Abstract
Autophagy is a conserved lysosomal pathway for the degradation of cytoplasmic components. Basal autophagy in kidney cells is essential for the maintenance of kidney homeostasis, structure and function. Under stress conditions, autophagy is altered as part of the adaptive response of kidney cells, in a process that is tightly regulated by signalling pathways that can modulate the cellular autophagic flux — mammalian target of rapamycin, AMP-activated protein kinase and sirtuins are key regulators of autophagy. Dysregulated autophagy contributes to the pathogenesis of acute kidney injury, to incomplete kidney repair after acute kidney injury and to chronic kidney disease of varied aetiologies, including diabetic kidney disease, focal segmental glomerulosclerosis and polycystic kidney disease. Autophagy also has a role in kidney ageing. However, questions remain about whether autophagy has a protective or a pathological role in kidney fibrosis, and about the precise mechanisms and signalling pathways underlying the autophagy response in different types of kidney cells and across the spectrum of kidney diseases. Further research is needed to gain insights into the regulation of autophagy in the kidneys and to enable the discovery of pathway-specific and kidney-selective therapies for kidney diseases and anti-ageing strategies.
Autophagy is a lysosomal degradation pathway that breaks down cytoplasmic components for clearance and reuse. Depending on the type of cargo to be degraded and the route of cargo delivery to lysosomes, autophagy can be classified as macroautophagy, microautophagy or chaperone-mediated autophagy. Macroautophagy refers to the engulfment of large cytoplasmic materials by autophagosomes, which then fuse with lysosomes for degradation1. Microautophagy involves the direct sequestration of small cytosolic components into an inward invagination of the lysosomal membrane without the formation of autophagosomes2. In chaperone-mediated autophagy, proteins containing the KFERQ motif are identified by cytosolic heat shock cognate 71 kDa protein (encoded by HSPA8) and then translocated across the lysosomal membrane via interactions with lysosome-associated membrane glycoprotein 2A (LAMP2) for delivery into the lysosomal lumen through multimerization of LAMP2 with other cofactors3.
Macroautophagy (hereafter termed autophagy) has a variety of physiological and pathophysiological roles1,4,5. Basal autophagy removes potentially dysfunctional organelles and long-lived proteins to maintain cellular homeostasis, whereas autophagy induced in response to environmental and intracellular stress might serve as an adaptive response to ensure cell survival. However, autophagy might also contribute to cellular dysfunction and organ damage.
In this Review, we provide an overview of the cellular and molecular basics of autophagy, analyse the physiological roles of basal autophagy in the major types of resident kidney cells and summarize current evidence on the regulation and pathological roles of autophagy in glomerular and tubulointerstitial diseases. The therapeutic potential and challenges of targeting autophagy for the prevention and treatment of kidney diseases are also discussed.
Molecular basics of autophagy
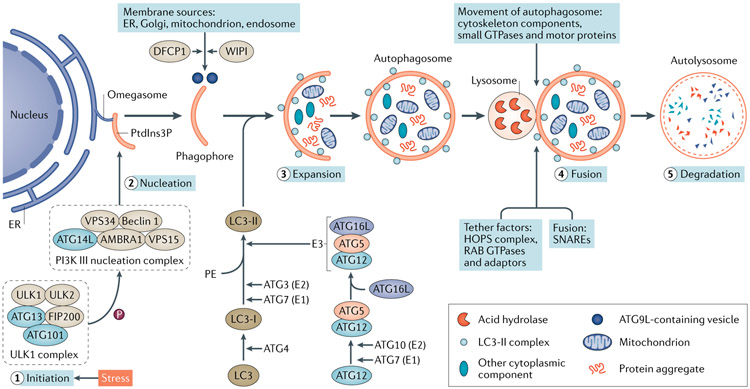
Autophagy is a multistep process that involves initiation, nucleation, expansion, fusion and degradation (FIG. 1). Several distinct complexes that are composed of autophagy-related proteins (ATGs) function co-ordinately with membrane trafficking components at different steps of autophagosome biogenesis (FIG. 1). The complex formed by serine/threonine protein kinases ULK1, ULK2 and other protein partners is a major component in the initiation of autophagy6-8, whereas the class III phosphoinositide 3-kinase (PI3K) complex regulates vesicle nucleation and phagophore formation9,10. Two ubiquitin (Ub)-like conjugation systems, the ATG12–ATG5–ATG16L system and the microtubule-associated protein 1 light chain 3 (MAP1LC3; also known as LC3) system control the expansion and completion of the autophagosome11 (FIG. 1). The regulation of autophagosome–lysosome fusion to form autolysosomes is less well understood. Cytoskeleton components and cytoskeleton-related motor proteins mediate the movement of autophagosomes to lysosomes for tethering, followed by soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated fusion12. Notably, autolysosomes are not permanent and disintegrate once autophagy is terminated. This process, called autophagic lysosome reformation (ALR), allows recycling of lysosomal membrane proteins and regeneration of lysosomes from autolysosomes through reformation tubules and vesicles.
Fig. 1 ∣. Autophagy dynamics and core machinery.
Autophagy is a muitistep process involving initiation, nucleation, expansion, fusion and degradation. Upon induction, the ULK1 complex — comprising the serine/threonine protein kinases ULK1 and ULK2, autophagy-related protein 13 (ATG13), FAK family kinase-interacting protein of 200 kDa (FIP200) and ATG101 — phosphorylates multiple substrates, including components of the phosphoinositide 3-kinase (PI3K) III nucleation complex to promote phagophore nucleation. The PI3K III complex comprises PI3K catalytic subunit type 3 (VPS34), PI3K regulatory subunit 4 (VPS15), beclin 1, beclin 1-associated autophagy-related key regulator (ATG14L) and activating molecule in beclin 1-regulated autophagy protein 1 (AMBRA1). This complex produces phosphatidylinositol-3-phosphate (PtdIns3P) at the omegasome, which recruits PtdIns3P-binding proteins for phagophore expansion. The delivery of membrane from other sources to the expanding phagophore requires PtdIns3P-binding proteins WD repeat domain phosphoinositide-interacting proteins (WIPIs) and zinc-finger FYVE domain-containing protein 1 (DFCP1), as well as cycling of the transmembrane protein ATG9L in the form of membrane vesicles and tubules. Two ubiquitin-like conjugation systems, the ATG12–ATG5–ATG16L system and the microtubule-associated protein 1 light chain 3 (LC3) system, participate in autophagosome elongation and completion. The conversion of cytosolic LC3-I into membrane-bound LC3-II is indicative of autophagy induction and autophagosome formation. The completed autophagosome then fuses with a lysosome to form an autolysosome, in which the autophagosome inner membrane and cargos are degraded by lysosomal hydrolases and eventually released for recycling. The fusion process is regulated by a large set of molecules, including cytoskeleton components and related motor proteins, tethering factors including the homotypic fusion and vacuole protein sorting (HOPS) complex, the RAB GTPases, and specific soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes. E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme; E3, ubiquitin ligase; ER, endoplasmic reticulum; PE, phosphatidylethanolamine.
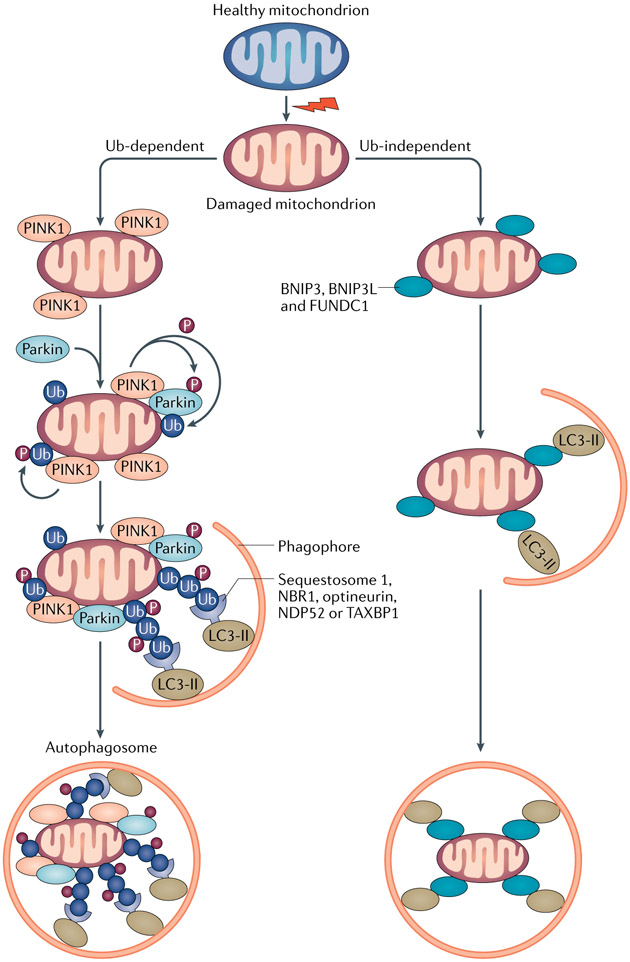
In addition to non-selective cytosolic bulk degradation, autophagy can also selectively remove specific cargoes, such as mitochondria by mitophagy, lysosomes by lysophagy, protein aggregates by aggrephagy and lipid droplets by lipophagy13. Mitophagy is the best-described form of selective autophagy and is predominantly mediated by LC3-associated autophagy receptors via both Ub-dependent and Ub-independent pathways (FIG. 2).
Fig. 2 ∣. Mitophagy pathways and cargo recognition.
Mitophagy is mainly mediated by microtubule-associated protein 1 light chain 3 (LC3)-associated autophagy receptors via both ubiquitin (Ub)-dependent and Ub-independent pathways. Ub-dependent mitophagy involves the mitochondrial serine/threonine protein kinase PINK1 and E3 Ub-protein ligase parkin (PINK1–parkin) pathway. Following loss of mitochondrial membrane potential, PINK1 accumulates on the outer mitochondrial membrane (OMM), where it recruits and phosphorylates parkin to add phospho–Ub chains on OMM proteins. Autophagy receptors, such as sequestosome 1, next to BRCA1 protein (NBR1), optineurin, calcium-binding and coiled-coil domain-containing protein 2 (NDP52) and TAX1-binding protein 1 (TAX1BP1), which contain both Ub-binding domains and LC3-interacting regions (LIRs), bridge the ubiquitylated mitochondria to LC3-associated autophagosomal membranes for sequestration. PINK1-mediated phosphorylation of ubiquitin might be sufficient to recruit NDP52 and optineurin and induce mitophagy independently of parkin. Ub-independent mitophagy is mediated by several OMM receptors such as BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3), BNIP3-like (BNIP3L), FUN14 domain-containing protein 1 (FUNDC1), which do not require interaction with Ub for cargo recognition. Peptidyl-prolyl cis-trans isomerase FKBP8 and BCL-2-like 13 (BCL2L13) are potential additional mitophagy receptors. By binding to LC3 via the LIRs at their cytosolic N terminus, mitophagy receptors link damaged mitochondria directly to autophagosomes.
Regulatory signalling pathways
Autophagy is tightly regulated to enable cells to adapt to or counteract cellular stress. Several studies have highlighted a complex signalling network that senses changes in nutrient and/or energy status to either stimulate or inhibit autophagy. Intracellular stress, which can be induced by reactive oxygen species (ROS), endoplasmic reticulum (ER) stress, hypoxia, DNA damage and immune signalling, has also been demonstrated as a potential stimulator of autophagy (reviewed elsewhere14).
Nutrient and/or energy signalling.
Autophagy induced by nutrient and/or energy deprivation is primarily regulated by signalling pathways that include the serine/threonine protein kinase mammalian target of rapamycin (mTOR), AMP-activated protein kinase (AMPK) and sirtuins (FIG. 3). mTOR, in combination with cofactors and regulators, forms the rapamycin-sensitive mTOR complex 1 (mTORC1) and the rapamycin-insensitive mTORC2 (REF.15). mTORC1 is a master regulator of autophagy that is activated by amino acids or growth factors, such as insulin and insulin-like growth factor-1, via upstream pathways15. Hamartin (encoded by TSC1)–tuberin (encoded by TSC2) complexes negatively regulate mTORC1 by inhibiting GTP binding protein RHEB15. Under nutrient-rich conditions, active mTORC1 suppresses autophagy by phosphorylating ULK1 and ATG13 to inhibit the ULK1–ULK2 complex. Active mTORC1 also stimulates ribosome biogenesis and mRNA translation by phosphorylating two downstream targets, ribosomal protein S6 kinase β1 (S6K1; also known as p70S6K) complexed with S6 ribosomal protein (S6RP) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1)15,16, which supports cell growth and proliferation. Under starvation, mTORC1 is suppressed and dissociates from the ULK1–ULK2 complex, allowing ULK1 to be activated by AMPK to induce autophagy17 (FIG. 3). Well-known inhibitors of mTORC1 include sirolimus (also known as rapamycin) and rapamycin analogues (also known as rapalogs), such as everolimus (also known as RAD001) and temsirolimus18. Unlike mTORC1, the function of mTORC2 in autophagy is controversial19,20.
Fig. 3 ∣. Signalling networks for the induction of autophagy.
Mammalian target of rapamycin complex 1 (mTORC1) is the major negative regulator of autophagy, whereas AMP-activated protein kinase (AMPK) and sirtuin 1 are positive regulators. mTOR is activated by amino acids via the RAG family of GTPases or by growth factors through two protein kinase pathways that involve RACα serine/threonine protein kinase (AKT) and mitogen-activated protein kinase kinase (MAPKK)–extracellular signal-regulated kinase (ERK). AKT and ERK1 or ERK2 can phosphorylate the hamartin–tuberin complex, which negatively regulates mTORC1 by inhibiting GTP-binding protein RHEB. AMPK is phosphorylated and activated by several upstream kinases, including serine/threonine protein kinase STK11, calcium/calmodulin-dependent protein kinase 2 (CAMKK2) and mitogen-activated protein kinase kinase kinase 7 (TAK1). Once activated, AMPK can directly phosphorylate serine/threonine protein kinase ULK1 to promote autophagy and/or phosphorylate hamartin–tuberin and regulatory-associated protein of mTOR (RAPTOR), which is one of the components of mTORC1, to inhibit mTORC1 and enable the induction of autophagy. Depletion of cellular energy stores increases NAD+ levels and activates sirtuin 1. Active sirtuin 1 induces autophagy by directly deacetylating several essential autophagy proteins such as autophagy-related protein 5 (ATG5), ATG7 and microtubule-associated protein 1 light chain 3 (LC3). During starvation, sirtuin 1 can deacetylate forkhead box protein O1 (FOXO1) to upregulate the GTPase RAB7 and promote autophagic flux. Sirtuin 1 can also deacetylate FOXO3 to enhance the expression of various genes that encode ATGs such as ULK2, beclin 1, phosphoinositide 3-kinase catalytic subunit type 3 (also known as VPS34), BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3), BNIP3-like (BNIP3L), ATG12, ATG4B and LC3. Moreover, sirtuin 1 promotes autophagy by interacting with hamartin–tuberin and deacetylating STK11. mTOR-independent pathways have also been implicated in autophagic regulation under conditions of nutrient shortage. Upon starvation, mitogen-activated protein kinase 8 (MAPK8) is activated, which phosphorylates the apoptosis regulator BCL-2 to liberate and activate beclin 1 to induce autophagy. Activated MAPK8 might also phosphorylate sirtuin 1 to promote its enzymatic activity. Phosphorylation of beclin 1 by death-associated protein kinases (DAPKs) facilitates its dissociation from the anti-apoptotic protein BCL2L1, which also promotes the induction of autophagy. Activation of eukaryotic translation initiation factor 2 subunit-α (eIF2α) and of inhibitor of NF-κB kinase (IKK) is also involved in starvation-induced autophagy. In addition, starvation stimulates the nuclear translocation of transcription factor EB (TFEB) to transactivate the expression of genes involved in autophagosome formation, lysosome biogenesis and lysosome function. By contrast, starvation stimulates the nuclear export of zinc-finger protein with KRAB and SCAN domains 3 (ZKSCAN3), which prevents its repression of ATG gene expression. ATF4, activating transcription factor 4; PI3K, phosphoinositide 3-kinase; RAF, RAF proto-oncogene serine/threonine-protein kinase.
As mentioned above, AMPK positively regulates autophagy. Under conditions of energy depletion, AMPK is activated by a high AMP:ATP ratio through upstream kinases including serine/threonine-protein kinase STK11 (also known as LKB1), calcium/calmodulin-dependent protein kinase 2 (CAMKK2), and mitogen-activated protein kinase kinase kinase 7 (also known as TAK1)21-23 (FIG. 3). Active AMPK induces autophagy through direct phosphorylation of ULK1, or through the phosphorylation of tuberin or regulatory-associated protein of mTOR (RAPTOR; encoded by RPTOR) to inhibit mTORC1 (REFS24-26) (FIG. 3). Notably, although they have opposing roles in the regulation of ULK1, both AMPK and mTORC1 can be phosphorylated and inhibited by ULK1, which creates a complex crosstalk27,28. Several drug agents can directly or indirectly activate AMPK, including metformin, 5-aminoimidazole-4-carboxamide riboside (AICAR), A-769662 and compound 911 (REF.29).
Sirtuins (SIRT1 to SIRT7) are NAD+-dependent class III histone deacetylases. Under conditions of energy depletion, SIRT1 is activated by increased NAD+ levels. Active SIRT1 can stimulate autophagy through the deacetylation of ATG proteins30,31 or of the transcription factors forkhead box protein O1 (FOXO1) and FOXO3a, which transactivates autophagy genes32. In addition, SIRT1 crosstalks with the mTOR and AMPK pathways by interacting with hamartin–tuberin and deacetylating LKB1, thus regulating different mechanisms involved in energy metabolism and cell survival, including autophagy33-35 (FIG. 3). Resveratrol and SRT compounds such as SRT1720 activate SIRT1 (REF.36).
Pathways indirectly associated with nutrient and/or energy signalling.
Pathways that are indirectly associated with those involved in nutrient and/or energy signalling have also been implicated in the regulation of autophagy during nutrient shortage (FIG. 3). For example, active mitogen-activated protein kinase 8 (MAPK8; also known as JNK1)37 and death-associated protein kinases (DAP kinases) promote the induction of autophagy via beclin 1 (REF.38) (FIG. 3). Phosphorylation of eukaryotic translation initiation factor 2 subunit-α (eIF2α)39, activation of inhibitor of NF-κB kinase (IKK)40, nuclear translocation of transcription factor EB (encoded by TFEB)41 and nuclear export of zinc-finger protein with KRAB and SCAN domains 3 (encoded by ZKSCAN3)42 are also involved in starvation-induced autophagy (FIG. 3).
Autophagy in kidney homeostasis
Autophagy is reportedly dispensable for kidney development43,44, but in adult kidneys, basal autophagy in resident kidney cells, including podocytes, proximal tubule epithelial cells (PTECs), glomerular mesangial cells (GMCs) and glomerular endothelial cells (GECs), seems to be crucial for maintaining kidney integrity and normal physiology (TABLE 1). In GMCs, for example, in vitro studies showed that autophagy regulates the turnover of collagen I45 and is cytoprotective in response to serum deprivation46. However, the role of autophagy in GMCs in vivo awaits further investigation.
Table 1 ∣.
Effects of kidney cell-specific deletion of autophagy genes on kidney integrity and physiology
| Animal model | Target kidney tissue | Kidney phenotypes | Refs |
|---|---|---|---|
| Atg5fl/fl; Six2–Cre or Atg7fl/fl; Six2–Cre | Entire nephron | Mild podocyte and tubular dysfunction within 2 months, dramatic glomerular and tubular changes resembling human FSGS by 4 months, and kidney failure by 6 months | 167 |
| Atg5fl/fl; Pax8–rtTA; TetO–Cre | Entire tubule system | Mild increase in serum creatinine, ultrastructural alterations and accumulation of p62+ and ubiquitin+ protein aggregates in tubular cells after 5 months of induction | 43 |
| Atg5fl/fl; Ksp–Cre | Distal tubules | Accumulation of oxidative stress markers, but no structural and functional changes up to 12 months | 43 |
| Atg5fl/fl; Nphs2–Cre | Podocytes | Glomerulosclerosis and podocyte loss accompanied by proteinuria, ER stress and accumulation of oxidized and ubiquitylated protein aggregates at 20 months of age | 44 |
| Vps34fl/fl; Nphs2–Cre or Vps34fl/fl; Pod–Cre | Podocytes | Early-onset proteinuria, podocyte degeneration and glomerulosclerosis accompanied by disruption of autophagic flux | 47,48 |
| Atg5fl/fl; Cdh5–Cre | Glomerular endothelial cells | Slightly dilated capillaries, discrete podocyte foot process effacements, loss of glomerular endothelial fenestrations and endothelial cytoplasmic thickening from 10 weeks of age |
60 |
| Atg5fl/fl; Tek–Cre | Pan-endothelial cells | Mild capillary damage and ROS accumulation in glomeruli at 4 weeks of age; glomerular capillary damage and mesangial matrix expansion at 8 weeks of age | 61 |
ER, endoplasmic reticulum; FSGS, focal segmental glomerulosclerosis; ROS, reactive oxygen species.
Podocytes
In transgenic mice with GFP-tagged LC3 (LC3–GFP), abundant punctate distribution of LC3–GFP was observed in podocytes, but staining was minimal in kidney tubules and in other glomerular cells under physiological conditions44, which suggests that podocytes have a high level of basal autophagy. Moreover, blockade of autophagosomal degradation with the lysosomal inhibitor chloroquine induced a rapid accumulation of LC3–GFP puncta in podocytes, suggesting that these cells had a normal autophagic flux44. In doxycycline-controlled podocyte-specific Atg5-knockout mice, the acute induction of Atg5 deletion in 12-week-old mice triggered a rapid onset of albuminuria, indicating that basal autophagy is crucial for the maintenance of podocyte function44. By contrast, up to 2–4 months after birth, kidney histology, glomerular ultrastructure and albuminuria were indistinguishable between mice with a constitutive podocyte-specific Atg5 deletion and their wild-type littermates44. Increased proteasome activity seemed to compensate for the constitutive loss of autophagy in these mice. Moreover, mice with lysosomal dysfunction in podocytes, induced by inducible podocyte-specific deletion of the genes encoding mTOR, prorenin or PI3K catalytic subunit type 3 (also known as VPS34) developed severe glomerulosclerosis and proteinuria47-50, highlighting the importance of an intact autophagic flux for the maintenance of podocyte homeostasis.
Although podocytes have high basal levels of autophagy, high mTORC1 activity is also required for these cells to maintain their function51,52. This seemingly contradictory finding suggests a potential interplay between these two pathways in podocytes. An early study suggested that podocytes form TOR–autophagy spatial coupling compartments (TASCCs), which are distinct cytoplasmic compartments enriched for mTORC1 that include autolysosomes but largely exclude autophagosomes53. Therefore, TASCCs provide cells with a mechanism for simultaneous activation of anabolic and catabolic processes, which facilitates mTOR-controlled synthesis of secretory proteins with sources of energy or building blocks provided by constitutive autophagy. Moreover, the products of autolysosomal degradation can reactivate mTORC1 within TASCCs, which might, in turn, initiate ALR54. Podocyte-specific Mtor-knockout mice accumulated autophagosomes and autolysosomes within podocytes, and developed severe proteinuria and kidney failure shortly after birth, suggesting that mTOR-associated ALR might be important for podocyte health49.
Another study revealed that AMPK, rather than mTORC1 signalling, controlled the high basal levels of autophagy in podocytes. The researchers showed that mice with podocyte-specific mTORC1 hyperactivation (induced by podocyte-specific deletion of Tsc1) and mTORC1 hypoactivation (induced by podocyte-specific deletion of Rptor) had high basal levels of autophagy that were comparable to those of wild-type mice55. These findings suggested that basal podocyte autophagy was independent of mTORC1 activity. In glomeruli, podocyte-specific AMPK activity was increased in mice with mTORC1 hyperactivation and suppressed in mice with mTORC1 hypoactivation55. AMPK activity might counteract the effects of mTORC1 activation to stabilize the levels of autophagy in these mice. Short-term (3 days) and long-term (3 weeks) treatment with AICAR resulted in similarly high levels of podocyte autophagy in mice with podocyte-specific mTORC1 hyperactivation or hypoactivation and in wild-type mice, compared with mice without AICAR treatment, suggesting that autophagy induction by AMPK is mTORC1-independent in podocytes55. Treatment with rapamycin for 3 days inhibited mTORC1 activity and concurrently activated AMPK in podocytes, which led to autophagy induction, whereas 3 weeks of treatment with rapamycin inactivated AMPK and counteracted the pro-autophagic effect achieved by mTORC1 inhibition and thus autophagy levels returned to the baseline observed in untreated mice55. These findings suggest that the inhibitory effect of mTORC1 on podocyte autophagy is limited to a short-term adaptive response and that AMPK signalling is the main regulatory pathway in the control of podocyte autophagy, which would allow concurrent high basal autophagy and high mTORC1 activity in podocytes55. Tyrosine protein kinase JAK2 was also reported to modulate basal autophagy in podocytes by facilitating the translocation of signal transducer and activator of transcription 1 (STAT1) to the nucleus, where it binds to the Tfeb promoter to induces its expression56.
Proximal tubule epithelial cells
Kidney PTECs have relatively low levels of autophagy under physiological conditions43. However, in mice, selective deletion of Atg5 or Atg7 in proximal tubules resulted in progressive kidney damage and premature kidney ageing, as demonstrated by an accumulation of damaged mitochondria, sequestosome 1 and Ub-positive aggregates, as well as elevated tubular cell apoptosis and kidney tubulointerstitial fibrosis compared with wild-type controls43,57. By contrast, the kidneys of mice with Atg5 deletion in distal tubules or collecting ducts were histologically similar to those of wild-type mice43. These findings suggest that low levels of basal autophagy are essential to the maintenance of PTEC function under normal conditions and that proximal tubules rely on basal autophagy more than other tubular segments.
Using the mito-quality control (mito-QC) mitophagy reporter mouse model, we examined the level of basal mitophagy in the kidney58. Mito-QC mice express a tandem mCherry–GFP protein fused to the outer mitochondrial membrane mitochondrial fission 1 protein (encoded by FIS1); the differential pH sensitivities between mCherry and GFP enable tracking of the delivery of mitochondria to lysosomes to form mitolysosomes59. Interestingly, under control conditions mice had a high level of mitophagy in some cortical proximal tubules, where mitolysosomes were predominantly observed near the apical membrane58. A high rate of mitochondrial turnover might be required to meet the high energy demand of the large number of ATP-dependent transporters that are involved in reabsorption and excretion at the apical membrane of kidney proximal tubules. By contrast, mitochondrial turnover was minimal in S3 segment proximal tubules under control conditions. Mitophagy was also observed at much lower levels in glomeruli compared with cortical proximal tubules58.
Glomerular endothelial cells
In mice with a GEC-specific Atg5 deletion (Atg5fl/fl; cadherin 5 (Cdh5)–Cre), researchers observed slight dilation of glomerular capillaries, podocyte foot process effacement and loss of glomerular endothelial fenestrations in the glomerular capillary loops from 10 weeks of age; however, functional defects, as assessed by urinary creatinine and blood urea nitrogen (BUN), were not apparent up to 20 weeks of age60. Similar changes in the capillary loops and glomerular endothelial fenestra were observed at 4 weeks of age in another conditional Atg5-knockout mouse model (Atg5fl/fl; angiopoietin 1 receptor (Tek)–Cre) in which Atg5 loss occurred in both endothelial and haematopoietic cells61. However, at 8 weeks of age, the capillaries of Tek–Atg5−/− mice had a lobular pattern, with thickening of the capillary loops and mesangial matrix expansion, and they died by 12 weeks of age, potentially owing to severe haematopoietic injury. Of note, 6-month-old Tek–Atg5−/− and wild-type mice that had received a bone marrow transplant from wild-type donors at 4 weeks of age, which would have rescued the loss of Atg5 in the haematopoietic compartment of Tek–Atg5−/− mice, had similar levels of urinary albumin and plasma urea nitrogen61. However, at 12 months of age, these transplanted Tek–Atg5−/− mice developed mesangiolysis and glomerulosclerosis accompanied by kidney dysfunction, as assessed by plasma creatinine and BUN, which suggests that autophagy deficiency in GECs but not in haematopoietic cells contributed to the glomerular defects observed in TekαAtg5−/− mice. Moreover, administration of the ROS scavenger N-acetyl-l-cysteine (NAC) protected against the glomerular lesions in Tek–Atg5−/− knockout mice61. This observation suggests that endothelial autophagy might protect glomeruli from oxidative stress and maintain the integrity of glomerular capillaries. The differences in glomerular defects observed between Tek–Atg5−/− and Cdh5–Atg5−/− mice might be attributable to the different activity and/or site specificity of the promoters used to drive Cre recombinase in these two mouse models60,61.
Autophagy in kidney ageing
Ageing is associated with structural and functional changes in the kidney that potentially compromise its capacity for repair and increase its susceptibility to damage — such effects might underlie the high incidence of chronic kidney disease (CKD) in elderly individuals62. One study reported that ageing in mouse tissues, including kidney tissues, was characterized by suppression of autophagy by the run domain beclin-1-interacting and cysteine-rich domain-containing protein (rubicon)63. Accordingly, the expression of the autophagy proteins LC3-I and ATG7 was dramatically reduced in the kidneys of 24-month-old rats compared with those of 3-month-old animals, and this decrease was accompanied by an accumulation of sequestosome 1, Ub-positive protein aggregates and damaged mitochondria, as well as the induction of oxidative stress64.
Autophagy deficiency in podocytes also seems to promote ageing. At 20–24 months of age, mice with a podocyte-specific Atg5-knockout displayed more obvious features of cellular ageing such as mitochondrial damage, ER stress and accumulation of oxidized and ubiquitylated protein aggregates and lipofuscin compared with age-matched wild-type controls44. Proteasome activity increased in 8-month-old Atg5−/− mice compared with wild-type controls, and this increase seemed to clear protein aggregates and compensate for the loss of autophagy. However, this increase in proteasome activity was significantly reduced in older mice compared with younger Atg5-knockouts44; podocyte loss and progressive glomerulosclerosis were also observed in older mice. Another study, in which researchers used a mouse model of podocyte-specific deletion of the lysosomal aspartic protease cathepsin D (Ctsd), further demonstrated the importance of lysosomal activity in podocyte maintenance during ageing65. Defective autolysosome degradation in podocytes triggered the accumulation of toxic lipofuscins, protein aggregates and other harmful cytoplasmic materials, eventually leading to apoptotic podocyte death. As a result, these podocyte-specific Ctsd−/− mice developed proteinuria at 5 months of age and kidney failure by 20–22 months of age65.
The mechanisms underlying the ageing-related reduction of autophagy in podocytes were further investigated. One study reported that podocyte SIRT1 expression was lower in 26–28-month-old mice than in 3-month-old mice and that podocyte-specific knockdown of Sirt1 aggravated age-associated kidney injury in ageing mice66. Another report indicated that ageing was accompanied by a reduction in the expression of CCAAT/enhancer-binding protein-α (CEBPα) and that podocyte-specific deletion of CEBPα accelerated kidney ageing, as indicated by the increased accumulation of senescence markers and ageing-induced kidney lesions in Cebpa-knockout mice compared with wild-type controls67. In vitro, exposure to adriamycin induced premature senescence in podocytes, which was accompanied by a reduction in phospho–AMPK and an increase in phospho–mTOR — overexpression of CEBPα restored the dysregulated activity of AMPK and mTOR67. These findings suggest that ageing-related changes in the expression of proteins such as SIRT1 and CEBPα might lead to reduced autophagy in podocytes and promote kidney ageing.
Unlike podocytes, compared with 2-month-old mice, PTECs of 24-month-old mice have a higher basal autophagic flux, but their response to starvation-induced autophagy is blunted68. In a mouse model of tamoxifen-inducible proximal tubule-specific Atg5 deletion, sequestosome 1 and Ub-positive aggregates accumulated at higher levels in aged kidneys than in those from younger mice, suggesting that aged kidneys are more reliant on autophagy for the removal of damaged proteins and organelles68. The researchers also demonstrated that disruption of autophagy in proximal tubules for 24 months caused significant kidney dysfunction, tubular atrophy and tubulointerstitial fibrosis, which were accompanied by an aggravation of ageing-related mitochondrial dysfunction as well as damage to mitochondrial and nuclear DNA. Furthermore, the researchers reported dysregulation of nutrient sensing pathways in the proximal tubules of aged mice, including an ageing-related decline in SIRT1 levels and the failure to further activate AMPK in response to starvation and mTOR hyperactivation68. Moreover, in doxycycline-inducible kidney tubule-specific Atg5-knockout mice treated with doxycycline from 2 months of age for 2 weeks, ageing-related markers, including accumulation of sequestosome-1 and ubiquitin-positive inclusion bodies, as well as concentric membrane bodies, appeared in renal tubular cells 4–5 months after induction of Atg5 loss in tubular segments43. These findings further suggested that autophagy impairment accelerated the ageing process and tubular damage in young mice.
Autophagy in acute kidney injury
Acute kidney injury (AKI) is common and is characterized by a rapid decline of kidney function. Research has demonstrated that autophagy has an important role in the development of AKI — autophagy-mediated removal of protein aggregates and damaged organelles to maintain cellular homeostasis might also protect cells and tissues against injury. Induction of autophagy in kidney tubular cells, particularly in PTECs, has been documented in rodent models of AKI induced by kidney ischaemia–reperfusion injury (IRI), by nephrotoxic drugs such cisplatin, by sepsis and other AKI risk factors69.
Earlier studies, which used mostly pharmacological approaches to inhibit autophagy, reported contradictory findings, but the subsequent use of mouse models with selective deletion of autophagy genes in proximal tubules has provided further insight into the role of autophagy in AKI. For example, selective Atg5 or Atg7 deletion in proximal tubules dramatically sensitized the kidney to kidney IRI, as demonstrated by more severe kidney dysfunction and tubular cell apoptosis in these mice compared with wild-type controls44,57,70. Similarly, proximal tubule-specific Atg7 or Atg5-knockout mice were more sensitive to cisplatin-induced AKI than control mice70,71. These and other related studies provide compelling evidence for a protective role of tubular cell autophagy in AKI induced by kidney IRI and cisplatin (TABLE 2).
Table 2 ∣.
Effects of autophagy or mitophagy defects on AKI and kidney interstitial fibrosis
| Animal model | Targeted tissues | Disease model | Kidney phenotype | Refs |
|---|---|---|---|---|
| Atg5fl/fl; Kap–Cre | Proximal tubules | Cisplatin and IRI | Increased sensitivity to AKI | 57,71 |
| Atg7fl/fl; Pepck–Cre | Proximal tubules | Cisplatin, IRI and LPS | Increased sensitivity to AKI | 70,74 |
| Atg5fl/fl; Pax8–rtTA; TetO–Cre | Entire kidney tubules | IRI | Increased sensitivity to AKI | 43 |
| Pink1 and Park2 single or double knockout | Global | Cisplatin, IRI, contrast agent | Increased sensitivity to AKI | 77,88,90 |
| Atg7fl/fl; Pepck–Cre | Proximal tubules | UUO | Reduced kidney fibrosis, fibroblast proliferation and activation, tubular atrophy, apoptosis, interstitial macrophage infiltration and production of pro-fibrotic factors | 108 |
| Atg5fl/fl; GGT:: Ert2–Cre | S3 segment of proximal tubules | IRI | Reduced interstitial fibrosis and tubular senescence and superior kidney function 30 days after AKI | 102 |
| Map1lc3a knockout or hemizygous deletion of Becn1 | Global | UUO | Increased collagen deposition and TGFβ1 levels in obstructed kidney | 118 |
| Atg5fl/fl; Kap–Cre | Proximal tubules | UUO | Increased interstitial fibrosis and cell cycle arrest at the G2/M phase |
119 |
| Atg7 deletion | Distal tubules | UUO | Increased tubulointerstitial fibrosis, accumulation of damaged mitochondria, expression of NLRP3 inflammasome, IL-1β and caspase 1 | 123 |
| Hif1afl/fl; Pax8–rtTA; TetO–Cre | Proximal tubules | IRI | Reduced autophagy in proximal tubules accompanied by increased tubular injury, interstitial fibrosis and functional damage to the kidney | 105 |
AKI, acute kidney injury; IRI, ischaemia–reperfusion injury; LPS, lipopolysaccharide; TGF-β1, transforming growth factor β1; UUO, unilateral ureteric obstruction.
The beneficial effect of autophagy was further confirmed in two experimental models of sepsis-induced AKI caused by caecal ligation and puncture, or lipopoly-saccharide exposure72-75. Autophagy and/or mitophagy were also protective in contrast-induced nephrotoxicity, another clinically relevant model of AKI76-78. Moreover, promoting autophagy through pharmacological inhibition of mTORC1, or activation of AMPK or sirtuins, has been shown to be renoprotective during AKI70,75,79.
Collectively, current evidence suggests that autophagy is active in kidney tubular cells and functions as a defence mechanism in AKI (FIG. 4). Notably, mTORC1 inhibits autophagy but also promotes cell growth and differentiation — mTORC1 might be essential for maintaining kidney tubular homeostasis in response to ischaemic stress80 and mTOR inhibitors should therefore be used with caution.
Fig. 4 ∣. Autophagy in AKI and kidney interstitial fibrosis.
Acute kidney injury (AKI) induces autophagy in proximal tubule epithelial cells (PTECs) to protect against kidney injury. Following the initial injury, autophagy in PTECs undergoes dynamic changes and mammalian target of rapamycin (mTOR)-mediated autophagy resolution during the injury recovery phase facilitates tubular cell proliferation and repair. Moreover, tightly regulated autophagy is required for phagocytosis of apoptotic cells by epithelial cells, which might also inhibit inflammatory responses and thus promote kidney repair. By contrast, persistent induction of autophagy in PTECs after AKI might induce cellular senescence and suppress cell proliferation. These autophagy-mediated changes stimulate the production and secretion of pro-fibrotic cytokines, leading to maladaptive repair and tubulointerstitial fibrosis. mTORC, mTOR complex.
In AKI, the initial insults induce intracellular stress in PTECs, including oxidative stress, ER stress, hypoxia, DNA damage, immunity-related signalling pathways and nutrient and/or energy deprivation69, all of which are known to stimulate autophagy. However, the precise mechanisms that regulate autophagy in tubular cells in response to these stress signals are yet to be elucidated in models of AKI.
In addition, emerging studies suggest a complex crosstalk between autophagy and various pathways of cell death, including apoptosis, necrosis and necroptosis, which also contribute to the complex regulation of autophagy (reviewed elsewhere69). In general, autophagy and apoptosis can be induced in response to the same stimuli and these two pathways might share common regulatory molecules enabling them to regulate and modify each other to coordinately determine the fate of the cell. The interplay between autophagy and apoptosis in AKI has been observed in a few studies. In a model of ischaemic AKI, overexpression of the anti-apoptotic protein BCL2L1 in kidney tubular cells significantly reduced beclin 1 expression and suppressed autophagy compared with controls81. In cultured PTECs, exposure to cisplatin at different doses stimulated distinct components of the unfolded protein response (UPR) — a 10-μM dose of cisplatin promoted autophagy, whereas a 50-μM dose induced apoptosis82. Moreover, in PTECs treated with 50 μM of cisplatin, inhibition of autophagy enhanced the activity of caspases 3, 6 and 7, whereas key autophagy proteins, such as beclin 1, ATG5 and ATG12 were cleaved and degraded in a caspase-dependent manner83.
One study suggested that autophagy has a major role in mediating the renoprotective effects of caloric restriction against kidney IRI in rats. In this experimental setting, preconditioning with caloric restriction stimulated autophagy in kidneys, potentially by increasing SIRT1 expression, which prevented the decrease in peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α) and endothelial nitric oxide synthase in kidney tubules, leading to renoprotection79. Ischaemic preconditioning (IPC) is a potential approach for tissue protection during subsequent ischaemic injury in various organs including the kidney, although it seems to be less effective in aged kidneys84. The loss of the beneficial effect of kidney IPC in aged kidneys is most likely due to defects in autophagy, which suggests that these two mechanisms are closely related84. Indeed, we demonstrated that kidney IPC promoted the clearance of damaged mitochondria via mitophagy to protect tubular cells and kidneys against ischaemic injury58. Moreover, in a rat model of ischaemic AKI, IPC protection was mediated by autophagy and regulated by a signalling pathway that involved a serine/threonine protein kinase SGK1–FOXO3a–hypoxia-inducible factor 1α (HIF1α) signalling axis85. These findings suggest that autophagy might interact with other defence mechanisms to protect the kidneys from AKI.
Selective autophagy in AKI
Growing evidence suggests that mitophagy is an important defence mechanism in AKI86,87. We reported a dramatic induction of mitophagy in proximal tubules during kidney IRI, which was partially abrogated in mice with a single or double knockout of the mitophagy genes Pink1 and Park2 (also known as Prkn). Importantly, compared with wild-type littermates, Pink1 and/or Park2-knockout mice had more severe kidney dysfunction and tissue damage, which were accompanied by an accumulation of damaged mitochondria and increased tubular cell death, ROS generation and kidney inflammation, which suggested an important role for mitophagy in the protection of tubular cells during ischaemic AKI88. We subsequently demonstrated that induction of BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3)-mediated mitophagy also provided renoprotection during ischaemic AKI89.
Consistent with these findings, mitophagy with pro-survival effects was also activated in PTECs during AKI induced by cisplatin90,91, contrast agents77,78 or sepsis92. For instance, deletion of Pink1 or Park2 in mice abolished contrast-induced mitophagy in kidney tubules and worsened kidney injury as assessed by kidney tubular damage, plasma creatinine and BUN, mitochondrial damage, ROS production and cell death77. In cultured PTECs, knockdown of Pink1 or Park2 prevented the induction of mitophagy in response to contrast media and this reduction in mitophagy enhanced PTEC death by activating the NLRP3 inflammasome77. These findings suggest that mitophagy might protect against AKI by decreasing inflammation.
Mitophagy induction in PTECs might represent a common mechanism for kidney tubular cell protection in various models of AKI. The mitophagy-mediated elimination of dysfunctional mitochondria might prevent excessive ROS accumulation and the activation of the mitochondrial apoptotic cascade, as well as inhibit the release of mitochondrial DNA and other damage-associated molecular patterns that might otherwise promote inflammation during AKI.
Lysosomes are also abundant in PTECs, where they are used for endocytosis-mediated tubular reabsorption. In a mouse model of hyperuricaemia-associated AKI induced by uric acid and oxalic acid, lysosome damage accompanied by lysophagy occurred in proximal tubules in wild-type mice93. Proximal tubule-specific Atg5-knockout impaired hyperuricaemia-induced lysophagy in kidney tubules and aggravated hyperuricaemia-induced kidney injury as assessed by plasma creatinine and BUN93. These findings suggest a renoprotective role of lysophagy in AKI.
Autophagy and the AKI–CKD transition
After AKI, surviving tubular cells proliferate, migrate and then differentiate to replace lost tubular cells and repair injured kidney tubules94,95. Tubular repair might be complete after mild AKI, but severe or episodic AKI are usually followed by incomplete or maladaptive repair that results in the development of kidney fibrosis and CKD96,97. Although the mechanisms that underlie the transition from AKI to CKD remain to be elucidated, emerging evidence suggests that autophagy in kidney tubules is involved in kidney repair.
In one study, researchers used autophagy-reporter mice that expressed a tandem red fluorescent protein (RFP)–enhanced GFP (EGFP)–LC3 fusion protein to investigate autophagy during IRI-induced AKI and subsequent recovery98. They reported that autophagy was induced in proximal tubules 1 day after reperfusion and that at day 3 autophagosomes fused with lysosomes for clearance, leading to the resolution of autophagy in those cells. The inhibition of mTOR in some proximal tubule cells led to persistent activation of autophagy and impaired proliferation, which indicated that mTOR activation contributed to the resolution of autophagy and tubular repair.
The importance of dynamic changes in autophagy to normal tubular repair after ischaemic AKI was further verified in proximal tubule-specific telomerase (TerC or TerT)-knockout mice99. Telomerases are involved in the elongation of telomeres and thus prevent telomere shortening. Studies demonstrated that telomere shortening induced autophagy100 and that the induction of autophagy reduced telomerase activity101. Kidney IRI caused comparable kidney injury at day 1 in both wild-type and telomerase (TerC or TerT)-knockout mice, but whereas wild-type mice showed kidney recovery 2–3 days after IRI and kidney function, as assessed by plasma creatinine and BUN, was restored to normal within 14 days, knockout mice had delayed and incomplete kidney recovery. In addition, compared with wild-type mice, in which autophagy was initiated at day 1 after reperfusion, peaked at day 3 and then reduced gradually within 7–14 days, proximal tubule-specific telomerase-knockout mice showed no induction of autophagy as late as 14 days after reperfusion99. These results suggest that, after ischaemic AKI, timely and tightly regulated autophagy is crucial for kidney tubules to survive the initial injury, regenerate and contribute to normal kidney repair (FIG. 4).
Interstitial fibrosis is a hallmark of maladaptive repair in the transition from AKI to CKD (FIG. 4) and autophagy might also have a pro-fibrotic role according to findings from a mouse model of post-ischaemic kidney fibrosis102. Following ischaemic AKI, researchers observed persistent activation of autophagy and pro-senescent changes in the S3 segments of proximal tubules, as well as kidney fibrosis. Atg5 deletion in the S3 segment inhibited tubular senescence, attenuated interstitial fibrosis and improved kidney function as assessed by transcutaneous measurement of the glomerular filtration rate, which suggested that autophagy in tubular cells contributed to post-ischaemic kidney fibrosis by promoting senescence102. Notably, compared with wild-type littermates, Atg5-knockout mice had more tubular cell death 2 h after reperfusion, but reduced tubular damage and inflammation at day 3, suggesting that autophagy enhances cell survival during the initial tubular injury but hinders normal repair during the recovery phase. The long-term pro-fibrotic effects of autophagy might be due its cytoprotective role — by protecting damaged tubular cells from cell death, autophagy enables compromised, senescent cells to persist and promote kidney interstitial fibrosis102.
Tubular cell arrest at the G2/M phase after severe AKI also contributes substantially to kidney fibrosis by stimulating the generation and secretion of pro-fibrotic cytokines103 — the formation of TASCCs in G2/M-arrested PTECs was a major contributor to pro-fibrotic changes in these cells104. Unlike in podocytes, TASCCs were undetectable in tubular cells of normal mice. However, many TASCC structures appeared in kidney tubules after severe kidney injury induced by aristolochic acid or prolonged kidney ischaemia. Of note, TASCC formation began 7 days after injury, increased remarkably between days 14 and 21 as kidney fibrosis progressed, and was sustained at a moderate level at day 42, which was the experimental end point. Over 80% of these TASCC+ tubular cells were also phospho-histone 3 (pH3)+, which indicated that they were arrested at the G2/M phase of the cell cycle. Cyclin G1 promoted G2/M arrest in PTECs and upregulated TASCC formation. Notably, TASCC disruption after kidney injury reduced the synthesis and secretion of pro-fibrotic cytokines in PTECs and suppressed kidney fibrosis after severe AKI104. These results suggest that in G2/M-arrested tubular cells, the autophagy pathway might participate in the formation of TASCCs to facilitate a change in the secretory phenotype of PTECs and promote fibrotic maladaptive repair after AKI.
Several studies have examined the potential signalling pathways underlying the persistent activation of kidney tubular autophagy after AKI. One report demonstrated that chronic hypoxia after severe ischaemic AKI activated FOXO3 in a HIF1α-dependent manner105. Compared with wild-type controls, mice with a proximal tubule-specific Foxo3 deletion failed to induce autophagy in kidney tubules after AKI. The expression of BNIP3, a known target of FOXO3 and a key component of the autophagy machinery, was also decreased in Foxo3-knockout mice, whereas ULK1 expression was only slightly reduced. These results suggest a potential role for the HIF1α–FOXO3 pathway in regulating tubular cell autophagy during the transition from AKI to CKD105.
In addition to hypoxia, we reported that the induction of autophagy in proximal tubules appeared to be mediated by ER stress. In a mouse model of unilateral kidney IRI injury, ER stress and autophagy were both induced in kidney tubular cells, and attenuation of ER stress by the chemical chaperones tauroursodeoxycholic acid and 4-phenylbutyric acid attenuated autophagy and the progression of kidney fibrosis106. Persistent autophagy during kidney repair or AKI-to-CKD transition might involve complex mechanisms that are still poorly understood.
Autophagy in tubulointerstitial fibrosis
The role of autophagy in kidney tubulointerstitial fibrosis has been extensively studied in experimental models of fibrosis such as unilateral ureteric obstruction (UUO) or treatment with transforming growth factor β1 (TGFβ1), but findings are controversial probably because of the multiple functions of autophagy in fibrosis (FIG. 4). In mice with tubule-specific inducible overexpression of TGFβ1, autophagy was activated along with tubular degeneration and widespread peritubular fibrosis. Of note, these degenerating cells were not positive for terminal deoxynucleotidyl transferase dUTP nick end labelling staining, which is used to identify apoptotic cells, suggesting that autophagy might drive tubular atrophy in TGFβ1-induced kidney interstitial fibrosis. Earlier studies showed that, in mice subjected to UUO, autophagy was induced along with apoptosis in obstructed kidney tubules and that both pathways promoted tubular atrophy and nephron loss107. We also reported that autophagy was persistently activated in obstructed kidney tubules in UUO mice108. Importantly, inhibition of autophagy by chloroquine, or by selective deletion of Atg7 in proximal tubules, reduced kidney fibrosis in UUO mice compared with untreated or wild-type controls, respectively, potentially by attenuating tubular apoptosis, macrophage infiltration and production of fibroblast growth factor108.
In addition, in UUO mice, pharmacological inhibition of autophagy markedly reduced the accumulation of lipids in kidney tubules, which was accompanied by reduced tubular cell apoptosis and degeneration, and suppressed kidney interstitial fibrosis109. Interestingly, in mouse models of liver fibrosis induced by thioacetamide or carbon tetrachloride, the induction of autophagy in hepatic stellate cells led to lipid degradation and activated these cells to promote liver fibrosis110,111. These results suggest that the regulation of lipid metabolism by autophagy might modulate fibrinogenesis. In the kidney, autophagy might have a pro-fibrotic effect by promoting the accumulation of lipids and inducing lipotoxicity in kidney tubular cells, as well as stimulating lipolysis to fuel liver fibroblasts. One study in UUO mice and in cultured kidney fibroblast cells exposed to TGFβ1 suggested that autophagy has pro-fibrotic effects and is involved in the activation of kidney fibroblasts112. However, the role of autophagy-mediated lipolysis in these processes remains undetermined.
The upstream signalling pathways potentially involved in the induction of autophagy in PTECs during kidney fibrosis have also been investigated. Following UUO, FOXO3 was activated and expressed in the nuclei of autophagic proximal tubules and the induction of autophagy was inhibited in Foxo3-knockout mice113. FOXO3 activation increased the expression of multiple core ATG genes and proteins in UUO kidneys of wild-type mice, suggesting that FOXO3 might induce autophagy through the transcriptional activation of key autophagy genes113. Other proteins involved in the induction of autophagy in models of kidney fibrosis include CCN family member 4 (also known as WNT1) in PTECs114 and protein kinase C α in kidney fibroblasts112 following UUO or treatment with TGFβ1, as well as DNA damage-inducible transcript 3 protein (DDIT3; also known as CHOP) in distal tubules and collecting ducts in the UUO model115.
By contrast, several studies have also reported that autophagy can have an anti-fibrotic role (TABLE 2). In a rat model of UUO, autophagy was induced through inhibition of the RACα serine/threonine protein kinase (also known as PKB or AKT)–mTOR pathway, but inhibition of autophagy by 3-methyladenine aggravated tubular cell apoptosis and tubulointerstitial fibrosis, suggesting that autophagy might exert an anti-fibrotic effect by suppressing tubular cell injury116.
Fibronectin can also be degraded through autophagy in PTECs. In cultured mouse PTECs, microtubule associated protein 1 S (MAP1S) interacted with LC3 and activated autophagy, which contributed to the suppression of fibrosis by increasing fibronectin turnover117. Map1s deletion impaired autophagic clearance of fibronectin and induced kidney fibrosis in aged mice. The researchers also observed reduced expression of MAP1S and accumulation of fibronectin in kidney biopsy samples from patients with kidney fibrosis compared with normal kidney tissues distant from tumour foci collected from patients with clear cell renal cell carcinoma117. Another study showed that inhibiting autophagy prevented the degradation of mature TGFβ1 in UUO kidneys and in TGFβ1-treated PTECs, suggesting that autophagy might also inhibit kidney interstitial fibrosis by negatively regulating TGFβ1 (REF.118).
The anti-fibrotic effects of autophagy might result from its role in cell cycle regulation. In the UUO model, cell cycle G2/M arrest in proximal tubules was more severe in proximal tubule-specific Atg5-knockout mice than in wild-type mice; this effect was associated with an accelerated development of kidney interstitial fibrosis119. G2/M arrest and production of collagen I were further elevated in angiotensin II-treated ATG5-deficient primary PTECs compared with angiotensin II-treated wild-type cells. Moreover, Atg5 overexpression elevated autophagy in angiotensin II-treated PTECs accompanied by a reduction in cells arrested in the G2/M phase and collagen 1 expression119. The same research group subsequently demonstrated that ATG5-mediated autophagy suppressed NF-κB signalling, which limited tubular cell inflammation in response to the kidney injury induced by UUO or angiotensin II120. Moreover, prohibitin 2-mediated mitophagy was induced by angiotensin II and attenuated kidney tubular cell injury by improving mitochondrial function and inhibiting NLRP3 inflammasome activation121.
Autophagy induced in distal tubules and pericytes during UUO also protected against kidney interstitial fibrosis by suppressing TGFβ and NLRP3-inflammasome signalling122,123. Other studies further demonstrated that UUO-induced kidney fibrosis was more severe in Pink1 and Park2 single knockout mice than in wild-type littermates, suggesting a protective role for mitophagy in kidney fibrosis124,125.
Collectively, these findings suggest that autophagy has a dual role in UUO-induced kidney fibrosis. Persistent activation of autophagy might induce tubular atrophy and thereby promote kidney fibrosis, whereas autophagy-mediated degradation of excessive collagen might prevent fibrosis. Of note, the UUO model is not ideal for the study of kidney repair, as fibrosis progresses rapidly, is irreversible and the injury phase cannot be easily distinguished from the repair phase.
Autophagy in diabetic kidney disease
Diabetic kidney disease (DKD) is one of the most common and life-threatening diabetic complications and a leading cause of CKD and kidney failure worldwide126. The pathological hallmark of DKD is persistent albuminuria or proteinuria accompanied by the decline of the glomerular filtration rate and pathological changes in all kidney compartments126. The pathogenesis of DKD involves a complex interplay of hyperglycaemia-induced metabolic alterations, haemodynamic changes and various types of intracellular stress. Collectively, these processes induce the production of a range of cytokines, chemokines and growth factors that promote cell injury, inflammation and kidney fibrosis. Dysregulated autophagy in major types of resident kidney cells has also been recognized as an important contributor to the progression of DKD (TABLE 3).
Table 3 ∣.
Effects of kidney cell-specific deletion of autophagy genes on chronic kidney disease
| Animal model | Kidney tissue with autophagy deficiency |
Kidney disease model | Kidney phenotype | Ref. |
|---|---|---|---|---|
| Atg5fl/fl; Six2–Cre or Atg7fl/fl; Six2–Cre | Entire nephron | None | Glomerular and tubular changes resembling human FSGS by 4 months | 167 |
| Atg5fl/fl; Nphs2–Cre | Podocytes | PAN, adriamycin | Increased sensitivity to glomerular diseases; increased sensitivity to diabetes-induced podocytopathy | 44 |
| STZ-induced DKD | Leaky filtration barrier and glomerulosclerosis | 60 | ||
| HFD-induced DKD | Increased sensitivity to diabetes-induced podocyte loss and proteinuria | 128 | ||
| Atg5fl/fl; Cdh5–Cre | Glomerular endothelial cells | STZ-induced DKD | Increased sensitivity to diabetes-induced dilation of glomerular capillaries and endothelial lesions | 60 |
| Atg5fl/fl; Kap–Cre | Proximal tubules | FFA/albumin overload | Increased sensitivity to proteinuria-induced tubulointerstitial lesions | 148 |
DKD, diabetic kidney disease; FFA, free fatty acid; FSGS, focal segmental glomerulosclerosis; HFD, high-fat diet; PAN, puromycin aminonucleoside; STZ, streptozotocin.
Podocyte autophagy in DKD
In DKD, alterations in podocyte autophagy seem to correlate with the duration of the exposure to hyperglycaemia, the severity of podocyte injury and glomerulopathy, and disease stage or severity of DKD. In a mouse kidney podocyte cell line, exposure to high glucose for 48 h induced autophagy, whereas exposure to high glucose for 15 days had a suppressive effect on autophagy60. In line with these in vitro findings, in streptozotocin (STZ)-induced type 1 diabetic mice, podocyte autophagy was induced at 4 weeks and suppressed at 8 weeks post-STZ injection. Of note, 4 weeks after STZ injections these mice were only hyperglycaemic and did not have glomerular lesions; however, podocyte injury was present at the 8-week time point. Compared with wild-type mice, STZ-induced glomerulopathy was more severe in podocyte-specific Atg5-knockout mice60. These results suggest that exposure of podocytes to hyperglycaemia, either short-term exposure in vitro or in the early stages of diabetes, might induce autophagy for renoprotection, whereas prolonged hyperglycaemia in vitro or in the late stages of diabetes, might suppress autophagy in podocytes and contribute to the progression of DKD.
Furthermore, transmission electron microscopy revealed that in mice treated with STZ, mesangial expansion and glomerulosclerosis were more pronounced in podocyte-specific Atg5-knockout mice than in wild-type mice, underscoring the interactions between podocytes and mesangial cells in diabetes60. Kidney biopsy samples from patients with diabetes also indicated that autophagy was reduced in podocytes compared with kidney specimens from patients with mild urinary protein excretion or only haematuria, which were characterized by minimal changes in histology127. Another study showed that podocyte autophagy was impaired in patients with type 2 diabetes, as well as in OLETF rats (that is, rats with obesity-induced diabetes), with severe proteinuria, but not in those with no or minimal proteinuria128. Moreover, a high-fat diet induced minimal proteinuria in wild-type mice, but podocyte-specific Atg5-knockout mice developed substantial albuminuria accompanied by severe tubulointerstitial lesions and injury to podocytes, which accumulated damaged lysosomes. Interestingly, stimulating cultured podocytes with serum from patients with type 2 diabetes or OLETF rats with severe proteinuria inhibited autophagy and induced lysosomal dysfunction and apoptosis, suggesting that serum-derived factors might negatively regulate podocyte autophagy as DKD progresses128.
The mechanisms underlying DKD-related impairment of podocyte autophagy are under intensive investigation. Dysregulation of nutrient and/or energy-sensing pathways and intracellular stress signals including ROS, ER stress and hypoxia have all been implicated in the regulation of podocyte autophagy in DKD129,130 (FIG. 5). One study also demonstrated that advanced glycation end-products (AGEs) impaired autophagy in podocytes by inducing lysosomal dysfunction127. Treatment with the antioxidants resveratrol and vitamin E restored lysosomal function and rescued the impairment in autophagy, thereby attenuating AGE-induced podocyte injury. Another study suggested that AGEs suppress the autophagic flux in podocytes by activating mTORC1 and inhibiting the nuclear translocation of TFEB131.
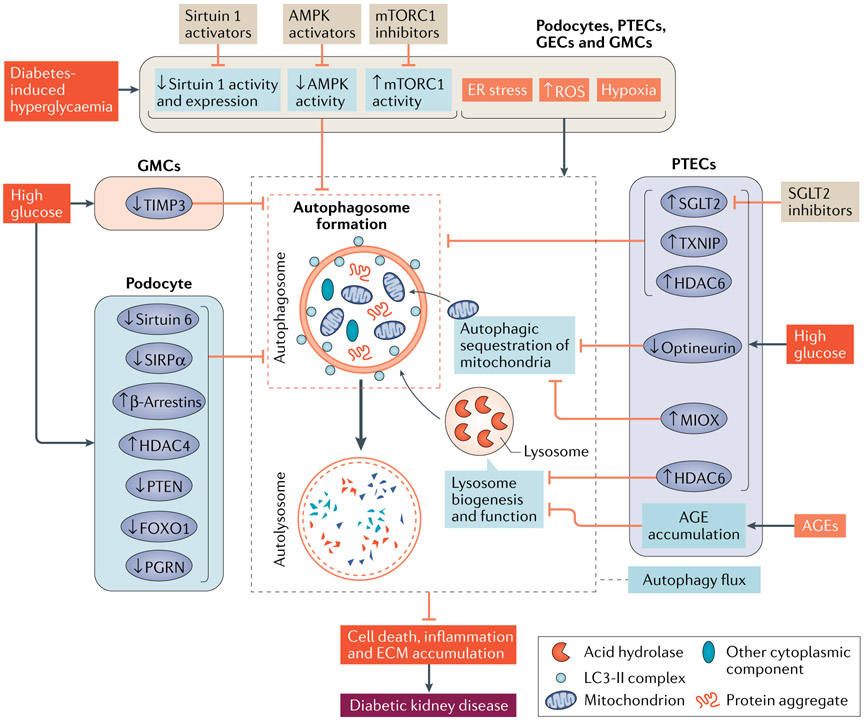
Fig. 5 ∣. Autophagy in diabetic kidney disease.
Diabetes-associated hyperglycaemia induces cellular stress, including an increase in reactive oxygen species (ROS), endoplasmic reticulum (ER) stress and hypoxia. In the kidney, the initial adaptive response seems to include the activation of autophagy in podocytes, proximal tubule epithelial cells (PTECs), glomerular endothelial cells (GECs) and glomerular mesangial cells (GMCs). By contrast, sustained disturbance of major nutrient and/or energy sensing pathways leads to activation of mammalian target of rapamycin complex 1 (mTORC1), and inhibition of AMP-activated protein kinase (AMPK) and sirtuin 1, which suppresses autophagy in these kidney cells. In podocytes exposed to high glucose, increased expression of β-arrestins or histone deacetylase 4 (HDAC4), or reduced expression of phosphatase and tensin homologue (PTEN), forkhead box protein O1 (FOXO1), progranulin (PGRN), sirtuin 6 or tyrosine-protein phosphatase non-receptor type substrate 1 (SIRPα) might result in autophagy impairment. In PTECs, exposure to high glucose increased the expression of sodium–glucose cotransporter 2 (SGLT2), thioredoxin-interacting protein (TXNIP), HDAC6 and inositol oxygenase (MIOX), or reduced expression of optineurin, all of which might also impair autophagy. Moreover, accumulation of advanced glycation end-products (AGEs) in PTECs might cause lysosome dysfunction and consequently disrupt auto-lysosome formation and function. In GMCs, reduced expression of metalloproteinase inhibitor 3 (TIMP3) might inhibit autophagy. Collectively, intracellular stress, disturbances of nutrient-sensing pathways and changes in these signalling pathways might lead to dysregulated autophagy and the progression of DKD. ECM, extracellular matrix; LC3, microtubule-associated protein 1 light chain 3.
β-Arrestins, which were upregulated in the kidneys of patients and mice with diabetes compared with their healthy counterparts, as well as in podocytes exposed to high glucose, might also regulate podocyte autophagy in DKD. β-Arrestins interacted with ATG7 and negatively regulated ATG12–ATG5 conjugation, which suppressed autophagy in podocytes132.
Histone deacetylase 4 (HDAC4) was also upregulated in cultured podocytes treated with high glucose, AGEs or TGFβ133. Under these conditions, HDAC4 suppressed podocyte autophagy by deacetylating STAT1 and sensitized podocytes to injury. HDAC4 inhibition restored autophagy and ameliorated podocyte injury133. In addition, overexpression of FOXO1 in podocytes activated PINK1–parkin-dependent mitophagy, which eliminated aberrant mitochondria and ameliorated podocyte injury in STZ-induced diabetic mice, suggesting a role for FOXO1 in the regulation of mitophagy in podocytes134. Renal progranulin (PGRN) was identified in 2019 as a novel upstream positive regulator of FOXO1. PGRN was significantly reduced in kidneys of STZ-induced diabetic mice and in kidney biopsy samples from patients with DKD compared with control mice and healthy human tissue, respectively135. Pgrn deficiency aggravated mitochondrial damage and dysfunction in podocytes from diabetic mice and worsened podocyte injury and proteinuria, whereas exposure to recombinant PGRN promoted mitophagy and mitochondrial biogenesis, thereby attenuating podocyte injury in diabetic mice. PGRN-related mitochondrial protection was mediated via PGRN–SIRT1–PGC1α regulation of FOXO1 and could be abrogated by the inhibition of mitophagy135.
The expression of phosphatase and tensin homologue (PTEN) was also reduced in podocytes from patients and mice with DKD compared with those from their healthy counterparts136. Compared with wild-type mice, overexpression of PTEN in inducible podocyte-specific Pten-knock-in mice reactivated autophagy and alleviated glomerular injury following STZ injection136. Moreover, SIRT6 was decreased in kidney biopsy samples from patients with podocytopathies, including DKD137. Podocyte-specific Sirt6 deletion accelerated podocyte injury and proteinuria in both STZ-induced diabetic mice and db/db mice. SIRT6 had an essential role in maintaining autophagy in podocytes treated with high glucose by inhibiting NOTCH signalling through deacetylation of acetylated histone 3 lysine 9 (H3K9ac) at Notch1 and Notch4 promoter sites137.
Tyrosine-protein phosphatase non-receptor type substrate 1 (SHPS1; encoded by Sirpa and also known as SIRPα) was another important regulator of podocyte autophagy in experimental models of nephropathy, including DKD138. SHPS1 was reduced in podocytes from STZ-induced diabetic mice and in cultured podocytes treated with high glucose. Sirpa deficiency inhibited podocyte autophagy in STZ-induced diabetic mice by suppressing AKT phosphorylation and subsequent glycogen synthase kinase 3β (GSK-3β)–β-catenin signalling — this effect exacerbated diabetes-induced proteinuria and podocyte injury compared with diabetic wild-type mice138. The observed autophagy impairment in diabetic podocytes seems to involve a complex interplay of various regulation mechanisms, including inhibition of autophagy due to excessive nutrient and/or energy, and activation of autophagy due to hyperglycaemia-induced intracellular stress.
PTEC autophagy in DKD
Hyperglycaemia, hyperinsulinemia and a high level of free fatty acids are major metabolic alterations observed in diabetes. Among them, hyperglycaemia inhibits autophagy in kidney tubules of diabetic animals and patients with diabetes mellitus139,140. Sodium–glucose cotransporter 2 (SGLT2) is the primary regulator of tubular glucose reabsorption and its expression is upregulated in diabetic kidneys. Sglt2 deletion alleviated STZ-induced accumulation of sequestosome 1 in the kidney, suggesting that SGLT2-mediated glucose uptake might contribute to the impairment of autophagy observed in PTECs under conditions of hyperglycaemia141. Pharmacological inhibition of SGLT2 might therefore contribute to kidney protection in DKD by attenuating the inhibitory effect of hyperglycaemia on autophagy.
One study reported that autophagy was impaired in high glucose-treated PTECs, diabetic kidneys of mice and kidney biopsy samples from patients with DKD140. Importantly, autophagy impairment in these conditions was shown to be mediated by the p53–microRNA (miR)-214–ULK1 axis, where p53 is activated in DKD to induce miR-214 in kidney tubules. Upon induction, miR-214 directly targets ULK1, leading to autophagy impairment140. p53 was also reported to induce miR-155 to repress SIRT1 resulting in autophagy suppression during high glucose treatment of cultured PTECs142. In diabetic rats and in PTECs exposed to high glucose, the upregulation of miR-22 was also shown to suppress autophagy by reducing PTEN expression in PTECs143. This regulation of gene expression mediated by miR-NAs might represent an integral part of the autophagy regulatory network in both podocytes and PTECs in response to hyperglycaemia. Thioredoxin-interacting protein (encoded by TXNIP) was also upregulated in the kidney of STZ-induced diabetic rats and in PTECs exposed to high glucose, where it activated mTORC1 and impaired autophagy144. The upregulation of TXNIP was also observed in patients with diabetes144.
High glucose also caused defective mitophagy in PTECs and accelerated tubular cell senescence. Overexpression of optineurin but not of PINK1 restored mitophagy and protected PTECs against premature senescence and activation of the NLRP3 inflammasome, which attenuated tubular injury145. Inositol oxygenase (encoded by Miox) was upregulated in PTECs exposed to high glucose and in the kidney tubules of diabetic mice; mitochondrial fragmentation and reduction in mitophagy were also observed. Inhibition of inositol oxygenase reactivated mitophagy and improved mitochondrial integrity139. Another study demonstrated that administration of the mitochondria-targeted antioxidant MitoQ ameliorated tubular injury in diabetic mice and reduced high-glucose-induced PTEC death by partially restoring mitophagy. This effect was potentially mediated by an increase in PINK1 expression induced by nuclear factor erythroid 2-related factor 2 (REF.140). These findings suggest that mitophagy in kidney tubules might be beneficial in DKD.
Of interest, one study identified a regulatory relationship between histone deacetylase 6 (HDAC6) and TFEB and its contribution to dysregulated autophagy in PTECs in various experimental models of CKD, including DKD146. In diabetic PTECs, deacetylation of TFEB by HDAC6 suppressed the activity and nuclear localization of this transcription factor, which led to the inhibition of autophagy and tubular cell death146. Dysregulated autophagy under hyperglycaemic conditions was also shown to be dependent on increased ubiquitylation of lysine 63 (Lys63–Ub), which is often observed in tubular cells from patients with DKD. Pharmacological inhibition of Lys63–Ub induced by high glucose significantly rescued autophagy impairment in PTECs and consequently abrogated tubular cell apoptosis147. Collectively, these results suggest that hyperglycaemia inhibits autophagy in kidney tubules and that different signalling pathways are involved in this effect.
The excess protein present in the glomerular filtrate owing to diabetes-induced proteinuria seems to activate autophagy and/or mitophagy in PTECs as an adaptive response148,149. However, prolonged or excessive albumin uptake might eventually impair autophagy in PTECs through an mTOR-dependent mechanism and induce progressive tubular injury150. For example, in cultured PTECs and mice, chronic albumin overload resulted in defective mitophagy, which was associated with decreased expression of BNIP3L151. Moreover, hyperactivation of mTOR might contribute to obesity-associated inhibition of autophagy in PTECs of patients and mice with type 2 diabetes, as the autophagy defect observed in mice with obesity induced by a high-fat diet could be ameliorated by treating the mice with rapamycin148. Similarly, in diabetic Wistar fatty rats, impaired autophagy in PTECs was reversed by a very-low-protein diet and by a daily 40% restriction of the food consumption, which suppressed mTORC1 and activated SIRT1 pathways, respectively, resulting in an attenuation of DKD-related tubular injury and kidney dysfunction152,153.
Of note, using cultured PTECs and different diabetic mouse models, researchers have suggested that type 1 and type 2 DKD, modelled by STZ injection and the db/db mouse models, respectively, differ in autophagic responses154. Autophagy in the proximal tubules seemed to be mainly regulated by insulin, instead of amino acids or glucose, which activated the mTOR pathway. Therefore, autophagy was suppressed in db/db mice owing to hyperactive mTOR, whereas high levels of autophagy were induced in STZ-injected mice, which eventually led to lysosomal stress. Notably, pretreatment with rapamycin caused stagnation of autophagy and exaggerated kidney IRI in STZ-injected mice, whereas rapamycin reactivated autophagy and attenuated kidney IRI in db/db mice154. These results highlight that understanding the distinct patterns of autophagy dysregulation in PTECs in different types of DKD is crucial to their therapeutic targeting.
AGEs generated in the hyperglycaemic state are generally endocytosed and degraded in the lysosomes of PTECs155, but high-glucose conditions seem to lead to defective autophagy, lysosomal dysfunction and the accumulation of AGEs in PTECs. An overload of AGEs disrupted lysosomal function and autophagic flux in PTECs156,157. Lysosome-associated membrane glycoprotein 1 (LAMP1) was upregulated in response to AGE overload in Atg5-competent primary PTECs, but was suppressed in ATG5-deficient cells. Similarly, the upregulation of LAMP1 was also suppressed in PTEC-specific Atg5-knockout STZ-induced diabetic mice compared with diabetic controls. Of note, AGEs also accumulated in the PTECs, glomeruli and kidney vasculature of these Atg5-knockout diabetic mice, in which inflammation and kidney fibrosis increased compared with controls156. These findings suggest that autophagy in PTECs has a role in regulating lysosomal biogenesis — impaired autophagy in diabetes might therefore reduce the clearance of damaged lysosomes and lead to AGE accumulation, which further disrupts lysosomal function. This vicious cycle might eventually lead to irreversible injury in the kidney tubules, glomeruli and tubulointerstitium, contributing to the progression of DKD.
Autophagy in GECs and GMCs in DKD
The role of autophagy in GECs and GMCs in DKD remains largely unclear. A 2010 study suggested that autophagy might be involved in regulating the degradation of bone morphogenetic protein and activin membrane bound inhibitor (BAMBI) in endothelial cells158. BAMBI was downregulated in glomeruli isolated from biopsy samples of patients with DKD compared with samples from healthy donors, and in the glomeruli of STZ-induced diabetic mice compared with wild-type controls159. STZ-induced diabetic mice with Bambi deletion displayed more severe glomerulopathy than diabetic wild-type mice159. BAMBI is a negative modulator of TGFβ signalling but canonical TGFβ signalling, as assessed by phosphorylation of Mothers against decapentaplegic homologue 3 (SMAD3) and expression of its target genes in glomeruli, was comparable in diabetic Bambi-knockout mice and diabetic wild-type mice. However, mitogen-activated protein kinase 3/1 (also known as ERK1/2) signalling and SMAD1/5 signalling in glomeruli were enhanced in diabetic Bambi-knockout mice compared with diabetic wild-type mice, which suggests that Bambi deletion enhances alternative TGFβ signalling in DKD159. Whether regulation of BAMBI by autophagy in endothelial cells is relevant to the development of DKD remains under investigation.
GEC-specific Atg5-knockout mice displayed mild lesions to the glomerular filtration barrier compared with wild-type mice under non-diabetic conditions, whereas STZ-induced diabetic mice with GEC-specific Atg5 deletion exhibited more severe glomerular endothelial injuries, including glomerular basement membrane thickening and podocyte effacement, than diabetic wild-type mice60. These observations suggest that autophagy in GECs preserves both endothelial integrity and podocyte homeostasis. In vitro experiments also revealed changes in GMC autophagy during DKD60. The metalloproteinase inhibitor 3 gene (Timp3) was downregulated in the kidney of STZ-induced diabetic mice and in patients with diabetes, as well as in GMCs treated with high glucose; Timp3 deletion exacerbated diabetic kidney injury in mice160. In cultured GMCs exposed to high-glucose, Timp3 deletion suppressed autophagy by reducing the expression of FOXO1a and its target ATG genes; restoration of TIMP3 expression in GMCs from Timp3−/− mice by transfection with a Timp3 adenovirus reversed the impairment in autophagy. A pro-survival role for autophagy was also demonstrated in GMCs treated with AGEs. Autophagy and mitophagy were activated in GMCs via ROS and prevented AGE-induced mitochondrial dysfunction and cell apoptosis161. Despite these in vitro findings, the role of autophagy in GMCs during DKD has not yet been verified in vivo.
Autophagy in FSGS
Focal segmental glomerulosclerosis (FSGS) is a fibrotic kidney disease characterized by glomerular lesions, particularly in podocytes — the formation of adhesions in the glomerular capillary tuft leads to capillary occlusion and nephron degeneration162. FSGS is either idiopathic or secondary to a number of other disorders, such as acquired immune deficiency syndrome and obesity; the cause of idiopathic FSGS remains unclear. Several studies indicate that podocyte autophagy has an important role in the development of FSGS.
Variants of the apolipoprotein L1 (APOL1) gene known to be associated with FSGS have been shown to interfere with endosomal trafficking and block autophagic flux, ultimately leading to podocyte injury163,164. Using puromycin aminonucleoside (PAN) treatment of differentiated podocytes and rats to model FSGS, researchers revealed a positive correlation between levels of LC3-II and the number of autophagic vacuoles in podocytes, as well as the recovery from the damage induced by PAN nephrosis165. This study presented the first evidence that podocyte autophagy might counter the development of FSGS. Another report demonstrated that podocyte-specific Atg5-knockout mice were more susceptible to PAN-induced glomerulosclerosis than wild-type controls44. We also demonstrated that autophagy activation was triggered by exposure to adriamycin in mouse podocytes in vitro and in vivo166. In vitro, podocyte apoptosis induced by adriamycin was reduced by rapamycin but increased by chloroquine. In vivo, adriamycin induced more severe podocyte injury, glomerulopathy and proteinuria in inducible podocyte-specific Atg7-knockout mice than in wild-type mice.
In another study, researchers deleted Atg5 or Atg7 in embryonic nephron progenitor cells and demonstrated that loss of autophagy in the entire nephron was sufficient to induce albuminuria, as well as glomerular and tubulointerstitial changes, that recapitulated the pathological features of human idiopathic FSGS167. This phenotype was potentially due to mitochondrial dysfunction and ER stress in podocytes and kidney tubules caused by the impairment in autophagy167. Activation of autophagy in podocytes might therefore represent a defence mechanism in FSGS and a potential therapeutic strategy.
Autophagy in podocytes of patients with FSGS has also been investigated. Electron microscopy and biochemical analyses revealed that patients with FSGS had a significantly lower percentage of autophagosome-positive podocytes and significantly lower levels of kidney beclin 1 than patients with minimal change disease (MCD)168. An analysis of repeated kidney biopsies from patients with MCD further revealed that patients with reduced podocyte autophagy were more likely to progress to FSGS than patients with relatively high levels of podocyte autophagy, who were more likely to have MCD168. A gradual decrease in podocyte autophagy might therefore underlie the possible progression of podocytopathies from MCD to FSGS. By contrast, one study reported high ATG3 mRNA levels in glomeruli from both patients with FSGS and patients with MCD44. Another study demonstrated a positive correlation between the number of autophagic vacuoles in podocytes and foot process effacement and proteinuria in patients with MCD169. The reasons for these discrepancies remain unknown and highlight the complexity of autophagy in human kidney cells, which complicates the use of autophagy biomarkers to predict the progression of kidney diseases.
The mechanisms underlying the activation of autophagy in FSGS podocytes remain largely unclear, but activation of mTORC1 (REFS166,170) and downregulation of SIRPα138 were observed in glomeruli from both patients with FSGS and in mouse models of FSGS.
Autophagy in polycystic kidney disease
Polycystic kidney disease (PKD) is the most common genetic form of CKD and is characterized by the development and growth of multiple kidney cysts, which leads to kidney damage and can cause kidney failure171. Autophagic flux was impaired in kidney epithelial cells in animal models of PKD and in patients with PKD172, suggesting that this impairment might contribute to PKD progression173. PKD is one of the most common ciliopathies and these disorders are associated with decreased autophagy174. The presence of primary cilia seems to be required for the activation of autophagy under conditions of starvation and fluid flow, and, in turn, autophagy defects suppress ciliagenesis175,176. In addition, many agents that protect against PKD, such as rapamycin and the mTOR-independent agents carbamazepine and minoxidil can also restore autophagy in animal models177-182, further suggesting that autophagy impairment has a pathogenic role in PKD. Mechanistically, aberrant mTOR activation has been linked to impaired autophagy and defective cilia in PKD183.
Autophagy as a therapeutic target
Given the crucial role of autophagy in the development of kidney diseases and ageing, pharmacological modulation of autophagy might be a promising strategy for the prevention and treatment of kidney diseases. Pharmacological enhancement of autophagy has been beneficial in several experimental models of kidney disease, including models of AKI, DKD, PKD, FSGS and kidney ageing. Notably, the precise function of autophagy in kidney fibrosis remains controversial.
In aged mice, long-term calorie restriction restored autophagic activity through the activation of the SIRT1–FOXO3 axis, which attenuated mitochondrial oxidative damage and protected against hypoxia-induced oxidative stress and mitochondrial damage in ageing kidneys79. Another study demonstrated that calorie restriction enhanced autophagy and mitigated most of the age-related damage in podocytes and convoluted proximal tubules184. Interestingly, the preservation of kidney structure in 11- or 21-month-old mice was better in animals fed lard as a source of dietary fat than in those fed soybean or fish oils as dietary fat. Another study demonstrated that short-term inhibition of mTORC1 with a low dose of everolimus in 24-month-old rats dramatically reduced age-related kidney disease and even counter-regulated age-related gene expression changes in the kidney185. However, the contribution of autophagy to the anti-ageing effects of everolimus was not investigated in this study.
Inhibition of mTORC1 and activation of AMPK or SIRT1 through genetic or pharmacological approaches have also been shown to prevent the progression of DKD in animal models186-188. However, the side effects of long-term or complete mTORC1 inhibition in kidney cells have been well-recognized, such as the early onset of proteinuria caused by specific inhibition of mTORC1 activity in podocytes in mice and the disruption of kidney tubular homeostasis caused by specific inhibition of mTORC1 activity in kidney tubular cells in mice51,80. Such inhibitors should therefore be used with caution in patients with DKD. In addition, Astragaloside IV, a saponin purified from the Astragalus root, prevented DKD progression by stimulating autophagy in diabetic mice189-191, whereas triptolide, the active component of the herb Tripterygium wilfordii Hook F, alleviated diabetic kidney fibrosis through the miR-141-3p-PTEN–AKT–mTOR pathway188. Long-term calorie restriction also improved glomerular damage in diabetic mice, at least partly by activating autophagy153.
The expanding list of chemical activators of autophagy includes mTOR inhibitors and mTOR-independent agents18,29,36,192. mTOR inhibitors, such as rapamycin, rapamycin analogues and mTORC1 kinase inhibitors, are already widely used as immunosuppressive agents in clinical settings18. mTOR-independent autophagy-inducing agents include activators of AMPK29 and SIRT136, chemicals that reduce intracellular inositol or IP3 levels (such as lithium, carbamazepine and sodium valproate), the K+-ATP channel opener minoxidil and the Tat–beclin 1 peptide, among others193-195. Some of these reagents have been shown to activate autophagy in kidneys in vivo with beneficial effects in animal models of AKI, DKD, FSGS or PKD69,175,178,196. Autophagy inhibitors, including 3-methyladenine and chloroquine, have also been widely tested in animal studies197.
Despite these encouraging findings, clinical translation of autophagy modulators remains challenging. First, the targets of these candidate agents might regulate cellular processes beyond autophagy and their pharmacological targeting might therefore result in unexpected harmful side effects. For example, mTOR has opposite effects on autophagy and cell growth and differentiation — mTOR inhibitors might provide renoprotection by activating autophagy, but they might also inhibit cell growth and therefore hinder kidney repair198,199. Second, in some disease settings, autophagy might have different roles depending on the cell types involved and the disease stage. For example, autophagy activation in PTECs during AKI is renoprotective, whereas autophagy induction in leukocytes might improve cell survival and promote inflammation200. In addition, sustained autophagy in PTECs after AKI might compromise kidney repair102. Therefore, the timing, duration and magnitude of autophagy modulation should be carefully considered to maximize its protective effect. Importantly, the cytoprotective effects of autophagy modulators have thus far only been reported in animal models of kidney disease, and evidence that these findings can be translated to humans is currently lacking.
Conclusions and perspectives
Substantial evidence supports an important role for autophagy in kidney physiology and pathology. Despite this progress, many unanswered questions remain. For example, the role of autophagy in GMCs, GECs and immune cells in different kidney disease conditions, as well as the mechanisms underlying the multifaceted role of autophagy in kidney tubulointerstitial fibrosis, remain unclear. Many signalling pathways participate in the regulation of autophagy in the kidney, but their precise mechanisms and signal relay in different types of kidney cells remain largely unknown. Moreover, in many cases, autophagy might interact with other cellular processes to influence the development of kidney diseases — such interactions and the regulatory mechanisms involved must also be investigated. The role of selective autophagy, such as mitophagy, in kidney diseases and ageing is also poorly understood. In addition, current evidence for autophagy dysfunction in kidney diseases is derived from hypothesis-driven studies and evidence from unbiased research, such as omics studies201, is still lacking. Finally, despite the obvious difficulties, future research must include the validation of preclinical findings in human samples and the testing of the potential therapeutic potential of compounds that modulate autophagy in clinical trials.
Key points.
Basal autophagy is essential to the maintenance of kidney homeostasis, structure and function.
Autophagy is suppressed in aged kidneys, which accelerates the progression of ageing and age-related kidney diseases.
In the acute injury phase of acute kidney injury (AKI), autophagy is induced in proximal tubules and acts as a protective mechanism. During the recovery phase, regulated autophagy is crucial for tubular repair.
Persistent activation of autophagy after AKI induces phenotypic changes in proximal tubule cells that might lead to maladaptive repair, contribute to interstitial fibrosis and promote transition from AKI to chronic kidney disease.
Autophagy defects in kidney cells of both tubular and glomerular compartments contribute to the development of diabetic kidney disease and focal segmental glomerulosclerosis.
Polycystic kidney disease is associated with autophagy defects in kidney tubules that might contribute to the development and formation of kidney cysts.
Acknowledgements
The authors were supported in part by grants from the National Natural Science Foundation of China (81870474), the National Institutes of Health (DK058831, DK087843) and the US Department of Veterans Affairs (BX000319). Z.D. is a recipient of the Senior Research Career Scientist award from the US Department of Veterans Affairs.
Glossary
- Vesicle nucleation
The process of mobilizing a small group of molecules to the phagophore assembly site to form a phagophore during autophagy.
- Phagophore
A cup-shaped double-membrane vesicle that expands and engulfs the components destined for digestion during autophagy.
- Autophagic flux
The whole process of autophagy, including autophagosome formation, maturation, fusion with lysosomes, subsequent breakdown and the release of macromolecules back into the cytosol.
- Lipofuscin
A yellowish brown, autofluorescent, lipidcontaining pigment that accumulates in the cytoplasm of cells during ageing.
- Ischaemic preconditioning
(IPC). An endogenous adaptive mechanism elicited by brief ischaemia that protects against a subsequent more sustained ischaemic insult.
- Chemical chaperones
Small molecules that enhance protein folding and/or stability.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Nephrology thanks C. Edelstein, D. Koya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dikic I & Elazar Z Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol 19, 349–364 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Oku M & Sakai Y Three distinct types of microautophagy based on membrane dynamics and molecular machineries. Bioessays 40, e1800008 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Kaushik S & Cuervo AM The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol 19, 365–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B & Kroemer G Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morishita H & Mizushima N Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol 35, 453–475 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Park JM et al. The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy 12, 547–564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JM et al. ULK1 phosphorylates Ser30 of BECN1 in association with ATG14 to stimulate autophagy induction. Autophagy 14, 584–597 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachari M & Ganley IG The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 61, 585–596 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kihara A, Noda T, Ishihara N & Ohsumi Y Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol 152, 519–530 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Y et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol 11, 468–476 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walczak M & Martens S Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 9, 424–425 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorincz P & Juhasz G Autophagosome-lysosome fusion. J. Mol. Biol 432, 2462–2482 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Gatica D, Lahiri V & Klionsky DJ Cargo recognition and degradation by selective autophagy. Nat. Cell Biol 20, 233–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroemer G, Marino G & Levine B Autophagy and the integrated stress response. Mol. Cell 40, 280–293 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu GY & Sabatini DM mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol 21, 183–203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Kundu M, Viollet B & Guan KL AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol 13, 132–141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosokawa N et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981–1991 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR & Kroemer G Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov 16, 487–511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang K et al. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy 10.1080/15548627.2019.1704117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aspernig H et al. Mitochondrial perturbations couple mTORC2 to autophagy in C. elegans. Cell Rep. 29, 1399–1409 e1395 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Shaw RJ et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero-Martin G et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28, 677–685 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson KA et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, Park S, Takahashi Y & Wang HG The association of AMPK with ULK1 regulates autophagy. PLoS One 5, e15394 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoki K, Zhu T & Guan KL TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Gwinn DM et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loffler AS et al. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 7, 696–706 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Dunlop EA, Hunt DK, Acosta-Jaquez HA, Fingar DC & Tee AR ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy 7, 737–747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Yang G, Kim Y, Kim J & Ha J AMPK activators: mechanisms of action and physiological activities. Exp. Mol. Med 48, e224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee IH et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl Acad. Sci. USA 105, 3374–3379 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang R et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456–466 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Hariharan N et al. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ. Res 107, 1470–1482 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan F, Cacicedo JM, Ruderman N & Ido Y SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem 283, 27628–27635 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou X et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem 283, 20015–20026 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh HS, McBurney M & Robbins PD SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5, e9199 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai H, Sinclair DA, Ellis JL & Steegborn C Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol. Ther 188, 140–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Y, Pattingre S, Sinha S, Bassik M & Levine B JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678–688 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zalckvar E et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 10, 285–292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.B’Chir W et al. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 41, 7683–7699 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Criollo A et al. The IKK complex contributes to the induction of autophagy. EMBO J. 29, 619–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Settembre C et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauhan S et al. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol. Cell 50, 16–28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8, 826–837 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Hartleben B et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest 120, 1084–1096 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SI et al. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J. Biol. Chem 287, 11677–11688 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Y et al. TGF-{beta}1 protects against mesangial cell apoptosis via induction of autophagy. J. Biol. Chem 285, 37909–37919 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bechtel W et al. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrol 24, 727–743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Chen MX, Fogo AB, Harris RC & Chen JK mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J. Am. Soc. Nephrol 24, 198–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cina DP et al. Inhibition of MTOR disrupts autophagic flux in podocytes. J. Am. Soc. Nephrol 23, 412–420 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oshima Y et al. Prorenin receptor is essential for normal podocyte structure and function. J. Am. Soc. Nephrol 22, 2203–2212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godel M et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest 121, 2197–2209 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoki K et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest 121, 2181–2196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narita M et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rong Y et al. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc. Natl Acad. Sci. USA 108, 7826–7831 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bork T et al. Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy 10.1080/15548627.2019.1705007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alghamdi TA et al. Janus kinase 2 regulates transcription factor EB expression and autophagy completion in glomerular podocytes. J. Am. Soc. Nephrol 28, 2641–2653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimura T et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol 22, 902–913 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livingston MJ et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 15, 2142–2162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McWilliams TG et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol 214, 333–345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenoir O et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 11, 1130–1145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda J et al. Antioxidant role of autophagy in maintaining the integrity of glomerular capillaries. Autophagy 14, 53–65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Sullivan ED, Hughes J & Ferenbach DA Renal aging: causes and consequences. J. Am. Soc. Nephrol 28, 407–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura S et al. Suppression of autophagic activity by Rubicon is a signature of aging. Nat. Commun 10, 847 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui J et al. Age-related changes in the function of autophagy in rat kidneys. Age 34, 329–339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto-Nonaka K et al. Cathepsin D in podocytes is important in the pathogenesis of proteinuria and CKD. J. Am. Soc. Nephrol 27, 2685–2700 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chuang PY et al. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am. J. Physiol. Renal Physiol 313, F621–F628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L et al. C/EBPalpha deficiency in podocytes aggravates podocyte senescence and kidney injury in aging mice. Cell Death Dis. 10, 684 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto T et al. Time-dependent dysregulation of autophagy: implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 12, 801–813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaushal GP & Shah SV Autophagy in acute kidney injury. Kidney Int. 89, 779–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang M et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 82, 1271–1283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi A et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol 180, 517–525 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Hsiao HW et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock 37, 289–296 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Leventhal JS et al. Autophagy limits endotoxemic acute kidney injury and alters renal tubular epithelial cell cytokine expression. PloS One 11, e0150001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mei S et al. Autophagy is activated to protect against endotoxic acute kidney injury. Sci. Rep 6, 22171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howell GM et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PloS One 8, e69520 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ko GJ, Bae SY, Hong YA, Pyo HJ & Kwon YJ Radiocontrast-induced nephropathy is attenuated by autophagy through regulation of apoptosis and inflammation. Hum. Exp. Toxicol 35, 724–736 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Lin Q et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 26, 101254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lei R et al. Mitophagy plays a protective role in iodinated contrast-induced acute renal tubular epithelial cells injury. Cell Physiol. Biochem 46, 975–985 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Lempiainen J et al. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1alpha-eNOS pathway and enhanced autophagy. Acta Physiol. 208, 410–421 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Grahammer F et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc. Natl Acad. Sci. USA 111, E2817–E2826 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chien CT, Shyue SK & Lai MK Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation 84, 1183–1190 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Rovetta F et al. ER signaling regulation drives the switch between autophagy and apoptosis in NRK-52E cells exposed to cisplatin. Exp. Cell Res 318, 238–250 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Herzog C, Yang C, Holmes A & Kaushal G P zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins but impairs autophagic flux and worsens renal function. Am. J. Physiol. Renal Physiol 303, F1239–F1250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jankauskas SS et al. The age-associated loss of ischemic preconditioning in the kidney is accompanied by mitochondrial dysfunction, increased protein acetylation and decreased autophagy. Sci. Rep 7, 44430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Y et al. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis. 9, 338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhatia D & Choi ME The emerging role of mitophagy in kidney diseases. J. Life Sci. 1, 13–22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Cai J, Tang C & Dong Z Mitophagy in acute kidney injury and kidney repair. Cells 9, 338 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang C et al. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14, 880–897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang C et al. Activation of BNIP3-mediated mitophagy protects against renal ischemia-reperfusion injury. Cell Death Dis. 10, 677 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y et al. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 9, 1113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao C et al. Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function. Oncotarget 8, 20988–21000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu JX et al. Disturbance of mitochondrial dynamics and mitophagy in sepsis-induced acute kidney injury. Life Sci. 235, 116828 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Maejima I et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang-Panesso M et al. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Invest 129, 5501–5517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kusaba T, Lalli M, Kramann R, Kobayashi A & Humphreys BD Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl Acad. Sci. USA 111, 1527–1532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosen S & Heyman S Concerning cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 94, 218 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Kumar S Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 93, 27–40 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Li L, Wang ZV, Hill JA & Lin F New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J. Am. Soc. Nephrol 25, 305–315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng H, Fan X, Lawson WE, Paueksakon P & Harris RC Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int. 88, 85–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nassour J et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 565, 659–663 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taji F et al. Autophagy induction reduces telomerase activity in HeLa cells. Mech. Ageing Dev 163, 40–45 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Baisantry A et al. Autophagy induces prosenescent changes in proximal tubular S3 segments. J. Am. Soc. Nephrol 27, 1609–1616 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang L, Besschetnova TY, Brooks CR, Shah JV & Bonventre JV Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med 16, 535–543 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Canaud G et al. Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci. Transl. Med 11, eaav4754 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li L et al. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J. Clin. Invest 129, 2374–2389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shu S et al. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 37, 269–280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li L, Zepeda-Orozco D, Black R & Lin F Autophagy is a component of epithelial cell fate in obstructive uropathy. Am. J. Pathol 176, 1767–1778 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Livingston MJ et al. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12, 976–998 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan Q et al. Autophagy activation contributes to lipid accumulation in tubular epithelial cells during kidney fibrosis. Cell Death Discov. 4, 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hernandez-Gea V et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142, 938–946 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thoen LF et al. A role for autophagy during hepatic stellate cell activation. J. Hepatol. 55, 1353–1360 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Xue X et al. Protein kinase Calpha drives fibroblast activation and kidney fibrosis by stimulating autophagic flux. J. Biol. Chem 293, 11119–11130 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li L et al. Forkhead box O3 (FoxO3) regulates kidney tubular autophagy following urinary tract obstruction. J. Biol. Chem 292, 13774–13783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang X et al. WNT1-inducible signaling protein-1 mediates TGF-beta1-induced renal fibrosis in tubular epithelial cells and unilateral ureteral obstruction mouse models via autophagy. J. Cell Physiol 235, 2009–2022 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Noh MR, Woo CH, Park MJ, In Kim J & Park KM Ablation of C/EBP homologous protein attenuates renal fibrosis after ureteral obstruction by reducing autophagy and microtubule disruption. Biochim. Biophys. Acta Mol. Basis Dis 1864, 1634–1641 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Kim WY et al. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology 17, 148–159 (2012). [DOI] [PubMed] [Google Scholar]
- 117.Xu G et al. Defects in MAP1S-mediated autophagy turnover of fibronectin cause renal fibrosis. Aging 8, 977–985 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ding Y et al. Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J. Am. Soc. Nephrol 25, 2835–2846 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H et al. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy 12, 1472–1486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng X et al. ATG5-mediated autophagy suppresses NF-kappaB signaling to limit epithelial inflammatory response to kidney injury. Cell Death Dis. 10, 253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu Y, Wang J, Xu W, Ding F & Ding W Prohibitin 2-mediated mitophagy attenuates renal tubular epithelial cells injury by regulating mitochondrial dysfunction and NLRP3 inflammasome activation. Am. J. Physiol. Renal Physiol 316, F396–F407 (2019). [DOI] [PubMed] [Google Scholar]
- 122.Nam SA et al. Autophagy in FOXD1 stroma-derived cells regulates renal fibrosis through TGF-beta and NLRP3 inflammasome pathway. Biochem. Biophys. Res. Commun 508, 965–972 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Nam SA et al. Autophagy attenuates tubulointerstital fibrosis through regulating transforming growth factor-beta and NLRP3 inflammasome signaling pathway. Cell Death Dis. 10, 78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhatia D et al. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight 4, e132826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li S et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic. Biol. Med 152, 632–649 (2020). [DOI] [PubMed] [Google Scholar]
- 126.Alicic RZ, Rooney MT & Tuttle KR Diabetic kidney disease: challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol 12, 2032–2045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu WJ et al. Lysosome restoration to activate podocyte autophagy: a new therapeutic strategy for diabetic kidney disease. Cell Death Dis. 10, 806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tagawa A et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 65, 755–767 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Yang D et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol. Life Sci 75, 669–688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ding Y & Choi ME Autophagy in diabetic nephropathy. J. Endocrinol 224, R15–R30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao X et al. Advanced glycation end-products suppress autophagic flux in podocytes by activating mammalian target of rapamycin and inhibiting nuclear translocation of transcription factor EB. J. Pathol 245, 235–248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu J et al. beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis. 7, e2183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 86, 712–725 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Li W et al. FoxO1 promotes mitophagy in the podocytes of diabetic male mice via the PINK1/Parkin pathway. Endocrinology 158, 2155–2167 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Zhou D et al. PGRN acts as a novel regulator of mitochondrial homeostasis by facilitating mitophagy and mitochondrial biogenesis to prevent podocyte injury in diabetic nephropathy. Cell Death Dis. 10, 524 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang H et al. Podocyte-specific knockin of PTEN protects kidney from hyperglycemia. Am. J. Physiol. Renal Physiol 314, F1096–F1107 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Liu M et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat. Commun 8, 413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li L et al. Signal regulatory protein alpha protects podocytes through promoting autophagic activity. JCIInsight 5, e124747 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhan M, Usman IM, Sun L & Kanwar YS Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol 26, 1304–1321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma Z et al. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney. J. Clin. Invest (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vallon V et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Renal Physiol 304, F156–F167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Zheng ZJ, Jia YJ, Yang YL & Xue YM Role of p53/miR-155-5p/sirt1 loop in renal tubular injury of diabetic kidney disease. J. Transl. Med 16, 146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y et al. MicroRNA-22 promotes renal tubulointerstitial fibrosis by targeting PTEN and suppressing autophagy in diabetic nephropathy. J. Diabetes Res. 2018, 4728645 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang C et al. Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci. Rep 6, 29196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen K et al. Optineurin-mediated mitophagy protects renal tubular epithelial cells against accelerated senescence in diabetic nephropathy. Cell Death Dis. 9, 105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brijmohan AS et al. HDAC6 inhibition promotes transcription factor EB activation and is protective in experimental kidney disease. Front. Pharmacol 9, 34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pontrelli P et al. Deregulation of autophagy under hyperglycemic conditions is dependent on increased lysine 63 ubiquitination: a candidate mechanism in the progression of diabetic nephropathy. J. Mol. Med 96, 645–659 (2018). [DOI] [PubMed] [Google Scholar]
- 148.Yamahara K et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J. Am. Soc. Nephrol 24, 1769–1781 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tan J, Wang M, Song S, Miao Y & Zhang Q Autophagy activation promotes removal of damaged mitochondria and protects against renal tubular injury induced by albumin overload. Histol. Histopathol 33, 681–690 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Nolin AC et al. Proteinuria causes dysfunctional autophagy in the proximal tubule. Am. J. Physiol. Renal Physiol 311, F1271–F1279 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu D et al. NIX-mediated mitophagy protects against proteinuria-induced tubular cell apoptosis and renal injury. Am. J. Physiol. Renal Physiol 316, F382–F395 (2019). [DOI] [PubMed] [Google Scholar]
- 152.Kitada M et al. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp. Diabetes Res 2011, 908185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kitada M et al. A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia 59, 1307–1317 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Sakai S et al. Proximal tubule autophagy differs in type 1 and 2 diabetes. J. Am. Soc. Nephrol 30, 929–945 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saito A et al. Significance of proximal tubular metabolism of advanced glycation end products in kidney diseases. Ann. NY Acad. Sci 1043, 637–643 (2005). [DOI] [PubMed] [Google Scholar]
- 156.Takahashi A et al. Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes 66, 1359–1372 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Liu WJ et al. Autophagy-lysosome pathway in renal tubular epithelial cells is disrupted by advanced glycation end products in diabetic nephropathy. J. Biol. Chem 290, 20499–20510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xavier S et al. BAMBI is expressed in endothelial cells and is regulated by lysosomal/autolysosomal degradation. PLoS One 5, e12995 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fan Y et al. BAMBI elimination enhances alternative TGF-beta signaling and glomerular dysfunction in diabetic mice. Diabetes 64, 2220–2233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fiorentino L et al. Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol. Med 5, 441–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu L, Fan Q, Wang X, Zhao X & Wang L Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis. 7, e2445 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rosenberg AZ & Kopp JB Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol 12, 502–517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumar V et al. Disrupted apolipoprotein L1-miR193a axis dedifferentiates podocytes through autophagy blockade in an APOL1 risk milieu. Am. J. Physiol. Cell Physiol 317, C209–C225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beckerman P et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat. Med 23, 429–438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Asanuma K et al. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 17, 1165–1167 (2003). [DOI] [PubMed] [Google Scholar]
- 166.Yi M et al. Autophagy is activated to protect against podocyte injury in adriamycin-induced nephropathy. Am. J. Physiol. Renal Physiol 313, F74–F84 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kawakami T et al. Deficient autophagy results in mitochondrial dysfunction and FSGS. J. Am. Soc. Nephrol 26, 1040–1052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zeng C et al. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J. Pathol 234, 203–213 (2014). [DOI] [PubMed] [Google Scholar]
- 169.Ogawa-Akiyama A et al. Podocyte autophagy is associated with foot process effacement and proteinuria in patients with minimal change nephrotic syndrome. PLoS One 15, e0228337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zschiedrich S et al. Targeting mTOR signaling can prevent the progression of FSGS. J. Am. Soc. Nephrol 28, 2144–2157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Torres VE & Harris PC Progress in the understanding of polycystic kidney disease. Nat. Rev. Nephrol 15, 70–72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Belibi F et al. Hypoxia-inducible factor-1alpha (HIF-1alpha) and autophagy in polycystic kidney disease (PKD). Am. J. Physiol. Renal Physiol 300, F1235–F1243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nowak KL & Edelstein CL Apoptosis and autophagy in polycystic kidney disease (PKD). Cell. Signal 68, 109518 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB & Christensen ST Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol 15, 199–219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pampliega O & Cuervo AM Autophagy and primary cilia: dual interplay. Curr. Opin. Cell Biol 39, 1–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang S, Livingston MJ, Su Y & Dong Z Reciprocal regulation of cilia and autophagy via the MTOR and proteasome pathways. Autophagy 11, 607–616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takiar V et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl Acad. Sci. USA 108, 2462–2467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu P, Sieben CJ, Xu X, Harris PC & Lin X Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum. Mol. Genet 26, 158–172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cornec-Le Gall E, Alam A & Perrone RD Autosomal dominant polycystic kidney disease. Lancet 393, 919–935 (2019). [DOI] [PubMed] [Google Scholar]
- 180.Rowe I et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med 19, 488–493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walz G et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med 363, 830–840 (2010). [DOI] [PubMed] [Google Scholar]
- 182.Li A et al. Rapamycin treatment dose-dependently improves the cystic kidney in a new ADPKD mouse model via the mTORC1 and cell-cycle-associated CDK1/cyclin axis. J. Cell Mol. Med 21, 1619–1635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kou P, Wei S & Xiong F Recent advances of mTOR inhibitors use in autosomal dominant polycystic kidney disease: is the road still open? Curr. Med. Chem 26, 2962–2973 (2019). [DOI] [PubMed] [Google Scholar]
- 184.Calvo-Rubio M et al. Dietary fat composition influences glomerular and proximal convoluted tubule cell structure and autophagic processes in kidneys from calorie-restricted mice. Aging Cell 15, 477–487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shavlakadze T et al. Short-term low-dose mTORC1 inhibition in aged rats counter-regulates age-related gene changes and blocks age-related kidney pathology. J. Gerontol. A Biol. Sci. Med. Sci 73, 845–852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hong Q et al. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 93, 1330–1343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ren H et al. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell Endocrinol. 500, 110628 (2020). [DOI] [PubMed] [Google Scholar]
- 188.Li XY et al. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol. Ther. Nucleic Acids 9, 48–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Song G et al. Astragaloside IV ameliorates early diabetic nephropathy by inhibition of MEK1/2-ERK1/2-RSK2 signaling in streptozotocin-induced diabetic mice. J. Int. Med. Res 46, 2883–2897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guo H et al. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKalpha-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci. Rep 7, 6852 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang X et al. Astragaloside IV represses high glucose-induced mesangial cells activation by enhancing autophagy via SIRT1 deacetylation of NF-kappaB p65 subunit. Drug Des. Devel. Ther 12, 2971–2980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Morel E et al. Autophagy: a druggable process. Annu. Rev. Pharmacol. Toxicol 57, 375–398 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Panda PK et al. Chemical screening approaches enabling drug discovery of autophagy modulators for biomedical applications in human diseases. Front. Cell Dev. Biol 7, 38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shoji-Kawata S et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams A et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol 4, 295–305 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lin TA, Wu VC & Wang CY Autophagy in chronic kidney diseases. Cells 8, 61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pasquier B Autophagy inhibitors. Cell Mol. Life Sci 73, 985–1001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fantus D, Rogers NM, Grahammer F, Huber TB & Thomson AW Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat. Rev. Nephrol 12, 587–609 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Viana SD, Reis F & Alves R Therapeutic use of mTOR inhibitors in renal diseases: advances, drawbacks, and challenges. Oxid. Med. Cell Longev 2018, 3693625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ & Carter AB Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44, 582–596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rinschen MM et al. A multi-layered quantitative in vivo expression atlas of the podocyte unravels kidney disease candidate genes. Cell Rep. 23, 2495–2508 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]