Abstract
Cancer Medications Enquiry Database (CanMED) is comprised of two interactive, nomenclature-specific databases within the Observational Research in Oncology Toolbox: CanMED-Healthcare Common Procedure Coding System (HCPCS) and CanMED-National Drug Code (NDC), described through this study. CanMED includes medications with a) a US Food and Drug Administration-approved cancer treatment or treatment-related symptom management indication, b) inclusion in treatment guidelines, or c) an orphan drug designation. To demonstrate the joint utility of CanMED, medication codes associated with female breast cancer treatment were identified and utilization patterns were assessed within Surveillance Epidemiology and End Results-Medicare (SEER) data. CanMED-NDC (11_2018 v.1.2.4) includes 6860 NDC codes: chemotherapy (1870), immunotherapy (164), hormone therapy (3074), and ancillary therapy (1752). Treatment patterns among stage I–IIIA (20 701) and stage IIIB–IV (2381) breast cancer patients were accordant with guideline-recommended treatment by stage and molecular subtype. CanMED facilitates identification of medications from observational data (eg, claims and electronic health records), promoting more standardized and efficient treatment-related cancer research.
Observational data from administrative claims and electronic health records are increasingly being used to assess medication treatment patterns and outcomes. In oncology, operationalizing medication data for research purposes is particularly complex. The increasing volume of medications available in practice to treat cancer (through breakthrough therapy, accelerated and regular approvals) and the therapeutic applications for these medications are rapidly changing (1). As such, cancer medications considered in treatment-related studies must be continuously updated to ensure appropriately focused assessments. Additionally, oncology therapies can be dispensed in multiple settings; therefore, complete capture of medication utilization frequently requires linkage across data sources (eg, practice and pharmacy claims) or healthcare systems and, thereby, an awareness of how medications are documented in each data source.
A primary medication classification system used for observational pharmacy data is the National Drug Code (NDC). An NDC is a unique identifier comprised of either nine digits (product NDC) divided into two segments where the first five digits identify the drug manufacturer and the next four digits identify the chemical agent, or 11 digits (package NDC), which includes the product NDC with an additional two-digit suffix to identify the package size (2,3). In observational data, NDCs are mainly used to document medications dispensed by a pharmacist in an outpatient setting as captured in pharmacy claims or in electronic health records.
Although NDCs have been required since 1972 for all commercially available, US Food and Drug Administration (FDA)–approved medications, a historical guide has not been readily available to the research community. In the absence of a comprehensive, longitudinal NDC database, challenges abound in identifying appropriate NDCs, particularly given NDCs are frequently created, discontinued, and even reassigned to completely different medications. Medications can also have multiple product and/or package NDCs, which can introduce variation and, thus, increased opportunities for medication under ascertainment via code omission. The accurate identification of oncology medications via NDCs for observational assessments of systemic cancer therapy (eg, chemotherapy or immunotherapy) is becoming increasingly important as the cancer treatment paradigm shifts from parenteral therapies primarily to the inclusion of oral therapeutics.
Recognizing the need for a comprehensive resource to provide standardized identification of cancer medications and relevant codes, the Cancer Medications Enquiry Database (CanMED) was created by the National Cancer Institute (NCI) Division of Cancer Control and Population Sciences. CanMED includes all US FDAapproved cancer medications and their associated codes in two queryable databases based on the primary pharmacy nomenclature classification systems: NDC and Healthcare Common Procedure Coding System (HCPCS). The development and utility of CanMED-HCPCS is described in a separate article (4). The primary aim of this article is to introduce CanMED-NDC and, through an example assessment of systemic breast cancer (BC) treatment, demonstrate the combined utility of CanMED-NDC and CanMED-HCPCS.
Methods
Development of CanMED- NDC
Oncology Medication Inclusion Strategy
The medication inclusion process and treatment assignment has previously been described (4). Briefly, medications were considered for inclusion in CanMED based on use as an agent in the treatment or management of cancer. As such, drugs must either a) have an FDA-approved labeled indication for cancer treatment or symptom management, b) be present in the National Comprehensive Cancer Network (NCCN) guidelines for the treatment or management of cancer, or c) carry an orphan drug indication for the treatment or management of cancer. Medications designated as over the counter (OTC) are not included; OTC medications are not approved for the treatment and management of cancer. All included medications are assigned to a mutually exclusive treatment category: chemotherapy, immunotherapy, hormonal therapy, and ancillary therapy. Included ancillary medications must be required for or directly associated with the administration or management of oncologic therapy. Adjacent therapies such as opioids are not included. The inclusion and categorization of each medication is adjudicated independently by two pharmacists. Discontinued medications and NDC codes are retained to allow for retrospective research.
NDC Identification
Initial Development of NDC Masterfile
In December 2017, the US FDA NDC Directory and the NDC Structured Product Labelling Data Elements File (2012–2017) were accessed to create an NDC Masterfile (5,6). All NDCs associated with oncology medications, as defined above, were selected by referencing this Masterfile. From the Masterfile, two subcategories of NDC were created: oncologic agents and nononcologic agents. Clinical expert assessment was employed to ensure the medication dosage and/or labeling for all NDCs included as oncologic agents were indicative of an oncology indication. All other NDCs were categorized as nononcologic agents (eg, medications not indicated to treat cancer or those that can be used to treat cancer but not in the form specified by the NDC, such as topical glucocorticoids). The Masterfile provides the infrastructure necessary to generate CanMED-NDC.
Automated Updates
NDCs for established (eg, previously included in the NDC Masterfile) medications can change due to the introduction, modification, or discontinuation of a medication product and/or package. Additionally, each time a new medication becomes commercially available, new NDCs are introduced. Therefore, in an effort to ensure CanMED-NDC remains timely, an automated strategy was developed.
An automated algorithm references and compares the US FDA NDC Directory with the Masterfile by brand and generic name for modifications. All new, modified, or discontinued NDCs are flagged for dual clinical expert review and evaluation. The algorithm is updated accordingly. For example, if the clinical experts determine the new NDC (eg, a new product or package) is indicative of cancer treatment, then the new NDC is classified as an oncologic in the Masterfile and available in CanMED-NDC. Conversely, if the clinical experts determine that the flagged NDC is not indicative of cancer treatment (eg, the product or package was not approved for use in oncology), then the NDC is categorized as a nononcologic in the Masterfile so that it will not be flagged for review in the future. If an oncologic agent NDC is no longer found to be included in the NDC Directory, the product and package NDCs are retained for historic analyses with a discontinuation date, when available.
Discovery of newly FDA-approved molecular entities (eg, medications not previously included in the Masterfile) is accomplished in a similar manner by referencing the NDC Directory weekly. Any NDC associated with a new medication, as identified by generic name, is flagged for dual clinical expert review and evaluation. If it is determined that the medication NDC is indicative of an oncology indication, then the NDC is included in the Masterfile as an oncologic agent and available in CanMED-NDC. Conversely, if it is deemed that the medication is not indicative of an oncology indication, then the NDC is included in the Masterfile as a nononcologic. Again, in future comparative referencing any NDCs that have been categorized as a nononcologic are no longer flagged for review.
Dual Review and Maintenance
The NDC Masterfile update process is electronically initiated when the automated algorithm identifies four categories of NDCs that need additional manual review: new entity NDC, new NDC, modified NDC, and removed NDC. A dual manual review process on each queue is then conducted which requires dual clinician agreement before changes are made to the Masterfile. The first reviewer conducts a comprehensive review of the medication and associated NDC, after which a recommendation is submitted to the second reviewer. The second reviewer assesses the submitted materials and has the authority to make changes to the NDC Masterfile. Content disagreements are resolved by consensus. The NDC Masterfile is reviewed and maintained through this process on a regular basis. System updates and algorithm refinement are ongoing to improve the quality and efficiency of the resource.
CanMED-NDC
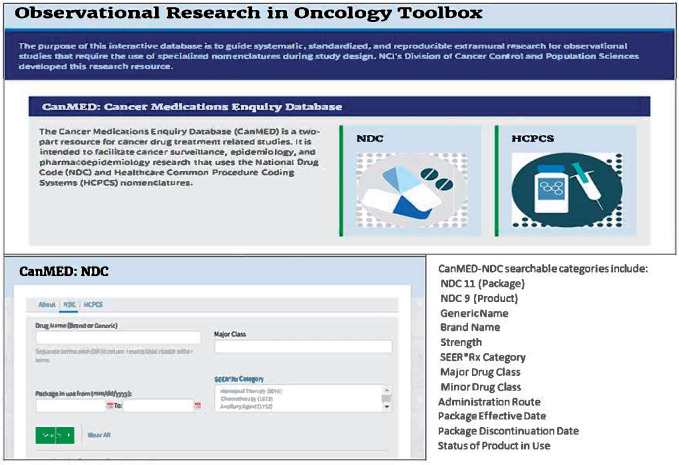
The data in CanMED-NDC include only those NDCs that have been reviewed and determined to be oncologic agents. The resulting database provides searchable features to find relevant NDCs for specific medications, minor and major drug classifications, or even treatment categories (eg, chemotherapy, hormonal therapy, immunotherapy, or ancillary therapy). Treatment categorization is based on Surveillance, Epidemiology, and End Results (SEER)*Rx, which is a tool developed for cancer registries to assign treatment (7). NDC searches can also be limited by year to identify codes that were in effect during a specific time period. Discontinued NDCs are retained with a discontinuation date, when known, to allow for historic analyses. Variables available in the CanMED-NDC include: NDC 11 (Package), NDC 9 (Product), Generic Name, Brand Name, Strength, SEER*Rx Treatment Category, Major Drug Class, Minor Drug Class, Administration Route, Package Effective Date, Package Discontinuation Date, and Status of Product in Use (Figure 1). The query results from searches are exportable to Excel for use with common statistical analysis packages such as SAS and R.
Figure 1.
Observational research in oncology toolbox, Cancer Medications Enquiry Database (CanMED)-National Drug Code (NDC) interface.
Case Example
As an example to showcase the potential application of CanMED-NDC along with CanMED-HCPCS in facilitating treatment-related observational research, SEER-Medicare, which is a linkage of the SEER Program population-based cancer registry data with Medicare enrollment and claims data, were analyzed to describe BC systemic therapy patterns (8). Medications assessed were identified using NCCN guidelines, which allowed for stage-specific and molecular subtype characterization.
Female patients were included if diagnosed at age 66 years or older with a first primary BC between 2010 and 2013. Patients were excluded from the analysis if they had in situ (stage 0) or unknown stage or if they had insufficient information to determine molecular subtype (eg, unknown estrogen receptor [ER], progesterone receptor [PR], or human epidermal growth factor receptor [HER] 2 status). Patients were also required to have continuous fee-for-service Medicare Parts A (inpatient), B (outpatient), and D (prescription drug) enrollment for at least 6 months post diagnosis, including the month of cancer diagnosis. Medicare claims were available through 2014.
CanMED was queried for NCCN guideline–recommended BC medications (Table 1) to identify appropriate NDC and HCPCS codes (9). Medicare claims were then reviewed to identify medication utilization based on NDCs (Part D) and HCPCS (Part B). Medications were analyzed based on treatment category: chemotherapy, immunotherapy, hormonal therapy, and ancillary therapy. Stage based on derived American Joint Committee on Cancer version 7 was dichotomized as stage I–IIIA and stage IIIB–IV; stage III, not otherwise specified, was included with stage IIIB–IV. Hormone receptor (HR) status was classified as positive, if ER or PR positive, and negative, if ER and PR negative. Borderline ER or PR status was considered positive. Molecular subtype was classified as luminal A [HR+/HER2−]), luminal B [HR+/HER2+], HER2 enriched [HR−/HER2+], and triple negative [HR−/HER2−]. Patients with borderline HER2 status (n = 575) were excluded from the molecular subtype analysis. Descriptive statistics were calculated by stage and molecular subtype to describe treatment patterns among BC patients within 6 months of diagnosis.
Table 1.
CanMED Case Example: FDA Indications for BC treatment and NCCN Guideline–Directed Therapies for Stage I–IV Breast Cancer Treatment, 2018
| Generic | Brand | Administration route | FDA approval | Off label use | Stage I–IIIA | Stage IIIB-IV* | FDA indication | NCCN guidelines | HER2 | HR: ER/PR |
|---|---|---|---|---|---|---|---|---|---|---|
| Ado-trastuzumab emtansine | Kadcyla | IV | 2013 | — | — | X | Yes | Yes | Yes | — |
| Anastrazole | Arimidex | Oral | 1995 | — | X | — | Yes | Yes | — | Yes |
| Capecitabine | Xeloda | Oral | 1998 | — | — | X | Yes | Yes | — | — |
| Carboplatin | Carboplatin | IV | 1989 | — | X | — | Yes | Yes | — | — |
| Cisplatin | Cisplatin | IV | 1978 | — | — | X | No | Yes | — | — |
| Cyclophosphamide | Cyclophosphamide | Both | 1959 | — | X | — | Yes | Yes | — | — |
| Denosumab | Prolia, Xgeva | IV | 2010 | Ancillary | — | X | Yes | Yes | — | — |
| Dexrazoxane | Totect, Zinecard | IV | 1995 | Ancillary, may be useful to identify multiple primaries | X | — | Yes | No | — | — |
| Docetaxel | Taxotere, Docefrez | IV | 1996 | — | X | — | Yes | Yes | — | — |
| Doxorubicin | Adriamycin | IV | 1989 | — | X | — | Yes | Yes | — | — |
| Doxorubicin liposomal | Doxil, Lipodox | IV | 1995 | — | X | — | Yes | Yes | — | — |
| Epirubicin | Ellence | IV | 1999 | — | X | — | Yes | Yes | — | — |
| Eribulin | Halaven | IV | 2010 | — | — | X | Yes | Yes | — | — |
| Everolimus | Afinitor | Oral | 2009 | — | — | X | Yes | Yes | — | — |
| Exemestane | Aromasin | Oral | 1999 | — | X | — | Yes | Yes | — | Yes |
| Fluorouracil | Adrucil | IV | 1962 | — | X | — | Yes | Yes | — | — |
| Fulvestrant | Faslodex | IM | 2002 | — | — | X | Yes | Yes | — | Yes |
| Gemcitabine | Gemzar | IV | 1996 | — | — | X | Yes | Yes | — | — |
| Goserelin acetate | Zoladex | IV | 1989 | — | X | — | Yes | Yes | — | — |
| Ixabepilone | ixempra | IV | 2007 | — | — | X | Yes | Yes | — | — |
| Lapatinib | Tykerb | Oral | 2007 | — | X | Yes | Yes | Yes | — | |
| Letrozole | Femara | Oral | 1997 | — | X | — | Yes | Yes | — | Yes |
| Leucovorin calcium | Wellcovorin | IV only indicated | 1952 | Ancillary, may be useful to identify multiple primaries | X | — | Yes | No | — | — |
| Levoleucovorin | Fusilev | IV | 2008 | Ancillary, may be useful to identify multiple primaries | X | — | Yes | No | — | — |
| Megestrol acetate | Megace | Oral | 1993 | — | — | X | Yes | Yes | — | Yes |
| Mesna | Mesnex | Oral, IV | 1988 | Ancillary | X | — | Yes | No | — | — |
| Methotrexate | Trexall | Oral, IV, IM | 1953 | — | X | — | Yes | Yes | — | — |
| Methyltestosterone | Android, Testred | Oral | 1935 | — | — | X | Yes | No | — | — |
| Paclitaxel | Taxol, Pacitaxel | IV | 1992 | — | X | — | Yes | Yes | — | — |
| Paclitaxel protein bound | Abraxane | IV | 1992 | — | X | — | Yes | Yes | — | — |
| Palbociclib | Ibrance | Oral | 2015 | — | — | X | Yes | Yes | — | — |
| Pamidronate | Aredia | IV | 1998 | Ancillary, may be useful to identify multiple primaries | X | Yes | Yes | — | — | |
| Pertuzumab | Perjeta | IV | 2012 | — | X | — | Yes | Yes | Yes | — |
| Raloxifene hydrocholride | Evista | Y | 1997 | — | X | — | Yes | Yes | — | Yes |
| Ribociclib | Kisqali | Oral | 2017 | — | — | X | Yes | Yes | — | — |
| Ribociclib/letrozole | Kisqali Femara Co-Pack | Oral | 2017 | — | — | X | Yes | Yes | — | — |
| Tamoxifen | Nolvadex, Soltamox | Oral | 1977 | — | X | Yes | Yes | — | Yes | |
| Testosterone enanthate | Delatestryl | IV | 1953 | — | — | X | Yes | No | — | — |
| Toremifene | Fareston | Oral | 1997 | — | X | — | Yes | Yes | — | Yes |
| Trastuzumab | Herceptin | IV | 1998 | — | X | — | Yes | Yes | Yes | — |
| Vinblastine | Velban | IV | 1965 | — | — | X | Yes | No | — | — |
| Vinorelbine | Navelbine | IV | 1994 | — | — | X | No | Yes | — | — |
Includes stage III, NOS. FDA = Food and Drug Administration; NCCN = National Comprehensive Cancer Network; HER2 = Human Epidermal Growth Factor Receptor 2; NOS = Not Otherwise Specified; HR: ER/PR = Hormone Receptor, Estrogen Receptor, Progesterone Receptor.
Results
CanMED-NDC (11_2018 v.1.2.4) is a comprehensive database that includes 6860 NDC codes for oncology medications: chemotherapy (1870), immunotherapy (164), hormonal therapy (3074), and ancillary therapy (1752).
Case Example
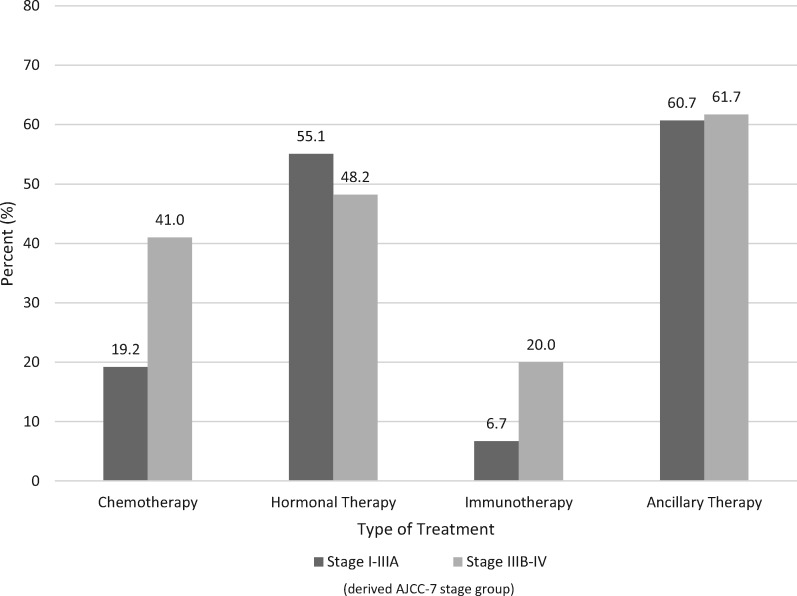
Among the defined SEER-Medicare cohort, treatment patterns were evaluated in two clinically meaningful methods: treatment by stage and treatment by molecular subtype. There were 23 082 BC patients included in the analyses by stage (stage I–IIIA: 20 701 and 2381 with stage IIIB–IV). Among patients with stage I–IIIA tumors, 19.2% received chemotherapy, 55.1% received hormonal therapy, 6.7% received immunotherapy, and 60.7% received ancillary therapy (Figure 2). Among patients with stage IIIB–IV tumors, 41.0% received chemotherapy, 48.2% received hormonal therapy, 20.0% received immunotherapy, and 61.7% received ancillary therapy during the study period.
Figure 2.
Percent of breast cancer patients receiving systemic therapy within 6 months of diagnosis by treatment type and stage group, Surveillance, Epidemiology, and End Results-Medicare, 2010–2014.
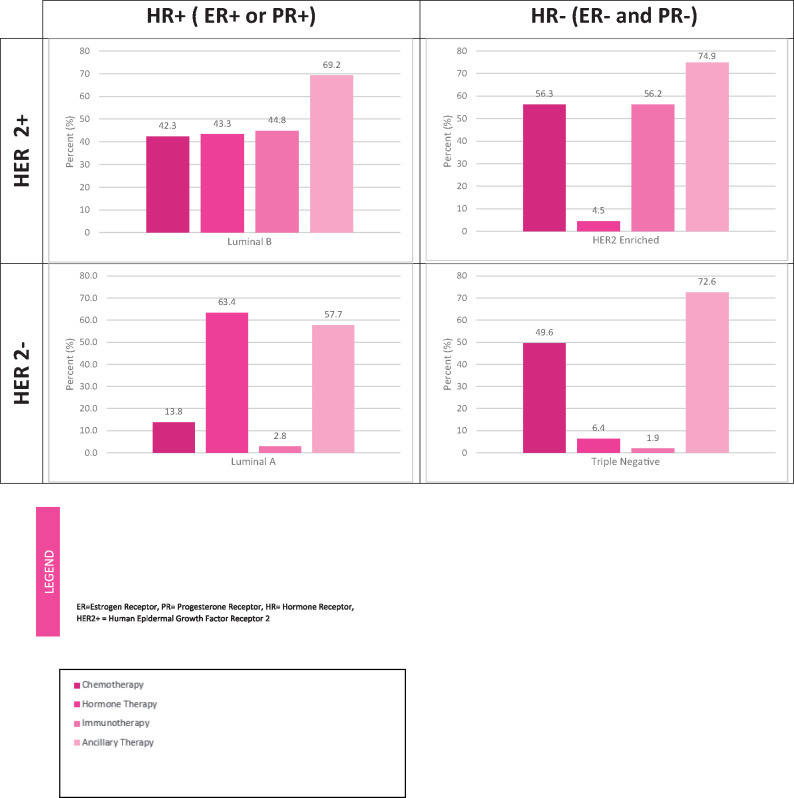
Treatment patterns were additionally analyzed in 22 507 patients by the four relevant BC molecular subtypes: luminal A, luminal B, HER enriched, and triple negative (Figure 3). The primary treatment administered varied by molecular subtype. The most prominent modality for patients with luminal A tumors was hormonal therapy (63.4%) and for patients with triple negative tumors was chemotherapy (49.6%). Roughly 43% of patients with luminal B tumors received either chemotherapy, hormonal therapy, or immunotherapy, and HER-enriched patients were primarily treated with chemotherapy (56.3%) and immunotherapy (56.2%).
Figure 3.
Breast cancer treatment utilization by molecular subtype, Surveillance, Epidemiology, and End Results-Medicare, 2010–2014. ER = Estrogen Receptor; PR = Progesterone Receptor; HR = Hormone Receptor; HER2+ = Human Epidermal Growth Factor Receptor 2.
Discussion
The primary objective of CanMED-NDC is to provide a current, comprehensive resource that provides a standardized method of identifying NDC codes for systemic oncology medications or, conversely, the identification of medications associated with NDC codes extracted from observational data. The database can be used by cancer registries to facilitate ascertainment of treatment from medical and pharmacy claims for case finding and collection of detailed treatment and among the broader research community to conduct assessments of treatment patterns. Utilization of the database(s) can also increase comparability, replication, and standardization across cancer surveillance, epidemiology, and pharmacoepidemiology studies. CanMED nomenclatures can also be further linked with other ontologies (eg, RxNorm) for additional applications.
The analysis of BC treatment trends by stage reflects what was expected. For example, in comparision with patients with stage I–IIIA tumors, clinically significantly more patients with stage IIIB–IV tumors received chemotherapy and immunotherapy; receipt of hormonal therapy and ancillary medications was comparable by stage group. The evaluation of the treatment by molecular subtype reflects the current approach to biomarker-guided BC treatment and is largely concordant with treatment guidelines. BC patients with the luminal A subtype, characterized by HR+, had the greatest receipt of hormonal therapy, and luminal B and HER 2-enriched subtypes had greater receipt of immunotherapy due to use of trastuzumab for HER2+ tumors.
The case example demonstrated how the CanMED databases can facilitate treatment-related research. In the current study, a broad understanding of the BC treatment landscape was of interest, which required the combiend use of both CanMED databases. The CanMED-NDC provided the ability to ascertain treatment in data categorized by NDC such as Part D oral therapy claims (eg, BC hormonal therapies), and the CanMED-HCPCS provided the ability to identify systemic therapies within data sourced by HCPCS codes such as Part B (eg, chemotherapies and immunotherapies). The use of databases was tailored to the study question. If the study objective was to evaluate either oral therapy or systemic therapy, then only the CanMED-NDC or CanMED-HCPCS database was used respectively. The development and utility of CanMED-HCPCS is described in a separate article (4). Consultation with a data expert and a clinician is recommended to decide which database(s) are most appropriate to use for a particular data source and observational research study question.
Similar to those previously described for CanMED-HCPCS (4), there are limitations to the CanMED-NDC that need to be considered. First, the development and maintenance of CanMED-NDC are reliant on publicly available historical (2012–2017) and regular data updates to online documentation provided by the FDA. Therefore, it is possible that some historical codes were not available, and some fields may be left blank if unavailable (eg, effective or discontinuation dates). However, we aim through our detailed processes to provide the most comprehensive, public resource for oncology drug codes. The constantly changing landscape of cancer treatment makes the prospective development of the CanMED-NDC both challenging and necessary. Because of the clinician review process, there may be a lag time between when a new therapy is FDA approved and when it is included in the CanMED-NDC. However, to stay as relevant as possible, the CanMED-NDC will be maintained through a dual review process by NCI clinicians and any changes will be regularly updated.
There are other limitations that researchers need to consider when using CanMED-NDC. Certain medications, such as methotrexate, can be used for cancer and for multiple other conditions unrelated to cancer (eg, treatment of rheumatoid and juvenile arthritis or recalcitrant psoriasis). Users should be cautious when including such medications in their cancer-related treatment analyses. It is recommended that medications with multiple indications should accompany a cancer diagnosis (International Classification of Diseases code), be a specific part of an administered cancer regimen (R-CHOP), or be used in a cancer-specific algorithm to be considered cancer-related treatment (10–12). For increased confidence, this recommendation may be extended to any medication assessed.
Researchers also need to be cognizant that identification of cancer-related therapies solely using NDC codes may lead to underascertainment because cancer medications are also often administered in an inpatient setting, which is billed used a different coding nomenclature: HCPCS (eg, Medicare Part B) (13). The CanMED-HCPCS was developed complementarily for a more complete view of medication use. The systems are interoperable. Medications that do not have an associated HCPCS code appear as HCPCS: “NA” in the search, and CanMED-NDC can then be queried to assess if any relevant NDCs for oral and outpatient medications are available depending on the study question. As mentioned, researchers conducting administrative claims–based studies of cancer treatment are advised to use both the CanMED-HCPCS and CanMED-NDC, when applicable, to identify a comprehensive list of relevant codes to understand exposure and longitudinal medication utilization.
The CanMED-NDC provides a comprehensive, clinician-reviewed resource for oncology drug codes that can be used for observational research. Along with CanMED-HCPCS, this database allows researchers to rigorously assess the codes required to capture systemic cancer treatment in the most complete resource currently available. The use case demonstrated how CanMED can be queried and translated into an actionable analysis of clinically relevant BC treatment trends by stage and molecular subtype using SEER-Medicare data. The interactively designed CanMED-NDC will be valuable to the research community and encourage standardization of medication identification for high-quality oncology treatment research.
Notes
Affiliations of authors: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD (DRR, AG, CJKL, LP, VIP); Health Care Delivery Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD (LE); Information Management Services, Inc., Calverton, MD (BO, TSM, SB).
The authors have no conflicts of interest reported.
References
- 1. Chen EY, Raghunathan V, Prasad V.. An overview of cancer drugs approved by the US Food and Drug Administration based on the surrogate end point of response rate. JAMA Intern Med. 2019;179(7):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slavin M. The National Drug Code. Am J Hosp Pharm. 1972;29(6):468–470. [PubMed] [Google Scholar]
- 3. Slavin M. Origin, development and application of the National Drug Code. J Am Pharm Assoc. 1969;9(9):460–462. [DOI] [PubMed] [Google Scholar]
- 4. Rivera DR, Lam CJK, Petkov VI.. Development and utility of the observational research in oncology toolbox: Cancer Medications Enquiry Database (CanMED) Healthcare Common Procedure Coding System (HCPCS). Cosubmission with supplement. J Natl Cancer Inst Monogr. 2020;2020(55):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. National Drug Code Directory. https://www.accessdata.fda.gov/scripts/cder/ndc/. Accessed August 2, 2017.
- 6.US Food and Drug Administration. Structured Product Labeling (SPL) Data Elements (NSDE). https://www.fda.gov/industry/structured-product-labeling-resources/nsde. Accessed August 2, 2017.
- 7.National Cancer Institute, Division of Cancer Control and Population Sciences. Surveillance Research Program. SEER*Rx Interactive Antineoplastic Drugs Database. February 8, 2019. https://seer.cancer.gov/seertools/seerrx/. Accessed April 17, 2018.
- 8. Warren J, Klabunde C, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(Supplement):IV–IV18. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network Breast Cancer Guidelines v. 2018. https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. Accessed April 17, 2018.
- 10. Ritzwoller DP, Hassett MJ, Uno H, et al. Development, validation, and dissemination of a breast cancer recurrence detection and timing informatics algorithm. J Natl Cancer Inst. 2018;110(3):273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clarke CL, Feigelson HS.. Developing an algorithm to identify history of cancer using electronic medical records. eGEMs. 2016;4(1):1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warren JL, Mariotto A, Melbert D, et al. Sensitivity of Medicare claims to identify cancer recurrence in elderly colorectal and breast cancer patients. Med Care. 2016;54(8):e47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lund JL, Sturmer T, Harlan LC, et al. Identifying specific chemotherapeutic agents in Medicare data: a validation study. Med Care. 2013;51(5):e27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]