors that may be specific to particular racial/ethnic groups is critical for guiding tailored approaches aimed at reducing BC survival disparities.
To address these gaps in knowledge, especially for Hispanic and Asian American women, we pooled multiethnic data from the California Breast Cancer Survivorship Consortium (CBCSC) (27) and the Northern California Breast Cancer Family Registry (NC-BCFR) (28). Using the wealth of cancer registry and questionnaire data that have been harmonized across the studies in CBCSC, we assessed associations of race/ethnicity with ER/PR-specific mortality and variations by selected healthcare, sociodemographic, and lifestyle characteristics.
Materials and Methods
Study sample
The CBCSC harmonized cancer registry and questionnaire data from six population-based BC studies conducted in California (27). The present analysis is based on three population-based case-control studies of BC [the Asian American Breast Cancer Study (AABCS) (29); the Women’s Contraceptive and Reproductive Experiences Study (CARE) (30); and the San Francisco Bay Area Breast Cancer Study (SFBCS) (31)] and two cohort studies [the California Teachers Study (CTS) (32); and the Multiethnic Cohort (MEC) (33)], and includes 9,701 women diagnosed from 1993-2007 with an invasive BC and more than 30 days of follow-up. In addition, we included data from the NC-BCFR which enrolled women newly diagnosed with BC into a prospective family study (28). After excluding women who also participated in SFBCS (n=320) or CTS (n=23), the NC-BCFR contributed data on 2,647 invasive BC cases diagnosed from 1995-2009 with more than 30 days of follow-up. Cases who did not self-identify as African American, Asian American, Hispanic, or NHW were excluded (N=80), leaving 12,268 in the pooled dataset. Of these, 15.5% had missing ER or PR status. ER/PR-specific analyses were based on 8,163 ER/PR+ cases and 2,203 ER−/PR− cases. We could not classify the BC cases by HER2 status, because the California Cancer Registry (CCR) did not collect data on HER2 until 1999, and data were substantially incomplete before 2005 (23,34).
BC cases were linked to the CCR to ascertain vital status and underlying cause of death, if deceased. The CCR conducts follow-up by linking cancer cases to state and national databases, including the National Death Index. Follow-up time was defined as the time from diagnosis to study end date (December 31, 2010), last known contact, or death, whichever occurred first. Mean follow-up time was 8.7 years. Study participants consented by written informed consent or receipt by mail of a completed questionnaire. The studies were approved by the Institutional Review Board of each participating institution and the California State Committee for the Protection of Human Subjects.
Study variables
Each parent study collected data using its own structured questionnaire. Questionnaire and cancer registry data were harmonized according to common definitions developed for the CBCSC (27) and applied to the NC-BCFR. CCR data included ER and PR status, age and year at diagnosis, American Joint Committee on Cancer stage, histology, grade, nodal involvement, tumor size, subsequent cancers, receipt of first-course treatment (surgery type, radiation, chemotherapy), hospital type, marital status and neighborhood socioeconomic status (SES) at diagnosis. Neighborhood SES is a composite measure at the census block-group level of seven SES indicators, including education, occupation, employment, household income, poverty, rent, and house value (35), which were linked to CCR geocodes of address at diagnosis. Neighborhood SES was based on 1990 U.S. Census data for cases diagnosed prior to 1996, and on 2000 Census data for cases diagnosed from 1996-2007. For NC-BCFR cases, those diagnosed from 2006-2009 were assigned neighborhood SES values based on 2010 Census data. Neighborhood SES was categorized into quintiles based on California state-wide distributions. The CCR records the first facility reporting each cancer case. As previously described (36), hospitals were categorized as i) National Cancer Institute-designated Cancer Centers (NCI-CC) as of 2010 (37), ii) American College of Surgeons Cancer Program (ACOS-CP; i.e., Academic Comprehensive Cancer Program, Comprehensive Community Cancer Program, Community Cancer Program) (38), or iii) other. Information was collected by structured questionnaires administered by interview or submitted by mail on self-identified race/ethnicity, education, and pre-diagnosis parity, weight, height, smoking history, and alcohol consumption. Body mass index (kg/m2, BMI) was calculated using reported or measured weight (kg) at least 6 months before BC diagnosis divided by height squared (m2).
Statistical analysis
Multivariable Cox proportional hazards models were fit to data to estimate hazard ratios (HR) and 95% confidence intervals (CI) for BC-specific mortality and all-cause mortality. Given that survival is relatively high for BC, we considered both mortality outcomes. We assessed mortality by race/ethnicity separately for i) all BC cases combined (including cases with unknown ER/PR status), ii) ER+ cases, iii) ER− cases, iv) PR+ cases, v) PR− cases, vi) ER/PR+ cases and stratified by stage (I/II vs. III/IV), and vii) ER−/PR− cases and stratified by stage. To examine the influence of different sets of prognostic factors on racial/ethnic survival disparities, we conducted three models in sequence. Model 1, the base model, included age and year of diagnosis. Model 2 included variables in Model 1 and histology, grade, nodal involvement, tumor size, subsequent tumors, and receipt of first course treatment (surgery, radiation, chemotherapy). Model 3 included variables in Model 2 and marital status, education, neighborhood SES, parity, BMI, smoking history, and alcohol consumption. The Cox models used attained age as the time scale, and were stratified by study and stage to allow the baseline hazard functions within each model to vary by study and stage. Covariates included in the models followed the analytic approach developed for the CBCSC analyses (27,36,39–42). All covariates included a category for missing data and were categorized as shown in the footnotes of Table 3. Heterogeneity in HR estimates by race/ethnicity was assessed using the Wald test.
Table 3.
Breast cancer-specific and all-cause mortality, for breast cancer overall and by tumor estrogen receptor and progesterone receptor status, stage at diagnosis, and race/ethnicity
| Breast cancer-specific mortality | All-cause mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Model 1 HR (95% CI) 1 |
Model 2 HR (95% CI) 2 |
Model 3 HR (95% CI) 3 |
Deaths | Model 1 HR (95% CI) 1 |
Model 2 HR (95% CI) 2 |
Model 3 HR (95% CI) 3 |
|
| n | n | N | |||||||
| All breast cancer 4 | 12,268 | 1,686 | 3,052 | ||||||
| Non-Hispanic White | 5,245 | 627 | 1.0 | 1.0 | 1.0 | 1,330 | 1.0 | 1.0 | 1.0 |
| Hispanic | 2,596 | 303 | 0.90 (0.76-1.07) | 0.88 (0.74-1.04) | 0.85 (0.70-1.02) | 512 | 0.87 (0.77-0.99) | 0.88 (0.77-1.00) | 0.76 (0.63-0.87) |
| African American | 2,403 | 514 | 1.54 (1.33-1.78) | 1.45 (1.25-1.68) | 1.27 (1.08-1.49) | 853 | 1.44 (1.29-1.60) | 1.38 (1.23-1.54) | 1.11 (0.98-1.25) |
| Asian American | 2,024 | 242 | 0.91 (0.71-1.17) | 0.87 (0.68-1.12) | 0.91 (0.70-1.18) | 357 | 0.83 (0.69-1.02) | 0.81 (0.66-0.99) | 0.84 (0.68-1.03) |
| P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | ||||
| ER+ breast cancer | 7,890 | 920 | 1,827 | ||||||
| Non-Hispanic White | 3,621 | 362 | 1.0 | 1.0 | 1.0 | 861 | 1.0 | 1.0 | 1.0 |
| Hispanic | 1,343 | 254 | 0.86 (0.68-1.09) | 0.83 (0.66-1.06) | 0.83 (0.64-1.07) | 458 | 0.89 (0.75-1.05) | 0.89 (0.75-1.05) | 0.74 (0.62-0.89) |
| African American | 1,656 | 153 | 1.72 (1.42-2.10) | 1.69 (1.39-2.07) | 1.42 (1.13-1.78) | 287 | 1.59 (1.38-1.83) | 1.57 (1.35-1.81) | 1.20 (1.02-1.41) |
| Asian American | 1,270 | 151 | 1.03 (0.76-1.41) | 1.00 (0.73-1.37) | 1.09 (0.79-1.51) | 221 | 0.94 (0.74-1.20) | 0.91 (0.71-1.16) | 0.96 (0.74-1.24) |
| P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | ||||
| ER− breast cancer | 2,521 | 475 | 693 | ||||||
| Non-Hispanic White | 875 | 169 | 1.0 | 1.0 | 1.0 | 252 | 1.0 | 1.0 | 1.0 |
| Hispanic | 648 | 149 | 0.92 (0.67-1.27) | 0.93 (0.67-1.28) | 0.89 (0.62-1.28) | 221 | 0.86 (0.66-1.12) | 0.90 (0.69-1.17) | 0.83 (0.61-1.12) |
| African American | 618 | 103 | 1.07 (0.80-1.42) | 1.13 (0.84-1.51) | 1.00 (0.73-1.37) | 144 | 1.10 (0.87-1.39) | 1.16 (0.91-1.47) | 0.97 (0.75-1.27) |
| Asian American | 380 | 54 | 0.87 (0.53-1.42) | 0.84 (0.51-1.38) | 0.86 (0.51-1.45) | 76 | 0.72 (0.48-1.09) | 0.71 (0.47-1.09) | 0.75 (0.48-1.16) |
| P=0.72 | P=0.47 | P=0.85 | P=0.08 | P=0.05 | P=0.38 | ||||
| PR+ breast cancer | 6,456 | 717 | 1,428 | ||||||
| Non-Hispanic White | 2,944 | 280 | 1.0 | 1.0 | 1.0 | 678 | 1.0 | 1.0 | 1.0 |
| Hispanic | 1,063 | 198 | 0.81 (0.62-1.05) | 0.82 (0.63-1.08) | 0.79 (0.59-1.06) | 347 | 0.87 (0.72-1.05) | 0.90 (0.75-1.09) | 0.74 (0.59-0.91) |
| African American | 1,390 | 121 | 1.54 (1.24-1.92) | 1.54 (1.23-1.93) | 1.30 (1.00-1.68) | 227 | 1.51 (1.29-1.78) | 1.51 (1.28-1.78) | 1.14 (0.95-1.37) |
| Asian American | 1,059 | 118 | 0.98 (0.69-1.38) | 0.95 (0.67-1.35) | 1.03 (0.72-1.49) | 176 | 0.91 (0.69-1.19) | 0.87 (0.66-1.15) | 0.95 (0.71-1.26) |
| P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | ||||
| PR− breast cancer | 3,428 | 625 | 966 | ||||||
| Non-Hispanic White | 1,349 | 235 | 1.0 | 1.0 | 1.0 | 387 | 1.0 | 1.0 | 1.0 |
| Hispanic | 774 | 183 | 0.92 (0.70-1.22) | 0.88 (0.67-1.18) | 0.80 (0.59-1.10) | 281 | 0.86 (0.69-1.08) | 0.86 (0.69-1.08) | 0.76 (0.59-0.98) |
| African American | 821 | 132 | 1.27 (0.99-1.63) | 1.23 (0.96-1.59) | 1.05 (0.79-1.38) | 193 | 1.24 (1.02-1.51) | 1.24 (1.02-1.52) | 1.00 (0.80-1.25) |
| Asian American | 484 | 75 | 0.95 (0.63-1.43) | 0.91 (0.60-1.37) | 0.89 (0.58-1.38) | 105 | 0.87 (0.62-1.23) | 0.85 (0.60-1.21) | 0.83 (0.58-1.19) |
| P=0.06 | P=0.06 | P=0.30 | P<0.01 | P<0.01 | P=0.07 | ||||
| ER/PR+ breast cancer | 8,163 | 964 | 1,890 | ||||||
| Non-Hispanic White | 3,709 | 381 | 1.0 | 1.0 | 1.0 | 888 | 1.0 | 1.0 | 1.0 |
| Hispanic | 1,703 | 161 | 0.85 (0.68-1.07) | 0.84 (0.67-1.06) | 0.84 (0.65-1.08) | 295 | 0.87 (0.74-1.03) | 0.88 (0.75-1.04) | 0.74 (0.61-0.88) |
| African American | 1,419 | 264 | 1.59 (1.31-1.92) | 1.58 (1.30-1.92) | 1.31 (1.05-1.63) | 475 | 1.51 (1.31-1.73) | 1.50 (1.30-1.72) | 1.14 (0.97-1.33) |
| Asian American | 1,332 | 158 | 0.98 (0.73-1.33) | 0.96 (0.71-1.30) | 1.06 (0.77-1.46) | 232 | 0.90 (0.71-1.15) | 0.88 (0.69-1.12) | 0.94 (0.73-1.20) |
| P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | ||||
| Stage I or II | 7,355 | 676 | 1,520 | ||||||
| Non-Hispanic White | 3,388 | 271 | 1.0 | 1.0 | 1.0 | 738 | 1.0 | 1.0 | 1.0 |
| Hispanic | 1,499 | 100 | 0.86 (0.66-1.13) | 0.80 (0.61-1.04) | 0.73 (0.54-0.98) | 217 | 0.88 (0.73-1.05) | 0.87 (0.73-1.05) | 0.70 (0.57-0.86) |
| African American | 1,246 | 189 | 1.84 (1.48-2.29) | 1.63 (1.31-2.04) | 1.27 (0.99-1.62) | 381 | 1.64 (1.41-1.91) | 1.57 (1.35-1.83) | 1.16 (0.97-1.37) |
| Asian American | 1,222 | 116 | 0.93 (0.66-1.31) | 0.85 (0.60-1.21) | 0.94 (0.65-1.35) | 184 | 0.87 (0.67-1.13) | 0.83 (0.63-1.08) | 0.90 (0.68-1.18) |
| P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | ||||
| Stage III or IV | 634 | 263 | 320 | ||||||
| Non-Hispanic White | 240 | 104 | 1.0 | 1.0 | 1.0 | 129 | 1.0 | 1.0 | 1.0 |
| Hispanic | 173 | 54 | 0.87 (0.57-1.33) | 1.04 (0.67-1.62) | 1.23 (0.75-2.02) | 70 | 0.90 (0.61-1.32) | 1.08 (0.72-1.60) | 1.05 (0.67-1.64) |
| African American | 127 | 65 | 1.34 (0.91-1.97) | 1.39 (0.92-2.11) | 1.28 (0.80-2.04) | 75 | 1.27 (0.89-1.82) | 1.27 (0.87-1.86) | 1.04 (0.68-1.61) |
| Asian American | 94 | 40 | 1.03 (0.55-1.92) | 1.08 (0.56-2.08) | 1.04 (0.52-2.08) | 46 | 0.91 (0.50-1.65) | 0.96 (0.51-1.79) | 0.89 (0.46-1.70) |
| P =0.14 | P =0.37 | P =0.74 | P =0.24 | P =0.58 | P =0.97 | ||||
| ER−/PR− breast cancer | 2,203 | 420 | 613 | ||||||
| Non-Hispanic White | 778 | 145 | 1.0 | 1.0 | 1.0 | 220 | 1.0 | 1.0 | 1.0 |
| Hispanic | 560 | 94 | 0.98 (0.69-1.39) | 0.98 (0.69-1.40) | 0.95 (0.64-1.42) | 134 | 0.91 (0.68-1.22) | 0.96 (0.72-1.29) | 0.90 (0.65-1.25) |
| African American | 553 | 135 | 1.25 (0.92-1.70) | 1.29 (0.94-1.77) | 1.16 (0.82-1.64) | 195 | 1.24 (0.96-1.60) | 1.31 (1.01-1.70) | 1.09 (0.82-1.45) |
| Asian American | 312 | 46 | 0.92 (0.55-1.55) | 0.88 (0.52-1.50) | 0.89 (0.51-1.55) | 64 | 0.79 (0.51-1.23) | 0.80 (0.51-1.25) | 0.80 (0.50-1.29) |
| P = 0.29 | P = 0.19 | P = 0.60 | P = 0.03 | P = 0.02 | P = 0.42 | ||||
| Stage I or II | 1,860 | 297 | 462 | ||||||
| Non-Hispanic White | 649 | 100 | 1.0 | 1.0 | 1.0 | 163 | 1.0 | 1.0 | 1.0 |
| Hispanic | 471 | 62 | 1.01 (0.68-1.51) | 1.00 (0.67-1.50) | 0.92 (0.59-1.44) | 97 | 0.91 (0.66-1.25) | 1.00 (0.72-1.38) | 0.86 (0.60-1.23) |
| African American | 463 | 101 | 1.51 (1.08-2.12) | 1.45 (1.03-2.05) | 1.27 (0.86-1.86) | 153 | 1.38 (1.05-1.81) | 1.45 (1.10-1.91) | 1.13 (0.83-1.55) |
| Asian American | 277 | 34 | 0.96 (0.55-1.69) | 0.91 (0.51-1.62) | 0.93 (0.50-1.72) | 49 | 0.78 (0.49-1.25) | 0.85 (0.52-1.38) | 0.87 (0.52-1.45) |
| P = 0.03 | P = 0.06 | P = 0.34 | P < 0.01 | P < 0.01 | P = 0.30 | ||||
| Stage III or IV | 285 | 113 | 131 | ||||||
| Non-Hispanic White | 106 | 43 | 1.0 | 1.0 | 1.0 | 48 | 1.0 | 1.0 | 1.0 |
| Hispanic | 77 | 28 | 0.95 (0.48-1.88) | 1.09 (0.52-2.28) | 1.20 (0.46-3.09) | 33 | 0.91 (0.66-1.25) | 1.25 (0.63-2.49) | 1.35 (0.55-3.34) |
| African American | 70 | 30 | 1.10 (0.58-2.08) | 1.26 (0.61-2.62) | 1.02 (0.42-2.48) | 35 | 1.05 (0.55-2.00) | 1.27 (0.64-2.52) | 0.92 (0.40-2.11) |
| Asian American | 32 | 12 | 0.66 (0.20-2.11) | 0.62 (0.18-2.20) | 0.54 (0.11-2.68) | 15 | 0.80 (0.28-2.32) | 0.67 (0.22-2.08) | 0.46 (0.11-1.95) |
| P = 0.84 | P = 0.71 | P = 0.84 | P = 0.85 | P = 0.64 | P = 0.56 | ||||
Abbreviations: AJCC, American Joint Committee on Cancer; BMI, body mass index; CCR, California Cancer Registry; CI, confidence interval; ER/PR+, estrogen receptor-positive or progesterone receptor-positive; ER−/PR−, estrogen receptor-negative and progesterone receptor-negative; HR, hazard ratio; NHW, non-Hispanic white; SES, socioeconomic status.
Model 1 used delayed entry Cox proportional hazards regression with attained age (days) as the time scale. Entry date into the risk set is the later of date of questionnaire completion or date of BC diagnosis. The exit date is earliest of date of death, last follow-up date in CCR, or December 31, 2010. The Cox model was stratified by study (AABCS, CARE, CTS, MEC, NC-BCFR, SFBCS) and AJCC stage (I, II, III, IV, unknown) and included age at diagnosis (years), log transformed age at diagnosis, and year of diagnosis.
Model 2 included Model 1 variables and histology (ductal, lobular, other), grade (I, II, III/IV, unknown), nodal involvement (no, yes, unknown), availability of tumor size as continuous measure (yes, no) and tumor size (continuous), diagnoses of subsequent cancers (yes, no), time between diagnoses of subsequent tumors (days), surgery type (none, mastectomy, breast conserving surgery, other), radiation therapy (yes, no), and chemotherapy (yes, no, unknown).
Model 3 included Model 2 variables and marital status at diagnosis (single or never married, married, separated or divorced, widowed, unknown), education (some high school or less, high school graduate, some college or technical school, college graduate or higher degree, unknown), neighborhood SES at diagnosis (quintiles, unknown), number of full-term pregnancies (nulliparous, 1, 2, 3, ≥4, unknown), BMI (<25, 25-29.9, ≥30 kg/m2, unknown) in year before diagnosis (case-control studies) or within 6 months of diagnosis (cohort studies), pre-diagnosis smoking history (never, past, current, unknown), and alcohol consumption (0, ≤2, >2 drinks per week, unknown) in year before diagnosis (case-control studies) or within 6 months of diagnosis (cohort studies).
Includes all women regardless of ER or PR status.
For both case groups (ER/PR+ BC and ER−/PR− BC), we evaluated associations of race/ethnicity with BC-specific and all-cause mortality and variations by healthcare factors (surgery type, hospital type), sociodemographic characteristics (age at diagnosis, marital status, education, neighborhood SES), and lifestyle factors (BMI, smoking history, alcohol consumption). Cases were classified jointly by race/ethnicity and each dichotomized explanatory variable (8 subgroups for each mortality outcome). In models for each factor, we examined racial/ethnic variation in mortality associated with each dichotomized factor (low vs. high risk) and estimated HRs and 95% CIs for each combination of race/ethnicity x factor, with NHW women and the lower-risk level of each factor as the referent category. Analyses were performed using SAS, version 9.4, software (SAS Institute, Inc., Cary, North Carolina).
Results
Patient characteristics
African American women were more likely to be diagnosed with BC stage II or higher and less likely to receive initial care at a NCI-CC or ACOS-CP hospital, whereas Asian American women were most likely to have received a mastectomy (Table 1). NHW women were more likely to have a college degree or higher alcohol consumption; Hispanic women were more likely to have lower education and higher parity; African American women were more likely to be unmarried at diagnosis, a current smoker, or live in lower SES neighborhoods; and Asian American women were more likely to be married, have a BMI <25 kg/m2, and not smoke or consume alcohol (Table 2). Differences in patient characteristics by joint ER/PR status are shown in Supplemental Tables 1 and 2.
Table 1.
Clinical and healthcare characteristics of women with breast cancer 1, by race/ethnicity
| Non-Hispanic White N=4487 |
Hispanic N=2,263 |
African American N=1,972 |
Asian American N=1,644 |
Total N=10,366 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Study 2 | ||||||||||
| AABCS | 0 | 0 | 0 | 0 | 0 | 0 | 817 | 50 | 817 | 8 |
| CARE | 428 | 10 | 61 | 3 | 374 | 19 | 0 | 0 | 863 | 8 |
| CTS | 2,701 | 60 | 79 | 3 | 62 | 3 | 85 | 5 | 2,927 | 28 |
| MEC | 301 | 7 | 467 | 21 | 558 | 28 | 116 | 7 | 1,442 | 14 |
| NC-BCFR | 568 | 13 | 684 | 30 | 530 | 27 | 626 | 38 | 2,408 | 23 |
| SFBCS | 489 | 11 | 972 | 43 | 448 | 23 | 0 | 0 | 1,909 | 18 |
| AJCC stage at diagnosis | ||||||||||
| I | 2,365 | 53 | 983 | 43 | 789 | 40 | 750 | 46 | 4,887 | 47 |
| II | 1,672 | 37 | 987 | 44 | 920 | 47 | 749 | 46 | 4,328 | 42 |
| III | 261 | 6 | 215 | 10 | 144 | 7 | 103 | 6 | 723 | 7 |
| IV | 85 | 2 | 35 | 2 | 53 | 3 | 23 | 1 | 196 | 2 |
| Unknown | 104 | 2 | 43 | 2 | 66 | 3 | 19 | 1 | 232 | 2 |
| Histology | ||||||||||
| Ductal | 3,214 | 72 | 1,754 | 78 | 1,519 | 77 | 1,281 | 78 | 7,768 | 75 |
| Lobular | 899 | 20 | 322 | 14 | 261 | 13 | 214 | 13 | 1,696 | 16 |
| Other | 374 | 8 | 187 | 8 | 192 | 10 | 149 | 9 | 902 | 9 |
| Grade | ||||||||||
| I | 1,040 | 23 | 363 | 16 | 276 | 14 | 240 | 15 | 1,919 | 19 |
| II | 1,798 | 40 | 845 | 37 | 634 | 32 | 682 | 41 | 3,959 | 38 |
| III or IV | 1,279 | 29 | 875 | 39 | 882 | 45 | 618 | 38 | 3,654 | 35 |
| Unknown | 370 | 8 | 180 | 8 | 180 | 9 | 104 | 6 | 834 | 8 |
| Nodal involvement | ||||||||||
| No nodes | 3,007 | 67 | 1,352 | 60 | 1,171 | 59 | 1,006 | 61 | 6,536 | 63 |
| Positive nodes | 1,363 | 30 | 862 | 38 | 731 | 37 | 608 | 37 | 3,564 | 34 |
| Unknown | 117 | 3 | 49 | 2 | 70 | 4 | 30 | 2 | 266 | 3 |
| Tumor size (cm) | ||||||||||
| <1 | 897 | 20 | 333 | 15 | 229 | 12 | 257 | 16 | 1,713 | 17 |
| 1-<5 | 3,146 | 70 | 1,670 | 74 | 1,480 | 75 | 1,221 | 74 | 7,517 | 73 |
| ≥5 | 274 | 6 | 187 | 8 | 185 | 9 | 135 | 8 | 781 | 8 |
| Unknown | 173 | 4 | 73 | 3 | 78 | 4 | 31 | 2 | 355 | 3 |
| Had 1 or more subsequent cancers | ||||||||||
| No | 3,677 | 82 | 1,977 | 87 | 1,650 | 84 | 1,387 | 84 | 8,691 | 84 |
| Yes | 810 | 18 | 286 | 13 | 322 | 16 | 257 | 16 | 1,675 | 16 |
| Surgery 3 | ||||||||||
| No surgery | 58 | 1 | 28 | 1 | 64 | 3 | 16 | 1 | 166 | 2 |
| Mastectomy | 1,652 | 37 | 941 | 42 | 753 | 38 | 840 | 51 | 4,186 | 40 |
| Breast-conserving surgery | 2,772 | 62 | 1,288 | 57 | 1,152 | 58 | 786 | 48 | 5,998 | 58 |
| Other | 5 | <1 | 6 | <1 | 3 | <1 | 2 | <1 | 16 | <1 |
| Radiation therapy 3 | ||||||||||
| No | 1,881 | 42 | 952 | 42 | 963 | 49 | 869 | 53 | 4,665 | 45 |
| Yes | 2,606 | 58 | 1,311 | 58 | 1,009 | 51 | 775 | 47 | 5,701 | 55 |
| Chemotherapy 3 | ||||||||||
| No | 2,659 | 59 | 991 | 44 | 1,001 | 51 | 745 | 45 | 5,396 | 52 |
| Yes | 1,761 | 39 | 1,246 | 55 | 939 | 48 | 872 | 53 | 4,818 | 46 |
| Unknown | 67 | 1 | 26 | 1 | 32 | 2 | 27 | 2 | 152 | 1 |
| Hospital type | ||||||||||
| NCI Cancer Center | 249 | 6 | 130 | 6 | 87 | 4 | 123 | 7 | 589 | 6 |
| ACOS Cancer Program | 2,040 | 45 | 828 | 37 | 484 | 25 | 683 | 42 | 4,035 | 39 |
| Other | 2,198 | 49 | 1,305 | 58 | 1,401 | 71 | 838 | 51 | 5,742 | 55 |
Abbreviations: ACOS, American College of Surgeons; AJCC, American Joint Committee on Cancer; ER, estrogen receptor status; PR, progesterone receptor; NCI, National Cancer Institute.
Analysis based on 8,163 women with ER/PR+ breast cancer and 2,203 women with ER−/PR− breast cancer.
AABCS, Asian American Breast Cancer Study; CARE, Women’s Contraceptive and Reproductive Experiences Study; CTS, California Teachers Study; MEC, Multiethnic Cohort Study; NC-BCFR, Northern California Breast Cancer Family Registry; SFBCS, San Francisco Bay Area Breast Cancer Study.
Receipt of first-course treatment.
Table 2.
Sociodemographic and lifestyle characteristics of women with breast cancer 1, by race/ethnicity
| Non-Hispanic White N=4487 |
Hispanic N=2,264 |
African American N=1,972 |
Asian American N=1,644 |
Total N=10,366 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Age at diagnosis (years) | ||||||||||
| <35 | 105 | 2 | 96 | 4 | 41 | 2 | 82 | 5 | 324 | 3 |
| 35-49 | 783 | 17 | 744 | 33 | 541 | 27 | 615 | 37 | 2,683 | 26 |
| 50-64 | 2,019 | 45 | 933 | 41 | 861 | 44 | 683 | 42 | 4,496 | 43 |
| 65-79 | 1,283 | 29 | 467 | 21 | 467 | 24 | 249 | 15 | 2,466 | 24 |
| ≥80 | 297 | 7 | 23 | 1 | 62 | 3 | 15 | 1 | 397 | 4 |
| Marital status 2 | ||||||||||
| Never married | 561 | 13 | 345 | 15 | 459 | 23 | 234 | 14 | 1,599 | 15 |
| Married | 2,822 | 63 | 1,372 | 61 | 776 | 39 | 1,212 | 74 | 6,182 | 60 |
| Separated/divorced | 514 | 11 | 281 | 12 | 415 | 21 | 82 | 5 | 1,292 | 12 |
| Widowed | 520 | 12 | 217 | 10 | 271 | 14 | 103 | 6 | 1,111 | 11 |
| Unknown | 70 | 2 | 48 | 2 | 51 | 3 | 13 | 1 | 182 | 2 |
| Education | ||||||||||
| Some high school or less | 78 | 2 | 812 | 36 | 244 | 12 | 108 | 7 | 1,242 | 12 |
| High school graduate | 336 | 7 | 493 | 22 | 431 | 22 | 180 | 11 | 1,440 | 14 |
| Some college or technical school | 622 | 14 | 566 | 25 | 774 | 39 | 392 | 24 | 2,354 | 23 |
| College graduate or higher degree | 3,445 | 77 | 383 | 17 | 514 | 26 | 962 | 59 | 5,304 | 51 |
| Unknown | 6 | <1 | 9 | <1 | 9 | <1 | 2 | <1 | 26 | <1 |
| Neighborhood SES (quintiles) 2 | ||||||||||
| 1 (low) | 125 | 3 | 257 | 11 | 452 | 23 | 91 | 6 | 925 | 9 |
| 2 | 375 | 8 | 416 | 18 | 501 | 25 | 209 | 13 | 1,501 | 14 |
| 3 | 699 | 16 | 479 | 21 | 413 | 21 | 272 | 17 | 1,863 | 18 |
| 4 | 1,188 | 26 | 524 | 23 | 366 | 19 | 415 | 25 | 2,493 | 24 |
| 5 (high) | 1,991 | 44 | 551 | 24 | 214 | 11 | 638 | 39 | 3,394 | 33 |
| Unknown | 109 | 2 | 36 | 2 | 26 | 1 | 19 | 1 | 190 | 2 |
| Number of full-term pregnancies | ||||||||||
| Nulliparous | 1,080 | 24 | 316 | 14 | 334 | 17 | 417 | 25 | 2,147 | 21 |
| 1 | 646 | 14 | 296 | 13 | 377 | 19 | 282 | 17 | 1,601 | 15 |
| 2 | 1,471 | 33 | 565 | 25 | 462 | 23 | 521 | 32 | 3,019 | 29 |
| 3 | 788 | 18 | 458 | 20 | 361 | 18 | 261 | 16 | 1,868 | 18 |
| ≥ 4 | 462 | 10 | 617 | 27 | 424 | 22 | 154 | 9 | 1,657 | 16 |
| Unknown | 37 | 1 | 11 | <1 | 14 | 1 | 12 | 1 | 74 | 1 |
| BMI (kg/m2) 3 | ||||||||||
| <25 | 2,514 | 56 | 729 | 32 | 563 | 29 | 1,118 | 68 | 4,924 | 48 |
| 25-29.9 | 1,169 | 26 | 735 | 32 | 645 | 33 | 392 | 24 | 2,941 | 28 |
| ≥30 | 655 | 15 | 754 | 33 | 712 | 36 | 116 | 7 | 2,237 | 22 |
| Unknown | 149 | 3 | 45 | 2 | 52 | 3 | 18 | 1 | 264 | 3 |
| Pre-diagnosis smoking history | ||||||||||
| Never | 2,231 | 50 | 1,173 | 52 | 780 | 40 | 1,351 | 82 | 5,535 | 53 |
| Past | 1,426 | 32 | 375 | 17 | 452 | 23 | 203 | 12 | 2,456 | 24 |
| Current | 366 | 8 | 162 | 7 | 323 | 16 | 82 | 5 | 933 | 9 |
| Unknown 4 | 464 | 10 | 553 | 24 | 417 | 21 | 8 | <1 | 1,442 | 14 |
| Pre-diagnosis alcohol consumption (drinks/week) | ||||||||||
| 0 | 1,452 | 32 | 1,380 | 61 | 1,176 | 60 | 1,409 | 86 | 5,417 | 52 |
| ≤2 | 813 | 18 | 345 | 15 | 302 | 15 | 76 | 5 | 1,536 | 15 |
| >2 | 2,080 | 46 | 511 | 23 | 447 | 23 | 155 | 9 | 3,193 | 31 |
| Unknown | 142 | 3 | 27 | 1 | 47 | 2 | 4 | <1 | 220 | 2 |
Abbreviations: BMI, body mass index; ER, estrogen receptor status; PR, progesterone receptor; SES, socioeconomic status.
Analysis based on 8,163 women with ER/PR+ breast cancer and 2,203 women with ER−/PR− breast cancer.
At diagnosis.
In year before diagnosis (case-control studies) or within 6 months of diagnosis (cohort studies).
Smoking history was not assessed in an early component of SFBCS and therefore unknown for 14% of cases.
Breast cancer-specific and all-cause mortality by race/ethnicity
For all BCs combined, compared to NHW women, BC-specific mortality was greater among African American women (HR 1.54, 95% CI 1.33-1.78) in a minimally adjusted model (Model 1, Table 3), but did not differ for Hispanic and Asian American women. Additional adjustment for tumor characteristics and treatment (Model 2), and for sociodemographic and lifestyle characteristics in addition to Model 2 factors (Model 3), had the biggest impact on mortality of African American women, reducing the HR to 1.27 (CI 1.08-1.49). Compared to NHW women, mortality was marginally lower among Hispanic women (HR 0.85, CI 0.70-1.02), but did not differ among Asian American women. In the fully adjusted Model 3, all-cause mortality was lower among Hispanic women (HR 0.76, CI 0.63-0.87) than among NHW women, but did not differ from NHW women for African American or Asian American women.
Mortality patterns across racial/ethnic groups were similar for women with ER+, PR+, or ER/PR+ BC. In Model 3, for ER/PR+ BC, BC-specific mortality was greater among African Americans (HR 1.31, CI 1.05-1.63), and all-cause mortality was lower among Hispanics (HR 0.74, CI 0.61-0.88). Analyses stratified by stage at diagnosis showed heterogeneity by race/ethnicity for women with stage I/II BC, but not for those with stage III/IV BC. For ER−/PR− BC, racial/ethnic mortality differences were less pronounced than for ER/PR+ BC, and there were no differences by race/ethnicity in the fully adjusted models.
ER/PR+ breast cancer: Mortality and modifying factors
For ER/PR+ BC, joint associations of race/ethnicity and selected healthcare, sociodemographic, and lifestyle factors with mortality are presented in Supplemental Table 3. For associations with each dichotomized factor presented below, we compared women in each racial/ethnic group who had that characteristic (e.g., obese) or did not have that characteristic (e.g., not obese) to the reference group of NHW women who did not have that characteristic (e.g., not obese). Several factors modified BC-specific mortality among African American women (Figure 1). Mortality was higher among African American women who had a mastectomy (HR 1.62, CI 1.18-2.23), received initial care at a non-accredited (HR 1.57, CI 1.21-2.04), were not married (HR 1.39, CI 1.07-1.80), were from lower SES neighborhoods (HR 1.48, CI 1.16-1.88), or were obese (HR 1.52, CI 1.14-2.03), ever smokers (HR 1.51, CI 1.10-2.07), or alcohol consumers (HR 1.53, CI 1.15-2.05), compared to NHW women without these characteristics, whereas BC-specific mortality was similar for NHW and African American women without these characteristics. BC-specific mortality was also higher among African American women who were married (HR 1.44, CI 1.08-1.91), more educated (HR 1.42, CI 1.11-1.82), from higher SES neighborhoods (HR 1.34, CI 1.00-1.80), or non-obese (HR 1.34, CI 1.05-1.73), than among NHW women with comparable characteristics.
Figure 1.
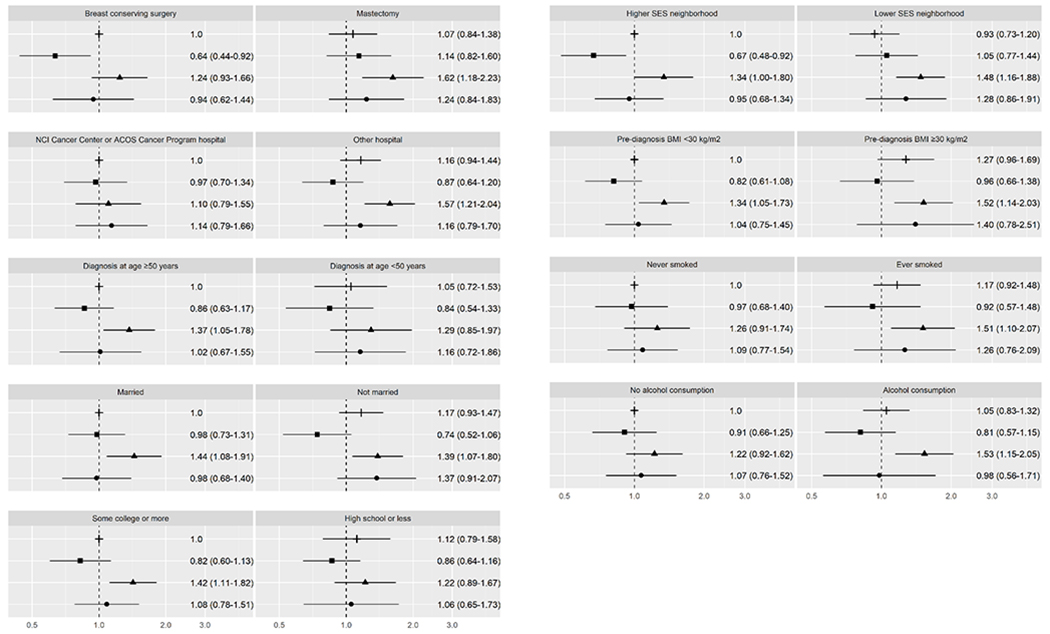
Breast cancer-specific mortality for ER+ or PR+ breast cancer. This figure depicts hazard ratios and 95% confidence intervals for joint associations of race/ethnicity and healthcare, sociodemographic, and lifestyle characteristics with breast cancer-specific mortality. The following symbols are used for each racial/ethnic group: ┼ for non-Hispanic Whites, ▪ for Hispanics, ▴ for African Americans, ● for Asian Americans.
Among Hispanic women, few factors modified BC-specific mortality. Women treated with breast-conserving surgery (HR 0.64, CI 0.44-0.92) or from higher SES neighborhoods (HR 0.67, CI 0.48-0.92) had better survival than NHW women with comparable characteristics. Among Asian American women, BC-specific mortality did not vary by any of the factors we examined. Among NHW women, BMI was the only characteristic that modified BC-specific mortality; mortality was marginally higher among obese compared to non-obese NHW women (HR 1.27, CI 0.96-1.69) (Supplemental Table 3).
For all-cause mortality (Figure 2), similar patterns emerged, although differences in HR estimates were not as pronounced. Characteristics associated with higher BC-specific mortality were also associated with higher all-cause mortality among African American women (i.e., treatment with mastectomy, receipt of initial care at a non-accredited hospital, unmarried, lower education, residence in lower SES neighborhood, obesity, and smoking history), compared to NHW women without these characteristics. HR estimates ranged from 1.24 (CI 1.03-1.49) for initial care at a non-accredited hospital to 1.47 (CI 1.17-1.85) for smoking history. All-cause mortality was also higher among African American women who were more educated (HR 1.18, CI 0.98-1.42) or non-obese (HR 1.31, CI 1.10-1.57) than NHW women with comparable characteristics. Compared to NHW women, Hispanic women had lower all-cause mortality, regardless of hospital type, age at diagnosis, marital status, or alcohol consumption. Furthermore, all-cause mortality was not higher among Hispanics who had a mastectomy, had lower education, or were obese or from lower SES neighborhoods, when compared to NHW women without these characteristics. Among Asian American women, all-cause mortality did not vary by any of the factors we examined. Among NHW women, all-cause mortality was higher among those who were not married (HR 1.18, CI 1.02-1.37), obese (HR 1.42, CI 1.18-1.70), or ever smokers (HR 1.26, CI 1.08-1.46) compared to NHW women without these characteristics (Table 2).
Figure 2.
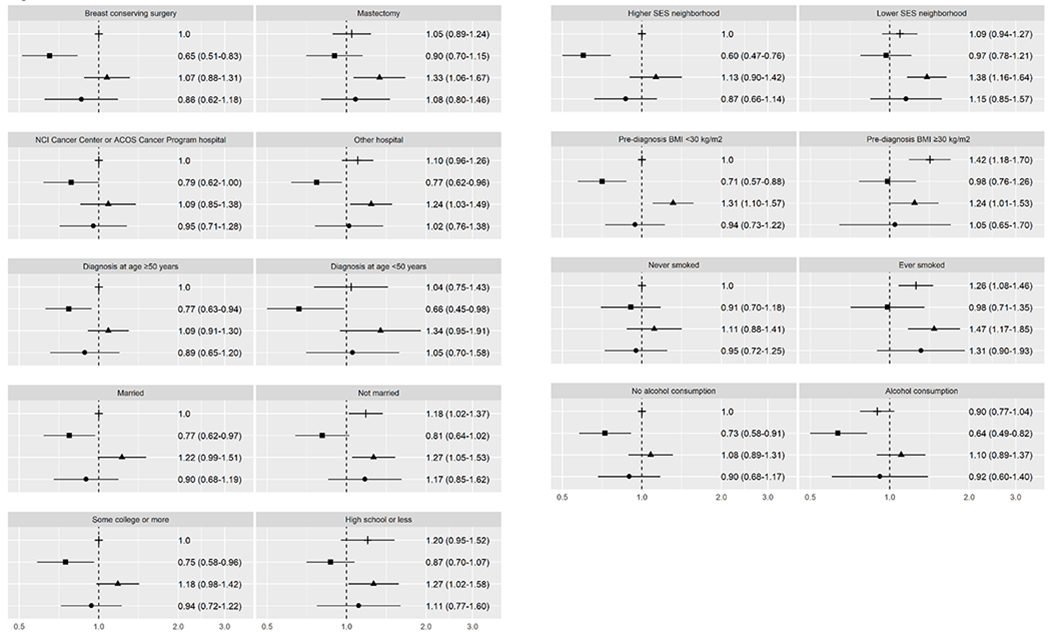
All-cause mortality for ER+ or PR+ breast cancer. This figure depicts hazard ratios and 95% confidence intervals for joint associations of race/ethnicity and healthcare, sociodemographic, and lifestyle characteristics with all-cause mortality. The following symbols are used for each racial/ethnic group: ┼ for non-Hispanic Whites, ▪ for Hispanics, ▴ for African Americans, ● for Asian Americans.
ER−/PR− breast cancer: Mortality and modifying factors
For women with ER−/PR− BC, the association of race/ethnicity with BC-specific and all-cause mortality varied by few factors (Supplemental Table 4). Compared to NHW women from higher SES neighborhoods, BC-specific mortality was higher among women from lower SES neighborhoods, both among NHW women (HR 1.39, CI 1.01-1.90) and African American women (HR 1.34, CI 0.96-1.85). BC-specific mortality was also higher among African American women (HR 1.62, CI 1.01-2.60) who never smoked compared to NHW never smokers (Figure 3). All-cause mortality (Figure 4) was higher among African American women (HR 1.42, CI 1.05-1.94) and NHW women (HR=1.45, CI 1.07-1.95) from lower SES neighborhoods compared to NHW women from higher SES neighborhoods.
Figure 3.
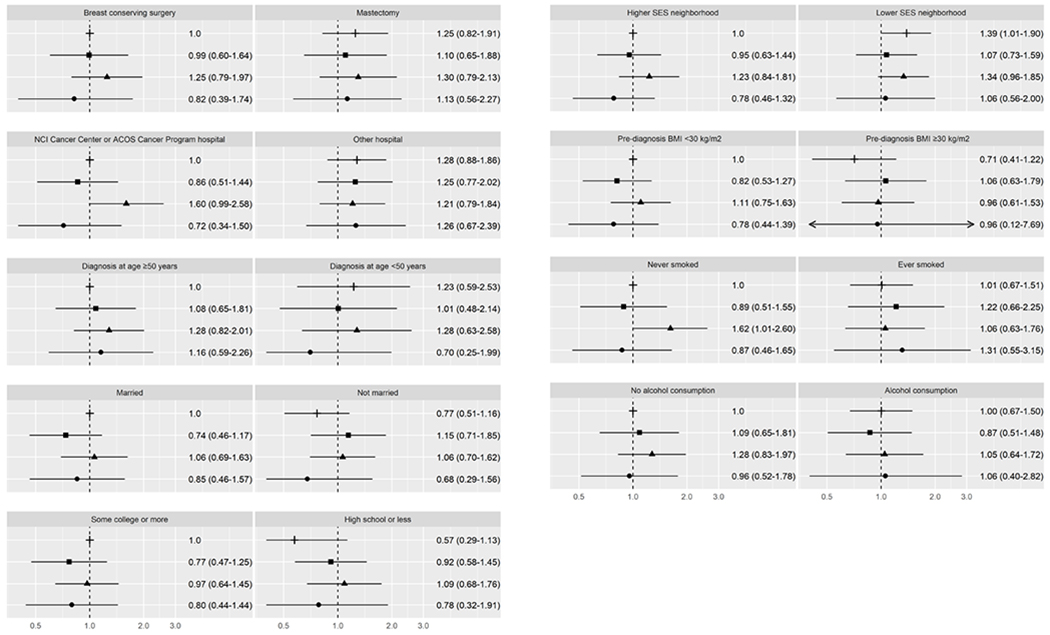
Breast cancer-specific mortality for ER− and PR− breast cancer. This figure depicts hazard ratios and 95% confidence intervals for joint associations of race/ethnicity and healthcare, sociodemographic, and lifestyle characteristics with breast cancer-specific mortality. The following symbols are used for each racial/ethnic group: ┼ for non-Hispanic Whites, ▪ for Hispanics, ▴ for African Americans, ● for Asian Americans.
Figure 4.
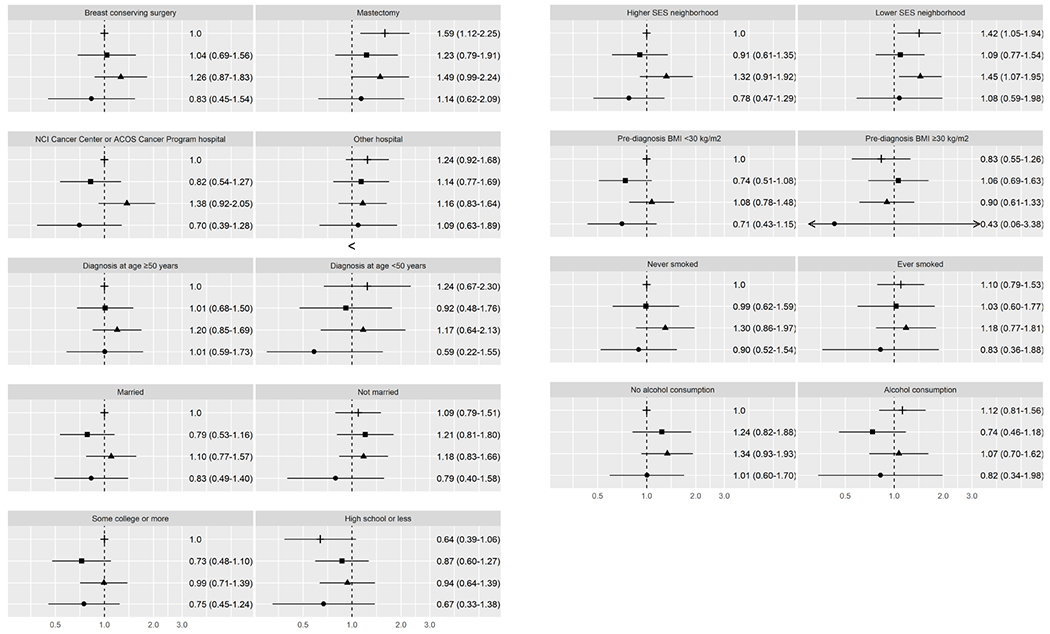
All-cause mortality for ER− and PR− breast cancer. This figure depicts hazard ratios and 95% confidence intervals for joint associations of race/ethnicity and healthcare, sociodemographic, and lifestyle characteristics with all-cause mortality. The following symbols are used for each racial/ethnic group: ┼ for non-Hispanic Whites, ▪ for Hispanics, ▴ for African Americans, ● for Asian Americans.
Discussion
In this study of over 10,000 Californian women with BC, enriched for racial/ethnic minority groups, we found differential racial/ethnic patterns in mortality by ER/PR status. For women with ER/PR+ BC, we found higher BC-specific mortality among African American women compared to NHW women and lower all-cause mortality among Hispanic women compared to NHW women, whereas for women with ER−/PR− BC, mortality did not differ by race/ethnicity. Assessing the joint associations of race/ethnicity and healthcare, sociodemographic, and lifestyle characteristics with mortality, we identified several factors (surgery type, marital status, neighborhood SES, BMI, smoking history, and alcohol consumption) that modified associations with race/ethnicity, except for Asian American women. In contrast, for ER−PR− BC, we found that associations of race/ethnicity with mortality varied only by neighborhood SES. Through analyses that considered the joint associations of race/ethnicity and healthcare, sociodemographic, and lifestyle characteristics, we gained additional insights into factors that may modify mortality differently across the four racial/ethnic groups, particularly in African American and Hispanic women.
Although the inclusion of sociodemographic and lifestyle characteristics attenuated the increased relative hazards for mortality among African American women, BC-specific mortality remained higher for BC overall and for ER/PR+ BC, which is consistent with other reports of higher mortality among African American women with BC (11,21–24). Among women with ER/PR+ BC, African American women with stage I/II disease had slightly higher BC-specific mortality than NHW women with the same stage disease (HR=1.27, CI 0.99-1.62). That finding is consistent with data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registries (43) and clinical trials (44) where African American women with stage I disease (all BCs combined) had higher BC-specific mortality, compared to NHW women. After excluding triple negative cases in the SEER-wide study (43), findings were similar and the authors partly attributed the higher mortality of African American women with stage I BC to more aggressive tumor features, such as a higher likelihood that African American women with small tumors (less than 2 cm) present with lymph node metastases or distant metastases.
The higher BC-specific mortality among African American women with stage I/II disease may also be related to differences in receipt of guideline-concordant treatment, although we were not able to directly assess this possibility in the SEER registry. African American women diagnosed with stage I/II BC have been shown to be less likely to receive breast-conserving surgery compared to NHW women (4). In our study, however, the proportion of women with stage I/II BC who had breast-conserving surgery was comparable among African American (65%), Hispanic (61%), and NHW (66%) women, but lower among Asian American women (52%). Compared to NHW women with breast-conserving surgery, BC-specific and all-cause mortality was higher among women who received a mastectomy among African American women only. Treatment with mastectomy may be a proxy for restricted care options among African American women: for example, care at centers with less expertise in coordinating multidisciplinary interventions such as breast conserving surgery and radiation, or limited access to the transportation or time off from work needed to complete radiation therapy. Other factors may also modify the higher mortality among African Americans, such as more extensive disease or comorbidities that may contraindicate breast-conserving surgery.
While we did not have information on access to health care after diagnosis, we were able to examine associations of mortality with hospital type. African American women diagnosed with ER/PR+ BC had BC-specific mortality that was similar to that of NHW women if initial care was at a hospital affiliated with NCI-designated Cancer Centers or the ACOS Cancer Program, whereas those who received initial care at other hospitals had higher BC-specific and all-cause mortality. This finding was unique to African American women, suggesting that lack of access to care or systemic barriers to high-quality care may disproportionately affect African American BC patients and their survival outcomes (45). We previously reported an association between hospital type and mortality for BC overall (36), and we show here the same association for ER/PR+ BC, but not for ER−/PR− BC. These findings suggest that interventions specific to the diagnosis and treatment of ER/PR+ BC might be delivered more effectively by accredited hospitals, which tend to have higher standards of adherence to treatment best practices for BC patients (46). Candidate interventions might include the quality of pathology laboratories in identifying ER/PR+ tumor status; referral to medical oncology for discussion and prescription of endocrine therapy; and clinical expertise in managing side effects of endocrine therapy, which may facilitate adherence. Research is needed to identify and implement key interventions that could improve access of African American women with ER/PR+ BC to higher quality care, thereby improving their survival.
Sociodemographic characteristics have been associated with survival of BC patients (9), including better survival of married women with BC (6,47). Consistent with those findings, we found for ER/PR+ BC that unmarried women, except among Hispanic women, had higher BC-specific mortality compared to married NHW women. A similar pattern was seen for all-cause mortality. However, married African American women also had higher BC-specific and all-cause mortality, whereas married Hispanic women had a greater overall survival benefit than married NHW women. Better survival of married BC patients may be related to greater social and/or economic support or other socially mediated factors (47–49). Our findings suggest that the mechanisms linking marital status to cancer survival may differ across racial/ethnic groups (50).
Better BC survival has also been associated with higher levels of education (51), and living in higher SES neighborhoods (6). However, we did not see such a pattern among African American women with ER/PR+ BC. BC-specific mortality was higher among those who were more educated or from higher SES neighborhoods than NHW women with comparable education or neighborhood SES. These findings are consistent with prior findings of higher BC-specific mortality among African American women than NHW women across all levels of census tract SES (52,53), and of lower BC-specific mortality associated with higher county-level income and education among NHW women, but not among African American women (54). Additionally, we found that more educated Hispanic women and those from higher SES neighborhoods had a greater survival benefit than NHW women with comparable education and neighborhood SES. These findings warrant a deeper understanding of the factors underlying education and neighborhood SES that might disproportionately affect survival of African American women with ER+/PR+ BC. Education and neighborhood SES may be related to quality of health care received and complex social determinants (9).
Consistent with other reports of higher mortality among obese women with BC (55,56), for ER/PR+ BC, we found a pattern of higher BC-specific mortality among obese women, except among Hispanic women, and higher all-cause mortality among obese NHW and African American women, compared to non-obese NHW women. However, BC-specific and all-cause mortality was also elevated among non-obese African American women relative to non-obese NHW women. As for other lifestyle-related factors, NHW and African American women who were never smokers or consumers of alcohol had similar mortality. While smoking and alcohol consumption were associated with higher BC-specific mortality, this was seen only among African American women. Other studies have found higher BC-specific mortality associated with current smoking (57,58), but evidence for alcohol consumption is inconclusive (59). The proportions of women who were current or past smokers or obese were highest among African American women, whereas the proportion of women consuming alcohol was highest among NHW women. The present findings suggest that certain lifestyle behaviors around the time of diagnosis were associated with better survival of women diagnosed with ER/PR+ BC. Because data on lifestyle factors after diagnosis were not available across all studies, we could not investigate the impact of post-diagnosis lifestyle factors on survival disparities.
In contrast to our findings for ER/PR+ BC, few factors modified all-cause mortality among women with ER−/PR− BC. Risk was greater among African American and NHW women from lower SES neighborhoods compared to NHW women from higher SES neighborhoods. This finding is consistent with a Michigan study of ER−/PR− BC, where clinical characteristics did not explain the higher all-cause mortality among African American women compared to NHW women, but there were no differences by race/ethnicity after adjustment for neighborhood SES (25).
In the U.S., African American and other communities of color are more likely to experience adverse conditions and toxic stressors throughout their life, and often need to exert more effort for basic daily activities. Effectively, the resulting increased and prolonged levels of social stress eventually impact emotional and physical health. Although the CBCSC has previously investigated neighborhood social and built environment factors (13,36,40,41), finding complex interactions with individual-level factors, research on cancer health disparities needs to acknowledge that health inequities are rooted in and continue to be maintained by structural factors as upstream social determinants of health. Research needs to focus on structural racism, interpersonal discrimination, and medical mistrust as drivers of cancer health inequities, and policies and measures to address disparities must fundamentally start with addressing structural factors.
Several limitations need to be considered when interpreting these results. They include: the relatively small sample size of ER−/PR− BC in each racial/ethnic group, the possibility of selection bias, as not all eligible women chose to participate in the parent case-control and cohort studies; incomplete cancer registry information on HER2 status, with only 669 triple negative (ER−, PR−, HER2−) cases in the pooled dataset; incomplete data on receipt of radiotherapy and chemotherapy (60), and limited to first-course treatment; and lack of data on receipt of endocrine therapy, guideline-concordant treatment, treatment delays, or adherence to treatment. Information on comorbidities, physical activity, health care access, health insurance, and behavioral factors such as diet was not available across all studies that were pooled (27). We had only limited data on social determinants of health, such as education and neighborhood SES (41). Other social determinants that may drive survival disparities for ER/PR+ BC warrant in-depth investigation (e.g., unemployment, income, neighborhood disadvantage, lack of social support, social isolation, racial discrimination, and systemic racism) (9). Nevertheless, our study has several important strengths, including a long follow-up of an average 8.7 years, a high follow-up rate in the CCR, population-based design, the highly racially/ethnically diverse study sample with a large number of African American, Hispanic, and Asian American women with BC accounting for 57% of the study sample. The sample size was sufficient for race/ethnicity-specific analyses by ER/PR status, and assessing associations of ER/PR+ BC mortality with a wide range of modifying factors, including lifestyle factors that are not available in cancer registries. However, larger multiethnic studies are warranted to investigate mortality for ER−/PR− BC and triple negative BC.
In conclusion, in this large multiethnic study of women diagnosed with invasive BC, BC-specific and all-cause mortality differed by race/ethnicity for BC overall and ER/PR+ BC, but not for ER−/PR− BC. We found that healthcare, sociodemographic, and lifestyle factors may contribute to racial/ethnic survival disparities among women with ER/PR+ BC.
Supplementary Material
Acknowledgments:
This work was supported by the California Breast Cancer Research Program (CBCRP; grant 16ZB-8001 to A.H. Wu, R. Sposto, C. Vigen; 16ZB-8002 to S.L. Gomez, T.H. Keegan, S. Shariff-Marco, J. Koo, J. Yang, A.W. Kurian, E.M. John; 16ZB-8003 to L. Bernstein, Y. Lu; 16ZB-8004 to M.L. Kwan; and 16ZB-8005 to K.R. Monroe, I. Cheng, B.E. Henderson). The John Cancer Research Program Fund supported the work by V. McGuire. The Asian American Breast Cancer Study was supported by CBCRP grants 1RB-0287, 3PB-0120, and 5PB-0018 to A.H. Wu. The San Francisco Bay Area Breast Cancer Study was supported by National Cancer Institute grants R01 CA063446 and R01 CA077305; by the U.S. Department of Defense (DOD) grant DAMD17-96-1-6071; and by the CBCRP grants 1RB-0125 to P.L. Horn-Ross and 7PB-0068 to E.M. John. The Women’s Contraceptive and Reproductive Experiences (CARE) Study was funded by the National Institute of Child Health and Human Development (NICHD), through a contract with USC (N01-HD-3-3175). The California Teachers Study was funded by the California Breast Cancer Act of 1993; National Cancer Institute grants (R01 CA77398 and K05 CA136967) to L. Bernstein and the California Breast Cancer Research Fund (contract 97-10500) to L. Bernstein. The Multiethnic Cohort Study is supported by National Cancer Institute grants R01 CA54281, R37CA54281, and U01 CA164973 to L. Le Marchand, L.R. Wilkens and C. Haiman. The Life After Cancer Epidemiology Study is supported by National Cancer Institute grant R01 CA129059 to B.J. Caan. The Breast Cancer Family Registry is supported by the National Cancer Institute grant U01 CA164920 to I. Andrulis, S. Colonna, M. Daly, J.L. Hopper, E.M. John, and M.B. Terry. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Abbreviations:
- AABCS
Los Angeles County Asian American Breast Cancer Study
- ACOS-CP
American College of Surgeons Cancer Program
- BC
breast cancer
- CARE
Women’s Contraceptive and Reproductive Experiences study
- CBCSC
California Breast Cancer Survivorship Consortium
- CCR
California Cancer Registry
- CI
confidence intervals
- CTS
California Teachers Study
- ER
estrogen receptor status
- HR
hazard ratio
- MEC
Multiethnic Cohort study
- NC-BCFR
Northern California Breast Cancer Family Registry
- NCI-CC
National Cancer Institute Cancer Center
- NHW
non-Hispanic White
- PR
progesterone receptor status
- SES
socioeconomic status
- SFBCS
San Francisco Bay Area Breast Cancer Study
- U.S.
United States
Footnotes
Conflict of interest:
The authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;701:7–30. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;676:439–48. [DOI] [PubMed] [Google Scholar]
- 3.Hunt BR, Hurlbert MS. Black:white disparities in breast cancer mortality in the 50 largest cities in the United States, 2005–2014. Cancer Epidemiol 2016. [DOI] [PubMed] [Google Scholar]
- 4.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med 2003;1631:49–56. [DOI] [PubMed] [Google Scholar]
- 5.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 2011;1273:729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol 2018;361:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenzie F, Jeffreys M. Do lifestyle or social factors explain ethnic/racial inequalities in breast cancer survival? Epidemiol Rev 2009;31:52–66. [DOI] [PubMed] [Google Scholar]
- 8.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas 2010;661:5–15. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat 2019. [DOI] [PubMed] [Google Scholar]
- 10.Dimick J, Ruhter J, Sarrazin MV, Birkmeyer JD. Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions. Health Aff (Millwood) 2013;326:1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol 2015;3320:2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keegan TH, Kurian AW, Gali K, Tao L, Lichtensztajn DY, Hershman DL, et al. Racial/ethnic and socioeconomic differences in short-term breast cancer survival among women in an integrated health system. Am J Public Health 2015;1055:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sposto R, Keegan TH, Vigen C, Kwan ML, Bernstein L, John EM, et al. The Effect of Patient and Contextual Characteristics on Racial/Ethnic Disparity in Breast Cancer Mortality. Cancer Epidemiol Biomarkers Prev 2016;257:1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;29521:2492–502. [DOI] [PubMed] [Google Scholar]
- 15.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J 2009;156:593–602. [DOI] [PubMed] [Google Scholar]
- 16.Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS, Hershman D, Neugut AI. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer 2005;10311:2241–51. [DOI] [PubMed] [Google Scholar]
- 17.Banegas MP, Tao L, Altekruse S, Anderson WF, John EM, Clarke CA, et al. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res Treat 2014;1443:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev 2018;276:619–26. [DOI] [PubMed] [Google Scholar]
- 19.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 2009;1132:357–70. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 2010;1624:6100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, et al. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer 2013;13:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast Cancer Mortality in African-American and Non-Hispanic White Women by Molecular Subtype and Stage at Diagnosis: A Population-Based Study. Cancer Epidemiol Biomarkers Prev 2015;247:1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauscher GH, Silva A, Pauls H, Frasor J, Bonini MG, Hoskins K. Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast Cancer Res Treat 2017;1632:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roseland ME, Schwartz K, Ruterbusch JJ, Lamerato L, Krajenta R, Booza J, et al. Influence of clinical, societal, and treatment variables on racial differences in ER−/PR− breast cancer survival. Breast Cancer Res Treat 2017;1651:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collin LJ, Jiang R, Ward KC, Gogineni K, Subhedar PD, Sherman ME, et al. Racial Disparities in Breast Cancer Outcomes in the Metropolitan Atlanta Area: New Insights and Approaches for Health Equity. JNCI Cancer Spectr 2019;33:pkz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu AH, Gomez SL, Vigen C, Kwan ML, Keegan TH, Lu Y, et al. The California Breast Cancer Survivorship Consortium (CBCSC): prognostic factors associated with racial/ethnic differences in breast cancer survival. Cancer Causes Control 2013;2410:1821–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John EM, Sangaramoorthy M, Koo J, Whittemore AS, West DW. Enrollment and biospecimen collection in a multiethnic family cohort: the Northern California site of the Breast Cancer Family Registry. Cancer Causes Control 2019;304:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu AH, Yu MC, Tseng CC, Pike MC. Body size, hormone therapy and risk of breast cancer in Asian-American women. Int J Cancer 2007;1204:844–52. [DOI] [PubMed] [Google Scholar]
- 30.Marchbanks PA, McDonald JA, Wilson HG, Folger SG, Mandel MG, Daling JR, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med 2002;34626:2025–32. [DOI] [PubMed] [Google Scholar]
- 31.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev 2005;1412:2905–13. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein L, Allen M, Anton-Culver H, Deapen D, Horn-Ross PL, Peel D, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States). Cancer Causes Control 2002;137:625–35. [DOI] [PubMed] [Google Scholar]
- 33.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;1514:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007;1099:1721–8. [DOI] [PubMed] [Google Scholar]
- 35.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;128:703–11. [DOI] [PubMed] [Google Scholar]
- 36.Shariff-Marco S, Ellis L, Yang J, Koo J, John EM, Keegan THM, et al. Hospital characteristics and breast cancer survival in the California Breast Cancer Survivorspip Consortium JCO Oncology Practice 2020;166:e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Cancer Institute Cancer Center designation (as of April 2010). http://www.cancer.gov/researchandfunding/extramural/cancercenters/find-a-cancer-center.
- 38.American College of Surgeons Cancer Programs. https://www.facs.org/quality-programs/cancer.
- 39.Kwan ML, John EM, Caan BJ, Lee VS, Bernstein L, Cheng I, et al. Obesity and mortality after breast cancer by race/ethnicity: The California Breast Cancer Survivorship Consortium. Am J Epidemiol 2014;1791:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng I, Shariff-Marco S, Koo J, Monroe KR, Yang J, John EM, et al. Contribution of the neighborhood environment and obesity to breast cancer survival: the California Breast Cancer Survivorship Consortium. Cancer Epidemiol Biomarkers Prev 2015;248:1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shariff-Marco S, Yang J, John EM, Kurian AW, Cheng I, Leung R, et al. Intersection of Race/Ethnicity and Socioeconomic Status in Mortality After Breast Cancer. J Community Health 2015;406:1287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan TH, et al. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California Breast Cancer Survivorship Consortium (CBCSC). Cancer Epidemiol Biomarkers Prev 2015;242:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015;3132:165–73. [DOI] [PubMed] [Google Scholar]
- 44.Albain KS, Unger JM, Crowley JJ, Coltman CA Jr., Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 2009;10114:984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 2015;653:221–38. [DOI] [PubMed] [Google Scholar]
- 46.Miller ME, Bleicher RJ, Kaufman CS, Kurtzman SH, Chang C, Wang CH, et al. Impact of Breast Center Accreditation on Compliance with Breast Quality Performance Measures at Commission on Cancer-Accredited Centers. Ann Surg Oncol 2019;265:1202–11. [DOI] [PubMed] [Google Scholar]
- 47.Gomez SL, Hurley S, Canchola AJ, Keegan TH, Cheng I, Murphy JD, et al. Effects of marital status and economic resources on survival after cancer: A population-based study. Cancer 2016;12210:1618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol Hematol 2010;752:122–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rendall MS, Weden MM, Favreault MM, Waldron H. The protective effect of marriage for survival: a review and update. Demography 2011;482:481–506. [DOI] [PubMed] [Google Scholar]
- 50.Martinez ME, Unkart JT, Tao L, Kroenke CH, Schwab R, Komenaka I, et al. Prognostic significance of marital status in breast cancer survival: A population-based study. PLoS One 2017;125:e0175515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herndon JE 2nd, Kornblith AB, Holland JC, Paskett ED. Effect of socioeconomic status as measured by education level on survival in breast cancer clinical trials. Psychooncology 2013;222:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000–2010. BMC Cancer 2013;13:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr 2014;201449:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal S, Ying J, Boucher KM, Agarwal JP. The association between socioeconomic factors and breast cancer-specific survival varies by race. PLoS One 2017;1212:e0187018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwan ML, Chen WY, Kroenke CH, Weltzien EK, Beasley JM, Nechuta SJ, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat 2012;1322:729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;2510:1901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boone SD, Baumgartner KB, Baumgartner RN, Connor AE, John EM, Giuliano AR, et al. Active and passive cigarette smoking and mortality among Hispanic and non-Hispanic white women diagnosed with invasive breast cancer. Ann Epidemiol 2015;2511:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parada H Jr., Sun X, Tse CK, Olshan AF, Troester MA, Conway K. Active smoking and survival following breast cancer among African American and non-African American women in the Carolina Breast Cancer Study. Cancer Causes Control 2017;289:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali AM, Schmidt MK, Bolla MK, Wang Q, Gago-Dominguez M, Castelao JE, et al. Alcohol consumption and survival after a breast cancer diagnosis: a literature-based meta-analysis and collaborative analysis of data for 29,239 cases. Cancer Epidemiol Biomarkers Prev 2014;236:934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;549:e55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


