Abstract
Small RNAs (sRNAs) are mainly classified into microRNAs (miRNAs) and small interfering RNAs (siRNAs) according to their origin. miRNAs originate from single-stranded RNA precursors, whereas siRNAs originate from double-stranded RNA precursors that are synthesized by RNA-dependent RNA polymerases. Both of single-stranded and double-stranded RNA precursors are processed into sRNAs by Dicer-like proteins. Then, the sRNAs are loaded into ARGONAUTE proteins, forming RNA-induced silencing complexes (RISCs). The RISCs repress the expression of target genes with sequences complementary to the sRNAs through the cleavage of transcripts, the inhibition of translation or DNA methylation. Here, we summarize the recent progress of sRNA pathway in the interactions of rice with various parasitic organisms, including fungi, viruses, bacteria, as well as insects. Besides, we also discuss the hormone signal in sRNA pathway, and the emerging roles of circular RNAs and long non-coding RNAs in rice immunity. Obviously, small RNA pathway may act as a part of rice innate immunity to coordinate with growth and development.
Keywords: MicroRNA, Small interfering RNA, ARGONAUTE, Dicer-like, RNA dependent RNA polymerase, Rice immunity, Hormone signal
Background
Plants employ a sophisticated immune system to protect themselves from biotic stress. The cell surface-located receptors surveil and recognize pattern molecules from pathogens to initiate immune responses, termed pattern-triggered immunity (PTI). Typical responses in PTI include the burst of reactive oxygen species (ROS), the induction of pathogenesis-related genes (PR genes), and the deposition of callose. Pathogens usually overcome PTI with effectors to facilitate their invasion and proliferation. In turn, plant resistance (R) proteins specifically recognize cognate effectors to activate immune responses, called effector-triggered immunity (ETI). Immune responses in ETI are much stronger than those in PTI and are often accompanied by the hypersensitive response (HR) at the invasion site (Boller and Felix 2009; Boller and He 2009; Jones and Dangl 2006).
Plant immune responses are tightly controlled by various immunity-associated regulators, such as transcription factors and small RNAs (sRNAs) (Chandran et al. 2018; Wang et al. 2018a). Plant endogenous sRNAs include miRNAs and siRNAs, both of them are processed by Dicer-like proteins (DCLs) and incorporated into ARGONAUTE proteins (AGOs) to form the RNA-induced gene silencing complexes (RISCs) (Baulcombe 2004). The RISCs specifically bind to DNA or RNA sequences of sRNAs target genes and repress their expression on transcriptional, post-transcriptional or translational levels through DNA methylation, mRNA cleavage or translational repression, respectively (Brodersen et al. 2008; Llave et al. 2002; Song et al. 2019; Wu et al. 2010). Obviously, DCLs and AGOs are two types of core components shared by miRNA and siRNA signaling pathway. DCLs are a kind of endoribonucleases that specifically slice double-stranded RNAs (dsRNAs) into sRNA duplexes (Carmell and Hannon 2004). AGOs recognize and incorporate the mature sRNAs to execute the sRNA-guided repression of target genes (Fabian et al. 2010; Hutvagner and Simard 2008; Wu et al. 2010). Besides, the biosynthesis of siRNAs requires the activity of RNA-dependent RNA polymerases (RDRs). The RDRs exploit the single-stranded RNA (ssRNA) transcripts as the templates to synthesize the long dsRNAs that act as the precursors of siRNAs (Wassenegger and Krczal 2006). The exogenous virus-derived siRNAs (vsiRNAs) are a type of well-studied triggers of antiviral defense. The biosynthesis and function of vsiRNAs are also dependent on host DCLs, RDRs and AGOs (Brosseau and Moffett 2015; Du et al. 2007; Qu et al. 2008). Plants employ the vsiRNAs to initiate gene silencing of virus transcripts, thus disturbing virus replication and proliferation, which is regarded as the plant self-defensive mechanism against viruses (Llave 2010; Yang and Li 2018). However, in some cases, vsiRNAs are indicated to direct RNA silencing of host genes to facilitate infection (Smith et al. 2011; Xia et al. 2018; Yang et al. 2020a).
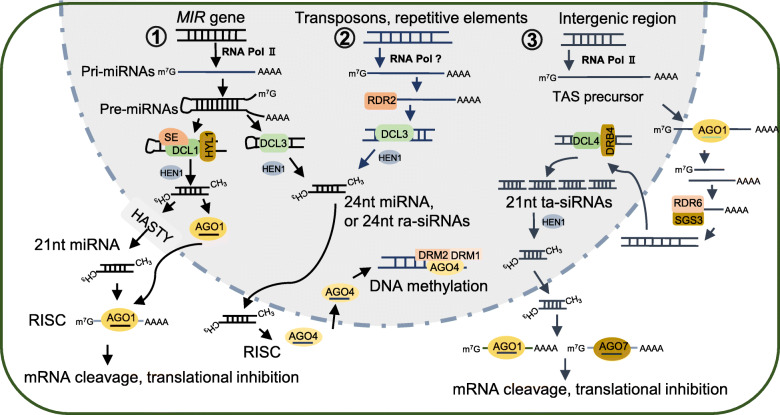
The biosynthesis of miRNAs is different from that of siRNAs (Fig. 1). miRNAs originate from the primary miRNA transcripts (pri-miRNAs) that are transcribed by RNA polymerase II (Pol II). Later, the single-stranded pri-miRNAs are processed into the precursor miRNAs (pre-miRNAs) that possess a stem-loop structure (He and Hannon 2004; Henderson et al. 2006). In contrast, siRNAs originate from multiple different transcripts that are transcribed by Pol II or Pol IV, such as TAS transcripts, cis-antisense overlapping coding transcripts, and transcripts derived from the retroelements or repetitive DNA (Hamilton et al. 2002; Katiyaragarwal and Jin 2010; Onodera et al. 2005; Vazquez 2006; Yoshikawa et al. 2005). All of these transcripts are exploited by RDRs to synthesize the double-stranded siRNA precursors (Wassenegger and Krczal 2006). The pre-miRNAs and the double-stranded siRNA precursors are sliced into sRNA duplexes by DCLs, then one strand of the sRNA duplex is incorporated into AGOs to form RISCs (Carmell and Hannon 2004; Song et al. 2019; Voinnet 2009). Besides, the processing of vsiRNAs is similar to that of the endogenous siRNAs. The production of vsiRNAs is divided into primary vsiRNAs and secondary vsiRNAs (Llave 2010). The primary vsiRNAs are derived from dsRNAs that are generated by viral RDRs during genome replication and transcription (RNA viruses) or via converging bidirectional transcription (DNA viruses), and the dsRNAs are perceived by the host DCLs to cut into sRNAs duplexes. Whereas, the secondary vsiRNAs are produced from dsRNAs that are synthesized by the host RDRs from the aberrant viral RNA cleavage products. Both the primary vsiRNAs and the secondary vsiRNAs are loaded into the host AGOs to form the RISCs (Aliyari et al. 2008; Llave 2010; Qu et al. 2008).
Fig. 1.
A simple model describes the biosynthesis and assembly of small RNAs. ① MIR genes are transcribed by RNA Polymerase II (RNA Pol II) into primary miRNAs (pri-miRNAs). Pri-miRNAs form an imperfect fold-back structure and then are processed into a stem-loop-structured precursor miRNAs (pre-miRNAs). The pre-miRNAs are further processed into 21- or 24-nt miRNA duplexes by the complex that mainly includes DCL1 or DCL3, HYPONASTICLEAVES1(HYL1) and SERRATE (SE). The overhang ends of miRNA duplex are methylated by HUA ENHANCER 1 (HEN1) to increase stability and then one strand of the miRNA duplex is loaded into AGO proteins to form RNA-induced gene silencing complex (RISC) in the cytoplasm or the nucleus. The 21-nt miRNAs exploit two pathways to incorporate into AGO1 protein. One, the miRNA duplex is exported to the cytoplasm via HASTY channel, then one strand of the duplex is loaded into AGO1 to form RISC. Two, some 21-nt miRNAs could be directly loaded into AGO1 to form RISC in the nucleus and the RISC is exported to the cytoplasm. Then, the RISCs repress gene expression via mRNA cleavage, or translational inhibition. Besides, 24-nt miRNAs are loaded into AGO4 in the cytoplasm and reenter into the nucleus to mediate DNA methylation by recruiting DOMAINS REARRANGED METHYLTRANSFERASE (DRM). ② The transposons or repetitive elements are transcribed by an unknown RNA Polymerase (RNA Pol?), then these transcripts are used as templates by RDR2 to synthesize double stranded RNAs (dsRNAs). Later, the dsRNAs are processed into 24-nt repeat-associated siRNAs (ra-siRNAs) by DCL3 and are methylated by HEN1 at 3′-terminal. Then one strand of ra-siRNAs is loaded into AGO4 to form RISC to mediate DNA methylation of target gene by recruiting the methyltransferases DRM1 and DRM2. ③ The specific intergenic regions are transcribed by RNA Pol II to generate the TAS precursors. The TAS precursors are targeted by some specific miRNA-AGO1 complex (such as miR390-AGO1 or miR173-AGO1) and are cleaved into fragments, then the 5′ or 3′ fragments of TAS precursors are exploited by RDR6 and SGS3 as the templates to synthesize the dsRNAs. The dsRNAs are imported into the nucleus and processed by DCL4 and DRB4 into 21-nt phased, trans-acting siRNAs (ta-siRNAs). After the methylation of ta-siRNAs at 3′-terminal, ta-siRNAs are exported into the cytoplasm and one strand of the ta-siRNAs is loaded into AGO1 or AGO7 to form RISC to suppress gene expression
Rice (Oryza sativa) is the most important staple crop in Asian countries and feeds more than half of the world population. Rice production is essential for global food security. However, rice production is threatened by diverse diseases, such as rice blast caused by Magnaporthe oryzae (synonymous with Pyricularia oryzae), rice sheath blight caused by Rhizoctonia solani (R. solani), rice leaf blight caused by Xanthomonas oryzae pv. oryzae (Xoo), rice dwarf disease caused by Rice dwarf virus (RDV), rice stripe disease caused by Rice stripe virus (RSV). In return, rice employs an efficient defensive system to resist these pathogens. Both PTI and ETI are indispensable for rice immune system against fungal and bacterial pathogens (Wang et al. 2014), and antiviral RNA silencing is a crucial weapon for defense against viruses (Yang and Li 2018). Plants also exploit ETI against nematodes (Kandoth and Mitchum 2013). Moreover, hormone signals contribute to rice immunity against fungi, bacteria, viruses and insects (De Vleesschauwer et al. 2013). In recent years, an increasing number of reports demonstrate that sRNAs act as the critical regulators of gene expression to fine-tune rice immune responses to different pathogens and insect pests. Here, we summarize the recent progresses on the studies of rice sRNA pathway in the interactions of rice with pathogens and insect pests (Table 1 and Fig. 2).
Table 1.
Characterized sRNAs involved in interactions of rice with pathogens and insect pests
| sRNAs | Target genes | Biotic stressors | Literature |
|---|---|---|---|
| miR7695 | OsNramp6.8 | Resistance to M. oryzae | (Campo et al. 2013; Sanchez-Sanuy et al. 2019) |
| miR398b | OsCSDs | Resistance to M. oryzae | (Li et al. 2019b) |
| miR162a | OsDCL1a | Resistance to M. oryzae | (Li et al. 2020a) |
| miR166k-5p | OsEIN2.1; OsEIN2.2 | Resistance to M. oryzae and F. fujikuroi | (Salvador-Guirao et al. 2018) |
| miR396 | OsGRFs | Susceptible to M. oryzae, BPH; Resistance to D. zeae | (Chandran et al. 2018; Dai et al. 2019; Li et al. 2019a) |
| miR156 | OsSPLs | Susceptible to M. oryzae, Xoo, BPH | (Ge et al. 2018; Liu et al. 2019; Zhang et al. 2020) |
| miR1873 | LOC_Os05g01790; | Susceptible to M. oryzae | (Zhou et al. 2020) |
| miR167 | OsARFs | Susceptible to M. oryzae | (Zhao et al. 2020) |
| miR319 | OsTCP21; OsGAmyb | Susceptible to M. oryzae, RRSV | (Zhang et al. 2016a; Zhang et al. 2018b) |
| miR169 | OsNF-YAs | Susceptible to M. oryzae, Xoo | (Li et al. 2017; Yu et al. 2018) |
| miR164a | OsNAC11; OsNAC60; |
Susceptible to M. oryzae, P. infestans, R. solani |
(Wang et al. 2018b) |
| miR444 | OsMADSs |
Susceptible to M. oryzae; Resistance to RSV |
(Wang et al. 2016; Xiao et al. 2017) |
| miR171b | OsSCL6-IIs | Resistance to RSV | (Tong et al. 2017) |
| miR528 | L-ascorbate oxidase; | Susceptible to RSV | (Wu et al. 2017) |
| TE-siR815 | OsWRKY45–1 | Susceptible to Xoo | (Zhang et al. 2016b) |
| siR109944 | OsFBL55 | Susceptible to R. solani | (Qiao et al. 2020) |
| miR168 | OsAGO1 | Susceptible to RSV | (Wu et al. 2015) |
| miR-14 | CsSpo; CsEcR | Resistance to RSB | (He et al. 2019) |
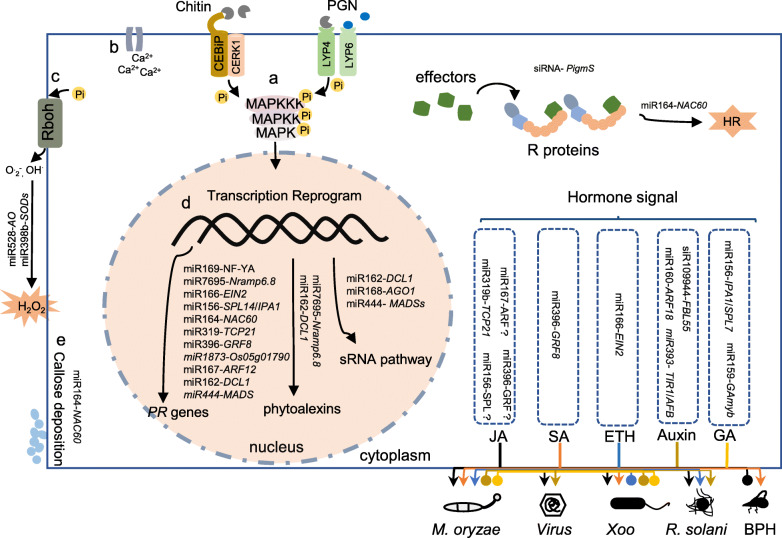
Fig. 2.
Small RNA regulatory modules in PTI, ETI, and hormone signal pathway. PAMPs derived from fungi or bacteria, such as chitin or peptidoglycan (PGN), are recognized by the pattern-recognition receptors (i.e. CEBiP and CERK1, or LYP4, and LYP6) to activate PTI responses including: a) activation of MAPK cascades, b) calcium influx, c) ROS burst, d) the induction of PR genes and phytoalexins genes, and e) callose deposition. The miR528-AO and miR398b-SODs modules contribute to the production of superoxide radicals (O·2−) and hydroxyl radical (OH·), and H2O2 accumulation. The modules regulating PR genes expression include miR169-NF-YA4/10/11, miR7695-Nramp6.8, miR166-EIN2, miR156-SPL14/IPA1, miR164-NAC60, miR319-TCP21, miR396-GRF8, miR1873-Os05g01790, miR167-ARF12, miR162-DCL1, and miR444-MADSs. The miR7695-Nramp6.8 and miR162-DCL1 module also participate in phytoalexins biosynthesis. The miR164-NAC60 module may be involved in callose deposition. Besides, the recognition between effectors and R proteins activates ETI that often leads to the hypersensitive response (HR). The siRNAs derived from the miniature transposons MITE1 and MITE2 regulate PigmS expression thus control the activity of PigmR; and the miR164-NAC60 module may contribute to the occurrence of HR. Hormone signal pathways differently regulate rice resistance to Magnaporthe oryzae (M. oryzae), viruses, Xanthomonas oryzae pv. oryzae (Xoo), Rhizoctonia solani (R. solani) and the planthopper (BPH). Jasmonic acid (JA) signal pathway positively regulates rice immunity against M. oryzae, viruses, Xoo, and R. solani but enhances susceptibility to BPH. Salicylic acid (SA) signal pathway positively regulates rice immunity against M. oryzae, Xoo and BPH. Ethylene (ET) signal pathway positively regulates rice immunity against M. oryzae, R. solani but enhances susceptibility to Xoo. Auxin signal pathway positively regulates rice immunity against virus and R. solani but increases susceptibility to M. oryzae and Xoo. Gibberellic acid (GA) signal pathway negatively regulates rice immunity against M. oryzae and Xoo. Four modules may block JA signal pathway to promote the infection of M. oryzae, or Xoo, including miR167-ARFs, miR396-GRFs, miR319-TCP21, and miR156-SPLs. The miR396-GRF8 module may impair SA signal pathway to promote the infestation of BPH. The miR166-EIN2 module may enhance ET signal pathway to interfere with the infection of M. oryzae. The siR109944-FBL55 module may impede auxin signal pathway to promote R. solani infection. The miR160-ARF18 and miR393-TIR1/AFB modules may act in auxin signal pathway. The miR156-IPA1/SPL7 module may enhance GA signal pathway to promote the infection of Xoo. The miR159-GAmyb module may act in GA signal pathway. Arrows indicate positive regulation, circles indicate negative regulation
Review
Roles of Core Components of sRNA Pathway in Rice Immunity
Small RNA pathway possesses three types of core components, namely DCLs, AGOs, and RDRs. Rice genome encodes eight DCLs, nineteen AGOs and five RDRs. The DCLs include OsDCL1a, OsDCL1b, OsDCL1c, OsDCL2a, OsDCL2b, OsDCL3a, OsDCL3b/OsDCL5, and OsDCL4 (Kapoor et al. 2008; Margis et al. 2006). Different OsDCLs are responsible for the production of different sRNAs. For example, OsDCL1 and OsDCL3a are involved in the biosynthesis of 21- or 24-nt miRNAs (Liu et al. 2005; Yan et al. 2011). OsDCL3b/OsDCL5 and OsDCL4 are more involvement with the production of 21- or 24-nt siRNAs (Shi et al. 2007; Song et al. 2012a; Song et al. 2012b; Wu et al. 2010). OsDCL2 contributes to the accumulation of vsiRNAs derived from the Oryza sativa endornavirus (Urayama et al. 2010). Rice AGOs are phylogenetically classified into 6 clades, namely, AGO1, AGO4, AGO7, MEL1 clades, and two singleton clades. AGO1 clade includes OsAGO1a, OsAGO1b, OsAGO1c, OsAGO1d, and OsAGO10. AGO4 clade contains OsAGO4a, OsAGO4b, OsAGO15, and OsAGO16. AGO7 clade includes OsAGO2, OsAGO3, and OsAGO7. MEL1 clade includes MEL1, OsAGO11, OsAGO12, OsAGO13, and OsAGO14. The two singletons are OsAGO17 and OsAGO18 (Kapoor et al. 2008). OsAGO1a, OsAGO1b and OsAGO1c have a strong binding preference for miRNAs initiated with an uracil. In contrast, OsAGO4a and OsAGO16 predominantly bind to 24-nt miRNAs that are initiated with an adenine, whereas OsAGO4b mainly accommodates 24-nt miRNAs with an uracil or guanine (Wu et al. 2009; Wu et al. 2010). Rice RDRs include OsRDR1, OsRDR2, OsRDR3a, OsRDR3b, and OsRDR6 (Kapoor et al. 2008). OsRDR1, OsRDR2, and OsRDR6 are responsible for the accumulation of 21–24-nt siRNAs (Song et al. 2012b; Wu et al. 2010; Yang et al. 2008b). OsRDR1 also contributes to the production of several miRNAs, such as Osa-miR1859, Osa-miR395p, and Osa-miR820a (Wang et al. 2014). To date, reports indicate that five OsDCLs, six OsAGOs, and two OsRDRs are involved in rice responses to different pathogens and insects.
Five OsDCLs have been reported to be involved in rice responses to M. oryzae and several viruses, including OsDCL1, OsDCL2, OsDCL3a, OsDCL3b/OsDCL5, and OsDCL4. OsDCL1 positively regulates rice resistance to RSV, but compromises resistance to M. oryzae. The infection of M. oryzae promotes the expression of OsDCL1 in the rice blast susceptible accession Lijiang xin Tuan Heigu (LTH), but reduces OsDCL1 expression in the rice blast resistant accessions International Rice Blast Line Pyricularia-Kanto51-m-Tsuyuake (IRBLkm-Ts) and Yahui2115 (Li et al. 2020a). Knockdown of OsDCL1 (dcl1) confers resistance to M. oryzae accompanying with high expression of multiple PR genes and two PTI related genes, OsKS4 and OsNAC4 (Li et al. 2020a; Zhang et al. 2015a). However, dcl1 mutants are more susceptible to RSV associating with higher levels of RSV genomic RNAs and the coat protein transcripts (Yang and Li 2018). In contrast, activating the expression of OsDCL1 results in suppressed expression of OsPR1a and OsPBZ1, and reduced accumulation of phytoalexin and tolerance to oxidative stress, thus compromising resistance against M. oryzae and F. fujikuroi, the causative agents of rice blast and bakanae disease, respectively (Salvador-Guirao et al. 2019). OsDCL2 is down-regulated by the infection of M. oryzae and Southern rice black-streaked dwarf virus (SRBSDV), but significantly up-regulated by RSV infection (Du et al. 2011; Xu and Zhou 2017). Knockdown of OsDCL2 (dcl2) abolishes the dsRNAs of the endogenous dsRNA virus Oryza sativa endornavirus. Besides, dcl2 mutants exhibit compromised resistance to RSV (Urayama et al. 2010; Yang and Li 2018). Furthermore, OsDCL3a, OsDCL3b/OsDCL5, and OsDCL4 are differentially responsive to M. oryzae, RSV, and RSBDV. Both OsDCL3a and OsDCL3b/OsDCL5 are down-regulated by RSV infection, and OsDCL4 is down-regulated by RBSDV infection, whereas both OsDCL3a and OsDCL4 are up-regulated by M. oryzae infection (Du et al. 2011; Salvador-Guirao et al. 2019; Xu and Zhou 2017). Consistently, knockdown of either OsDCL3a, OsDCL3b/OsDCL5 or OsDCL4 leads to enhanced susceptibility to RSV (Yang and Li 2018). In conclusion, rice DCLs contribute to rice immunity against M. oryzae and viruses, and they play different roles in response to different parasitic organisms.
To date, six AGO genes are identified to be involved in rice responses to RSV or M. oryzae, including OsAGO1a, OsAGO1b, OsAGO4a, OsAGO18, OsAGO2, and OsAGO11. OsAGO2 and OsAGO11 are up-regulated in RSV- or RBSDV-infected rice plant, suggesting their potential roles in response to viruses (Du et al. 2011; Xu and Zhou 2017). OsAGO18 cooperates with OsAGO1 to enhance rice immunity against RSV. Inoculation with RSV improves the expression of OsAGO1a, OsAGO1b, and OsAGO18 (Wu et al. 2015). Mutations in OsAGO1a/b/c/d (ago1) or OsAGO18 (ago18) result in enhanced susceptibility to RSV, whereas overexpression of OsAGO18 enhances resistance to RSV. Besides, OsAGO1a and OsAGO1b but not OsAGO18 protein could bind vsiRNAs to mediate antiviruses. Because OsAGO18 competes with OsAGO1 for binding miR168 during RSV infection, increase of OsAGO18 level leads to decreased the cleavage of OsAGO1 transcripts mediated by miR168, thus keeping a high level of OsAGO1 protein and improving efficiency of antiviral RNA silencing to enhance rice immunity against RSV (Wu et al. 2015). OsAGO4a fine-tunes the expression of PigmS and PigmR from the Pigm locus to regulate rice blast resistance together with OsRDR2 and OsDCL3a (Deng et al. 2017). Overexpression of PigmR confers a broad-spectrum resistance to M. oryzae with yield penalty, whereas overexpression of PigmS leads to susceptibility to M. oryzae but increased yield under blast disease-free conditions. PigmS interacts with PigmR to attenuate immune responses mediated by PigmR via abrogating the formation of PigmR homodimer. OsAGO4a precisely regulates the expression of PigmS via mediating DNA methylation of two tandem miniature transposons (MITE1, MITE2) located in the promoter of PigmS. As a result, high levels of methylation in MITE1 and MITE2 lead to repressed expression of PigmS and releases of PigmR, thus resulting in a strong immunity at vegetative stage; whereas reduced methylation levels in MITE1 and MITE2 in pollen lead to enhanced expression of PigmS, thus rescuing seed production to counteract yield penalty induced by PigmR (Deng et al. 2017). Therefore, OsAGO1a, OsAGO1b and OsAGO18 positively regulate rice immunity against viruses, and OsAGO4 plays a critical role in the trade-off between immunity against M. oryzae and yield productivity.
RDRs are mainly involved in antiviral functions. OsRDR1 and OsRDR6 have been reported to regulate resistance against RSV and RDV. The expression of OsRDR1 is enhanced following the inoculation with SRBSDV and RDV (Du et al. 2011; Xu and Zhou 2017). OsRDR1 is required for antiviral RNA silencing triggered by the ssRNA virus Brome mosaic bromovirus, but not the ssDNA virus wheat dwarf geminivirus (Chen et al. 2010). Moreover, knockout of OsRDR1 reduces rice resistance against RSV accompanying with increased accumulation of RSV RNA and CP proteins (Wang et al. 2016). Similarly, silencing of OsRDR6 (rdr6) is more susceptible to RSV and RDV. Moreover, rdr6 mutants show a reduced vsiRNAs derived from RSV and RDV, implying the important role of RDR6 in producing vsiRNAs (Hong et al. 2015b; Jiang et al. 2012). Whereas, overexpression of OsRDR6 (OXRDR6) shows a comparable resistance with wild type (WT) plants. Further studies show that RDV-infection reduces the transcripts of OsRDR6 and abolishes the protein accumulation of RDR6 in the OXRDR6 transgenic plants (Hong et al. 2015b), suggesting that OsRDR6 may be targeted by viruses to facilitate infection. Indeed, some viral proteins have been proposed to disturb with the functions of OsRDR6. RDV Pns10 protein could repress the expression of OsRDR6, and may bind vsiRNAs to prevent the initiation of antiviral RNA silencing (Ren et al. 2010). Additionally, the P6 protein encoded by Rice yellow stunt rhabdovirus could interact with OsRDR6 thus interfering with its function in the production of vsiRNAs, eventually leading to suppressed rice immunity (Guo et al. 2013).
In conclusion, some core components of sRNA pathway are essential for antiviral RNA silencing signals, such as OsDCL1, OsDCL2, OsDCL3a, OsDCL3b/OsDCL5, OsDCL4, OsAGO1, OsAGO18, OsRDR1, and OsRDR6. Besides, OsDCL1 negatively, but OsAGO4a positively regulates rice resistance against M. oryzae. OsRDR6 is targeted by several viruses to disturb with the antiviral RNA silencing. As the core components of sRNA pathway, DCLs, AGOs and RDRs may work synergistically or antagonistically to fine-tune rice responses to different pathogens. Therefore, it is worthy to further study the roles of the biotic-responsive members of OsDCLs, OsAGOs, and OsRDRs in rice immunity.
Small RNAs in Rice-M. oryzae Pathological System
Rice-M. oryzae pathological system has become an ideal model for the study of plant-fungus interactions. More than 70 miRNAs respond to M. oryzae or its elicitors (Li et al. 2019c). Among these miRNAs, five have been identified as positive regulators in rice blast resistance, namely miR160a, miR162a, miR166k-h, miR398b, and miR7695, whereas eight have been identified as negative regulators, namely miR156, miR164a, miR167d, miR169a, miR319b, miR396, miR1873, and miR444b.2(Chandran et al. 2018; Li et al. 2020a; Li et al. 2019b; Li et al. 2014; Li et al. 2017; Salvador-Guirao et al. 2018; Sanchez-Sanuy et al. 2019; Wang et al. 2018b; Xiao et al. 2017; Zhang et al. 2018b; Zhang et al. 2020; Zhao et al. 2020; Zhou et al. 2020). Obviously, many miRNAs play a role in regulating rice-M. oryzae interactions.
Four miRNAs are well-studied in regulating rice-M. oryzae interactions. First, miR7695 is a rice-specific miRNA that positively regulates rice blast resistance via suppressing the expression of OsNramp6.8, an iron transporter that negatively regulates rice immunity (Campo et al. 2013; Perisperis et al. 2017). Overexpression of miR7695 results in increased iron accumulation, enhanced innate immune responses, and increased accumulation of phytoalexins. Further studies revealed that high concentration of iron improves the expression of OsCPS2 and OsCPS4, which function in the first cyclization steps of phytoalexin biosynthesis, suggesting that miR7695 may promote phytoalexins accumulation via controlling the transport of iron (Campo et al. 2013; Sanchez-Sanuy et al. 2019; Toyomasu et al. 2015). Second, miR398b is a well-elucidated positive regulator in rice resistance against M. oryzae. miR398b targets several members of the superoxide dismutase (SOD) family genes, including copper-binding SOD1 (OsCSD1), OsCSD2, OsSODX, and chaperone of CSD (OsCCSD). SODs catalyze the reduction of superoxide radicals (O·2−) into hydrogen peroxide (H2O2), which is an indispensable component in plant immunity (Kaur et al. 2014; Mittler et al. 2011). The transgenic lines overexpressing miR398b, and mutants of OsCSD1, OsCSD2, and OsSODX display enhanced resistance against M. oryzae accompanying with enhanced SOD enzyme activity and increased H2O2 accumulation. Conversely, blocking miR398, or overexpression of OsCSD2, or mutation of OsCCSD facilitates the infection of M. oryzae, which is associated with suppressed SOD enzyme activity and decreased H2O2 accumulation (Li et al. 2019b). Therefore, miR398b coordinately controls the SODs enzyme activity via manipulating different target genes to enhance the production of H2O2 (Li et al. 2019b). Different from miR7695 and miR398b, both miR164a and miR319b facilitate M. oryzae infection. miR164a targets two transcription factor genes, OsNAC11 and OsNAC60, which belongs to NAC (NAM/ATAF/CUC) transcription factor family that play critical roles in plant development and stress-induced responses (Puranik et al. 2012). Overexpressing miR164a or silencing OsNAC60 leads to repressed expression of OsNPR1 and OsPBZ1, two defense marker genes for innate immunity and SA signaling pathway (Lu et al. 2015). In contrast, overexpression of OsNAC60 in Nicotiana benthamiana triggers strong autoimmunity phenotypes that could be abolished by co-expressing of miR164a. Furthermore, overexpression of miR164a leads to compromised host resistance to Phytophthora infestans and R. solani, two destructive filamentous pathogens in potato, tomato and rice, respectively, indicating that miR164a may play a conserved role in plant immunity against different filamentous pathogens (Wang et al. 2018b). Similarly, miR319 also negatively regulates rice resistance to M. oryzae. Overexpression of miR319b (OX319b) or interfering with the expression of OsTCP21 (TCP21i), one target gene of miR319, promotes infection of M. oryzae, which is associated with suppressed induction of basal immune responses and impaired JA signal. The plant hormone JA is necessary for resistance against hemi-biotrophic and necrotrophic pathogens (De Vleesschauwer et al. 2013). In contrast, overexpression of OsTCP21 impedes infection of M. oryzae presumably due to enhanced basal immune responses and JA signal. Considering miR319b is up-regulated during infection, miR319b may be manipulated by M. oryzae to suppress rice immunity through repressing basal immune responses and JA signal (Zhang et al. 2018b).
Besides the miRNA regulatory modules mentioned above, quite a few miRNA regulatory modules have been functionally characterized in rice immunity against M. oryzae (Table 1). The negative regulatory modules generally impair the accumulation of H2O2 and the induction of defense-related genes in transgenic lines overexpressing miRNAs or mutants of target genes, such as miR167d-OsARF12, miR396a/c/d/h-OsGRF6/7/8/9, miR169a-OsNF-YAs, miR444b.2-OsMADS, miR1873-Os05g01790, and miR156-OsSPL14 (Chandran et al. 2018; Li et al. 2017; Xiao et al. 2017; Zhang et al. 2020; Zhao et al. 2020; Zhou et al. 2020). Conversely, the positive regulatory modules enhance defense responses, such as miR162a-OsDCL1 and miR166k-OsEIN2.1 modules (Li et al. 2020a; Salvador-Guirao et al. 2018). Furthermore, MITE1- and MITE2-derived siRNAs trigger DNA methylation of the promoter of PigmS and repress its expression, thus to positively regulate rice blast resistance (Deng et al. 2017) (Table 1 and Fig. 2).
In addition to sRNA pathway in rice, sRNA pathway of M. oryzae also contributes to rice-M. oryzae interactions. Several studies have identified some core components of sRNA pathway and a lot of miRNA-like RNAs (milRNAs) in M. oryzae. MoDCL2, MoRdRP2, and MoAGO3 are core components of sRNA pathway in M. oryzae. Mutation of MoDCL2, MoRdRP2, and MoAGO3 results in defects in fungal growth, development, and virulence, suggesting their indispensability in the pathogenicity of M. oryzae (Raman et al. 2017; Raman et al. 2013). Besides, there are 171 non-redundant milRNAs identified to be differentially expressed among wild type strain, and the appressorium-defective strains ΔMorgs1, ΔMorgs3, and ΔMorgs7 (Li et al. 2020b), implying their roles in appressorial formation and virulence. Indeed, overexpression of milR236 leads to delayed appressorium formation and impaired virulence. milR236 is one of the differentially expressed milRNAs, its overexpression leads to suppression of the target gene MoHat1, a histone acetyltransferase that is required for the formation of appressorium (Li et al. 2020b). Furthermore, there are 366 sRNAs up-regulated in M. oryzae during infection and fourteen of them are predicted to target rice genes (Zhang et al. 2019). This report implies the existence of cross-kingdom RNA silencing in rice-M. oryzae interactions.
The cross-kingdom RNA silencing has been discovered in the interaction between Arabidopsis plants and Botrytis cinerea (B. cinerea), and in the interaction between cotton and Verticillium dabliae. For example, Bc-siR3.2 is up-regulated in B. cinerea-infected plants and targets two host genes, AtMPK2 and AtMPK1. Ectopically expressing of Bc-siR3.2 in Arabidopsis leads to more susceptible to B. cinerea and significantly reduces the mRNA levels of AtMPK2 and AtMPK1, whose double mutants show enhanced susceptibility to B. cinerea (Wang et al. 2017b; Weiberg et al. 2013). In return, plants also export sRNAs into pathogens to interfere with B. cinerea infection. During infection of B. cinerea, Arabidopsis cells secrete exosome-like extracellular vesicles to deliver sRNAs into fungal cells of B. cinerea. For example, the two tasiRNAs TAS1c-siR483 and TAS2-siR453, target three genes Bc-Vsp51, Bc-DCTN1, and Bc-SAC1, which are involved in vesicle-trafficking pathways important for fungal virulence (Cai et al. 2018). Therefore, cross-kingdom RNA silencing in rice-M. oryzae interactions could be a future research focus.
Small RNAs in Rice-Viruses Pathological System
Rice production are challenged by many different viruses, such as Rice stripe virus (RSV), Rice dwarf virus (RDV), Rice ragged stunt virus (RRSV), Rice black-streaked dwarf virus (RBSDV), Southern rice black-streaked dwarf virus (SRBSDV), Rice tungro bacilliform virus (RTBV), and rice tungro spherical virus (RTSV). A lot of sRNAs has been identified during different viruses infection, including RSV, RDV, RBSDV, SRBSDV, RTBV, and RTSV (Du et al. 2011; Guo et al. 2015; Guo et al. 2012; Lian et al. 2016; Xu and Zhou 2017; Yang et al. 2016; Zarreen et al. 2018). Specifically, the roles of rice miRNAs are the most studied in the rice-RSV interaction. Two miRNAs act as positive regulators and one miRNA acts as a negative regulator in the regulation of anti-RSV responses. RSV is a brown planthopper (BPH)-transmitted single-stranded RNA virus (Wang et al. 2008). A series of rice miRNAs are differentially expressed at the RSV-infected plants (Du et al. 2011; Guo et al. 2012; Lian et al. 2016; Yang et al. 2016). Among these miRNAs, miR444 and miR171 are reported to positively regulate rice resistance to RSV, whereas miR528 negatively regulates resistance to RSV. miR444 is induced in RSV-infected plants, leading to suppressed expression of three target genes, namely OsMADS23, OsMADS27a, and OsMADS57 that belong to the MADS transcription factor family. Plant MADS box proteins regulate genes expression by binding the CArG motif, one conserved motif that exists in the promoter of OsRDR1 (de Folter and Angenent 2006). Further studies pointed that OsMADS23, OsMADS27a, and OsMADS57 interact with each other to form homodimers or heterodimers to repress the expression of OsRDR1, whose knockout mutants exhibit enhanced susceptibility to RSV. Therefore, miR444-OsMADS modules enhance antiviral ability via promoting antiviral RNA silencing signal (Wang et al. 2016). Conversely, RSV-infection represses the accumulation of miR171b. Blocking the function of miR171b leads to phenotypes similar to those of RSV-infected rice plants, containing a stunted growth and reduced chlorophyll content in leaves. Moreover, overexpression of miR171b enhances resistance to RSV accompanying with repressed expression of three target genes, including OsSCL6-IIa, OsSCL6-IIb, and OsSCL6-IIc (Tong et al. 2017). Different from miR444 and miR171b, miR528 negatively regulates rice resistance to RSV. During RSV infection, miR528 is sequestered by OsAGO18, thus elevating the expression of L-ascorbate oxidase (AO), one target gene of miR528 (Wu et al. 2015; Yao et al. 2019). AO protein regulates the apoplast redox state in plants by oxidizing L-ascorbic acid (AsA), leading to decelerate the detoxification of reactive oxygen species (ROS) (Mittler et al. 2011; Pignocchi et al. 2006). Mutation of miR528, or overexpression of AO improves basal ROS levels as a result of enhanced rice immunity. Conversely, overexpression of miR528 impedes antiviral ability presumably due to reduced basal ROS levels (Wu et al. 2017).
In addition to participate in the rice-RSV interaction, miRNAs are also found to respond to other viruses. There are 14 miRNAs in leaves and 16 miRNAs in roots differentially respond to RBSDV-infection. Among them, five miRNAs show a similar expression pattern, including miR166a-f, miR169h-m, miR408, miR827, and miR1428e; and the other miRNAs display a diverse expression patterns, including miR394, miR397, miR530, miR162, miR398, miR1862d, miR159ab, miR171, and miR1432 (Sun et al. 2015). Moreover, a total of 54 responsive rice miRNAs are identified after inoculating with RTBV and RTSV. Among them, miR5493, miR159e, and miR1875 are up-regulated, whereas down-regulated miRNAs are belonging to the families of miR167, miR164, miR156, miR815, miR171, miR444, miR166, miR1439, miR396, miR169, miR818, miR172, and miR408. In contrast, RDV-infection barely disturbs the homeostasis of rice miRNAs, only a few miRNAs are respond to RDV, including miR167a, miR171, miR1863, and miR393 (Du et al. 2011). These reports suggest that rice miRNAs are more involved in regulating rice responses to RSBDV, RTBV, and RTSV than to RDV. Furthermore, miR319 has also been identified to negatively regulate anti-RRSV resistance. RRSV is a double-stranded RNA virus that is transmitted by BPH (Ling et al. 1978). miR319 targets four TCP genes, including OsPCF5, OsPCF6, OsPCF8 and OsTCP21. miR319 is induced during RRSV infection associating with a significant reduction of OsTCP21 expression. Overexpressing miR319 (OX319) or silencing OsTCP21 (TCP21 IR) results in plant phenotypes similar to RRSV-infected rice plants that showed stunted growth, excess tillering, and dark green and crinkle leaves. Besides, OX319 and TCP21 IR transgenic plants support more RRSV duplication accompanying with decreased JA content. Conversely, overexpression of OsTCP21 results in decreased RRSV proliferation associating with increased JA content (Martintrillo and Cubas 2010; Zhang et al. 2016a).
Furthermore, viruses are spread by the viral vector insects, some of the viral vector insects also cause significant loss on rice production independent of viruses. The brown planthopper (BPH) is a rice-specific herbivore and causes direct mechanical damage to rice phloem using the stylet (Sōgawa 1982). A total of 55 differentially expressed miRNAs (DEMs) are identified between the BPH resistant rice BPH6G and wild type following the invasion of BPH. Among these DEMs, 29 are oppositely expressed in BPH6G and wild type, containing miRNAs from families of miR169, miR166, miR160, miR156, miR396, miR319, and miR1861, indicating that these DEMs may contribute to the immune responses triggered in BPH6G (Tan et al. 2020). Moreover, miR156 and miR396 are further proved to negatively regulate rice resistance against BPH. Silencing of miR156 improves tolerance to BPH, and leads to a suppressed expression of multiple JA biosynthesis genes and decreased accumulation of JA/JA-Ile. Because the JA signal pathway is considered to negatively regulate BPH resistance in rice, miR156 acts as a negative regulator in rice resistance against BPH via promoting JA pathway (Dai et al. 2019; Li et al. 2015; Zhou et al. 2009). miR396 is a well-studied negative regulator in antiviruses and targets 12 growth regulating factor (GRF) genes (Gao et al. 2015). Overexpression a target mimic of miR396 (MIM396) leads to enhanced resistance to BPH accompanying with down-regulated JA signal genes OsCoia and OsCoib and up-regulated SA marker gene OsNPR1 (Dai et al. 2019). Consistently, transgenic plants overexpressing OsGFR8, one target gene of miR396, results in phenotypes similar to those of MIM396. Later studies found that OsGRF8 directly activates the expression of OsF3H, one gene that responses to BPH and is involved in flavonoid biosynthesis (Wang et al. 2012). Flavonoid is an important secondary metabolite against biotic or abiotic stress (Pourcel et al. 2007). Overexpression of OsF3H enhances flavonoid contents and improves tolerance to BPH; whereas silencing of OsF3H compromises resistance to BPH, indicating that miR396-OsGRF8 module may be utilized by BPH to suppress rice immunity via manipulating downstream hormone signal and flavonoid biosynthesis (Dai et al. 2019). Intriguingly, JA signal exhibits two-faced roles between rice-viruses interaction and rice-BPH interaction. Usually, JA is considered to be a positive regulator of virus resistance whereas a negative regulator of BPH resistance in rice. Given that some viruses are transmitted with the help of BPH in field, many questions can be research foci in the future. For example, how do plants/viruses avoid or exploit multi-faceted roles of JA during infection? Whether do sRNAs play an important role in fine-tuning the contradictory relationship during infection?
Moreover, the sRNAs of viruses and insects also contribute to the interactions of rice with viruses and insects. After inoculating with SRBDV, 366 vsiRNAs are detected in the 14-dpi samples and 28-dpi samples. The vsiRNAs are mostly in 21- and 22-nt, implying that OsDCL2 and OsDCL4 are the predominant enzymes for the biogenesis of SRBDV-derived siRNAs. A total of 151 vsiRNAs are predicted to target 844 rice genes related to disease/stress responses and RNA silencing components, suggesting the contribution of vsiRNAs in viral pathogenicity (Lan et al. 2018; Xu and Zhou 2017). Besides, miR-14 is a conserved miRNA in rice stem borers (RSB) and planthoppers. miR-14 of Chilo suppressalis (Csu-miR-14) targets CsSpo and CsEcR, two genes that act in ecdysone signal network. Overexpression of Csu-miR-14 in RSB leads to abnormal development. Consistently, overexpression of Csu-miR-14 (OX-14) in rice leads to enhanced resistance to RSB (He et al. 2019). Feeding RSB with OX-14 transgenic plants causes developmental defects and high mortality. Therefore, miR-14 could be a potent candidate tool in engineering resistance against RSB in rice.
The Involvement of sRNAs in Other Rice Diseases
In addition to rice blast disease and rice virus diseases, sRNAs are also involved in regulating immune responses to the other rice diseases, such as rice bacterial blight, rice foot rot, and rice sheath blight disease.
Rice leaf blight is one of the most devastating bacterial diseases of rice caused by Xanthomonas oryzae pv. oryzae (Xoo). Several sRNAs are identified to be differentially expressed between the Xoo-susceptible accession MDJ8 and the Xoo-resistant accession Rb49 that carries the Xoo-resistance genes Xa3/Xa6 (Hong et al. 2015a), suggesting that these sRNAs may contribute to the Xa3/Xa6-mediated resistance. Among these Xoo-responsive sRNAs, miR156, miR169o, and TE-siR815 are identified as negative regulators in rice resistance against Xoo (Liu et al. 2019; Yu et al. 2018; Zhang et al. 2015b). miR156 is induced following the invasion of Xoo, and overexpression of miR156 boosts Xoo infection accompanying with decreased expression of OsPR1b, OsPR1a, and OsWRKY45. Conversely, overexpression of a miR156 target mimic (MIM156), or OsIPA1, or OsSPL7, the target genes of miR156, enhances rice resistance to Xoo associating with improved expression of OsPR1b, OsPR1a, and OsWRKY45 (Liu et al. 2019). Furthermore, OsIPA1 and OsSPL7 block GA signaling pathway via stabilizing OsSLR1, a repressor of GA signaling pathway. Because GA promotes rice susceptibility to Xoo, and mutation of OsSLR1 in MIM156 background reduces resistance to Xoo, miR156 most likely acts as a negative regulator in rice immunity against Xoo via promoting GA signal pathway and suppressing the basal immune responses (Liu et al. 2019; Yang et al. 2008a). In contrast, miR169o is suppressed following the inoculation of Xoo (Yu et al. 2018). Overexpression of miR169o causes a much greater lesion associating with suppressed expression of OsPR1b, OsPR10a, and OsPAL. Consistently, overexpression of the target genes of miR169o leads to enhanced expression of OsPR1b, OsPR10a and OsPAL, implying that miR169o may suppress the innate immune responses to negatively regulate rice resistance against Xoo (Yu et al. 2018). Similar to miR156 and miR169o, TE-siR815 also negatively regulates rice immunity against Xoo. TE-siR815 is derived from the intron region of WRKY45–1 locus. WRKY45 has two allelic loci, including WRKY45–1 and WRKY45–2. Overexpression of WRKY45–2 enhances rice resistance to Xoo, while overexpression of WRKY45–1 leads to susceptibility to Xoo. Further studies found that TE-siR815 targets and represses the expression of OsST1, an important downstream component in WRKY45-mediated resistance against Xoo (Zhang et al. 2016b). Obviously, TE-siR815 blocks the OsWRKY45-mediated immune responses to facilitate the proliferation of Xoo.
Rice sheath blight also ranks one of the most devastating diseases of rice caused by the fungal pathogen Rhizoctonia solani. Many miRNAs are differentially expressed between the rice sheath resistant line YSBR1 and the susceptible line Xudao3 following the inoculation of R. solani, including osa-miR398a, osa-miR1881, osa-miR530, osa-miR444, osa-miR812, osa-miR1861, osa-miR3980, osa-miR531f families, osa-miR171f-5p, osa-miR2863a, osa-miR3979-3p, osa-miR1428e-3p, and osa-miR156 (Cao et al. 2020). Moreover, 14 long siRNAs (lsiRNAs) in length from 25- to 30-nt and several sRNAs are also identified to be responsive to R. sonali. Among them, some siRNAs-target modules show a tight conversely co-expression relationship, including lsiRNA51031-Os08g15322, lsiRNA73750-Os09g14490, lsiRNA118183-Os08g06220, lsiRNA194568-Os06g38990, miR167h-OsARF8, miR171a-Os02g44360, and miR160c-OsARF10, suggesting their involvement in regulating rice response to R. solani (Niu et al. 2018; Qiao et al. 2020). Among these differentially expressed sRNAs, siR109944 is further proved as a negative regulator in rice resistance against R. solani. siR109944 is derived from a precursor containing DNA transposons and targets OsFBL55 that encodes a protein containing a typical TIR domain and sharing 65.57% similarity with the IAA receptor OsTIR1 (Dharmasiri et al. 2005; Yu et al. 2013). siR109944 is repressed upon the infection of R. solani. Overexpression of siR109944 facilitates the growth of R. solani companying with reduced IAA accumulation, whereas overexpression of OsFBL55 significantly suppresses R. solani growth associating with increased IAA accumulation. Consistently, IAA application improves resistance to R. solani, suggesting that IAA positively regulates rice sheath blight resistance and siR109944 manipulates auxin signal pathway to negatively regulate rice resistance to R. solani (Qiao et al. 2020). Furthermore, sRNAs derived from R. solani are also found to be involved in rice-R. solani interaction. A total of 109 conserved milRNAs and 68 novel milRNAs are identified during R. solani infection in the AG1 IA strain and they are predicted to target genes involved in pathogenicity (Lin et al. 2016).
Rice foot rot is another emerging bacterial disease caused by Dickeya zeae (D. zeae). A total of 79 miRNAs is differentially expressed following the infection of D. zeae in a foot rot resistant accession Nanjing 40, including miR2118, miR393, miR166, miR171, miR156, miR159, miR396. Among them exists 29 co-expressed miRNA-target modules including miR396-OsGRFs, indicating their involvement in rice-D. Zeae interaction. Furthermore, overexpression of miR396f enhances rice resistance to D. zeae associating with a milder symptom in roots (Li et al. 2019a).
Small RNA Interconnects Hormone Signaling and the Other Non-coding RNAs
Small RNA seems to post its roles largely via a network of transcription factors that may act in different hormone signaling pathways. On one hand, rice sRNAs could manipulate hormone signal pathways to confer or compromise rice immunity against M. oryzae, Xoo, R. solani, or viruses (Fig. 2). For examples, miR319-OsTCP21 module negatively regulates rice resistance against M. oryzae and RRSV via blocking JA signal pathway; miR156-OsSPL7/OsIPA1 module negatively regulates rice resistance via promoting GA signal pathway (Liu et al. 2019; Zhang et al. 2016a; Zhang et al. 2018b). Whereas, miR166k-OsEIN2 module positively regulates resistance to M. oryzae by activating ET signal pathway (Salvador-Guirao et al. 2018). On the other hand, hormone signal pathways could regulate the sRNA pathway in return. For example, the expression of pre-miR166k-166 h is rapidly increased following the treatment with ethylene precursor ACC (1-aminocyclopropane-1-carboxylic acid), which suggests a positive regulation loop between miR166k and ethylene signal pathway. Besides, several M. oryzae-responsive miRNAs carry the hormone-related cis-elements in the promoters. For example, the classical Auxin responsive element TGTCTC exists in the promoters of miR167d and miR160a, which both target the ARF family members that are downstream components of Auxin signal pathway. Furthermore, recent reports pointed that JA positively regulates the expression of OsAGO18 and enhances rice antiviral activity (Wu et al. 2015; Yang et al. 2020b). Therefore, the sRNA pathway may manipulate the hormone signal to regulate rice resistance against the pathogenic organisms, and at the same time, the expression of some sRNAs may be controlled by the transcription factors that act downstream of some specific hormone signal.
In addition to sRNAs, other non-coding RNAs, such as long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), have been emerging to take part in regulating rice-pathogen interactions (Wang et al. 2017a). LncRNAs are longer than 200-nt and could execute their function to interact with sRNAs in two ways: (1) act as the target mimic of miRNAs to block miRNAs from binding their target genes, and (2) serve as sRNAs precursors to produce sRNAs (Wang et al. 2017a). For examples, AtIPS1 acts as a target mimic of miR399 to protect miR399’s target gene AtPHO2 from the cleavage by miR399 under phosphate deficiency condition (Franco-Zorrilla et al. 2007). In Blumeria graminis f. sp. tritici-infected wheat, TalncRNA5 and TapmlnRNA19 are identified as the precursors of miR2004, and TapmlnRNA8 is the precursor of miR2066 (Xin et al. 2011). Xoo infection triggers differential expression of 576 lncRNAs and these lncRNAs are predicted to target genes enriched in JA signal pathway. One lncRNA, ALEX1, is proved to up-regulate the expression of JA synthesis genes and JA signal genes, thus positively regulating resistance to Xoo (Yu et al. 2020). Except for responding to Xoo, lncRNAs are also responsive for D. zeae. A total of 4709 lncRNAs are identified upon D. zeae infection, and their predicted target genes are involved in multiple signal pathways related to defense responses (Li et al. 2018a). CircRNAs are formed by pre-mRNAs through back-splicing in which the upstream 3′ splicing acceptor site is joined to the downstream 5′ splicing donor site (Ashwal-Fluss et al. 2014). Similar to lncRNAs, circRNAs could block the function of miRNAs by acting as the miRNA sponges (Li et al. 2018b). In rice, the amount of circRNAs is more abundant in the rice blast resistance accession IRBLkm-Ts than the rice blast susceptible accession LTH, and a total of 636 circRNAs specifically expressed after the infection of M. oryzae, indicating the involvement of circRNAs in rice responses to M. oryzae (Fan et al. 2020). Moreover, circR5g05610 is further proved to enhance rice immunity against M. oryzae (Fan et al. 2020). Given that lncRNAs and circRNAs are capable of disturbing miRNAs function by competing miRNA with miRNA targets, or producing miRNAs, or acting as miRNA sponges via masking miRNA binding sites (Huang et al. 2017; Li et al. 2018b), it is possible that sRNAs, lncRNAs, and circRNAs could cooperate or antagonize with each other to mediate rice immunity.
Conclusions
The core components of sRNA pathway and varieties of sRNAs act as critical regulators of gene expression to fine-tune rice immunity against parasitic organisms, including fungi, bacteria, viruses, and insects. Some specific sRNA-target modules are involved and may act consistently or conversely in the interactions of rice with different biotic stressors. For examples, miR396a/c/d/h-OsGRF6/OsGRF7/OsGRF8/OsGRF9 modules negatively regulate resistance to M. oryzae but miR396-OsGRF8 module acts as a negative regulator in rice resistance against BPH. The miR319-OsTCP21 module negatively regulates rice resistance to RRSV and M. oryzae. miR444-OsMADS23/OsMADS27a/OsMADS57 modules positively regulate rice resistance to RSV, but overexpression of miR444 leads to more susceptible to M. oryzae. Besides, some sRNAs also coordinate responses to biotic stressors with the regulation of growth and development. For examples, miR156-OsIPA1 module negatively regulates rice resistance to M. oryzae and it also regulates seed dormancy and germination; miR396-OsGRFs modules negatively regulate rice resistance to M. oryzae and they also regulate plant height, leaf morphology and seed size (Guo et al. 2016; Tang et al. 2018; Zhang et al. 2018a; Zhang et al. 2020). Therefore, sRNA pathway may act as a part of innate immunity to tightly coordinate rice growth, development, and immunity, thus could be used as a candidate to balance the trade-off between yield and resistance in rice breeding.
Currently, our understanding is fragmentary on the roles of sRNA pathway in the interactions of rice with pathogens and insect pests and limited to several important pathogens, such as M. oryzae and some viruses. Even to these pathogens, many more pathogen-responsive sRNAs need further functional characterization. Because some sRNAs that function in rice response to one pathogen may also function in response to the other, priority of future research should be focused on those sRNAs characterized in one rice-pathogen interaction. For example, miR168, miR171, and miR528 act in rice-virus interaction, they might also function in rice-M. oryzae or rice-Xoo interaction. Consistently, miR7695 and siR109944 contribute to rice-fungi interactions, they might function in rice-virus interaction. Priority may also be focused on identifying the upstream regulators that determine the accumulation of sRNAs during the invasion of biotic stressors and on dissecting the down-stream components that relay the regulatory signal mediated by the sRNA-target modules. Besides, no studies have identified sRNAs that function in cross-kingdom RNA silencing during infection in rice. Thus, it is worthy to explore whether sRNAs derived from rice or biotic stressors contribute to cross-kingdom regulation.
Acknowledgements
Not applicable.
Abbreviations
- PTI
pattern-triggered immunity
- HR
hypersensitive response
- DCL
Dicer-like
- RDR
RNA-dependent RNA polymerase
- AGO
ARGONAUTE
- PTGS
post-transcriptional gene silencing
- TGS
transcriptional gene silencing
- vsiRNA
virus-derived siRNA
- VSRs
suppressors of RNA silencing
- siRNAs
small interfering RNAs
- pri-miRNA
primary miRNA
- miRNA
microRNA
- PoI II
RNA polymerase II
- pre-miRNA
precursor miRNA
- HYL1
HYPONASTICLEVES1
- SE
SERRATE
- lncRNA
long non-coding RNA
- M. oryzae
Magnaporthe oryzae
- P. infestans
phytophthora infestants
- R. solani
Rhizoctonia solani
- H2O2
hydrogen peroxide
- Rbohs
respiratory burst oxidase homologs
- SOD
superoxide dismutase
- ARF
auxin responsive factor
- GRF
growth regulating factor
- NF-YA
nuclear transcription factor Y
- SPL
SQUAMOSA promoter-binding protein-like transcription factor
- EIN2
ethylene-insensitive 2
- FBL55
F-Box domain and LRR-containing protein 55
- RSV
Rice stripe virus
- RDV
Rice dwarf virus
- RBSDV
Rice black streaked dwarf virus
- SRBSDV
Southern rice black-streaked dwarf virus
- Nramp6
natural resistance-associated macrophage protein 6
- ssRNA
single-stranded RNA
- dsRNA
double-stranded RNA
- AO
L-ascorbate oxidase
- BPH
brown planthopper
- Xoo
Xanthomonas oryzae pv. oryzae
- D. zeae
Dickeya zeae
- JA
jasmonic acid
- SA
salicylic acid
- GA
gibberellin acid
- ET
ethylene
Authors’ Contributions
Qin-Feng searched the literature and drafted the manuscript. Wen-Ming Wang supervised the revision, together with Yan-Li and Zhi-Xue Zhao proof read and edited the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China grants (31430072 and 31672090 to W-MW, and 31471761 to YL) and the Sichuan Applied Fundamental Research Foundation (2020YJ0332 to W-MW).
Availability of Data and Materials
Not applicable.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qin Feng, Email: 1247931645@qq.com.
Yan Li, Email: jiazaihy@163.com.
Zhi-Xue Zhao, Email: 1005795703@qq.com.
Wen-Ming Wang, Email: j316wenmingwang@163.com.
References
- Aliyari R, Wu Q, Li HW, et al. Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in drosophila. Cell Host Microbe. 2008;4:387–397. doi: 10.1016/j.chom.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–744. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, et al. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320:1185–1190. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- Brosseau C, Moffett P. Functional and genetic analysis identify a role for Arabidopsis ARGONAUTE5 in antiviral RNA silencing. Plant Cell. 2015;27:1742–1754. doi: 10.1105/tpc.15.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Qiao L, Wang M, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo S, Peris-Peris C, Sire C, et al. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (Natural resistance-associated macrophage protein 6) gene involved in pathogen resistance. New Phytol. 2013;199:212–227. doi: 10.1111/nph.12292. [DOI] [PubMed] [Google Scholar]
- Cao W, Cao X, Zhao J, et al. Comprehensive characteristics of MicroRNA expression profile conferring to Rhizoctonia solani in Rice. Rice Sci. 2020;27:101–112. doi: 10.1016/j.rsci.2019.04.007. [DOI] [Google Scholar]
- Carmell MA, Hannon GJ. RNase III enzymes and the initiation of gene silencing. Nat Struct Mol Biol. 2004;11:214–218. doi: 10.1038/nsmb729. [DOI] [PubMed] [Google Scholar]
- Chandran V, Wang H, Gao F, et al. miR396-OsGRFs module balances growth and Rice blast disease-resistance. Front Plant Sci. 2018;9:1999. doi: 10.3389/fpls.2018.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Tamai A, Mori M, et al. Analysis of rice RNA-dependent RNA polymerase 1 (OsRDR1) in virus-mediated RNA silencing after particle bombardment. J Gen Plant Pathol. 2010;76:152–160. doi: 10.1007/s10327-010-0226-5. [DOI] [Google Scholar]
- Dai Z, Tan J, Zhou C, et al. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa) Plant Biotechnol J. 2019;17:1657–1669. doi: 10.1111/pbi.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Folter S, Angenent GC. Trans meets cis in MADS science. Trends Plant Sci. 2006;11:224–231. doi: 10.1016/j.tplants.2006.03.008. [DOI] [PubMed] [Google Scholar]
- De Vleesschauwer D, Gheysen G, Hofte M. Hormone defense networking in rice: tales from a different world. Trends Plant Sci. 2013;18:555–565. doi: 10.1016/j.tplants.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhai K, Xie Z, et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science. 2017;355:962–965. doi: 10.1126/science.aai8898. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Du P, Wu J, Zhang J, et al. Viral infection induces expression of novel phased microRNAs from conserved cellular microRNA precursors. PLoS Pathog. 2011;7:e1002176. doi: 10.1371/journal.ppat.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du QS, Duan CG, Zhang ZH, et al. DCL4 targets cucumber mosaic virus satellite RNA at novel secondary structures. J Virol. 2007;81:9142–9151. doi: 10.1128/JVI.02885-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Fan J, Quan W, Li GB, et al. circRNAs are involved in the Rice-Magnaporthe oryzae interaction. Plant Physiol. 2020;182:272–286. doi: 10.1104/pp.19.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- Gao F, Wang K, Liu Y, et al. Blocking miR396 increases rice yield by shaping inflorescence architecture. Nat Plants. 2015;2:15196. doi: 10.1038/nplants.2015.196. [DOI] [PubMed] [Google Scholar]
- Ge Y, Han J, Zhou G, et al. Silencing of miR156 confers enhanced resistance to brown planthopper in rice. Planta. 2018;248:813–826. doi: 10.1007/s00425-018-2942-6. [DOI] [PubMed] [Google Scholar]
- Guo C, Li L, Wang X, et al. Alterations in siRNA and miRNA expression profiles detected by deep sequencing of transgenic rice with siRNA-mediated viral resistance. PLoS One. 2015;10:e0116175. doi: 10.1371/journal.pone.0116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Han N, Xie Y, et al. The miR393a/target module regulates seed germination and seedling establishment under submergence in rice (Oryza sativa L.) Plant Cell Environ. 2016;39:2288–2302. doi: 10.1111/pce.12781. [DOI] [PubMed] [Google Scholar]
- Guo H, Song X, Xie C, et al. Rice yellow stunt rhabdovirus protein 6 suppresses systemic RNA silencing by blocking RDR6-mediated secondary siRNA synthesis. Mol Plant-Microbe Interact. 2013;26:927–936. doi: 10.1094/MPMI-02-13-0040-R. [DOI] [PubMed] [Google Scholar]
- Guo W, Wu G, Yan F, et al. Identification of novel Oryza sativa miRNAs in deep sequencing-based small RNA libraries of rice infected with Rice stripe virus. PLoS One. 2012;7:e46443. doi: 10.1371/journal.pone.0046443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A, Voinnet O, Chappell L, et al. Two classes of short interfering RNA in RNA silencing. EMBO J. 2002;21:4671–4679. doi: 10.1093/emboj/cdf464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Xiao H, Sun Y, et al. Transgenic microRNA-14 rice shows high resistance to rice stem borer. Plant Biotechnol J. 2019;17:461–471. doi: 10.1111/pbi.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Henderson IR, Zhang X, Lu C, et al. Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat Genet. 2006;38:721–725. doi: 10.1038/ng1804. [DOI] [PubMed] [Google Scholar]
- Hong H, Liu Y, Zhang H, et al. Small RNAs and gene network in a durable disease resistance gene--mediated defense responses in Rice. PLoS One. 2015;10:e0137360. doi: 10.1371/journal.pone.0137360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Qian D, Sun R, et al. OsRDR6 plays role in host defense against double-stranded RNA virus, Rice Dwarf Phytoreovirus. Sci Rep. 2015;5:11324. doi: 10.1038/srep11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Feurtado JA, Smith MA, et al. Long noncoding miRNA gene represses wheat β-diketone waxes. Proc Natl Acad Sci U S A. 2017;114:201617483. doi: 10.1073/pnas.1617483114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- Jiang L, Qian D, Zheng H, et al. RNA-dependent RNA polymerase 6 of rice (Oryza sativa) plays role in host defense against negative-strand RNA virus, Rice stripe virus. Virus Res. 2012;163:512–519. doi: 10.1016/j.virusres.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kandoth PK, Mitchum MG. War of the worms: how plants fight underground attacks. Curr Opin Plant Biol. 2013;16:457–463. doi: 10.1016/j.pbi.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Arora R, Lama T, et al. Genome-wide identification, organization and phylogenetic analysis of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analysis during reproductive development and stress in rice. BMC Genomics. 2008;9:451. doi: 10.1186/1471-2164-9-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyaragarwal S, Jin H. Role of small RNAs in host-microbe interactions. Annu Rev Phytopathol. 2010;48:225–246. doi: 10.1146/annurev-phyto-073009-114457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Sharma A, Guruprasad K, et al. Versatile roles of plant NADPH oxidases and emerging concepts. Biotechnol Adv. 2014;32:551–563. doi: 10.1016/j.biotechadv.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Lan Y, Li Y, EZ, et al. Identification of virus-derived siRNAs and their targets in RBSDV-infected rice by deep sequencing. J Basic Microbiol. 2018;58:227–237. doi: 10.1002/jobm.201700325. [DOI] [PubMed] [Google Scholar]
- Li R, Zhang J, Li J et al (2015) Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 4. 10.7554/eLife.04805.001 [DOI] [PMC free article] [PubMed]
- Li W, Jia Y, Liu F et al (2019a) Integration analysis of small RNA and Degradome sequencing reveals MicroRNAs responsive to Dickeya zeae in resistant Rice. Int J Mol Sci 20. 10.3390/ijms20010222 [DOI] [PMC free article] [PubMed]
- Li WQ, Jia YL, Liu FQ, et al. Genome-wide identification and characterization of long non-coding RNAs responsive to Dickeya zeae in rice. RSC Adv. 2018;8:34408–34417. doi: 10.1039/c8ra04993a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71:428–442. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Li XP, Ma XC, Wang H, et al. Osa-miR162a fine-tunes rice resistance to Magnaporthe oryzae and yield. Rice (N Y) 2020;13:38. doi: 10.1186/s12284-020-00396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cao XL, Zhu Y, et al. Osa-miR398b boosts H2O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019;222:1507–1522. doi: 10.1111/nph.15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jeyakumar JMJ, Feng Q et al (2019c) The roles of rice microRNAs in rice-Magnaporthe oryzae interaction. Phytopathology Research 1. 10.1186/s42483-019-0040-8
- Li Y, Liu X, Yin Z, et al. MicroRNA-like milR236, regulated by transcription factor MoMsn2, targets histone acetyltransferase MoHat1 to play a role in appressorium formation and virulence of the rice blast fungus Magnaporthe oryzae. Fungal Genet Biol. 2020;137:103349. doi: 10.1016/j.fgb.2020.103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu YG, Shi Y, et al. Multiple rice microRNAs are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiol. 2014;164:1077–1092. doi: 10.1104/pp.113.230052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao SL, Li JL, et al. Osa-miR169 negatively regulates Rice immunity against the blast fungus Magnaporthe oryzae. Front Plant Sci. 2017;8:2. doi: 10.3389/fpls.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian S, Cho WK, Kim SM, et al. Time-course small RNA profiling reveals Rice miRNAs and their target genes in response to Rice stripe virus infection. PLoS One. 2016;11:e0162319. doi: 10.1371/journal.pone.0162319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, He L, He J, et al. Comprehensive analysis of microRNA-Seq and target mRNAs of rice sheath blight pathogen provides new insights into pathogenic regulatory mechanisms. DNA Res. 2016;23:415–425. doi: 10.1093/dnares/dsw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling KC, Tiongco ER, Aguiero VM. Rice ragged stunt, a new virus disease. Plant disease reporter. 1978;62:701–705. [Google Scholar]
- Liu B, Li P, Li X, et al. Loss of function of OsDCL1 affects microRNA accumulation and causes developmental defects in rice. Plant Physiol. 2005;139:296–305. doi: 10.1104/pp.105.063420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Shi Z, Zhang X, et al. Inducible overexpression of ideal plant Architecture1 improves both yield and disease resistance in rice. Nat Plants. 2019;5:389–400. doi: 10.1038/s41477-019-0383-2. [DOI] [PubMed] [Google Scholar]
- Llave C. Virus-derived small interfering RNAs at the core of plant-virus interactions. Trends Plant Sci. 2010;15:701–707. doi: 10.1016/j.tplants.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Llave C, Xie Z, Kasschau KD, et al. Cleavage of scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- Lu F, Wang H, Wang S, et al. Enhancement of innate immune system in monocot rice by transferring the dicotyledonous elongation factor Tu receptor EFR. J Integr Plant Biol. 2015;57:641–652. doi: 10.1111/jipb.12306. [DOI] [PubMed] [Google Scholar]
- Margis R, Fusaro AF, Smith NA, et al. The evolution and diversification of dicers in plants. FEBS Lett. 2006;580:2442–2450. doi: 10.1016/j.febslet.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Martintrillo M, Cubas P. TCP genes: a family snapshot ten years later. Trends Plant Sci. 2010;15:31–39. doi: 10.1016/j.tplants.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Niu D, Zhang X, Song X, et al. Deep sequencing uncovers Rice long siRNAs and its involvement in immunity against Rhizoctonia solani. Phytopathology. 2018;108:60–69. doi: 10.1094/PHYTO-03-17-0119-R. [DOI] [PubMed] [Google Scholar]
- Onodera Y, Haag JR, Ream T, et al. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell. 2005;120:613–622. doi: 10.1016/j.cell.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Perisperis C, Serracardona A, Sanchezsanuy F, et al. Two NRAMP6 isoforms function as Iron and manganese transporters and contribute to disease resistance in Rice. Mol Plant-Microbe Interact. 2017;30:385–398. doi: 10.1094/MPMI-01-17-0005-R. [DOI] [PubMed] [Google Scholar]
- Pignocchi C, Kiddle G, Hernandez I, et al. Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol. 2006;141:423–435. doi: 10.1104/pp.106.078469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L, Routaboul JM, Cheynier V, et al. Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci. 2007;12:29–36. doi: 10.1016/j.tplants.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Puranik S, Sahu PP, Srivastava PS, et al. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17:369–381. doi: 10.1016/j.tplants.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Qiao L, Zheng L, Sheng C, et al. Rice siR109944 suppresses plant immunity to sheath blight and impacts multiple agronomic traits by affecting auxin homeostasis. Plant J. 2020;102:948–964. doi: 10.1111/tpj.14677. [DOI] [PubMed] [Google Scholar]
- Qu F, Ye X, Morris TJ. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc Natl Acad Sci. 2008;105:14732–14737. doi: 10.1073/pnas.0805760105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman V, Simon SA, Demirci F, et al. Small RNA functions are required for growth and development of Magnaporthe oryzae. Mol Plant-Microbe Interact. 2017;30:517–530. doi: 10.1094/MPMI-11-16-0236-R. [DOI] [PubMed] [Google Scholar]
- Raman V, Simon SA, Romag A, et al. Physiological stressors and invasive plant infections alter the small RNA transcriptome of the rice blast fungus, Magnaporthe oryzae. BMC Genomics. 2013;14:326. doi: 10.1186/1471-2164-14-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Guo Y, Gao F, et al. Multiple functions of Rice dwarf phytoreovirus Pns10 in suppressing systemic RNA silencing. J Virol. 2010;84:12914–12923. doi: 10.1128/JVI.00864-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Guirao R, Baldrich P, Tomiyama S, et al. OsDCL1a activation impairs phytoalexin biosynthesis and compromises disease resistance in rice. Ann Bot. 2019;123:79–93. doi: 10.1093/aob/mcy141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Guirao R, Hsing YI, San Segundo B. The Polycistronic miR166k-166h positively regulates Rice immunity via post-transcriptional control of EIN2. Front Plant Sci. 2018;9:337. doi: 10.3389/fpls.2018.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Sanuy F, Peris-Peris C, Tomiyama S, et al. Osa-miR7695 enhances transcriptional priming in defense responses against the rice blast fungus. BMC Plant Biol. 2019;19:563. doi: 10.1186/s12870-019-2156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Wang J, Wan X, et al. Over-expression of rice OsAGO7 gene induces upward curling of the leaf blade that enhanced erect-leaf habit. Planta. 2007;226:99–108. doi: 10.1007/s00425-006-0472-0. [DOI] [PubMed] [Google Scholar]
- Smith NA, Eamens AL, Wang MB. Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog. 2011;7:e1002022. doi: 10.1371/journal.ppat.1002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sōgawa K. The Rice Brown Planthopper: feeding physiology and host plant interactions. Annu Rev Entomol. 1982;27:49–73. doi: 10.1146/annurev.en.27.010182.000405. [DOI] [Google Scholar]
- Song X, Li P, Zhai J, et al. Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. Plant J. 2012;69:462–474. doi: 10.1111/j.1365-313X.2011.04805.x. [DOI] [PubMed] [Google Scholar]
- Song X, Li Y, Cao X, et al. MicroRNAs and their regulatory roles in plant-environment interactions. Annu Rev Plant Biol. 2019;70:489–525. doi: 10.1146/annurev-arplant-050718-100334. [DOI] [PubMed] [Google Scholar]
- Song X, Wang D, Ma L, et al. Rice RNA-dependent RNA polymerase 6 acts in small RNA biogenesis and spikelet development. Plant J. 2012;71:378–389. doi: 10.1111/j.1365-313X.2012.05001.x. [DOI] [PubMed] [Google Scholar]
- Sun Z, He Y, Li J, et al. Genome-wide characterization of rice black streaked dwarf virus-responsive microRNAs in rice leaves and roots by small RNA and degradome sequencing. Plant Cell Physiol. 2015;56:688–699. doi: 10.1093/pcp/pcu213. [DOI] [PubMed] [Google Scholar]
- Tan J, Wu Y, Guo J, et al. A combined microRNA and transcriptome analyses illuminates the resistance response of rice against brown planthopper. BMC Genomics. 2020;21:144. doi: 10.1186/s12864-020-6556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Liu H, Guo S, et al. OsmiR396d affects gibberellin and Brassinosteroid signaling to regulate plant architecture in Rice. Plant Physiol. 2018;176:946–959. doi: 10.1104/pp.17.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A, Yuan Q, Wang S, et al. Altered accumulation of Osa-miR171b contributes to rice stripe virus infection by regulating disease symptoms. J Exp Bot. 2017;68:4357–4367. doi: 10.1093/jxb/erx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T, Usui M, Sugawara C, et al. Transcripts of two ent-copalyl diphosphate synthase genes differentially localize in rice plants according to their distinct biological roles. J Exp Bot. 2015;66:369–376. doi: 10.1093/jxb/eru424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama S, Moriyama H, Aoki N, et al. Knock-down of OsDCL2 in rice negatively affects maintenance of the endogenous dsRNA virus, Oryza sativa endornavirus. Plant Cell Physiol. 2010;51:58–67. doi: 10.1093/pcp/pcp167. [DOI] [PubMed] [Google Scholar]
- Vazquez F. Arabidopsis endogenous small RNAs: highways and byways. Trends Plant Sci. 2006;11:460–468. doi: 10.1016/j.tplants.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Wang H, Jiao X, Kong X, et al. A signaling Cascade from miR444 to RDR1 in Rice antiviral RNA silencing pathway. Plant Physiol. 2016;170:2365–2377. doi: 10.1104/pp.15.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HD, Chen JP, Zhang HM, et al. Recent Rice stripe virus epidemics in Zhejiang Province, China, and experiments on sowing date, disease–yield loss relationships, and seedling susceptibility. Plant disease reporter. 2008;92:1190–1196. doi: 10.1146/annurev.en.27.010182.000405. [DOI] [PubMed] [Google Scholar]
- Wang J, Meng X, Dobrovolskaya OB, et al. Non-coding RNAs and their roles in stress response in plants. Genomics, Proteomics, Bioinformatics. 2017;15:301–312. doi: 10.1016/j.gpb.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou L, Shi H, et al. A single transcription factor promotes both yield and immunity in rice. Science. 2018;361:1026–1028. doi: 10.1126/science.aat7675. [DOI] [PubMed] [Google Scholar]
- Wang M, Weiberg A, Dellota E, Jr, et al. Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017;14:421–428. doi: 10.1080/15476286.2017.1291112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Zhang D, Wang Z, et al. Mutation of the RDR1 gene caused genome-wide changes in gene expression, regional variation in small RNA clusters and localized alteration in DNA methylation in rice. BMC Plant Biol. 2014;14:177. doi: 10.1186/1471-2229-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li H, Si Y, et al. Microarray analysis of broad-spectrum resistance derived from an indica cultivar Rathu Heenati. Planta. 2012;235:829–840. doi: 10.1007/s00425-011-1546-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xia Y, Lin S et al (2018b) Osa-miR164a targets OsNAC60 and negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. Plant J:584–597. 10.1111/tpj.13972 [DOI] [PubMed]
- Wassenegger M, Krczal G. Nomenclature and functions of RNA-directed RNA polymerases. Trends Plant Sci. 2006;11:142–151. doi: 10.1016/j.tplants.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yang R, Yang Z, et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat Plants. 2017;3:16203. doi: 10.1038/nplants.2016.203. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang Z, Wang Y, et al. Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. Elife. 2015;4:e05733. doi: 10.7554/eLife.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhang Q, Zhou H, et al. Rice MicroRNA effector complexes and targets. Plant Cell. 2009;21:3421–3435. doi: 10.1105/tpc.109.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhou H, Zhang Q, et al. DNA methylation mediated by a microRNA pathway. Mol Cell. 2010;38:465–475. doi: 10.1016/j.molcel.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Xia Z, Zhao Z, Jiao Z et al. (2018) Virus-Derived Small Interfering RNAs Affect the Accumulations of Viral and Host Transcripts in Maize Viruses 10. 10.3390/v10120664 [DOI] [PMC free article] [PubMed]
- Xiao Z-y, Wang Q-x, Sheng-li Z, Wang H, Jin-lu L, Jing F, Yan L, Wang W-m. MiR444b.2 regulates resistance to Magnaporthe oryzae and tillering in rice. Acta phytopathologica Sinica. 2017;4:82–93. [Google Scholar]
- Xin M, Wang Y, Yao Y, et al. Identification and characterization of wheat long non-protein coding RNAs responsive to powdery mildew infection and heat stress by using microarray analysis and SBS sequencing. BMC Plant Biol. 2011;11:61. doi: 10.1186/1471-2229-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhou G. Characteristics of siRNAs derived from southern rice black-streaked dwarf virus in infected rice and their potential role in host gene regulation. Virol J. 2017;14:27. doi: 10.1186/s12985-017-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zhang Y, Yang K, et al. Small RNAs from MITE-derived stem-loop precursors regulate abscisic acid signaling and abiotic stress responses in rice. Plant J. 2011;65:820–828. doi: 10.1111/j.1365-313X.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Li Q, Deng Y, et al. Altered disease development in the eui mutants and Eui Overexpressors indicates that gibberellins negatively regulate Rice basal disease resistance. Mol Plant. 2008;1:528–537. doi: 10.1093/mp/ssn021. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang F, Li J, et al. Integrative analysis of the microRNAome and Transcriptome illuminates the response of susceptible Rice plants to Rice stripe virus. PLoS One. 2016;11:e0146946. doi: 10.1371/journal.pone.0146946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang T, Li J, et al. Chinese wheat mosaic virus-derived vsiRNA-20 can regulate virus infection in wheat through inhibition of vacuolar- (H(+) )-PPase induced cell death. New Phytol. 2020;226:205–220. doi: 10.1111/nph.16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Seo HH, Han SJ, et al. Phytohormone abscisic acid control RNA-dependent RNA polymerase 6 gene expression and post-transcriptional gene silencing in rice cells. Nucleic Acids Res. 2008;36:1220–1226. doi: 10.1093/nar/gkm1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Huang Y, Yang J, et al. Jasmonate signaling enhances RNA silencing and antiviral defense in Rice. Cell Host Microbe. 2020;28(89–103):e108. doi: 10.1016/j.chom.2020.05.001. [DOI] [PubMed] [Google Scholar]
- Yang Z, Li Y. Dissection of RNAi-based antiviral immunity in plants. Curr Opin Virol. 2018;32:88–99. doi: 10.1016/j.coviro.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Yao S, Yang Z, Yang R, et al. Transcriptional regulation of miR528 by OsSPL9 orchestrates antiviral response in Rice. Mol Plant. 2019;12:1114–1122. doi: 10.1016/j.molp.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Peragine A, Park MY, et al. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 2005;19:2164–2175. doi: 10.1101/gad.1352605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cao Y, et al. Overexpression of miR169o, an overlapping MicroRNA in response to both nitrogen limitation and bacterial infection, promotes nitrogen use efficiency and susceptibility to bacterial blight in Rice. Plant Cell Physiol. 2018;59:1234–1247. doi: 10.1093/pcp/pcy060. [DOI] [PubMed] [Google Scholar]
- Yu H, Moss BL, Jang SS, et al. Mutations in the TIR1 Auxin receptor that increase affinity for Auxin/Indole-3-acetic acid proteins result in Auxin hypersensitivity. Plant Physiol. 2013;162:295–303. doi: 10.1104/pp.113.215582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Zhou YF, Feng YZ, et al. Transcriptional landscape of pathogen-responsive lncRNAs in rice unveils the role of ALEX1 in jasmonate pathway and disease resistance. Plant Biotechnol J. 2020;18:679–690. doi: 10.1111/pbi.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarreen F, Kumar G, Johnson AMA, et al. Small RNA-based interactions between rice and the viruses which cause the tungro disease. Virology. 2018;523:64–73. doi: 10.1016/j.virol.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ding Z, Wu K, et al. Suppression of Jasmonic acid-mediated defense by viral-inducible MicroRNA319 facilitates virus infection in Rice. Mol Plant. 2016;9:1302–1314. doi: 10.1016/j.molp.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu M, Tang M, et al. Repression of microRNA biogenesis by silencing of OsDCL1 activates the basal resistance to Magnaporthe oryzae in rice. Plant Sci. 2015;237:24–32. doi: 10.1016/j.plantsci.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu S, Chang H, et al. Mining Magnaporthe oryzae sRNAs with potential Transboundary regulation of Rice genes associated with growth and defense through expression profile analysis of the pathogen-infected Rice. Front Genet. 2019;10:296. doi: 10.3389/fgene.2019.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tao Z, Hong H, et al. Transposon-derived small RNA is responsible for modified function of WRKY45 locus. Nat Plants. 2016;2:16016. doi: 10.1038/nplants.2016.16. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Srivastava AK, et al. Knockdown of Rice MicroRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018;176:2082–2094. doi: 10.1104/pp.17.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Li Y, Zheng YP, et al. Expressing a target mimic of miR156fhl-3p enhances Rice blast disease resistance without yield penalty by improving SPL14 expression. Front Genet. 2020;11:327. doi: 10.3389/fgene.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Mei J, Wang T, et al. Identification and expression analysis of OsmiR1861k in rice leaves in response to Xanthomonas oryzae pv. oryzae. J Gen Plant Pathol. 2015;81:108–117. doi: 10.1007/s10327-015-0579-x. [DOI] [Google Scholar]
- Zhang X, Bao Y, Shan D, et al. Magnaporthe oryzae induces the expression of a MicroRNA to suppress the immune response in Rice. Plant Physiol. 2018;177:352–368. doi: 10.1104/pp.17.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZX, Feng Q, Cao XL, et al. Osa-miR167d facilitates infection of Magnaporthe oryzae in rice. J Integr Plant Biol. 2020;62:702–715. doi: 10.1111/jipb.12816. [DOI] [PubMed] [Google Scholar]
- Zhou G, Qi J, Ren N, et al. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009;60:638–648. doi: 10.1111/j.1365-313X.2009.03988.x. [DOI] [PubMed] [Google Scholar]
- Zhou SX, Zhu Y, Wang LF, et al. Osa-miR1873 fine-tunes rice immunity against Magnaporthe oryzae and yield traits. J Integr Plant Biol. 2020;62:1213–1226. doi: 10.1111/jipb.12900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.