Abstract
Background
Pulmonary sarcomatoid carcinoma (PSC) is a rare lung cancer subtype. Studies concerning PSC are limited and controversial; therefore, we analyzed the treatment and outcomes of PSC utilizing a relatively large single-institution database.
Methods
From January 2003 to December 2018, 262 consecutive PSC patients treated at our institution were retrospectively reviewed. The clinical characteristics, treatments, and outcomes were analyzed.
Results
The median survival time (MST) was 22.0 months, with 1-, 3-, and 5-year overall survival (OS) rates of 59.9%, 40.1%, and 36.1%, respectively. Patients who underwent surgery had a significantly better prognosis than patients who received nonsurgical treatment (MST, 23.0 vs. 11.0 months, P=0.016). The use of surgery followed by adjuvant therapy significantly prolonged survival in stage III patients (MST, 17.0 vs. 8.0 months, P=0.003) but not in stage I and II patients. Multivariate analysis showed that a systemic inflammation-immune index (SII) value >430.8, TNM stage and necrosis were independent prognostic predictors of OS and disease-free survival (DFS) in radically resected PSC patients (P<0.05). In addition, SII and necrosis were independent risk factors for recurrence after the radical resection of PSC (P<0.05).
Conclusions
PSC is aggressive and has a poor prognosis. Surgery should be the mainstay treatment for operable cases, and adjuvant therapy is recommended for locally advanced disease. A novel potential biomarker, SII, which is an integrated parameter based on preoperative lymphocyte, neutrophil, and platelet counts, may be useful for prognostic prediction and the identification of resected PSC patients at high risk for recurrence.
Keywords: Pulmonary sarcomatoid carcinoma (PSC), survival, prognostic biomarker, systemic immune-inflammation index (SII)
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare malignancy with an incidence rate less than 1.0% (1,2). Based on the 2015 World Health Organization classification, the PSC was subcategorized into five types: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma (3). Several previous studies have shown that the prognosis of PSC is worse than that of other subtypes of lung cancer (1,4-6). The optimal treatment strategies for PSC remain controversial due to its rarity and highly malignant nature. Surgery is currently considered the primary treatment for PSC. PSC was reported to be insensitive to conventional platinum-based chemotherapy (7). Recent studies have demonstrated potentially targetable driving mutations in the MET gene, which renewed hope for improved treatment outcomes for PSC (8). In addition to the unfavorable prognosis, no effective prognostic indicator has been developed for SPC according to the previous reports. In recent decades, several systematic inflammation and immune parameters have been verified as biomarkers to predict survival and recurrence in a variety of malignant tumors (9-12), these parameters include neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and more integrated variable of systemic inflammation-immune index (SII). Recent studies have reported that higher of these biomarkers indicate a worse prognosis in lung cancer (13,14). Regrettably, the existing studies concerning PSC remain limited and most of them were retrospective studies with small sample sizes, besides, none of them have studied the related prognostic indicator. In this study, we report the largest cohort of PSC patients from a single institution and analyze the prognosis, therapeutic modalities, and relevant prognostic factors of PSC. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-960).
Methods
Patient cohort
The Research Ethics Committee of the Cancer Institute and Hospital of the Chinese Academy of Medical Sciences (CICAMS) approved this study and waived the requirement for patient informed consent due to the retrospective nature of the study. From January 2003 to December 2018, 262 patients with histologically confirmed PSC were reviewed from the database of the CICAMS.
All patients provided a detailed clinical history and underwent a physical examination. Complete blood count (CBC), chemistry analysis, pulmonary function test, computed tomography of the chest and upper abdomen, bronchoscopy, brain magnetic resonance imaging, emission computed tomography bone scans or positron emission tomography of the whole body were routinely performed prior to treatment at our institution. Demographic and clinical data were retrieved from the medical records database; this data included age, sex, pretreatment laboratory test results, tumor characteristics, TNM stage, pathological features, and treatment information. The staging of PSC was according to the American Joint Committee on Cancer (AJCC) 8th edition TNM staging manual (15).
The baseline values of neutrophils (N), lymphocytes (L), and platelets (P) were retrieved from the CBC, which was routinely performed within 1 week before surgery. As defined previously, three indices were calculated according to these baseline values: the SII, which was calculated as P×N/L; the NLR, which was calculated as N/L; and the PLR, which was calculated as P/L (9).
Regular follow-up and examinations after treatment were recommended for all patients. Patients were evaluated at return visits every 3 months for the first 2 years after treatment, every 6 months for the following 3 years, and annually thereafter. For patients who received radical resection, information of the first recurrence confirmed by radiological or pathological assessment was also reviewed and then subclassified into locoregional or distant recurrence (16). Locoregional recurrence refers to recurrence within the same lobe, bronchial stump of the original tumor or ipsilateral second lobe, hilum or mediastinum. Distant recurrence was defined as any metastatic disease beyond the locoregional sites. The follow-up and relapse information were obtained by contacting patients or requesting the information from our follow-up center.
Statistical analysis
The continuous variables were portrayed by the median and range and the categorical variables were described as frequency and percentage. Differences between variables were calculated using the Chi-square test or Fisher’s exact test. Overall survival (OS) was calculated from the date of surgery or diagnosis to the date of death or end of follow-up. For patients who underwent radical resection, disease-free survival (DFS) was defined as the interval from the date of surgery to the date of recurrence or death from any cause. The median follow-up time was calculated using the inverse Kaplan-Meier method. The survival curve was plotted using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards models were used to evaluate the independent prognostic factors. The cumulative incidence of recurrence (CIR) was used to evaluate the probability of recurrence in a competing risks model, and comparisons were made with Gray’s test. The Fine-Gray method was used for multivariate analysis of associations between variables and the CIR. A two-sided P value less than 0.05 was considered statistically significant. Statistical tests were performed using SPSS 23.0 (IBM crop, Armonk, NY, USA) and R (version 3.6.1, R Foundation for Statistical Computing), with the “survival” (version 2.44-1.1), “survminer” (version 0.4.5), “cmprsk” (version 2.2.8) and “survivalROC” (version 1.0.3) packages. The function surv_cutpoint (R package survival) was used for the bioinformatic analysis of the continuous data (including SII, NLR, PLR, and number of lymph nodes dissected) to determine the optimal discriminatory cut-off values for survival. The area under curve (AUC) was calculated by the time-dependent receiver operating characteristic (ROC) curve created by R package survival ROC.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Research Ethics Committee of the CICAMS approved this study (18-224/1782) and waived the requirement of the patient’s informed consent for the retrospective nature of the study. Patients’ data were anonymized and maintained with confidentiality.
Results
Patient characteristics
The baseline clinical data of the 262 patients are shown in Table 1. The median age was 61 years (range, 26–89 years), with 211 men (80.5%) and 51 women (19.5%). Most patients were current or former smokers (176 of 262, 67.2%), and 127 patients (48.5%) had a Brinkman index (the number of cigarettes smoked per day multiplied by the number of years of smoking) greater than 500. In total, 35.9% of the patients were found to have obstructive pneumonia based on radiological features. Tumors were most commonly found in the peripheral field of the lung, with a median size of 4.7 cm (range, 0.8–16 cm). Seventeen of the 262 patients (6.5%) had metastatic disease at the initial diagnosis.
Table 1. Demographic and clinical characteristics of 262 PSC patients.
| Variables | n | % |
|---|---|---|
| Sex | ||
| Female | 51 | 19.5 |
| Male | 211 | 80.5 |
| Age, y, median [range] | 61.0 [26–89] | |
| Smoking history | ||
| No | 86 | 32.8 |
| Yes | 176 | 67.2 |
| Brinkman index | ||
| ≤500 | 135 | 51.5 |
| >500 | 127 | 48.5 |
| Personal cancer history | ||
| Yes | 248 | 94.7 |
| No | 14 | 5.3 |
| Year of diagnosis | ||
| 2003–2010 | 49 | 18.7 |
| 2011–2018 | 213 | 81.3 |
| Obstructive pneumonia | ||
| No | 168 | 64.1 |
| Yes | 94 | 35.9 |
| Laterality | ||
| Left lung | 108 | 41.2 |
| Right lung | 154 | 58.8 |
| Tumor site | ||
| Upper lobe | 67 | 25.6 |
| Middle lobe | 11 | 4.2 |
| Lower lobe | 79 | 30.2 |
| Tumor location | ||
| Central | 79 | 30.2 |
| Peripheral | 183 | 69.8 |
| Tumor size, cm, median [range] | 4.7 [0.8–16] | |
| Tumor size, cm | ||
| ≤5 | 152 | 58 |
| >5 | 110 | 42 |
| Histology | ||
| Pleomorphic carcinoma | 79 | 30.2 |
| Spindle cell carcinoma | 44 | 16.8 |
| Giant cell carcinoma | 7 | 2.7 |
| Carcinosarcoma | 3 | 1.1 |
| Pulmonary blastoma | 1 | 0.4 |
| PSC, NOS | 128 | 48.8 |
| TNM stage | ||
| I | 52 | 19.8 |
| II | 70 | 26.7 |
| III | 123 | 46.9 |
| IV | 17 | 6.5 |
| T category | ||
| 1 | 19 | 7.3 |
| 2 | 111 | 42.4 |
| 3 | 73 | 27.9 |
| 4 | 59 | 22.5 |
| N category | ||
| 0 | 130 | 49.6 |
| 1 | 46 | 17.6 |
| 2 | 77 | 29.4 |
| 3 | 9 | 3.4 |
| M category | ||
| 0 | 245 | 93.5 |
| 1 | 17 | 6.5 |
PSC, pulmonary sarcomatoid carcinoma; NOS, not otherwise specified.
Treatment patterns
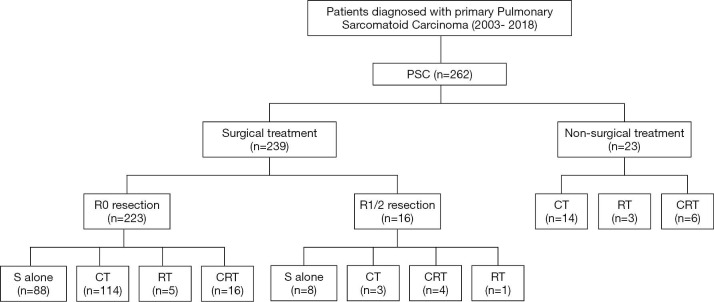
In the present study, most patients (239 of 262, 91.2%) received surgical treatment, and the detailed treatment patterns are summarized in Figure 1. Of the 239 surgically resected patients, 223 (93.3%) underwent a radical R0 resection, and 16 (6.7%) patients underwent an R1/R2 resection.
Figure 1.
Treatment modalities in pulmonary sarcomatoid carcinoma. PSC, pulmonary sarcomatoid carcinoma; CT, chemotherapy; RT, radiotherapy; CRT, chemoradiotherapy.
The surgical procedures included lobectomy (n=200), pneumonectomy (n=24), and sublobectomy (n=15, including wedge resection or segmentectomy). A total of 117 patients (49.0%) received surgery plus adjuvant chemotherapy (CT), and 96 (40.2%) received surgery alone. Only 8 patients received neoadjuvant chemotherapy or chemoradiotherapy (CRT) followed by surgery. Of the 23 nonsurgical patients, 14 received CT, 3 received radiotherapy (RT), and 6 received CRT.
Survival outcome and analysis
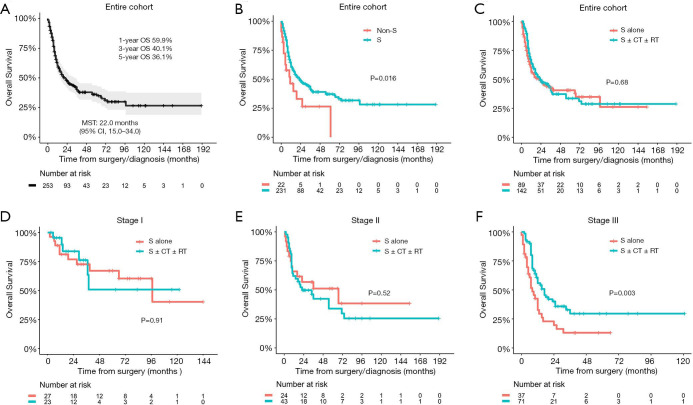
After the exclusion of 9 patients who were lost to follow-up, a total of 253 patients (231 in the surgical group and 22 in the nonsurgical treatment group) were included in the survival analysis. In the entire cohort, the median follow-up time was 40 months [95% confidence interval (CI), 34.0–58.0 months]. The median survival time (MST) was 22.0 months (95% CI, 15.0–34.0 months), with 1-, 3-, and 5-year OS rates of 59.9%, 40.1%, and 36.1%, respectively (Figure 2A). Compared with patients who received nonsurgical treatment, patients who underwent surgical treatment had a significantly better prognosis (MST, 11.0 vs. 23.0 months, P=0.016, Figure 2B), however, patients who received surgery alone had a similar MST to that of patients who underwent surgery with adjuvant therapy (22.0 vs. 23.0 months, P=0.680, Figure 2C). The clinical characteristics of the three groups are presented in Table S1. In the subgroup analysis stratified by TNM stage, stage III patients who received surgery with adjuvant therapy had a significantly longer MST than those who underwent surgery alone (17.0 vs. 8.0 months, P=0.006); however, the differences between two groups failed to reach significance in patients with stage I or stage II disease (Figure 2D,E,F).
Figure 2.
Overall survival curve of the entire PSC cohort and comparison of overall survival among different treatment groups. PSC, pulmonary sarcomatoid carcinoma; OS, overall survival; MST, median survival time; CI, confidence interval; S, surgery; CT, chemotherapy; RT, radiotherapy.
Survival of 215 patients with radical resection
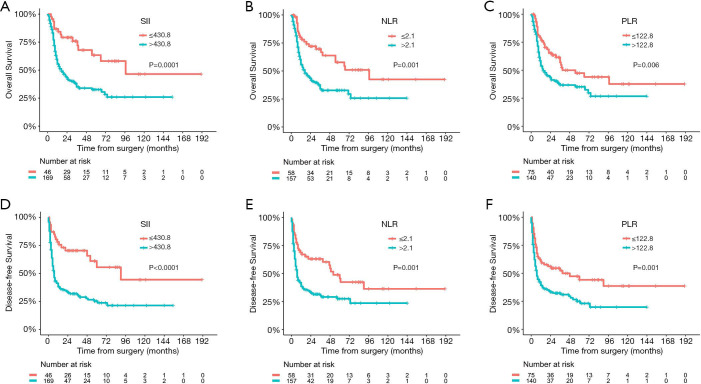
The 215 patients who received radical resection was then further analyzed for survival and recurrence. The optimal cut-off values for those continuous variables were identified using OS as the primary treatment outcome. As shown in Figure S1, the optimal cut-off values were 430.8 for SII, 2.1 for the NLR, 122.8 for the PLR, and 30.0 for the number of lymph nodes dissected. The corresponding variables were then grouped into high or low categories based on these cut-off values. In addition, these predetermined cut-off values were also able to perfectly divide patients into distinct DFS subgroups, which further verified their reliability and reproducibility (Figure 3).
Figure 3.
The Kaplan-Meier curves for overall survival and disease-free survival in patients stratified by optimal cut-off values of SII, the NLR, and the PLR. SII, systemic inflammation-immune index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
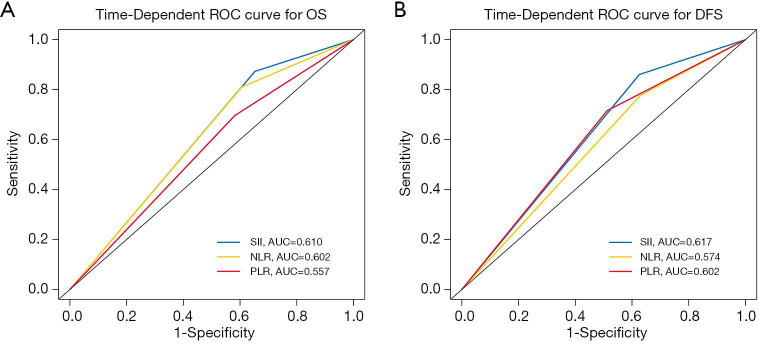
In univariate Cox regression analysis, SII, the NLR, the PLR, tumor size, the TNM stage, necrosis, and visceral pleura invasion (VPI) were revealed to be significantly correlated with OS (P<0.05, Table 2). Compared with patients who had superior survival outcomes, those who had inferior survival outcomes had higher SII values (P<0.001), higher NLRs (P=0.001), higher PLRs (P=0.006), larger tumor sizes (P<0.001), more advanced TNM stages (P<0.001), the presence of necrosis (P=0.001), and the presence of VPI (P=0.041). In addition, SII, the NLR, the PLR, the TNM stage, and tumor necrosis were also identified as independent prognostic factors for DFS (P<0.05; Table 2). We then performed multivariate Cox regression analysis to adjust for the confounding factors. As indicated in Table 3, SII, NLR, PLR, the TNM stage, and necrosis were further identified as independent prognostic predictors of OS and DFS. Based on the time-dependent ROC curves, the AUCs of the dichotomized SII, NLR and PLR were 0.610, 0.602, 0.557 for OS, and 0.617, 0.574, 0.602 for DFS (Figure 4).
Table 2. Univariate analysis of variables associated with OS and DFS in 215 radically resected PSC patients.
| Variables | OS | DFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (male vs. female) | 1.044 (0.656–1.662) | 0.855 | 0.867 (0.578–1.300) | 0.490 | |
| Age (>60 vs. ≤60) | 1.028 (0.715–1.477) | 0.883 | 1.067 (0.761–1.496) | 0.705 | |
| Smoking history (yes vs. no) | 1.060 (0.716–1.569) | 0.773 | 0.843 (0.592–1.200) | 0.343 | |
| Brinkman index (>500 vs. ≤500) | 0.708 (0.491–1.023) | 0.066 | 0.686 (0.462–1.017) | 0.057 | |
| SII (>430.8 vs. ≤430.8) | 2.695 (1.564–4.646) | <0.001 | 2.831 (1.699–4.716) | <0.001 | |
| NLR (>2.1 vs. ≤2.1) | 2.219 (1.399–3.518) | 0.001 | 2.004 (1.324–3.034) | 0.001 | |
| PLR (>122.8 vs. ≤122.8) | 1.754 (1.175–2.619) | 0.006 | 1.856 (1.274–2.705) | 0.001 | |
| Personal cancer history (yes vs. no) | 0.506 (0.161–1.594) | 0.245 | 0.538 (0.199–1.455) | 0.222 | |
| Year of diagnosis (2003–2010 vs. 2011–2018) | 0.764 (0.501–1.166) | 0.213 | 0.905 (0.603–1.359) | 0.631 | |
| Obstructive pneumonia (yes vs. no) | 0.897 (0.614–1.313) | 0.577 | 0.997 (0.704–1.412) | 0.986 | |
| Laterality (right vs. left) | 1.051 (0.726–1.522) | 0.792 | 1.105 (0.784–1.558) | 0.567 | |
| Tumor site | |||||
| Middle vs. upper | 0.658 (0.241–1.796) | 0.414 | 0.845 (0.370–1.930) | 0.689 | |
| Lower vs. upper | 1.046 (0.689–1.589) | 0.833 | 1.177 (0.806–1.718) | 0.399 | |
| Tumor location (peripheral vs. central) | 0.974 (0.665–1.427) | 0.893 | 1.006 (0.704–1.437) | 0.976 | |
| Tumor size (>5 vs. ≤5) | 2.098 (1.458–3.019) | <0.001 | 1.854 (1.323–2.598) | <0.001 | |
| Histology | |||||
| Pleomorphic carcinoma | Reference | Reference | |||
| Spindle cell carcinoma | 1.023 (0.604–1.732) | 0.934 | 0.985 (0.601–1.614) | 0.953 | |
| Giant cell carcinoma | 1.185 (0.464–3.029) | 0.723 | 1.068 (0.422–2.699) | 0.890 | |
| Carcinosarcoma | NA | 0.959 | 0.434 (0.060–3.154) | 0.409 | |
| Pulmonary blastoma | 8.894 (1.181–67.002) | 0.034 | 2.363 (0.324–17.249) | 0.397 | |
| PSC, NOS | 1.144 (0.747–1.751) | 0.537 | 1.113 (0.754–1.643) | 0.591 | |
| Resection type | |||||
| Pneumectomy vs. lobectomy | 1.183 (0.675–2.073) | 0.558 | 0.995 (0.580–1.705) | 0.984 | |
| Sublobectomy vs. lobectomy | 0.950 (0.386–2.339) | 0.912 | 0.751 (0.306–1.843) | 0.532 | |
| No. of lymph nodes dissected (>30 vs. ≤30) | 1.216 (0.803–1.841) | 0.355 | 1.226 (0.832–1.807) | 0.304 | |
| TNM stage | |||||
| II vs. I | 2.144 (1.176–3.908) | 0.013 | 2.149 (1.238–3.730) | 0.007 | |
| III vs. I | 3.531 (2.002–6.228) | <0.001 | 3.260 (1.939–5.483) | <0.001 | |
| Necrosis (yes vs. no) | 1.861 (1.294–2.677) | 0.001 | 1.705 (1.217–2.388) | 0.002 | |
| VPI (yes vs. no) | 1.488 (1.016–2.180) | 0.041 | 1.354 (0.955–1.921) | 0.089 | |
| LVI (yes vs. no) | 1.306 (0.746–2.285) | 0.350 | 1.139 (0.675–1.922) | 0.625 | |
| Treatment pattern (surgery ± CT ± RT vs. surgery alone) | 1.038 (0.713–1.510) | 0.846 | 1.090 (0.768–1.546) | 0.631 | |
NA indicates the failure of calculation of the HR and 95% CI due to the limited cases of carcinosarcoma. PSC, pulmonary sarcomatoid carcinoma; OS, overall survival; DFS, disease-free survival; HR, hazards ratio; CI, confidence interval; SII, systemic inflammation-immune index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio; NOS, not otherwise specified; VPI, visceral pleura invasion; LVI, lymphovascular invasion; CT, chemotherapy; RT, radiotherapy.
Table 3. Multivariate cox regression analysis of variables associated with OS and DFS in 215 radically resected patients.
| Variables | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Overall survival | ||||||||
| SII (>430.8 vs. ≤430.8) | 2.239 (1.285–3.899) | 0.004 | NA | NA | NA | NA | ||
| NLR (>2.1 vs. ≤2.1) | NA | NA | 1.821 (1.141–2.904) | 0.012 | NA | NA | ||
| PLR (>122.8 vs. ≤122.8) | NA | NA | NA | NA | 1.570 (1.036–2.380) | 0.033 | ||
| Tumor size (>5 vs. ≤5) | 1.383 (0.925–2.067) | 0.114 | 1424 (0.954–2.126) | 0.084 | 1.365 (0.910–2.049) | 0.133 | ||
| TNM stage | ||||||||
| II vs. I | 1.630 (0.884–3.007) | 0.118 | 1.601 (0.849–3.017) | 0.146 | 1.693 (0.915–3.132) | 0.093 | ||
| III vs. I | 2.657 (1.488–4.744) | 0.001 | 2.388 (1.277–4.464) | 0.006 | 2.795 (1.560–5.008) | 0.001 | ||
| Necrosis (yes vs. no) | 1.753 (1.214–2.531) | 0.003 | 1.615 (1.117–2.333) | 0.011 | 1.815 (1.251–2.634) | 0.002 | ||
| VPI (yes vs. no) | 1.217 (0.823–1.799) | 0.326 | 1.169 (0.790–1.729) | 0.435 | 1.218 (0.826–1.798) | 0.319 | ||
| Disease-free survival | ||||||||
| SII (>430.8 vs. ≤430.8) | 2.425 (1.435–4.097) | 0.001 | NA | NA | NA | NA | ||
| NLR (>2.1 vs. ≤2.1) | NA | NA | 1.725 (1.132–2.629) | 0.011 | NA | NA | ||
| PLR (>122.8 vs. ≤122.8) | NA | NA | NA | NA | 1.598 (1.077–2.371) | 0.020 | ||
| Tumor size (>5 vs. ≤5) | 1.237 (0.856–1.787) | 0.257 | 1.254 (0.868–1.813) | 0.228 | 1.226 (0.846–1.777) | 0.281 | ||
| TNM stage | ||||||||
| II vs. I | 1.469 (0.816–2.644) | 0.200 | 1.684 (0.940–3.017) | 0.080 | 1.548 (0.859–2.786) | 0.146 | ||
| III vs. I | 2.022 (1.133–3.607) | 0.017 | 2.307 (1.304–4.081) | 0.004 | 2.204 (1.237–3.929) | 0.007 | ||
| Necrosis (yes vs. no) | 1.608 (1.142–2.264) | 0.006 | 1.511 (1.075–2.125) | 0.018 | 1.664 (1.177–2.353) | 0.004 | ||
NA indicates not included in the model due to interference with the three indices, SII, the NLR and the PLR. OS, overall survival; DFS, disease-free survival; HR, hazards ratio; CI, confidence interval; SII, systemic inflammation-immune index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio; VPI, visceral pleura invasion.
Figure 4.
Predictive ability of SII, the NLR, and the PLR for survival in radically resected PSC patients. The sensitivity and specificity values of the three variables were compared by ROC curves. PSC, pulmonary sarcomatoid carcinoma; ROC, receiver operating characteristic; OS, overall survival; DFS, disease-free survival; SII, systemic inflammation-immune index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio; AUC, area under curve.
Recurrence analysis of 215 patients with curative resection
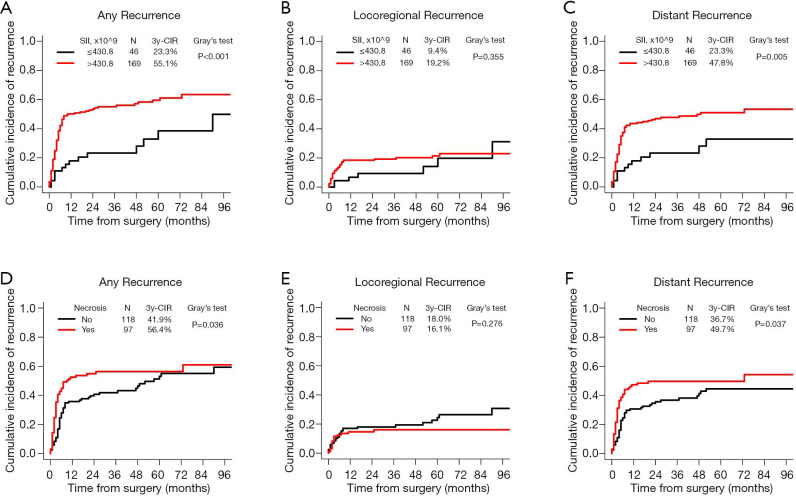
A total of 111 (51.6%) patients were confirmed to have tumor recurrence at the final follow-up, with a median recurrence-free time (duration from surgery to the date of recurrence) of 4.0 months. The first recurrence site was locoregional in 16 patients, distant in 69 patients, and both locoregional and distant in 26 patients. It revealed that patients with early recurrence had worse survival than patients with late recurrence (P=0.048, Figure S2) after dividing the patients into two subgroups based on the median recurrence-free time. Results from our univariate competing-risks regression analysis for developing any recurrence showed that SII, the NLR, the PLR, the Brinkman index, tumor size, the TNM stage, the presence of necrosis, and treatment modality were significantly correlated with recurrence (P<0.05, Table S2). Based on the multivariate model, SII and the presence of necrosis were finally identified as independent risk factors for recurrence (P<0.05, Table 4). As shown in Figure 5, compared with patients with lower SII values, patients with higher SII values had a significantly increased risk of developing any recurrence (3-year CIR, 55.1% vs. 23.3%; P<0.001; Figure 5A). Similarly, patients with the presence of necrosis had a significantly increased risk of developing any recurrence compared with patients without necrosis (3-year CIR, 56.4% vs. 41.9%; P=0.036; Figure 5D). Moreover, when the CIR curves were stratified by different types of recurrence, SII and the presence of necrosis were revealed to be significantly correlated with distant recurrence but not locoregional recurrence.
Table 4. Multivariate competing risk analysis of variables associated with recurrence in 215 radically resected patients.
| Variables | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Brinkman index (>500 vs. ≤500) | 0.717 (0.489–1.050) | 0.088 | 0.729 (0.497–1.070) | 0.100 | 0.743 (0.509–1.080) | 0.120 | ||
| SII (>430.8 vs. ≤430.8) | 2.198 (1.258–3.840) | 0.005 | NA | NA | NA | NA | ||
| NLR (>2.1 vs. ≤2.1) | NA | NA | 1.414 (0.919–2.180) | 0.120 | NA | NA | ||
| PLR (>122.8 vs. ≤122.8) | NA | NA | NA | NA | 1.449 (0.950–2.210) | 0.085 | ||
| Tumor size (>5 vs. ≤5) | 1.393 (0.924–2.100) | 0.110 | 1.403 (0.930–2.120) | 0.110 | 1.381 (0.918–2.080) | 0.120 | ||
| TNM stage | ||||||||
| II vs. I | 1.230 (0.669–2.260) | 0.500 | 1.417 (0.779–2.580) | 0.250 | 1.309 (0.709–2.420) | 0.390 | ||
| III vs. I | 1.062 (0.559–2.020) | 0.850 | 1.261 (0.682–2.330) | 0.460 | 1.171 (0.620–2.210) | 0.630 | ||
| Necrosis (yes vs. no) | 1.442 (1.003–2.070) | 0.048 | 1.371 (0.951–1.980) | 0.091 | 1.463 (1.016–2.110) | 0.041 | ||
| Treatment pattern (surgery ± CT ± RT vs. surgery alone) | 1.525 (0.979–2.380) | 0.062 | 1.461 (0.943–2.260) | 0.090 | 1.504 (0.973–2.320) | 0.066 | ||
NA indicates not included in the model due to interference with the three indices, SII, the NLR and the PLR. HR, hazard ratio; CI, confidence interval; SII, systemic inflammation-immune index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio; CT, chemotherapy; RT, radiotherapy.
Figure 5.
CIR curves by SII and necrosis in radically resected PSC patients. (A and D) CIR for any recurrence; (B and E) CIR for any locoregional recurrence; (C and F) CIR for any distant recurrence. CIR, cumulative incidence of recurrence; PSC, pulmonary sarcomatoid carcinoma; SII, systemic inflammation-immune index.
Discussion
PSC is a particularly rare histological type of lung cancer that is characterized by a poor prognosis. The 262 PSC patients in this study accounted for approximately 1.0% of all 26,000 lung cancer patients at our institution during the study period. Due to its rarity, most publications have been retrospective studies with small sample sizes. To the best of our knowledge, our study includes the largest PSC cohort to date from a single-institution database.
In our study, we found a predominance of male patients, those in their sixth decade of age, and smokers among PSC patients, which was similar to the characteristics reported by previous studies (6,17,18). While size was heterogeneous, tumors mostly involved the peripheral field of the lung. Compared with the other studies (6,18-20), our study reported a relatively lower rate of metastatic disease (6.5%) at the initial diagnosis, which may be due to the fact that some metastatic patients were identified in district hospitals were not referred to our hospital. The total cohort had an MST of 22.0 months; however, prior published series reported a relatively short MST ranging from 3.5 to 19.1 months (5,6,18-23). This may be largely due to the higher proportions of early-stage and surgically resected patients in our cohort.
To date, due to the lack of relevant studies, the optimal treatment strategy for PSC remains unestablished. Surgery remains the primary treatment approach for PSC, and surgery was performed in most of our patients (91.2%). In line with previous reports (20,21), our study verified the prolongation of survival with surgical treatment compared with nonsurgical treatment (MST, 23.0 vs. 11.0 months, P=0.016). In terms of adjuvant therapy, however, our study found no survival benefit from adjuvant therapy after surgical resection. Maneenil et al. (6) also reported no survival benefit of surgery plus neoadjuvant or adjuvant therapy versus surgery alone. As shown in Table S1, the treatment approaches among various stages of disease were different. Hence, we performed a subgroup analysis stratified by TNM stage to eliminate the stage and selection bias. It is worth noting that surgery followed by adjuvant therapy resulted in prolonged survival in patients with stage III disease but not in those with stage I or II disease. However, it is premature to conclude that adjuvant therapy brings no benefit to patients of stage I or II disease. The limited sample size in the subgroup analysis may restrict the statistical power. Besides, further analysis of the detailed adjuvant treatment pattern is still warranted. In summary, radical surgery should be recommended as the mainstay treatment for nonmetastatic PSC, and adjuvant therapies should be considered for select cases. Unfortunately, due to the limited number of cases, we were unable to evaluate the effect of neoadjuvant therapy on survival or the difference in prognosis according to the modality of nonsurgical treatment. Given that conventional treatment does not obtain a satisfactory outcome for PSC patients, advanced targeted therapy or immunotherapy is warranted in clinical practice. The recent identification of alternations in the MET gene, namely, an exon 14 skipping mutation, has been reported in PSC and renewed hope for the development of targeted therapy for PSC (8,24). In addition, PD-L1 expression was also reported to have a high incidence in PSC patients (25-28), and several case reports showed a survival benefit from immunotherapy with PD-1 blockade in PSC patients (6,29).
Risk factors for prognosis were analyzed in the cohort of resected PSC patients. Similar to other non-small cell lung cancer (NSCLC), the TNM stage was revealed to be the main determinant for prognosis, and an advanced stage represented a worse survival. In addition, of all histological variables, necrosis was found to be the only independent parameter influencing OS and DFS. Vieira et al. (17) reported distinct survival outcomes between groups of patients with the presence and absence of necrosis in univariate analysis (P=0.04); however, this difference failed to reach a significant level in multivariate analysis. This may largely due to the heterogeneity in ethnicities between East Asian and European populations.
It is notable that we revealed for the first time that the SII is an important independent prognostic index for PSC. An increasing number of studies have revealed the prognostic values of inflammation-related indices in several types of malignancies (9,11,13,30). Hu et al. (9) reported that a high SII value was associated with adverse survival outcomes and a high circulating tumor cell (CTC) level in patients with hepatocellular carcinoma. Jomrich et al. (11) found that an elevated SII value was an independent adverse prognostic factor in patients with resectable gastroesophageal adenocarcinomas. In NSCLC, Wang et al. (13) discovered that the preoperative SII had superior sensitivity in the prediction of survival compared to most of the conventional clinical variables. However, the optimal cut-off value for this prognostic predictor is cancer-specific, due to the diverse genetic backgrounds and histological origins. The cut-off value for SII in our study (430.8) was identified for the first time in PSC patients; the value is similar to those identified in other types of solid cancer, such as 330 in hepatocellular carcinoma (9), 410 in esophageal squamous cell carcinoma (31), 484.5 in oral squamous cell carcinoma (10), and 419.6 in NSCLC (14). In addition to SII, accumulating evidence has also revealed the prognostic value of the NLR and PLR in diverse solid tumors. We similarly found that high values for the inflammatory immune-related indices, namely, the NLR and PLR, are associated with reduced OS and DFS; however, the HRs for both the NLR and PLR were lower than that of the more integrated variable, SII.
We reported for the first time in our study the patterns of recurrence in radically resected PSC patients and analyzed the correlated factors in detail. Our study found that 51.6% of all patients developed recurrence, with a median recurrence-free time of 4.0 months, and the most common pattern was distant recurrence (85.6%). Previous studies conducted by Yuki et al. (32), Park et al. (33), and Seong et al. (34) reported similar recurrence rates and recurrence-free times. These studies together indicate that PSC is highly aggressive and is prone to early recurrence, especially distant metastasis. In addition, an earlier recurrence implies a more rapid progression and inferior survival. Regrettably, neither of the previous studies further analyzed the potential factors influencing recurrence. In the multivariate competing risk model, SII and necrosis were identified as independent risk factors for recurrence. More interestingly, we found that SII and necrosis were correlated with distant recurrence but not locoregional recurrence. Previous studies similarly confirmed the predictive value of SII for tumor recurrence in various cancers. Aziz et al. (35) reported in their multicenter study that SII was an independent predictor of recurrence in patients with resectable pancreatic ductal adenocarcinoma, Hu et al. (9) revealed SII to be the strongest predictor for cancer recurrence in patients with curative resection of hepatocellular carcinoma. Wang et al. (13) demonstrated that higher pretreatment SII values indicate an inferior distant metastasis-free survival in patients with stage III-N2 NSCLC. These findings indicate that SII is a vital parameter in PSC, and it influences not only survival but also recurrence.
SII, as an integrated indicator, reflects a combination of nonspecific inflammation and immune function. An elevated preoperative SII value usually indicates thrombocytopenia, neutrophilia, or lymphopenia, which reflects an elevated inflammatory response and dysfunctional immune status in patients. Previous studies have revealed that an unbalanced inflammatory status or a deficiency in immune response triggers tumor proliferation, invasion, and metastasis (36,37), which could be explained by the innate function of the three types of blood cells, as previously reported (9,10,13). Hu et al. (9) also revealed an elevated CTC level in patients with high SII values, which may explain the corresponding high recurrence rate. Moreover, a high SII value was also reported to be associated with aggressive features of tumors, such as larger tumor size, poor differentiation, and advanced stages (9,10,13,35). In line with previous reports, our study also revealed correlations of the SII value with tumor size and TNM stage in PSC patients, which further confirmed that PSC is an aggressive phenotype (Table S3). In summary, after performing an integrated evaluation of neutrophils, platelets, and neutrophils, our study suggests that the SII value is a strong predictor of survival and recurrence in PSC patients, and its predictive sensitivity and specificity were both superior to those of the NLR and PLR. In addition, SII is inexpensive, easy to determine, and reproducible, making it a potential biomarker for the precise determination of prognosis and identification of patients at high risk of recurrence, which may help clinicians seeking to apply individualized treatment modalities and intensive follow-up strategies. The decision for the application of postoperative adjuvant treatment depends on the TNM stage, pathological risk factors, as well as physical condition. Patients with advanced disease or multiple risk factors are conventionally recommended with the introduction of adjuvant therapy, however, special consideration should also be given for the early stage disease due to the highly aggressive entity of PSC. As a robust prognostic predictor, we assumed that SII may contribute one of the indicators for the application of adjuvant therapy, especially for those with early stage disease. However, these results also warrant further validation in larger prospective studies.
Certain limitations remain in our study. First, the retrospective nature of this study failed to eliminate the inherent biases of patient selection and heterogeneity, which restricts the applicability of our results. Second, due to the limited sample size and lack of complete information, we were unable to perform a subanalysis of nonsurgical treatments, which also constitute an important portion of the treatment for PSC. Third, the genetic analysis of malignancies was not routinely performed until recently; thus, we also failed to report detailed information regarding the driving genetic mutations in PSC. Moreover, comorbid diabetes mellitus, hypertension, or renal failure may potentially influence the blood cell levels, and this possibility was not fully analyzed in our study.
Conclusions
As a unique subtype of lung cancer, PSC is rare and has a very poor prognosis. Surgery remains the mainstay therapy and resulted in superior survival compared with nonsurgical treatment. However, adjuvant therapy should be considered for select patients with locally advanced disease. In addition, a novel index, SII, which is based on the lymphocyte, neutrophil, and platelet counts, was revealed for the first time to be a strong independent predictor of survival and recurrence in patients with resected PSC. Although this was the largest PSC cohort to date, we still need further verification of our results in properly designed multicenter prospective clinical trials.
Supplementary
The article’s supplementary files as
Acknowledgments
We sincerely thank all authors, investigators, sponsors, and patients for their effort and participation in our included studies.
Funding: This work was supported by the National Key Basic Research Development Plan (2018YFC1312105), the CAMS Innovation Fund for Medical Sciences (2016-I2M-1-001, 2017-I2M-1-005), the Fundamental Research Funds for the Central Universities (3332018070), the National Natural Science Foundation of China (81802299, 81502514).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Research Ethics Committee of the CICAMS approved this study (18-224/1782) and waived the requirement of the patient’s informed consent for the retrospective nature of the study. Patients’ data were anonymized and maintained with confidentiality.
Footnotes
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-960
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-960
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-960
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-960). The authors have no conflicts of interest to declare.
References
- 1.Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results database analysis. Surgery 2012;152:397-402. 10.1016/j.surg.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 2.Chaft JE, Sima CS, Ginsberg MS, et al. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J Thorac Oncol 2012;7:1400-5. 10.1097/JTO.0b013e3182614856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 4.Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg 2007;84:973-80. 10.1016/j.athoracsur.2007.03.099 [DOI] [PubMed] [Google Scholar]
- 5.Rahouma M, Kamel M, Narula N, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg 2018;53:828-834. 10.1093/ejcts/ezx417 [DOI] [PubMed] [Google Scholar]
- 6.Maneenil K, Xue Z, Liu M, et al. Sarcomatoid carcinoma of the lung: the mayo clinic experience in 127 patients. Clin Lung Cancer 2018;19:e323-33. 10.1016/j.cllc.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 7.Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013;8:1574-7. 10.1097/01.JTO.0000437008.00554.90 [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Jia Y, Stoopler MB, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol 2016;34:794-802. 10.1200/JCO.2015.62.0674 [DOI] [PubMed] [Google Scholar]
- 9.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 10.Diao P, Wu Y, Li J, et al. Preoperative systemic immune-inflammation index predicts prognosis of patients with oral squamous cell carcinoma after curative resection. J Transl Med 2018;16:365. 10.1186/s12967-018-1742-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg 2019. [Epub ahead of print]. doi: . 10.1097/SLA.0000000000003370 [DOI] [PubMed] [Google Scholar]
- 12.Shi M, Zhao W, Zhou F, et al. Neutrophil or platelet-to-lymphocyte ratios in blood are associated with poor prognosis of pulmonary large cell neuroendocrine carcinoma. Transl Lung Cancer Res 2020;9:45-54. 10.21037/tlcr.2020.01.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Men Y, Kang J, et al. Systemic inflammation-immune status predicts survival in stage III-N2 non-small cell lung cancer. Ann Thorac Surg 2019;108:1701-9. 10.1016/j.athoracsur.2019.06.035 [DOI] [PubMed] [Google Scholar]
- 14.Guo W, Cai S, Zhang F, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer 2019;10:761-8. 10.1111/1759-7714.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin MB, Edge S, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017:185-202. [Google Scholar]
- 16.Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806-14. 10.1097/JTO.0000000000000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira T, Antoine M, Ruppert AM, et al. Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer 2014;85:276-81. 10.1016/j.lungcan.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 18.Ung M, Rouquette I, Filleron T, et al. Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin Lung Cancer 2016;17:391-7. 10.1016/j.cllc.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 19.Steuer CE, Behera M, Liu Y, et al. Pulmonary sarcomatoid carcinoma: an analysis of the national cancer data base. Clin Lung Cancer 2017;18:286-92. 10.1016/j.cllc.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Xing L, Yuan Y. A clinical analysis of 114 cases of sarcomatoid carcinoma of the lung. Clin Exp Med 2018;18:555-62. 10.1007/s10238-018-0517-2 [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Yang H, Cai Q, et al. Characteristics and prognostic analysis of 69 patients with pulmonary sarcomatoid carcinoma. Am J Clin Oncol 2016;39:215-22. 10.1097/COC.0000000000000101 [DOI] [PubMed] [Google Scholar]
- 22.Gu L, Xu Y, Chen Z, et al. Clinical analysis of 95 cases of pulmonary sarcomatoid carcinoma. Biomed Pharmacother 2015;76:134-40. 10.1016/j.biopha.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 23.Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol 2013;11:252. 10.1186/1477-7819-11-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saffroy R, Fallet V, Girard N, et al. MET exon 14 mutations as targets in routine molecular analysis of primary sarcomatoid carcinoma of the lung. Oncotarget 2017;8:42428-37. 10.18632/oncotarget.16403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698-707. 10.1016/j.ejca.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 26.Vieira T, Antoine M, Hamard C, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016;98:51-8. 10.1016/j.lungcan.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 27.Pécuchet N, Vieira T, Rabbe N, et al. Molecular classification of pulmonary sar- comatoid carcinomas suggests new therapeutic opportunities. Ann Oncol 2017;28:1597-604. 10.1093/annonc/mdx162 [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Xu J, Li R, et al. PD-L1 and CD47 co-expression in pulmonary sarcomatoid carcinoma: a predictor of poor prognosis and potential targets of future combined immunotherapy. J Cancer Res Clin Oncol 2019;145:3055-65. 10.1007/s00432-019-03023-w [DOI] [PubMed] [Google Scholar]
- 29.Sukrithan V, Sandler J, Gucalp R, et al. Immune checkpoint blockade is associated with durable responses in pulmonary sarcomatoid carcinoma. Clin Lung Cancer 2019,20:e242-6. 10.1016/j.cllc.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 30.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 31.Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore) 2017;96:e5886. 10.1097/MD.0000000000005886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuki T, Sakuma T, Ohbayashi C, et al. Pleomorphic carcinoma of the lung: a surgical outcome. J Thorac Cardiovasc Surg 2007;134:399-404. 10.1016/j.jtcvs.2007.04.018 [DOI] [PubMed] [Google Scholar]
- 33.Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology 2011;81:206-13. 10.1159/000333095 [DOI] [PubMed] [Google Scholar]
- 34.Seong YW, Han SJ, Jung W, et al. Perioperative change in neutrophil-to-lymphocyte ratio (NLR) is a prognostic factor in patients with completely resected primary pulmonary sarcomatoid carcinoma. J Thorac Dis 2019;11:819-26. 10.21037/jtd.2019.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aziz MH, Sideras K, Aziz NA, et al. The Systemic-immune-inflammation index independently predicts survival and recurrence in resectable pncreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg 2019;270:139-46. 10.1097/SLA.0000000000002660 [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as