Abstract
Background and Objectives:
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first described during a pneumonia outbreak in Wuhan, has attracted tremendous attention in a short period of time as the death toll and the number of confirmed cases is growing unceasingly. Although molecular testing is the gold standard method of SARS-CoV-2 detection, the existence of the false-negative results presents a major limitation to this method.
Materials and Methods:
This retrospective Double-Centre study was conducted on 1320 COVID-19 patients recruited at Taleghani and Shohadae Tajrish Hospitals in Tehran, Iran. We analyzed the leukocyte, lymphocyte and neutrophil counts of hospitalized cases both on admission and at discharge. We also evaluated the alteration of these parameters within a seven-day follow-up.
Results:
Of the whole, 1077 (81.6%) neither were admitted to intensive care unit (ICU) nor experienced death, and were defined as the mild-moderate group. Of 243 severe cases, while 59 (24.3%) were admitted to ICU and cured with the intensive care services, 184 (75.7%) patients died of the disease, either with or without ICU admission. Calculation of neutrophil-to-lymphocyte ratio (NLR) revealed that the mild-moderate cases had a lower ratio at discharge. On the other hand, the ratio was significantly higher in the death group as compared to the ICU group; highlighting the fact that patients with a higher degree of neutrophilia and a greater level of lymphopenia have a poor prognosis.
Conclusion:
We suggest that NLR greater than 6.5 may reflect the progression of the disease towards an unfavorable clinical outcome, with this notion that the ratios higher than 9 may strongly result in death.
Keywords: COVID-19, SARS-CoV-2, Prognosis, Neutrophil-to-lymphocyte ratio, Hematological parameters
INTRODUCTION
The severe acute respiratory syndrome corona-virus 2 (SARS-CoV-2), which was first described during a pneumonia outbreak in Wuhan (1), has attracted tremendous attention in a short period of time as the death toll and the number of confirmed cases is growing unceasingly. Despite being lower in its mortality rate compared to SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome), its long incubation period together with the relatively low pathogenicity increases the risk of contagion of SARS-CoV-2 and facilitates its spread (2). Taking advantage of this, rapid identification of healthy carriers looks to be a great deal not only to diminish viral spread also to impede disease progression (3). Although the containment measures carried out in China have, at least for the moment, decreased the risk of contagion significantly, most countries have not taken the first and the last steps properly, both steps requiring truthful diagnostic testing (4). Even though molecular testing of pharyngeal swab specimens is the gold standard method for the etiological detection of SARS-CoV-2, false-negative results missing 30% to 50% of infected cases present a major limitation to this method (5, 6). In a scramble to fix this challenge before it is too late, an urgent need to find an alternative approach is felt much more than before; one that would identify COVID-19 cases in a simple as well as a rapid way. Earlier studies showed that the alteration in the hematologic parameters not only may help to identify patients with SARS-CoV-2 infection but also may assist clinicians to economically discriminate between the severe and non-severe cases in a timely fashion (7–11). In this Double-Centre study, we retrospectively analyzed the prognostic value of the white blood cells (WBC), neutrophil and lymphocyte counts in 1320 hospitalized COVID-19 cases both on admission and at discharge. We also evaluated the alteration trend of the indicated parameters in a 7-day follow-up.
MATERIALS AND METHODS
Patients and procedures.
This retrospective double-Centre study was conducted on a large cohort of 1320 COVID-19 patients recruited at Taleghani and Shohadae Tajrish Hospitals in Tehran, Iran from February 20 to May 20, 2020. This study was approved by the Shahid Beheshti University of Medical Sciences Ethics Committee (IR.SBMU.RETECH. REC.1399.133) and written informed consent was waived from patients. Based on clinical symptoms including fever, cough, dyspnea and pleuritic chest pain as well as coarse crackles on auscultation, chest computed tomography (CT) was requested for all the patients admitted to the hospital. All imaging features including pure ground-glass opacity (GGO), pure consolidation, mixed GGO and consolidation, reversed halo, intralesion altraction bronchiectasis, crazy-paving, intralesional vascular enlargement, linear opacities, pleural effusion, and pericardial effusion were reviewed and evaluated by an expert radiologist. A thin-section CT involvement score was assigned based on all abnormal areas involved. The number of affected lung lobes was also counted, and the location of the lesion was considered as peripheral if it was in the outer one-third of the lung; otherwise, it was considered as central. Other radiological patterns were also evaluated. Also, throat swab specimens from the upper respiratory tract were obtained and maintained in a virus-transport medium. The presence of SARS-CoV2 in pharyngeal swab specimens was detected by RT-PCR analysis using the Liferiver Novel Coronavirus (2019-nCoV) real-time multiplex RT-PCR Kit on the ABI real-time PCR cycler. Each 25-μl reaction mixture contained 19 μl of SARS-CoV2 Super Mix, 1 μl of RT-PCR Enzyme Mix, and 5 μl of nucleic acid extract. Conditions for the amplifications were 45°C for 10 min, 95°C for 15 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Data on the leukocyte, lymphocyte, and neutrophil counts, obtained from routinely drawn peripheral venous blood on admission, were retrospectively extracted from patients’ electronic medical records. The percentage of leukocytosis (WBC >11), lymphopenia (lymphocyte <1.1), and neutrophilia (neutrophil >6.3) was also calculated in the studied population. Besides, we serially (every two days) monitored the alteration of these parameters in hospitalized patients within a 7-day follow-up. Either admission to the intensive care unit (ICU) or death was defined as criteria for the severity of the disease. Those admitted to ICU and cured with the intensive care services, defined as the “ICU” group in Table 2. While patients died of the disease, either with or without ICU admission, defined as the “death” group in Table 2.
Table 2.
Baseline characteristics and hematologic findings of patients with severe COVID-19.
| Severe (ICU & Death) | ICU | Death | P Value | |
|---|---|---|---|---|
| N (%); Mean (±SD) | N (%); Mean (±SD) | N (%); Mean (±SD) | ||
| Baseline features | ||||
| Sample size | 243 (100) | 59 (24.3%) | 184 (75.7%) | |
| Age (Year) | 66.47 (±17.70) | 56.03 (±18.84) | 69.82 (±15.98) | 0.000 |
| ≤ 39 | 19/243 (7.8) | 14/59 (23.7) | 5/184 (2.7) | 0.000 |
| 40–49 | 20/243 (8.2) | 8/59 (13.6) | 12/184 (6.5) | |
| 50–59 | 32/243 (13.2) | 9/59 (15.3) | 23/184 (12.5) | |
| 60–69 | 46/243 (18.9) | 11/59 (18.6) | 35/184 (19) | |
| ≥ 70 | 126/243 (51.9) | 17/59 (28.8) | 109/184 (59.2) | |
| Sex | ||||
| Female | 102 (42) | 28 (47.5) | 74 (40.2) | 0.327 |
| Male | 141 (58) | 31 (52.5) | 110 (59.8) | |
| Systolic BP | 120.1 (±26.22) | 123.5 (±22.78) | 118.96 (±27.23) | 0.241 |
| Diastolic BP | 74.1 (±13.25) | 76.74 (±11.04) | 73.21 (±13.87) | 0.078 |
| Heart rate | 92.4 (±20.51) | 90.5 (±17.60) | 93.0 (±21.41) | 0.371 |
| Respiratory rate | 20.4 (±6.24) | 18.88 (±4.85) | 20.9 (±6.57) | 0.018 |
| Temperature | 37.2 (±0.81) | 37.2 (±0.76) | 37.2 (±0.83) | 0.920 |
| Hospitalization (Day) | 8.16 (±11.03) | 10.91 (±9.67) | 7.27 (±11.31) | 0.027 |
| Hematologic findings | ||||
| WBC count | 9.74 (±6.23) | 6.78 (±3.42) | 10.80 (±6.66) | 0.000 |
| < 3.5 | 10% | 15.5% | 8% | 0.000 |
| > 11 | 34.1% | 12.1% | 42% | |
| Neutrophil count | 8.16 (±5.50) | 5.42 (±3.13) | 9.15 (±5.83) | 0.000 |
| < 1.8 | 3% | 7.5% | 1.4% | 0.000 |
| > 6.3 | 54.8% | 26.4% | 65.1% | |
| Lymphocyte count* | 1.05 (0.72–1.49) | 1.12 (0.75–1.54) | 1.05 (0.70–1.40) | 0.808 |
| < 1.1 | 54% | 48.1% | 56.1% | 0.317 |
| Neu/Lymph ratio | 8.17 (±5.96) | 5.30 (±4.42) | 9.23 (±6.12) | 0.000 |
Median (IQR); BP: Blood pressure; WBC: White blood cell; Neu: Neutrophil; Lymph: Lymphocyte.
Statistical analysis.
The continuous variables were examined to determine the normality of the distribution using histograms, measures of skewness and kurtosis, and Kolmogorov–Smirnov test. The normally distributed variables were described as the means ± standard deviation (SD) and the skewed distributed variables were expressed as the median and interquartile range (25–75%). Categorical variables were summarized as frequencies (percentage). The normally distributed continuous variables were compared between non-severe and severe groups using the two independent sample t-test and non-normally distributed variables with the Mann–Whitney U test. Comparisons of categorical variables between groups were conducted using Chi-square test of independence. All tests were two-sided, and a P value of less than 0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed using the IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
Role of the funding source.
The funder of the study had no role in study design, data collection, data analysis, and interpretation, or writing of the manuscript. The corresponding authors had full access to all the data in this study and had final responsibility for the decision to submit for publication.
RESULTS
Baseline characteristics and hematologic findings of patients with COVID-19.
Of 1320 COVID-19 patients, 516 (39.1) were female and 804 (60.9) were male. In total, 1077 (81.6%) patients neither admitted to ICU nor experienced death and were defined as the mild-moderate (non-severe) group. As represented in Table 1, there was a significant difference concerning age between patients with the severe and non-severe disease (mean age of 48.9 Vs. 66.4; P: <0.001). While most of the patients (36%) in the non-severe group were less than 40 years old, 51.9% were older than 70 in the severe group; further emphasizing the fact that the old age is among the significant factors affecting the severity of COVID-19. Heart rate and respiratory rate were also significantly higher in patients with severe disease as compared to those with a non-severe condition (P: <0.001). As expected, the duration of hospitalization was significantly higher in severe COVID-19 cases. Of 243 severe cases, 59 (24.3%) were admitted to ICU and cured with the intensive care services (defined as the “ICU” group in Table 2). On the other hand, the remaining 184 cases (75.7%) who died of the disease were defined as the “death” group in this Table. Notably, among patients with severe disease, we also found an older age range in patients who died compared to the ICU group (mean age of 69.8 Vs. 56.0; P: <0.001) (Table 2). A greater respiratory rate and a longer hospitalization was noted in the death group (P: <0.05).
Table 1.
Baseline characteristics and hematologic findings of patients with COVID-19.
| All Patients | Mild-Moderate | Severe (ICU & Death) | P Value | |
|---|---|---|---|---|
| N (%); Mean (±SD) | N (%); Mean (±SD) | N (%); Mean (±SD) | ||
| Baseline features | ||||
| Sample size | 1320 (100) | 1077 (81.6) | 243 (18.4) | |
| Age (Year) | 52.15 (±19.22) | 48.92 (±18.04) | 66.47 (±17.70) | 0.000 |
| ≤ 39 | 406 (30.8) | 387 (36) | 19 (7.8) | 0.000 |
| 40–49 | 216 (16.4) | 196 (18.2) | 20 (8.2) | |
| 50–59 | 201 (15.2) | 169 (15.7) | 32 (13.2) | |
| 60–69 | 196 (14.9) | 150 (13.9) | 46 (18.9) | |
| ≥ 70 | 301 (22.7) | 175 (16.2) | 126 (51.9) | |
| Sex | ||||
| Female | 516 (39.1) | 414 (38.4) | 102 (42) | 0.308 |
| Male | 804 (60.9) | 663 (61.6) | 141 (58) | |
| Systolic BP | 118.1 (±17.17) | 117.7 (±14.37) | 120.1 (±26.22) | 0.172 |
| Diastolic BP | 74.5 (±10.31) | 74.6 (±9.57) | 74.1 (±13.25) | 0.616 |
| Heart rate | 88.5 (±14.96) | 87.6 (±13.27) | 92.4 (±20.51) | 0.001 |
| Respiratory rate | 18.4 (±14.12) | 18.0 (±3.39) | 20.4 (±6.24) | 0.000 |
| Temperature | 37.2 (±0.77) | 37.2 (±0.77) | 37.2 (±0.81) | 0.943 |
| Hospitalization (Day) | 1.91 (±3.76) | 2.89 (±4.34) | 8.16 (±11.03) | 0.000 |
| Hematologic findings | ||||
| WBC count | 7.96 (±6.50) | 7.47 (±6.20) | 9.74 (±6.23) | 0.000 |
| < 3.5 | 9.4% | 9.2% | 10% | 0.000 |
| > 11 | 16.3% | 11.4% | 34.1% | |
| Neutrophil count | 5.98 (±4.75) | 5.42 (±4.37) | 8.16 (±5.50) | 0.000 |
| < 1.8 | 4.4% | 4.7% | 3% | 0.000 |
| > 6.3 | 32.8% | 27.2% | 54.% | |
| Lymphocyte count* | 1.29 (0.90–1.82) | 1.34 (0.96—1.90) | 1.05 (0.72–1.49) | 0.004 |
| < 1.1 | 37.9% | 33.7% | 54% | 0.000 |
| Neu/Lymph ratio | 5.21 (±4.78) | 4.45 (±4.11) | 8.17 (±5.96) | 0.000 |
Median (IQR); BP: Blood pressure; WBC: White blood cell; Neu: Neutrophil; Lymph: Lymphocyte.
The mean white blood cells (WBC) count was 7.96 (±6.50) in all COVID-19 patients, which among them 9.4% and 16.3% displayed leukopenia (<3.5) and leukocytosis (<11), respectively. The mean count of neutrophils was 5.98 (±4.75) and the median count of lymphocytes was 1.29 (0.90–1.82), providing a neutrophil-to-lymphocyte ratio (NLR) of 5.21 in COVID-19 cases. As represented in Table 1, while the WBC and neutrophil counts were significantly higher in severe cases (P: <0.001), the number of lymphocytes was significantly lower with a meaningful NLR of 8.17 (±5.96) in these patients. Such a finding concerning the number of WBC and neutrophil was found in the severe cases between the ICU and the death groups, revealing that the mean WBC and neutrophil counts were higher in the death group as compared to the ICU cases (Table 2). Notably, there was no significant difference in the number of lymphocytes between the indicated group (P: 0.317).
Alteration in the WBC, neutrophil, and lymphocyte counts on admission and at discharge.
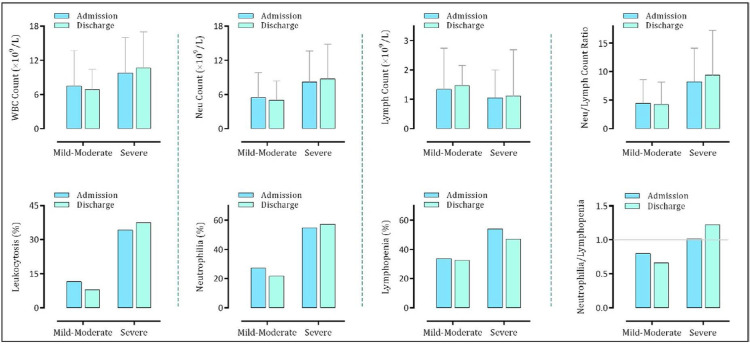
As it is evident in Fig. 1, while the WBC and neutrophil counts were higher in the severe cases compared to the mild-moderate group both on admission and at discharge, the number of lymphocytes was lower in patients with severe disease. Accordingly, the percentages of leukocytosis, neutrophilia, and lymphopenia were higher in the severe group. Comparing the admission and discharge values of these parameters revealed that the WBC and neutrophil counts in patients with a mild-moderate status were decreased at discharge, whereas they showed a higher number of lymphocytes when compared with admission lymphocyte count. Notably, the calculation of NLR revealed that patients with mild-moderate disease had a lower ratio at discharge; indicating that a decrease in the number of neutrophils together with an increment in the lymphocytes has occurred in these patients during hospitalization. In the severe group, on the other hand, since the number of neutrophils was not decreased at discharge, we could find no difference in NLR between admission and discharge. Taking a look at the ratio of neutrophilia/lymphopenia shows that the probability of lymphopenia is higher than neutrophilia in the mild-moderate group, especially at discharge. Notably, this finding was quite different in the severe group. As presented in Fig. 1, while the rate of the emergence of neutrophilia in the severe COVID-19 cases was equal to the rate of lymphopenia on admission, it was significantly greater at discharge.
Fig. 1.
Alteration in the WBC, neutrophil, and lymphocyte counts in patients with severe and non-severe disease on admission and at discharge.
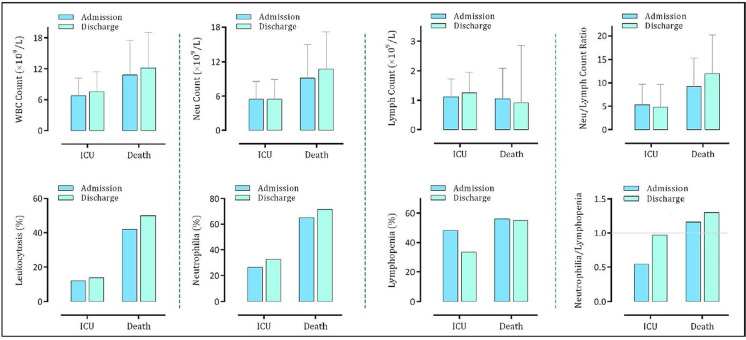
By comparing the numbers of WBC and neutrophils as well as the percentages of leukocytosis and neutrophilia within severe cases, we found that the values of these parameters were higher in the death group as compared to the ICU ones (Fig. 2). Notably, the emergence of lymphopenia ___both on admission and at discharge___was also higher in those who died of the disease. Calculation of NLR in the severe cases revealed that the value of this ratio is significantly higher in the death group, especially at discharge; highlighting the fact that COVID-19 patients with a higher degree of neutrophilia and a greater level of lymphopenia will progress towards unfavorable outcomes.
Fig. 2.
Alteration in the WBC, neutrophil, and lymphocyte counts in the severe COVID-19 patients (either admitted to ICU or died of the disease) on admission and at discharge.
Alteration in the WBC, neutrophil, and lymphocyte counts during a 7-day follow-up.
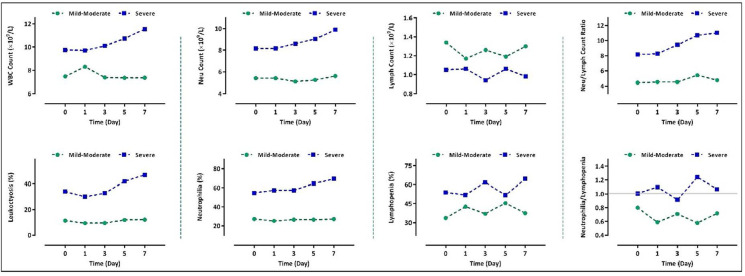
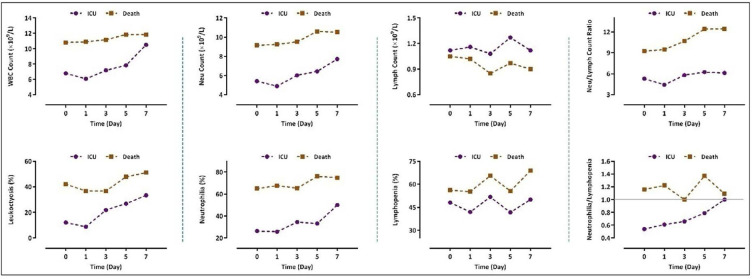
The results of a 7-day follow-up in the mild-moderate group revealed that the WBC and neutrophil counts, as well as the percentages of leukocytosis and neutrophilia, were nearly constant during the hospitalization, whereas monitoring of the values of these parameters displayed a curve with an increasing slope in the severe group (Fig. 3). Even though the number of lymphocytes in the severe cases was lower when compared to the mild-moderate group, we could find no constant trend in the lymphocyte counts both in the severe and non-severe groups. Generally, NLR in the severe group followed an increasing trend with the maximal ratio observed on the seventh day. Of particular importance, while the ratio was less than 5.5 in the mild-moderate group, it was higher than 8 in patients with a severe condition. Alteration analysis of these parameters showed the same results when we compared data between the death and the ICU groups. In agreement with the results represented in Fig. 3, we found that COVID-19 patients who died of the disease had experienced a greater degree of leukocytosis, neutrophilia, and lymphopenia during a 7-day follow-up (Fig. 4). It is worth mentioning that NLR was greater than 9 in the death group and followed an increasing trend during hospitalization; whereas all the values for the indicated ratio were lower than 6.5 in the ICU group (Fig. 4). Notably, as represented in this figure, while the percentage of lymphopenia was higher in the ICU group, the emergence of neutrophilia was more common in those who died.
Fig. 3.
Alteration in the WBC, neutrophil, and lymphocyte counts in patients with severe and non-severe disease within a 7-day follow-up.
Fig. 4.
Alteration in the WBC, neutrophil, and lymphocyte counts in the severe COVID-19 patients (either admitted to ICU or died of the disease) within a 7-day follow-up.
DISCUSSION
Although most of patients with COVID-19 are recovering, infection with SARS-CoV-2 still continues to take its toll (12). At the time of writing this article, over 7,500,000 infected cases were diagnosed with sorrowful statistics of more than 420,000 related deaths (https://www.who.int/). In Iran, infection of over 180,000 people with more than 8,500 deaths ranked this country as the 10th-highest number of SARS-CoV-2 cases as of June 11, 2020. Albeit molecular testing is the gold standard method of in vitro diagnostic, the probability of the false-negative results and the often long-waiting time together with the prerequisite of certified laboratories, expensive equipment and skilled personnel denote major limitations to the PCR-based diagnostic method (5, 6). There are several studies that reported the diagnostic and prognostic value of laboratory parameters in COVID-19 cases (13–15). Ferrari et al. suggested routine blood tests as a potential diagnostic tool for SARS-CoV-2 infection and indicated that combining appropriate cutoffs for certain hematological parameters could help in identifying these patients (11, 16).
In this retrospective study, we evaluated the results of the WBC, neutrophil, and lymphocyte counts of 1320 COVID-19 patients among which 516 (39.1) were female and 804 (60.9) were male. Not only heart rate and respiratory rate were significantly higher in patients with severe disease as compared to those with a non-severe condition, we also found that most of the patients (52%) in the severe group were older than 70 years old. Among patients with severe disease, we also found an older age range in patients who died of the disease as compared to the ICU group; further emphasizing the fact that the old age is among the significant factors affecting the severity of COVID-19. The mean count of neutrophils was 5.98 (±4.75) and the median count of lymphocytes was 1.29 (0.90–1.82), providing a neutrophil-to-lymphocyte ratio (NLR) of 5.21 in COVID-19 cases. We also found that the WBC and neutrophil counts were significantly higher in severe cases as compared to those with non-severe disease (P: <0.001); proposing that patients with severe disease may experience an increased rate of neutrophils release from bone marrow storage to the blood to more effectively battle with the virus. The number of lymphocytes on admission was also significantly lower in patients who admitted to ICU or those who died of the disease, providing a meaningful NLR of 8.17vs. 4.45 in patients with a mild-moderate condition. In a recent study, the number of 3.3 was suggested as the cut-off for NLR in patients aged more than 50. In fact, they reported that the incidence of critical illness in this age group was 9.1% (1/11) for patients having NLR < 3.13, and 50% (7/14) for those with NLR ≥ 3.13 (17). Consistently, Yang et al. reported that the elevated NLR may serve as an independent factor for poor clinical outcome of COVID-19 (18). In another study, Liu et al. proposed NLR as an independent risk factor of the in-hospital mortality and reported that there was an 8% higher risk of in-hospital mortality for each unit increase in NLR (19). Our results showed that the mean WBC and neutrophil counts were higher in the death group as compared to those who admitted to ICU. However, there was no significant difference in the number of lymphocytes between the indicated groups, proposing that an increased number of neutrophils may probably reflect the progression of the disease towards an unfavorable outcome stronger than a decreased lymphocyte counts may do. While most of the earlier studies reported neutrophil and lymphocyte counts of COVID-19 cases on admission, we analyzed the alteration in the WBC, neutrophil, and lymphocyte counts within a 7-day follow-up. We found that the WBC and neutrophil counts, as well as the percentages of leukocytosis and neutrophilia, were nearly constant during the hospitalization in the mild-moderate group, whereas alteration of these parameters within the indicated period of time generated a curve with an increasing slope in the severe group. Our findings were in agreement with a recent study conducted by Wang et al. who reported that non-survivor COVID-19 cases presented a higher level of leukocytosis, neutrophilia, and lymphopenia during hospitalization than those who survived (20). While the analysis of NLR during hospitalization revealed a ratio of less than 5.5 in the mild-moderate group, we found this ratio to be higher than 8 in patients with a severe condition. We also found that COVID-19 patients who died of the disease had experienced a greater ratio than those who admitted to ICU. In this regard, NLR was greater than 9 in the death group and followed an increasing trend during hospitalization, whereas all the values for the indicated ratio were lower than 6.5 in the ICU group. Taken together, our results suggest that NLR greater than 6.5 may reflect the progression of the disease towards an unfavorable clinical outcome, with this notion that the ratios higher than 9 may strongly predict death.
ACKNOWLEDGEMENTS
The authors would like to thank the Shahid Beheshti University of Medical Sciences for supporting this study (Grant No.: 24331).
REFERENCES
- 1. Lu H, Stratton CW, Tang YW. The Wuhan SARS-CoV-2-What’s next for China. J Med Virol 2020; 92: 546– 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239– 1242. [DOI] [PubMed] [Google Scholar]
- 3. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. N Engl J Med 2020; 382: 2158– 2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang Y-W, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 2020; 58 (6): e00512– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 2020: 296: E41– E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020: 296: E32– E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol 2020; 95: 834– 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol 2020; 95: E131– E134. [DOI] [PubMed] [Google Scholar]
- 9. Casini A, Alberio L, Angelillo-Scherrer A, Fontana P, Gerber B, Graf L, et al. Suggestions for thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19. Swiss Med Wkly 2020; 150: w20247. [DOI] [PubMed] [Google Scholar]
- 10. Frater JL, Zini G, d’Onofrio G, Rogers HJ. COVID-19 and the clinical hematology laboratory. Int J Lab Hematol 2020; 42 Suppl 1: 11– 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta 2020; 510: 475– 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis 2020; 20: 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020; 58: 1131– 1134. [DOI] [PubMed] [Google Scholar]
- 14. Favaloro EJ, Lippi G. Recommendations for minimal laboratory testing panels in patients with COVID-19: potential for prognostic monitoring. Semin Thromb Hemost 2020; 46: 379– 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020; 58: 1021– 1028. [DOI] [PubMed] [Google Scholar]
- 16. Ferrari D, Motta A, Strollo M, Banfi G, Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin Chem Lab Med 2020; 58: 1095– 1099. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 2020; 18: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang A-P, Liu J, Tao W, Li H-m. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 2020: 84: 106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HH, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020; 81: e6– e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061– 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]