Abstract
NaCT/SLC13A5 is a Na+-coupled transporter for citrate in hepatocytes, neurons, and testes. It is also called mINDY (mammalian ortholog of ‘I'm Not Dead Yet’ in Drosophila). Deletion of Slc13a5 in mice leads to an advantageous phenotype, protecting against diet-induced obesity, and diabetes. In contrast, loss-of-function mutations in SLC13A5 in humans cause a severe disease, EIEE25/DEE25 (early infantile epileptic encephalopathy-25/developmental epileptic encephalopathy-25). The difference between mice and humans in the consequences of the transporter deficiency is intriguing but probably explainable by the species-specific differences in the functional features of the transporter. Mouse Slc13a5 is a low-capacity transporter, whereas human SLC13A5 is a high-capacity transporter, thus leading to quantitative differences in citrate entry into cells via the transporter. These findings raise doubts as to the utility of mouse models to evaluate NaCT biology in humans. NaCT-mediated citrate entry in the liver impacts fatty acid and cholesterol synthesis, fatty acid oxidation, glycolysis, and gluconeogenesis; in neurons, this process is essential for the synthesis of the neurotransmitters glutamate, GABA, and acetylcholine. Thus, SLC13A5 deficiency protects against obesity and diabetes based on what the transporter does in hepatocytes, but leads to severe brain deficits based on what the transporter does in neurons. These beneficial versus detrimental effects of SLC13A5 deficiency are separable only by the blood-brain barrier. Can we harness the beneficial effects of SLC13A5 deficiency without the detrimental effects? In theory, this should be feasible with selective inhibitors of NaCT, which work only in the liver and do not get across the blood-brain barrier.
Keywords: blood-brain barrier, brain, EIEE25/DEE25, liver, metabolic syndrome, NACT/SLC13A5/mINDY
Introduction
Transport proteins (transporters) are involved in the movement of several biological molecules (e.g. nutrients, metabolites) across the plasma membrane and membranes of subcellular organelles (e.g. mitochondria, lysosomes) [1,2]. Although expressed throughout the body, transport proteins are particularly expressed at higher density and in a broader spectrum in the liver, intestine, kidney, and placenta where several nutrients and metabolites are secreted or absorbed/reabsorbed [1,2]. Transporters are divided into two major groups: ATP-binding cassette (ABC) proteins and the solute carrier (SLC) proteins [1]. ABC proteins include transporters such as the multidrug resistance protein 1 (MDR1) and the breast cancer resistance protein (BCRP), which all function, at least in mammals, as efflux transporters for the elimination of xenobiotics, toxins, and selective endogenous substrates via a transport mechanism that is coupled to ATP hydrolysis [1]. As such, ABC proteins profoundly affect the absorption, distribution, and elimination of several drugs, thus contributing to drug–drug interactions and possibly drug efficacy and toxicity. In contrast, SLC transporters most often mediate the influx of endogenous and exogenous substrates into cells, and their transport mechanism may or may not involve the participation of a driving force. When it is involved, the driving force is not ATP as in the case of ABC transporters but transmembrane ion gradients (Na+ gradient, H+ gradient, Cl− gradient, and K+ gradient). Some SLC transporters do participate in the efflux of their substrates, most often associated with vectorial transcellular movement across barrier structures (e.g., intestinal barrier, renal tubular barrier, blood–brain barrier, blood–retinal barrier, placental barrier) and facilitated wither by the transmembrane concentration gradient for the substrates or by exchange with another substrate. SLC transporters recognize as their substrates a wide variety of biologically and pharmacologically active compounds, including sugars, amino acids, peptides, vitamins, inorganic ions, nucleosides, and metabolites. Currently, there are more than 400 SLC transporters identified in humans, which are organized into more than 65 gene families [3–5]. For a majority of these transporters, the transportable substrates and the modes of transport have been elucidated. However, there are still a significant number of SLC transporters whose transport function and transportable substrates remain unknown; these transporters are referred to as ‘orphan’ transporters. Given their role in providing cells with important nutrients and metabolites to feed into multiple biochemical pathways, loss-of-function mutations in many of these transporters have been identified as either the sole cause (single-gene disorders) or modifiers (multifactorial disorders) of disease processes involving almost every tissue in the body.
In recent years, the SLC13 transporter family has received increasing attention because of the involvement of one of its members as the single-gene cause of a severe metabolic disease. The involved transporter, known as SLC13A5, is a Na+-coupled citrate transporter (named NaCT based on its transport function), and the associated disease is called early infantile epileptic encephalopathy-25 (EIEE25) or developmental epileptic encephalopathy-25 (DEE25). The members of the SLC13 transport family are found in both prokaryotes and eukaryotes, including gram-negative bacteria, cyanobacteria, archaea, plant chloroplasts, yeast, and mammals [3]. As the initially identified members of this family are transporters for divalent anions such as sulfate and dicarboxylates and use a transmembrane Na+ gradient as the driving force, this family is sometimes referred to as divalent anion sodium symporter (DASS) superfamily [6]. With the discovery of the most recent member of this family, SLC13A5/NaCT, which transports the tricarboxylate anion citrate, the rationality of the name DASS to the family comes into question.
Based on the nature of the substrates, the members of the SLC13 family fall into two categories, one for the inorganic anion sulfate and the other for organic anions (dicarboxylates and tricarboxylates) [7,8]. To date, there are five members in this family: Na+-dependent sulfate transporter-1 (NaS1/SLC13A1), Na+-dependent sulfate transporter-2 (NaS2/SLC13A4), Na+-dependent dicarboxylate transporter-1 (NaC1/NADC1/SDCT1/SLC13A2), Na+-dependent dicarboxylate transporter-3 (NaC3/NaDC3/SDCT2/SLC13A3), and Na+-dependent citrate transporter (NaC2/NaCT/SLC13A5) (Table 1). A recent report identified another putative transporter as the potential newest member of the SLC13 family [9], but it is not clear whether the transporter, classified as SLC13A6, actually belongs to this family because its amino acid sequence bears very low resemblance to that of the other members of the family. Furthermore, there is no information on the transport function or the substrates of this transporter. Therefore, it remains to be seen if this new transporter protein qualifies to be included as an authentic member of the SLC13 family. It is important to note, however, that SLC13A6 does have significant similarity to the CitMHS (citrate-Mg2+ : H+/citrate-Ca2+ : H+ symporter) family of citrate transporters in bacteria [10], but these prokaryotic transporters do not exhibit any similarity in amino acid sequence with the authenticated members of the SLC13 family either.
Table 1. Functional characteristics of the members of the SLC13 family in humans.
| HUGO Name TCDB ID UniProt ID |
Common name(s) | Substrates | Tissue expression | Chromosome location | Disease connection |
|---|---|---|---|---|---|
| SLC13A1 2.A.47.1.16 Q9BZW2 |
Na+/sulfate cotransporter NaS1 |
Sulfate Thiosulfate Selenate |
Kidney (BBM) | 7q31.31 | None |
| SLC13A2 2.A.47.1.17 Q13183 |
Na+/dicarboxylate cotransporter (low affinity) NaDC1/NaC1 |
Succinate α-Ketoglutarate Fumarate Malate Citrate |
Kidney (BBM) Intestine (BBM) |
17q11.2 | None |
| SLC13A3 2.A.47.1.15 Q8WWT9 |
Na+/dicarboxylate cotransporter (high-affinity) NaDC3/NaC3 |
Succinate α-Ketoglutarate Fumarate Malate Citrate |
Kidney (BBM) Liver (SM/BLM) Placenta (BBM) |
20q13.12 | None |
| SLC13A4 2.A.47.1.14 Q9UKG4 |
Na+/sulfate cotransporter SUT1/NaS2 |
Sulfate | Placenta (BBM) Testis Heart |
7q33 | None |
| SLC13A5 2.A.47.1.9 Q86YT5 |
Na+/citrate cotransporter NaCT/NaC2 |
Citrate Succinate Pyruvate |
Liver (SM/BLM) Brain (neuron) Testis (germ cell) |
17p13.1 | EIEE25 (DEE25) OMIM # 615905 |
BBM, lumen-facing brush-border membrane in kidney and intestinal epithelial cells and maternal-facing brush-border membrane in placental trophoblasts; SM/BLM, blood-facing sinusoidal membrane, also called basolateral membrane, in hepatocytes; SLC, solute-linked carrier; NaS, Na+/Sulfate cotransporter; NaDC, Na+/Dicarboxylate Cotransporter; SUT1, Sulfate Transporter; NaCT, Na+-coupled Citrate Transporter; NaC, Na+/Carboxylate transporter; EIEE25, Early Infantile Epileptic Encephalopathy-25; DEE25, Developmental Epileptic Encephalopathy; HUGO, Human Genome Organization; TCDB, Transporter Classification Database; UniProt, UniProt database for protein sequences; ID, Identification number. Each of the transporter is identified by its SLC classification according to the Human Genome Organization (e.g. SLC13A1) or by its transporter classification according to the Transporter Classification Database (e.g. 2.A.47.1.16) and the corresponding protein is identified by its amino acid sequence ID according to the UniProt database for protein sequences (e.g. Q9BZW2).
Every member of the SLC13 family functions as an electrogenic transporter, meaning that their transport function is associated with membrane depolarization. Since all of them are coupled to Na+ symport, the stoichiometry of Na+ to the organic anionic substrate is 3 : 1 for sulfate (NaS1 and NaS2) and divalent organic anions such as succinate or monoprotonated citrate (NaDC1 and NaDC3) and 4 : 1 for trivalent citrate (NaCT) [7,8]. This coupling stoichiometry means a net transfer of one positive charge into the cells per transport cycle, thus providing the molecular basis for the electrogenic nature of the transport process. The SLC13 transport proteins consist of 572–627 amino acids with 8–13 transmembrane domains; they also contain several consensus sites for phosphorylation and N-myristoylation. In addition, there is a 17-amino acid consensus sequence motif, known as the ‘sodium : sulfate symporter family signature’, in each of these proteins [11]. In this review, we focus on NaCT/SLC13A5, one particular member of this family, with emphasis on the biological consequences of loss-of-function mutations in the transporter in humans versus mice.
Discovery of INDY as a lifespan-modifying transporter protein in Drosophila
The discovery of NaCT in mammals was directly linked to the identification of a specific transporter as a lifespan modifier in Drosophila [12]. Rogina et al. [12] discovered certain mutant flies which showed significantly extended lifespan, and subsequently identified the gene responsible for this phenomenon. The mutant flies with extended lifespan harbored heterozygous deletion of the gene. Homozygous deletion of the gene did not confer the increase in lifespan; it actually had the opposite effect, decreasing the lifespan to a small, but significant, extent. The amino acid sequence of the protein product of the gene was deduced and was found to bear the structural motifs of a transporter protein (e.g. multiple transmembrane domains). Comparison of its amino acid sequence with the sequences of the transporters in mammals known at the time of this discovery indicated close resemblance to two plasma membrane transporters, namely NaDC1/SLC13A2 and NaDC3/SLC13A3 (Figure 1), which transport several dicarboxylate intermediates of the citric-acid cycle in a Na+-coupled manner [7,8]. When the original discovery of this gene in Drosophila was reported, the actual transport function of the protein product was not established [12]. But, based on the structural similarity of this putative transporter protein with NaDC1/SLC13A2 and NaDC3/SLC13A3, the authors postulated that the Drosophila protein was also a transporter for the dicarboxylate intermediates of the citric-acid cycle. It was surmised that as these intermediates are important energy-rich metabolites which can readily enter the citric-acid cycle to generate metabolic energy, partial deficiency of the putative transporter in Drosophila due to heterozygous deletion of the gene would mimic caloric restriction in the organism. It was already known that caloric restriction enhances lifespan in Drosophila, Caenorhabditis elegans, rodents, and primates [13,14]. Therefore, it made sense that partial deficiency of the putative transporter in Drosophila was associated with the extension of lifespan if it indeed is the transporter for the citric-acid cycle intermediates. It also seemed logical as to why the total absence of the transporter did not result in lifespan extension; if caloric restriction goes beyond a certain limit, it will have detrimental effects on the organ function and on the organism as a whole. With this rationale, Rogina et al. [12] named this life-extending gene Indy and the protein product INDY (I'm Not Dead Yet).
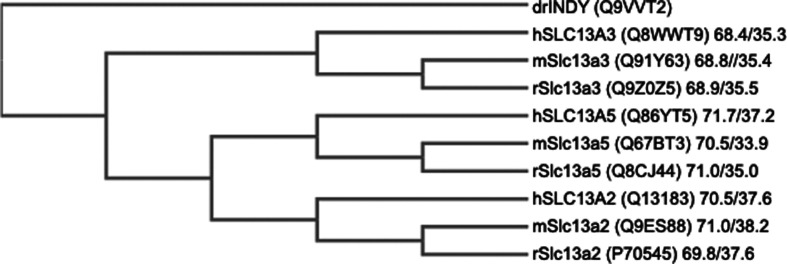
Figure 1. Phylogenetic tree of the members of the SLC13 family.
The values for amino acid sequence similarity/identity given for each member is between Drosophila INDY and the respective member. Protein sequences were obtained from Uniport and were aligned using Clustal Omega Software to construct the phylogram. The Lalign program was used to obtain the values for similarity and identity in amino acid sequence for each member in comparison with Drosophila INDY.
Establishment of the transport function of Drosophila INDY and its relevance to lifespan
Even though the impact of INDY on lifespan in Drosophila was established unequivocally with strong experimental evidence, the function of the INDY protein as a transporter for citric-acid cycle intermediates was only a speculation, solely based on the amino acid sequence similarity of INDY to the Na+-coupled dicarboxylate transporters in mammals [7,8]. By the time INDY was discovered in Drosophila, there were two Na+/dicarboxylate cotransporters in mammals, NaDC1 and NaDC3 (Figure 1; Table 1). NaDC1 (SLC13A2) was first cloned from kidney [15] and NaDC3 (SLC13A3) was first cloned from placenta [16]. NaDC1 is primarily expressed in the lumen-facing brush-border membrane of the renal and intestinal absorptive epithelial cells, whereas NaDC3 is expressed in the basolateral membrane of renal epithelial cells, sinusoidal (basolateral) membrane of hepatocytes, the maternal-facing brush-border membrane of placental trophoblast cells, and astrocytes in the brain. Both transporters exhibit similar substrate selectivity, favoring dicarboxylates. The major difference in the transport function between the two transporters is in substrate affinity; NaDC1 is a low-affinity transporter while NaDC3 is a high-affinity transporter. If Drosophila INDY is indeed a dicarboxylate transporter, does it resemble NaDC1 or NaDC3 in transport function? Drosophila INDY is expressed in fat body, oenocytes, and certain specific cell types in the midgut, all of which are capable of metabolic processing of nutrients in terms of energy production or storage of excess calories [12]. Based on this tissue distribution and biological function relevant to caloric restriction, we first thought that Drosophila INDY might be equivalent to mammalian NaDC3. To test this idea, we cloned Drosophila INDY and studied its transport function using succinate as a substrate [17]. These studies did show that INDY was indeed a transporter for this dicarboxylate. However, when the transport function was analyzed in detail, it became obvious that the transport features and substrate specificity were not similar to those of either NaDC3 or NaDC1. INDY turned out to be a transporter with a preference for the tricarboxylate citrate than for dicarboxylates [17]. Furthermore, it worked as an exchanger and its transport function was not dependent on Na+. These findings were confirmed and corroborated independently by the investigators who first identified INDY in Drosophila [18,19]. Based on these data, neither NaDC1 nor NaDC3 seemed the likely candidate to represent the mammalian counterpart of Drosophila INDY.
Now that the transport function of INDY has been established as a transporter for citric-acid cycle intermediates, it becomes easier to explain the relationship between INDY and lifespan [20,21]. The substrates for INDY include almost all intermediates in the citric-acid cycle, namely citrate, α-ketoglutarate, succinate, fumarate, and malate. Pyruvate, though not a dicarboxylate, appears to be a substrate while the other biologically important monocarboxylates, lactate, and β-hydroxybutyrate, are not. Based on the caloric values of these intermediates and their concentrations in the circulation, the amount of energy that is available in blood from these compounds can be estimated (Table 2). Among the substrates of INDY, citrate is present at the highest levels (150–200 μM). The hemolymph, the plasma counterpart in Drosophila, contains similar levels of citrate (101 ± 54 μM) [22]. The normal physiologic function of INDY might be to provide these calorie-rich metabolites to metabolically active oenocytes and cells of the fat body and midgut. Therefore, partial deficiency of INDY as expected in Drosophila with heterozygous deletion of Indy might mimic a biologic condition similar to that caloric restriction. One of the mechanisms by which high caloric intake elicits detrimental effects on the organism is by maintaining the mitochondrial electron transport chain activity at high levels. Even though ATP generation from this fundamental biologic process is obligatory for the organismal survival, which involves the complete transfer of four electrons to molecular oxygen thus producing water, the same process also generates proportionately high levels of reactive oxygen species (superoxide, hydrogen peroxide, and hydroxyl radical) as the accidental byproducts of incomplete electron transfer to molecular oxygen: O2 + 1e → O2• (superoxide); O2 + 2e → O22− (peroxide); O2 + 3e → O2− (oxide) + O• (hydroxyl radical in the presence of H+) [23,24]. These oxygen species are highly reactive, capable of oxidizing and damaging a variety of macromolecules (proteins, nucleic acids, lipids), compromising their normal function. Cumulative accumulation of such damages enhances the aging process and decreases lifespan. As such, heterozygous deletion of Indy, which causes a partial deficiency of INDY, reduces the entry of the citric-acid cycle intermediates into cells, consequently suppressing the mitochondrial electron transport chain and hence the generation of the reactive oxygen species. This represents at least one of the molecular mechanisms by which partial deficiency of INDY could increase the lifespan in an organism.
Table 2. . Citric-acid cycle intermediates and related metabolites in plasma: concentration and energy content.
| Metabolite | Concentration (μM) | Energy content (Calories) |
|---|---|---|
| Tricarboxylates | ||
| Citrate | 150–200 | 225–300 |
| Isocitrate | <10 | |
| Dicarboxylates | ||
| Succinate | 80–100 | 70–90 |
| Malate | 35–100 | 35–100 |
| α-Ketoglutarate | 10–15 | 10–15 |
| Fumarate | <10 | |
| Oxaloacetate | <10 | |
| Monocarboxylates | ||
| Lactate | 500–2000 | 480–1900 |
| Pyruvate β-Hydroxybutyrate |
80–160 50–100 |
75–150 55–110 |
Energy content was calculated from the plasma concentrations of these metabolites, assuming a plasma volume of 3 l in humans. The caloric value for organic acids is in the range of 2.5–3.5 Calories/g. The caloric value used for lactate, pyruvate, and β-hydroxybutyrate was 3.5; for the remaining metabolites, a value of 2.5 was used (https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Classics/ah74.pdf). The energy contents for the metabolites whose plasma concentrations are less than 10 μM are not included. 1 Calorie = 1 kcal.
Discovery of NaCT/SLC13A5 as the mammalian ortholog of INDY (mINDY)
The establishment of Drosophila INDY as a Na+-independent transporter for citric-acid cycle intermediates functioning as an exchanger rather than as a symporter with Na+ or a uniporter revealed that neither NaDC1/SLC13A2 nor NaDC3/SLC13A3 could be the mammalian ortholog of Drosophila INDY. This was puzzling because the amino acid sequence of Drosophila INDY showed significant similarity to only the aminoacid sequences of these two mammalian transporters known at that time. This raised a question as to whether there was a mammalian counterpart of Drosophila INDY that had not been identified yet. To answer this question, we undertook a systematic approach to examine the known ESTs (established sequence tags) for their predicted amino acid sequences to see if any of them exhibited similarity to the sequences of NaDC1 or NaDC3 but was not identical with either of them. ESTs are partial sequences of mRNAs; therefore, neither the full-length sequences nor the biological functions of the protein products is known. The rationale for our approach was simple: if we identify an EST in a given species whose predicted aminoacid sequence is similar to that of NaDC1 or NaDC3 but not identical with either of these transporters in the same species, the EST would most likely represent a new, not yet characterized, member of the SLC13 family. If such an EST exists in any mammalian species, we should be able to clone the full-length version of the EST and characterize its transport function to determine if its functional features are similar to those of Drosophila INDY. We took this unbiased approach to identify the mammalian ortholog of Drosophila INDY. This search led to the identification of an EST in rat brain which met all the criteria set forth in the approach. With this EST as the probe, we screened a rat brain cDNA library and cloned the full-length version of the EST. Functional characterization of the clone in heterologous expression systems showed that the full-length cDNA encoded a transporter that was distinct from rat NaDC1 and rat NaDC3 but catalyzed Na+-coupled transport of citrate [25].
Unlike NaDC1 and NaDC3 which have a preference for dicarboxylates over the tricarboxylate citrate, the newly identified transporter from rat brain showed a preference for citrate. Dicarboxylates and monocarboxylates interacted with the transporter with much lower affinities. The affinity for succinate for the newly discovered transporter was 6-fold less than that for citrate, and the affinities for other dicarboxylates such as fumarate and α-ketoglutarate were even lower. Stereochemistry seemed to play a critical role in the interaction of citrate with the transporter because isocitrate, which contains the hydroxyl group on a different carbon than in citrate, was not a substrate. This was the first transporter in mammals that functions specifically in the entry of citrate into cells. Based on these functional features, we named the new transporter NaCT (Na+-coupled citrate transporter). According to the Human Genome Nomenclature, NaCT is referred to as SLC13A5, the newest member of the SLC13 family. In substrate selectivity, NaCT resembled Drosophila INDY. However, NaCT and Drosophila INDY differed in Na+-dependence and transport mechanism: NaCT is a Na+/citrate symporter, whereas Drosophila INDY is not only Na+-independent but also an exchanger for citrate and other dicarboxylates. As such, NaCT did not function exactly identical with Drosophila INDY. Nonetheless, among the three transporters for citric-acid cycle intermediates identified thus far in mammals, NaCT was the closest to Drosophila INDY in terms of transport function. Since no transporter other than NaCT has been identified till this day in mammals that is functionally closer to Drosophila INDY, NaCT has been widely accepted as the de facto mammalian ortholog of Drosophila INDY (mINDY). It is interesting to note, however, that Drosophila INDY does not show any preferential similarity/identity with mammalian INDY in amino acid sequence; the similarity/identity of the amino acid sequence of Drosophila INDY is similar for all three dicarboxylate/tricarboxylate transporters in mammals (Figure 1). This is not surprising given the fact that the functional features of Drosophila INDY bear no great resemblance to those of the mammalian transporters except for the substrate specificity.
Based on northern blot data with rat tissues, NaCT is expressed at the highest level in the liver and testes and at a much lower level in the brain, the tissue from which the transporter was originally cloned [25]. Other tissues showed evidence of little or no expression. The subcellular localization of NaCT in the liver is of interest and importance, particularly with regard to its recognition as mINDY. NaCT is expressed exclusively in the sinusoidal (basolateral) membrane of the hepatocytes in rat liver [26], which means that NaCT functions to deliver citrate and other citric-acid cycle intermediates from the circulation into hepatocytes. It is important to note that Drosophila INDY is expressed in cells of fat body, the tissue analogous to liver in mammals, thus suggesting a similar physiological function for NaCT in rat and INDY in Drosophila. Liver is a unique organ capable of carrying out most of the biochemical pathways related to catabolism and anabolism. It can modulate the rate of energy-consuming anabolic pathways and energy-generating catabolic pathways as needed under conditions of caloric deficiency (i.e. starvation) or caloric sufficiency (i.e. fed state). Therefore, the fact that liver is the major site of NaCT expression can be taken as supporting evidence for NaCT being the mammalian ortholog of Drosophila INDY because of the connection of partial deficiency of INDY to the state of caloric restriction.
Since the original identification of rat NaCT, the mouse and human orthologs were cloned and functionally characterized [27,28]. As in rat, the mouse and human NaCTs are also expressed primarily in the liver and testes, with significant expression detectable in brain. The substrate selectivity and the obligate requirement for Na+ are also the same as with rat NaCT. The transport mode of NaCT in all three species involves an electrogenic mechanism with the transport process associated with membrane depolarization. Since then, the transporter has been cloned from several other species (rabbit, dog, monkey, chimpanzee, zebrafish, and C. elegans) [29]. Interestingly, the transporter is Na+-coupled and electrogenic even in C. elegans, a feature that distinguishes it from Drosophila INDY. In mammals, the requirement for Na+ is obligatory for NaCT function; no other monovalent cation (K+, H+, or Li+) could support the transport function in the total absence of Na+. There is no involvement of any anion (e.g. Cl−) in NaCT-mediated transport process.
Species-specific differences in functional features of NaCT in mammals
One of the most notable differences in the functional features of rodent (mouse or rat) and human NaCTs is in the affinity for citrate. When expressed ectopically in human retinal pigment epithelial cells, rat [25] and mouse [27] NaCTs exhibit a Michaelis constant for citrate in the range of 20–40 μM. With the same expression system and identical experimental conditions for uptake measurement, human NaCT shows a Michaelis constant of ∼650 μM [28]. This represents a 15–30 fold difference in substrate affinity. Interestingly, the corresponding value for the constitutively expressed NaCT in human liver cell line HepG2 is even higher, in the low millimolar range (∼5 mM) [26]. Whether the observed difference in substrate affinity for human NaCT between transient expression and constitutive expression is also true for the rodent NaCTs is not known because there are no published reports on substrate affinity for constitutively expressed mouse NaCT or rat NaCT. The reasons for the difference in substrate affinity between transiently expressed human NaCT and constitutively expressed human NaCT are also not known. These findings nonetheless show that rodent NaCTs are high-affinity/low capacity transporters, whereas human NaCT is a low-affinity/high-capacity transporter. We contend that this difference between human NaCT and rodent NaCTs in citrate affinity is of physiologic significance, given that the physiologic concentration of citrate in circulation is in the range of 150–200 µM. With Michaelis constant at 20–40 μM, mouse and rat NaCTs are expected to be almost totally saturated, meaning that their ability to mediate the entry of citrate from the blood into cells will not vary much despite significant physiological or pathological fluctuations in citrate levels in the blood. In contrast, with a Michaelis constant of 650–5 000 μM, human NaCT is not saturated with citrate under physiologic conditions. This means that the ability of human NaCT to mediate the entry of citrate from the blood into cells will vary proportionately with changes in circulating levels of citrate. With the values for Michaelis constant and maximal velocity deduced from the heterologous expression system in mammalian cells, we calculated that the amount of citrate delivered into cells via mouse NaCT is only ∼5% of the corresponding value for human NaCT at physiologic concentrations of citrate found blood.
A second important difference between the rodent NaCTs and human NaCT is in the impact of lithium on their transport function. The mammalian Na+/dicarboxylate cotransporters NaDC1 and NaDC3 are obligatorily dependent on Na+ for their transport function [7,8]. Na+ cannot be substituted by Li+ to support their transport function. However, in the presence of Na+, the transport functions of NaDC1 and NaDC3 are inhibited by Li+ [30,31]. Since the Na+ : dicarboxylate stoichiometry for both these transporters is 3 : 1, at least one of the three Na+-binding sites might interact with Li+. However, it is not clear why Li+ would interfere with the transport function if it substitutes for Na+. The interaction of Li+ with these two transporters seems to be complex because one of the mutants of NaDC1, but not the wild type NaDC1, is actually stimulated by Li+ in the presence of Na+ [32]. The observed inhibition of NaDC1 by Li+ has clinical significance. Urinary excretion of dicarboxylates is increased in patients undergoing lithium therapy for psychiatric disorders [33]. Since NaDC1 plays a major role in the reabsorption of filtered dicarboxylates in the kidney [34], the inhibition of this transporter by Li+ offers a molecular mechanism for this clinical finding [35]. Because of these published reports on the inhibition of Na+/dicarboxylate cotransporters by Li+, we asked whether NaCT, a closely related member of the same family, behaves in a similar manner with regard to Li+ inhibition. Unexpectedly, our experiments showed that human NaCT is not inhibited, but actually activated markedly, by Li+ in the presence of Na+ [36]. Subsequent studies showed a surprising species difference in this phenomenon; only primate NaCTs (monkey, chimpanzee, and human) are activated by Li+, whereas NaCTs from non-primates (mouse, rat, rabbit, and dog) are inhibited by Li+ [29]. The Li+-induced activation of primate NaCTs involves a marked increase in citrate affinity, meaning that Li+ converts the primate NaCTs from a low-affinity/high-capacity type into a high-affinity/low-capacity type [36]. Interestingly, one particular amino acid in human NaCT, namely F500, is an important determinant of substrate affinity [36]. Substitution of Phe at this position with Leu does exactly what Li+ does, i.e. converting the low-affinity/high-capacity transporter into a high-affinity/low-capacity transporter. This is accompanied with loss of sensitivity to Li+-dependent activation [36]. The stimulation of human NaCT by Li+ can provide a mechanistic explanation for the dyslipidemia and weight gain experienced by patients undergoing lithium therapy [37,38]. The therapeutically relevant concentration of Li+ in circulation is 1.5–2 mM, a concentration that elicits a two-to-three fold increase in the transport activity of human NaCT [29,36]. Since human NaCT is expressed in the sinusoidal membrane of hepatocytes [26], Li+ stimulation can increase intracellular citrate levels, which can be subsequently shuttled towards the synthesis of fatty acids and generation of low-density lipoproteins. The experimental evidence as a proof-of-concept comes from studies with the human liver cell line HepG2 which showed Li+-induced enhancement in the incorporation of extracellular citrate into cellular lipids [36]. Based on this stark difference in the effect of Li+ on the transport function of NaCT between primates and non-primates, it is obvious that mice or rats cannot be used as animal models to interrogate in vivo the connection between lithium therapy and dyslipidemia observed in humans.
Structural differences between murine and human NaCTs based on modeling approach
At the protein level, mouse NaCT and human NaCT exhibit fairly high similarity and identity in amino acid sequence (Figure 1). Therefore, it is surprising to note such marked differences in functional features between the two species. Analysis of the primary structure of both proteins using the PredictProtein server [39,40] reveals a very similar topology; both proteins possess 11 transmembrane domains, with the amino terminus facing the cytoplasmic side and the carboxy terminus facing the exoplasmic side. In addition, both proteins contain two hairpin loops which are hydrophobic and reside within the lipid bilayer; one faces inward towards the cytoplasm and present between transmembrane domains 4 and 5 while the other faces outward towards the exoplasmic side and present between transmembrane domains 9 and 10. Despite this similarity, the observed functional differences indicate that the two proteins most likely have differences in the active site responsible for the binding of citrate and Na+ because this is where the observed differences reside, namely the affinity for citrate and the effect of Li+. If the two proteins differ in amino acid residues at the active site that are involved in the binding of citrate and Li+, that could explain the functional differences. For this, we need information on the crystal structure of the proteins to deduce the three-dimensional layout of the substrate (citrate/Na+)-binding site. Unfortunately, neither mouse NaCT nor human NaCT has been crystallized and structural features elucidated. The closest relative whose crystal structure is known at present is the bacterial Na+/dicarboxylate cotransporter VcINDY [41]. Ironically, even though this bacterial transporter is called INDY, it is capable of transporting only dicarboxylates; citrate is not a transportable substrate [42]. In this respect, the bacterial transporter is more similar to NaDC1 and NaDC3 than to Drosophila INDY or mammalian INDY. Recognizing this deficiency, a human variant of VcINDY was generated, crystallized, and structural features elucidated [43]. In this human variant, the amino acids found in the substrate-binding site of VcINDY were replaced with amino acids found in the corresponding positions of human NaCT. This is the closest model template available at present to predict the amino acid residues involved in the binding of citrate and Na+ in mouse and human NaCTs. Recently, we performed homology modeling of mouse and human NaCTs using the humanized variant of VcINDY as the template to provide a structural basis for the functional differences in NaCT between the two species [44,45]. This modeling approach, though not perfect, did predict the presence of a greater number of amino acid residues in mouse NaCT than in human NaCT that interact with citrate and stabilize its binding at the active site. This could explain, at least to some extent, the ∼30-fold higher affinity of mouse NaCT for citrate compared with human NaCT. As mentioned in a previous section, phenylalanine at position 500 in human NaCT seems to play a critical role in Li+ binding and consequent activation of the transport function. The homology modeling approach does predict the presence of this particular amino acid residue in the substrate-binding site. Nonetheless, many caveats exist in this modeling because the analysis is solely based on the structure of a distantly related bacterial transporter. Definitive conclusions as to the exact molecular basis for the differences in functional features between mouse NaCT and human NaCT must wait for the availability of the structural information for NaCT in a mammalian species.
Hormonal and pharmacologic regulation of NaCT expression
Given the importance of citrate in cellular metabolism, both in the cytoplasm and in the mitochondria, several studies have focused on the regulation of NaCT expression and function and on the signaling pathways involved therein. Understandably, the liver is the principal tissue investigated in these studies because of its role as the master metabolic organ. The glucogenic/hyperglycemic hormone glucagon is a positive regulator of NaCT expression in the liver [46]. The molecular mechanism involved in this process is the canonical cAMP signaling pathway. Glucagon interacts with its cell-surface receptor to increase intracellular cAMP levels in hepatocytes, which then binds and activates the transcription factor cAMP-responsive element-binding protein (CREBP). The binding of CREBP to the promoter of NaCT gene is facilitated in liver cells upon exposure to glucagon. These findings have also been corroborated with animal studies where experimentally induced diabetes enhances NaCT expression in liver via CREBP [46]. The induction of NaCT in the liver by glucagon or diabetes makes perfect metabolic sense. Glucagon influences multiple metabolic pathways in hepatocytes, and one of the final outcomes of this process is an increased the hepatic output of glucose into the blood. Citrate in the cytoplasm functions as an inhibitor of glycolysis and a stimulator of gluconeogenesis (Figure 2). Therefore, the induction of NaCT by glucagon would increase intracellular levels of citrate in liver cells, which would subsequently inhibit glucose breakdown and facilitate glucose synthesis from non-carbohydrate precursors. Collectively, this would increase the hepatic output of glucose into the circulation. Similarly, diabetes is associated with an increased glucagon/insulin ratio, thus involving glucagon-induced NaCT expression in increased release of glucose from the liver into the blood, thus contributing to hyperglycemia seen in diabetes. A related phenomenon is the recent finding in our laboratory that the anti-diabetic drug metformin suppresses the expression of NaCT in liver cells [47]. One of the metabolic pathways targeted for inhibition by metformin is gluconeogenesis. This occurs via multiple mechanisms [48], and our studies have identified the suppression of NaCT expression as one of these mechanisms. The signaling pathway for the metformin-induced silencing of NaCT expression involves activation of AMPK, which in turn inhibits mTORC1 and CREBP.
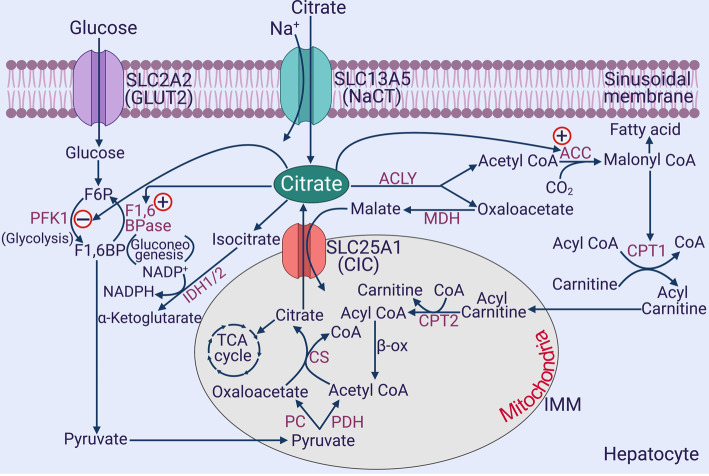
Figure 2. Involvement of citrate in multiple biochemical pathways in the liver.
PFK-1, phosphofructokinase-1; F1,6-BPase, fructose-1,6-bisphosphatase; ACLY, ATP:citrate lyase; ACC, acetyl-CoA carboxylase; IDH, isocitrate dehydrogenase; ME, malic enzyme; CPT, carnitine palmitoyl transferase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase; CS, citrate synthase; MDH, malate dehydrogenase; β-ox, β-oxidation; CIC, citrate carrier; TCA cycle, tricarboxylic acid cycle; IMM, inner mitochondrial membrane. Only the inner mitochondrial membrane is depicted for the mitochondria. CPT1 is associated with the outer mitochondrial membrane, whereas CPT2 is associated with the inner mitochondrial membrane. All other enzymes shown within the mitochondria, except for the succinate dehydrogenase associated with the TCA cycle, are present in the matrix.
Further studies using human and rat primary liver cells have shown that the nuclear receptor PXR (pregnane X receptor) and AhR (arylhydrocarbon receptor) regulate NaCT expression [49,50]. Rifampicin activates PXR, which then induces the expression of NaCT by transcriptional activation; this results in lipid accumulation in hepatocytes [49]. Similarly, the benzo[a]pyrene activates AhR, which then dimerizes with ARNT, translocates into the nucleus, and causes transcriptional induction of NaCT [50]. These findings are relevant to non-alcoholic fatty liver induced by cigarette smoking and western diets (e.g. barbecued and fried meats) because these conditions increase the exposure to benzo[a]pyrene, which activates AhR and enhance fatty acid and cholesterol synthesis in liver, thus contributing to fatty liver. Similarly, inflammatory cytokines associated with obesity promote the development of non-alcoholic fatty liver. Accordingly, IL-6 released from adipocytes induces NaCT in the liver [51]. Activation of IL-6 receptor leads to phosphorylation and consequent nuclear translocation of the transcription factor STAT3, which then leads to transcriptional induction of NaCT.
Taken collectively, the findings on the regulation of NaCT expression in the liver underline the important role of this transporter in hepatic metabolism, primarily related to carbohydrate and lipid metabolism. Increased expression and activity of NaCT in the liver essentially means increased glucose production and increased fatty acid and cholesterol synthesis. Such changes in hepatic metabolism would promote insulin resistance, diabetes, obesity, and metabolic syndrome. On the other hand, decreased expression and activity of NaCT in the liver would mean decreased risk for the afore-mentioned metabolic disorders.
Metabolic and biologic phenotype of Slc13a5 (NaCT)-null mice
The original publication that reported on the discovery of INDY in Drosophila described the biologic consequences of INDY deficiency on the organism [12]. The phenotype of the organism with a partial deficiency of INDY mimics the physiologic condition of caloric restriction and reflects alterations in metabolic pathways that are advantageous to the overall health of the organism [12,18,52,53]. INDY deficiency doubles the lifespan without compromising fertility and physical activity. This metabolic phenotype is mostly related to the changes in biochemical pathways associated with citrate and other intermediates of the citric-acid cycle caused by INDY deficiency in fat body, oenocytes, and a subset of cells in the midgut, all of which are associated with the uptake, utilization and storage of nutrients [18]. The liver carries out similar functions in mammals, and this is where NaCT/SLC13A5, the mammalian INDY, is expressed the most; an organ equivalent to fat body in Drosophila. Therefore, it was of interest to determine the consequences of NaCT deficiency in mammalian organisms. Towards this goal, Birkenfeld et al. [54] generated Slc13a5-null mice and assessed the consequences. Homozygous deletion of Slc13a5 in mice was compatible with life, and there was no indication of any effect on reproductive capability. Uptake of circulating citrate into the liver was reduced significantly in Slc13a5-null mice. Most importantly, the null mice were resistant to diet-induced obesity and diabetes. Analysis of hepatocytes from the null mice showed an increase in mitochondrial density as well as several genes related to the electron transport chain, citric-acid cycle, and oxidative phosphorylation. The hepatic sterol regulatory element-binding protein (SREBP)-1c, a lipogenic master regulator, was decreased. In addition, there was the reduced generation of ATP and increased levels of AMP with consequent activation of AMPK, which promoted mitochondrial biogenesis, insulin sensitivity, and fatty acid oxidation. Activated AMPK does affect several metabolic pathways, primarily facilitating catabolic reactions, and blocking anabolic reactions. Slc13a5-null mice on a high-fat diet showed a 90% decrease in the incorporation of circulating citrate into intracellular sterols and fatty acids in the liver. Oil-Red-O staining and electron microscopy of liver sections of the null mice also showed a marked reduction in triglycerides and diacylglycerol. In addition, fasting blood glucose was lower, the glucose tolerance test revealed improved clearance of blood glucose, endogenous glucose production was reduced in the null mice. Essentially similar results were obtained in mice when Slc13a5 was silenced by other means such as RNAi [55] and antisense oligonucleotides [56].
An interesting and important difference between Drosophila and mice was readily detectable in terms of the consequences of INDY deficiency. In Drosophila, only heterozygous deletion of INDY elicited beneficial effects. Homozygous deletion produced detrimental effects on the organism; though the effects were small, they were significant. In contrast, heterozygous mice for Slc13a5 deletion were similar to wild type mice, and no protection against diet-induced obesity or diabetes was observed in mice with the deletion of one copy of the Slc13a5 gene. Only when both copies were deleted, the beneficial consequences became noticeable.
Biology of NaCT in liver and its connection to metabolic pathways, steatosis, and cancer
The liver is a master metabolic organ capable of almost every biochemical pathway known to exist in mammals. Citrate is a pivotal metabolite which is at the intersection of most of these pathways, including glycolysis, gluconeogenesis, fatty acid synthesis, fatty acid oxidation, cholesterol synthesis, citric-acid cycle, post-translational modification, and redox homeostasis (Figure 2). These are the pathways that are intimately related to diabetes, obesity, insulin resistance/sensitivity, and metabolic syndrome. Interestingly, the involvement of citrate in all of these pathways, except for citric-acid cycle, occurs mostly in the cytoplasm; thus making cytoplasmic levels of citrate as the major determinant of changes in these pathways influenced by citrate. It is widely thought that cytoplasmic citrate arises solely from its transfer from the mitochondrial matrix via the citrate carrier (CIC) present in the inner mitochondrial membrane. This mitochondrial transporter, known as CIC or SLC25A1, belongs to the SLC25 family, which functions as an exchanger for citrate and malate [57]. Citrate is generated within the mitochondrial matrix from acetyl-CoA and oxaloacetate via citrate synthase, which initiates the citric-acid cycle. However, when citrate production by this pathway exceeds its utilization in the citric-acid cycle, it is transported out of the mitochondria into the cytoplasm via SLC25A1 in exchange for cytoplasmic malate. Once citrate enters the cytoplasm, it can participate in the aforementioned metabolic pathways. This is the mechanism by which excess carbohydrate gets converted into fat where glycolysis metabolizes glucose into pyruvate, and pyruvate enters the mitochondria for subsequent sequential conversion into acetyl-CoA and citrate. Then, citrate comes out of the mitochondria into the cytoplasm for subsequent conversion into fatty acids. Thus, the entire biochemistry of cytoplasmic citrate centered around its mitochondrial origin. The potential participation of blood-borne citrate in this process was never considered. The discovery of the plasma membrane citrate transporter NaCT/SLC13A5, which is expressed at the highest level in the liver and mediates the active transport of citrate from the circulation into hepatocytes, represents an important paradigm shift in the biology of citrate in the liver. Given that the plasma levels of citrate are in the range of 150–200 μM [58,59], SLC13A5 has the ability to impact on the cytoplasmic concentration of citrate in liver cells to a significant extent. Based on the plasma concentration and the caloric value, the energy content of citrate in circulation in humans is second only to that of lactate (Table 2). However, lactate is used for energy by almost every tissue in the body because of the ubiquitous expression of the monocarboxylate transporters (MCT1–MCT4), whereas all the energy in citrate is available only for selective tissues (e.g. liver) that express SLC13A5.
The role of cytoplasmic citrate in influencing various metabolic pathways in liver can be summarized as follows (Figure 2). Citrate is a potent inhibitor of glycolysis because it is an allosteric inhibitor of the rate-limiting enzyme phosphofructokinase-1, and at the same time it is also a stimulator of gluconeogenesis because fructose-1,6-bisphosphatase is activated by citrate allosterically. Citrate is broken down to acetyl-CoA and oxaloacetate by the enzyme ATP : citrate lyase (ACLY); acetyl CoA serves as the building block for the synthesis of fatty acids and cholesterol while oxaloacetate either gets into gluconeogenesis via phosphoenolpyruvate carboxykinase or gets converted to malate for the subsequent generation of NADPH by malic enzyme. Acetyl-CoA is not only the carbon source for fatty acid/cholesterol synthesis but also an allosteric activator of acetyl-CoA carboxylase, which catalyzes the first step in the conversion of acetyl-CoA into fatty acids and cholesterol. Malonyl-CoA generated by acetyl-CoA carboxylase not only feeds into fatty acid synthesis but also blocks the entry of long-chain fatty acids into mitochondria by inhibiting carnitine palmitoyl transferase-1, thus inhibiting the oxidation of fatty acids which occurs within the mitochondrial matrix. In addition, acetyl CoA in the cytoplasm is obligatory for the post-translational acetylation of proteins and enzymes, which has a far-ranging potential in influencing a variety of cellular processes. Citrate in the cytoplasm can also be converted into isocitrate by cytoplasmic aconitase; isocitrate is then subjected to reactions mediated by cytoplasmic isocitrate dehydrogenases 1 and 2, which generate NADPH. Cytoplasmic NADPH serves two important purposes; first, it provides reducing equivalents for the synthesis of fatty acids and cholesterol, and second, it protects cells from oxidative stress by serving as a coenzyme for glutathione reductase. NADPH could also provide electrons for the conversion of molecular oxygen into the reactive oxygen species superoxide via NADPH oxidase under certain conditions. Since the citrate transporter SLC25A1 in the inner mitochondrial membrane is an exchanger, cytoplasmic citrate could also enter the mitochondrial matrix, depending on the concentration gradient for citrate across the inner mitochondrial membrane, to feed into the citric-acid cycle, which will increase ATP production and decrease AMP, consequently increasing the energy content of the cell and at the same time decreasing the activity of the regulatory enzyme AMPK.
A recently published report by Hu et al. [60] focused on comparative proteomic analysis in HepG2 cells with and without SLC13A5 knockdown. Approximately 300 proteins showed significant changes (up-regulation and down-regulation) between the two groups. Interestingly, the most prominent change was found in the ketogenic enzyme HMG-CoA lyase, which breaks down 3-hydroxy-3-methylglutaryl-CoA into acetoacetate and acetyl-CoA. In accordance with this proteomic data, the cellular levels of the ketone bodies acetoacetate and β-hydroxybutyrate were found to be increased SLC13A5-knockdown cells. These findings are interesting because hepatic ketogenesis is a hallmark of caloric restriction. When the caloric intake is restricted as in fasting and starvation, blood glucose levels go down and the liver begins to oxidize fatty acids via β-oxidation, thus generating increased amounts of acetyl-CoA within the mitochondria. A portion of acetyl-CoA thus generated enters the citric-acid cycle to generate energy while the remaining portion is used to generate ketone bodies to serve as an energy source, alternative to glucose, for tissues such as the heart, skeletal muscle, kidney, and brain. This phenomenon is particularly important for the maintenance of neuronal function when glucose availability becomes limited.
In summary, the function of NaCT/SLC13A5 in the liver is linked to increased synthesis of fatty acids and cholesterol, decreased fatty acid oxidation, decreased glucose breakdown, increased glucose production, increased ketogenesis, and increased ATP/AMP ratio. These metabolic changes provide the molecular basis for the dyslipidemia, obesity, diabetes, and metabolic syndrome observed in Slc13a5-null mice. It is, therefore, understandable as to why Slc13a5 deficiency protects against obesity, fatty liver, and diabetes. Interestingly, the same biochemical pathways also play a critical role in cancer. Cancer cells have an obligatory need to synthesize fatty acids and cholesterol to support membrane biogenesis and increase ATP/AMP ratio to promote anabolism for macromolecular synthesis. Accordingly, the expression of NaCT/SLC13A5 is increased in hepatocellular carcinoma, and silencing of the transporter in liver cancer cells suppress their proliferation [61,62]. Similarly, blocking the activity of ACLY, which channels cytoplasmic citrate into fatty acid/cholesterol synthesis, induces growth arrest and apoptosis and decreases tumor growth in preclinical model systems [63–65].
There is also some emerging evidence indicating a role for citrate in iron homeostasis. The liver is the principal site for the production of the iron-regulatory hormone hepcidin, and citrate and the activity of NaCT/SLC13A5 which increases the cellular levels of citrate induce the expression of this hormone [66]. While hepcidin is expected to reduce the circulating levels of iron, it will increase cellular levels of iron by blocking the function of the iron exporter ferroportin (SLC40A1) [67]. It is also of interest to note that the cytoplasmic enzyme aconitase (ACO1), which converts citrate into isocitrate, is also an iron-regulatory protein. When iron levels are low in the cell, this protein functions as IRP-1 (iron-regulatory protein-1), which binds to the iron-responsive elements (IREs) in the 5′-untranspated regions (UTR) of the mRNAs coding for proteins involved in iron storage (ferritin), heme synthesis (δ-aminolevulinate synthetase-2) and erythropoiesis (hypoxia-inducible factor-2α) [68]. The binding of IRP-1 to IREs in the 5′-UTR of these mRNAs suppresses translation. When cellular iron levels are high, it increases the biogenesis of Fe-S clusters that are needed for aconitase to function as an enzyme in converting citrate into isocitrate. Citrate itself is a potent iron chelator, and non-transferrin-bound iron in circulation exists mostly in the form of citrate-iron chelate [69]. This citrate-iron connection might be of importance not only for iron homeostasis under physiologic conditions but also for cancer. Excess iron is a promoter of cancer, and cancer cells reprogram iron-regulatory pathways to accumulate iron to support their growth [70]. This is evident from the increased incidence of liver cancer in patients with hemochromatosis, a genetic disease of iron overload [71]. In fact, the connection between excess iron and cancer could go beyond the liver as our recent studies have demonstrated the connection between hemochromatosis and colon cancer in preclinical mouse models [72]. The publicly available TCGA (the cancer genome atlas) database indicates that SLC13A5 is also up-regulated several fold in pancreatic adenocarcinoma and that the expression levels have a negative correlation with patient survival. A unique feature of SLC13A5 that is of direct relevance to cancer is the marked activation of this transporter by acidic pH [29]. In this regard, H+ does exactly what Li+ does; at least one of the Na+-binding sites must have a higher affinity for H+ than for Na+. That is how acidic pH is able to activate the transport function of SLC13A5. The connection of this functional feature to cancer comes from the fact that the tumor microenvironment is acidic, this creating an inwardly directly H+ gradient across the plasma membrane of tumor cells. This transmembrane H+ gradient might energize the uptake of citrate from blood into tumor cells, thus driving anabolic pathways and supporting cell proliferation. The possible connection of SLC13A5 to cancer might also be of special relevance to metastatic cancer that grows in bone because citrate is present in abundance in this tissue. When the metastatic cancer grows within the bone, it leads to osteolysis; then, the cancer cells have direct access to citrate released from bone mineral.
Biology of NaCT in brain and its connection to epilepsy, encephalopathy, and cognitive function
Even though NaCT was first cloned from brain, very little information was available until recently on the biological consequences of NaCT deficiency in terms of neuronal function. Since deletion of Slc13a5 in mice resulted in beneficial effects, most of which are due to changes in metabolic pathways in the liver, the question arises as to the impact of Slc13a5 deficiency on brain function. But, surprisingly there were no readily discernable signs of compromised brain function in Slc13a5-null mice [54]. Nonetheless, it is important to emphasize the fact that NaCT mRNA is expressed in several regions of the brain [25] and NaCT protein is present exclusively in neurons [73]. The first indication that NaCT does play a role in the brain came from a recent report by Henke et al. [74]. This study showed that Slc13a5-null mice had an increased propensity for epileptic seizures and increased excitability in hippocampal neurons upon administration of pro-epileptic agents such as pentylenetetrazol. Proteomic analysis of the hippocampal neurons of Slc13a5-null showed a decrease in proteins associated with the synthesis, release, reuptake, and degradation of the neurotransmitters GABA and serotonin. Metabolic pathways related to the electron transport chain, Complex I biogenesis, oxidative phosphorylation, and lipid metabolism were also disrupted. This phenotype was accompanied with an increase in extracellular citrate in cerebrospinal fluid, implying that the loss of cellular uptake of citrate due to Slc13a5 deletion underlies this phenotype.
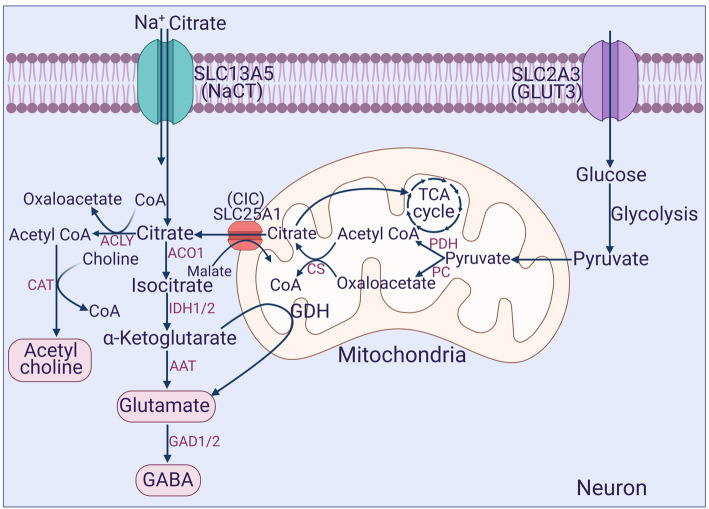
The functions of citrate in neurons are very different from those in the liver. Most of the metabolic pathways in which citrate participates in the liver are not operational in neurons. This includes synthesis of fatty acids and cholesterol, fatty acid oxidation, ketogenesis, and gluconeogenesis. Citrate does, however, play an essential role in neurons in energy production via the citric-acid cycle, an important function considering the fact that neuronal activity relies heavily on ATP generated in oxidative metabolism. The other pathways involved in the generation of NADPH for the maintenance of the cellular antioxidant machinery and in acetyl-CoA-dependent post-translational modification are most likely operative in neurons as in the liver (Figure 2). A more important role of citrate in neurons is in the synthesis of the neurotransmitters acetylcholine, glutamate and GABA (Figure 3). Choline acetyltransferase, which synthesizes acetylcholine from acetyl-CoA and choline is a cytoplasmic enzyme. Therefore, cytoplasmic citrate is necessary for this pathway because it is the sole source of acetyl-CoA outside the mitochondria. Citrate could also be converted into glutamate and GABA (Figure 3). All these three neurotransmitters are critical for neuronal function with control over numerous physiological functions, and disruption of their synthesis could lead to a wide spectrum of diseases. Cholinergic neurons are critical for motor control and for memory and cognitive function. Glutamatergic neurons are excitatory and also play a role in memory and cognition. GABAergic neurons are inhibitory. An imbalance in acetylcholine, glutamate, and GABA could be etiologic in the pathogenesis of Alzheimer's disease, epilepsy, loss of muscle coordination, and delayed development of brain functions. Given this wide range of possible involvement of citrate in physiological and pathological processes, it is surprising that Slc13a5-null mice do not exhibit more serious neurological symptoms except for the decreased threshold for pharmacologically induced seizures reported recently [74].
Figure 3. Involvement of citrate in the synthesis of neurotransmitters in neurons.
CAT, choline acetyl transferase; ACLY, ATP:citrate lyase; ACO1, cytoplasmic aconitase; IDH, isocitrate dehydrogenase; GDH, glutamate dehydrogenase; AAT, amino acid transaminases; GAD, glutamic acid decarboxylase.
Some of the detrimental consequences of NaCT deficiency predicted from the known functions of citrate in multiple metabolic pathways in neurons are probably mitigated to some extent by the metabolic reprogramming that occurs in the liver in the absence of the transporter. The most prominent change observed in the liver of Slc13a5-null mice is enhanced ketogenesis [54,60]. The metabolic phenotype of the liver in Slc13a5-null mice is similar to that under conditions of caloric restriction or ketogenic diet, with elevated levels of the ketone body β-hydroxybutyrate in circulation. This metabolite is readily available to the brain. β-Hydroxybutyrate has multiple functions, the most recognized among them being its role as the energy source. Even though glucose is the preferred energy substrate for the neurons, ketone bodies are readily used by these cells when glucose availability becomes limited as in starvation. Relatively less recognized, but biologically important nonetheless, functions of β-hydroxybutyrate include inhibition of class I and III histone deacetylases [75,76] and activation of the G-protein-coupled receptor GPR109A (also known as hydroxycarboxylic acid receptor 2 or HCA2) [77,78]. It has been well established that ketogenic diet protects against epilepsy [76], and the provision of β-hydroxybutyrate to neurons as an energy substrate under this dietary condition is at least partly responsible for this beneficial therapeutic effect. Similarly, pharmacologic activation of GPR109A has been shown to protect neuronal death in pathological conditions [79]. Related to this phenomenon is the recent report on the impaired cognitive function in aged Slc5a8-knockout mice, which was ascribed to decreased availability of β-hydroxybutyrate to neurons [80]. Studies from our laboratory have established a role for the transporter SLC5A8 in Na+-coupled uptake of β-hydroxybutyrate into neurons [81]. SLC5A8 has a similar role in facilitating reabsorption of β-hydroxybutyrate from the tubular filtrate. Therefore, deletion of Slc5a8 in mice leads to increased urinary loss of this important energy-rich metabolite, thus eventually compromising neuronal function. Corroborating this line of thinking with regard to the potential beneficial implications of enhanced ketogenesis in Slc13a5-null mice, a recent study by Wang et al. [82] demonstrated increased hippocampal neurogenesis and dendritic spine formation in dentate granule cells in these null mice, which were associated with enhanced memory performance. This process appeared to be mediated by increased expression of brain-derived neurotrophic factor (BDNF). The connection between these findings and β-hydroxybutyrate becomes obvious from other studies that show the induction of BDNF expression in cortical and hippocampal neurons by β-hydroxybutyrate-mediated inhibition of histone deacetylases [83]. This is further supported by ample evidence in the literature demonstrating the positive effects of caloric restriction on memory and cognitive function [84–86].
Consequences of SLC13A5 deficiency in humans: early infantile epileptic encephalopathy-25 (EIEE25)
Neither Drosophila nor mouse as model organisms provided any indication that Slc13a5/mINDY deficiency is deleterious to brain function. In the former, heterozygous deletion of the Indy gene proved to be biologically beneficial in terms of lifespan, whereas in the latter homozygous deletion of Slc13a5 resulted in an advantageous phenotype with resistance to diet-induced obesity and diabetes. To date, the only detrimental effect observed in Slc13a5-null mice is the increased susceptibility to chemically induced epilepsy. In stark contrast with these findings, Thevenon et al. [87] reported on the first and genetically inheritable case of SLC13A5 deficiency in humans which was associated with severe neuronal dysfunction. The condition is characterized by early onset of epilepsy within the first 24 h of life, which persists at least throughout childhood. Since this original report in 2014, more than 60 families with affected children have been reported in the literature. This is an autosomal recessive disease with the discernable clinical phenotype seen only in homozygous or compound heterozygous mutations in SLC13A5 with a loss of transport function. Carriers of these mutations appear normal. There are several genetic diseases with the overlapping clinical presentation of neonatal epilepsy and encephalopathy, collectively called EIEE (early infantile epileptic encephalopathy) or DEE (developmental epileptic encephalopathy), and the disease caused by loss-of-function mutations in SLC13A5 is designated EIEE25/DEE25. To date, there have been 10 reports in the literature describing different mutations that cause EIEE25 and the associated metabolic, biochemical, and clinical phenotype [88–97]. There is also a non-profit Foundation, called TESS (treatments for epilepsy and symptoms of SLC13A5) Foundation, which maintains a diligent record of patient families, details of the individual mutations, summary of clinical presentations associated with specific mutations, and updated information on related publications (https://www.tessresearch.org). The details of the disease can also be obtained from another publicly available database, Online Mendelian Inheritance in Man (https://www.omim.org). The first and readily noticeable clinical feature in patients with SLC13A5 deficiency is the presentation of seizures within the first few days of life. The seizures persist and do not generally respond to generally used anti-epileptic medications. It can be tonic or multifocal type. Ketogenic diet might be of some benefit in controlling the seizures, and the severity and frequency of seizures generally tend to improve with age. Other symptoms include microcephaly, delayed myelination, abnormal electroencephalogram, severe expressive language delay, weak muscle tone (hypotonia), lack of motor control (ataxia) and peripheral hypertonia, subtle changes in the white matter in the brain with evidence of gliosis, and a distinctive tooth phenotype (hypodontia, delayed eruption, defective enamel, yellow discoloration). The biochemical findings include increased levels of citrate in blood and cerebrospinal fluid, as expected from the defective SLC13A5-mediated uptake of citrate into liver cells and neurons. The mechanistic connection between SLC13A5 deficiency and neuronal dysfunction as well as the clinical hallmarks of the disease have been recently reviewed [98,99]. Since the clinical presentation overlaps among different types of EIEE, it is challenging to make specific diagnosis of SLC13A5 involvement in patients presenting with seizures in early neonatal period without any information on genetic testing. However, delayed development of teeth might represent a specific hallmark of EIEE25 because of the special role of citrate in the formation of tooth enamel (see below). It is, therefore, potentially feasible to use this phenotype in the differential diagnosis of SLC13A5 deficiency [100].
It is interesting to note that almost the entire focus of research on SLC13A5 mutations in humans centers on the biology of the transporter in the brain. Nothing is known on the potential impact of these mutations on liver function where the transporter is expressed the most. It is understandable that the neurological complications in EIEE25 are so severe that studies related to this aspect naturally take precedence. It is nonetheless necessary to point out the stark difference in the biological outcome of NaCT deficiency in mice versus humans. The primary biological phenotype in Slc13a5-null mice is related to the beneficial effects of changes that occur in the liver due to Slc13a5 deficiency; if there are neurological consequences in these null mice, they seem to be negligible or minor. In contrast, the major outcome in humans due to the loss of function in SLC13A5 is the neurological dysfunction. What happens to the biochemical pathways in the liver in patients with EIEE25 is not known and still remains to be investigated.
Disease-causing mutations in human SLC13A5
To date, 41 different disease-causing mutations in SLC13A5 have been reported in a total of 104 patients [44; https://www.tessresearch.org). Boys and girls are equally affected. Disease arises either from the inheritance of the same loss-of-function mutations on both copies of the chromosome (homozygosity) or from the inheritance of two different loss-of-function mutations, one on each copy of the chromosome (compound heterozygosity). Of the 41 mutations, 8 of them result in either no protein or a truncated protein. The remaining 33 mutations result in single-amino acid substitutions. Interestingly, these disease-causing mutations are not concentrated in any specific topological region of the transporter protein; they are distributed throughout the protein. The most predominant mutation (23 out of 104 patients) is Gly219Arg, which is located in the 5th transmembrane domain. The other top four predominant mutations are Thr227Met (transmembrane domain 5), Arg333ter, Ser427Leu (transmembrane domain 9), and Leu488Pro (transmembrane domain 10). Recently, we proposed a new classification of these mutations based on the predicted structural consequences: Class I contains mutations that affect the binding of substrates to the transporter (e.g. Thr227Met, Thr142M); Class II mutations are located in the scaffold domain of the transporter that is needed for proper folding and hence for proper trafficking of the protein to the plasma membrane (e.g. Gly219Arg, Ser427Leu), and Class III mutations cause the defective synthesis of the protein or premature stop codons. It is of interest to note that the most predominant mutation, Gly219Arg, belongs to the group of trafficking defect mutations. This is important because in theory, it should be possible to promote the trafficking of such mutant proteins to the plasma membrane and restore the transport function as has been successfully shown for certain specific mutants of the cystic fibrosis gene CFTR [101]. It is, therefore, worthwhile to investigate if effective pharmacoperones can be developed to correct the disease-causing trafficking defects in SLC13A5. It also remains to be seen whether this can be achieved with non-specific chaperones such as phenylbutyrate or target-specific chaperones need to be developed for the purpose.
NaCT in bone mineralization and tooth enamalization: relevance to bone- and tooth-related clinical consequences of NaCT deficiency in humans
EIEE25 patients show characteristic tooth development defects, such as hypodontia, teeth hypoplasia, widely spaced teeth, gingival hyperplasia, and amelogenesis imperfecta [100]. The Slc13a5-null mice show similar deficits in tooth development, including the absence of mature enamel, aberrant enamel matrix, and fragile teeth with predisposition to tooth abscesses [91]. The teeth of the null mice also show a decrease in bone mineral density and impaired bone formation as well as decreased growth and shorter body length [91]. In some respect, these features are not totally surprising given that citrate is essential for the formation of bone and teeth and that ∼70% of citrate in the body resides in these two tissues [102,103]. Specifically, citrate is essential for the formation and stabilization of apatite crystals in bone. The exact mechanism by which citrate accumulates in bone and teeth remains controversial. It has been proposed that citrate in bone-forming osteoblasts arise almost entirely from within the cell due to truncation of the citric-acid cycle rather than via uptake from blood [104]. In contrast, other studies using transcriptome profiling have provided evidence of NaCT expression in bone [105,106]. Similarly, studies by Pemberton et al. [107] and Hsu et al. [108] using microarray gene expression have shown up-regulation of NaCT during tooth development in mice. Furthermore, citrate transport via increased expression of NaCT supports the differentiation of human mesenchymal stem cells into osteoblasts for proper bone development [109]. Together, these findings demonstrate the importance of NaCT in proper bone and teeth development. It is, therefore, not surprising that NaCT deficiency results in impaired bone and teeth formation in humans and mice.
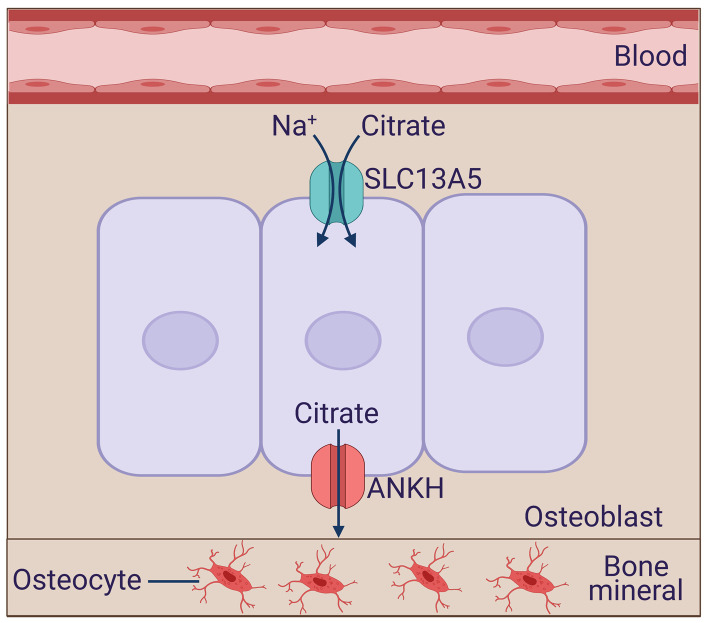
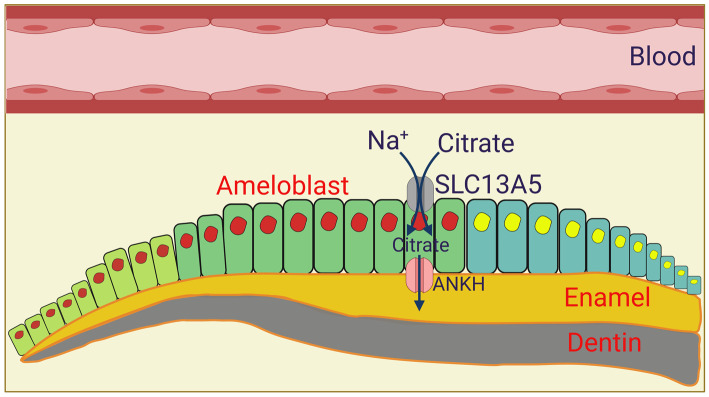
Both in osteoblasts which are involved in bone formation and mineralization (Figure 4) and in ameloblasts which are involved in tooth enamalization (Figure 5), the actual function of citrate in chelating calcium and forming the mineral is in the extracellular space. Accordingly, osteoblasts and ameloblasts are polarized cells with their basolateral membrane facing the blood side and the apical membrane facing the side of mineral formation. Both cell types express NaCT, but the exact location of the transporter has not been investigated. We predict that NaCT is expressed on the basolateral membrane in these two cell types, thus capable of transporting citrate from blood into cells. This blood-borne citrate along with endogenously generated citrate are transferred across the apical membrane to the opposite side where it chelates calcium to form the mineral or enamel. Since NaCT is a Na+-coupled active transporter for citrate, its postulated presence in the basolateral membrane makes physiologic sense because it will facilitate the entry of citrate into the cell. The question then arises as to the identity of the transport mechanism in the apical membrane that is responsible for the release of citrate from these cells onto the other side. The most likely candidate responsible for this process is the ANKH protein, which has been recently shown to function as a citrate transporter, releasing citrate from cells to the extracellular medium [110]. Interestingly, loss-of-function mutations or deletions of this transporter show severe defects in bone development, known as ankylosing spondylitis, both in humans and in mice [111,112]. Based on these findings, we propose a model for the vectorial transfer of citrate across osteoblasts (Figure 4) and ameloblasts (Figure 5) from blood to the side of mineralization and enamalization, respectively, with the polarized distribution of NaCT and ANKH.
Figure 4. Potential role of NaCT/SLC13A5 in osteoblasts in bone mineralization.
ANKH, human ankylosis gene product.
Figure 5. Potential role of NaCT/SLC13A5 in ameloblasts in tooth enamalization.
Ameloblasts differentiate from primordial cells, first into a secretory epithelium type, then to a differentiated epithelium type, and finally to a reduced enamel epithelium type (from left to right). We postulate that the vectorial transfer of citrate from blood-side to the enamal side occurs in differentiated ameloblasts. ANKH, humna ankylosis gene product. The figure was adapted and modified from ref. [120].
Small-molecule inhibitors of NaCT
Even though the biological consequences of NaCT deficiency seem to be different in mice versus humans, it is safe to conclude that the loss of the transporter function has differential impact on the brain and the liver. Based on the evidence from Slc13a5-null mice and EIEE25 patients, the impact on the brain is detrimental, whereas the impact on the liver is beneficial. It has to be noted that the beneficial consequences of NaCT loss in the liver became known from Slc13a5-null mice several years before the negative influence of NaCT loss on neuronal function became evident from EIEE25 patients. This fueled research to identify inhibitors of NaCT with the idea that such inhibitors could have therapeutic potential in the prevention/treatment of metabolic diseases such as diabetes and obesity and also in the extension of healthy lifespan by blocking the function of the transporter in the liver [113–117].
The first generation of NaCT inhibitors was developed using the Docking module of Molecular Operating Environment [113]. This model used a 3D structure of NaCT deduced from a homology modeling of LeuT, a bacterial amino acid transporter. Obviously, this was not an ideal template for structural analysis of NaCT because LeuT transports amino acids and NaCT transports carboxylate anions, and there is no homology in amino acid sequence between the two transporters. In fact, LeuT bears structural resemblance to neurotransmitter transporters in the SLC6 family, whereas NaCT belongs to the SLC13 family. But, the structure of LeuT was the best option available at that time because there was no information on the structure of any other transporter more closely related to NaCT. Despite the flaw in the suitability of the template, the search yielded two compounds, identified as 39396 and 4180643, which inhibited human NaCT in a noncompetitive manner with Ki values of 0.34 mM and 0.30 mM, respectively [113]. Subsequently, the inhibitor PF-06649298 was developed by Pfizer using virtual searches for structural similarities with citrate [114–116]. Studies with human NaCT showed that PF-06649298 was a potent inhibitor with an IC50 value of 0.4–10 μM. PF-06649298 has no species selectivity and inhibits both mouse and human NaCTs. Further modifications to PF-06649298 allowed for a four-fold increase in the inhibitor potency for human NaCT [117]. Interestingly, the inhibitory potency was higher for PF-06649298 and its derivative PF-06761281 with increasing citrate concentrations. The results indicated that PF-06649298 and PF-06761281 were allosteric inhibitors whose interaction with NaCT depends on the state of the transporter with regard to citrate binding [115]. Administration of PF-06649298 to mice showed a decrease in hepatic intracellular citrate and plasma glucose levels, and an increase in insulin sensitivity [114]. This pharmacological outcome of the inhibitor was as expected from the inhibition of NaCT in the liver. Recently, a new molecule, identified as BI01383298, has been shown as a highly potent inhibitor of human NaCT with an IC50 of 25–60 nM, which is significantly more potent than PF-06649298. Interestingly, this new compound inhibits only human NaCT and has no effect on mouse NaCT. Moreover, unlike the Pfizer compounds, BI0138328 has no structural resemblance to citrate. This information on BI01383298 is available only from the Structural Genomics Consortium, a public-private partnership that supports the discovery of new medicines through open-access research. To the best of our knowledge, there was no published report on this inhibitor. We have recently characterized the inhibition of human NaCT by BI01383298 in detail [45]. Our studies were able to corroborate the inhibitory features and the species specificity of this inhibitor available at the Structural Genomics Consortium. But our studies have also unveiled an interesting aspect of this inhibitor which was not known before. The inhibition of human NaCT by BI01383298 is irreversible. This represents the first irreversible blocker of NaCT. This feature, combined with the specificity and nanomolar affinity of the inhibitor for human NaCT, makes BI01383298 unique for future investigations to evaluate the biology of NaCT and its potential as a pharmacologic target in humans.
The small molecules described above inhibit the function of SLC13A5 by acting at the transporter protein level. The biological consequences of inhibiting the transport function could also be achieved by pharmacologically suppressing the expression of the transporter. Most studies on the regulation of NaCT expression, primarily in the liver, focused on the signaling pathways involved in the up-regulation of the transporter expression; this includes pathways involving cAMP, AhR, and PXR [46,48–51]. However, since it is the suppression of NaCT activity in the liver that produces biologically beneficial effects, identification of signaling pathways that silence the expression of the transporter in the liver might have therapeutic relevance. In this regard, our recent studies with the anti-diabetic drug metformin might be of interest [47].
NaCT as a therapeutic target: potential beneficial and detrimental effects of NaCT inhibitors and the relevance of blood-brain barrier to the phenomenon
Pharmacologic blockade of NaCT in the liver results in reprogramming of metabolic pathways that mimic caloric restriction and consequently reduce the risk of diet-induced obesity, diabetes, and metabolic syndrome. Findings from long-lived Indy mutant Drosophila [21] and Slc13a5-null mice [118] have provided strong convincing evidence in support of this idea. There are no data from EIEE25 patients which support or repudiate the possible beneficial effects of NaCT deficiency on the metabolic phenotype of the liver. The biological consequences of NaCT deficiency in the brain are diagonally opposite of what happens in the liver. Loss of function of NaCT in humans cause EIEE25 associated with severe neurological dysfunction. This detrimental brain phenotype is not readily apparent in Slc13a5-null mice. The marked differences in the functional features of human NaCT and mouse NaCT might explain as to why the biochemical and biological consequences differ between Slc13a5-null mice and EIEE25 patients [44,98]. Mouse NaCT (Slc13a5) functions as a high-affinity/low-capacity transporter for citrate, whereas human NaCT (SLC13A5) functions as a low-affinity/high-capacity transporter for citrate. This means that the amount of citrate that enters cells via NaCT-mediated transport markedly varies in humans versus mice. We have estimated that, at physiological concentrations of citrate in circulation, the amount of citrate transported by mouse NaCT is only ∼5% of the amount of citrate transported by human NaCT [44]. We have also attempted to provide a structural basis for these functional differences between mouse and human NaCTs [44]. Notwithstanding this plausible explanation, the fact remains undisputable that the manifestations of NaCT deficiency are different between mice and humans. This raises doubts as to the rationality of using mice as an animal model to study what NaCT does in humans. Nonetheless, it seems fairly convincing that pharmacologic blockade of NaCT produces beneficial effects in the liver but detrimental effects in the brain. But, it is possible, at least in theory, to develop NaCT inhibitors that function only in the periphery and do not enter the brain across the blood–brain barrier. If this can be achieved, such agents would cause NaCT deficiency only in the liver, thereby eliciting primarily the beneficial effects. Several inhibitors of NaCT have been identified, but whether or not these inhibitors cross the blood–brain barrier has not been determined. The issue of potential transfer across the blood-brain barrier needs to be addressed for any NaCT inhibitor/blocker prior to assessing its potential as a therapeutic agent for the prevention or treatment of diet-induced obesity and diabetes. Thus, the ability of any NaCT inhibitor to get across the blood-brain barrier would be the overriding criterion to differentiate between the ‘good’ consequences of NaCT blockade in the liver and the ‘evil’ consequences of NaCT blockade in the brain.
Perspectives and conclusions
One of the caveats in this hypothetical ‘good’ versus ‘evil’ outcome of pharmacological use of NaCT inhibitors is that the severe disease-related phenotype of NaCT deficiency in the brain arises in EIEE25 patients with the loss of the transporter function at the time of conception. What will happen if the transporter function is compromised with pharmacologic agents in adults well after the brain development is mostly completed? There is no information available addressing this question. Furthermore, the magnitude of NaCT deficiency in the brain induced by pharmacologic agents in a dose-dependent manner could be a game changer in this phenomenon. EIEE25 patients have almost complete absence of the transporter function, and that too from their fetal life during early neuronal development. The clinical outcome might not be the same if the deficiency is only partial as is evident from the recessive nature of this disease. Heterozygous carriers of loss-of-function mutations in SLC13A5 do not have any clinical phenotype. Therefore, the ‘beneficial’ versus the ‘detrimental’ effects of NaCT inhibitors in adults might be variable depending on the dosage of the inhibitor. This raises another interesting and important issue. What if carriers with an already existing partial deficiency of the transporter are exposed to pharmacologic agents that further compromise the transport function? Recently, we reported a case of a heterozygous carrier for a loss-of-function mutation in SLC13A5 who developed seizures only when exposed to metformin, valproic acid, and starvation [119]. We found the clinical phenotype of this individual very interesting because our studies have previously found that metformin silences the expression of NaCT in the human liver cell line HepG2 [47]. This raises the possibility that heterozygous carriers of NaCT mutations might be at risk of developing at least some of the symptoms of EIEE25 if the activity of the transporter from the normal allele is compromised with drugs (pharmaco-synergistic heterozygosity).
This also brings up the issue of potential age-related changes in the expression and activity of NaCT in the brain. What if aging decreases the expression and activity of NaCT in the brain? What would such changes mean to heterozygous carriers of loss-of-function mutations in NaCT? This is particularly important considering the possibility that NaCT-mediated delivery of citrate into neurons might be critical for the synthesis of the critical neurotransmitters acetylcholine, glutamate, and GABA. Age-related decline in neuronal function and plasticity is related, at least to some extent, to age-dependent changes in the levels of these neurotransmitters. Since the heterozygous carriers of SLC13A5 mutations already have a partial deficiency in transporter expression, any further decline in the transporter activity due to age-dependent decrease in the expression/activity of the transporter from the normal allele could have clinical consequences. Further research is needed to address these caveats and questions.
Acknowledgement
We acknowledge the use of pre-made icons and templates from BioRender's web-based software in preparation of some of the figures included in this manuscript.
Abbreviations
- NaCT
Na+-coupled citrate transporter
- SLC13A5
solute-linked carrier gene family 13A member 5
- INDY
I'm Not Dead Yet
- mINDY
mammalian INDY
- EIEE25
early infantile epileptic encephalopathy-25
- DEE25
developmental epileptic encephalopathy-25
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Welch Endowed Chair in Biochemistry, Grant No. BI-0028, at Texas Tech University Health Sciences Center.
Open Access
Open access for this article was enabled by the participation of the Texas Tech University Health Sciences Center in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with EBSCO.
Author Contribution
J.J.K. prepared the initial draft of this review. Y.D.B. was responsible for the preparation of the figures. S.S. contributed to the analysis of amino acid sequence similarity between Drosophila INDY and mammalian Na+-coupled transporters for di/tricarboxylates. V.G. was responsible for the initial outline of the review and for the writing and editing of the manuscript. J.J.K. and Y.D.B. also contributed to the preparation of the manuscript.
References
- 1.Lin, L., Yee, S.W., Kim, R.B. and Giacomini, K.M. (2015) SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 14, 543–560 10.1038/nrd4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumann, T., König, J., Henke, C., Willmes, D.M., Bornstein, S.R., Jordan, J.et al. (2019) Solute carrier transporters as potential targets for the treatment of metabolic disease. Pharmacol. Rev. 72, 343–379 10.1124/pr.118.015735 [DOI] [PubMed] [Google Scholar]
- 3.Saier, M.H., Eng, B.H., Fard, S., Garg, J., Haggerty, D.A., Hutchinson, W.et al. (1999) Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim. Biophys. Acta 1422, 1–56 10.1016/S0304-4157(98)00023-9 [DOI] [PubMed] [Google Scholar]
- 4.Schlessinger, A., Yee, S.W., Sali, A. and Giacomini, K.M. (2013) SLC classification: an update. Clin. Pharmacol. Ther. 94, 19–23 10.1038/clpt.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaller, L. and Lauschke, V.M. (2019) The genetic landscape of the human solute carrier (SLC) transporter superfamily. Hum. Genet. 138, 1359–1377 10.1007/s00439-019-02081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu, M. (2019) Structure and mechanism of the divalent anion/Na+ symporter. Int. J. Mol. Sci. 20, 440 10.3390/ijms20020440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajor, A.M. (2000) Molecular properties of sodium/dicarboxylate cotransporters. J. Membr. Biol. 175, 1–8 10.1007/s002320001049 [DOI] [PubMed] [Google Scholar]
- 8.Markovich, D. and Murer, H. (2004) The SLC13 gene family of sodium sulphate/carboxylate cotransporters. Pflugers Archiv. 447, 816–817 10.1007/s00424-003-1207-8 [DOI] [PubMed] [Google Scholar]
- 9.Sreedharan, S., Stephansson, O., Schiöth, H.B. and Fredriksson, R. (2011) Long evolutionary conservation and considerable tissue specificity of several atypical solute carrier transporters. Gene 478, 11–18 10.1016/j.gene.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 10.Lensbouer, J.J. and Doyle, R.P. (2010) Secondary transport of metal-citrate complexes: the CitMHS family. Crit. Rev. Biochem. Mol. Biol. 45, 453–462 10.3109/10409238.2010.504701 [DOI] [PubMed] [Google Scholar]
- 11.Markovich, D. (2001) Physiological roles and regulation of mammalian sulfate transporters. Physiol. Rev. 81, 1499–1533 10.1152/physrev.2001.81.4.1499 [DOI] [PubMed] [Google Scholar]
- 12.Rogina, B., Reenan, R.A., Nilsen, S.P. and Helfand, S.L. (2000) Extended life-span conferred by cotransporter gene mutations in Drosophila. Science 290, 2137–2140 10.1126/science.290.5499.2137 [DOI] [PubMed] [Google Scholar]
- 13.Picca, A., Pesce, V. and Lezza, A.M.S. (2017) Does eating less make you live longer and better? An update on calorie restriction. Crit. Interv. Aging 12, 1887–1902 10.2147/CIA.S126458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwangbo, D.S., Lee, H.Y., Abozaid, L.S. and Min, K.J. (2020) Mechanisms of lifespan regulation by calorie restriction and intermittent fasting in model organisms. Nutrients 12, 1194 10.3390/nu12041194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pajor, A.M. (1995) Sequence and functional characterization of a renal sodium/dicarboxylate cotransporter. J. Biol. Chem. 270, 5779–5785 10.1074/jbc.270.11.5779 [DOI] [PubMed] [Google Scholar]
- 16.Kekuda, R., Wang, H., Huang, W., Pajor, A.M., Leibach, F.H., Devoe, L.D.et al. (1999) Primary structure and functional characteristics of a mammalian sodium-coupled high affinity dicarboxylate transporter. J. Biol. Chem. 274, 3422–3429 10.1074/jbc.274.6.3422 [DOI] [PubMed] [Google Scholar]
- 17.Inoue, K., Fei, Y.J., Huang, W., Zhuang, L., Chen, Z. and Ganapathy, V. (2002) Functional identity of Drosophila melanogaster Indy as a cation-independent, electroneutral transporter for tricarboxylic acid-cycle intermediates. Biochem. J. 367, 313–319 10.1042/bj20021132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knauf, F., Rogina, B., Jiang, Z., Aronson, P.S. and Helfand, S.L. (2002) Functional characterization and immunolocalization of the transporter encoded by the life-extending gene Indy. Proc. Natl. Acad. Sci. U.S.A. 99, 14315–14319 10.1073/pnas.222531899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knauf, F., Mohebbi, N., Teichert, C., Herold, D., Rogina, B., Helfand, S.et al. (2006) The life-extending gene Indy encodes an exchanger for Krebs-cycle intermediates. Biochem. J. 397, 25–29 10.1042/BJ20060409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helfand, S.L. and Rogina, B. (2003) Genetics of aging in the fruit fly, Drosophila melanogaster. Annu. Rev. Genet. 37, 329–348 10.1146/annurev.genet.37.040103.095211 [DOI] [PubMed] [Google Scholar]
- 21.Rogna, B. (2017) INDY – a new link to metabolic regulation in animals and humans. Front. Genet. 8, 66 10.3389/fgene.2017.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudry, B., de Goeij, E., Mineo, A., Gasper, P., Hadjieconomou, D., Studd, C.et al. (2019) Sex differences in intestinal carbohydrate metabolism promote food intake and sperm maturation. Cell 178, 901–918 10.1016/j.cell.2019.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kauppila, T.E.S., Kauppila, J.H.K. and Larsson, N.G. (2017) Mammalian mitochondria and aging: an update. Cell Metab. 25, 57–71 10.1016/j.cmet.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 24.Rottenberg, H. and Hoek, J.B. (2017) The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 16, 943–955 10.1111/acel.12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue, K., Zhuang, L., Maddox, D.M., Smith, S.B. and Ganapathy, V. (2002) Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J. Biol. Chem. 277, 39469–39476 10.1074/jbc.M207072200 [DOI] [PubMed] [Google Scholar]
- 26.Gopal, E., Miyauchi, S., Martin, P.M., Ananth, S., Srinivas, S.R., Smith, S.B.et al. (2007) Expression and functional features of NaCT, a sodium-coupled citrate transporter, in human and rat livers and cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G402–G408 10.1152/ajpgi.00371.2006 [DOI] [PubMed] [Google Scholar]
- 27.Inoue, K., Fei, Y.J., Zhuang, L., Gopal, E., Miyauchi, S. and Ganapathy, V. (2004) Functional features and genomic organization of mouse NaCT, a sodium-coupled transporter for tricarboxylic acid cycle intermediates. Biochem. J. 378, 949–957 10.1042/bj20031261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue, K., Zhuang, L. and Ganapathy, V. (2002) Human Na+ -coupled citrate transporter: primary structure, genomic organization, and transport function. Biochem. Biophys. Res. Commun. 299, 465–471 10.1016/S0006-291X(02)02669-4 [DOI] [PubMed] [Google Scholar]
- 29.Gopal, E., Babu, E., Ramachandran, S., Bhutia, Y.D., Prasad, P.D. and Ganapathy, V. (2015) Species-specific influence of lithium on the activity of SLC13A5 (NaCT): lithium-induced activation is specific for the transporter in primates. J. Pharmacol. Exp. Ther. 353, 17–26 10.1124/jpet.114.221523 [DOI] [PubMed] [Google Scholar]
- 30.Pajor, A.M., Hirayama, B.A. and Loo, D.D. (1998) Sodium and lithium interactions with the Na+/dicarboxylate cotransporter. J. Biol. Chem. 273, 18923–18929 10.1074/jbc.273.30.18923 [DOI] [PubMed] [Google Scholar]
- 31.Wang, H., Fei, Y.J., Kekuda, R., Yang-Feng, T.L., Devoe, L.D., Leibach, F.H.et al. (2000) Structure, function, and genomic organization of human Na+-dependent high-affinity dicarboxylate transporter. Am. J. Physiol. Cell Physiol. 278, C1019–C1030 10.1152/ajpcell.2000.278.5.C1019 [DOI] [PubMed] [Google Scholar]
- 32.Pajor, A.M. and Sun, N.N. (2010) Role of isoleucine-554 in lithium binding by the Na+/dicarboxylate cotransporter NaDC1. Biochemistry 49, 8937–8943 10.1021/bi100600j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond, P.A., Jenner, F.A., Lee, C.R., Lenton, E., Pollitt, R.J. and Sampson, G.A. (1972) The effect of lithium salts on the urinary excretion of α-oxoglutarate in man. Br. J. Pharmacol. 46, 116–123 10.1111/j.1476-5381.1972.tb06854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pajor, A.M. (1999) Citrate transport by the kidney and intestine. Semin. Nephrol. 19, 195–200 PMID: [PubMed] [Google Scholar]
- 35.Wright, E.M., Wright, S.H., Hirayama, B. and Kippen, I. (1982) Interactions between lithium and renal transport of Krebs cycle intermediates. Proc. Natl. Acad. Sci. U.S.A. 79, 7514–7517 10.1073/pnas.79.23.7514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue, K., Zhuang, L., Maddox, D.M., Smith, S.B. and Ganapathy, V. (2003) Human sodium-coupled citrate transporter, the orthologue of Drosophila Indy, as a novel target for lithium action. Biochem. J. 374, 21–26 10.1042/bj20030827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baptista, T., Teneud, L., Contreras, Q., Alastre, T., Burguera, J., de Burguera, M.et al. (1995) Lithium and body weight gain. Pharmacopsychiatry 28, 35–44 10.1055/s-2007-979586 [DOI] [PubMed] [Google Scholar]
- 38.Chen, Y. and Silverstone, T. (1990) Lithium and weight gain. Int. Clin. Psychopharmacol. 5, 217–226 10.1097/00004850-199007000-00007 [DOI] [PubMed] [Google Scholar]
- 39.Rost, B., Yachdav, G. and Liu, J. (2004) The PredictProtein server. Nucl. Acids Res. 32, W321–W326 10.1093/nar/gkh377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yachdav, G., Kloppmann, E., Kajan, L., Hecht, M., Goldberg, T., Hamp, T.et al. (2014) PredictProtein - an open resource for online prediction of protein structural and functional features. Nucl. Acids Res. 42, W337–W343 10.1093/nar/gku366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mancusso, R., Gregorio, G.G., Liu, Q. and Wang, D.N. (2012) Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter. Nature 491, 622–626 10.1038/nature11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulligan, C., Fitzgerald, G.A., Wang, D.N. and Mindell, J.A. (2014) Functional characterization of a Na+-dependent dicarboxylate transporter from Vibrio cholerae. J. Gen. Physiol. 143, 745–759 10.1085/jgp.201311141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nie, R., Stark, S., Symersky, J., Kaplan, R.S. and Lu, M. (2017) Structure and function of the divalent anion/Na+ symporter from Vibrio cholerae and a humanized variant. Nat. Commun. 8, 15009 10.1038/ncomms15009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaramillo-Martinez, V., Urbatsch, I.L. and Ganapathy, V. (2020) Functional distinction between human and mouse sodium-coupled citrate transporters and it biologic significance: an attempt for structural basis using a homology modeling approach. Chem. Rev. 10.1021/acs.chemrev.0c00529 [DOI] [PubMed] [Google Scholar]
- 45.Higuchi, K., Kopel, J.J., Sivaprakasam, S., Jaramillo-Martinez, V., Sutton, R.B., Urbatsch, I.L.et al. (2020) Functional analysis of a species-specific inhibitor selective for human Na+-coupled citrate transporter (NaCT/SLC13A5/mINDY). Biochem. J. 477, 4149–4165 10.1042/BCJ20200592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuschafer-Rube, F., Lieske, S., Kuna, M., Henkel, J., Perry, R.J., Erion, D.M.et al. (2013) The mammalian INDY homolog is induced by CREB in a rat model of type 2 diabetes. Diabetes 63, 1048–1057 10.2337/db13-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopel, J., Higuchi, K., Ristic, B., Sato, T., Ramachandran, S. and Ganapathy, V. (2020) The hepatic plasma membrane citrate transporter NaCT (SLC13A5) as a molecular target for metformin. Sci. Rep. 10, 8536 10.1038/s41598-020-65621-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rena, G., Hardie, D.G. and Pearson, E.R. (2017) The mechanisms of action of metformin. Diabetologia 60, 1577–1585 10.1007/s00125-017-4342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, L., Li, H., Garzel, B., Yang, H., Sueyoshi, T., Li, Q.et al. (2015) SLC13A5 is a novel transcriptional target of the pregnane X receptor and sensitizes drug-induced steatosis in human liver. Mol. Pharmacol. 87, 674–682 10.1124/mol.114.097287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neuschäfer-Rube, F., Schraplau, A., Schewe, B., Lieske, S., Krützfeldt, J.-M., Ringel, S.et al. (2015) Arylhydrocarbon receptor-dependent mIndy (Slc13a5) induction as possible contributor to benzo[a]pyrene-induced lipid accumulation in hepatocytes. Toxicology 337, 1–9 10.1016/j.tox.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 51.von Loeffelholz, C., Lieske, S., Neuschäfer-Rube, F., Willmes, D.M., Raschzok, N., Sauer, I.M.et al. (2017) The human longevity gene homolog INDYand interleukin-6 interact in hepatic lipid metabolism. Hepatology 66, 616–630 10.1002/hep.29089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neretti, N., Wang, P.Y., Brodsky, A.S., Nyguyen, H.H., White, K.P., Rogina, B.et al. (2009) Long-lived Indy induces reduced mitochondrial reactive oxygen species production and oxidative damage. Proc. Natl. Acad. Sci. U.S.A. 106, 2277–2282 10.1073/pnas.0812484106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, P.Y., Neretti, N., Whitaker, R., Hosier, S., Chang, C., Lu, D.et al. (2009) Long-lived Indy and calorie restriction interact to extend life span. Proc. Natl. Acad. Sci. U.S.A. 106, 9262–9267 10.1073/pnas.0904115106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birkenfeld, A.L., Lee, H.Y., Guebre-Egziabher, F., Alves, T.C., Jurczak, M.J., Jornayvaz, F.R.et al. (2011) Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 14, 567 10.1016/j.cmet.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brachs, S., Winkel, A.F., Tang, H., Birkenfeld, A.L., Brunner, B., Jahn-Hofmann, K.et al. (2016) Inhibition of citrate cotransporter Slc13a5/mINDY by RNAi improves hepatic insulin sensitivity and prevents diet-induced non-alcoholic fatty liver disease in mice. Mol. Metab. 5, 1072–1082 10.1016/j.molmet.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pesta, D.H., Perry, R.J., Guebre-Egziabher, F., Zhang, D., Jurczak, M., Fischer-Rosinsky, A.et al. (2015) Prevention of diet-induced hepatic steatosis and hepatic insulin resistance by second generation antisense oligonucleotides targeted to the longevity gene mIndy (Slc13a5). Aging 7, 1086–1093 10.18632/aging.100854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruprecht, J.J. and Kunji, E.R.S. (2020) The SLC25 mitochondrial carrier family: structure and mechanism. Trends Biochem. Sci. 45, 244–258 10.1016/j.tibs.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mycielska, M.E., Patel, A., Rizaner, N., Mazurek, M.P., Keun, H., Patel, A.et al. (2009) Citrate transport and metabolism in mammalian cells: prostate epithelial cells and prostate cancer. BioEssays 31, 10–20 10.1002/bies.080137 [DOI] [PubMed] [Google Scholar]
- 59.Mycielska, M., Milenkovic, V., Wetzel, C., Rümmele, P. and Geissler, E. (2015) Extracellular citrate in health and disease. Curr. Mol. Med. 15, 884–891 10.2174/1566524016666151123104855 [DOI] [PubMed] [Google Scholar]
- 60.Hu, T., Huang, W., Li, Z., Kane, M.A., Zhang, L., Huang, S.M.et al. (2020) Comparative proteomic analysis of SLC13A5 knockdown reveals elevated ketogenesis and enhanced cellular toxic response to chemotherapeutic agents in HepG2 cells. Toxicol. Appl. Pharmacol. 402, 115117 10.1016/j.taap.2020.115117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li, Z., Li, D., Choi, E.Y., Lapidus, R., Zhang, L., Huang, S.M.et al. (2017) Silencing of solute carrier family 13 member 5 disrupts energy homeostasis and inhibits proliferation of human hepatocarcinoma cells. J. Biol. Chem. 292, 13890–13901 10.1074/jbc.M117.783860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peters, J.M. (2017) Flipping a citrate switch on liver cancer cells. J. Biol. Chem. 292, 13902–13903 10.1074/jbc.H117.783860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatzivassiliou, G., Zhao, F., Bauer, D.E., Andreadis, C., Shaw, A.N., Dhanak, D.et al. (2005) ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8, 311–321 10.1016/j.ccr.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 64.Zaidi, N., Royaux, I., Swinnen, J.V. and Smans, K. (2012) ATP citrate lyase knockdown induces growth arrest and apoptosis through different cell- and environment-dependent mechanisms. Mol. Cancer Ther. 11, 1925–1935 10.1158/1535-7163.MCT-12-0095 [DOI] [PubMed] [Google Scholar]
- 65.Menendez, J.A. and Lupu, R. (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 7, 763–777 10.1038/nrc2222 [DOI] [PubMed] [Google Scholar]
- 66.da Silva, A.R., Neves, J., Mleczko-Sanecka, K., Tandon, A., Sauer, S.W., Hentze, M.et al. (2017) Cellular citrate levels establish a regulatory link between energy metabolism and the hepatic iron hormone hepcidin. J. Mol. Med. 95, 851–860 10.1007/s00109-017-1551-3 [DOI] [PubMed] [Google Scholar]
- 67.Drakesmith, H., Nemeth, E. and Ganz, T. (2015) Ironing out ferroportin. Cell Metab. 22, 777–787 10.1016/j.cmet.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkinson, N. and Pantopoulos, K. (2014) The IRP/IRE system in vivo: insights from mouse models. Front. Pharmacol. 5, 176 10.3389/fphar.2014.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koppenol, W.H. and Hider, R.H. (2019) Iron and redox cycling. Do's and don'ts. Free Radic. Biol. Med. 133, 3–10 10.1016/j.freeradbiomed.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 70.Torti, S.V. and Torti, F.M. (2020) Iron: the cancer connection. Mol. Aspects Med. 75, 100860 10.1016/j.mam.2020.100860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowdley, K.V. (2004) Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 127, S79–S86 10.1016/j.gastro.2004.09.019 [DOI] [PubMed] [Google Scholar]
- 72.Sivaprakasam, S., Ristic, B., Mudaliar, N., Hamood, A.N., Colmer-Hamood, J., Wachtel, M.S.et al. (2020) Hereditary hemochromatosis promotes colitis and colon cancer and causes bacterial dysbiosis in mice. Biochem. J. 477, 3867–3883 10.1042/BCJ20200392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yodoya, E., Wada, M., Shimada, A., Katsukawa, H., Okada, N., Yamamoto, A.et al. (2006) Functional and molecular identification of sodium-coupled dicarboxylate transporters in rat primary cultured cerebrocortical astrocytes and neurons. J. Neurochem. 97, 162–173 10.1111/j.1471-4159.2006.03720.x [DOI] [PubMed] [Google Scholar]
- 74.Henke, C., Töllner, K., van Dijk, R.M., Miljanovic, N., Cordes, T., Twele, F.et al. (2020) Disruption of the sodium-dependent citrate transporter SLC13A5 in mice causes alterations in brain citrate levels and neuronal network excitability in the hippocampus. Neurobiol. Dis. 143, 105018 10.1016/j.nbd.2020.105018 [DOI] [PubMed] [Google Scholar]
- 75.Newman, J.C. and Verdin, E. (2014) Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 25, 42–52 10.1016/j.tem.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poff, A.M., Rho, J.M. and D'Agostino, D.P. (2019) Ketone administration for seizure disorders: History and rationale for ketone esters and metabolic alternatives. Front. Neurosci. 13, 1041 10.3389/fnins.2019.01041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taggart, A.K.P., Kero, J., Gan, X., Cai, T.Q., Cheng, K., Ippolito, M.et al. (2005) (D0-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 280, 26649–26652 10.1074/jbc.C500213200 [DOI] [PubMed] [Google Scholar]
- 78.Ristic, B., Bhutia, Y.D. and Ganapathy, V. (2017) Cell-surface G-protein-coupled receptors for tumor-associated metabolites: a direct link to mitochondrial dysfunction in cancer. Biochim. Biophys. Acta Rev. Cancer 1868, 246–257 10.1016/j.bbcan.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahman, M., Muhammad, S., Khan, M.A., Chen, H., Ridder, D.A., Muller-Fielitz, H.et al. (2014) The (-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 5, 3944 10.1038/ncomms4944 [DOI] [PubMed] [Google Scholar]
- 80.Suissa, L., Flachon, V., Guigonis, J.M., Olivieri, C.V., Burel-Vandenbos, F., Guglielmi, J.et al. (2020) Urinary ketone body loss leads to degeneration of brain white matter in elderly SLC5A8-deficient mice. J. Cereb. Blood Flow Metab. 40, 1709–1723 10.1177/0271678X19873662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin, P.M., Gopal, E., Ananth, S., Zhuang, L., Itagaki, S., Prasad, B.M.et al. (2006) Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of L-lactate and ketone bodies in the brain. J. Neurochem. 98, 279–288 10.1111/j.1471-4159.2006.03878.x [DOI] [PubMed] [Google Scholar]
- 82.Wang, P.Y., Sung, C.W., Tsai, Y.H., Yeh, S.R., Lin, W.S. and Fan, S.Z. (2020) Nervous system deletion of mammalian INDY in mice mimics dietary restriction-induced memory enhancement. J. Gerontol. A Biol. Sci. Med. Sci. 76, 50–56 10.1093/gerona/glaa203 [DOI] [PubMed] [Google Scholar]
- 83.Sleiman, S.F., Henry, J., Al-Haddad, R., El Hayek, L., Abou Haidar, E., Stringer, T.et al. (2016) Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 5, e15092 10.7554/eLife.15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy, T., Dias, G.P. and Thuret, S. (2014) Effects of diet on brain plasticity in animal and human studies: ming the gap. Neural Plast. 2014, 563160 10.1155/2014/563160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cauwenberghe, C.V., Vandendriessche, C., Libert, C. and Vandenbroucke, R.E. (2016) Caloric restriction: beneficial effects on brain aging and Alzheimer's disease. Mamm. Genome 27, 300–31986 10.1007/s00335-016-9647-6 [DOI] [PubMed] [Google Scholar]
- 86.Buntwal, L., Sassi, M., Morgan, A.H., Andrews, Z.B. and Davies, J.S. (2019) Ghrelin-mediated hippcampal neurogenesis: implications for health and disease. Trends Endocrinol. Metab. 30, 844–859 10.1016/j.tem.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 87.Thevenon, J., Milh, M., Feillet, F., St-Onge, J., Duffourd, Y., Jugé, C.et al. (2014) Mutations in SLC13A5 cause autosomal-recessive epileptic encephalopathy with seizure onset in the first days of life. Am. J. Hum. Genet. 95, 113–120 10.1016/j.ajhg.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hardies, K., de Kovel, C.G.F., Weckhuysen, S., Asselbergh, B., Geuens, T., Deconinck, T.et al. (2015) Recessive mutations inSLC13A5result in a loss of citrate transport and cause neonatal epilepsy, developmental delay and teeth hypoplasia. Brain 138, 3238–3250 10.1093/brain/awv263 [DOI] [PubMed] [Google Scholar]
- 89.Klotz, J., Porter, B.E., Colas, C., Schlessinger, A. and Pajor, A.M. (2016) Mutations in the Na+/citrate cotransporter NaCT (SLC13A5) in pediatric patients with epilepsy and developmental delay. Mol. Med. 22, 310–321 10.2119/molmed.2016.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schossig, A., Bloch-Zupan, A., Lussi, A., Wolf, N.I., Raskin, S., Cohen, M.et al. (2016) SLC13A5is the second gene associated with Kohlschütter–Tönz syndrome. J. Med. Genet. 54, 54–62 10.1136/jmedgenet-2016-103988 [DOI] [PubMed] [Google Scholar]
- 91.Irizarry, A.R., Yan, G., Zeng, Q., Lucchesi, J., Hamang, M.J., Ma, Y.L.et al. (2017) Defective enamel and bone development in sodium-dependent citrate transporter (NaCT) Slc13a5 deficient mice. PLoS One 12, e0175465 10.1371/journal.pone.0175465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anselm, I., MacCuaig, M., Prabhu, S.B. and Berry, G.T. (2017) Disease heterogeneity in Na+/citrate cotransporter deficiency. JIMD Rep. 31, 107–111 10.1007/8904_2016_546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bainbridge, M.N., Cooney, E., Miller, M., Kennedy, A.D., Wulff, J.E., Donti, T.et al. (2017) Analyses of SLC13A5-epilepsy patients reveal perturbations of TCA cycle. Mol. Genet. Metab. 121, 314–319 10.1016/j.ymgme.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weeke, L.C., Brilstra, E., Braun, K.P., Zonneveld-Huijssoon, E., Salomons, G.S., Koeleman, B.P.et al. (2017) Punctate white matter lesions in full-term infants with neonatal seizures associated with SLC13A5 mutations. Eur. J. Paediatr. Neurol. 21, 396–403 10.1016/j.ejpn.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 95.Selch, S., Chafai, A., Sticht, H., Birkenfeld, A.L., Fromm, M.F. and König, J. (2018) Analysis of naturally occurring mutations in the human uptake transporter NaCT important for bone and brain development and energy metabolism. Sci. Rep. 8, 11330 10.1038/s41598-018-29547-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alhakeem, A., Alshibani, F. and Tabarki, B. (2018) Extending the use of stiripentol to SLC13A5-related epileptic encephalopathy. Brain Dev. 40, 827–829 10.1016/j.braindev.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 97.Yang, Q.Z., Spelbrink, E.M., Nye, K.L., Hsu, E.R. and Porter, B.E. (2020) Epilepsy and EEG phenotype of SLC13A5 citrate transporter disorder. Child Neurol. Open 7, 2329048X2093136 10.1177/2329048X20931361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhutia, Y.D., Kopel, J.J., Lawrence, J.J., Neugebauer, V. and Ganapathy, V. (2017) Plasma membrane Na+-coupled citrate transporter (SLC13A5) and neonatal epileptic encephalopathy. Molecules 22, 378 10.3390/molecules22030378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matricardi, S., De Liso, P., Freri, E., Costa, P., Castellotti, B., Magri, S.et al. (2020) Neonatal developmental and epileptic encephalopathy due to autosomal recessive variants in SLC13A5 gene. Epilepsia 61, 2474–2485 10.1111/epi.16699 [DOI] [PubMed] [Google Scholar]
- 100.Kopel, J., Bhutia, Y.D., Ramanchandran, S., Lawrence, J.J., Neugebauer, V. and Ganapathy, V. (2017) Tooth hypoplasia for differential diagnosis of childhood epilepsy associated with SLC13A5 mutations. Int. J. Neurol. Disord. 1, 33–37 10.29328/journal.jnnd.1001006 [DOI] [Google Scholar]
- 101.Lopes-Pacheco, M. (2020) CFTR modulators: The changing face of cystic fibrosis in the are of precision medicine. Front. Pharmacol. 10, 1662 10.3389/fphar.2019.01662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dickens, F. (1941) The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem. J. 35, 1011–1023 10.1042/bj0351011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Costello, L.C., Franklin, R.B., Reynolds, M.A. and Chellaiah, M. (2012) The important role of osteoblasts and citrate production in bone formation: “Osteoblast citration” as a new concept for an old relationship. Open Bone J. 4, 10.2174/1876525401204010027 10.2174/1876525401204010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Franklin, R.B., Chellaiah, M., Zou, J., Reynolds, M.A. and Costello, L.C. (2014) Evidence that osteoblasts are specialized citrate-producing cells that provide the citrate for incorporation into the structure of bone. Open Bone J. 6, 1–7 10.2174/1876525401406010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mantila Roosa, S.M., Liu, Y. and Turner, C.H. (2010) Gene expression patterns in bone following mechanical loading. J. Bone Miner. Res. 26, 100–112 10.1002/jbmr.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu, S., Tang, W., Fang, J., Ren, J., Li, H., Xiao, Z.et al. (2009) Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol. Endocrinol. 23, 1505–1518 10.1210/me.2009-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pemberton, T.J., Li, F.Y., Oka, S., Mendoza-Fandino, G.A., Hsu, Y.H., Bringas, P.et al. (2007) Identification of novel genes expressed during mouse tooth development by microarray gene expression analysis. Dev. Dyn. 236, 2245–2257 10.1002/dvdy.21226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsu, W.B., Hsu, W.H., Hung, J.S., Shen, W.J. and Hsu, R.W.W. (2018) Transcriptome analysis of osteoblasts in an ovariectomized mouse model in response to physical exercise. Bone Joint Res. 7, 601–608 10.1302/2046-3758.711.BJR-2018-0075.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma, C., Tian, X., Kim, J.P., Xie, D., Ao, X., Shan, D.et al. (2018) Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc. Natl. Acad. Sci. U.S.A. 115, E11741–E11750 10.1073/pnas.1813000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szeri, F., Lundkvist, S., Donnelly, S., Engelke, U.F.H., Rhee, K., Williams, C.J.et al. (2020) The membrane protein ANKH is crucial for bone mechanical performance by mediating cellular export of citrate and ATP. PLoS Genet. 16, e1008884 10.1371/journal.pgen.1008884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abhishek, A. and Doherty, M. (2011) Pathophysilogy of articular chondrocalcinosis – role of ANKH. Nat. Rev. Rheumatol. 7, 96–104 10.1038/nrrheum.2010.182 [DOI] [PubMed] [Google Scholar]
- 112.Zhang, Y., Shi, S., Ciurli, C. and Poole, A.R. (2002) Animal models of ankylosing spondylitis. Curr. Rheumatol. Rep. 4, 507–512 10.1007/s11926-002-0058-1 [DOI] [PubMed] [Google Scholar]
- 113.Sun, J., Aluvila, S., Kotaria, R., Mayor, J.A., Walters, D.E. and Kaplan, R.S. (2010) Mitochondrial and plasma membrane citrate transporters: Discovery of selective inhibitors and application to structure/function analysis. Mol. Cell. Pharmacol. 2, 101–110 PMID: [PMC free article] [PubMed] [Google Scholar]
- 114.Huard, K., Brown, J., Jones, J.C., Cabral, S., Futatsugi, K., Gorgoglione, M.et al. (2015) Discovery and characterization of novel inhibitors of the sodium-coupled citrate transporter (NaCT or SLC13A5). Sci. Rep. 5, 17391 10.1038/srep17391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rives, M.L., Shaw, M., Zhu, B., Hinke, S.A. and Wickenden, A.D. (2016) State-dependent allosteric inhibition of the human SLC13A5 citrate transporter by hydroxysuccinic acids, PF-06649298 and PF-06761281. Mol. Pharmacol. 90, 766–774 10.1124/mol.116.106575 [DOI] [PubMed] [Google Scholar]
- 116.Pajor, A.M., de Oliveira, C.A., Song, K., Huard, K., Shanmugasundaram, V. and Erion, D.M. (2016) Molecular basis for inhibition of the Na+/citrate transporter NaCT (SLC13A5) by dicarboxylate inhibitors. Mol. Pharmacol. 90, 755–765 10.1124/mol.116.105049 [DOI] [PubMed] [Google Scholar]
- 117.Huard, K., Gosset, J.R., Montgomery, J.I., Gilbert, A., Hayward, M.M., Magee, T.V.et al. (2016) Optimization of a dicarboxylic series for in vivo inhibition of citrate transport by the solute carrier 13 (SLC13) family. J. Med. Chem. 59, 1165–1175 10.1021/acs.jmedchem.5b01752 [DOI] [PubMed] [Google Scholar]
- 118.Willmes, D.M., Kurzbach, A., Henke, C., Schumann, T., Zahn, G., Heifetz, A.et al. (2018) The longevity gene INDY (I'm Not Dead Yet) in metabolic control: potential as pharmacological target. Pharmacol. Ther. 185, 1–11 10.1016/j.pharmthera.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 119.Kopel, J., Grooms, A., Ganapathy, V. and Clothier, J. (2020) Metformin, valproic acid, and starvation induce seizures in a patient with partial SLC13A5 deficiency: a case of pharmaco-synergistic heterozygosity. Psychiatr. Genet. 1, 32–35 10.1097/YPG.0000000000000269 [DOI] [PubMed] [Google Scholar]
- 120.Wright, J. T. (2018) Developmental defects of enamel In Reference Module in Biomedical Sciences, Elsevier [Google Scholar]