Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality and is characterized by episodes of acute exacerbations. Finding a systemic biomarker that reliably predicts outcome after an acute exacerbation remains a major challenge. Heat shock protein 27 (HSP27) has been previously studied in COPD, however, urine excretion trajectory and prognostic value after an exacerbation is unknown.
Methods
In this retrospective post hoc analysis of a prospective study that included 253 COPD patients who were hospitalized for acute exacerbation, 207 patients were analyzed. Urine and serum were sampled at admission, discharge, and 180 days after discharge; urine excretion trajectory was analyzed and correlated with clinicopathological and survival data.
Results
HSP27 urine excretion increased after an exacerbation episode [1.8% admission, 1.8% discharge, 2.3% 180 days after discharge (P=0.091)]. In severely ill patients (GOLD IV) this course was even more distinct [1.6% admission, 2.1% discharge, 2.8% 180 days after discharge (P=0.007)]. Furthermore, fractional HSP27 urine excretion at discharge was increased in GOLD IV patients (P=0.031). In Kaplan-Meier and univariable Cox proportional hazard models patients with HSP27 urine excretion below 0.845% showed significantly worse survival at 30, 90 and 180 days after discharge. In a multivariable Cox proportional hazard model including established COPD outcome parameters fractional HSP27 urine excretion remained a significant predictor of survival at 30 and 90 days after discharge. Comparing this model to our already published model that includes HSP27 serum concentration we could show that fractional HSP27 urine excretion performs better in short-term survival.
Conclusions
Our findings provide novel information about fractional HSP27 urine excretion trajectory in acute exacerbation of COPD. Fractional HSP27 urine excretion may be significantly reduced during an episode of acute exacerbation in COPD patients and may be used as a predictor of short-term all-cause mortality.
Keywords: Biomarkers, heat shock protein 27 heat-shock proteins (HSP27 heat-shock proteins), pulmonary disease, chronic obstructive, urine
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death worldwide (1). It is characterized by acute exacerbations (AECOPD) that significantly affect morbidity and mortality both short- and long-term after an exacerbation episode (2). AECOPD, defined as acute worsening of respiratory symptoms associated with physiological deterioration beyond day-to-day variations, is an event in the natural disease progression (3). Hitherto COPD has been understood as a pulmonary disease that is characterized by structural abnormalities of airways and pulmonary parenchyma. However, recent studies indicate systemic inflammatory reactions to be associated with these pathologies. T cell mediated autoimmunity has been suggested in COPD patients. An increased number of CD28nullCD8+ and CD28nullCD4+ has been reported in COPD and this T cell senescence is associated with the loss of HSP90 (4-6). As a consequence, this immune activation can also be detected systemically by elevation of inflammatory mediators (7).
Diagnosis and monitoring of COPD patients is mainly performed using spirometry, however, in acute exacerbation this method may not be recommended as it is too difficult to perform (8). Apart from a variety of clinical parameters (9-11) several blood biomarkers e.g., procalcitonin (12), CRP (13), uric acid (14), cardiac troponin T (cTnT) and N-terminal pro-brain natriuretic protein (NT-proBNP) (15) have been studied in AECOPD. Finding a systemic marker that offers a prognostic value is of enormous importance as identifying COPD patients at high risk of poor outcome could help intensifying interventions to improve quality of life and reduce morbidity in those patients. However, identifying such a biomarker is difficult, as no gold standard to definitively clarify its value has been established, yet.
Heat shock proteins (HSPs) are a group of highly preserved stress proteins that are usually found intracellularly. As part of the intracellular stress response HSPs are released from cells to interact with adjacent cells or may enter the blood stream. They are directly involved in chaperoning and regulating cell death pathways (16). Heat shock protein 27 (HSP27) is a member of the small HSP family with a molecular weight of approximately 27 kDa. It is involved in a multitude of cellular functions such as stress tolerance, protein degradation and cell death (17). It further provides protective mechanisms against oxidative-inflammatory conditions (18). Extracellular HSP27 can act anti-inflammatory by increasing the level of the anti-inflammatory cytokine interleukin-10 and attenuating migration and adhesion of macrophages (19,20). It is thought to exert an important role in many different conditions such as cancer and cardiovascular disease (21,22). Its role as a diagnostic and prognostic marker in COPD has already been studied extensively with partly contradictory results (23-28).
As a consequence of observations on the expression of HSP27 in COPD a possible role of this protein as a useful marker also in episodes of acute exacerbation is suggested as HSP27 is released from PBMCs (29). Previous work on HSP27 in serum and plasma included only a small set of patients. Recently published data showed increased serum concentration during hospitalization for an acute exacerbation, further elevated serum HSP27 was associated with worse survival (28). Nonetheless no data are available on fractional HSP27 urine excretion in AECOPD. We hypothesize that fractional HSP27 urine excretion might provide prognostic value in those patients. To test this hypothesis, we performed serum and urine measurements and correlated them with clinicopathological and survival data.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3683).
Methods
Patient population
The study has been approved by the National Ethics Committee of the Republic of Slovenia (EC number: 65/02/09), was conducted according to the declaration of Helsinki (as revised in 2013) and is registered at ClinicalTrials.gov (NCT01225627). Informed consent was provided by all participants. Study protocol and main findings were published previously (30,31). In brief, 253 patients that were admitted for AECOPD between 2009 and 2011 at the University Clinic of Pulmonary and Allergic Diseases Golnik, Slovenia were prospectively enrolled. After informed consent was given, patients were screened for inclusion and exclusion criteria as described previously (30) and enrolled according to their eligibility. Blood and urine samples were collected at admission, discharge, and 180 days after discharge. A post hoc analysis of 207 patients was performed, as samples were not available for 28 patients and 18 patients were excluded from the final analyses due to missing values. HSP27 serum levels have recently been analyzed in this patient cohort (28), in this re-analysis four patients were excluded due to missing urine samples. 3-year-mortality data (all cause) were available for all patients. Blood was taken at admission for full blood count, CRP, cTnT, NT-proBNP and other measurements as per good clinical practice.
Quantification of urine HSP27
A commercially available ELISA Kit (DYC1580, R&D Systems, Minneapolis, MN, USA) was used to assess serum and urine total HSP27 concentrations according to the manufacturer’s protocol. This kit was previously compared to other commercial kits and showed superior performance of diagnostic accuracy in diagnosis of pathologies of the lung (32). Inter- and intraassay variability for urine samples of this ELISA kit has been previously assessed (33).
Fractional HSP27 excretion was calculated as previously described (33) with the formula: [(urine HSP27 × serum creatinine)/(serum HSP27 × urine creatinine) ×100 to eliminate varying water excretion and resorption as confounding factors as well as to compensate the influence of comprised renal capillary endothelial permeability during exacerbation (34).
HSP27 cut off determination
Cut off values of HSP27 urine excretion at admission for Kaplan-Meier curve analysis and Cox proportional hazard models for all cause mortality calculations were identified using Cutoff Finder version 2.1, a freely available R functions based web application (35).
Statistics
Data obtained were evaluated statistically using IBM SPSS Statistics version 23 (SPSS Inc., Chicago, USA) and GraphPad Prism 6 software (GraphPad Software Inc., LA Jolla, CA, USA). Mann-Whitney U test and Kruskal-Wallis test with each pairwise P value correction were used to compare non-parametric, unpaired variables and Friedman test with each pairwise P value correction was used to compare non-parametric paired variables and expressed as median and interquartile range (IQR). Kaplan-Meier curves and log-rank test were used to evaluate event-free (time to death) interval. Cox proportional hazard models were built to evaluate prognostic significance of established factors and fractional HSP27 urine excretion at 30 days, 90 days, 180 days, 1 year and 3 years and were expressed as hazard ratio (HR) with corresponding 95% confidence intervals (CI). As this is an explorative reanalysis of an already published dataset, we aimed to compare this model to the published one (HSP27 serum concentration as a prognostic predictor) (28). Harrell’s c-Index was assessed for both models at all above given time points. Binary logistic regression was performed to obtain predicted probability values of the models and ROC curves with those values were plotted. Area under the curve (AUC) was used to compare both models at all time points. All tests were performed in a two-sided manner. P values equal or below 0.05 were considered as statistically significant.
Results
Study population
All 207 patients were included into this explorative re-analysis. HSP27 urine excretion values were available at admission of 207 patients, at discharge of 189 patients and at 180 days after discharge of 107 patients. A summary of clinicopathological parameters of all included patients from admission is depicted in Table 1. Patients are predominately male (72%) and suffered from advanced COPD (GOLD II–IV). Median age was 72 years (IQR: 64–77). All patients received COPD specific optimal medical therapy. Follow up time was 1095 days, the number of deaths at 30 days after discharge was 7 (3.4%), 13 (6.3%) at 90 days, 25 (12.1%) at 180 days, 70 (33.8%) at 2 years and 98 (47.3%) at 3 years.
Table 1. Patients’ baseline demographic and laboratory values at admission.
| Baseline | All patients, n=207 | HSP27 high (>0.854%), n=169 | HSP27 low (≤0.854%), n=38 | P value | Survivors at 180 days, n=186 | Decedents at 180 days, n=26 | P value |
|---|---|---|---|---|---|---|---|
| Gender (male/female) | 148 (72%)/59 (28%) | 113 (70%)/56 (33%) | 35 (92%)/3 (8%) | 0.002 | 130 (71%)/52 (29%) | 18 (72%)/7 (28%) | 0.953 |
| Age (years; median ± IQR) | 72.4 (63.5–77.3) | 71.9 (63.4–77.1) | 74.4 (66.0–78.4) | 0.284 | 71.2 (62.8–76.9) | 76.1 (71.2–81.2) | 0.009 |
| GOLD | 0.491 | 0.064 | |||||
| GOLD II | 25 (12%) | 22 (13%) | 3 (8%) | 24 (13%) | 1 (4%) | ||
| GOLD III | 93 (45%) | 73 (43%) | 20 (53%) | 85 (47%) | 8 (32%) | ||
| GOLD IV | 89 (43%) | 74 (44%) | 15 (39%) | 73 (40%) | 16 (64%) | ||
| BMI (kg/m2; median ± IQR) | 25.7 (23.3–30.4) | 25.0 (23.3–30.3) | 28.0 (23.2–32.5) | 0.401 | 25.8 (23.4–30.4) | 24.2 (21.2–30.0) | 0.252 |
| Tiffeneau index (%) | 41 (32.0–52.0) | 41 (33.0–53.0) | 38 (30.0–50.0) | 0.364 | 41 (32.0–51.0) | 42 (30–54) | 0.748 |
| LTOT (%) | 46 (22%) | 39 (23%) | 7 (18%) | 0.533 | 38 (21%) | 8 (32%) | 0.210 |
| Comorbidities | |||||||
| Heart failure | 54 (26%) | 43 (25%) | 11 (29%) | 0.657 | 43 (24%) | 11 (44%) | 0.030 |
| Arterial fibrillation | 19 (16%) | 14 (16%) | 5 (16%) | 0.985 | 17 (17%) | 2 (13%) | 0.662 |
| Ischemic heart disease | 17 (15%) | 13 (15%) | 4 (13%) | 0.764 | 14 (14%) | 3 (19%) | 0.606 |
| Arterial hypertension | 51 (44%) | 35 (41%) | 5 (16%) | 0.293 | 44 (44%) | 7 (44%) | 0.989 |
| Diabetes mellitus | 27 (23%) | 20 (23%) | 7 (23%) | 0.939 | 23 (23%) | 4 (25%) | 0.844 |
| CRP (mg/L, median ± IQR) | 23.1 (3.8–77.2) | 22.3 (4.2–87.6) | 32.7 (3.4–67.3) | 0.988 | 21.5 (3.7–75.6) | 37.9 (14.0–77.2) | 0.312 |
| cTnT (ng/L, median ± IQR) | 0.0 (0.0–0.017) | 0.0 (0.0–0.015) | 0.0 (0.0–0.029) | 0.854 | 0.0 (0.0–0.015) | 0.0 (0.0–0.03) | 0.140 |
| NT-proBNP (pg/mL, median ± IQR) | 471.2 (138.7–1,692.0) | 489.5 (132.8–1,643.0) | 451.9 (171.0–2,378.0) | 0.472 | 363.4 (120.0–1,493.0) | 1323.0 (471.2–3789.0) | 0.002 |
| Serum creatinine-admission (mg/dL, median ± IQR) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.9 (0.8–1.0) | 0.710 | 0.9 (0.7–1.1) | 0.8 (0.6–1.1) | 0.525 |
| Urine creatinine-admission (mg/dL, median ± IQR) | 72.9 (32.5–128.4) | 63.3 (29.3–114.4) | 130.4 (100.2–198.4) | <0.001 | 71.3 (32.5–125.8) | 90.5 (33.4–132.4) | 0.748 |
| Urea–admission (mmol/L, median ± IQR) | 6.9 (5.3–9.7) | 6.7 (5.3–9.7) | 7.35 (5.4–9.6) | 0.613 | 6.7 (5.3–9.7) | 7.5 (5.7–9.2) | 0.429 |
| eGFR (CDK-EPI) (mL/min/1.73 m2, median ± IQR) | 81.7 (65.2–93.5) | 81.7 (64.9–93.6) | 83.1 (72.5–91.3) | 0.767 | 82.3 (66.8–93.3) | 76.0 (63.6–93.5) | 0.667 |
| HSP27 urine excretion: admission (%, median ± IQR) | 1.8 (1.0–4.1) | 2.2 (1.3–5.1) | 0.6 (0.4–0.7) | <0.001 | 1.8 (1.0–4.4) | 1.6 (0.8–2.4) | 0.145 |
| HSP27 urine excretion: discharge (%, median ± IQR) | 1.8 (1.1–3.9) | 2.0 (1.2–4.7) | 1.2 (0.8–1.9) | 0.001 | 1.8 (1.1–3.9) | 1.7 (1.1–3.2) | 0.606 |
| HSP27 urine excretion: 180 days after discharge (%, median ± IQR) | 2.3 (1.4–4.7) | 2.7 (1.6–5.3) | 0.9 (0.7–1.8) | 0.000 | 2.3 (1.4–4.7) | – | – |
| HSP27 serum: admission (pg/mL, median ± IQR) | 2,204.0 (1,545.7–3,013.1) | 2,030.7 (1,487.9–2,719.2) | 2,817.6 (2,226.6–3,543.0) | 0.001 | 2,099.9 (1,521.3–2,802.4) | 3100.1 (2286.7–3462.6) | 0.003 |
| HSP27 serum: discharge (pg/mL, median ± IQR) | 1,993.6 (1,504.4–2,686.5) | 1,948.0 (1,520.4–2,675.5) | 2,224.9 (1,500.4–2,872.0) | 0.670 | 1,931.1 (1,500.4–2,675.5) | 2458.7 (1680.7–2827.5) | 0.195 |
| HSP27 serum: 180 days after discharge (pg/mL, median ± IQR) | 1,635.6 (1,333.5–2,405.2) | 1,653.6 (1,333.5–2,126.8) | 1,991.4 (1,416.6–2,881.0) | 0.215 | 1,635.6 (1,333.5–2,405.2) | – | – |
| HSP27 urine–admission (pg/mL, median ± IQR) | 3,330.6 (2,160.1–4,939.2) | 3,592.4 (2,372.4–5,243.6) | 2,211.7 (1,077.5–3,484.0) | <0.001 | 3,363.2 (2,160.1–4,977.8) | 3129.9 (2178.1–4417.2) | 0.784 |
| HSP27 urine: discharge (pg/mL, median ± IQR) | 2,857.3 (1,491.9–4,783.1) | 2,959.5 (1,533.4–5,511.7) | 1,914.7 (926.6–3,466.1) | 0.014 | 2,884.1 (1,413.2–4,783.1) | 2074.8 (1528.3–4979.1) | 0.720 |
| HSP27 urine: 180 days after discharge (pg/mL, median ± IQR) | 2,533.9 (1,557.4–4,836.8) | 2,629.7 (1,670.7–5,340.0) | 1,764.1 (1,173.9–3,818.2) | 0.038 | 2,533.9 (1,557.4–4,836.8) | – | – |
HSP27, heat shock protein 27; IQR, interquartile range; GOLD, global initiative for chronic obstructive lung disease; BMI, body mass index; LTOT, long-term oxygen therapy; CRP, C-reactive protein; cTnT, cardiac Troponin T; NT-proBNP, n-terminal pro-brain natriuretic peptide.
HSP27 urine excretion in an episode of acute exacerbation in COPD
Both HSP27 urine and serum concentration were measured at admission, discharge, and 180 days after discharge. Both showed decreasing values during the observed time frame. HSP27 urine concentration (median) was 3,300.6 pg/mL (IQR: 2,160.1–4,939.2 pg/mL) at admission, 2,857.3 pg/mL (IQR: 1,491.9–4,783.1 pg/mL) at discharge and 2,533.9 pg/mL (IQR: 1,557.4–4,836.8 pg/mL) 180 days after discharge (P=0.091, Figure 1A). HSP27 serum concentration (median) was 2,204.0 pg/mL (IQR: 1,545.7–3,013.1 pg/mL) at admission, 1,993.6 pg/mL (IQR: 1,504.4–2,686.5 pg/mL) at discharge and 1,635.6 pg/mL (IQR: 1,333.5–2,405.2 pg/mL) 180 days after discharge (P=0.101, Figure 1A). Serum and urine concentration of HSP27 did not significantly correlate at admission (r=−0.05, P=0.471).
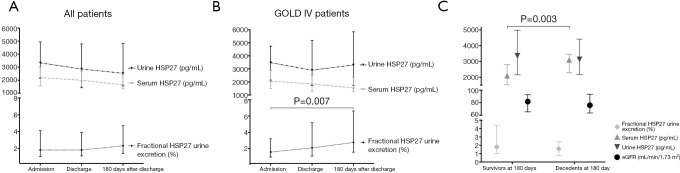
Figure 1.
Time course of HSP27 urine and serum concentration as well as fractional HSP27 urine excretion in all patients (A) and GOLD IV patients (B). Differences of HSP27 urine and serum concentration, fractional HSP27 urine excretion and eGFR between survivors and decedents at 180 days after admission (C). HSP27, heat shock protein 27; eGFR, estimated glomerular filtration rate.
Levels of proteins in urine are highly dependent on individually varying water excretion and resorption as well as renal capillary endothelial permeability during an episode of exacerbation. To eliminate those confounding factors fractional urine excretion of HSP27 was calculated. Fractional HSP27 urine excretion exhibited a trend towards significantly decreased values during and immediately after an episode of acute exacerbation. Fractional HSP27 urine excretion (median) was 1.8% (IQR: 1.0–4.1%) at admission, 1.8% (IQR: 1.1–3.9%) at discharge and 2.3% (OQR: 1.4–4.7%) 180 days after discharge (P=0.091, Figure 1A). Further admission serum HSP27 and fractional HSP27 urine excretion correlated significantly (r=−0.241, P<0.001).
If only severely ill patients (GOLD IV) are included, a more distinct course could be demonstrated. Fractional HSP27 urine excretion (median) in GOLD IV patients was 1.6% (IQR: 1.0–3.2%) at admission, 2.1% (IQR: 1.1–5.2%) at discharge and 2.8% (IQR: 1.6–6.6%) 180 days after discharge (P=0.007, Figure 1B). A statistical difference could be detected between admission and 180 days after discharge (P=0.007). HSP27 serum and urine concentration did not differ in GOLD IV patients (Figure 1B).
As serum HSP27 allows discrimination between mild and severe COPD (24), but no data on fractional HSP27 urine excretion in different GOLD stages is available we compared HSP27 urine excretion between GOLD stages. Fractional HSP27 urine excretion was elevated in GOLD IV patients after an episode of acute exacerbation (GOLD II vs. GOLD IV, P=0.031) and 180 days after admission (GOLD III vs. GOLD IV, P=0.035), however not at admission (P=0.819). However, HSP27 urine excretion did not correlate significantly with age at any time point.
By comparing fractional HSP27 urine excretion, serum, and urine HSP27 and eGFR in decedents and survivors at 180 days after admission we observed significantly increased serum HSP27 levels in decedents (Figure 1C).
Fractional HSP27 urine excretion at admission as a predictor of survival
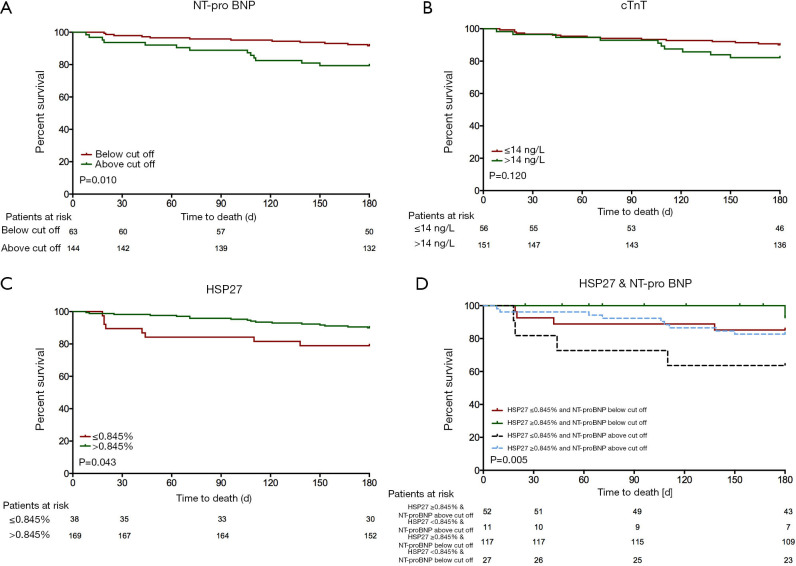
To assess admission fractional HSP27 urine excretion’s impact as a biomarker that predicts all-cause mortality we performed Kaplan Meier curve analyses and built a Cox proportional hazard model. Survival data were available for all patients for up to 3 years after admission. Using the R functions-based web application Cutoff Finder 2.1 we could identify a cut off level for admission fractional HSP27 urine excretion that predicts survival within the first 180 days after discharge (0.845%). We further compared HSP27 to the well-known biomarkers NT-proBNP (Figure 2A) and cTnT (Figure 2B). Median survival time for patients with values below the cut off was 1309 days (95% CI: 806–1,812 days) and for patients with values above the cut off was 1185 days (95% CI: 943–1,427 days). Patients with fractional HSP27 urine excretion at admission below or equal 0.845% had significantly worse survival 30 days (P=0.007, Log rank =7.292), 90 days (P=0.006, Log rank =7.608) and 180 days (P=0.043, Log rank =4.100) after discharge (Figure 2C). There was no significant difference of survival for longer follow up periods (1 year: P=0.219, Log rank =1.511; 3 years: P=0.952, Log rank =0.004). Combination of fractional HSP27 urine excretion and NT-proBNP in a Kaplan-Meier survival analysis showed a worse survival in the NT-proBNP-high/HSP27-low group after 180 days (log-rank test =12.709, P=0.005) (Figure 2D).
Figure 2.
Kaplan-Meier survival stratified by (A) NT-proBNP, (B) cTnT, (C) HSP27 and (D) combined concentrations of HSP27 and NT-proBNP at admission. NT-proBNP, n-terminal pro-brain natriuretic peptide; HSP27, heat shock protein 27; cTnT, cardiac troponin T.
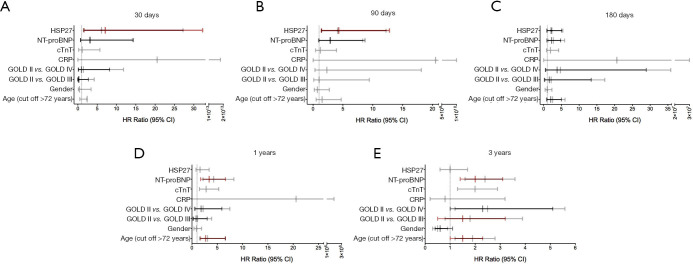
Adjusted Cox proportional hazard models were built using established COPD outcome parameters (age, gender, GOLD class, CRP, cTnT and NT-proBNP, Tables 2,3). In a univariable analysis admission fractional HSP27 urine excretion did significantly predict 30 day (HR: 6.1, 95% CI: 1.4–27.2, Figure 3A), 90 day (HR: 4.1, 95% CI: 1.4–12.2, Figure 3B) and 180-day mortality (HR: 2.3, 95% CI: 1.0–5.4, Figure 3C). For the timepoints 1 year and 3 years HSP27 did not predict mortality (Figure 3D,E). In a multivariable analysis fractional HSP27 urine fraction remained significant at 30 days (HR: 7.1, 95% CI: 1.6–32.3, Figure 3A) and 90 days (HR: 4,3, 95% CI: 1.4–12.8, Figure 3B) after admission.
Table 2. Univariable Cox proportional hazards analysis for prognostic factor of outcome data are presented as hazard ratios and 95% CI.
| Factor | Mortality at 30 days | Mortality at 90 days | Mortality at 180 days | Mortality at 1 year | Mortality at 3 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Age (≥72 years) | 2.4 (0.5–2.3) | 0.300 | 1.5 (0.5–4.7) | 0.458 | 2.5 (1.0–6.0) | 0.040 | 3.1 (1.5–6.6) | 0.003 | 1.9 (1.2–2.8) | 0.003 | ||||
| Gender (male vs. female) | 0.4 (0.1–3.4) | 0.413 | 0.7 (0.2–2.7) | 0.648 | 1.0 (0.4–2.3) | 0.929 | 0.9 (0.4–1.9) | 0.817 | 0.5 (0.3–0.9) | 0.016 | ||||
| GOLD | ||||||||||||||
| GOLD II vs. III | 0.3 (0.0–4.2) | 0.341 | 1.0 (0.1–9.4) | 0.966 | 2.1 (0.3–17.2) | 0.472 | 1.1 (0.3–3.9) | 0.896 | 1.8 (0.8–3.9) | 0.164 | ||||
| GOLD II vs. IV | 1.4 (0.2–11.8) | 0.767 | 2.3 (0.3–18.1) | 0.442 | 4.7 (0.6–35.8) | 0.131 | 2.2 (0.7–7.5) | 0.188 | 2.5 (1.2–5.6) | 0.021 | ||||
| CRP (≥0.5 mg/L) | 20.5 (0.0–1.8×1013) | 0.830 | 20.5 (0.0–1.0×1010) | 0.767 | 20.6 (0.0–3.0×107) | 0.677 | 20.6 (0.0–2.0×106) | 0.606 | 0.8 (0.2–3.2) | 0.731 | ||||
| Cardiac TnT (≥14 ng/L) | 1.1 (0.2–5.7) | 0.912 | 1.2 (0.4–3.9) | 0.750 | 1.9 (0.8–4.2) | 0.127 | 2.8 (1.5–5.3) | 0.002 | 2.0 (1.3–2.9) | 0.001 | ||||
| NT-proBNP (age-adjusted) | 3.2 (0.7–14.1) | 0.132 | 2.8 (0.9–8.3) | 0.066 | 2.7 (1.2–5.9) | 0.014 | 4.3 (2.2–8.3) | <0.001 | 2.4 (1.6–3.6) | <0.001 | ||||
| Fraction HSP27 urine excretion (<0.845%) | 6.1 (1.4–27.2) | 0.0018 | 4.1 (1.4–12.2) | 0.011 | 2.3 (1.0–5.4) | 0.049 | 1.6 (0.8–3.4) | 0.223 | 1.0 (0.6–1.7) | 0.952 | ||||
GOLD, global initiative for chronic obstructive lung disease; CRP, C-reactive protein; cTnT, cardiac Troponin T; NT-proBNP, n-terminal pro-brain natriuretic peptide; HSP27, heat shock protein 27.
Table 3. Multivariable Cox proportional hazard analysis for 180 days mortality (backward LR selection).
| Factor | Mortality at 30 days | Mortality at 90 days | Mortality at 180 days | Mortality at 1 year | Mortality at 3 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Age (≥72 years) | – | n.s. | – | n.s. | 2.0 (0.8–5.0) | 0.127 | 2.7 (1.7–6.6) | 0.013 | 1.5 (1.0–2.3) | 0.073 | ||||
| Gender (male vs. female) | – | n.s. | – | n.s. | – | n.s. | 0.6 (0.4–1.1) | 0.084 | ||||||
| GOLD | ||||||||||||||
| GOLD II vs. III | 0.2 (0.0–2.7) | 0.207 | – | n.s. | 1.6 (0.2–13.4) | 0.631 | 0.8 (0.2–3.0) | 0.772 | 1.5 (0.5–3.2) | 0.418 | ||||
| GOLD II vs. IV | 0.9 (0.1–8.2) | 0.908 | – | n.s. | 3.8 (0.5–28.9) | 0.196 | 1.8 (0.5–5.9) | 0.360 | 2.3 (1.0–5.1) | 0.041 | ||||
| CRP (≥ 0.5 mg/L) | – | n.s. | – | n.s. | – | n.s. | ||||||||
| TnT (≥14 ng/L) | – | n.s. | – | n.s. | – | n.s. | ||||||||
| NT-proBNP (age-adjusted) | 3.1 (0.7–14.4) | 0.153 | 2.9 (1.0–8.7) | 0.054 | 2.2 (0.9–4.8) | 0.067 | 3.4 (1.7–6.6) | <0.001 | 2.0 (1.4–3.1) | 0.001 | ||||
| Fraction HSP27 urine excretion cut off (<0.845%) | 7.1 (1.6–32.3) | 0.011 | 4.3 (1.4–12.8) | 0.009 | 2.1 (0.9–5.1) | 0.080 | ||||||||
Data are presented as hazard ratios and 95% CI. GOLD, global initiative for chronic obstructive lung disease; CRP, C-reactive protein; cTnT, cardiac Troponin T; NT-proBNP, n-terminal pro-brain natriuretic peptide; HSP27, heat shock protein 27.
Figure 3.
HR and 95% CI in univariable (grey bar) and multivariable (black and red bar, for the latter P<0.05) Cox regression analysis after 30 days (A), 90 days (B), 180 days (C), 1 year (D) and 3 years (E). Variables remaining in the model after multivariable analyses are presented in red/black. HSP27, heat shock protein 27; NT-proBNP, n-terminal pro-brain natriuretic peptide; cTnT, cardiac troponin T; CRP, C-reactive protein; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratios.
We have previously analyzed HSP27 serum concentration as a prognosis predictor in this patient subset and have identified its power in mid-term survival (90 days to 1 year follow-up time) (28). In contrast HSP27 fractional urine excretion seems to reveal its strength in short-term survival (30 days). We compared both Cox proportional hazard models and observed higher c-indices of the models including HSP27 fractional urine excretion compared to the serum model in short-term survival (urine vs. serum at 30 days: c-index: 0.850 vs. 0.500). This is supported by the AUC of both models (urine vs. serum at 30 days: AUC: 0.701 vs. 0.500). In contrast both c-index and AUC were superior in the serum model on year 1 (Table 4).
Table 4. Comparison of c-indices and AUC of the cox proportional hazard model including HSP27 urine excretion and the previously published cox proportional hazard model including HSP27 serum excretion (28).
| Time period | HSP27 fractional urine excretion | HSP27 serum excretion | |||
|---|---|---|---|---|---|
| C-index | AUC | C-index | AUC | ||
| 30 days | 0.850 | 0.701 | 0.500 | 0.500 | |
| 90 days | 0.717 | 0.720 | 0.657 | 0.664 | |
| 180 days | 0.735 | 0.726 | 0.699 | 0.724 | |
| 1 year | 0.740 | 0.773 | 0.758 | 0.790 | |
| 3 years | 0.669 | 0.701 | 0.665 | 0.701 | |
AUC, area under the curve; HSP27, heat shock protein 27.
Association with other markers
Increased NT-proBNP concentrations at admission were significantly associated with fatal outcome over the follow-up period (Figure 2A), in contrast elevated cTnT levels were not associated with worse outcome (Figure 2B). As CRP, NT-proBNP and cTnT have already been identified as prognostic markers in AECOPD we correlated them with admission fractional HSP27 urine excretion (15,36-40). We did not observe a correlation of admission fractional HSP27 urine excretion with any marker (CRP: r=−0.060, P=0.394; NT-proBNP: r=−0.052, P=0.454, cTnT: r=0.106, P=0.130).
Discussion
In the present study we demonstrated decreased factional HSP27 urine excretion at admission due to an episode of acute exacerbation in COPD patients and an increase after 180 days after discharge. This course was even more distinct in advanced COPD patients (GOLD IV). Furthermore, we could identify an admission fractional HSP27 urine excretion cut off value serving as predictors for short-term mortality in Kaplan-Meier curve analyses and a univariable and multivariable Cox proportional hazard model including established outcome parameters. Recently published data on HSP27 serum concentration in this patient cohort suggest a role as a predictor of all cause survival for mid- and long-term follow up (up to 1 year). We hypothesized that fractional HSP27 urine excretion might be a superior prognosis marker especially for the period of 30 days after discharge. Therefore, we compared both models and observed higher Harrell’s c-index and AUC in the model including fractional HSP27 urine excretion for 30-day all-cause mortality, suggesting a superior performance of the fractional HSP27 urine excretion-based model. However, in 1-year survival analysis the model including HSP27 serum concentration was superior. Survival data up to 3 years were available for our patient cohort. In none of the models HSP27 was a predictor of 3-year mortality, in contrast, we observed NT-proBNP, a well-known cardiac marker, as the main predictor in a multivariable Cox proportional hazard model.
Several previous studies attempted to identify biomarkers that predict clinical outcome after hospitalization for AECOPD. Acute exacerbations are both common and serious conditions in COPD patients and frequency and severity of exacerbation episodes are a major factor limiting survival and quality of life (3). Prevention of exacerbations is generally considered a key strategy in managing COPD (1). Thus, identifying patients who are at high risk for further exacerbations or death is of particular importance (3).
The heat shock response is characterized by increased expression of HSPs due to stress to ensure cell integrity and minimize the extent of apoptosis (17). HSPs can be spilled into the blood system by cell damage to mediate a response by the innate immune system but also by immune cells themselves (29,41). A variety of syndromes e.g., stable COPD (24), sepsis (42) and coronary artery disease (43) are associated with altered HSP27 concentration. Elevated extracellular HSP27 levels are found under inflammatory conditions. Especially oxidative stress induces HSP27 expression. Copper (Cu2+), a toxic metal ion that is contained in cigarette smoke, results in increased expression of HSP27. Hence, HSP27 binds Cu2+ and inhibits Cu2+ mediated generation of reactive oxygen species and oxidative stress (17,44,45). Oxidative stress derived from cigarette smoke or air pollutants plays a pivotal role in the pathogenesis of COPD and especially during an exacerbation episode the burden of oxidative stress increases (46). Increased HSP27 expression and extracellular secretion in COPD may reflect mechanisms to protect bronchial epithelial cells from apoptosis under oxidative stress (47). Even though previous studies clearly implicate a role for HSP27 in the pathogenesis of COPD it is still unclear. Increased HSP27 levels in serum of stable COPD patients were demonstrated (24,48) whereas Cui et al. showed decreased plasma HSP27 concentrations but increased lymphocyte HSP27 levels in coal miners suffering from COPD compared to healthy coal miners (25). In another study increased expression of HSP27 in bronchial epithelium of COPD patients was seen (26). In contrast, Cappello et al. could not detect any differences in HSP27 expression in bronchial epithelium of COPD patients and control subjects (27). Elevated intracellular HSP27 expression may result in spillage into the vascular bed. Thus, it is not surprising that we could detect a trend towards increased serum and urine HSP27 concentrations during exacerbation and decreasing concentrations in the stable phase. As HSP27 belongs to the family of small HSPs and is of a size of about 27 kDa, renal elimination of HSP27 may be suggested (17). Fractional HSP27 urine excretion reflects the percentage of filtered HSP27 that is excreted. Lebherz-Eichinger et al. demonstrated increased fractional HSP27 urine excretion in severe kidney injury (33). Patients included in our cohort did not suffer from kidney injury and did not observe a difference in eGFR in both survivors and decedents (Figure 1C) as well as in fractional HSP27 urine excretion low or high group. Increased fractional HSP27 urine excretion in patients suffering from kidney injury may be a result of direct release of the failing kidney. Thus, we hypothesize that increasing HSP27 urine excretion and simultaneously decreasing serum and urine HSP27 concentrations after an exacerbation represent less production of HSP27 due to decreasing stress and successful renal elimination of HSP27. This dynamic may reflect a persistent stable condition of those patients.
Additionally to increased HSP27 serum concentration in COPD patients compared to healthy smokers and non-smokers Hacker et al. demonstrated also continuous increase with disease severity (24). In accordance with these findings, we could detect a significant increase of HSP27 urine excretion in GOLD IV patients compared to GOLD II patients. Increased HSP27 urine excretion in GOLD IV patients may reflect augmented excretion by the kidney to eliminate elevated HSP27 production due to increasing stress levels in severely ill patients.
We provide significant evidence that fractional HSP27 urine excretion could serve as a biomarker to identify COPD patients that are at high risk for death within 180 days after discharge after an episode of acute exacerbation with superior performance especially in the first 30 days after discharge in comparison to our already published serum HSP27 based model. Nevertheless, some limitations of our study should be mentioned. First of all, we conducted a single-center study and one must carefully translate our results into other patient groups. Second, we included only GOLD II–IV patients. No intervention apart from standard care was performed so we can only hypothesize that the availability of fractional HSP27 urine excretion to physicians offers a useful tool for the management of AECOPD.
Conclusions
In conclusion, we found that fractional HSP27 urine excretion is decreased during an episode of acute exacerbation and can serve as a mortality predictor within a short follow up time. We propose that obtaining HSP27 urine and serum concentration at admission and calculating fraction HSP27 urine excretion might offer valuable information for the management of COPD patients admitted to a hospital due to an acute exacerbation within the first year. Whereas fractional HSP27 urine excretion is superior within the first 30 days after discharge, HSP27 serum concentration might provide valuable information for patient management up to 1 year after discharge. Nevertheless, further studies including a larger sample size are needed to confirm our results.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by Christian Doppler Laboratory for Cardiac and Thoracic Diagnosis and Regeneration, Medical University of Vienna, Austria.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study has been approved by the National Ethics Committee of the Republic of Slovenia (EC number: 65/02/09) and conducted according to the Declaration of Helsinki (as revised in 2013). Informed consent was provided by all participants.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3683
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3683
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3683). MF reports personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, outside the submitted work. The other authors have no conflicts of interest to declare.
References
- 1.Januzzi JL, Peacock WF, Maisel AS, et al. Measurement of the Interleukin Family Member ST2 in Patients With Acute Dyspnea. J Am Coll Cardiol 2007;50:607-13. 10.1016/j.jacc.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 2.Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-67. 10.1164/ajrccm.154.4.8887592 [DOI] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786-96. 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodge G, Mukaro V, Reynolds PN, et al. Role of increased CD8/CD28null T cells and alternative co-stimulatory molecules in chronic obstructive pulmonary disease. Clin Exp Immunol 2011;166:94-102. 10.1111/j.1365-2249.2011.04455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambers C, Hacker S, Posch M, et al. T cell senescence and contraction of T cell repertoire diversity in patients with chronic obstructive pulmonary disease. Clin Exp Immunol 2009;155:466-75. 10.1111/j.1365-2249.2008.03835.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodge G, Roscioli E, Jersmann H, et al. Steroid resistance in COPD is associated with impaired molecular chaperone Hsp90 expression by pro-inflammatory lymphocytes. Respir Res 2016;17:135. 10.1186/s12931-016-0450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med 2013;45:291-300. 10.3109/07853890.2012.732703 [DOI] [PubMed] [Google Scholar]
- 8.Welniak TJ, Panzenbeck A, Koyfman A, et al. Chronic obstructive pulmonary disease: Emergency care in acute exacerbation. Afr J Emerg Med 2015;5:75-84. 10.1016/j.afjem.2014.08.002 [DOI] [Google Scholar]
- 9.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995;107:1193-8. 10.1378/chest.107.5.1193 [DOI] [PubMed] [Google Scholar]
- 10.Antonelli Incalzi R, Fuso L, De Rosa M, et al. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997;10:2794-800. 10.1183/09031936.97.10122794 [DOI] [PubMed] [Google Scholar]
- 11.Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med 1995;98:272-7. 10.1016/S0002-9343(99)80374-X [DOI] [PubMed] [Google Scholar]
- 12.Rammaert B, Verdier N, Cavestri B, et al. Procalcitonin as a prognostic factor in severe acute exacerbation of chronic obstructive pulmonary disease. Respirology 2009;14:969-74. 10.1111/j.1440-1843.2009.01597.x [DOI] [PubMed] [Google Scholar]
- 13.Peng C, Tian C, Zhang Y, et al. C-reactive protein levels predict bacterial exacerbation in patients with chronic obstructive pulmonary disease. Am J Med Sci 2013;345:190-4. 10.1097/MAJ.0b013e318253c921 [DOI] [PubMed] [Google Scholar]
- 14.Bartziokas K, Papaioannou AI, Loukides S, et al. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur Respir J 2014;43:43-53. 10.1183/09031936.00209212 [DOI] [PubMed] [Google Scholar]
- 15.Marcun R, Sustic A, Brguljan PM, et al. Cardiac biomarkers predict outcome after hospitalisation for an acute exacerbation of chronic obstructive pulmonary disease. Int J Cardiol 2012;161:156-9. 10.1016/j.ijcard.2012.05.044 [DOI] [PubMed] [Google Scholar]
- 16.Calderwood SK, Mambula SS, Gray PJ, Jr, et al. Extracellular heat shock proteins in cell signaling. FEBS Lett 2007;581:3689-94. 10.1016/j.febslet.2007.04.044 [DOI] [PubMed] [Google Scholar]
- 17.Bakthisaran R, Tangirala R, Rao Ch M. Small heat shock proteins: Role in cellular functions and pathology. Biochim Biophys Acta 2015;1854:291-319. 10.1016/j.bbapap.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 18.Arrigo AP. The cellular "networking" of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol 2007;594:14-26. 10.1007/978-0-387-39975-1_2 [DOI] [PubMed] [Google Scholar]
- 19.De AK, Kodys KM, Yeh BS, et al. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol 2000;165:3951-8. 10.4049/jimmunol.165.7.3951 [DOI] [PubMed] [Google Scholar]
- 20.Rayner K, Chen YX, McNulty M, et al. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res 2008;103:133-41. 10.1161/CIRCRESAHA.108.172155 [DOI] [PubMed] [Google Scholar]
- 21.Ghayour-Mobarhan M, Saber H, Ferns GA. The potential role of heat shock protein 27 in cardiovascular disease. Clin Chim Acta 2012;413:15-24. 10.1016/j.cca.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 22.Lianos GD, Alexiou GA, Mangano A, et al. The role of heat shock proteins in cancer. Cancer Lett 2015;360:114-8. 10.1016/j.canlet.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 23.Jan Ankersmit H, Nickl S, Hoeltl E, et al. Increased serum levels of HSP27 as a marker for incipient chronic obstructive pulmonary disease in young smokers. Respiration 2012;83:391-9. 10.1159/000336557 [DOI] [PubMed] [Google Scholar]
- 24.Hacker S, Lambers C, Hoetzenecker K, et al. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab 2009;55:31-40. [PubMed] [Google Scholar]
- 25.Cui X, Xing J, Liu Y, et al. COPD and levels of Hsp70 (HSPA1A) and Hsp27 (HSPB1) in plasma and lymphocytes among coal workers: a case-control study. Cell Stress Chaperones 2015;20:473-81. 10.1007/s12192-015-0572-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu R, Ouyang Q, Dai A, et al. Heat shock protein 27 and cyclophilin A associate with the pathogenesis of COPD. Respirology 2011;16:983-93. 10.1111/j.1440-1843.2011.01993.x [DOI] [PubMed] [Google Scholar]
- 27.Cappello F, Caramori G, Campanella C, et al. Convergent sets of data from in vivo and in vitro methods point to an active role of Hsp60 in chronic obstructive pulmonary disease pathogenesis. PLoS One 2011;6:e28200. 10.1371/journal.pone.0028200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann M, Traxler D, Bekos C, et al. Heat shock protein 27 as a predictor of prognosis in patients admitted to hospital with acute COPD exacerbation. Cell Stress Chaperones 2020;25:141-9. 10.1007/s12192-019-01057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corsini E, Galbiati V, Papale A, et al. The role of HSP27 in RACK1-mediated PKC activation in THP-1 cells. Immunol Res 2016;64:940-50. 10.1007/s12026-016-8802-1 [DOI] [PubMed] [Google Scholar]
- 30.Farkas J, Kadivec S, Kosnik M, et al. Effectiveness of discharge-coordinator intervention in patients with chronic obstructive pulmonary disease, study protocol of a randomized controlled clinical trial. Respir Med 2011;105:S26-30. 10.1016/S0954-6111(11)70007-5 [DOI] [PubMed] [Google Scholar]
- 31.Lainscak M, Kadivec S, Kosnik M, et al. Discharge coordinator intervention prevents hospitalizations in patients with COPD: a randomized controlled trial. J Am Med Dir Assoc 2013;14:450 e1-6. [DOI] [PubMed]
- 32.Zimmermann M, Mueller T, Dieplinger B, et al. Circulating heat shock protein 27 as a biomarker for the differentiation of patients with lung cancer and healthy controls--a clinical comparison of different enzyme linked immunosorbent assays. Clin Lab 2014;60:999-1006. 10.7754/Clin.Lab.2013.130526 [DOI] [PubMed] [Google Scholar]
- 33.Lebherz-Eichinger D, Ankersmit HJ, Hacker S, et al. HSP27 and HSP70 serum and urine levels in patients suffering from chronic kidney disease. Clin Chim Acta 2012;413:282-6. 10.1016/j.cca.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 34.Cogo A, Ciaccia A, Legorini C, et al. Proteinuria in COPD patients with and without respiratory failure. Chest 2003;123:652-3; author reply 3. 10.1378/chest.123.2.652 [DOI] [PubMed] [Google Scholar]
- 35.Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 2012;7:e51862. 10.1371/journal.pone.0051862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brekke PH, Omland T, Holmedal SH, et al. Troponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbation. Eur Respir J 2008;31:563-70. 10.1183/09031936.00015807 [DOI] [PubMed] [Google Scholar]
- 37.Hoiseth AD, Neukamm A, Karlsson BD, et al. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011;66:775-81. 10.1136/thx.2010.153122 [DOI] [PubMed] [Google Scholar]
- 38.Hoiseth AD, Omland T, Hagve TA, et al. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD - a prospective cohort study. Respir Res 2012;13:97. 10.1186/1465-9921-13-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina AM, Marteles MS, Saiz EB, et al. Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur J Intern Med 2011;22:167-71. 10.1016/j.ejim.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 40.Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011;66:764-8. 10.1136/thx.2010.155333 [DOI] [PubMed] [Google Scholar]
- 41.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002;20:395-425. 10.1146/annurev.immunol.20.100301.064801 [DOI] [PubMed] [Google Scholar]
- 42.Hashiguchi N, Ogura H, Tanaka H, et al. Enhanced expression of heat shock proteins in activated polymorphonuclear leukocytes in patients with sepsis. J Trauma 2001;51:1104-9. 10.1097/00005373-200112000-00015 [DOI] [PubMed] [Google Scholar]
- 43.Jin C, Phillips VL, Williams MJ, et al. Plasma heat shock protein 27 is associated with coronary artery disease, abdominal aortic aneurysm and peripheral artery disease. Springerplus 2014;3:635. 10.1186/2193-1801-3-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernhard D, Rossmann A, Wick G. Metals in cigarette smoke. IUBMB Life 2005;57:805-9. 10.1080/15216540500459667 [DOI] [PubMed] [Google Scholar]
- 45.Hawse JR, Cumming JR, Oppermann B, et al. Activation of metallothioneins and alpha-crystallin/sHSPs in human lens epithelial cells by specific metals and the metal content of aging clear human lenses. Invest Ophthalmol Vis Sci 2003;44:672-9. 10.1167/iovs.02-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Eeden SF, Sin DD. Oxidative stress in chronic obstructive pulmonary disease: a lung and systemic process. Can Respir J 2013;20:27-9. 10.1155/2013/509130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merendino AM, Paul C, Vignola AM, et al. Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: possible implication in asthma. Cell Stress Chaperones 2002;7:269-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unver R, Deveci F, Kirkil G, et al. Serum Heat Shock Protein Levels and the Relationship of Heat Shock Proteins with Various Parameters in Chronic Obstructive Pulmonary Disease Patients. Turk Thorac J 2016;17:153-9. 10.5152/TurkThoracJ.2016.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as