Abstract
With 1.5–2.0 million new cases annually worldwide, corneal injury represents a common cause of vision loss, often from irreversible scarring due to surface corneal defects. In this study, we assessed the use of hepatocyte growth factor (HGF) loaded into an in situ photopolymerizable transparent gelatin-based hydrogel for the management of corneal defects. In vitro release kinetics showed that, in regard to the total amount of HGF released over a month, 55±11% was released during the first 24 hours, followed by a slow release profile for up to one month. The effect of HGF was assessed using an ex vivo model of pig corneal defect. After three days of organ culture, epithelial defects were found to be completely healed for 89% of the corneas treated with HGF, compared to only 11% of the corneas that had fully re-epithelialized when treated with the hydrogel without HGF. The thickness of the epithelial layer was found to be significantly higher for the HGF-treated group compared to the group treated with hydrogel without HGF (p=0.0012). Finally, histological and immunostaining assessments demonstrated a better stratification and adhesion of the epithelial layer in the presence of HGF. These results suggest that the HGF-loaded hydrogel system represents a promising solution for the treatment of persistent corneal defects at risk of scarring.
Keywords: cornea, defect, hydrogel, growth factor, wound healing
INTRODUCTION
The cornea is the 500 μm-thick dome-shaped tissue in the front of the eye, which separates the inside of the eye from the external environment. Its transparency and refractive power allow the light to be transmitted to the retina, enabling vision. The corneal surface is covered by the epithelium, a stratified cell layer, which acts as a barrier against external insults. The majority of the cornea is composed of stromal tissue that is mainly composed of water (78%).
The cornea can get damaged in response to a wide variety of insults, including surgical, infectious, immune-mediated, and traumatic, among other causes. When the damage is severe, the cornea can get ‘ulcerated’. A corneal ulcer is defined as an epithelial (superficial) defect of the cornea associated with subjacent stromal necrosis and thinning that can severely alter the integrity of the cornea. Corneal ulcers are not very infrequent and are most commonly seen in the context of microbial infections and immune-mediated disorders 1. It is estimated that between 30,000 and 75,000 cases of corneal ulcer occur annually in the United States 2. These are usually treated by topical administration of antimicrobial and anti-inflammatory medications, with the treatment targeting primarily the underlying (e.g. infectious) etiology. In the most serious cases, complications like corneal scarring or perforation may occur. If the risk of perforation is recognized, the application of cyanoacrylate glue is accepted despite its rough surface and high toxicity to the eye 3,4. Severely thinned corneal ulcers can be treated using amniotic membranes or corneal transplantation. However, this technique requires access to tissue banks and skilled surgeons.
Hepatocyte growth factor (HGF) is a heterodimeric molecule consisting of a 69 kDa α-chain and a 34 kDa β-chain linked by a disulfide bond. HGF is one of the growth factors which mediates regeneration in different organs. Secreted by mesenchymal cells, HGF stimulates morphogenesis, migration, proliferation, and survival of epithelial cells that express the receptor c-Met 5. Recently, it has been shown that HGF can suppress inflammation and promote epithelium repair in corneal injuries, suggesting that HGF-based therapies represent a promising strategy for corneal defects 6,7. However, beneficial use of growth factors like HGF requires an efficient method of drug delivery to provide sufficient local concentrations and reduce their waste to offload their cost.
In the recent decades, hydrogel systems have been used to successfully deliver drugs for many biomedical applications including cardiology, oncology, and wound healing 8. Hydrogels containing HGF have already been used for recruitment of mesenchymal stem cells 9, liver regeneration 10–12, cardial tissue remodeling 13–17 and engineering 18, treatment of vascular diseases 19,20 and brain tissue regeneration 21. Recently, our team developed an in situ photopolymerizable gelatin-based hydrogel, called GelCORE (Gel for corneal regeneration) (patent WO 2017/139318 Al). Before photopolymerization, GelCORE hydrogel is liquid, making it easy to apply to any shape and size of corneal defect. After photopolymerization with visible light, GelCORE hydrogel forms a solid and adhesive gel mimicking properties of the corneal stroma in terms of transparency, stiffness, elasticity and water content 22.
In this study, we aim to further investigate the capability of GelCORE bioadhesive to regenerate the cornea by loading recombinant human HGF into the bioadhesive hydrogel. We assessed in vitro physical properties (transparency and weight loss) as well as in vitro HGF-release kinetics. We also assessed the effect of HGF eluted by GelCORE hydrogels on an ex vivo model of corneal defect and compared it with the bioadhesive without HGF.
MATERIALS AND METHODS
HGF-loaded GelCORE hydrogel preparation
Gelatin methacryloyl (GelMA) was synthetized following a previously described protocol 23 (Figure 1A). Briefly, 15 g of skin porcine gelatin (Instagel®, PB Leiner USA, Davenport, IA) was dissolved in 150 mL of 0.1 M carbonate-bicarbonate buffer (pH=9) under vigorous stirring at 50°C for 1 h. Then, 8 mL of methacrylic anhydride (Sigma) was added dropwise under continuous stirring at 50°C for 3.5 h. During the reaction, the pH was kept constant by using 1.2 M HCl or 1 M NaOH. The reaction was stopped by diluting three times the solution with Phosphate Buffered Saline (PBS). The solution was finally purified via dialysis for three days and lyophilized. Photoinitiator (PI) solution was prepared as previously reported 22, by dissolving 1.875% (w/v) triethanolamine (Sigma), 1.25% (w/v) N-vinylcaprolactam (Sigma) and 0.5 mM eosin Y disodium salt in PBS. Different GelCORE precursor solutions were prepared by dissolving 20% of GelMA and two different concentrations of recombinant human HGF (100 and 500 ng/mL) (294-HG-005, R&D Systems) in the PI solution.
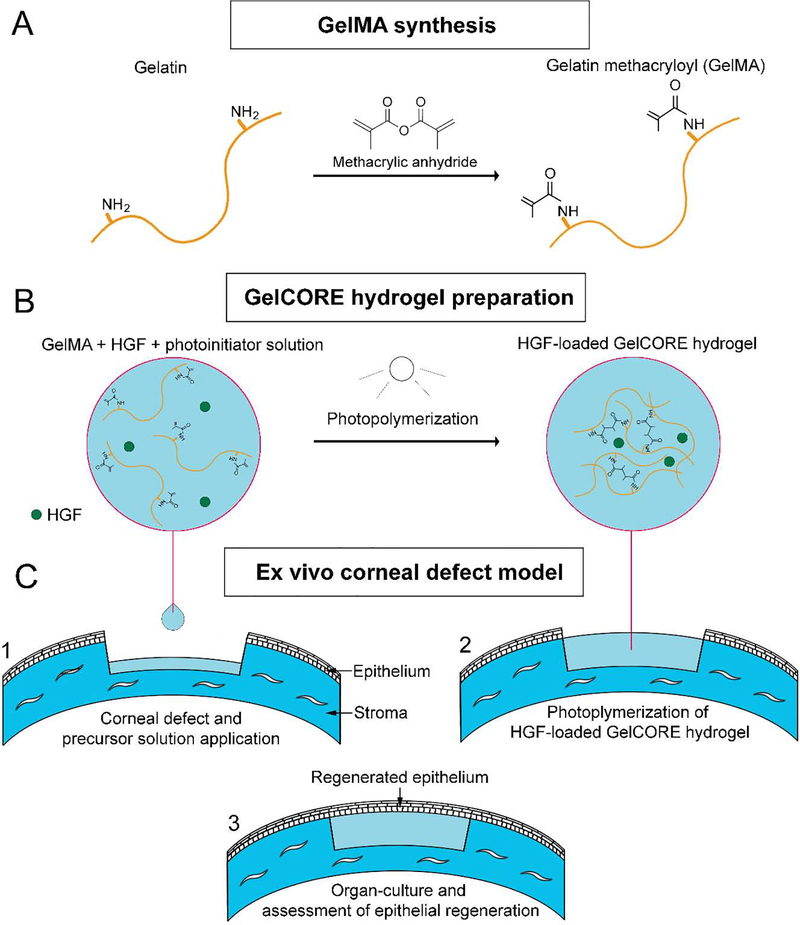
Figure 1.
Schematic of study. (A) GelMA synthesis, (B) HGF-loaded GelCORE hydrogel preparation and (C) Ex vivo corneal defect model. (1) Application of the HGF-loaded GelCORE precursor solution on the pig corneal stromal defect. (2) Photopolymerization of HGF-loaded GelCORE precursor using visible light into a solid hydrogel by chemically binding methacrylate groups. (3) Re-epithelialization over the HGF-loaded GelCORE hydrogel of organ-cultured pig corneas.
Measurement of the degree of methacrylation of GelMA
Degree of methacrylation (DM) of GelMA was calculated as described previously 24. Briefly, 20 mg of lyophilized GelMA or Instagel® gelatin powder was dissolved in 1 ml of deuterium oxide (Fisher Scientific). Proton nuclear magnetic resonance (1H NMR) spectra were obtained by using a 400 MHz spectrometer (Bruker, Billerica, MA, USA). Then, the area under the peaks related to lysine methylene signals (2.95–2.8 ppm) for both samples were normalized and integrated with respect to the phenylalanine signals (6.9–7.5ppm) as reference. The DM of GelMA was calculated according to Equation (1).
| (Equation 1) |
In vitro characterization of weight loss, transparency and HGF release
GelCORE sample preparation and incubation.
70 μL of each GelCORE precursor solution was placed into polydimethylsiloxane cylindrical molds (6-mm diameter; 2.5-mm height). A dental blue light (450-nm wavelength, 300-mW intensity, with 1 cm between the light and the sample) was used to photopolymerize the precursor solutions for 4 min until formation of solid GelCORE hydrogels (Figure 2B). The final concentration of HGF was 5 ng/sample for low[HGF] group (100ng/mL) and 35 ng/sample for high[HGF] group (500ng/mL). Each sample was stored during 28 days at 37°C in 2 mL of PBS containing 0.1% bovine serum albumin (Sigma) and 1% penicillin-streptomycin solution (Sigma). Weight losses and transparency were calculated after 1h, 2h, 6h, 24h, 2 days, 7 days, 14 days and 28 days (n=3).
Figure 2.
In vitro transparency of HGF-loaded GelCORE hydrogels. Transparency assessment (A) and representative photographies (B) of HGF-loaded GelCORE hydrogels at different time points after PBS incubation. (C). Data are represented as means ± SEM (n=3).
Transparency assessment.
At different time points after PBS incubation, transparency of the hydrogels was assessed using a method adapted from previously published photographic-based methods25. Briefly, a screen (387 dpi, 100% brilliance intensity) displaying a black-white stripe pattern (1 mm-width stripe each) was placed under a stereomicroscope equipped with CMOS camera (S9i, Leica). Petri dishes containing GelCORE samples were placed on the stripe pattern and a photograph of each sample was taken. Photographs were then analyzed using ImageJ software. For this, a 3×2-mm2 region of interest (ROI) of the GelCORE sample was selected and the minimal and maximal pixel intensity of this ROI was measured. Contrast was calculated using Equation (2), where Imax and Imin are the maximal and the minimal pixel intensity of the ROI, respectively.
| (Equation 2) |
For each photograph, a similar ROI of the petri dish was selected, and contrast was calculated with the same Equation (2). Transparency was finally calculated using Equation (3), where CGelCORE and CPetri Dish are the contrast of the GelCORE sample and the Petri dish, respectively.
| (Equation 3) |
Weight loss assessment.
All the GelCORE samples were weighed at each time point and weight loss was calculated using Equation (4), where W0 is the weight of the sample just after photopolymerization and Wt is the weight of the sample at different time points.
| (Equation 4) |
HGF release.
HGF concentration after incubation of the sample in PBS at 37°C was determined after 1h, 2h, 6h, 24h, 2 days, 3 days, 7 days, 14 days, 21 days and 28 days. At each time point, 120 μL of PBS sample was withdrawn and replaced with fresh PBS containing 0.1% bovine serum albumin (BSA) and 1% penicillin-streptomycin solution. The HGF concentration in PBS sample was analyzed by ELISA (Quantikine® ELISA, Human HGF, DHG00B, R&D systems).
Ex vivo assessment of the HGF-loaded GelCORE hydrogels in an injured porcine cornea model
Fresh (harvested within 48 hours) pig eyeballs were purchased from Sierra for Medical Science. After removal of extraocular muscles and conjunctival tissue, eyeballs were decontaminated for 1 min in 2.5% povidone-iodine solution (Betadine; Purdie Products, Stamford, CT) and rinsed with PBS supplemented with 10% penicillin-streptomycin solution. A partial trephination with approximatively 50% of the corneal depth was performed in the central cornea by using a 6-mm corneal trephine. Then, a crescent knife was used to dissect the anterior lamella of the trephined portion. Corneas were then excised with approximatively 4 mm of the limbal conjunctiva and placed on a concave silicon support, epithelial side down. The endothelial side was filled with 2%-agar (Sigma) Dulbecco’s Modified Eagle’s medium (DMEM; Sigma) supplemented with 10% Fetal Bovine Serum (FBS; Atlanta Biologicals, Flowery Branch, GA) and 1% penicillin-streptomycin solution. Once the agar solidified, the corneas were placed in a Petri dish, epithelial side up, and DMEM, supplemented with 10% FBS and 1% penicillin-streptomycin solution, was added until the limbus, leaving the epithelium exposed to air. Corneas were then divided in 3 groups (n=9 per group). For the control group, 20 μL of GelCORE precursor solution without HGF was applied into the stromal defect (no HGF). For the 2 other groups, 20 μL of GelCORE precursor solution with 100 ng/mL (low [HGF]) or 500 ng/mL (high [HGF]) of HGF were used to fill the stromal defect. For all groups, hydrogel was photopolymerized for 4 min with a dental blue light until complete solidification. Two corneas with no injury and no hydrogel were also used as controls for native tissue. Corneas were finally cultivated for five days in a humidified 5%-CO2 incubator at 37°C (Figure 1C). Each day, all corneas were stained with fluorescein and photographed under blue light to assess the re-epithelialization rate. Dimensional stability and retention time of the hydrogel as well as regenerated epithelial thickness were investigated by OCT at day 5. Then, the regenerated epithelium of corneas was assessed by hematoxylin and eosin (H&E) staining (n=3 per group) and immunostaining (n=6 per group). For H&E staining, corneas were fixed with paraformaldehyde 4% for 24 h and embedded in paraffin to maintain the corneal structure. Corneas were then cut into 6-μm-thick cross-sections, rehydrated and stained with H&E. Images of cross-sections were finally acquired with a bright-field microscope (Eclipse E800, Nikon). Immunostainings were performed following a previously described protocol 26. Briefly, corneas were embedded in optical cutting compound (Tissue-Tek®, Sakura® Finetek, Torrance, CA) and immediately frozen with dry ice. Corneas were cut into 8-μm-thick cross-sections and then rehydrated in distilled water for 15 min. Nonspecific binding sites were blocked with PBS supplemented by 1% BSA (Sigma) and 1% deactivated goat serum (GS) (Abcam) for 30 min at 37°C. Cross-sections were then incubated with rabbit anti-laminin-5 (1/200) (ab14509, Abcam) and mouse anti-cytokeratin 3 (1/200) (ab68260, Abcam) primary antibodies for 1 h at 37°C. After three rinses with PBS, cross-sections were incubated with anti-rabbit AlexaFluor 494 (1/500) (A-11012, Thermofisher Scientific) and anti-mouse AlexaFluor 488 (1/500) (A-11001, Thermo Fisher Scientific) secondary antibodies for 1h at 37°C. Cross-sections were then counterstained with Hoechst 33342 (H1399, Thermofisher Scientific) for 5 min at room temperature. Images of cross-sections were finally acquired with a fluorescence microscope (Eclipse E800, Nikon).
Statistical analysis
For in vitro experiments (transparency, weight loss and HGF release kinetics), three samples were tested per group (low [HGF] and high [HGF]) and a two-way analysis of variance (ANOVA) were performed. For ex vivo experiments, at least 7 samples were tested per group (No HGF, low [HGF] and high [HGF]). Log-rank test was performed for assessment of ex vivo re-epithelialization rate and Mann-Whitney tests for assessment of the thickness of the regenerated epithelium. All statistical analyses and figures were realized using GraphPad Prism 6.0 (GraphPad Software). All data were presented as means ± SEM.
RESULTS
Degree of methacrylation of GelMA
Degree of methacrylation (DM) of GelMA was measured by using 1H NMR analysis, DM was determined by comparing the amino lysine signals in GelMA and Instagel® gelatin. Accordingly, the DM was measured to be 89.2 ± 1.9, which can be considered as a high DM. Generally, by increasing the DM, the hydrogel crosslinking density can increase, which can result in an increase in stiffness and a decrease in the swelling ratio of the resulting hydrogels27.
In vitro assessment of transparency of HGF-loaded GelCORE hydrogels
Immediately after photopolymerization, transparency ratios of the hydrogels were found at 0.86 ± 0.05 and at 0.89 ± 0.05 when loaded with low HGF concentration (low[HGF] = 5 ng/sample) and high HGF concentration (high[HGF] = 35 ng/sample), respectively. No significant difference in hydrogel transparency was observed after 28 days of incubation in PBS (p=0.98), or between low[HGF] and high[HGF] hydrogels (p=0.12) (Figure 2A–B).
In vitro assessment of the weight loss and HGF release
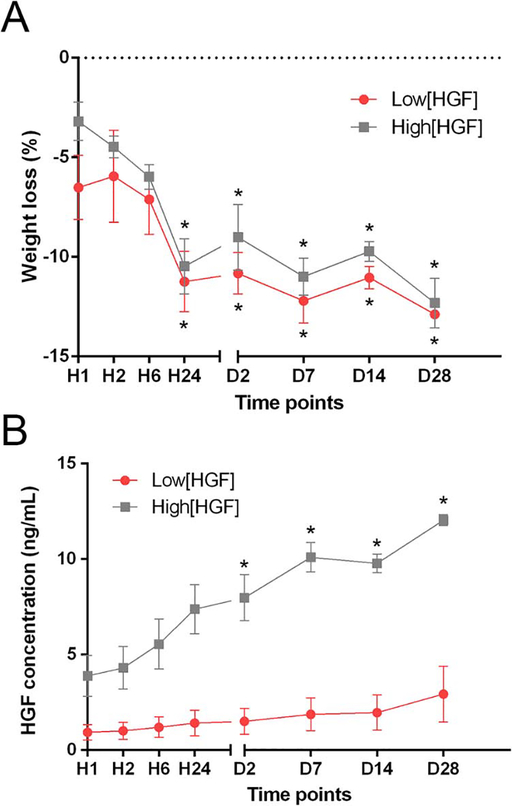
After 28 days of incubation in PBS at 37°C, the weight of GelCORE hydrogels significantly decreased by 12.88 ± 0.96% (p<0.0001) and 12.31 ± 1.76% (p<0.0001) for low[HGF] group and high[HGF], respectively (Figure 3A). More weight loss was observed during the first 24 h for all tested groups. No significant difference in the weight loss was observed between low[HGF] and high[HGF] hydrogels (p=0.4020). During PBS incubation of GelCORE hydrogels, HGF concentration in PBS solution increased continuously with time for both groups (Figure 3B). A fast release of HGF (55 ± 11%) was observed during the first 24 h of PBS incubation. HGF release was found 4.80 ± 0.56 times higher for high[HGF] group compared with low[HGF] group, which is similar to the difference of HGF concentration initially loaded in GelCORE hydrogels (5 ng/sample for low[HGF] and 35 ng/sample for high[HGF]).
Figure 3.
In vitro assessment of weight loss and HGF release kinetics. (A) Weight loss of HGF-loaded GelCORE hydrogels at different time points following PBS incubation. (B) Concentration of HGF released in PBS at different time points. Data are represented as means ± SEM (n=3). * significantly different (p<0.05) compared with H1.
Ex vivo assessment of re-epithelialization rate
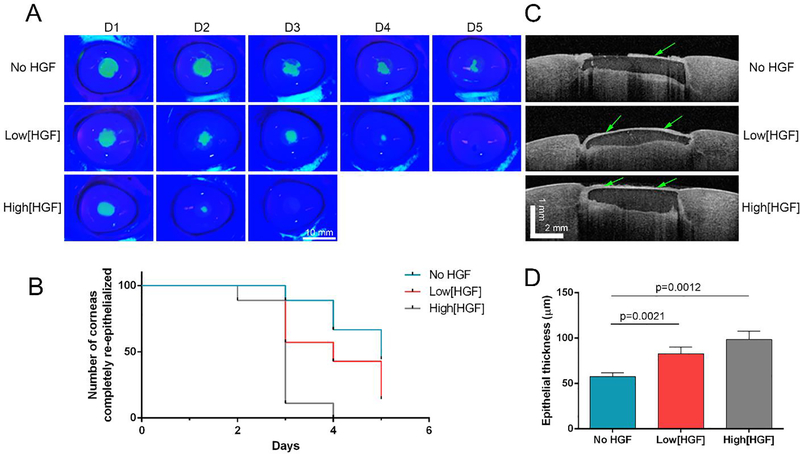
HGF-loaded GelCORE hydrogels were used on an ex vivo model of porcine corneal defect. After hydrogel application and photopolymerization, pig corneas were stored in organ-culture for five days. Fluorescein staining assessment showed significantly faster re-epithelialization for the corneas treated with the high[HGF]-loaded hydrogels compared with low[HGF]-loaded hydrogel and hydrogel without HGF (p=0.0012) (Figure 4A–B). 89% of the corneas treated with high[HGF] were completely re-epithelialized in three days, while only 42% and 11% of the corneas achieved complete re-epithelialization when treated with low[HGF] or no HGF, respectively. For the group with no HGF, 44% of the corneas were not completely re-epithelialized after five days of organ-culture. OCT imaging was performed after five days of organ-culture to assess the retention and thickness of the regenerated epithelium over the gel (Figure 4C–D). Epithelial thickness was found significantly higher for high[HGF] group (98.5 ± 21.9 μm, p=0.0012, n=7) and for low[HGF] group (82.8 ± 17.6 μm, p=0.0021, n=7) as compared with no HGF group (57.6 ± 11.9 μm, n=9).
Figure 4.
Corneal re-epithelialization after ex vivo application of the GelCORE hydrogels loaded with different concentration of HGF to corneal defects in pig cornea stored 5 days in organ-culture. (A) Representative photography of porcine corneas stained with fluorescein and illuminated with blue light at different time points. (B) Survival graph representing the number of corneas completely re-epithelialized at different times points. (C) Central OCT images of porcine corneas after 5 days in organ-culture. (D) Quantification of epithelial thickness from OCT imaged by ImageJ after 5 days in organ-culture. Data are represented as mean ± SEM (n=9 per group, n=7 for low[HGF] group).
Ex vivo assessment of regenerated epithelium
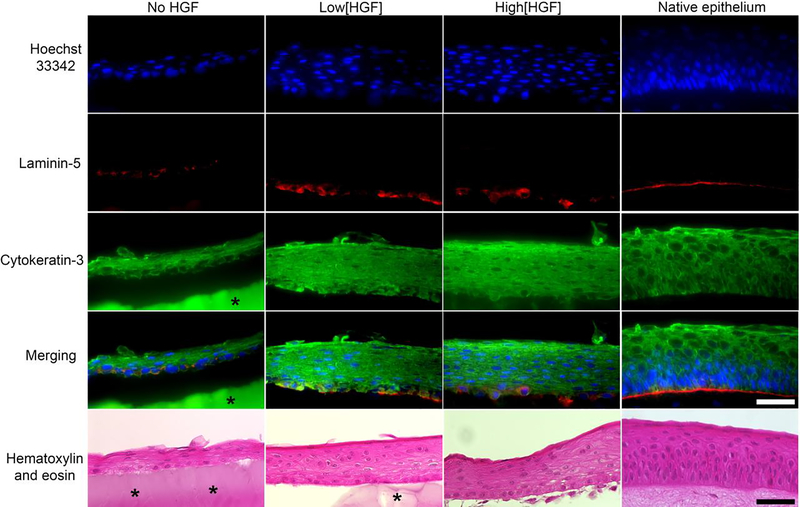
After 5 days of organ-culture, histology and immunostaining studies were performed to assess the quality of the regenerated epithelium over the hydrogels and to compared with native epithelium (Figure 5). Cross-sections of the pig corneas showed that the regenerated epithelium layer appeared thicker for high[HGF] group and low[HGF] group compared with no HGF group, and thus closer to the normal thickness of the native epithelium. Laminin-5 is a major component of the basal membrane of corneal epithelial cells and is used as a marker of adhesion and cohesion of these cells. Both high[HGF] and low[HGF] groups showed a laminin-5 immunostaining closer to the native epithelium as compared with no HGF group. Cytokeratin 3, which is a marker of differentiated epithelial cells, are similarly expressed in the three groups treated as well as for the native epithelium. Finally, higher cell stratification was observed after H&E staining for high[HGF] and low[HGF] compared with no HGF group. This higher stratification also appeared to be more similar to the normal stratification of the native epithelium. These results suggest that the addition of HGF can improve thickness, adhesion (laminin expression) and stratification of the regenerated epithelium, that makes it closer to the native epithelium.
Figure 5.
Immunostaining and histologic cross sections (hematoxylin and eosin) of central epithelium after 5 days of organ-culture in a porcine corneal stromal defect model and in a model without injury and treatment (native epithelium). Nuclei were stained with Hoechst 33342 (blue). Laminin-5 (red) and K3/K12 (green). Asterisks highlight GelCORE hydrogels. For some sample, hydrogels have been partially or totally detached during the cross-sectioning process. Scale bar – 50 μm.
DISCUSSION
In this study, we assessed the use of HGF-loaded gelatin-based hydrogel for the management of corneal defects. A common problem of using biomaterials for corneal bioengineering and wound healing is the lack of transparency or the loss of transparency with time 28. Here, we show that HGF can be easily loaded in a clear gelatin-based hydrogel, while maintaining transparency for at least one month. We also demonstrate that the weight of the HGF-loaded GelCORE hydrogels decreases with time, especially during the first day after photopolymerization, which results in faster release of HGF during the same period. Another study described a shrinkage of GelMA-based hydrogel, such that with higher GelMA concentrations, greater shrinkage is observed 29. Therefore, the weight loss observed in this study may be due to a shrinkage of GelCORE hydrogel, that can potentially increase HGF release.
Our ex vivo pig corneal defect model shows that the addition of HGF to GelCORE significantly improves epithelial regeneration in a dose-dependent manner. Normal pig epithelium maintains an approximatively 6–8 cell layer epithelium 30. Histology and immunostaining studies of HGF-loaded GelCORE hydrogels show the regeneration of these epithelial layers that are absent in the cornea treated without HGF. Therefore, HGF promotes better stratification and adhesion of the epithelium, improving its protective function. Epithelial cells are typically proliferative and able to resurface any wound quickly. However, epithelial defects can persist in some pathological conditions, including limbal stem cell deficiency, dry eye disease, or pathologies that lead to loss of normal neurosensory input such as diabetes mellitus 31.
In our previous study, it has been shown that GelCORE hydrogels, applied on an in vivo rabbit model, progressively degraded and had been replaced by corneal stromal tissue over 14 days 22. Since humans have a larger eye size, larger volume of GelCORE hydrogels can be used that can delay this degradation time. The degradation time can modify the HGF release measured in vitro in this study and should be investigated in vivo. Another study reported that small corneal defects (3-mm diameter) can heal in three days in an in vivo rabbit model 32. However, in our previous study, the healing of a 6-mm defect was not fully complete until seven days 22. This difference of healing time can be explained by the difference of the injury size. By using HGF, in GelMA hydrogels, a faster regeneration of the epithelium could be achieved, which could be particularly beneficial for patients with large defects or persistent defects.
Notwithstanding the need for these in vivo proof-of-concept studies, there are several reasons to suggest that HGF-loaded GelCORE hydrogels could represent a promising solution for the treatment of persistent corneal defects. First, GelCORE precursor solution can be easily applied to the ocular tissue with a syringe to cover defects of any size or shape. Second, photopolymerization can also easily be performed using a simple portable visible LED system. Unlike chemical or thermal polymerization, photopolymerization is entirely controllable by the operator, permitting a precise application of the precursor solution without time limitations. Third, the HGF loaded in GelMA hydrogel may accelerate wound healing, while preventing severe inflammatory reactions and scarring in ocular surface. The release of HGF from GelMA is likely beneficial due to the fact that wound healing and inflammatory processes are particularly active in the early period after tissue injury; this is in accord with standards of clinical care, which provides maximal frequency and dose of medications early after surgery or injury. Moreover, the sustained release of HGF for up to one month may help control inflammation and support the wound healing process for a more prolonged period.
Finally, these data suggest that our hydrogel system could possibly be utilized to incorporate other therapeutics, ranging from peptides to small molecules (in addition to recombinant proteins as shown herein) such as antimicrobial, anti-inflammatory or immunosuppressive medications. As such, the GelCORE hydrogel system could represent a promising platform for drug elution for different ocular surface disorders.
Supplementary Material
Highlights.
Loading of Hepatocyte Growth Factor (HGF) into Gelatin-based adhesive hydrogels
Sustained release of HGF from hydrogels for up to one month
HGF-loaded hydrogels promote corneal epithelial regeneration ex vivo
Potential solution for management of persistent corneal defects
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health (NIH) (R01EB023052, R01HL140618, R01EY024602 and P30EY003790), Department of Defense Vision Research Program Technology/Therapeutic Development Award (W81XWH-18-1-0654) and Research to Prevent Blindness (RPB) Stein Innovation Award.
Footnotes
Conflict of interest Statement
The following authors declare equity interest: Dr. Chauhan and Dr. Dana have equity interest in Claris biotherapeutics. Dr. Dana and Dr. Annabi has equity interest in Gelmedix.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Byrd LB and Martin N, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2019. [Google Scholar]
- 2.Pepose JS and Wilhelmus KR, Am. J. Ophthalmol, 1992, 114, 630–632. [DOI] [PubMed] [Google Scholar]
- 3.Golubović S and Parunović A, Fortschr Ophthalmol, 1990, 87, 378–381. [PubMed] [Google Scholar]
- 4.Yin J, Singh RB, Al Karmi R, Yung A, Yu M and Dana R, Cornea, DOI: 10.1097/ICO.0000000000001919. [DOI] [Google Scholar]
- 5.Miyagi H, Thomasy SM, Russell P and Murphy CJ, Experimental Eye Research, 2018, 166, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omoto M, Suri K, Amouzegar A, Li M, Katikireddy KR, Mittal SK and Chauhan SK, Molecular Therapy, 2017, 25, 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, Shah DI, Sahu SK and Chauhan SK, Stem Cell Reports, 2016, 7, 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J and Mooney DJ, Nature Reviews Materials, DOI: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill HS, Herron CC, Hastings CL, Deckers R, Lopez Noriega A, Kelly HM, Hennink WE, McDonnell CO, O’Brien FJ, Ruiz-Hernández E and Duffy GP, Acta Biomaterialia, 2017, 48, 110–119. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Liu S, Zhang L, Cheng J and Lu Y, International Journal of Nanomedicine, 2016, Volume 11, 4875–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang C-H, Wu W-W, Li H-Y, Chien Y, Sun C-C, Peng C-H, Lin AT-L, Huang C-S, Lai Y-H, Chiou S-H, Hung S-I, Chang Y-L, Lan Y-T, Liu D-M, Chien C-S, Huo T-I, Lee S-D and Wang C-Y, Cell Transplantation, 2015, 24, 541–559. [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Lee JY, Jones CN, Revzin A and Tae G, Biomaterials, 2010, 31, 3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonnenberg SB, Rane AA, Liu CJ, Rao N, Agmon G, Suarez S, Wang R, Munoz A, Bajaj V, Zhang S, Braden R, Schup-Magoffin PJ, Kwan OL, DeMaria AN, Cochran JR and Christman KL, Biomaterials, 2015, 45, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano J, Marui A, Muranaka H, Masumoto H, Noma H, Tabata Y, Ido A, Tsubouchi H, Ikeda T and Sakata R, Interactive CardioVascular and Thoracic Surgery, 2014, 18, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salimath AS, Phelps EA, Boopathy AV, Che P, Brown M, García AJ and Davis ME, PLoS ONE, 2012, 7, e50980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruvinov E, Harel-Adar T and Cohen S, Journal of Cardiovascular Translational Research, 2011, 4, 559–574. [DOI] [PubMed] [Google Scholar]
- 17.Ruvinov E, Leor J and Cohen S, Biomaterials, 2010, 31, 4573–4582. [DOI] [PubMed] [Google Scholar]
- 18.Karam J-P, Muscari C, Sindji L, Bastiat G, Bonafè F, Venier-Julienne M-C and Montero-Menei NC, Journal of Controlled Release, 2014, 192, 82–94. [DOI] [PubMed] [Google Scholar]
- 19.Kim P-H, Yim H-G, Choi Y-J, Kang B-J, Kim J, Kwon S-M, Kim B-S, Hwang NS and Cho J-Y, Journal of Controlled Release, 2014, 187, 1–13. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan WS, Grant OB, Duffy GP and Murphy BP, Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2014, 102, 1700–1710. [DOI] [PubMed] [Google Scholar]
- 21.Nakaguchi K, Jinnou H, Kaneko N, Sawada M, Hikita T, Saitoh S, Tabata Y and Sawamoto K, Stem Cells International, 2012, 2012, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sani ES, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A, Khademhosseini A, Dana R and Annabi N, Science Advances, 2019, 5, eaav1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirahama H, Lee BH, Tan LP and Cho N-J, Sci Rep, 2016, 6, 31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Tian Z, Menard F and Kim K, Biofabrication, 2017, 9, 044101. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Andrades M, de la J, Cardona C, Ionescu AM, Mosse CA and Brown RA, PLoS ONE, 2015, 10, e0142099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guindolet D, Crouzet E, He Z, Herbepin P, Jumelle C, Perrache C, Dumollard JM, Forest F, Peoc’h M, Gain P, Gabison E and Thuret G, Investigative Ophthalmology & Visual Science, 2017, 58, 5907–5917. [DOI] [PubMed] [Google Scholar]
- 27.Annabi N, Zhang Y-N, Assmann A, Sani ES, Cheng G, Lassaletta AD, Vegh A, Dehghani B, Ruiz-Esparza GU, Wang X, Gangadharan S, Weiss AS and Khademhosseini A, Science Translational Medicine, 2017, 9, eaai7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palchesko RN, Carrasquilla SD and Feinberg AW, Advanced Healthcare Materials, 2018, 7, 1701434. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Ma L, Wang C and Gao C, Macromol Biosci, 2009, 9, 1194–1201. [DOI] [PubMed] [Google Scholar]
- 30.Crespo-Moral M, García-Posadas L, López-García A and Diebold Y, PLOS ONE, 2020, 15, e0227732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katzman LR and Jeng BH, Saudi J Ophthalmol, 2014, 28, 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Lu C, Wang L, Chen M, White J, Hao X, McLean KM, Chen H and Hughes TC, ACS Appl. Mater. Interfaces, 2018, 10, 13283–13292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.