Abstract
Background
Epidemiological studies have reported that dietary mineral intake plays an important role on lung cancer risk, but the association of sodium, potassium intake is still unclear.
Methods
We determined the association between dietary sodium, potassium intake and lung cancer risk based on the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial and the Women’s Health Initiative (WHI). Totally 165,409 participants who completed the baseline questionnaire (BQ) and diet history questionnaire (DHQ) were included into the analytical dataset, including 92,984 (44,959 men and 48,025 women) from the PLCO trial and 72,425 (women only) from the WHI cohort. Multivariable Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of incident lung cancer associated with dietary potassium and sodium intake. The dose-response relationship was also described using the spline smoothed curve after adjusting covariates.
Results
After the median follow-up of 8.55 and 18.56 years, 1,278 and 1,631 new cases of lung cancer were identified in the PLCO trial and WHI cohort, respectively. Intake of sodium was significantly associated with the incidence of lung cancer in the PLCO trial after multivariate adjustment for men (HR: 1.19, 95% CI: 1.05–1.35; P for linear trend =0.044). There was a suggestion that lung cancer risk had a quadratic curve correlation with the increase of potassium intake for women (third vs. lowest quintile: HR: 0.72, 95% CI: 0.54–0.96; P for quadratic trend =0.042). The similar results showing an inverse association between potassium intake and lung cancer risk were also observed in the WHI cohort for women (highest vs. lowest quintile: HR: 0.82, 95% CI: 0.70–0.97; P for linear trend =0.009).
Conclusions
Appropriate intake of potassium has a protective effect against lung cancer, while high consumption of sodium is associated with an increased risk of lung cancer.
Keywords: Lung cancer, sodium, potassium, cancer screening, Women’s Health Initiative
Introduction
Lung cancer is the most frequent malignant cancer and the leading cause of cancer mortality worldwide (1), with 2.1 million new cases accounting for 11.6% of the total new cancer cases and 1.8 million deaths accounting for 18.4% of the total cancer deaths predicted in 2018 (2). Previous studies have identified the risk factors contributing to the occurrence of lung cancer, such as cigarette smoking, gender and air pollution (3).
Recently, emerging evidence has suggested that intake of dietary minerals may have an impact on the incidence of lung cancer. It was found that both a higher zinc and iron intake was associated with a reduced risk of lung cancer (4,5). Similar protective effects of zinc were also reported in three large case-control studies. For the association between calcium intake and lung cancer risk, however, the findings were inconsistent. An increased risk of lung cancer risk on calcium intake was identified in a case-control study by Zhou et al., while an inverse association was found in the National Cancer Institute (NIH)-American Association of Retired Persons Diet and Health study (6). Moreover, several studies also showed that both copper and selenium may decrease the risk of lung cancer (7).
Potassium and sodium are two of the necessary trace elements in the human body, which are mainly derived from diet. Previously, several studies reported that high intake of potassium was negatively related to the occurrence of colorectal cancer, and high sodium consumption could increase the risk of renal cell cancer, colorectal cancer, and gastric cancer (8-11). However, there are few studies on the relationship between potassium, sodium intake and the risk of lung cancer.
In the present study, we aimed to evaluate whether dietary potassium, sodium intake was associated with lung cancer risk using the data from both the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial and the observational study of the Women’s Health Initiative (WHI).
The authors present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-870).
Methods
Study population
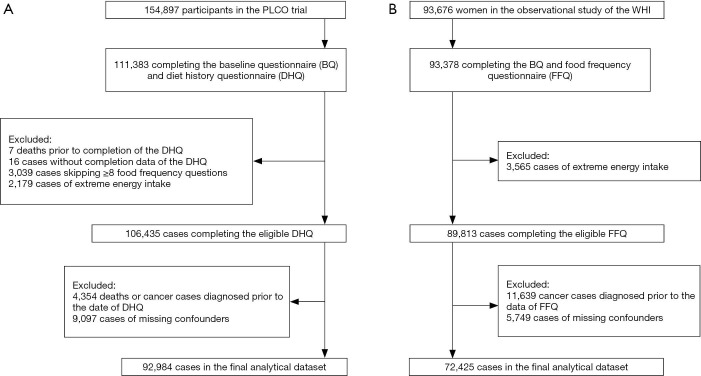
Totally 111,383 out of 154,897 participants in the PLCO cancer screening trial conducted at 10 study centers and sponsored by the National Cancer Institute (NCI) which was carried out to evaluate whether selected screening methods could reduce the mortality from prostate, lung, colorectal, and ovarian cancer completed the baseline questionnaire (BQ) and diet history questionnaire (DHQ) (12). After excluding 7 deaths prior to completion of the DHQ, 16 cases without the completion date of the DHQ, 3,039 cases skipping ≥8 food frequency questions and 2,179 cases of extreme energy intake, there were 106,435 participants completing the eligible DHQ. Finally 92,984 cases were included into the analytical dataset after excluding 4,354 deaths or cancer cases diagnosed prior to the date of DHQ and 9,097 cases of missing necessary covariates (alcohol intake, smoking status, pack-years of smoking, body mass index (BMI), education, diabetes history, and family history of lung cancer) (13). The flow diagram describing the population selection process was listed in Figure 1A.
Figure 1.
The flow diagram describing the population selection process: the PLCO trial (A) and the observational study of the WHI (B).
A total of 93,676 postmenopausal females aged 50–79 years were enrolled in the observational study (a sub-cohort) of the WHI which was a long-term national health study sponsored by the National Heart, Lung, and Blood Institute (NHLBI), with the purpose of exploring new risk indicators and biomarkers of morbidity and mortality in postmenopausal females (14). A similar selection procedure was performed in the WHI cohort (Figure 1B). Finally, 165,409 participants were included into the overall analytical dataset, including 92,984 (44,959 males and 48,025 females) from the PLCO trial and 72,425 (females only) from the WHI cohort.
The studies were approved by the corresponding institutional review boards and all the participants provided written informed consent (12,15).
Data collection
In the PLCO trial, all the participants completed a self-administered baseline questionnaire with demographic information, such as age, gender, race, education, family history of cancer, smoking history, medical history, and past screenings. BMI was computed as weight (kg)/height (m)2. Dietary data were collected through Dietary History Questionnaire (DHQ) which included 156 questions about the consumption frequencies of various foods, drinks, supplements, and other items. Furthermore, the daily intake of nutrients including potassium and sodium was derived from the frequency and portion-size responses to the food frequency questionnaire (FFQ), in which the relevant nutrient values were multiplied by the frequency and summed across. The nutrient composition databases were calculated by DietCalc software, in conjunction with a nutrient database based on the national dietary data [USDA’s 1994–96 Continuing Survey of Food Intakes by Individuals (CSFII), available from the USDA Food Survey Research Group, or the Nutrition Data Systems for Research (NDS-R) from the University of Minnesota] (16).
The demographic information was collected for every participant in the WHI cohort. Dietary data were collected through FFQ with 145 items which consisted of three basic components: a food list, a frequency response section for subjects to report how often each food was eaten and portion-size information. Additionally, the nutrient database used in the FFQ analysis software was based on the University of Minnesota's Nutrition Coordinating Center's (UM-NCC) nutrient database.
Confirmation of lung cancer
In both cohorts, the reports of lung cancer were collected through various methods including but not limited to the annual questionnaires which were mailed to all participants to determine the incidence of lung cancer and the diagnostic data within one year, family reports and death certificates. All the reports of lung cancer diagnosis were followed up and confirmed through abstraction of medical records (12,15).
Statistical analysis
When analyzing the association between dietary sodium, potassium intake and lung cancer risk, the mineral intake was firstly energy-adjusted by the residual method (17). For the primary analysis, participants were divided into quintiles of average intake of sodium or potassium in each cohort. The associations between mineral intake and risk of incidence were analyzed separately for different genders as the overall dietary mineral intake were significantly different between male and female. In addition, a pooled analysis was performed on all the females from the two cohorts.
Multivariable Cox proportional hazards regression was used to calculate the hazard ratios (HR) and its 95% confidence intervals (CI) for incident lung cancer associated with dietary potassium and sodium intake. Follow-up time was defined as the interval between the recruitment date to that of lung cancer diagnosis or withdrawal (death or the end of follow-up). Covariates adjusted in the Cox model included age, gender, BMI at baseline, energy from diet, education level (low: high school or below, intermediate: post high school or junior college, high: college or above), alcohol consumption, smoking status (never smokers, former smokers <20 pack-years, former smokers ≥20 pack-years, current smokers <20 pack-years, current smokers ≥20 pack-years), previous history of diabetes mellitus, and family history of lung cancer. The family history of cancer, but not lung cancer, was adjusted in the multivariable regression model for the WHI cohort because the information of lung cancer was not collected. When testing for trend across quintiles of mineral intake, median values were assigned to denote the corresponding quintiles. Furthermore, the dose-response relationship between dietary sodium or potassium intake and the lung cancer risk was described in a Cox proportional hazard model using the spline smoothed curve after adjusting covariates mentioned previously (18).
We further performed extensive sensitivity analysis to evaluate the robustness of our findings. Some studies suggested that yogurt rich in potassium was inversely associated with lung cancer risk after adjustment for other risk factors (19). In our study, it was found the average yogurt intake of females was twice as much as that of males in the PLCO trial. Besides, the ethnic differences may also have an impact on the results. Therefore, sensitivity analyses were conducted by including yogurt intake or race in the multivariable model. In order to reduce the bias due to measurement errors, we applied the “calibration” equations, details of the study have been described in (20), to FFQ data to estimate calibrated intake and performed the same Cox proportional hazards regression as another sensitivity analysis for the WHI cohort.
Additional analysis was performed after excluding the participants who were diagnosed with cancers other than lung cancer. We also evaluate the association between potassium and sodium intake and lung cancer risk at the different stratum determined by smoking status and classification [non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC)].
All statistical analyses were performed using R software (version 3.5.1). A value of P≤0.05 was considered statistically significant.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The usage of the lung cancer database in the PLCO and WHI studies was authorized by the ethics committees of the data providers and Nanjing Medical University.
Results
Description of the study population
Table 1 showed the baseline characteristics of lung cancer cases and controls in the PLCO trial and WHI cohort. After the median follow-up of 8.55 and 18.56 years, 1,278 and 1,631 new cases of lung cancer were identified in the PLCO trial and WHI cohort, respectively. Significant differences were exhibited between confirmed and non-confirmed lung cancer cases in the PLCO trial and WHI cohort regarding age, BMI, educational level, smoking status, alcohol consumption and family history of cancer (P<0.05). The cases in the PLCO trial were more likely to be male (P<0.001) and had a higher total energy intake (P=0.005), while those in the WHI cohort had a lower intake of potassium (P=0.001). Baseline characteristics of the study population according to quintiles of mineral intake and the correlation coefficients between minerals and dietary food sources were described in Tables S1-S3. It is important to notice that the distributions of mineral intake were significantly different between male and female. Males in the PLCO cohorts had higher sodium and potassium intakes than females in both the PLCO and WHI cohorts (P<0.001), which highlight the importance of making gender-specific analysis on the lung cancer risk on dietary mineral intakes (Figure S1).
Table 1. Baseline characteristics of lung cancer cases and controls in the PLCO trial and the WHI cohort.
| Baseline characteristics (mean ± SD) | The PLCO trial | The WHI cohort | |||||
|---|---|---|---|---|---|---|---|
| Confirmed LC | Non-confirmed LC | P valueb | Confirmed LC | Non-confirmed LC | P valueb | ||
| Total (N) | 1,278 | 91,706 | 1,631 | 70,794 | |||
| Potassium intake (g/day)a | 3.25±0.83 | 3.26±0.76 | 0.965 | 2.62±0.63 | 2.66±0.64 | 0.001 | |
| Sodium intake (g/day)a | 2.71±0.70 | 2.74±0.56 | 0.171 | 2.65±0.49 | 2.64±0.49 | 0.349 | |
| Age (year) | 64.04±5.15 | 62.30±5.26 | <0.001 | 64.89±6.72 | 63.34±7.33 | <0.001 | |
| Gender (%) | |||||||
| Female | 42.5 | 51.8 | <0.001 | 100 | 100 | ||
| Male | 57.5 | 48.2 | – | – | |||
| Body mass index (kg/m2) | 26.57±4.37 | 27.27±4.81 | <0.001 | 26.66±5.47 | 27.19±5.82 | <0.001 | |
| Education (%) | |||||||
| Low | 35.4 | 28.6 | <0.001 | 20.3 | 20.9 | 0.041 | |
| Middle | 40.4 | 34.4 | 39.2 | 36.2 | |||
| High | 24.2 | 37.0 | 40.5 | 42.9 | |||
| Cigarette smoking (%) | |||||||
| Never | 8.2 | 48.4 | <0.001 | 18.3 | 53.1 | <0.001 | |
| Former <20 pack-years | 6.7 | 18.8 | 17.3 | 27.0 | |||
| Former ≥20 pack-years | 44.5 | 24.0 | 39.6 | 14.1 | |||
| Current <20 pack-years | 1.1 | 0.8 | 4.4 | 2.4 | |||
| Current ≥20 pack-years | 39.5 | 8.0 | 20.4 | 3.4 | |||
| Alcohol intake (g/day) | 14.76±37.39 | 9.72±25.40 | <0.001 | 7.63±12.92 | 5.53±11.10 | <0.001 | |
| Energy intake (kcal/day) | 1,815.8±800.3 | 1,740.0±733.1 | 0.005 | 1,569.1±576.8 | 1,570.0±596.2 | 0.636 | |
| Diabetes (%) | 7.12 | 6.51 | 0.408 | 5.15 | 5.24 | 0.910 | |
| Family history of lung cancer (%) | 18.78 | 10.59 | <0.001 | ||||
| Family history of cancer (%) | 60.5 | 54.7 | <0.001 | 67.87 | 63.99 | 0.001 | |
SD, standard deviation; LC, lung cancer. a, intake was energy-adjusted. b, P value for difference was tested using Wilcox rank sum test (continuous) or Chi-square test (categorical).
Gender-specific association between mineral intake and risk of lung cancer
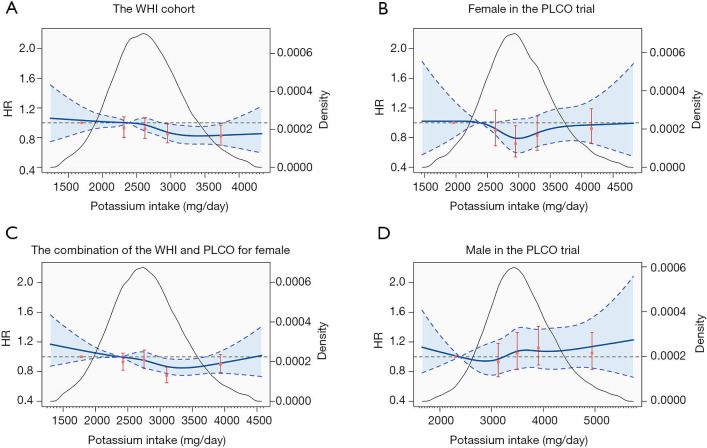
As shown in Table 2, higher intake of potassium was associated with a decreased risk of lung cancer in the WHI cohort. Compared with the subgroup with the lowest potassium intake (median intake 1,914 mg/day), the HRs and 95% CIs for subgroups with the second highest (median intake 2,935 mg/day) and highest intake (median intake 3,450 mg/day) were 0.85 (0.73, 0.99) and 0.82 (0.70, 0.97). We also identified a significant linear trend between potassium intake and lung cancer risk (P=0.009). Furthermore, the spline smoothed curve analysis demonstrated a significant dose-response association between potassium intake and risk of incident lung cancer (Figure 2A). For females in the PLCO trial, although not significant, higher potassium intake groups showed protective effects against lung cancer. As the spline smoothed curve shown in Figure 2B, the trend of HRs with potassium intake were in quadratic, subgroup with median potassium intake was associated with 26% reduction in the risk of lung cancer (compared with the lowest: HR: 0.72, 95% CI: 0.54–0.96). This was verified by fitting a quadratic regression with a squared term of potassium intake (P for quadratic trend =0.04). Similar trend was also observed in the pooled analysis (Figure 2C). However, the association was not significant for potassium intake in males (HR: 1.06, 95% CI: 0.97–1.17, Figure 2D).
Table 2. Gender-specific hazard ratios and 95% confidence intervals of lung cancer based on categories of dietary mineral intake.
| Continuous | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
|---|---|---|---|---|---|---|
| Potassium | ||||||
| WHI (female only) | ||||||
| Dietary intakea | 2,619.5 | <2,153.6 | 2,153.6–2,473.8 | 2,473.8–2,768.2 | 2,768.2–3,139.5 | ≥3,139.5 |
| Cases/person-years | 1,631/1,123,470 | 368/207,916 | 336/220,409 | 332/228,708 | 304/231,972 | 291/234,465 |
| Multivariable model HRb | 0.94 (0.87, 1.02) | 1.00 | 0.93 (0.80, 1.08) | 0.92 (0.79, 1.07) | 0.85 (0.73, 0.99) | 0.82 (0.70, 0.97) |
| P value | 0.124 | Ptrendc =0.009 | ||||
| Female in the PLCO | ||||||
| Dietary intakea | 2,943.8 | <2,463.4 | 2,463.4–2,797.0 | 2,797.0–3,096.8 | 3,096.8–3,487.7 | ≥3,487.7 |
| Cases/person-years | 543/390,992 | 123/77,876 | 106/77,994 | 87/77,975 | 103/78,589 | 124/78,558 |
| Multivariable model HRb | 0.96 (0.85, 1.09) | 1.00 | 0.90 (0.69, 1.17) | 0.72 (0.54, 0.96) | 0.83 (0.63, 1.09) | 0.92 (0.72, 1.19) |
| P value | 0.546 | Ptrendc =0.553 | ||||
| Male in the PLCO | ||||||
| Dietary intakea | 3,489.9 | <2,935.9 | 2,935.9–3,319.7 | 3,319.7–3,670.0 | 3,670.0–4,142.0 | ≥4,142.0 |
| Cases/person-years | 735/343,836 | 152/68,795 | 127/68,496 | 145/68,642 | 159/68,905 | 152/68,998 |
| Multivariable model HRb | 1.06 (0.97, 1.17) | 1.00 | 0.93 (0.73, 1.18) | 1.05 (0.83, 1.33) | 1.12 (0.89, 1.41) | 1.05 (0.83, 1.33) |
| P value | 0.188 | Ptrendc =0.348 | ||||
| Sodium | ||||||
| WHI (female only) | ||||||
| Dietary intakea | 2,620.2 | <2,291.5 | 2,291.5–2,522.5 | 2,522.5–2,722.4 | 2,722.4–2,980.3 | ≥2,980.3 |
| Cases/person-years | 1,631/1,123,470 | 337/221,914 | 293/224,463 | 343/224,825 | 313/226,959 | 345/225,309 |
| Multivariable model HRb | 1.10 (0.99, 1.21) | 1.00 | 0.94 (0.80, 1.11) | 1.15 (0.99, 1.35) | 1.05 (0.90, 1.23) | 1.12 (0.96, 1.30) |
| P value | 0.074 | Ptrendc =0.080 | ||||
| Female in the PLCO | ||||||
| Dietary intakea | 2,361.2 | <2,083.6 | 2,083.6–2,280.3 | 2,280.3–2,442.9 | 2,442.9–2,656.4 | ≥2,656.4 |
| Cases/person-years | 543/390,992 | 143/77,508 | 101/78,109 | 106/78,154 | 100/78,802 | 93/78,418 |
| Multivariable model HRb | 0.83 (0.68, 1.01) | 1.00 | 0.80 (0.62, 1.04) | 0.91 (0.70, 1.18) | 0.82 (0.63, 1.07) | 0.71 (0.54, 0.93) |
| P value | 0.057 | Ptrendc =0.022 | ||||
| Male in the PLCO | ||||||
| Dietary intakea | 3,108.9 | <2,732.1 | 2,732.1–2,996.7 | 2,996.7–3,229.6 | 3,229.6–3,551.6 | ≥3551.6 |
| Cases/person-years | 735/343,836 | 155/68,264 | 127/68,502 | 149/68,646 | 159/69,025 | 145/69,399 |
| Multivariable model HRb | 1.19 (1.05, 1.35) | 1.00 | 1.00 (0.78, 1.28) | 1.24 (0.97, 1.57) | 1.33 (1.05, 1.68) | 1.19 (0.93, 1.53) |
| P value | 0.008 | Ptrendc =0.044 | ||||
a, mineral intake was energy-adjusted (mg/day), median intake for continuous and range for quintiles. b, Cox proportional hazard models were used to adjust age, body mass index (kg/m2), energy intake (kcal/day), educational level (3 categories), alcohol consumption (g/day), smoking status (never smokers, former smokers <20 pack-years, former smokers ≥20 pack-years, current smokers <20 pack-years, current smokers ≥20 pack-years), history of diabetes (yes or no), and family history of lung cancer (yes or no) for the PLCO trial or family history of cancer (yes or no) for the WHI cohort. c, test for linear trend was estimated by assigning the median value of sodium or potassium intake in each quintile.
Figure 2.
Dose-response association between dietary potassium intake (energy-adjusted) and lung cancer risk for females in the WHI cohort (A), females in the PLCO trial (B), the combination of females (C), and males in the PLCO trial (D) using spline smoothed model. Multivariable risk estimate was calculated to adjust all covariates by spline regression with three knots (20th percentiles as the reference, 2,381 mg/day). Blue solid line represented point estimates, dashed lines and shadow area represented 95% confidence intervals. Red solid points and vertical lines represented HRs and 95% CIs of lung cancer based on categories of dietary potassium intake (compared with the lowest quintile), respectively. Black curve showed the kernel density estimates of participants (right y-axis) consuming of potassium.
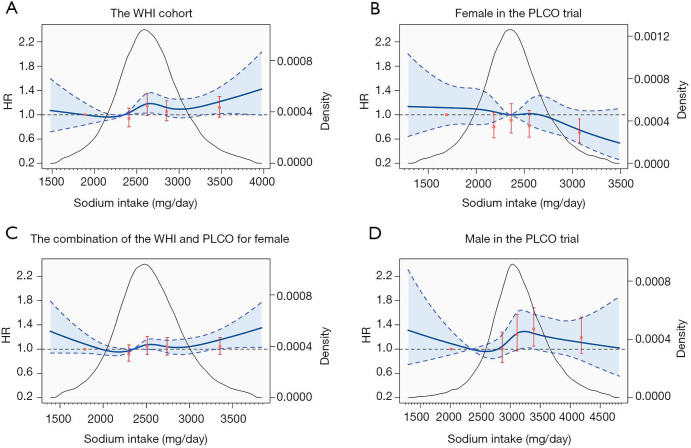
As for sodium intake, higher sodium intake was significantly associated with increased lung cancer risk for males in the PLCO trial (HR: 1.19, 95% CI: 1.05–1.35, P for linear trend =0.044). For females, we observed a linear trend for sodium intake against lung cancer in the PLCO (P for trend =0.022), while only a suggestive but not significant association in the WHI cohort (HR: 1.10, 95% CI: 0.99–1.21; P for trend =0.080). Corresponding spline smoothed curves were shown in Figure 3.
Figure 3.
Dose-response association between dietary sodium intake (energy-adjusted) and lung cancer risk for females in the WHI cohort (A), females in the PLCO trial (B), the combination of females (C), and males in the PLCO trial (D) using spline smoothed model. Multivariable risk estimate was calculated to adjust all covariates by spline regression with three knots (20th percentiles as the reference, 2,349 mg/day). Blue solid line represented point estimates, dashed lines and shadow area represented 95% confidence intervals. Red solid points and vertical lines represented HRs and 95% CIs of lung cancer based on categories of dietary sodium intake (compared with the lowest quintile), respectively. Black curve showed the kernel density estimates of participants (right y-axis) consuming of sodium.
Pooled analysis on females in both cohorts
A pooled analysis was performed by combining females in both cohorts. Participants were divided based on the quintiles of mineral intakes in the combined population. The results suggested a quadric relationship between potassium intake and lung cancer risk (Figure 2C). The HR with the second highest (median intake 3,079 mg/day) versus the lowest quintiles (median intake 1,998 mg/day) of potassium intake was 0.75 (95% CI: 0.65–0.86), but with no significant difference for the highest quintile (HR: 0.90, 95% CI: 0.79–1.03) (Table 3). As for sodium intake, although the association was not significant (HR: 1.03, 95% CI: 0.94–1.13), an upward trend was observed at the end of the spline curve (Figure 3C).
Table 3. Hazard ratios and 95% confidence intervals of lung cancer based on categories of dietary potassium intake for female.
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Ptrendc | |
|---|---|---|---|---|---|---|
| Dietary potassium intakea | <2,253.6 | 2,253.6–2,597.5 | 2,597.5–2,906.6 | 2,906.6–3,297.8 | ≥3,297.8 | |
| PLCO (N=48,025) | ||||||
| Cases/person-years | 78/44,846 | 80/60,993 | 108/78,777 | 106/93,023 | 171/113,353 | |
| Multivariable model HRb | 1.00 | 0.87 (0.63, 1.19) | 0.87 (0.65, 1.18) | 0.71 (0.53, 0.97) | 0.87 (0.66, 1.15) | 0.359 |
| WHI (N=72,425) | ||||||
| Cases/person-years | 461/269,115 | 379/256,249 | 342/228,387 | 230/204,040 | 219/165,679 | |
| Multivariable model HRb | 1.00 | 0.94 (0.82, 1.08) | 0.98 (0.85, 1.13) | 0.75 (0.64, 0.89) | 0.90 (0.76, 1.06) | 0.023 |
| ALL (N=120,450) | ||||||
| Cases/person-years | 539/313,961 | 459/317,242 | 450/307,165 | 336/297,063 | 390/279,032 | |
| Multivariable model HRb | 1.00 | 0.93 (0.82, 1.05) | 0.96 (0.84, 1.09) | 0.75 (0.65, 0.86) | 0.90 (0.79, 1.03) | 0.017 |
| Dietary sodium intakea | <2,186.3 | 2,186.3–2,409.5 | 2,409.5–2,610.6 | 2,610.6–2,871.8 | ≥2,871.8 | |
| PLCO (N=48,025) | ||||||
| Cases/person-years | 184/114,533 | 141/103,569 | 101/80,981 | 75/55,696 | 42/36,213 | |
| Multivariable model HRb | 1.00 | 1.01 (0.81, 1.27) | 0.93 (0.72, 1.19) | 0.95 (0.72, 1.25) | 0.76 (0.54, 1.07) | 0.150 |
| WHI (N=72,425) | ||||||
| Cases/person-years | 234/151,282 | 222/175,731 | 333/220,300 | 387/269,553 | 455/306,604 | |
| Multivariable model HRb | 1.00 | 0.91 (0.75, 1.09) | 1.14 (0.96, 1.36) | 1.10 (0.93, 1.30) | 1.12 (0.95, 1.32) | 0.05 |
| ALL (N=120,450) | ||||||
| Cases/person-years | 418/265,815 | 363/279,300 | 434/301,281 | 462/325,249 | 497/342,817 | |
| Multivariable model HRb | 1.00 | 0.92 (0.80, 1.07) | 1.05 (0.91, 1.21) | 1.04 (0.90, 1.19) | 1.04 (0.91, 1.19) | 0.284 |
a, mineral intake was energy-adjusted (mg/day), median intake for continuous and range for quintiles. b, Cox proportional hazard models were used to adjust age, body mass index (kg/m2), energy intake (kcal/day), educational level (3 categories), alcohol consumption (g/day), smoking status (never smokers, former smokers <20 pack-years, former smokers ≥20 pack-years, current smokers <20 pack-years, current smokers ≥20 pack-years), history of diabetes (yes or no), and family history of cancer (yes or no). c, test for linear trend was estimated by assigning the median value of potassium intake in each quintile.
Results from the sensitivity analysis
After additional adjustment for yogurt intake and race, the results were basically consistent with the above (Tables S4,S5). In the sensitivity analysis using calibrated potassium and sodium intake, consistent dose-response relationship was observed in the WHI cohort (data not shown). Besides, we observed that stratified analyses by smoking status or cancer type showed consistent results with the main analysis, although the association is not significant for SCLC patients due to limited sample size (Tables S6-S9).
Discussion
To the best of our knowledge, this was the first study to investigate the association between dietary sodium and potassium intake and lung cancer risk. Based on two prospective cohort studies, the association between mineral intake and lung cancer risk was analyzed systematically in our study. The main findings suggested that appropriate intake of potassium was associated with a reduced risk of lung cancer, but once excessive, the protective effect would be weakened. High-level intake of sodium was related to a higher risk of lung cancer for males. However, no significant correlation was observed for females due to the low overall consumption of sodium.
Principal findings and biological rationale
In the present study, a protective effect against lung cancer risk was observed in females with appropriate high-level intake of potassium in both cohorts. As the spline regression plots shown, the HRs had a quadratic curve correlation with the increase of potassium intake in the PLCO trial, but a linear downward trend in the WHI cohort. A possible explanation for different patterns in the two cohorts maybe related to different levels of potassium intake. Additionally, the median intake for females in the WHI cohort was less than that in the PLCO trial. Thus, the ascending part of the curve could possibly be unobserved due to lack of participants with high-level consumption of potassium.
Emerging evidence suggests that the necrosis of tumor cells can release an intracellular ion and potassium into extracellular fluid. The increased concentration of extracellular potassium leads to increased intracellular potassium within T-cells, which acts to impair T-cells receptor (TCR)-driven Akt-mTOR phosphorylation and effector programs. This can cause a profound suppression of T-cells effector function (21,22). T-cells can recognize the neoantigens, generated by the tumor cells, and then target and kill the cells that may be cancerous. In addition, T-cells also produce chemicals to help regulate the immune response and protect against tumors (23). Lacking of potassium intake is associated with the lower lung function (24). And there are evidences suggesting that potatoes, tomatoes, bananas and yogurt which are all rich in potassium can reduce the incidence of lung cancer effectively (25,26). In combination with the above-mentioned studies, our results indicated that appropriately increasing potassium intake could strengthen the lung function and decrease the incidence of lung cancer. However, once potassium intake exceeded the daily requirement, the concentration of potassium in microenvironment would increase, which could suppress the activity of T-cells and eventually reduce the protective effect on the occurrence of lung cancer.
The biological mechanisms underlying the association between lung cancer risk and intake of sodium remain unclear. Zhang et al. found that high salt intake, a major source of dietary sodium, increased the proinflammatory gene expression of mouse alveolar macrophages and aggravated inflammation in lungs, which was associated with the risk of lung cancer (27). Several cohort studies exhibited that high salt dietary was associated with an increased risk and mortality of lung cancer (28-30). In the present study, an association was found between an increased risk of lung cancer and higher sodium consumption for males in the PLCO trial and a borderline effect in the WHI cohort. An inconsistent borderline effect was also shown for females in the PLCO trial. It was worth noting that the overall energy-adjusted sodium intake of females in the PLCO trial was less than that of females in the WHI cohort and males in the PLCO trial. And females in the PLCO trial, especially those with higher quintiles, had a high proportion of ibuprofen use, which may contribute to lung cancer prevention (31). In addition, it was plausible that some potential lung cancer patients may not be diagnosed due to the shorter follow-up time for the PLCO trial than WHI cohort, with the median follow-up of 8.55 and 18.56 years respectively.
To date, a growing number of studies show the role of dietary mineral intake in the development of cancer. For instance, copper and magnesium can maintain the integrity of DNA by preventing oxidative DNA damage (32,33). Calcium plays an important role in the processes of cell proliferation and carcinogenesis by affecting the cell cycle regulation (34). Minerals participate in various processes of DNA repair, RNA expression, protein synthesis, immune function and so on (21,35-37). In the present study, sodium and potassium, two of the most important minerals in diet, was shown to involve in the cancer genesis.
Lacking of potassium intake and excessive intake of sodium can elevate the blood pressure and the risk of cardiovascular disease, stroke and coronary heart disease (38). A recent study has shown that replacement of regular salt with potassium-enriched substitutes is conductive to reducing the blood pressure (39). The World Health Organization suggests a potassium intake of at least 3,510 mg per day and a reduction of sodium to less than 2,000 mg per day for adults (40). These all recommend a healthy dietary pattern of high potassium and low sodium, further verifying the reliability of our conclusion.
Strengths and limitations of this study
There were several strengths in our study. First, this study was made based on the PLCO trial and the WHI which were large-scale, prospective, cohort studies with a relative complete follow-up information (41). Secondly, the combination of the two large-scale cohorts provided sufficient power to detect the association. Nevertheless, several limitations needed to be noted, including only description of the dietary baseline information, possible residual confounding or confounding by unmeasured factors, and the accuracy of dietary intake measurement.
Conclusions
Our results suggest that appropriate consumption of potassium has a protective effect against lung cancer; high consumption of sodium is related to an increased risk of lung cancer, along with the presence of a dose-response relationship despite the modest magnitude of estimate.
Supplementary
The article’s supplementary files as
Acknowledgments
We gratefully thank all PLCO and WHI participants and staff for their time and commitment to these studies. The funders had no role in study design, collection, analysis, interpretation of data, writing of the report or decision to publish or preparation of the manuscript.
Funding: This work is supported by the National Natural Science Foundation of China (Project No. 81872709 to YZ, 81530088 to FC, 81830100 to ZBH, 81820108028 to H.B. S., 81922061 to HXM); Key Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA110004 to YZ); and the Qing-lan Project of Jiangsu Province and Excellent Young Faculty Program of NMU.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The usage of the lung cancer database in the PLCO and WHI studies was authorized by the ethics committees of the data providers and Nanjing Medical University.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-870
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-870
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-870). The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Mao Y, Yang D, He J, et al. Epidemiology of Lung Cancer. Surg Oncol Clin N Am 2016;25:439-45. 10.1016/j.soc.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 4.Muka T, Kraja B, Ruiter R, et al. Dietary mineral intake and lung cancer risk: the Rotterdam Study. Eur J Nutr 2017;56:1637-46. 10.1007/s00394-016-1210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahabir S, Forman MR, Dong YQ, et al. Mineral intake and lung cancer risk in the NIH-American Association of Retired Persons Diet and Health study. Cancer Epidemiol Biomarkers Prev 2010;19:1976-83. 10.1158/1055-9965.EPI-10-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Park S, Liu G, et al. Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology 2005;16:772-9. 10.1097/01.ede.0000181311.11585.59 [DOI] [PubMed] [Google Scholar]
- 7.Zhuo H, Smith AH, Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev 2004;13:771-8. [PubMed] [Google Scholar]
- 8.Meng Y, Sun J, Yu J, et al. Dietary Intakes of Calcium, Iron, Magnesium, and Potassium Elements and the Risk of Colorectal Cancer: a Meta-Analysis. Biol Trace Elem Res 2019;189:325-35. 10.1007/s12011-018-1474-z [DOI] [PubMed] [Google Scholar]
- 9.Kune GA, Kune S, Watson LF. Dietary sodium and potassium intake and colorectal cancer risk. Nutr Cancer 1989;12:351-9. 10.1080/01635588909514036 [DOI] [PubMed] [Google Scholar]
- 10.Deckers IA, van den Brandt PA, van Engeland M, et al. Long-term dietary sodium, potassium and fluid intake; exploring potential novel risk factors for renal cell cancer in the Netherlands Cohort Study on diet and cancer. Br J Cancer 2014;110:797-801. 10.1038/bjc.2013.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peleteiro B, Barros S, Castro C, et al. Worldwide burden of gastric cancer in 2010 attributable to high sodium intake in 1990 and predicted attributable burden for 2030 based on exposures in 2010. Br J Nutr 2016;116:728-33. 10.1017/S0007114516002518 [DOI] [PubMed] [Google Scholar]
- 12.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273S-309S. 10.1016/S0197-2456(00)00098-2 [DOI] [PubMed] [Google Scholar]
- 13.Xu X. Processed Meat Intake and Bladder Cancer Risk in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cohort. Cancer Epidemiol Biomarkers Prev 2019;28:1993-7. 10.1158/1055-9965.EPI-19-0604 [DOI] [PubMed] [Google Scholar]
- 14.Langer RD, White E, Lewis CE, et al. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13:S107-21. 10.1016/S1047-2797(03)00047-4 [DOI] [PubMed] [Google Scholar]
- 15.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials 1998;19:61-109. 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- 16.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol 2000;152:279-86. 10.1093/aje/152.3.279 [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S-1228S; discussion 1229S-1231S. 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 18.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol 2009;62:511-7. 10.1016/j.jclinepi.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 19.Yang JJ, Yu D, Xiang YB, et al. Association of Dietary Fiber and Yogurt Consumption with Lung Cancer Risk: A Pooled Analysis. JAMA Oncol 2020;6:e194107. 10.1001/jamaoncol.2019.4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Van Horn L, Tinker LF, et al. Measurement error corrected sodium and potassium intake estimation using 24-hour urinary excretion. Hypertension 2014;63:238-44. 10.1161/HYPERTENSIONAHA.113.02218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eil R, Vodnala SK, Clever D, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016;537:539-43. 10.1038/nature19364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vodnala SK, Eil R, Kishton RJ, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019;363:eaau0135. 10.1126/science.aau0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Berhane KT, Li YF, et al. Dietary magnesium, potassium, sodium, and children's lung function. Am J Epidemiol 2002;155:125-31. 10.1093/aje/155.2.125 [DOI] [PubMed] [Google Scholar]
- 25.Ruano-Ravina A, Figueiras A, Dosil-Diaz O, et al. A population-based case-control study on fruit and vegetable intake and lung cancer: a paradox effect? Nutr Cancer 2002;43:47-51. 10.1207/S15327914NC431_5 [DOI] [PubMed] [Google Scholar]
- 26.Agudo A, Esteve MG, Pallares C, et al. Vegetable and fruit intake and the risk of lung cancer in women in Barcelona, Spain. Eur J Cancer 1997;33:1256-61. 10.1016/S0959-8049(97)00050-6 [DOI] [PubMed] [Google Scholar]
- 27.Zhang WC, Zheng XJ, Du LJ, et al. High salt primes a specific activation state of macrophages, M(Na). Cell Res 2015;25:893-910. 10.1038/cr.2015.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suadicani P, Hein HO, Gyntelberg F. ABO phenotypes and inflammation-related predictors of lung cancer mortality: the Copenhagen Male Study - a 16-year follow-up. Eur Respir J 2007;30:13-20. 10.1183/09031936.00062506 [DOI] [PubMed] [Google Scholar]
- 29.Hu J, La Vecchia C, Morrison H, et al. Salt, processed meat and the risk of cancer. Eur J Cancer Prev 2011;20:132-9. 10.1097/CEJ.0b013e3283429e32 [DOI] [PubMed] [Google Scholar]
- 30.Wakai K, Ohno Y, Genka K, et al. Risk modification in lung cancer by a dietary intake of preserved foods and soyfoods: findings from a case-control study in Okinawa, Japan. Lung Cancer 1999;25:147-59. 10.1016/S0169-5002(99)00051-3 [DOI] [PubMed] [Google Scholar]
- 31.Bittoni MA, Carbone DP, Harris RE. Ibuprofen and fatal lung cancer: A brief report of the prospective results from the Third National Health and Nutrition Examination Survey (NHANES III). Mol Clin Oncol 2017;6:917-20. 10.3892/mco.2017.1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med 2005;26:268-98. 10.1016/j.mam.2005.07.015 [DOI] [PubMed] [Google Scholar]
- 33.Mahabir S, Wei Q, Barrera SL, et al. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis 2008;29:949-56. 10.1093/carcin/bgn043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res 2009;29:3687-98. [PubMed] [Google Scholar]
- 35.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem 2004;15:572-8. 10.1016/j.jnutbio.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 36.Beckett EL, Yates Z, Veysey M, et al. The role of vitamins and minerals in modulating the expression of microRNA. Nutr Res Rev 2014;27:94-106. 10.1017/S0954422414000043 [DOI] [PubMed] [Google Scholar]
- 37.Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 2009;12:646-52. 10.1097/MCO.0b013e3283312956 [DOI] [PubMed] [Google Scholar]
- 38.Chmielewski J, Carmody JB. Dietary sodium, dietary potassium, and systolic blood pressure in US adolescents. J Clin Hypertens (Greenwich) 2017;19:904-9. 10.1111/jch.13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernabe-Ortiz A, Sal YRV, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med 2020;26:374-8. 10.1038/s41591-020-0754-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guideline: Potassium Intake for Adults and Children. Geneva: World Health Organization, 2012. [PubMed] [Google Scholar]
- 41.Andriole GL, Levin DL, Crawford ED, et al. Prostate Cancer Screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst 2005;97:433-8. 10.1093/jnci/dji065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as