Abstract
In 1939, Robinson and Brucer first proposed the concept of prehypertension (PHTN), which was defined as a systolic blood pressure of 120–139 mmHg and/or diastolic blood pressure of 80–89 mmHg. PHTN is a major global health risk that adversely affects human health, especially the cardiovascular system. People with PHTN have a higher risk of developing cardiovascular diseases, including stroke, coronary heart disease, myocardial infarction and total cardiovascular events. However, there are few systematic summaries of the relationship between PHTN and the cardiovascular system. Furthermore, because the definition of ‘normal BP’ and the advantages of more intensive BP control remain unclear, there is no consensus on optimal interventions. In an attempt to provide information for clinicians or professionals who are interested in reducing the risk associated with PHTN, we review the existing studies to provide references for them with the effects of PHTN on the cardiovascular system and the potential pathogenic mechanisms of PHTN, including inflammatory responses, insulin resistance, endothelial dysfunction, sympathovagal imbalance, activation of the renin-angiotensin system and others. PHTN is highly prevalent and has adverse effects on health. An effective public health strategy is important to prevent the progression of PHTN. We envisage that this information will increase the public attention of PHTN and help to provide more strategies to reduce the risk of cardiovascular events.
Keywords: Prehypertension (PHTN), cardiovascular system, pathogenic mechanism
Introduction
In 1939, Robinson and Brucer (1) first proposed the concept of prehypertension (PHTN), which was defined as a systolic blood pressure (SBP) of 120–139 mmHg and/or diastolic blood pressure (DBP) of 80–89 mmHg. On the basis of clinical data accumulated over the following 60 years, the risk of cardiovascular events is now known to be higher in patients with PHTN than in those with an optimal BP (≤120/80 mmHg). In 2003, the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) (2) defined the range of PHTN as an SBP of 120–139 mmHg and/or a DBP of 80–89 mmHg. Furthermore, the ACC/AHA (American College of Cardiology/American Heart Association) 2017 guidelines (3) consider an SBP within 130–139 mmHg or a DBP within 80–89 mmHg as stage 1 hypertension (HTN). However, this classification is controversial. The range of high normal BP defined in the 2018 Chinese HTN guidelines (4) is the same as that for PHTN defined by the JNC7. The PHTN discussed in this review is consistent with the JNC7.
The prevalence of PHTN in China (5) exceeds 30% and is affected by numerous factors including age, sex, body mass index, body fat percentage, waist circumference, education, ethnicity, geographical position (6), and others (7). Many studies have explored the impact of PHTN on cardiovascular events (8). For example, in the settings of a BP of 120–129/80–84 mmHg as the first stage of PHTN and 130–139/85–89 mmHg as the second stage of PHTN, the risk of cardiovascular events was reported to be higher in second stage PHTN patients (9). However, there are few systematic summaries of the relationship between PHTN and the cardiovascular system. Furthermore, because the definition of ‘normal BP’ and the advantages of more intensive BP control remain unclear, there is no consensus on optimal interventions.
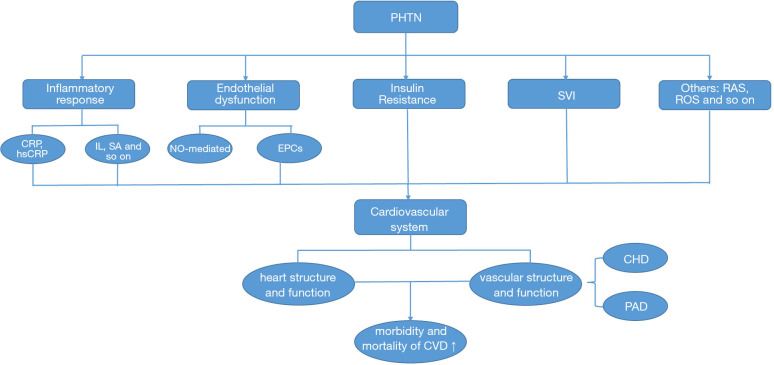
To provide information for professionals interested in reducing the risk of PHTN, this review summarizes existing clinical studies examining the effects of PHTN on the cardiovascular system and the potential pathogenic mechanisms (Figure 1). We envisage that this information will increase the public attention of PHTN and help to provide more strategies to reduce the risk of cardiovascular events. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5482).
Figure 1.
The relationship between prehypertension and the cardiovascular system: effects and potential pathogenic mechanisms.
Methods and materials
Published articles in the scientific literature examining the effects and potential pathogenic mechanisms of PHTN on the cardiovascular system were identified and reviewed. We searched for relevant publications including retrospective cohort studies, prospective studies, randomized controlled trials, systematic reviews, and meta-analyses using PubMed, Embase, and the Web of Science. Results of the literature search relevant to the present topic were screened by title, keywords, abstract, and then the whole publication. All searches were limited to English language. Articles were not restricted by the date of publication or region of origin.
Effects of PHTN on cardiovascular diseases
The number of patients suffering from cardiovascular diseases (CVD) increased to more than 485.6 million in 2017 (10). CVD is the leading cause of global mortality, with more than 17.79 million people dying of CVD in 2017, accounting for 31.8% of deaths worldwide (11). CVD is the main cause of death in both urban and rural China (12). Thus, it is essential to establish preventive measures to control this serious condition. When BP is within the PHTN range, the morbidity and mortality of CVD is increased (13). A meta-analysis of approximately 1 million people showed that the mortality of ischemic heart disease and stroke in middle-aged or older people (40–69 years old) was associated with increased BP (14). When BP exceeded 115/75 mmHg, the mortality of ischemic heart disease and stroke increased double exponentially. People with PHTN have a higher risk of developing CVD, including stroke, coronary heart disease (CHD), myocardial infarction (MI), and total cardiovascular events. Furthermore, the risk of CVD in people with PHTN was 1.44 times higher than in those with an optimal BP (<120/80 mmHg).
HTN
Patients with PHTN have a higher risk of developing HTN than those with an optimal BP (15,16). A higher baseline BP is also associated with a greater probability of developing HTN. People with first-stage PHTN have a 2–3-fold increased risk of developing HTN compared with those with an optimal BP (15), while people with second-stage PHTN have a 5–12-fold increased risk. Older people are more prone to developing HTN. For example, among patients with second-stage PHTN, 37% of those <65 years old and 50% of those >65 years old progressed to HTN compared with 5% and 16%, respectively, of those with an optimal BP (15). Of interest, there were no significant sex-related differences in that study. Adolescents with PHTN were also more likely to develop HTN than those with a BP <120/80 mmHg (17). In that study, PHTN was diagnosed in adolescents when their BP at the first measurement session was at the ≥90th percentile (or 120/80 mmHg) to the <95th percentile, or when their BP at the first measurement session was at the ≥95th percentile but their lowest follow-up BP was at the ≥90th percentile to the <95th percentile. Finally, individuals with PHTN were reported to have a higher incidence of HTN than those with an optional BP (hazard ratio: 2.98; 95% CI: 0.77–11.56) (17).
These studies confirm that both adults and adolescents with PHTN are more likely to progress to HTN. Researchers have identified some clinical variables to predict which individuals with PHTN are most likely to develop HTN. For example, black ethnicity, older age, higher body mass index (BMI), and the presence of diabetes or chronic kidney disease are all independently and positively associated with HTN (18). Timely intervention in people with PHTN may reduce the prevalence of HTN. Further clinical trials are required to determine the effects of different classes of antihypertensive drugs or different population characteristics on this relationship.
Effects of PHTN on arrhythmia
Atrial fibrillation (AF) is the most common heart arrhythmia and is associated with an increased risk of ischemic stroke, heart failure, and premature death. AF is associated with a 1.5–1.9-fold mortality risk after adjusting for related cardiovascular conditions (19). After correcting for influencing factors, PHTN was found to be an important risk factor for AF, for which the predictive effect of DBP was stronger than that for SBP (HR: 1.11; P=0.045) (20). Additionally, both men and women with PHTN had a higher risk of AF compared with subjects with an optimal BP. In a study of middle-aged men (40–59 years old), Grundvold et al. (21) reported that subjects with an SBP of 128–138 mmHg had a 1.5-fold increased risk of AF compared with those with an SBP <128 mmHg (95% CI: 1.10–2.03, P<0.001), while men with a DBP ≥80 mmHg had a 1.79-fold increased risk of AF (95% CI: 1.28–2.59; P<0.001) compared with the control group. Conen et al. (22) reported that women ≥45 years old with PHTN had a significantly increased risk of incident AF (P<0.05). Nevertheless, these findings cannot be generalized to people of different ethnicities, ages, or with concomitant diseases.
PHTN can increase the risk of AF and the incidence of adverse cardiovascular events. Future studies are required to confirm whether there is a BP threshold below which the risk of incident AF is not increased. Furthermore, few studies have examined the effects of PHTN in AF subjects of different ethnicities, ages, genders, or with concomitant diseases. Finally, most current research is focused on the relationship between PHTN and AF, while studies of other arrhythmias remain limited.
Effects of PHTN on heart structure
Changes in the structure of the heart play an important role in the development of CVD. Systolic and diastolic function of the left ventricle can be influenced by geometrical patterns, which are important to cardiovascular prognosis. For example, poor prognostic implications of left ventricular hypertrophy on stroke, serious arrhythmias, and sudden cardiac death have been reported (23).
The incidence of cardiac hypertrophy is higher in subjects with PHTN than those with optimal BP. A cross-sectional study of subjects ≥35 years old without CVD revealed left ventricular geometrical changes in those with PHTN, with eccentric hypertrophy being the main type of geometric alteration (P<0.001) (24). In that study, logistic regression analysis showed that both SBP and DBP were independent risk factors for left cardiac structural changes, while DBP was the strongest indicator for concentric remodeling, concentric hypertrophy, and eccentric hypertrophy (P<0.001). Furthermore, compared with individuals with an optimal BP, the left ventricular wall thickness (P<0.001) and left ventricular mass (P=0.006) of patients with PHTN increased, and persistent PHTN accelerated the development of cardiac hypertrophy and diastolic dysfunction (P<0.05) (25). In another study, left ventricular diastolic parameters including the E (early)/A (late) ratio, tissue Doppler imaging, early diastolic (Ea) velocity, and the E/Ea ratio were also impaired in subjects with PHTN (all P<0.001) (26), suggesting that subtle alterations in cardiovascular structure and function were already present at the prehypertensive stage. Whether such subtle alterations convey an increased risk of cardiovascular events and whether the changes are reversible with treatment warrant further study.
There are also contrasting data on the impact of PHTN. According to a study by Norton et al. (27), PHTN was not an independent predictor of left ventricular hypertrophy after adjustment for traditional cardiovascular risk factors in young to middle-aged individuals of African descent. Differences in the study design, sample size, statistical methods, and ethnic origin may explain some of the discrepancies between these studies. However, future studies are required to examine these potential effects. Furthermore, current studies are predominantly in adults, while there is limited information on adolescents and children.
Effects of PHTN on vascular structure and function
A normal structure and function of blood vessels is critical for normal cardiovascular physiology. However, PHTN can negatively influence vascular structure and function. For example, reduced coronary flow reserve (28) and increased aortic stiffness (29) are observed more frequently in subjects with PHTN compared with those with an optimal BP. Coronary atherosclerosis is also more severe in PHTN patients and coronary blood flow reserve is impaired in PHTN patients (P<0.01) (28). Furthermore, the persistence of PHTN is associated with accelerated structural stiffening of the large- to middle-sized arteries (P<0.05) and progressive vascular damage, which may be more pronounced in middle-aged and elderly subjects (29). Nevertheless, there are conflicting data on the impact of PHTN on aortic stiffness (27). Additionally, the rate of coronary artery calcification was significantly higher in patients with PHTN [relative risk (RR): 1.29; 95% CI: 0.97–1.72] (30). It is well established that dyslipidemia is associated with coronary atherosclerosis, while lipid changes have been found in PHTN patients (31). Thus, controlling serum lipid levels may play a role in preventing PHTN progression.
Compared with patients with an optimal BP, PHTN patients were found to have an increased risk of CHD (RR: 1.43; 95% CI: 1.26–1.63, P<0.001) (32), which was higher in Western (RR: 1.70; 95% CI: 1.49–1.94) than in Asian (RR: 1.25, 95% CI: 1.12–1.38; ratio of RRs: 1.36; 95% CI: 1.15–1.61) subjects. These results support the heterogeneity of target-organ damage caused by PHTN in different ethnicities. Han et al. (8) and Huang et al. (32) also confirmed that PHTN was associated with CHD. However, a general population trial cohort study suggested that PHTN was not associated with the incidence of acute CHD (33). Thus, more studies are clearly required to clarify the relationship between PHTN and CHD.
Finally, PHTN patients are at greater risk of peripheral arterial disease (PAD) than subjects with an optimal BP. For example, Tomiyama et al. (34) summarized several prospective studies and demonstrated that an abnormal pressure-wave reflection was a risk factor for developing HTN in PHTN patients. The ankle-brachial index (ABI) is a noninvasive and quick office measurement method for diagnosing PAD, with an ABI <0.9 having a high sensitivity and specificity for diagnosis of lower extremity atherosclerosis. Interestingly, the ABI of PHTN patients >19 years old was lower compared with subjects with a normal BP (0.90±0.14 vs. 1.023±0.21, respectively; P=0.00012) (35). Furthermore, an increase in the intima thickness of the radial artery was reported in prehypertensive patients (P<0.05) (36). These findings suggest that PHTN patients are prone to developing PAD. However, the effects of PHTN on other vessels require further exploration, as do experimental studies examining the effects of different BPs on vascular structure and function.
Potential pathogenic mechanisms of PHTN
There are a number potential pathogenic mechanisms underlying CVD in PHTN patients, as detailed below.
Inflammatory response
Inflammatory cytokines are linked with CVD via regulation of acute-phase proteins. Furthermore, the inflammatory cytokine levels in people with PHTN are significantly altered, which is associated with the occurrence of subsequent adverse events.
C-reactive protein (CRP) is a marker of systemic inflammation. CRP is a predictor of mortality and morbidity in CVD patients, including an increased risk of MI and stroke. Elevated CRP can damage vascular endothelial cells, reduce nitric oxide (NO) release, and thus cause vasoconstriction. CRP concentrations are also increased in PHTN patients. In the ATTICA study, increased SBP and DBP in PHTN patients was positively correlated with the CRP concentration (37). Furthermore, in 5-year follow-up study, elevated CRP was associated with an increased risk of developing HTN in PHTN patients (odds ratio per 1-mg/L increase: 1.12; 95% CI: 1.05–1.20) (38). In another study, the high sensitivity CRP (hsCRP) concentration was also increased in PHTN patients and was a sensitive index for predicting arteriosclerosis (39). By contrast, no significant association between hsCRP and PHTN (P>0.05) was reported in the Yi population (40), these differences may relate to differences in the ethnicity of the patients. Finally, a prospective study from the United States showed that subjects with an elevated CRP had an increased risk of developing HTN regardless of the BP range (41). These findings suggest that elevated BP and CRP levels may interact with each other to form a vicious circle that accelerates the occurrence of cardiovascular events. However, it should be noted that only females were examined in that study. Thus, the characteristics of the male population require further study.
Interleukin (IL)-17 is released by T cells and can generate more severe HTN by increasing peripheral artery resistance and arterial remodeling. These findings provide support for a relationship between vascular inflammation and PHTN. A higher median IL-17 level was also observed in 40- to 70-year-old patients with PHTN when compared with an optimal BP group (7.0 vs. 5.10 ng/L, respectively; P<0.01) (42). In that study, the rate of developing PHTN was higher when the IL-17 concentration was >3.5 ng/L, while linear regression modeling showed a positive relationship between increased IL-17 and SBP after controlling for other variables (P<0.05).
Sialic acid (SA) is an acute-phase protein associated with increased cardiovascular morbidity. Increased SA concentrations were reported in various CVD, including MI and essential HTN (43). Further studies have shown a positive association between increased SA concentrations and PHTN (P<0.01) (43), as well as higher SA concentrations in prehypertensive subjects compared with healthy controls (P=0.015), with SA being independently associated with SBP (P=0.01) and DBP (P=0.04) (44). Increased tumor necrosis factor-α, IL-6, amyloid-a, and homocysteine concentrations, and white blood cell counts, were also confirmed in PHTN patients compared with a normotensive group (37).
Endothelial dysfunction
The balance in the production the endothelium-derived relaxing factors (e.g., NO) and endothelium-derived constricting factors (e.g., endothelin 1) typically favors basal vasodilatation, and thus the maintenance of normal BP. Dysfunction of the vascular endothelium is involved in the pathogenesis of atherosclerotic vascular disease and atherogenesis (45). Endothelial injury and dysfunction are thought to contribute to cardiovascular risk in PHTN patients.
NO-mediated endothelial dysfunction
Vascular endothelial function is mainly regulated by NO production. Diminished NO-mediated endothelium-dependent vasodilation is linked to an increased risk of future CVD. The dysfunctional NO synthesis in PHTN may be a source of oxygen free radicals or reactive oxygen species, which may be an additive factor for developing overt HTN (46). The contribution of NO to endothelium-dependent vasodilation is also significantly diminished in adults with PHTN compared with normotensive adults. For example, forearm blood flow responses to acetylcholine, determined in the absence or presence of the endothelial NO synthase inhibitor NG-monomethyl-L-arginine (5 mg/min), were significantly lower (~30%) in PHTN patients (from 4.2±0.3 to 11.4±0.7 mL/100 mL tissue/min, respectively) compared with normotensive subjects (from 4.6±0.2 to 14.5±0.7 mL/100 mL tissue/min, respectively; P<0.05) (47). These findings suggest that PHTN is associated with NO-mediated impairment of vasodilatation. Interestingly, high levels of endothelin 1, angiotensin, arginine, and vasopressin were also reported in PHTN patients, which can also lead to NO-mediated impairment of vasodilatation. Moreover, fibrinolytic capacity is reduced in this population, leading to greater atherothrombotic risk (48).
Endothelial progenitor cell dysfunction
Endothelial progenitor cells (EPCs) are markers of cardiovascular health. EPC impairment has been linked to endothelial dysfunction and CVD. In PHTN, senescence of EPCs is substantially increased while NO production is markedly reduced. This pathophysiological process leads to impaired endothelial function, and thus represents an early event in the development of arterial HTN (49). The endothelial repair capacity of EPCs is also impaired in subjects with PHTN compared with healthy subjects (P<0.05) (50). Furthermore, EPC colony formation is impaired in adults with an SBP >130 mmHg (P<0.05) (51), while the negative influence of PHTN on EPC function is a consequence of elevated SBP rather than DBP.
Insulin resistance
Insulin resistance is a well-known characteristic of several cardiovascular disorders. Recent reports suggest the existence of insulin resistance in PHTN, which may play a role in the etiopathogenesis of HTN. For example, the Strong Heart Study conducted a 4-year follow-up of 625 PHTN patients and showed that those with developing HTN had a higher plasma glucose, insulin resistance, and HbA1c (hemoglobulin A1c) compared with participants without follow-up HTN (P<0.001) (52). In addition, a follow-up study of 1176 middle-aged and elderly participants showed a significantly higher fasting plasma glucose level in PHTN patients compared with those with an optimal BP (P<0.01) (53).
Sympathovagal imbalance
The autonomic nervous system (ANS) controls major functions of the body including circulation, respiration, digestion, immunity, and metabolism. The ANS executes its functions via the sympathetic and parasympathetic systems. As ANS activity contributes to the regulation of cardiac output and BP during rest, exercise, and in CVD, abnormalities in autonomic physiology, especially increased sympathetic activity, attenuated vagal tone, and delayed heart rate recovery, have been associated with increased mortality (54).
Sympathovagal imbalance (SVI) contributes to cardiovascular dysfunction in PHTN, whereby vagal withdrawal is associated with sympathetic overactivity. Sympathetic activation leads to vasoconstriction that increases vascular resistance and systemic arterial pressure. Vasoconstriction increases cardiac afterload, resulting in ventricular hypertrophy and an increase in the myocardial demand for oxygen and nutrition. Persistent sympathetic overactivity causes vascular damage and contributes to increased BP. Vascular hypertrophy facilitates the process of atherosclerosis and decreases blood flow to end organs. This predisposes the individual to ischemic organ damage such as CHD. Vagal withdrawal increases heart rate and decreases heart rate variability (HRV), a sensitive marker of SVI. Decreased HRV is known to cause CV morbidity and mortality (55). Heart rate recovery was also reported to be significantly lower in PHTN patients compared with a normal BP group (P<0.05), confirming the development of ANS dysfunction in PHTN patients (56). Additionally, a sex-related difference exists in the SVI (57), where the ratio of the low-frequency to high-frequency powers of the HRV spectrum was significantly higher in men with PHTN than in women (P<0.001). The vagal tone of patients with PHTN should be maintained at a higher level to prevent further increases in BP (57). Thus, PHTN patients should consider lifestyle modifications to achieve effective sympathovagal homeostasis. Future research should address the impact of these non-pharmacological therapies on the quality of improvement in SVI for the management of PHTN.
Others
Chronic activation of the renin-angiotensin system (RAS) was shown to underlie HTN, insulin resistance, and cardiac diseases (58,59). In addition, the RAS may contribute to the atherosclerotic process via angiotensin II, which acts as a proinflammatory mediator to induce atherosclerotic plaque development and exacerbate endothelial dysfunction (60). Blockade of the RAS can delay the transition of PHTN to HTN (61). Overall, these findings suggest that activation of the RAS plays a role in the progression of PHTN.
There is also evidence that reactive oxygen species can contribute to the development of overt PHTN. The rationale for antioxidant trials in PHTN was reviewed by Nambiar et al. (62). Compared with controls, prehypertensive subjects were reported to show depletion of erythrocyte glutathione but elevated malondialdehyde concentrations (63), the increase in plasma malondialdehyde suggests that oxygen free radicals may already have exerted their cytotoxic effects. In that study, plasma protein carbonyl levels were also significantly higher in prehypertensive subjects, implying that free radical-mediated oxidative protein damage occurs at the PHTN stage.
Is it appropriate to treat PHTN?
Recent guidelines recommend lifestyle adjustments as the first step for the control of PHTN (64,65). For example, PHTN patients following the DASH (dietary approaches to stop hypertension) diet showed improved SBP and reduced development of HTN (66). Although the 2018 European Society of Cardiology (ESC) guidelines (65) do not recommend physical activity in patients with a BP >180/110 mmHg, exercise recommendations should be personalized and take into account the patient’s medical history, risk factors, and ability to exercise. Although lifestyle modifications can effectively lower BP and have favorable effects on reducing cardiovascular-associated morbidity, a healthier everyday lifestyle may be difficult to maintain in the long term.
The effectiveness and benefits of treatment on PHTN are controversial. Brunström et al. reported that treatment was associated with reduced risk for major cardiovascular events (RR: 0.90; 95% CI: 0.84–0.97), but not with survival (RR: 0.98; 95% CI: 0.89–1.07), in subjects with prior CHD (67). Furthermore, in trials on patients with a baseline SBP <140 mmHg, treatment was not associated with mortality (RR: 0.98; 95% CI: 0.90–1.06) or major cardiovascular events (RR: 0.97; 95% CI: 0.90–1.04). The ACC/AHA 2017 guidelines suggest assessing the 10-year ASCVD (atherosclerotic cardiovascular disease) risk for patients with a BP ≥130/80 mmHg (3). Drug therapies should also be initiated immediately for high-risk patients with an ASCVD risk ≥10%. Further studies in PHTN patients are required to determine the effects of pharmacological treatment on cardiovascular-related morbidity and mortality or other target organ damage, to determine the cost-effectiveness of medical interventions, and to determine the most effective drug. Several clinical studies examining the benefits of medications in PHTN patients are currently underway, including the Chinese High Normal Blood Pressure Study (CHINOM). We keenly anticipate their findings.
Conclusion
PHTN is highly prevalent and has adverse effects on health. An effective public health strategy is important to prevent the progression of PHTN. There are many options for concerned clinicians and patients to reduce the risk of PHTN. The aim of this review was to familiarize clinicians, health care professionals, and policymakers with the cardiovascular effects of PHTN and its potential pathogenic mechanisms. This information will help to guide policy and health care delivery approaches to improve disease prevention for the population with PHTN.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding: This work was supported by the the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320057), CAMS Innovation Fund for Medical Sciences (2016-I2M-1-002), the National Key Research and Development Program of China (2016YFC1300100).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5482
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-5482
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5482). The authors have no conflicts of interest to declare.
References
- 1.Robinson SC, Brucer M. Range of normal blood pressure: a statistical and clinical study of 11,383 persons. Arch Intern Med 1939;64:409-44. 10.1001/archinte.1939.00190030002001 [DOI] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018;138:e426-e483. [DOI] [PubMed] [Google Scholar]
- 4.Joint Committee for Guideline R. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol 2019;16:182-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng XJ, Dong GH, Wang D, et al. Epidemiology of prehypertension and associated risk factors in urban adults from 33 communities in China--the CHPSNE study. Circ J 2012;76:900-6. 10.1253/circj.CJ-11-1118 [DOI] [PubMed] [Google Scholar]
- 6.Fan Z, Liao Z, Zong X, et al. Differences in prevalence of prehypertension and hypertension in children and adolescents in the eastern, central and western regions of China from 1991-2011 and the associated risk factors. PLoS One 2019;14:e0210591. 10.1371/journal.pone.0210591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Yuan Z. Prevalence and risk factors for prehypertension and hypertension among adults in Central China from 2000-2011. Clin Exp Hypertens 2018;40:734-43. 10.1080/10641963.2018.1431252 [DOI] [PubMed] [Google Scholar]
- 8.Han M, Li Q, Liu L, et al. Prehypertension and risk of cardiovascular diseases: a meta-analysis of 47 cohort studies. J Hypertens 2019;37:2325-32. 10.1097/HJH.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Su L, Cai X, et al. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J 2014;167:160-8.e1. 10.1016/j.ahj.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 10.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Benjamin EJ, MacMahon S. Prevention and Control of Cardiovascular Disease in the Rapidly Changing Economy of China. Circulation 2016;133:2545-60. 10.1161/CIRCULATIONAHA.115.008728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan W, Wu J, Liu S, et al. Impact of prehypertension on the risk of major adverse cardiovascular events in a Chinese rural cohort. Am J Hypertens 2020;33:465-70. 10.1093/ajh/hpaa019 [DOI] [PubMed] [Google Scholar]
- 14.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 15.Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet 2001;358:1682-6. 10.1016/S0140-6736(01)06710-1 [DOI] [PubMed] [Google Scholar]
- 16.Faselis C, Doumas M, Kokkinos JP, et al. Exercise capacity and progression from prehypertension to hypertension. Hypertension 2012;60:333-8. 10.1161/HYPERTENSIONAHA.112.196493 [DOI] [PubMed] [Google Scholar]
- 17.Redwine KM, Acosta AA, Poffenbarger T, et al. Development of hypertension in adolescents with pre-hypertension. J Pediatr 2012;160:98-103. 10.1016/j.jpeds.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 18.Selassie A, Wagner CS, Laken ML, et al. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension 2011;58:579-87. 10.1161/HYPERTENSIONAHA.111.177410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 20.Lee SS, Ae Kong K, Kim D, et al. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J 2017;38:2599-607. 10.1093/eurheartj/ehx316 [DOI] [PubMed] [Google Scholar]
- 21.Grundvold I, Skretteberg PT, Liestøl K, et al. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertension 2012;59:198-204. 10.1161/HYPERTENSIONAHA.111.179713 [DOI] [PubMed] [Google Scholar]
- 22.Conen D, Tedrow UB, Koplan BA, et al. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation 2009;119:2146-52. 10.1161/CIRCULATIONAHA.108.830042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart MH, Lavie CJ, Shah S, et al. Prognostic Implications of Left Ventricular Hypertrophy. Prog Cardiovasc Dis 2018;61:446-55. 10.1016/j.pcad.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 24.Li T, Yang J, Guo X, et al. Geometrical and functional changes of left heart in adults with prehypertension and hypertension: a cross-sectional study from China. BMC Cardiovasc Disord 2016;16:114. 10.1186/s12872-016-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markus MR, Stritzke J, Lieb W, et al. Implications of persistent prehypertension for ageing-related changes in left ventricular geometry and function: the MONICA/KORA Augsburg study. J Hypertens 2008;26:2040-9. 10.1097/HJH.0b013e328308da55 [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Cho GY, Baik I, et al. Early abnormalities of cardiovascular structure and function in middle-aged Korean adults with prehypertension: The Korean Genome Epidemiology study. Am J Hypertens 2011;24:218-24. 10.1038/ajh.2010.213 [DOI] [PubMed] [Google Scholar]
- 27.Norton GR, Maseko M, Libhaber E, et al. Is prehypertension an independent predictor of target organ changes in young-to-middle-aged persons of African descent? J Hypertens 2008;26:2279-87. 10.1097/HJH.0b013e328311f296 [DOI] [PubMed] [Google Scholar]
- 28.Erdogan D, Yildirim I, Ciftci O, et al. Effects of normal blood pressure, prehypertension, and hypertension on coronary microvascular function. Circulation 2007;115:593-9. 10.1161/CIRCULATIONAHA.106.650747 [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama H, Hashimoto H, Matsumoto C, et al. Effects of aging and persistent prehypertension on arterial stiffening. Atherosclerosis 2011;217:130-4. 10.1016/j.atherosclerosis.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 30.Lehmann N, Erbel R, Mahabadi AA, et al. Accelerated progression of coronary artery calcification in hypertension but also prehypertension. J Hypertens 2016;34:2233-42. 10.1097/HJH.0000000000001080 [DOI] [PubMed] [Google Scholar]
- 31.Kim M, Yoo HJ, Kim M, et al. Associations among oxidative stress, Lp-PLA2 activity and arterial stiffness according to blood pressure status at a 3.5-year follow-up in subjects with prehypertension. Atherosclerosis 2017;257:179-85. 10.1016/j.atherosclerosis.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Cai X, Liu C, et al. Prehypertension and the risk of coronary heart disease in Asian and Western populations: a meta-analysis. J Am Heart Assoc 2015;4:e001519. 10.1161/JAHA.114.001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glasser SP, Khodneva Y, Lackland DT, et al. Prehypertension and incident acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Hypertens 2014;27:245-51. 10.1093/ajh/hpt200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomiyama H, Yamashina A. Arterial stiffness in prehypertension: a possible vicious cycle. J Cardiovasc Transl Res 2012;5:280-6. 10.1007/s12265-011-9345-4 [DOI] [PubMed] [Google Scholar]
- 35.Rubio-Guerra AF, Garro-Almendaro AK, Lozano-Nuevo JJ, et al. Prehypertension is associated with peripheral arterial disease and low ankle-brachial index. Indian Heart J 2018;70:502-5. 10.1016/j.ihj.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myredal A, Gan LM, Osika W, et al. Increased intima thickness of the radial artery in individuals with prehypertension and hypertension. Atherosclerosis 2010;209:147-51. 10.1016/j.atherosclerosis.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 37.Chrysohoou C, Pitsavos C, Panagiotakos DB, et al. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens 2004;17:568-73. 10.1016/j.amjhyper.2004.03.675 [DOI] [PubMed] [Google Scholar]
- 38.Pitsavos C, Chrysohoou C, Panagiotakos DB, et al. Abdominal obesity and inflammation predicts hypertension among prehypertensive men and women: the ATTICA Study. Heart Vessels 2008;23:96-103. 10.1007/s00380-007-1018-5 [DOI] [PubMed] [Google Scholar]
- 39.Mozos I, Jianu D, Gug C, et al. Links between High-Sensitivity C-Reactive Protein and Pulse Wave Analysis in Middle-Aged Patients with Hypertension and High Normal Blood Pressure. Dis Markers 2019;2019:2568069. 10.1155/2019/2568069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan L, Li G, Wan S, et al. The association between high-sensitivity C-reactive protein and blood pressure in Yi people. BMC Public Health 2019;19:991. 10.1186/s12889-019-7324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. JAMA 2003;290:2945-51. 10.1001/jama.290.22.2945 [DOI] [PubMed] [Google Scholar]
- 42.Yao W, Sun Y, Wang X, et al. Elevated Serum Level of Interleukin 17 in a Population With Prehypertension. J Clin Hypertens (Greenwich) 2015;17:770-4. 10.1111/jch.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sathiyapriya V, Selvaraj N, Nandeesha H, et al. Association between protein bound sialic acid and high sensitivity C-reactive protein in prehypertension: a possible indication of underlying cardiovascular risk. Clin Exp Hypertens 2008;30:367-74. 10.1080/10641960802275106 [DOI] [PubMed] [Google Scholar]
- 44.Jinghua L, Tie Z, Ping W, et al. The relationship between serum sialic acid and high-sensitivity C-reactive protein with prehypertension. Med Sci Monit 2014;20:551-5. 10.12659/MSM.890314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diehl KJ, Weil BR, Greiner JJ, et al. Impaired endogenous fibrinolytic capacity in prehypertensive men. J Hum Hypertens 2015;29:468-72. 10.1038/jhh.2014.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cosentino F, Patton S, d'Uscio LV, et al. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest 1998;101:1530-7. 10.1172/JCI650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weil BR, Stauffer BL, Greiner JJ, et al. Prehypertension is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation in sedentary adults. Am J Hypertens 2011;24:976-81. 10.1038/ajh.2011.88 [DOI] [PubMed] [Google Scholar]
- 48.Valente FM, Vespasiano P, Barbosa JA, et al. Endothelial Changes in Individuals with Prehypertension. Curr Hypertens Rev 2016;12:134-8. 10.2174/1573402111666150812143827 [DOI] [PubMed] [Google Scholar]
- 49.Duprez D, Toleuova A. Prehypertension and the cardiometabolic syndrome: pathological and clinical consequences. Expert Rev Cardiovasc Ther 2013;11:1725-33. 10.1586/14779072.2013.857272 [DOI] [PubMed] [Google Scholar]
- 50.Giannotti G, Doerries C, Mocharla PS, et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension 2010;55:1389-97. 10.1161/HYPERTENSIONAHA.109.141614 [DOI] [PubMed] [Google Scholar]
- 51.MacEneaney OJ, DeSouza CA, Weil BR, et al. Prehypertension and endothelial progenitor cell function. J Hum Hypertens 2011;25:57-62. 10.1038/jhh.2010.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Marco M, de Simone G, Roman MJ, et al. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension: the Strong Heart Study. Hypertension 2009;54:974-80. 10.1161/HYPERTENSIONAHA.109.129031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao W, Sun Y, Wang X, et al. High prevalence of metabolic syndrome in a middle-aged and elderly population with prehypertension in Tianjin. Clin Exp Hypertens 2015;37:369-74. 10.3109/10641963.2014.977487 [DOI] [PubMed] [Google Scholar]
- 54.Rosenwinkel ET, Bloomfield DM, Arwady MA, et al. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin 2001;19:369-87. 10.1016/S0733-8651(05)70223-X [DOI] [PubMed] [Google Scholar]
- 55.Pal GK, Pal P, Nanda N, et al. Cardiovascular dysfunctions and sympathovagal imbalance in hypertension and prehypertension: physiological perspectives. Future Cardiol 2013;9:53-69. 10.2217/fca.12.80 [DOI] [PubMed] [Google Scholar]
- 56.Shin K, Shin K, Hong S. Heart rate recovery and chronotropic incompetence in patients with prehypertension. Minerva Med 2015;106:87-94. [PubMed] [Google Scholar]
- 57.Pal GK, Pal P, Nanda N, et al. Sympathovagal imbalance in young prehypertensives: importance of male-female difference. Am J Med Sci 2013;345:10-7. 10.1097/MAJ.0b013e31824ba080 [DOI] [PubMed] [Google Scholar]
- 58.Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev 2012;13:136-49. 10.1111/j.1467-789X.2011.00942.x [DOI] [PubMed] [Google Scholar]
- 59.Ren J, Pulakat L, Whaley-Connell A, et al. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88:993-1001. 10.1007/s00109-010-0663-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vašků A, Bienertová-Vašků J, Pařenica J, et al. ACE2 gene polymorphisms and invasively measured central pulse pressure in cardiac patients indicated for coronarography. J Renin Angiotensin Aldosterone Syst 2013;14:220-6. 10.1177/1470320312460291 [DOI] [PubMed] [Google Scholar]
- 61.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006;354:1685-97. 10.1056/NEJMoa060838 [DOI] [PubMed] [Google Scholar]
- 62.Nambiar S, Viswanathan S, Zachariah B, et al. Oxidative stress in prehypertension: rationale for antioxidant clinical trials. Angiology 2009;60:221-34. 10.1177/0003319708319781 [DOI] [PubMed] [Google Scholar]
- 63.Sathiyapriya V, Nandeesha H, Bobby Z, et al. Perturbation of oxidant-antioxidant status in non-obese prehypertensive male subjects. J Hum Hypertens 2007;21:176-8. 10.1038/sj.jhh.1002121 [DOI] [PubMed] [Google Scholar]
- 64.2018 Chinese Guidelines for Prevention and Treatment of Hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol 2019;16:182-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 66.Hashemi R, Rahimlou M, Baghdadian S, et al. Investigating the effect of DASH diet on blood pressure of patients with type 2 diabetes and prehypertension: Randomized clinical trial. Diabetes Metab Syndr 2019;13:1-4. 10.1016/j.dsx.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 67.Brunstrom M, Carlberg B. Association of Blood Pressure Lowering With Mortality and Cardiovascular Disease Across Blood Pressure Levels: A Systematic Review and Meta-analysis. JAMA Intern Med 2018;178:28-36. 10.1001/jamainternmed.2017.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as