Abstract
Important questions about mechanisms of physiological adaptation concern the role of phenotypic plasticity and the extent to which acclimatization responses align with genetic responses to selection. Such questions can be addressed in experimental studies of high-altitude vertebrates by investigating how mechanisms of acclimatization to hypoxia in lowland natives may influence genetic adaptation to hypoxia in highland natives. Evidence from high-altitude mammals suggest that evolved changes in some physiological traits involved canalization of the ancestral acclimatization response to hypoxia (genetic assimilation), a mechanism that results in an evolved reduction in plasticity. In addition to cases where adaptive plasticity may have facilitated genetic adaptation, evidence also suggests that some physiological changes in high-altitude natives are the result of selection to mitigate maladaptive plastic responses to hypoxia (genetic compensation). Examples of genetic compensation involve the attenuation of hypoxic pulmonary hypertension in Tibetan humans and other mammals native to high altitude. Here we discuss examples of adaptive physiological phenotypes in high-altitude natives that may have evolved by means of genetic assimilation or genetic compensation.
1. Introduction
The role of phenotypic plasticity in adaptive evolution is a subject of longstanding debate, and a key question concerns the extent to which plasticity generally facilitates or hinders directional natural selection (Ghalambor et al., 2007). This question can be profitably addressed in studies of high-altitude adaptation because physiological mechanisms of hypoxia acclimation and acclimatization have been intensively studied in humans and other vertebrates that have predominantly lowland ancestries. Insights into mechanisms of plasticity in hypoxia-responsive phenotypes of lowland natives suggest testable hypotheses about genetic mechanisms of evolved phenotypic changes in high-altitude natives.
High-altitude mammals and birds exhibit a characteristic suite of derived changes in respiratory, cardiovascular, and metabolic traits compared to their closest low-altitude relatives (Ivy and Scott, 2015; McClelland and Scott, 2019; Storz et al., 2019; Storz and Scott, 2019; Storz et al., 2010). In most cases we do not know what fraction of the observed trait differentiation is genetically based and what fraction is environmentally induced (during development or adult life). For example, comparative studies of human populations suggest that highland natives in the Andean altiplano and the Tibetan Plateau have generally higher aerobic performance capacities in hypoxia relative to lowland natives, but it is not clear to what extent such differences are attributable to genetic effects (Brutsaert, 2016; Brutsaert, 2008). In the case of deer mice (Peromyscus maniculatus), genetic crosses and common-garden experiments have revealed that highland natives have evolved a genetically based enhancement of aerobic capacity in hypoxia relative to lowland conspecifics (Chappell and Snyder, 1984; Cheviron et al., 2012; Cheviron et al., 2013; Cheviron et al., 2014; Lau et al., 2017; Lui et al., 2015; Storz et al., 2019; Tate et al., 2017; Tate et al., 2020). The superior aerobic performance of highlanders relative to lowlanders is attributable to accentuated plasticity in response to chronic hypoxia in some subordinate traits in conjunction with evolved changes in the mean values of other non-plastic traits (Cheviron et al., 2012; Cheviron et al., 2014; Lau et al., 2017; Lui et al., 2015; Tate et al., 2017; Tate et al., 2020). However, hypoxia-induced responses in subordinate traits do not necessarily make uniformly positive contributions. The acclimatization response at the level of whole-animal performance may reflect a mosaic of positive and negative effects of plasticity in underlying subordinate traits. Key questions concern the nature of interactions between plastic and evolved changes in different traits and whether such interactions are synergistic or antagonistic with regard to whole-animal performance capacities.
A plastic response to a given environmental stressor is adaptive if the induced change moves the mean phenotype closer to the environment-specific optimum favored by selection. Consider the new selection pressures encountered by members of a low-altitude species that colonize a high-altitude environment. Due to the lower PO2 at high altitude, the phenotypic optima of some physiological traits (e.g., pulmonary ventilation, pulmonary O2 diffusing capacity, cardiac output, etc.) may be shifted relative to the optimum in the ancestral, lowland environment (Fig. 1). For a given trait, if plasticity alone is sufficient to match the mean phenotype to the optimum of the newly colonized highland environment, then the environmentally induced change precludes an opportunity for selection to produce genetically based adaptation (Fig. 1B). Another possibility is that the plastic response moves the mean phenotype part way towards the new optimum, and selection on genetically based trait variation then closes the remaining gap (Fig. 1C). Plasticity is maladaptive if the induced change moves the mean phenotype further from the optimum, which then creates selection pressure to counteract the environmentally induced effect (Fig. 1D). Let us now explore how these different forms of plasticity might influence genetic mechanisms of hypoxia adaptation.
Figure 1.
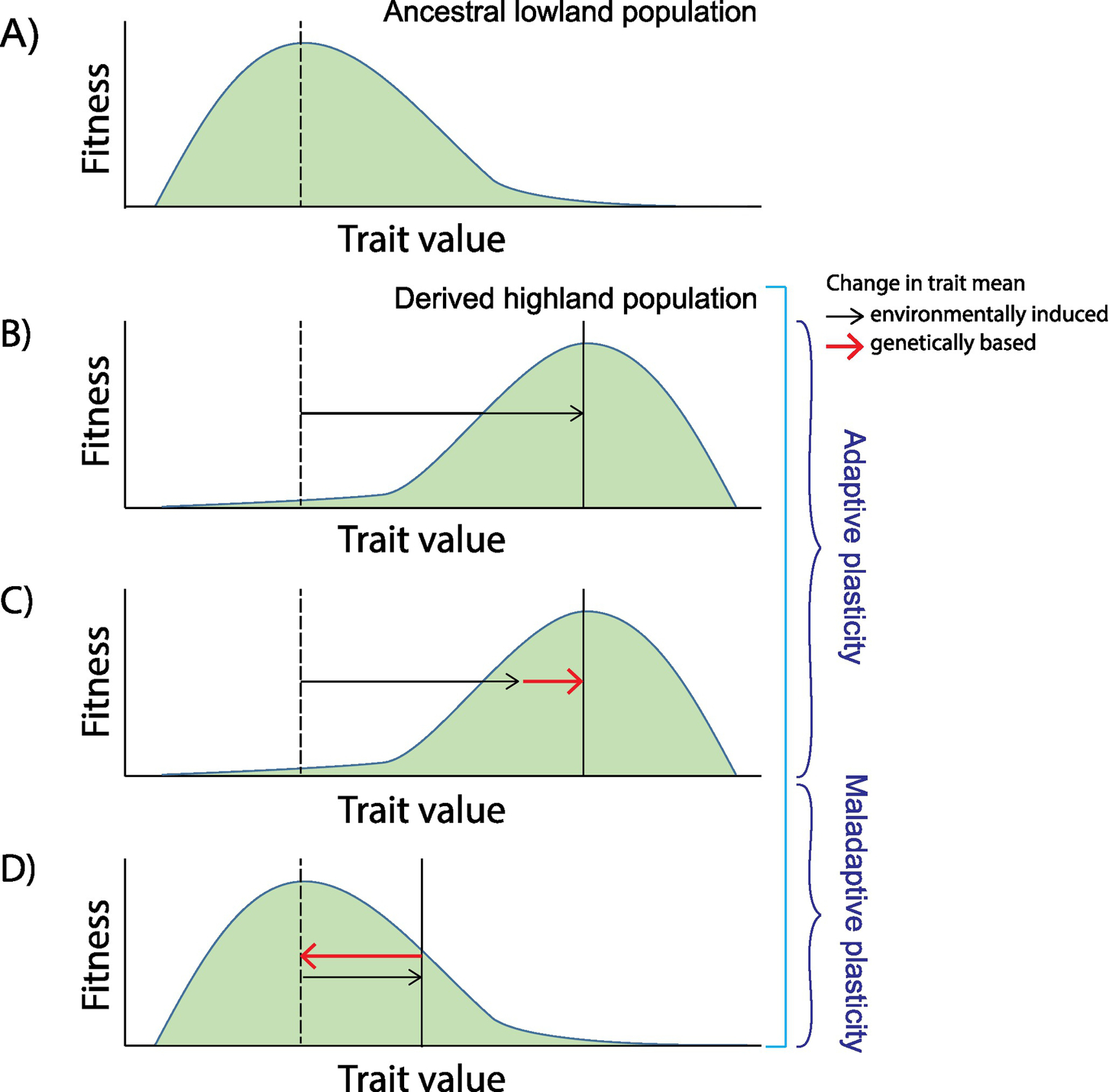
Role of phenotypic plasticity in adaptation to a newly colonized environment. (A) Hypothetical scenario in which fitness varies as a function of phenotypic trait values in an ancestral, lowland environment. The trait value that confers the highest fitness is denoted by a vertical dashed line. (B) In the high-altitude environment, the fitness function of the phenotype is shifted relative to that in the lowland environment. The high-altitude phenotypic optimum is denoted by the solid vertical line. Upon colonization of the high-altitude environment, a plastic response (black arrow) moves the population mean phenotype to the new optimum. In this scenario, adaptive plasticity in the fitness-related phenotype eliminates the opportunity for selection on genetically based trait variation. (C) A plastic response (black arrow) moves the population mean phenotype part way to the new optimum, and selection on genetically based variation (orange arrow) then shifts it the rest of the way. (D) A maladaptive plastic response (black arrow) moves the population mean further from the new optimum, and selection on genetically based variation (orange arrow) then compensates the environmentally induced change in phenotype.
If the hypoxia-induced expression of a given phenotype enables members of a lowland species to survive and function in a newly colonized high-altitude environment, constitutive expression of that same phenotype may be adaptive for permanent high-altitude residents, especially if plasticity has a cost. In such cases, the environmentally induced response can become canalized (genetically fixed) by selection, a phenomenon known as genetic assimilation. In the case of a colonizing population that is forced to adapt to chronic hypoxia, genetic assimilation of an adaptive plastic response results in a reduction or elimination of plasticity in the selected trait (Fig. 2A). By contrast, other physiological phenotypes may be characterized by a single global optimum across the altitudinal range of the species. In such cases, hypoxia-induced deviations from the global phenotypic optimum would be maladaptive, creating selection pressure to counteract the plastic response and restore the ancestral (lowland) phenotype (Fig. 2B,C). The process whereby selection on genetically based trait variation mitigates a maladaptive plastic response is known as genetic compensation (Grether, 2005). At high-altitude, mis-directed responses to hypoxia may occur in lowland species whose ancestors never encountered a situation where arterial hypoxemia and tissue hypoxia were caused by a reduced PO2 of atmospheric air (environmental hypoxia). In such cases, the sensing of reduced cellular O2 may be misinterpreted as a problem associated with pulmonary O2 exchange or convective O2 transport (e.g., hemorrhage or anemia). The misdirected physiological response to the hypoxic stimulus may be ineffectual, at best, or pathologically counterproductive, at worst (Dempsey and Morgan, 2015; Storz and Scott, 2019; Storz et al., 2010).
Figure 2.

Evolutionary changes in phenotypic plasticity and consequences for observed patterns of trait variation. (A) Genetic assimilation. The fitness function for the trait in question is different in the ancestral lowland environment and the newly colonized highland environment. The hypoxia-induced plastic response is adaptive (reaction norm illustrated with solid line) and becomes canalized by selection, resulting in the loss of plasticity. Far right panel: Hypothetical outcome of reciprocal-transplant experiment showing expression of the phenotype when highlanders and lowlanders are reared in native and nonnative environments. Arrow heads denote outcomes of reciprocal transplants (phenotypes expressed in the non-native environment). Highlanders and lowlanders exhibit a pronounced difference in phenotype (ΔP) when observed in their native habitats, and the reciprocal transplant reveals the loss of plasticity in highlanders. (B) Genetic compensation with canalization. Fitness function for the trait is the same in the lowland and highland environments. The hypoxia-induced plastic response shifts the mean trait value away from the global optimum. In highland natives, selection on genetically based trait variation counteracts the plastic change, thereby restoring the ancestral phenotype (i.e., the same phenotype expressed by lowland natives in the ancestral environment). Far right panel: Highlanders and lowlanders exhibit no difference in phenotype when observed in their native habitats (ΔP=0), and the reciprocal transplant reveals the loss of plasticity in highlanders. (C) Genetic compensation without canalization. Same scenario as in B, but the highland population evolves a new reaction norm without a concomitant loss of plasticity (change in Y-intercept, but no change in slope). Far right panel: Highlanders and lowlanders exhibit no difference in phenotype when observed in their native habitats (ΔP=0), and the reciprocal transplant reveals that they exhibit plastic responses in opposite directions when reared in nonnative environments.
In principle, genetic compensation can involve canalization of the ancestral phenotype (the slope of the reaction norm changes, but not the Y-intercept)(Fig. 2B). In this scenario, the canalized phenotype is unconditionally beneficial (conferring highest fitness in both the high- and low-altitude environments) and the result is an evolutionary loss of plasticity. The other possibility is that genetic compensation involves an adaptive shift in the reaction norm without a net loss of plasticity (the Y-intercept of the reaction norm changes, but not the slope)(Fig. 2C). In this scenario, the induced phenotype confers highest fitness in the high-altitude environment, but the plastic response would be detrimental upon return to normoxic conditions at low altitude. Genetic compensation without canalization therefore represents an adaptive solution suited to high-altitude specialists, but not generalist species that would potentially be exposed to a range of altitudinal zones (either within individual lifetimes or across generations).
Genetic assimilation and genetic compensation of hypoxia-responsive phenotypes result in patterns of altitudinal variation that can make it difficult to detect evolved changes in phenotype between populations. In the case of genetic assimilation, comparisons in the field would reveal a difference in phenotype between highland and lowland natives when measured in their native environments. However, a reciprocal-transplant experiment would be needed to reveal that the observed difference is attributable to an evolved loss of plasticity in the highland population (i.e., phenotypic differences would be manifest if highland and lowland natives were both raised at sea-level but not if they were both raised at high altitude; Fig. 2A, far-right panel). In the case of genetic compensation with canalization, no phenotypic differences between highland and lowland natives would be apparent under field conditions. However, the reciprocal-transplant experiment would unveil a cryptic difference in phenotype that is only manifest if highland and lowland natives are raised at high-altitude (because the hypoxia-induced response is retained in lowland natives but is blunted in highland natives; Fig. 2B, far-right panel). When the induced phenotype is not canalized, the reciprocal transplant experiment would unveil an even more pronounced difference in phenotype since highland and lowland natives would respond in opposite directions to changes in ambient PO2 in their nonnative environments (Fig. 2C, far-right panel).
These theoretical possibilities suggest that high-altitude adaptation may often involve a combination of genetic assimilation of favorable plastic responses and genetic compensation of detrimental responses (McClelland and Scott, 2019; Storz and Cheviron, 2021; Storz and Scott, 2019; Storz et al., 2010). With these possibilities in mind, let us now consider possible examples of adaptive traits in high-altitude natives that may have evolved by means of genetic assimilation or genetic compensation.
2. Genetic assimilation of adaptive acclimatization responses to hypoxia
Physiological studies have revealed numerous examples where highland natives constitutively express phenotypes that are similar to those expressed by hypoxia-acclimatized lowlanders, suggesting the possibility of genetic assimilation. For example, in lowland humans and other mammals, the typical acclimatization response to hypoxia involves an acute increase in ventilation that is amplified over several weeks of chronic exposure (Ivy and Scott, 2015; Pamenter and Powell, 2016). However, regardless of prior acclimation, deer mice native to high altitude maintain a breathing pattern that is similar to that of hypoxia-acclimated lowland mice, characterized by high resting ventilation and tidal volume across a range of inspired PO2 (Ivy and Scott, 2017; Ivy and Scott, 2018). Likewise, metabolic features of skeletal muscle in Tibetan humans native to high altitude are similar to those of acclimatized lowlanders (e.g., lower muscle mitochondrial densities and capacities for oxidative phosphorylation)(Horscroft et al., 2017).
The possible examples of genetic assimilation mentioned above are currently nothing more than suggestive hypotheses, as causal mechanisms have yet to be elucidated. One example in which a mechanism of genetic assimilation has been clearly documented involves the evolution of an increased hemoglobin (Hb)-O2 affinity in the Tibetan antelope, Pantholops hodgsonii, a highly athletic species that is endemic to the Tibetan Plateau and lives at altitudes of 3600–5500 m (Signore and Storz, 2020). Under conditions of severe hypoxia, an increased Hb-O2 affinity can help safeguard arterial O2 saturation at low inspired PO2, thereby improving circulatory O2 delivery (Storz, 2016, 2019).
When exposed to hypoxia, adult bovid mammals such as goats and sheep upregulate a juvenile Hb isoform (with relatively high O2-affinity) at the expense of the normal adult isoform (with relatively low O2-affinity), an isoform switch that produces a putatively beneficial increase in Hb-O2 affinity. A study that integrated comparative genomics and biochemical physiology revealed that Tibetan antelope evolved a derived increase in Hb-O2 affinity by truncating the ancestral ontogeny of globin gene expression such that the high-affinity juvenile Hb isoform completely supplanted the lower-affinity adult isoform that is expressed in the adult red blood cells of other bovids (Signore and Storz, 2020). This juvenilization of blood properties in Tibetan antelope – a form of ‘biochemical pedomorphosis’ – illustrates how alteration of an existing mode of developmental regulation can provide a ready mechanism of adaptive evolutionary change. Comparative genomic analysis revealed that the developmental switch in Hb isoform expression became canalized via deletion of a ~45 kb chromosomal region that spans the 3’ end of the Tibetan antelope β-globin gene cluster (Fig. 3). As a result of this chromosomal deletion, the adult-expressed β-globin gene was eliminated while the closely linked juvenile β-globin gene was left intact. Consequently, the high-affinity juvenile Hb isoform became the sole-expressed isoform in adult red cells. The chromosomal deletion in Tibetan antelope produced a drastic regulatory switch in Hb isoform expression such that a reversible acclimatization response to acute hypoxia (upregulation of the juvenile isoform at the expense of the adult isoform) became genetically assimilated as an irreversible adaptation to chronic hypoxia (Signore and Storz, 2020).
Figure 3.

Large-scale deletion in the β-globin gene cluster of Tibetan antelope is revealed by analysis of pairwise sequence matches with homologous chromosomal regions in other bovids. Cyan, green, and dark blue boxes represent members of triplicated gene blocks containing the genes that encode the β-type subunits of juvenile (βC), adult (βA), and fetal (βF) Hb isoforms, respectively. (a) Gray shading denotes percent sequence identity between homologous β-globin gene clusters. (b) A ~45 kb chromosomal deletion in the β-globin gene cluster of Tibetan antelope resulted in secondary loss of the βA-containing gene block. Modified with permission from Signore and Storz (2020).
3. Genetic compensation of maladaptive acclimatization responses to hypoxia
Studies of high-altitude humans and other mammals have documented several apparent examples of genetic compensation (McClelland and Scott, 2019; Storz and Cheviron, 2021; Storz and Scott, 2019; Storz et al., 2010; Velotta et al., 2018). One well-studied example in high-altitude natives is the attenuation of hypoxic pulmonary hypertension (HPH), a maladaptive response to chronic hypoxia with pathological effects in humans and other lowland mammals (Monge et al., 1992). In lowlanders at sea level, pulmonary arterial vessels constrict in response to low O2, which helps match regional variation in ventilation and blood flow across the lungs (‘V-Q matching’), and thus enhances gas-exchange efficiency. However, this vasoconstriction is counterproductive at high altitude because hypoxia occurs throughout the entirety of the lungs, leading to remodeling and thickening of pulmonary vessels that makes them less distensible, and increasing the pressure of pulmonary arterial blood (Shimoda and Laurie, 2014; Sylvester et al., 2012). HPH can impair O2 uptake and in severe cases can lead to life threatening pulmonary edema, right-ventricle hypertrophy, and heart failure (Sylvester et al., 2012). These pathological responses to chronic hypoxia do not occur in Tibetan humans nor in several other high-altitude taxa, which exhibit pulmonary arterial pressures in the range of lowlanders at sea level (Groves et al., 1993; Monge et al., 1992). In many previous studies of HPH, high-altitude taxa were studied in their native environment, so it has been difficult to distinguish whether there has been an evolved loss of plasticity (Fig. 2B) versus a maintenance of plasticity with a downward shift in the trait mean (Fig. 2C). However, recent common-garden experiments with deer mice provide support for both possibilities (Velotta et al., 2018; West et al., 2021). When exposed to chronic hypoxia, deer mice native to low altitude exhibit robust increases in pulmonary arterial pressure (reflected by systolic pressures in the right ventricle; Fig. 4A) that lead to right-ventricle hypertrophy (Fig. 4B). These responses are attenuated in deer mice native to high altitude, as they exhibit a shallower reaction norm for both traits in combination with a downshifted reaction norm for pulmonary arterial pressure. Highlanders also maintain V-Q matching in chronic hypoxia, unlike the greater V-Q mismatch experienced by lowlanders (West et al., 2021). This provides a physiological example of how highland natives appear to have evolved a means of mitigating maladaptive plastic responses to chronic hypoxia, although the underlying genetic mechanism has yet to be characterized.
Figure 4.

Exposure to chronic hypoxia led to (A) pulmonary hypertension (increased right-ventricle systolic pressure) and (B) right-ventricle hypertrophy (increased right ventricle mass relative to the combined mass of the left ventricle and septum) in deer mouse populations from low altitude, but these effects were attenuated in populations from high altitude. Data are means ± SE. * Significant differences between populations within an environment (P<0.05). Modified with permission from West et al. (2021).
4. Conclusions
The role of phenotypic plasticity in genetic adaptation is a topic of longstanding interest in evolutionary biology and is highly relevant to the study of hypoxia adaptation in high-altitude animals. Common-garden experiments on species like deer mice have revealed that physiological adaptation to high-altitude hypoxia involves complex interactions among functionally integrated traits that exhibit varying levels of plasticity (Tate et al., 2017, 2020). Physiological studies of high-altitude humans and other mammals provide potential examples of genetic assimilation of adaptive plasticity as well as genetic compensation of maladaptive plasticity. Experimental research on these underexplored modes of trait evolution is needed to identify and characterize causative genetic mechanisms, while comparative phylogenetic approaches can potentially aid inferences about possible historical paths of phenotypic change.
Highlights.
The role of phenotypic plasticity in genetic adaptation is a topic of longstanding interest in evolutionary physiology.
Evidence from high-altitude mammals suggest that evolved changes in some physiological traits involved canalization of the ancestral acclimatization response to hypoxia (genetic assimilation), a mechanism that results in an evolved reduction in plasticity.
Evidence also suggests that some physiological changes in high-altitude natives are the result of selection to mitigate maladaptive plastic responses to hypoxia (genetic compensation).
Acknowledgments
JFS acknowledges grant support from the National Institutes of Health (HL087216) and the National Science Foundation (OIA-1736249) and GRS acknowledges grant support from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2018–05707).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We hereby state that we have no conflicts of interest.
References
- Brutsaert T, 2016. Why are high altitude natives so strong at high altitude? Nature vs. nurture: genetic factors vs. growth and development. Advances in Experimental Medicine and Biology 903, 101–112. [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, 2008. Do high-altitude natives have enhanced exercise performance at altitude? Applied Physiology Nutrition and Metabolism 33, 582–592. [DOI] [PubMed] [Google Scholar]
- Chappell MA, Snyder LRG, 1984. Biochemical and physiological correlates of deer mouse α-chain hemoglobin polymorphisms. Proceedings of the National Academy of Sciences of the United States of America 81, 5484–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF, 2012. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proceedings of the National Academy of Sciences of the United States of America 109, 8635–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Bachman GC, Storz JF, 2013. Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. Journal of Experimental Biology 216, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Connaty AD, McClelland GB, Storz JF, 2014. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68, 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Morgan BJ, 2015. Humans in hypoxia: a conspiracy of maladaptation?! Physiology 30, 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN, 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21, 394–407. [Google Scholar]
- Grether GF, 2005. Environmental change, phenotypic plasticity, and genetic compensation. American Naturalist 166, E115–E123. [DOI] [PubMed] [Google Scholar]
- Groves BM, Droma T, Sutton JR, McCullough RG, McCullough RE, Zhuang JG, Rapmund G, Sun SF, Janes C, Moore LG, 1993. Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. Journal of Applied Physiology 74, 312–318. [DOI] [PubMed] [Google Scholar]
- Horscroft JA, Kotwica AO, Laner V, West JA, Hennis PJ, Levett DZH, Howard DJ, Fernandez BO, Burgess SL, Ament Z, Gilbert-Kawai ET, Vercueil A, Landis BD, Mitchell K, Mythen MG, Branco C, Johnson RS, Feelisch M, Montgomery HE, Griffin JL, Grocott MPW, Gnaiger E, Martin DS, Murray AJ, 2017. Metabolic basis to Sherpa altitude adaptation. Proceedings of the National Academy of Sciences of the United States of America 114, 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy CM, Scott GR, 2015. Control of breathing and the circulation in high-altitude mammals and birds. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology 186, 66–74. [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR, 2017. Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta Physiologica 221, 266–282. [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR, 2018. Evolved changes in breathing and CO2 sensitivity in deer native to high altitudes. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 315, R1027–R1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Connaty A, Mahalingam S, Wall N, Cheviron ZA, Storz JF, Scott GR, McClelland GB, 2017. Acclimation to hypoxia increases carbohydrate use during exercise in high-altitude deer mice. American Journal of Physiology - Regulatory, Integrative, and Comparative Physiology 312, R400–R411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR, 2015. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 308, R779–R791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland GB, Scott GR, 2019. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annual Review of Physiology 81, 561–583. [DOI] [PubMed] [Google Scholar]
- Monge C, Arregui CA, Leonvelarde F, 1992. Pathophysiology and epidemiology of chronic mountain sickness. International Journal of Sports Medicine 13, S79–S81. [DOI] [PubMed] [Google Scholar]
- Pamenter ME, Powell FL, 2016. Time domains of the hypoxic ventilatory response and their molecular basis. Comprehensive Physiology 6, 1345–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Laurie SS, 2014. HIF and pulmonary vascular responses to hypoxia. Journal of Applied Physiology 116, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signore AV, Storz JF, 2020. Biochemical paedomorphosis and genetic assimilation in the hypoxia adaptation of Tibetan antelope. Science Advances 6, eabb5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, 2016. Hemoglobin-oxygen affinity in high-altitude vertebrates: Is there evidence for an adaptive trend? Journal of Experimental Biology 219, 3190–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, 2019. Hemoglobin: Insights into Protein Structure, Function, and Evolution. Oxford University Press, Oxford. [Google Scholar]
- Storz JF, Cheviron ZA, 2021. Physiological genomics of adaptation to high-altitude hypoxia. Annual Review of Animal Biosciences ( 10.1146/annurev-animal-072820-102736). [DOI] [PMC free article] [PubMed]
- Storz JF, Cheviron ZA, McClelland GB, Scott GR, 2019. Evolution of physiological performance capacities and environmental adaptation: insights from high-elevation deer mice (Peromyscus maniculatus). Journal of Mammalogy 100, 910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR, 2019. Life ascending: Mechanism and process in physiological adaptation to high-altitude hypoxia. Annual Review of Ecology, Evolution, and Systematics 50, 503–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR, Cheviron ZA, 2010. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. Journal of Experimental Biology 213, 4125–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester JT, Shimoda LA, Aaronson PI, Ward JPT, 2012. Hypoxic pulmonary vasoconstriction. Physiological Reviews 92, 367–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate KB, Ivy CM, Velotta JP, Storz JF, McClelland GB, Cheviron ZA, Scott GR, 2017. Circulatory mechanisms underlying adaptive increases in thermogenic capacity in high-altitude deer mice. Journal of Experimental Biology 220, 3616–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate KB, Wearing OH, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR, 2020. Coordinated changes across the O2 transport pathway underlie adaptive increases in thermogenic capacity in high-altitude deer mice. Proceedings of the Royal Society B 287, 20192750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velotta JP, Ivy CM, Wolf CJ, Scott GR, Cheviron ZA, 2018. Maladaptive phenotypic plasticity in cardiac muscle growth is suppressed in high-altitude deer mice. Evolution 72, 2712–2727. [DOI] [PubMed] [Google Scholar]
- West CM, Wearing OH, Rhem RG, Scott GR, 2021. Pulmonary hypertension is attenuated and ventilation-perfusion matching is maintained during chronic hypoxia in deer mice native to high altitude. (in review). [DOI] [PubMed]


