Abstract
Background:
Anticoagulation increases the risk of intracerebral hemorrhage (ICH) in patients with cerebral amyloid angiopathy (CAA), so the management of stroke-risk in patients with both atrial fibrillation (AF) and CAA is controversial. Advances in left atrial appendage closure (LAAC) techniques provide a stroke-risk-reduction option which avoids long-term oral anticoagulation (OAC). We aimed to evaluate the safety of this intervention in patients with CAA.
Methods:
This is an observational cohort study of patients with severe CAA (with or without ICH) and AF who were treated with LAA closure. The Watchman™ and Amulet® LAAC devices, Lariat procedure or open surgical closure of the LAA were all considered acceptable means of closure. Patients with symptomatic ICH and those naive to anticoagulation were placed on clopidogrel and/or aspirin for 6 weeks after the procedure; patients who previously tolerated anticoagulation remained on warfarin or a DOAC for 6 weeks post-procedure. All antiplatelet and anticoagulation therapy was discontinued after confirmation of LAAC. All patients had aggressively optimized blood pressure and fall precautions in addition to surgical intervention. Safety, tolerability, stroke and hemorrhage rates were documented.
Outcome:
Twenty-six patients with a mean CHA2DS2-VASc score of 4.6 were treated, 13 with a history of symptomatic lobar hemorrhage and 13 without. All patients who completed LAAC tolerated device implantation. There were no documented ischemic strokes or symptomatic ICH during the 30 days after device implantation. Patients were followed for an average of 25 months. One patient who underwent LARIAT LAAC had an ischemic stroke in follow-up, but recovered well; there were no other thromboemboli in this cohort.
Conclusions:
This cohort study provides evidence that LAAC appears to be a safe and tolerable treatment to reduce stroke risk in patients with CAA. Because of the small size of the cohort and relatively short follow-up, the efficacy for stroke and ICH prevention is not conclusive, but the preliminary results are encouraging. LAA closure may be a good alternative to anticoagulation in patients with CAA and atrial fibrillation.
Keywords: Intracerebral hemorrhage, stroke, Watchman, secondary prevention, left atrial appendage closure, cerebral amyloid angiopathy
Introduction
Cerebral amyloid angiopathy (CAA) is a cerebral vasculopathy caused by β-amyloid deposition in cerebral arterioles and capillaries [1]. It is not uncommon after age 60 and predisposes patients to intracerebral hemorrhage (ICH) and cognitive impairment [2,3]. It is closely linked to Alzheimer’s disease (AD), but contributes to cognitive impairment independently from classical AD pathologies, particularly by reducing processing speed [4]. Patients with CAA are prone to lobar hemorrhages with a high rate of recurrent ICH. Roughly 25% of patients with CAA-related lobar hemorrhage have a recurrence within 5 years [5,6]. Hypertension, anticoagulation, antiplatelet therapy, head trauma and apolipoprotein E4 genotype are thought to increase the risk of ICH in this population [7–9]. It is not uncommon for patients with CAA to have comorbid atrial fibrillation (AF). Because of the associated cardioembolic stroke risk, AF is often treated with oral systemic dose adjusted warfarin or direct oral anticoagulants such as factor Xa inhibitors or direct thrombin inhibitors (DOAC) [10] and it is recommended in professional guidelines for both primary and secondary prevention.[11] In patients with CAA the risk of ICH makes this a difficult therapeutic decision. Greater than 90% of thromboemboli in AF form within the left arterial appendage (LAA) [12]. Closure of the LAA for the prevention of emboli in AF was first presented in the literature in 1949 and the modern concept was introduced in 1996 [13,14]. Since then, left atrial appendage closure (LAAC) has been shown to be safe and effective for mitigating stroke-risk in patients at high risk of bleeding [15–17]. Importantly, LAAC can be performed to decrease the stroke risk of AF without increasing hemorrhage risk as long-term anticoagulation is not required. Post-operative anticoagulation is generally indicated after LAAC for six to twelve weeks to allow the implanted device to fully endothelialize and confirm complete LAAC, though even this relatively short duration of anticoagulation in patients with very high risk of ICH this may pose an unacceptable risk. The safety and efficacy of LAAC has not been formally tested in patients with CAA. We evaluated the safety of LAAC in patients with CAA and in high-risk patients performed the closure without post-implantation anticoagulation, using anti-platelet therapy alone instead.
Methods
This is a multi-center, observational study that included patients from 2016 to 2020. At Vanderbilt University Medical Center, the cohort is derived from a collaboration between the Observational Study of CAA and Related Disorders (OSCAAR study) and the prospective Vanderbilt Left Atrial Appendage Registry (VaLAAR). The OSCAAR study follows a cohort of 134 patients with CAA in the Vanderbilt Cerebral Microvascular Diseases Clinic and was approved by the Institutional Review Board, approval # 180287. VaLAAR follows a cohort of over 600 patients who have undergone LAAC at Vanderbilt University Medical Center and was approved by the Institutional Review Board, approval #150110. An additional cohort was included from Rhode Island Hospital/Brown University, IRB# 1313000. This workflow is shown in figure 1. Inclusion criteria were a documented history of AF and a history of probable CAA by the Boston criteria or, for patients for whom MRI was not obtainable, the presence of one or more lobar hemorrhage in a clinical context typical of CAA and without severe hypertension or another clear cause [18,19]. Figure 2 shows typical neuroimaging diagnostic features. The decision to proceed with LAAC was made by the patients and their physicians on clinical grounds; those who declined LAAC were excluded. The Watchman™ (Boston Scientific Inc., Natick MA, USA) and Amulet® devices (Abbott Medical, St. Paul MN, USA), Lariat procedure (Atricure Inc, West Chester OH, USA) or endocardial surgical ligation using oversewing (proline), resection (excision), stapling or clipping (Atriclip, Atricure Inc.) of the LAA were all considered acceptable means of LAAC. Device based or Lariat LAAC was performed in the cardiac electrophysiology laboratory under general anesthesia with continuous transesophageal echocardiographic (TEE) imaging (see Figure 2). The approach to bridging thromboprophylaxis after LAAC was matched to our assessment of individual patient’s hemorrhage risk. Patients with a history of symptomatic ICH or who were otherwise felt to be high risk were placed on single or dual antiplatelet therapy for 6 weeks after the closure procedure while patients who had previously tolerated anticoagulation were continued on OAC or DOAC for 6 weeks after the procedure (bridging regimen was determined by the team of treating physicians). After 6 weeks and confirmatory imaging by transesophageal echocardiography (TEE) or gated contrast cardiac CT angiogram, antiplatelet or OAC/DOAC therapy was discontinued in the highest risk patients. Low-dose aspirin was continued in lower ICH risk patients or in those mandated by investigational device exemption (IDE) clinical trial protocols. All patients had optimized blood pressure to a goal of normotension and fall precautions in addition to surgical intervention. Safety, tolerability and stroke (both ischemic and hemorrhagic) incidence were documented. Individual subject-level data is presented. Neuroimaging was reviewed and quantified by MSS or BM using previously published criteria; these readers have previously demonstrated good intra-reader reliability [20]. Follow-up duration was calculated from the time of the LAAC or the time of CAA diagnosis, whichever was longer. Outcome events included surgical complications, symptomatic ischemic stroke, transient ischemic attack, intracerebral hemorrhage, new or unstable arrhythmia or death. Perioperative events were those occurring within 30 days of closure and a proven or probable link to the implant procedure. Post-operative events occurred after 30 days from the time of the procedure. From this data we calculated the stroke/TIA rate per 100 patient years.
Figure 1.
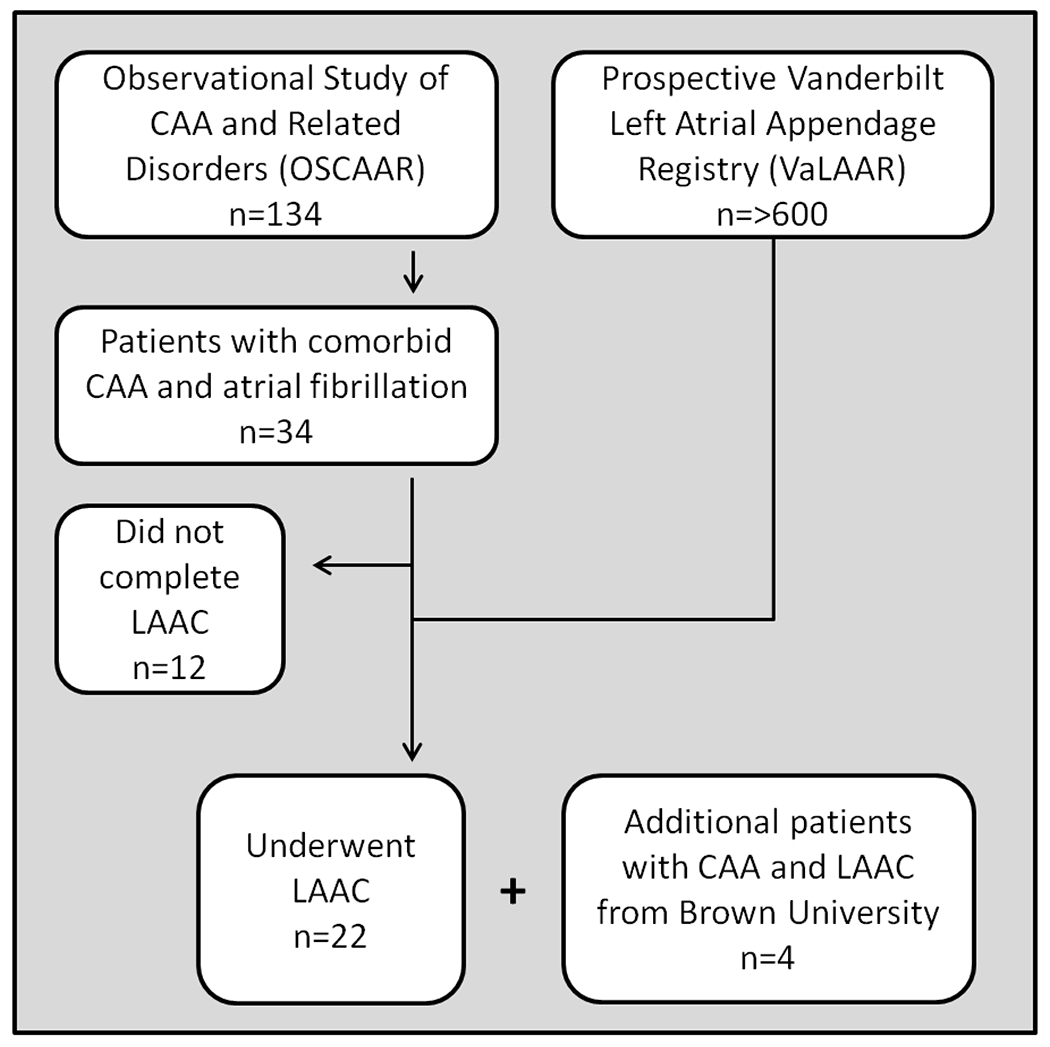
Workflow for patients selection in the study. CAA= cerebral amyloid angiopathy; LAAC= left atrial appendage closure.
Figure 2.
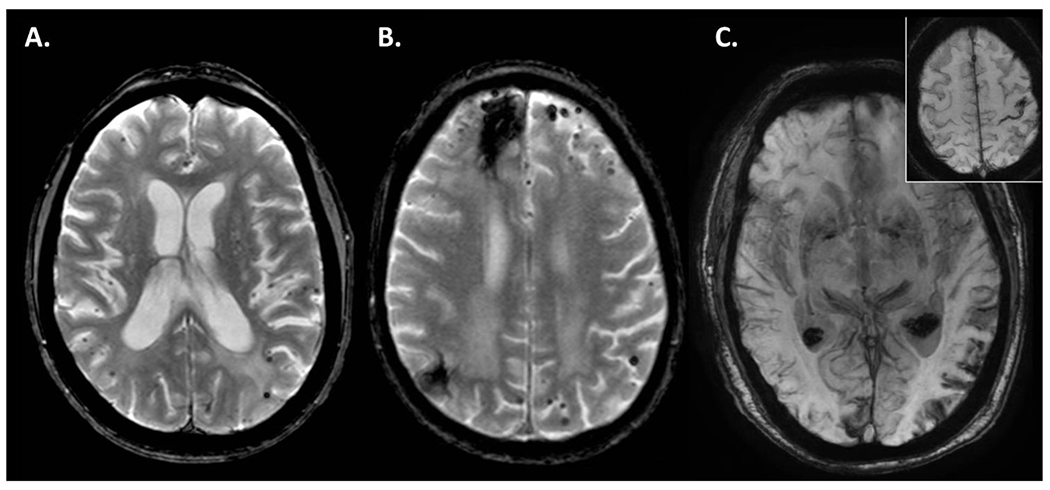
Imaging characteristics of selected cases illustrating diffuse cortical microhemorrhages, lobar intraparenchymal hemorrhage and cortical superficial siderosis, the typical hemorrhagic pathologies of cerebral amyloid angiopathy. A. Case V-4 is a 50 year old woman with early onset cerebral amyloid angiopathy who had hundreds of exclusively cortical cerebral microhemorrhages on this gradient echo T2* image. B. Case V-9 is an 84 year old woman with two prior intracerebral hemorrhages and cortical cerebral microhemorrhages who underwent LAAC with the Lariat procedure. C. Case V-18 is a 78 year old man with diffuse cortical superficial siderosis shown on this minimum intensity projection of a susceptibility weighted sequence who initially presented with focal motor seizures in the right hand correlating with a focal area of siderosis (see Inset).
Results
Of the 134 patients with CAA followed in the Vanderbilt Microvascular Disease Clinic, 34 had AF (25.4%). Twenty-six (n=26) patients were enrolled in this study, 22 from VUMC and an additional 4 from Brown University. Twenty-three were referred for LAAC on the basis of a diagnosis of CAA. One patient was referred for LAAC due to GI bleeding and was incidentally discovered to have CAA around the time of LAAC and two others had prophylactic surgical LAAC during cardiac procedures and were later diagnosed with CAA. Patient demographics are shown in Table 1. Twenty-four patients met the modified Boston criteria for a diagnosis of probable CAA. Two were unable to have an MRI due to a having an MRI-incompatible pacemaker, but were clinically diagnosed with probable CAA on the basis of one or more lobar intracerebral hemorrhages, their age and the absence of uncontrolled hypertension or other etiology. Of the twelve patients with CAA and atrial fibrillation who did not complete LAAC, one is currently planning to undergo LAAC, one died suddenly of unknown cause prior to LAAC, two had a history of hypercoagulability in addition to AF so closure would not adequately address their risk, two had single episodes of atrial fibrillation and another underwent an ablation (these latter three opted for continuous observation with implantable loop recorders). One patient opted out of the procedure due to advanced dementia and the procedure was not consistent with goals of care. Four patients opted out of the procedure due to personal preference; of these, two were treated with clopidogrel, one with low-dose apixaban and one remained on warfarin. This last patient died suddenly of an intracerebral hemorrhage.
Table 1:
Patient demographics
| Number | 26 |
| Sex | 10F/16M |
| Mean age (years +/− SD) | 73.0 +/− 8.5 |
| Percent with ICH | 50% |
| Percent with ischemic stroke or TIA | 23% |
| Percent with cognitive impairment | 32% |
| Percent with hypertension | 81% |
| Percent with diabetes | 31% |
| Percent with hyperlipidemia | 65% |
| Percent with coronary artery disease | 42% |
| Mean CHA2DS2VASc (mean +/− SD) | 4.6 +/− 1.5 |
| Mean HASBLED (mean +/− SD) | 3.8 +/− 1.0 |
| Mean follow-up time | 25 months |
Our approach to immediate post LAAC thromboprophylaxis was to attempt the maximum intensity therapy considered safe based on an individual clinical assessment of their ICH risk. The approach used in each case is shown in Table 2. Nine patients were treated with full-intensity OAC or DOAC for three months (current standard of care approach following device based LAAC). Two patients were treated with half dose apixaban (2.5mg BID for 45-90 days) while thirteen were bridged with dual or single antiplatelet therapy and in one case no bridging thromboprophylaxis was used. After a bridging period, 19 patients received long-term anti-platelet therapy while seven high-risk patients had all thromboprophylaxis stopped.
Table 2:
Case data
| Case # | Age at LAAC & sex | Clinical / Neuroimaging findings | CHA2DS2 VASC | HAS BLED | Device used | Perioperative events | Bridging therapy | Long-term therapy | Follow-up duration | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICH | CMHs | Siderosis | Cognitive impairment | ||||||||||
| V-1 | 71 M | − | 22 | 1 gyrus | + | 3 | 4 | Watchman | None | SAPT | SAPT | 22m | Well |
| V-2 | 72 M | − | 19 | − | − | 4 | 4 | Watchman | None | DAPT | SAPT | 22m | Well |
| V-3 | 80 M | 2 | Pacemaker | + | 4 | 6 | Watchman | None | Warf | SAPT | 41m | Well | |
| V-4 | 50 F | − | >50 | − | + | 5 | 2 | Watchman | None | SAPT | SAPT | 30m | Well |
| V-5 | 76 F | 2 | >50 | 1 gyrus | − | 5 | 4 | Amulet | None | SAPT | None | 24m | Died1 |
| V-6 | 57 F | − | 22 | − | − | 4 | 4 | Ligation | None | SAPT | SAPT | 23m | Well |
| V-7 | 78 M | 1 | 5 | − | − | 4 | 4 | Watchman | None | SAPT | SAPT | 28m | Well |
| V-8 | 65 F | 1 | 21 | 3 gyri | − | 4 | 3 | Amulet | None | SAPT | SAPT | 28m | Well |
| V-9 | 82 F | − | >50 | − | + | 7 | 3 | Lariat | None | SAPT | SAPT | 13m | Stroke2 |
| V-10 | 81 M | 1 | Pacemaker | − | 4 | 5 | Watchman | None | DAPT | None | 33m | Well | |
| V-11 | 76 F | 1 | 3 | − | − | 8 | 5 | Watchman | None | Id-NOAC | SAPT | 22m | Well |
| V-12 | 69 M | − | 7 | − | − | 4 | 3 | Watchman | None | SAPT | SAPT | 42m | Well |
| V-13 | 58 M | 1 | 3 | − | − | 4 | 3 | Watchman | None | NOAC | DAPT | 40m | Well |
| V-14 | 70 M | 1 | 3 | − | − | 4 | 4 | Watchman | None | SAPT | SAPT | 15m | Well |
| V-15 | 65 M | − | 31 | − | + | 2 | 3 | Lariat | None | None | None | 13m | Well |
| V-16 | 75 F | − | 2 | − | − | 6 | 3 | Lariat | None | NOAC | SAPT | 11m | Well |
| V-17 | 79 F | − | 2 | − | − | 6 | 2 | Watchman | None | NOAC | None | 4m | Well |
| V-18 | 78 M | − | 4 | Diffuse | − | 7 | 4 | Lariat | None | SAPT | None | 15m | Well |
| V-19 | 77 M | − | 2 | Diffuse | − | 3 | 3 | Watchman | None | Id-NOAC | SAPT | 3m | Well |
| V-20 | 81 M | 1 | >50 | − | + | 4 | 3 | Watchman | None | SAPT | None | 4m | Died4 |
| V-21 | 73 M | − | 10 | 1 gyrus | + | 3 | 4 | Ligation | None | Warf | SAPT | 46m | Well |
| V-22 | 68 F | 1 | 0 | 2 gyri | − | 4 | 5 | Atriclip | None | SAPT | None | 72m | Well |
| B-23 | 75 F | 1 | 2 | − | − | 3 | 4 | Watchman | None | Warf | SAPT | 35m | Well |
| B-24 | 77 M | 1 | >50 | 2 gyri | − | 6 | 5 | Watchman | None | Warf | SAPT | 31m | Well |
| B-25 | 86 M | − | 7 | − | − | 7 | 5 | Watchman | Arrhyth.5 | Warf | SAPT | 24m | Well |
| B-26 | 80 M | 1 | 21 | − | + | 5 | 5 | Watchman | None | Warf | SAPT | 17m | ICH6 |
Abbreviations: LAAC = left atrial appendage occlusion, ICH = intracerebral hemorrhage, CMH = cerebral microhemorrhage, SAPT = single antiplatelet therapy, DAPT = dual antiplatelet therapy, Warf = warfarin, NOAC = novel oral anti-coagulant, Id-NOAC = low dose NOAC, m = month.
The patient died from heart failure.
The patient was treated with tPA for a stroke at 48 days post-procedure and a thrombus was visualized near the site of closure. Clinical outcome after this event was outstanding. The patient had end-stage Alzheimer’s disease and died shortly after suffering a concussion in a motor vehicle accident 13 months post-closure.
The patient died of localization related status epilepticus related to a previous intracerebral hemorrhage.
The patient had a brief episode of pulseless ventricular tachycardia without an adverse long-term outcome.
The patient suffered a traumatic, polycompartmenal intracranial hemorrhage 18 months after the procedure while on aspirin monotherapy. He quickly recovered to his baseline.
We observed one perioperative adverse event which the patient survived without increase of baseline disability. The patient (B-25) had a brief episode of pulseless ventricular tachycardia and fully recovered. One additional patient (V-20) suffered a small ischemic stroke shortly after AF was discovered, but prior to LAAC; this was not considered a perioperative adverse event, but is included as an intent to treat.
Within the LAA-CAA cohort, we observed one incident ischemic stroke with an average of 26 months of follow-up (1.8 strokes / 100 person years). The individual with the stroke developed right hemiplegia and aphasia 48 days after LAAC and was treated with intravenous tissue plasminogen activator and made a full recovery (V-9 in Table 2). The bridging thromboprophylaxis regimen in this patient was three days of apixaban followed by clopidogrel alone due to high fall risk with unstable gait. Trans-esophageal echocardiography demonstrated a mobile thrombus near the site of closure, but repeat echocardiography after 6 weeks of anticoagulation showed resolution of the thrombus and confirmed complete LAA closure. Three patients in the cohort died; one due to complications related to status epilepticus (4 months after LAAC), one due to complications of a concussion in the context of Alzheimer’s (12 months after LAAC), and one due to heart failure (23 months after LAAC). No deaths were related to LAAC. Additionally, one patient experienced traumatic ICH 381 days after the procedure after a mechanical fall. Figure 2 demonstrates baseline LAA by TEE or 3-D TEE imaging intra-operatively and post implant LAA closure imaging for subjects V-4 Watchman™ LAAC and V-5 Amulet® LAA occluder.
Discussion
Patients with CAA and AF present a difficult clinical management problem to balance the ischemic stroke risk [21] with the risk of intracranial bleeding [22], particularly with respect to the decision to administer OAC or DOAC. The choice between anticoagulation and accepting the risk of ICH or omitting anticoagulation and accepting the risk of cardioembolic stroke can be avoided with the use of LAA closure. LAAC is an FDA approved treatment alternative to chronic anticoagulant therapy to suppress LAA thrombus formation and associated stroke risk in AF. Multiple published registries and two large randomized clinical trials have found that LAAC is well tolerated and effective without long term anticoagulant therapy [23,24], therefore mitigating the risk of bleeding reemergence with anticoagulant therapy. Patients with CAA and AF are a particularly high-risk group for whom the safety and efficacy of LAAC needs to be prospectively evaluated [25,26]. AF is not uncommon in CAA, affecting about 25% of patients in our clinic. As a referral center, this percentage may somewhat overestimate the true incidence, although a similar percentage was reported in another cohort with CAA [27]. In the patients who underwent LAAC, we found that treatment with a reduced intensity thromboprophylaxis regimen in the 30 days post LAAC was well tolerated. A recent observational study by Hucker et al., has shown safe and effective stroke risk reduction of LAAC with a short term OAC or DOAC post implant drug regimen in AF patients with a previous ICH [24]. This finding supports utilizing LAAC procedures even in high-risk patients who may not tolerate short-term anticoagulation. We observed one ischemic stroke during the observation period; while this manuscript is not powered to evaluate efficacy, based on the CHA2DS2VASc score we would have expected 3.4 thromboembolic events in our cohort during the follow-up period which reflects a rate of 1.9 per 100 patient years compared to an expected rate of 6.2 per 100 patient years [28]. This low event rate is consistent with previous work demonstrating the efficacy of LAAC for stroke prevention in AF (the mean CHA2DS2VASc score in the major LAAC studies were: PROTECT-AF 3.6, PREVAIL 4.0, CAP 3.8, CAP-2 4.5) [29].
A recent, pooled analysis of three observational cohorts of patients with ICH and an indication for anticoagulation addressed the issue of how to balance the risks of ICH and ischemic strokes in patients who have both CAA and AF [30]. The authors reached the controversial conclusion that restarting anticoagulation, even in the context of CAA with recent symptomatic hemorrhage, was associated with improved functional outcome at 90 days as well as decreased mortality and stroke risk, but the study was vulnerable to several biases which are critical in interpreting the literature around this topic. Non-randomized or non-systematic assignment to anticoagulation creates a selection bias as sicker patients are less likely to re-start anticoagulation. Additionally, the study considered all lobar ICH a manifestation of underlying CAA and few patients restarted on anticoagulation in the cohorts had a magnetic resonance imaging-based diagnosis of probable CAA based on the pattern of microhemorrhagic changes. Importantly, lobar ICH is not equivalent to a clinical diagnosis of CAA; in fact, in pathological case series over 50% of lobar hemorrhages did not have CAA [31,32]. More importantly, this study illustrates a false dichotomy which is widespread in current literature relating to accepting either the risk of hemorrhage or the risk of stroke in patients with CAA and AF. LAAC in this context may effectively mitigate the ischemic stroke risk associated with AF without the need for long term OAC or DOAC which is dangerous in patients with CAA [13,14]. The findings from the Biffi study directly influenced the initiation of the ASPIRE trial which is currently in progress [33]. The researchers will evaluate stroke and ICH rate in survivors of ICH with AF treated with either aspirin or apixaban. This study would be greatly enhanced by including an arm with LAAC as a guideline directed therapy to reduce AF related stroke risk now considered a standard of care consideration for high bleeding risk patients [34].
Anticoagulation in patients with severe CAA, indicated by the presence symptomatic intracerebral hemorrhage and/or a large number of cerebral microhemorrhages, places them at high-risk of ICH [22,35]. LAAC poses lower exacerbating pressure upon intracranial bleeding in CAA and should be a priority research topic in this space. Our study is limited by its small size and observational structure, but demonstrates the safety, feasibility and early clinical efficacy of LAAC in patients with CAA. Because of the compelling evidence of risk associated with anticoagulation in patients with CAA and the number of different LAAC device options that are FDA-approved for potential use alone with anticipation of Watchman FLX and Amulet LAAO device approvals, the efficacy of this intervention in CAA can reasonably be assessed in a multi-center prospective registry.
Conclusions
This LAA-CAA cohort study provides evidence that LAAC appears to be a safe and tolerable treatment to reduce stroke risk in patients with CAA. Preliminary results are encouraging as to the efficacy for stroke and ICH prevention. LAA closure may be a reasonable alternative to anticoagulation in patients with CAA and AF.
Figure 3.
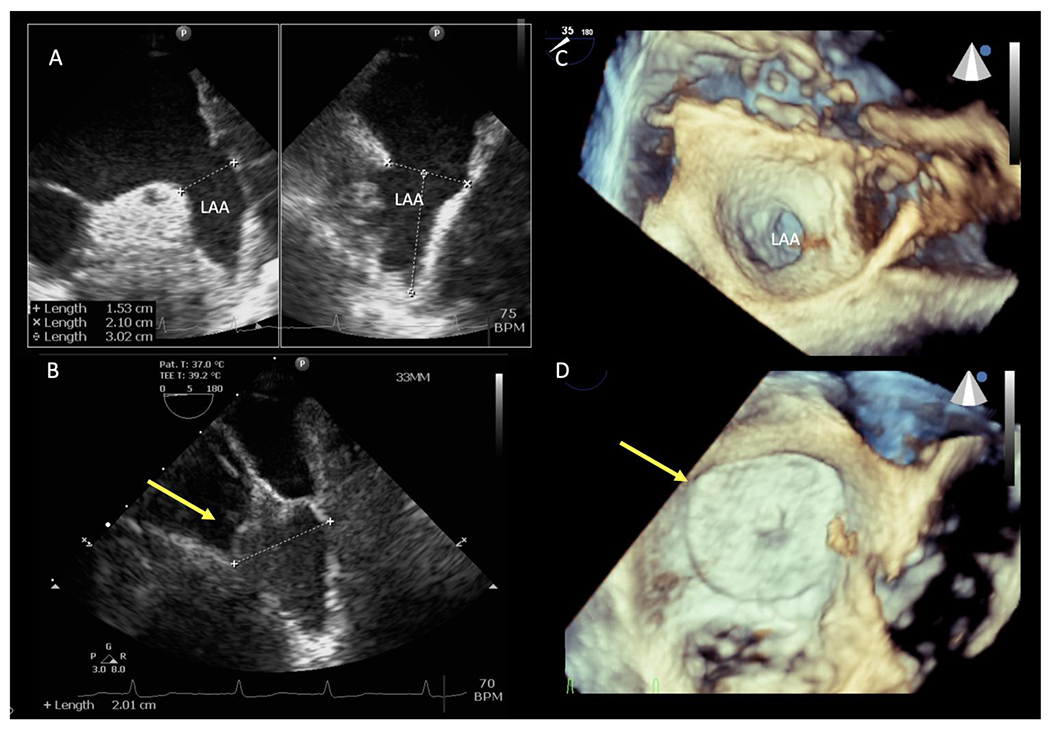
A. Two views from a transesophageal echocardiogram at 45 and 135 degree showing large baseline ‘windsock’ left atrial appendage. B. Placement of a 33mm Watchman left atrial appendage closure device at the left atrial appendage ostium with complete closure. C. 3D transesophageal echocardiogram view of the left atrial appendage ostium. D. Occlusion of left atrial appendage with Amulet occluder (this patient was enrolled in Amulet IDE clinical trial).
Acknowledgments
Funding: This study received no funding.
Conflicts of interest/Competing interests: Matthew Schrag is funded by the NIH (NINDS 1R03NS111486, 1R21NS106510and NIA 1K76AG060001) and serves on the DSMB for the REVISION trial. Shadi Yaghi has received research funding from Medtronic. Christopher Ellis receives research funding and serves as a consultant for Medtronic Inc. He also serves as a consultant for Boston Scientific Inc, Atriculture Inc and Abbot Medical Inc.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Availability of data and material: The data contained in this manuscript will be made available to other researchers upon request and approval by the relevant oversight committees.
Code availability: N/A
References
- 1.Schrag M, McAuley G, Pomakian J, Jeffry A, Tung S, Mueller C, Vinters HV, Haacke EM, Holshouser B, Kido D, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol. 2010;119(3):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Etten ES, Auriel E, Haley KE, Ayres A, Vashkevich A, Schwab K, Rosand J, Viswanathan A, Greenberg S, Gurol ME. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke. 2014;45(8):2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle PA, Yu L, Nag S, Wilson RS, Bennet DA, Schneider JA. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrag M, Kirshner H. Neuropsychological Effects of Cerebral amyloid angiopathy. Curr Neurol Neuroscl Rep. 2016;16(8):76. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke. 1983;14(6):915–923. [DOI] [PubMed] [Google Scholar]
- 6.Boulouis G, Charidimou A, Pasi M, Roongpiboonsopit D, Xiong L, Auriel E, van Etten E, Martinez-Ramirez SS, Ayres A, Vashkevich A, et al. Hemorrhage recurrence risk factors in cerebral amyloid angiopathy: Comparative analysis of the overall small vessel disease severity score versus individual neuroimaging markers. J Neurol Sci 2017;380:64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arima H, Tzourio C, Anderson C, Woodward M, Bousser MG, MacMahon S, Neal B, Chalmers J. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010;41(2):394–396. [DOI] [PubMed] [Google Scholar]
- 8.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55(7):947–951. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq PD, Murray LS, Smith C, Graham DI, Nicoll JAR, Gentleman SM. Cerebral amyloid angiopathy in traumatic brain injury: association with apolipoprotein E genotype. J Neurol Neurosurg Psychiatry 2005;76(2):229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns JGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 11.Kernan WN, Ovbiagele B, Black HR, Bravata D, Chimowitz M, Ezekowitz MD, Fang MC, Fisher M, Furie K, Heck DV, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. [DOI] [PubMed] [Google Scholar]
- 12.Yarmohammadi H, Klosterman T, Grewal G, Alraies MC, Lindsay BD, Bhargava M, Tang WHW, Klein A. Transesophageal echocardiography and cardioversion trends in patients with atrial fibrillation: a 10-year survey. J Am Soc Echocardiogr. 2012. September;25(9):962–8. [DOI] [PubMed] [Google Scholar]
- 13.Madden JL. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J Am Med Assoc. 1949; 140(9) :769–772. [DOI] [PubMed] [Google Scholar]
- 14.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61(2):755–759. [DOI] [PubMed] [Google Scholar]
- 15.Price MJ, Reddy VY, Valderrabano M, Halperin JL, Gibson DN, Gordon N, Huber KC, Homes DR. Bleeding outcomes after left atrial appendage closure compared with long-term warfarin: a pooled, patient-level analysis of the WATCHMAN randomized trial experience. JACC Cardiovasc Interv. 2015;8(15):1925–1932. [DOI] [PubMed] [Google Scholar]
- 16.Holmes DR, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL Trial. Journal of the American College of Cardiology. 2014;64(1):1–12. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen-Kudsk JE, Johnsen SP, Wester P, Airaksinen J, Lund J, De Backer O, Pakarinen S, Odenstedt J, Vikman S, Settergren M, et al. Left atrial appendage occlusion versus standard medical care in patients with atrial fibrillation and intracerebral haemorrhage: a propensity score-matched follow-up study. Eurointervention. 2017;13(3):371–378. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-lsla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38(2):254–259. [DOI] [PubMed] [Google Scholar]
- 19.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, van Buchem MA, Bruckman H, Greenberg SM.. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74(17):1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra A, Schindler J, Mac Grory B, Chu S, Youn T, Matouk C, Greer DM, Schrag M. Cerebral microhemorrhages and meningeal siderosis in infective endocarditis. Cerebrovasc Dis. 2017;43(l-2):59–67. [DOI] [PubMed] [Google Scholar]
- 21.Walker AM, Bennett D. Epidemiology and outcomes in patients with atrial fibrillation in the United States. Heart Rhythm. 2008;5(10):1365–1372. [DOI] [PubMed] [Google Scholar]
- 22.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34(7):1710–1716. [DOI] [PubMed] [Google Scholar]
- 23.Hutt E, Wazni OM, Saliba WI, Kanj M, Tarakji K, Aguilera J, Barakat AF, Rasmussen P, Uchino K, Russman A, et al. Left atrial appendage closure device implantation in patients with prior intracranial hemorrhage. Heart Rhythm 2019;16(5):663–668. [DOI] [PubMed] [Google Scholar]
- 24.Hucker WJ, Cohen JA, Gurol ME, Heist EK, Gianni C, Galvin J, Atkins D, Bommana S, Di Biase L, Ruskin J, et al. WATCHMAN implantation in patients with a history of atrial fibrillation and intracranial hemorrhage. J Interv Card Electrophysiol. 2019; 10.1007/s10840-019-00678-w [DOI] [PubMed] [Google Scholar]
- 25.Reddy VY, Doshi SK, Kar S, Gibson D, Price MJ, Huber K, Horton R, Buchbinder M, Neuzil P, Gordon NT, et al. Five-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J. Am. Coll. Cardiol 2017;70(24):2964–2975. [DOI] [PubMed] [Google Scholar]
- 26.Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, Gori T, Meincke F, Protopopov AV, Betts T, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm 2017;14(9):1302–1308. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser J, Schebesch KM, Brawanski A, Linker RA, Schlachetzki F, Wagner A. Long-Term Follow-Up of Cerebral Amyloid Angiopathy-Associated Intracranial Hemorrhage Reveals a High Prevalence of Atrial Fibrillation. J Stroke Cerebrovasc Dis. 2019;28(11):104342. [DOI] [PubMed] [Google Scholar]
- 28.Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur. Heart J 2012;33(12):1500–1510. [DOI] [PubMed] [Google Scholar]
- 29.Holmes DR, Reddy VY, Gordon NT, Delurgio D, Doshi S, Desai A, Stone JE, Kar S. Long-term safety and efficacy in continued access left atrial appendage closure registries. J. Am. Coll. Cardiol 2019;74(23):2878–2889. [DOI] [PubMed] [Google Scholar]
- 30.Biffi A, Kuramatsu JB, Leasure A, Kamel H, Kourkoulis C, Schwab K, Ayres AM, Elm J, Gurol ME, Greenberg SM, et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann. Neurol 2017;82(5):755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J. Neurol. Sci 1993;116(2):135–141. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara T, Takahashi M, Yokota T, Yamashita Y, Gondo T, Uchino F, Iwamoto N. The significance of cerebrovascular amyloid in the aetiology of superficial (lobar) cerebral haemorrhage and its incidence in the elderly population. J. Pathol 1991; 165(3) :229–234. [DOI] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov [Internet]. Identifier: NCT03907046. Anticoagulation for Stroke Prevention and Recovery After ICH (ASPIRE) 2019;[cited 2020 Mar 20 ] Available from: https://clinicaltrials.gov/ct2/show/NCT03907046
- 34.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz M, Field M, Furie K, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. [DOI] [PubMed] [Google Scholar]
- 35.Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, Smith EE, Greenberg SM, Rosand J, Viswanathan A.. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75(8):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]


