Abstract
Background
The neutrophil-lymphocyte ratio (NLR) has been recognized as a useful marker of poor prognosis in non-cardiac surgery patients. But, the prognostic function of NLR in cardiovascular surgery patients still largely unknown. The aim of this study was to explore the relationship between postoperative NLR and mortality in cardiac surgery patients.
Methods
Clinical data were extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) database. Postoperative day 1 (POD-1) NLR of the patients was calculated. All patients were divided into two groups according to the cut-off value of NLR, which was determined using the receiver operating characteristic (ROC) curve and Youden Index. The primary death outcomes were 30-day, 90-day and 1-year mortality. Cox proportional hazard analysis was performed to assess the associations between NLR and 1-year mortality. Logistic analysis was performed to assess the associations between NLR and other outcomes. Multivariate analyses were used to control for confounders.
Results
A total of 2,707 cardiac surgery patients were included in this study. The cut-off value of postoperative NLR was 7.28. Elevated postoperative NLR was associated with increased death outcomes including 30-day mortality [adjusted odds ratio (OR) 2.25, P=0.019], 90-day mortality (adjusted OR 2.49, P=0.001) and 1-year mortality [adjusted hazard ratio (HR) 1.58, P=0.03] of cardiac surgery in cox proportional hazard model. Elevated NLR was also associated with increased risk of continuous renal replacement therapy (CRRT) rate (adjusted OR 3.01, P=0.004), prolonged ICU stays (adjusted OR 2.55, P<0.001), prolonged hospital stays (adjusted OR 3.32, P<0.001) and duration of ventilatory support (adjusted OR 4.16, P<0.001) after adjusting confounders.
Conclusions
Elevated postoperative NLR was significantly associated with increased short-term and long-term mortality, CRRT rate, longer ICU and hospital stay, prolonged ventilation in patients undergoing cardiac surgery. NLR is promising to be a readily available and independent prognostic biomarker for patients with cardiac surgery.
Keywords: Cardiac surgery, neutrophil-lymphocyte ratio (NLR), mortality, complications
Introduction
Cardiac surgery with cardiopulmonary bypass (CPB) remains the standard treatment for most valvular heart disease, severe coronary heart disease and severe congenital heart disease. Although the procedure of CPB has been greatly improved, the incidence of complications and mortality of cardiac surgery is still significantly higher than that of non-cardiac surgery (1-3). The inflammatory reaction had been considered as a leading cause of the adverse postoperative outcomes after cardiac surgery (4,5).
Neutrophil-lymphocyte ratio (NLR), an inexpensive and readily available biomarker originated from routine blood examination, has been reported as a useful marker of system inflammation in sepsis, tumor, cardiovascular disease and non-cardiac surgery (6-9). The NLR has also been considered as a prognostic marker of atrial fibrillation, acute kidney injury (AKI), low output syndrome, prolonged mechanical ventilation among cardiac surgery (10-12). However, there was only a few studies about the association between postoperative NLR and outcomes of cardiac surgery, which was still debatable due to favourable and unfavourable results (13-16). The purpose of this study was to evaluate the relationship between NLR and postoperative outcomes in cardiac surgery patients from Medical Information Mart for Intensive Care (MIMIC) database. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2593).
Methods
Sources of data
All data of this retrospective cohort study were extracted from MIMIC-III version 1.4 (MIMIC-III v1.4) database. The MIMIC-III, a large critical care database for publicly and freely use, contains health data for more than 60,000 critical care patients stay at Beth Israel Deaconess Medical Center (BIDMC) (Boston, USA) from 2001 to 2012. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). In order to gain access to the database, the National Institutes of Health’s web-based course “Protecting Human Research Participants” have been completed. This project was both approved by BIDMC and the Institutional Review Boards of Massachusetts Institute of Technology (MIT) (Record ID 37938207) and individual consent for this retrospective analysis was waived.
Data collection and definitions
We used PostgreSQL tool for data extraction. The following data were extracted or calculated: ICU identification number, age, body mass index (BMI), gender, type of cardiac surgery, comorbidities, vital sign, laboratory parameters, continuous renal replacement therapy (CRRT) rate, the time of admission and discharge of hospital and ICU, the day of death, and the time of mechanical ventilation. The comorbidities included hypertension, congestive heart failure (CHF), diabetes, chronic pulmonary diseases, chronic renal disease. The laboratory parameters included white blood cell (WBC) counts, neutrophil and lymphocyte counts, hemoglobin, platelet, sodium, potassium, lactate, creatinine, blood urea nitrogen (BUN), activated partial thromboplastin time (APTT), prothrombin time (PT), anion gap, bicarbonate, chloride and glucose. The vital signs included heart rate, mean arterial pressure (MAP), respiratory rate, temperature and SPO2. The sequential organ failure assessment (SOFA) score was calculated. NLR, which means the ratio of neutrophil count to lymphocyte count, was calculated based on the neutrophil counts and lymphocyte counts on postoperative day 1 (POD-1).
Patients with cardiac surgery were selected. The exclusion criteria were that: missing the neutrophil or lymphocyte count data within the first 24 h of ICU admission, age <18 years old, data missing >5%. The primary outcomes were all-cause 30-day, 90-day and 1-year mortality post-cardiac surgery. Other outcomes were collected, including CRRT rate, length of ICU stays, length of hospital stays and the duration of ventilatory support.
Statistical methods
To evaluate the optimal cut-off value of NLR in predicting 1-year mortality of cardiac surgery, receiver operating characteristic (ROC) curve was performed and Youden Indexes were computed. The highest Youden Index was used to determine the optimal cut-off value of NLR. All study patients were divided into two groups according to the cut-off value of NLR.
Continuous variables were presented as mean ± standard deviation. Categorical variables were presented as frequency and percentage. The differences between two groups were compared using the Mann-Whitney U-test for continuous variables and the χ2 test or Fisher exact test for categorical variables.
Univariable (non-adjusted) and multivariable (adjusted) cox proportional hazards model were performed to assess the association between NLR and the 1-year mortality post-cardiac surgery. Method of forward: Wald was used to select variables in the multivariable cox proportional hazards analysis. The following covariates were adjusted as confounders: cardiac surgery type, age, gender, BMI, comorbidities (hypertension, CHF, diabetes, chronic pulmonary diseases, renal failure), laboratory tests (WBC, platelet, hemoglobin, lactate, creatinine, BUN, APTT, PT, anion gap, bicarbonate, chloride, glucose), vital signs (heart rate, MAP, respiratory rate, temperature, SPO2) and SOFA score. The results of cox proportional hazards analysis were presented as hazard ratios (HRs) with 95% confidence intervals (CIs). The survival curves of cox proportional hazards model were constructed. The 1-year Kaplan-Meier survival curve of two groups were constructed use log rank method. Univariable (non-adjusted) and multivariable (adjusted) logistic regression analysis were performed to investigate the association between NLR and other outcomes using the same method as above. The continuous data including length of ICU stays, length of hospital stays and the duration of ventilatory support were transformed into dichotomous data as ICU stay ≥3 days, hospital stay ≥14 days and ventilation time ≥48 h for the convenience to perform logistic analysis.
SPSS version 26.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Two-tailed P value <0.05 was considered statistically significant.
Results
Subject characteristics
A total of 2,707 cardiac surgery patients were selected in this study. The procedure of data selection and criteria of data exclusion is presented in Figure 1. The AUC of ROC curves of NLR and 1-year postoperative mortality was 0.687 (P<0.001) (Figure 2). The NLR value with the highest Youden Index in this study cohort was 7.28 (https://cdn.amegroups.cn/static/public/JTD-20-2593-1.docx), which was considered as the cut-off value of NLR. According to the cut-off value, all patients were divided into two groups, including high NLR group (NLR ≥7.28, N=974) and low NLR group (NLR <7.28, N=1,733).
Figure 1.
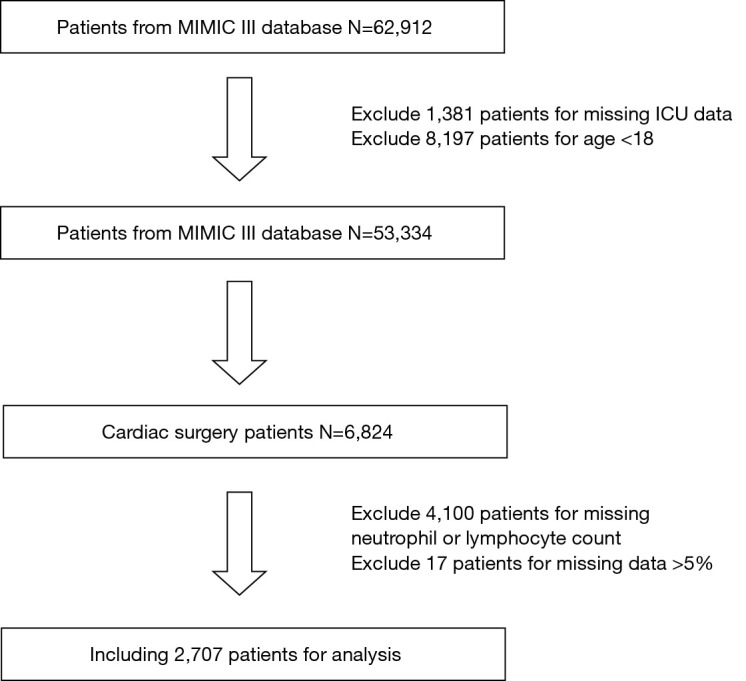
The procedure of data selection and exclusion. MIMIC-III, Medical Information Mart for Intensive Care III.
Figure 2.
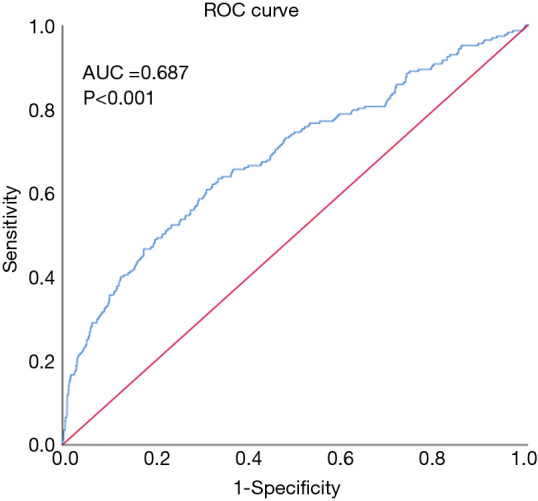
The ROC curves of NLR and 1-year postoperative mortality. ROC, receiver operating characteristic; NLR, neutrophil-lymphocyte ratio.
Baseline characteristics of the subjects are presented in Table 1. Compared with patients in low NLR group, patients in high NLR group tend to be female, older, lower rate of coronary artery bypass graft (CABG), lower BMI, lower rate of hypertension, diabetes, higher rate of CHF, renal failure, higher value of WBC counts, platelet counts, lactate, creatinine, BUN, APTT, PT, anion gap, glucose, heart rate, respiratory rate, lower value of hemoglobin, bicarbonate, chloride, MAP, SPO2, and higher SOFA scores.
Table 1. Baseline and clinical characteristics of the study population.
| Variables | NLR <7.28 (N=1,733) | NLR ≥7.28 (N=974) | P value |
|---|---|---|---|
| NLR | 3.9±1.6 | 17.1±15.3 | <0.001 |
| Cardiac surgery | <0.001 | ||
| CABG | 1,185 (72.3) | 455 (27.7) | |
| Valvular surgery | 317 (50.2) | 315 (49.8) | |
| CABG + valvular surgery | 231 (53.1) | 204 (46.9) | |
| Age, years | 66.5±12.3 | 68.6±12.8 | <0.001 |
| Gender, male | 1,221 (70.5) | 643(66.0) | 0.017 |
| BMI, kg/m2 | 28.9±6.0 | 28.2±6.1 | <0.001 |
| Comorbidities | |||
| Hypertension | 1,282 (74.0) | 619 (63.6) | <0.001 |
| Congestive heart failure | 551 (31.8) | 487 (50.0) | <0.001 |
| Diabetes | 625 (36.1) | 294 (30.2) | 0.002 |
| Chronic pulmonary diseases | 256 (14.8) | 154 (15.8) | 0.468 |
| Renal failure | 170 (9.8) | 157 (16.1) | <0.001 |
| Laboratory tests | |||
| WBC, 109/L | 14.4±6.6 | 16.3±7.2 | <0.001 |
| Platelet, 109/L | 210.9±73.0 | 227.1±97.0 | 0.001 |
| Hemoglobin, g/dL | 12.6±1.6 | 12.1±1.7 | <0.001 |
| Lactate, mmol/L | 3.0±1.6 | 3.8±2.9 | <0.001 |
| Creatinine, mg/dL | 1.2±1.0 | 1.5±1.4 | <0.001 |
| BUN, mg/dL | 19.7±10.9 | 25.8±16.3 | <0.001 |
| APTT, s | 49.9±27.2 | 56.9±33.5 | <0.001 |
| PT, s | 16.0±2.8 | 17.1±6.6 | <0.001 |
| Anion gap, mmol/L | 12.8±2.9 | 14.34±3.7 | <0.001 |
| Bicarbonate, mmol/L | 25.1±2.7 | 25±3.2 | <0.001 |
| Chloride, mmol/L | 109.3±4.3 | 108.2±5.3 | <0.001 |
| Sodium, mmol/L | 139.4±2.8 | 139.6±3.7 | 0.171 |
| Potassium, mmol/L | 5.4±0.8 | 5.4±0.9 | 0.104 |
| Glucose, mg/dL | 131.9±23.3 | 136.7±29.5 | <0.001 |
| Vital signs | |||
| Heart rate, beats/min | 84.7±10.4 | 86.3±11.7 | 0.003 |
| MAP, mmHg | 75.4±7.0 | 74.0±7.7 | <0.001 |
| Respiratory rate, beats/min | 17.2±2.8 | 17.9±3.6 | <0.001 |
| Temperature, °C | 36.9±0.5 | 36.9±0.6 | 0.945 |
| SPO2, % | 97.9±1.3 | 97.7±1.9 | 0.026 |
| SOFA score | 4.8±2.6 | 5.6±3.1 | <0.001 |
Values are expressed as mean ± SD or n (%). NLR, neutrophil-lymphocyte ratio; CABG, coronary artery bypass graft; BMI, body mass index; WBC, white blood cell; APTT, activated partial thromboplastin time; PT, prothrombin time; MAP, mean arterial pressure; SOFA, sequential organ failure assessment; SD, standard deviation.
Association between NLR and postoperative mortality and other outcomes
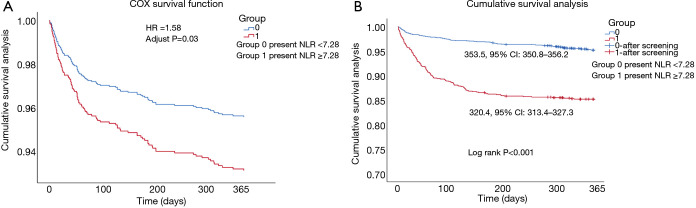
The postoperative mortality and other outcomes between two groups were presented in Table 2. The high NLR group had significant higher 30-day mortality rate (1.6% vs. 5.9%, P<0.001), 90-day mortality rate (2.5% vs. 11.1%, P<0.001) and 1-year mortality rate (4.8% vs. 14.8%, P<0.001). In both univariable and multivariable cox proportional hazards models (Table 3), higher NLR was associated with increased risk of 1-year mortality (adjusted HR 1.58, 95% CI: 1.05–2.38, P=0.030). The 1-year survival curves of cox proportional hazards model were presented in Figure 3A. The 1-year Kaplan-Meier survival curve (Figure 3B) of two groups showed the higher NLR patients had a lower mean survival time (320.4, 95% CI: 313.4–327.3 vs. 353.5, 95% CI: 350.8–356.2, log rank P<0.001). In both univariable and multivariable logistic regression model, elevated NLR was significantly increased the risk of 30-day mortality [adjusted odds ratio (OR) 2.25, 95% CI: 1.14–4.43, P=0.019], 90-day mortality (adjusted OR 2.49, 95% CI: 1.44–4.28, P=0.001) (Table 4).
Table 2. Clinical outcomes between study cohorts.
| Outcomes | NLR <7.28 (N=1,733) | NLR ≥7.28 (N=974) | P value |
|---|---|---|---|
| First outcome | |||
| 30-day mortality | 28 (1.6) | 57 (5.9) | <0.001 |
| 90-day mortality | 43 (2.5) | 108 (11.1) | <0.001 |
| One-year mortality | 83 (4.8) | 144 (14.8) | <0.001 |
| Second outcome | |||
| CRRT | 22 (1.3) | 84 (8.6) | <0.001 |
| Length of ICU stay, days | 3.6±4.1 | 9.5±13.2 | <0.001 |
| Length of hospital stay, days | 10.7±6.4 | 18.3±14.4 | <0.001 |
| Length of ventilation time, hours | 24.9±65.3 | 121.9±258.3 | <0.001 |
| ICU stay ≥3 days | 660 (39.1) | 643 (67.4) | <0.001 |
| Hospital stay ≥14 days | 348 (20.2) | 493 (51.1) | <0.001 |
| Ventilation time ≥48 hours | 157 (9.5) | 325 (35.6) | <0.001 |
Values are expressed as mean ± SD or n (%). NLR, neutrophil-lymphocyte ratio; CRRT, continuous renal replacement therapy; SD, standard deviation.
Table 3. The cox regression model of NLR and mortality.
| Outcomes | Non-adjusted model | Adjusted model | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| 30-day mortality | 3.70 (2.35, 5.81) | <0.001 | 2.18 (1.15, 4.14) | 0.017 | |
| 90-day mortality | 4.66 (3.27, 6.64) | <0.001 | 2.21 (1.33, 3.69) | 0.002 | |
| One-year mortality | 3.29 (2.51, 4.32) | <0.001 | 1.58 (1.05, 2.38) | 0.030 | |
Adjusted for the confounders: cardiac surgery type, age, gender, BMI, comorbidities (hypertension, congestive heart failure, diabetes, chronic pulmonary diseases, renal failure), laboratory tests (WBC, platelet, hemoglobin, lactate, creatinine, BUN, APTT, PT, anion gap, bicarbonate, chloride, glucose), vital signs (heart rate, MAP, respiratory rate, temperature, SPO2), SOFA score. NLR, neutrophil-lymphocyte ratio; HR, hazard ratio; CI, confidence interval; BMI, body mass index; WBC, white blood cell; BUN, blood urea nitrogen; APTT, activated partial thromboplastin time; PT, prothrombin time; MAP, mean arterial pressure; SOFA, sequential organ failure.
Figure 3.
The survival curves of cox function analysis and Kaplan-Meier survival analysis. (A) The 1-year survival curves of cox function analysis between two NLR groups; (B) the 1-year Kaplan-Meier survival curve of two groups. Group 0 present NLR <7.28, group 1 present NLR ≥7.28. NLR, neutrophil-lymphocyte ratio.
Table 4. The logistics regression of NLR and other clinical outcomes.
| Outcomes | Non-adjusted model | Adjusted model | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| 30-day mortality | 3.79 (2.40, 5.99) | <0.001 | 2.25 (1.14, 4.43) | 0.019 | |
| 90-day mortality | 4.90 (3.41, 7.04) | <0.001 | 2.49 (1.44, 4.28) | 0.001 | |
| CRRT | 7.34 (4.56, 11.82) | <0.001 | 3.01 (1.41, 6.42) | 0.004 | |
| ICU stay ≥3 days | 3.22 (2.72, 3.80) | <0.001 | 2.55 (1.61, 4.03) | <0.001 | |
| Hospital stay ≥14 days | 4.13 (3.48, 4.91) | <0.001 | 3.32 (2.45, 4.49) | <0.001 | |
| Ventilation time ≥48 hours | 5.24 (4.24, 6.49) | <0.001 | 4.16 (2.93, 5.89) | <0.001 | |
Adjusted for the confounders: cardiac surgery type, age, gender, BMI, comorbidities (hypertension, congestive heart failure, diabetes, chronic pulmonary diseases, renal failure), laboratory tests (WBC, platelet, hemoglobin, lactate, creatinine, BUN, APTT, PT, anion gap, bicarbonate, chloride, glucose), vital signs (heart rate, MAP, respiratory rate, temperature, SPO2), SOFA score. NLR, neutrophil-lymphocyte ratio; OR, odds ratio; CI, confidence interval; CRRT, continuous renal replacement therapy; BMI, body mass index; WBC, white blood cell; BUN, blood urea nitrogen; APTT, activated partial thromboplastin time; PT, prothrombin time; MAP, mean arterial pressure; SOFA, sequential organ failure.
The CRRT rate, length of ICU stays, length of hospital stays and the duration of ventilatory support were significantly increased in the high NLR group (Table 2). The rates of length of ICU stay ≥3 days, length of hospital stay ≥14 days and length of ventilation time ≥48 h were also higher in the high NLR group (Table 2). In both univariable (non-adjusted) and multivariable (adjusted) logistic regression model, elevated NLR was significantly increased the risk of CRRT rate (adjusted OR 3.01, P=0.004), ICU stay ≥3 days (adjusted OR 2.55, 95% CI: 1.61–4.03, P<0.001), hospital stay ≥14 days (adjusted OR 3.32, 95% CI: 2.45–4.49, P<0.001) and ventilation time ≥48 h (adjusted OR 4.16, 95% CI: 2.93–5.89, P<0.001) (Table 4).
Discussion
Inflammation plays an important part in the pathogenesis of complications related to cardiac surgery. NLR, a readily available and effective biomarker originated from routine blood examination, is an integrated biomarker of inflammation (6,7). NLR have emerged as a potential biomarker for predictor lethal outcomes and adverse events of patients with cardiac surgery.
In this large retrospective study, we investigated the relationship between NLR and short-term (30 days), medium-term (90 days) and long-term (1 year) mortality of cardiac surgery. The principle finding of the study was that the POD-1 NLR with the cut-off value of 7.28 was an independent predictor for short-term and long-term death, high risk of CRRT rate, longer ICU and hospital stay, prolonged ventilation in patients undergoing cardiac surgery.
The result of our study consistent with the current research for the association between NLR and mortality and complications post-cardiac surgery. A study by Kim et al. in 2015 assessed 600 adult patients underwent CPB surgery in their institution. They found that immediately elevated postoperative NLR with the cut-off value 10 was associated with development of AKI, prolonged hospital stays, and increased in-hospital death and 1-year death in the multivariate logistic regression analysis (17). A study of 906 cardiac surgery patients by Weedle et al. in 2019 investigated the association between postoperative complications and preoperative, POD-1 and POD-2 NLR, which found that elevated postoperative NLR was associated with postoperative atrial fibrillation and AKI (18). According to a large retrospective study by Silberman et al. including 3,027 cardiac surgery patients, preoperative NLR >2.6 was an independent predictor of operative mortality and late mortality, pleural effusion, low output syndrome, and prolonged ventilation among post-cardiac surgery (10). Recent literature of Gurbuz et al. reported the 7.8-year follow-up results. In this 751-CABG-patient study, the elevated NLR with the cut-off value of 4.32 was associated with long-term adverse cardiac events (19).
NLR reflects the relationship between neutrophil and lymphocyte of WBC count and considered as a valuable indicator of system inflammatory response. It has been well documented that the cardiac surgery with CPB was closely related with system inflammatory response. System inflammatory response plays an important role in multiple organ dysfunction syndrome (MODS) and immune system suppression post-cardiac surgery (4,20). Previous report has reported that the incidence rate of MODS post-cardiac surgery with CPB was 11% and the death rate of these patients was about 41% which was significantly higher than non-MODS patients (21). In our study, patients with elevated NLR had higher SOFA scores (4.8±2.6 vs. 5.6±3.1, P<0.001) and higher lactate level (3.0±1.6 vs. 3.8±2.9 mmol/L, P<0.001), which indicated that higher NLR reflect more serious organ dysfunction. The lung was the most vulnerable organ in the process of inflammatory reaction of CPB. The incidence of lung injury, including impaired oxygenation index, reduced lung compliance, increased ventilation/perfusion mismatch, was up to 12% after CPB (22). The results of present study had shown that the ventilation time was significantly increased in high NLR group (24.9±65.3 vs. 121.9±258.3 h, P<0.001), and NLR ≥7.28 increased the risk of ventilation time ≥48 h by 4.16 folds (P<0.001). This results manifest elevated NLR was related to sever lung damage and prolonged ventilation support. Kidney was another vulnerable organ during CPB procedure, the postoperative acute kidney injure rate was up to 55% and might cause 60% mortality among cardiac surgery patients (23). Previous studies have confirmed that the inflammation play a critical role in the development of AKI after cardiac surgery (24,25). The creatinine and BUN level, and CRRT rate were significantly increased in the elevated NLR patients of our study, NLR >7.28 increased the risk of CRRT by 3 folds (P=0.004). The system inflammatory reaction also contributes to other poor outcomes after cardiac surgery including cardiovascular complications, dysfunction of neural system, postoperative infection, coagulation disorder and death (26,27). The NLR was a valuable predictor that associate systemic inflammation and postoperative complications of cardiac surgery according to previous and present studies.
In current, risk stratification in cardiac surgery is dependent on clinical assessment or predictive models rather than common biomarkers. The NLR is an inexpensive and easily acquired parameter generated from blood routine, so NLR can be widely used as an effective clinical biomarker to enhance patient risk stratification after cardiac surgery. This prognostic utility of NLR is independent of other well-recognized individual risk factors and the EuroSCORE.
Limitation
There are several limitations in this study. First, this is a single center retrospective study based on MIMIC-III database, so selection bias is unavoidable existed. Secondly, some important clinical information may influence the final results, such as preoperative left ventricular ejection fraction, duration of CPB, and infection were missing. Thirdly, our study only evaluated the relationship between NLR and poor prognosis of cardiac surgery, but was not look into the possible mechanisms of the association. Further study should be performed to explain the immunological and pathophysiological mechanism of the association between NLR, systemic inflammation and clinical complications in cardiac surgery. Finally, we only investigated POD-1 NLR in this study, without evaluating the dynamic evolution of NLR during the whole perioperative period, which could helpful to get a comprehensive result. A further investigation is necessary to explore the relationship between the dynamic changes of NLR and mortality and morbidity of cardiac surgery in the future.
Conclusions
The results of this large retrospective study based on MIMIC-III database showed that elevated postoperative NLR with a cut-off value of 7.28 was an independent predictor for mortality and adverse events in patients undergoing cardiac surgery. The data of our study enhanced the evidence that NLR could be considered as a useful cardiac surgery risk stratification biomarker n the future.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81573234 and 81773445) and “333” Project of Jiangsu Province (No. LGY2016006).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This project was both approved by BIDMC and the institutional review boards of Massachusetts Institute of Technology (MIT) (Record ID 37938207) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2593
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2593
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2593). The authors have no conflicts of interest to declare.
References
- 1.Senst B, Kumar A, Diaz RR. Cardiac Surgery. Treasure Island (FL): StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 2.Krawczeski CD. Cardiopulmonary Bypass and AKI: AKI Is Bad, So Let's Get Beyond the Diagnosis. Front Pediatr 2019;7:492. 10.3389/fped.2019.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranucci M, Baryshnikova E. Inflammation and coagulation following minimally invasive extracorporeal circulation technologies. J Thorac Dis 2019;11:S1480-8. 10.21037/jtd.2019.01.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squiccimarro E, Labriola C, Malvindi PG, et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J Cardiothorac Vasc Anesth 2019;33:1682-90. 10.1053/j.jvca.2019.01.043 [DOI] [PubMed] [Google Scholar]
- 5.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 2002;97:215-52. 10.1097/00000542-200207000-00030 [DOI] [PubMed] [Google Scholar]
- 6.Najjar M, Agrawal S, Emond JC, et al. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma 2018;5:17-28. 10.2147/JHC.S86792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balta S, Celik T, Mikhailidis DP, et al. The Relation Between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin Appl Thromb Hemost 2016;22:405-11. 10.1177/1076029615569568 [DOI] [PubMed] [Google Scholar]
- 8.Kong W, He Y, Bao H, et al. Diagnostic Value of Neutrophil-Lymphocyte Ratio for Predicting the Severity of Acute Pancreatitis: A Meta-Analysis. Dis Markers 2020;2020:9731854. 10.1155/2020/9731854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Silva M, Cleghorn MC, Elnahas A, et al. Postoperative day one neutrophil-to-lymphocyte ratio as a predictor of 30-day outcomes in bariatric surgery patients. Surg Endosc 2017;31:2645-50. 10.1007/s00464-016-5278-y [DOI] [PubMed] [Google Scholar]
- 10.Silberman S, Abu-Yunis U, Tauber R, et al. Neutrophil-Lymphocyte Ratio: Prognostic Impact in Heart Surgery. Early Outcomes and Late Survival. Ann Thorac Surg 2018;105:581-6. 10.1016/j.athoracsur.2017.07.033 [DOI] [PubMed] [Google Scholar]
- 11.Iliopoulos I, Alder M, Cooper D, et al. Pre-operative neutrophil-lymphocyte ratio predicts low cardiac output in children after cardiac surgery. Cardiol Young 2020;30:521-5. 10.1017/S1047951120000487 [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Nguyen Khuong J, Borg Caruana C, et al. The Prognostic Value of Elevated Perioperative Neutrophil-Lymphocyte Ratio in Predicting Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Heart Lung Circ 2020;29:1015-24. 10.1016/j.hlc.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Sun Y, Zhang S. The Relationship Between Neutrophil to Lymphocyte Ratio and Clinical Outcome in Pediatric Patients After Cardiopulmonary Bypass Surgery: A Retrospective Study. Front Pediatr 2019;7:308. 10.3389/fped.2019.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaoud E, Mohamed A, Elkot MA. The Role of the Platelet/Lymphocyte Ratio and Neutrophil/Lymphocyte Ratio in Predicting High-Risk Heart Score in Patients Admitted with Non-ST Elevation Acute Coronary Syndrome. Pulse (Basel) 2020;8:66-74. 10.1159/000508592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob KA, Buijsrogge MP, Frencken JF, et al. White blood cell count and new-onset atrial fibrillation after cardiac surgery. Int J Cardiol 2017;228:971-6. 10.1016/j.ijcard.2016.11.038 [DOI] [PubMed] [Google Scholar]
- 16.Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 2010;105:186-91. 10.1016/j.amjcard.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Kim WH, Park JY, Ok SH, et al. Association Between the Neutrophil/Lymphocyte Ratio and Acute Kidney Injury After Cardiovascular Surgery: A Retrospective Observational Study. Medicine 2015;94:e1867. 10.1097/MD.0000000000001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weedle RC, Da Costa M, Veerasingam D, et al. The use of neutrophil lymphocyte ratio to predict complications post cardiac surgery. Ann Transl Med 2019;7:778. 10.21037/atm.2019.11.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurbuz O, Kumtepe G, Ozkan H, et al. Predictive Value of Neutrophil-Lymphocyte Ratio for Long-Term Cardiovascular Event Following Coronary Artery Bypass Grafting. Braz J Cardiovasc Surg 2020;35:274-84. 10.21470/1678-9741-2018-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corral-Velez V, Lopez-Delgado JC, Betancur-Zambrano NL, et al. The inflammatory response in cardiac surgery: an overview of the pathophysiology and clinical implications. Inflamm Allergy Drug Targets 2015;13:367-70. 10.2174/1871528114666150529120801 [DOI] [PubMed] [Google Scholar]
- 21.Kollef MH, Wragge T, Pasque C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest 1995;107:1395-401. 10.1378/chest.107.5.1395 [DOI] [PubMed] [Google Scholar]
- 22.Rong LQ, Di Franco A, Gaudino M. Acute respiratory distress syndrome after cardiac surgery. J Thorac Dis 2016;8:E1177-86. 10.21037/jtd.2016.10.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobson CE, Yavas S, Segal M, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444-53. 10.1161/CIRCULATIONAHA.108.800011 [DOI] [PubMed] [Google Scholar]
- 24.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 2004;66:480-5. 10.1111/j.1523-1755.2004.761_2.x [DOI] [PubMed] [Google Scholar]
- 25.Kim WH, Park J, Ok S, et al. Association Between the Neutrophil/Lymphocyte Ratio and Acute Kidney Injury After Cardiovascular Surgery: A Retrospective Observational Study. Medicine 2015;94:e1867. 10.1097/MD.0000000000001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thudium M, Heinze I, Ellerkmann RK, et al. Cerebral Function and Perfusion during Cardiopulmonary Bypass: A Plea for a Multimodal Monitoring Approach. Heart Surg Forum 2018;21:E028-E035. 10.1532/hsf.1894 [DOI] [PubMed] [Google Scholar]
- 27.Khuri SF, Wolfe JA, Josa M, et al. Hematologic changes during and after cardiopulmonary bypass and their relationship to the bleeding time and nonsurgical blood loss. J Thorac Cardiovasc Surg 1992;104:94-107. 10.1016/S0022-5223(19)34841-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as