Abstract
Immune checkpoint inhibitors have revolutionised cancer treatment; however, immune-related adverse events do occur, with up to 7% developing inflammatory arthritis. Common rheumatoid arthritis therapies such as methotrexate, prednisolone and biologics have been used to treat this arthritis in small, uncontrolled case series with varying success. In this case of personalised medicine, we report the first use of tofacitinib, a small molecular inhibitor of the Janus kinase-signal transducer and activator of transcription pathway, to treat checkpoint inhibitor-related inflammatory arthritis. This resulted in a rapid clinical response and complete, sustained remission of the arthritis with associated marked reduction in synovial molecular and cellular immune response.
Keywords: malignant disease and immunosuppression, drugs: musculoskeletal and joint diseases
Background
Immune checkpoint inhibitors (ICIs) have dramatically improved the prognosis of many cancers.1 2 ICI-related adverse events (irAEs) include systemic inflammatory reactions, most often affecting the skin and gastrointestinal tract. Inflammatory arthritis is reported in up to 7% of ICI-treated patients.3 4 We report the clinical and immunological characteristics of ICI-induced inflammatory arthritis in a patient with pulmonary adenocarcinoma and successful, sustained remission following tofacitinib therapy.
Case presentation
A 56-year-old man presented in 2018 with haemoptysis and changes in cognitive function. He was diagnosed with programmed death-ligand 1 (PD-L1) positive pulmonary adenocarcinoma with temporal and parietal lobe brain metastases. He reported a sister with rheumatoid arthritis. The lung cancer was treated successfully with the programmed cell death protein 1 (PD-1) inhibitor, pembrolizumab, and he received stereotactic radiotherapy for the metastatic brain lesions.
Six months after commencing pembrolizumab, the patient developed acute pain and swelling in the small joints of his hands and in his knees consistent with a polyarthritis. The patient had no other irAEs. Given his complete tumour response and significant irAEs, pembrolizumab treatment was stopped on the advice of the oncology team. However, the joint symptoms remained severe and incapacitating. Treatment with etoricoxib (COX-2 specific inhibitor, 90 mg once daily), weekly oral methotrexate 10 mg and sulfasalazine 1 g two times per day was commenced but with little clinical benefit. Prednisolone 20 mg daily was then added, but again there was minimal clinical response. Given the poor response to the conventional antirheumatic medications, treatment with targeted therapies were considered. Tofacitinib effectively reduces inflammation in synovial tissue, especially in targeting pathogenic CD4+ T cells as demonstrated in the biopsy, in addition to a potential rapid onset of action (<2 weeks),5 the patient consented to off-licence treatment with tofacitinib at a dose of 5 mg two times per day.
Initially, severe metacarpal joint synovitis was apparent, and there was a dramatic reduction in erythema and swelling following tofacitinib treatment (figure 1A). A rapid decrease of tender joint count, swollen joint count, C-reactive protein (CRP) and a DAS28-CRP (figure 1B) indicative of disease remission were also observed. Additionally, proximal muscle strength improved from 4/5 to 5/5 in all limbs. At 2 weeks, the patient had a dramatic clinical response. He entered complete clinical disease remission (DAS28-CRP <2.6) at week 20.
Figure 1.
Clinical course and remission before and after treatment with tofacitinib. Images of joint inflammation with bilateral metacarpophalangeal joint synovitis (arrows) before and after treatment with tofacitinib (A). Reduction in TJC, SJC, DAS28-CRP and CRP scores from commencement of tofacitinib treatment at time 0 and remission at week 31 (B). Dashed red line (DAS28-CRP) indicates clinical remission (>2.6) at week 20. CRP, C-reactive protein; SJC, swollen joint count; TJC, tender joint count.
Investigations
Routine investigations revealed a highly elevated CRP (217 mg/dL), a negative rheumatoid factor (1.2 IU/mL) and anticitrullinated protein antibodies (1.7 U/mL), but significantly raised antinuclear antibody titre (1:400). Following fully informed written consent, serial peripheral blood mononuclear cell (PBMCs) isolates and needle arthroscopy using a 2.7 mm (Karl Storz, Germany) were performed under local anaesthetic with full sterile procedure. Synovial tissue biopsies were obtained using a 2 mm diameter grasping forceps as previously reported.6 Routine histology and immunohistochemical analysis (CD3+ cells and PD-L1 expression) of the original adenocarcinoma biopsy of the lung and the joint synovial tissue, before and after treatment, are compared in figure 2.
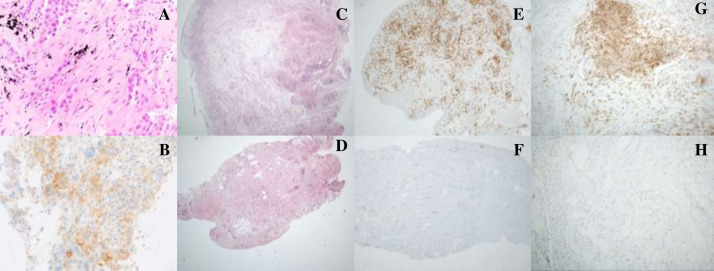
Figure 2.
Histological analysis of synovial tissue before and after treatment with tofacitinib. Needle core biopsy of the left lower lobe lung nodule showed acinar pattern pulmonary adenocarcinoma (A). PD-L1 immunohistochemistry staining showed focal membranous staining in 30% of tumour cells (B). Synovial biopsy H&E staining before tofacitinib treatment demonstrated a hyperplastic synovium with dense lymphoplasmacytic infiltrate (C) comprising predominantly CD3+ T-lymphocytes (446 lymphocytes/hpf, D) with 30% immunostaining positive for PD-L1 (E). Repeat synovial biopsy, following response to tofacitinib treatment demonstrated stromal fibrosis with a reduced inflammatory infiltrate (F) with rare CD3 expression (<1%, G) and negative staining for PD-L1 (H).
Single cell synovial tissue immune cell analysis was performed using flow cytometry on a 15-colour-BD LSRFortessa with specific antibody panels to identify cytokine expression, T-cell responses and polyfunctionality was further assessed, before and after tofacitinib, by Simplified Presentation Of Incredibly Complex Evaluations (SPICE) algorithm (figure 3).7
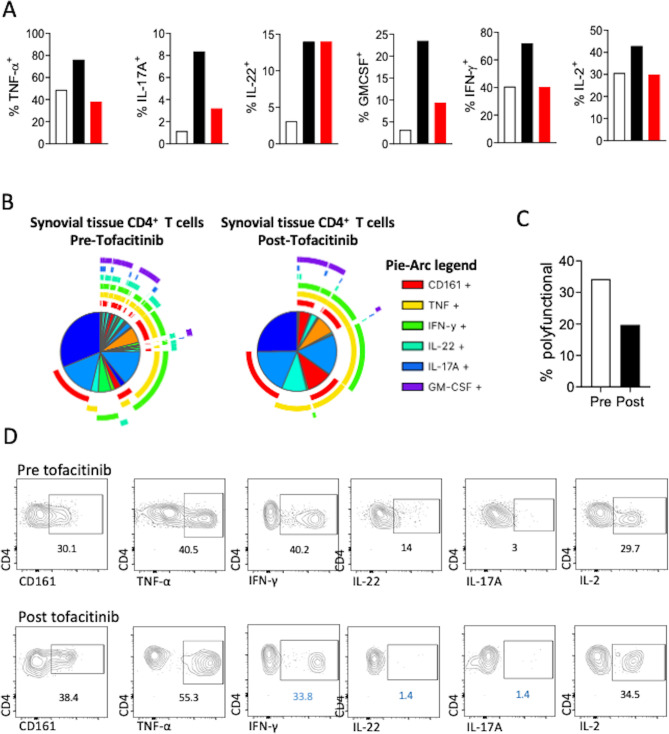
Figure 3.
Analysis of synovial tissue T cell polyfunctionality using SPICE algorithm. Flow cytometric analysis for the frequency of key proinflammatory cytokine-producing synovial tissue CD4 T cells pretreatment and post-treatment with tofacitinib. Frequencies in red denote increase and frequencies highlighted in blue, decrease in the corresponding proinflammatory cytokine post tofacitinib treatment compared with pretreatment responses (A). Following polyparametric flow cytometric analysis of synovial tissue derived T cells, the supervised data visualisation algorithm SPICE was used (B). The pie segments represent the percentage of cells and the pie arcs the cytokine or marker that is being expressed. Pie segments surrounded by overlapping pie arcs denote CD4 T cell populations expressing multiple cytokines simultaneously. Data presented for synovial tissue CD4 T cells at first arthroscopy—prior to the initiation of tofacitinib treatment and second arthroscopy—post tofacitinib treatment. Frequency of polyfunctional synovial tissue CD4 T cells expressing more than three proinflammatory cytokines simultaneously at time of first and second arthroscopy (C).
CT-guided biopsy of the right lung nodule showed alveolar infiltrated by a non-small-cell lung carcinoma with solid and acinar architecture (figure 2A). Immunohistochemistry was positive for markers of pulmonary adenocarcinoma (TTF-1 and Napsin) and negative for squamous differentiation markers (p63 and CK5/6). Molecular studies did not detect an epidermal growth factor receptor gene mutation and immunohistochemistry for anaplastic lymphoma kinase was negative. PD-L1 immunohistochemistry was positive (figure 2B) with a tumour proportion score of 30%.
Pretofacitinib synovial biopsy H&E staining demonstrated a hyperplastic synovium with dense lymphoplasmacytic infiltrate (figure 2C) comprising predominantly CD3+ T-lymphocytes (446 lymphocytes/hpf, figure 2D) with 30% immunostaining positive for PD-L1 (figure 2E). Repeat synovial biopsy, 30 days after commencing tofacitinib treatment demonstrated stromal fibrosis with a reduced inflammatory infiltrate (figure 2F) with rare CD3 expression (<1%, figure 2G) and negative staining for PD-L1 (figure 2H).
Single cell synovial tissue analysis
Gating strategy and flow plots for T cell (figure 4A), T regulatory (Treg) (figure 4B, C), T follicular helper (Tfh) (figure 5A–C) and B cells in PBMC, synovial fluid mononuclear cell and synovial tissue are shown in figure 6A, B. An increase in the frequency of synovial tissue CD4+ T cells producing IL-17A, IL-22 and Granulocyte-macrophage colony-stimulating factor (GMCSF) was demonstrated compared with peripheral blood CD4+ T cells (figure 3A), reflecting the inflammation observed at a clinical and histological level. While the frequency of Treg (CD4+CD127CD25±FOXP3+) cells was comparable between peripheral blood (6.7%), synovial fluid (7%) and synovial tissue (11%), qualitative differences, where observed, with absence of the more stable naive (CD45RO−CCR7+) Treg cells in the synovial fluid/tissue compared with periphery (figure 4B, C) suggesting a possible defect in their ability to supress T-cell function. At baseline PD-1 expressing CD4+ T cells were not detectable in the periphery (figure 5B), potentially due to stereochemical blockade of epitope recognition following treatment with pembrolizumab. Interestingly, however, PD-1 expression was detectable (37%) in synovial fluid CD4+ T cells (figure 5B). Tfh (CXCR5+) cell frequency was comparable between the periphery and synovial fluid, although synovial tissue Tfh cells were predominantly positive for the expression of the chemokine receptors CCR6 and CXCR3 indicative of a polyfunctional pathogenic Th1/Th17 mixed phenotype (figure 5C). Frequency of CD19+CD20±B cell was low in synovial fluid (0.6%) and tissue (0.1%) compared with periphery (3.5%), with predominance of potentially pathogenic PD-1 expressing memory B cells (34.5% vs 0.35%) in the synovial fluid (figure 6A, B). The frequency of key proinflammatory cytokine-producing synovial tissue CD4+ T cells decreased following tofacitinib treatment (figure 3A). Thus, immune cell analysis of pretofacitinib synovial tissue and fluid suggests that pembrolizumab induces inflammatory phenotypes that are known to significantly contribute to synovial pathogenesis.
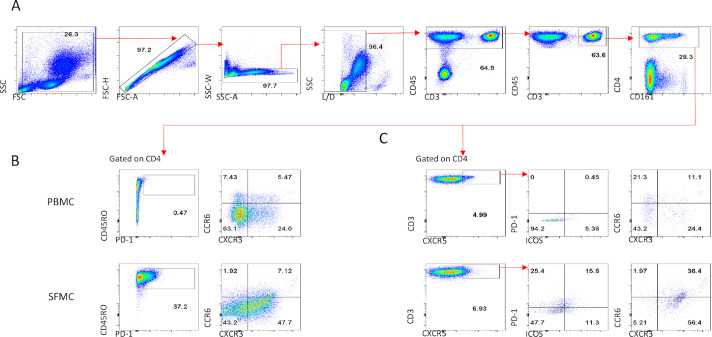
Figure 4.
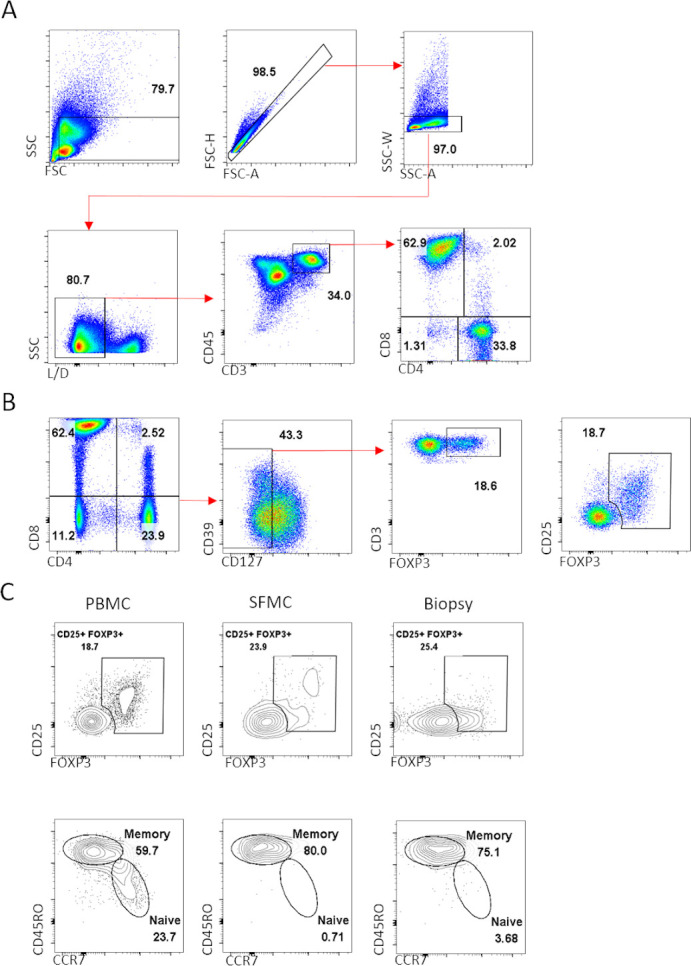
Gating strategy to identify cytokine expressing CD4 T cells and regulatory T cells. Gating strategy followed for the identification of CD4 T cells for the subsequent analysis of proinflammatory cytokine profile (A). Example for the identification of regulatory T cells. in PBMC and SFMC, in addition to expression of the Treg-specific transcription factor FOXP3, CD25 was also used to verify gating of Treg cells. In the synovial tissue due to loss of CD25 during the enzymatic digestion of synovial biopsies Treg cells were defined as CD4+CD127 CD39±FOXP3+ (B). Treg cells can be divided into naive and memory Treg subpopulations, note the absence of naive Treg in the SFMC and synovial tissue compared with peripheral blood (C). Data shown from paired PBMC, SFMC and synovial biopsies collected pretofacitinib. PBMC, peripheral blood mononuclear cell; SFMC, synovial fluid mononuclear cell; Treg cells, T regulatory cells.
Figure 5.
Tfh cell phenotype in the peripheral blood and synovial fluid. Gating strategy followed for the identification of CD4 T cells (A). Expression of T cell memory marker CD45RO and PD-1 as well as distribution of the chemokine receptors CCR6 and CXCR3 in peripheral blood and synovial fluid CD4 T cells (B). Identification of peripheral blood and synovial fluid Tfh cells based on expression of CXCR5 and subsequent characterisation of ICOS, PD-1, CCR6 and CXCR3 expression pretofacitinib (C). Tfh cell, T follicular helper cell.
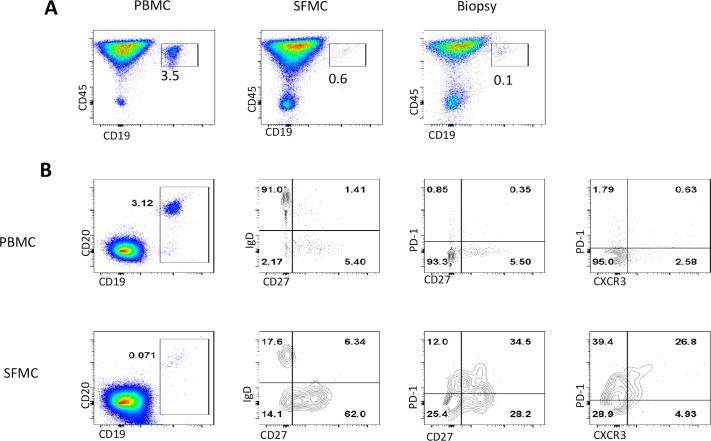
Figure 6.
Phenotype of peripheral blood and synovial fluid B cells at first arthroscopy. Frequency of B cells in PBMC, SFMCs and single synovial biopsy tissue cells (A). while the percentage of synovial fluid B cells is a fraction of the peripheral blood B cell population, the vast majority of synovial fluid B cells belong to the switched memory B cell compartment (CD27+IgD–). Interestingly, synovial fluid B cells had marked expression of PD-1 and the chemokine receptor CXCR3 compared with peripheral blood B cells. data shown are from paired PBMC and SFMC pretofacitinib (B). SFMC, synovial fluid mononuclear cells; PBMCs, peripheral blood mononuclear cells.
SPICE analysis of pretofacitinib synovial tissue showed marked CD4+ T cell polyfunctionality with 34.2% of cells expressing three or more proinflammatory cytokines simultaneously (figure 3B). Post-tofacitinib synovial biopsy analysis demonstrated a marked decrease in the frequency of polyfunctional CD4+ T cells (34.2% vs 19.7%, figure 3C), with specific reductions in Th1 and Th17 associated proinflammatory cytokines interferon-γ (IFN-γ, 40.2% vs 33.8%), IL-17A (3% vs 1.4%) and IL-22 (14% vs 1.4%, figure 3D).
Treatment
Maintenance treatment now includes Tofacitinib 5 mg twice daily, Methotrexate 10 mg and Folic acid 5 mg weekly. All other medication, including ICI and steroids, has been stopped.
Outcome and follow-up
The patient remains in complete, sustained arthritis remission after 24 months, and complete remission of lung adenocarcinoma 28 months since diagnosis. The patient had complete tumour response before and after tofacitinib. The patient remains in full remission from arthritis and cancer, 75 weeks after commencing tofacitinib.
Discussion
ICI have dramatically improved prognosis in many cancers through manipulation of T-cell pathways involved in cellular activation/deactivation. A 2018 meta-analysis of 23 RCT examined 13 721 patients on ICI for a range of malignancies.4 They demonstrated a significant survival benefit for immunotherapy compared with other systemic therapies (HR, 0.75, 95% CI, 0.70 to 0.81; p < 0.001; I2 = 61%).4
ICIs can trigger inflammation in almost any organ. Indeed, ICI-induced colitis, pneumonitis, hepatitis, neurotoxicity, hypophysitis and myocarditis can cause mortality.3 4 T-cell antibody and cytokine responses appear to contribute to the disruption of immune homeostasis and irAEs linked with ICIs.3 The PD-1 pathway regulates thymic T cell development and provides an activation threshold for T cell receptor (TCR) mediated signals.8 Several studies have demonstrated the key role of PD-1 in regulating autoreactive T cell response in the periphery by limiting activation and proliferation of autoreactive T cells.9 Different ICIs are associated with specific irAEs, colitis and hypophysitis are more frequent with CTLA-4 inhibition and pneumonitis and thyroiditis more frequent with PD-1 inhibitors.3 The mechanisms underlying the aforementioned distinction remain elusive, therefore further characterisation of the immune response in patients receiving ICIs will be crucial in minimising irAEs prevalence in the future.
Inflammatory arthritis is characterised by immune cell infiltration, activation leading to synovial hyperplasia, with subsequent joint destruction and disability.10 We have recently reported increased levels of PD-1 in the serum and synovial tissue of RA patients, with down-regulation of its ligand PD-L1 preventing overactivity of the PD-1 pathway.11 In this patient, PD-L1 inhibition triggered arthritis, as evident at both the clinical, macroscopic and microscopic level. This was further confirmed following single cell analysis of synovial tissue which showed an increase in immune cell infiltrates in the joint, particularly polyfunctional T cells and pathogenic Tfh cells, with defects observed for Treg phenotypes also. There remain no RCTs to determine the optimal treatment for ICI-induced synovitis.
Evidence for methotrexate, Non-steroidal anti-inflammatory drugs (NSAIDs), prednisolone, Tumour Necrosis Factor (TNF) blockade, and interleukin-6 (IL-6) inhibition is limited to small, uncontrolled case series.12 13 The recently published ‘EULAR points to consider’ provides significant clarity and a stepwise symptomatic treatment approach using glucocorticoids, conventional synthetic disease-modifying antirheumatic drugs and biologic agents.14 However, some patients will not respond to these therapies or experience significant adverse effects thus, other treatment strategies do need to be explored.
We and others have previously shown tofacitinib to regulate synovial inflammation and cellular metabolism in inflammatory arthritis.15 16 Janus kinase/signal transducer and activator of transcription (JAK/STAT) blockade regulates pro-inflammatory responses, invasive mechanisms and cellular bioenergetics that we and others have shown to be highly pathogenic.17 18 Furthermore, polyfunctional synovial T cells and not single cytokine producing T cells positively correlate with disease activity,19 suggesting they significantly contribute in propagating joint inflammation. Based on the evidence above and the patients molecular and cellular phenotype with CD4 +polyfunctional T-cell predominance on synovial biopsy, it was concluded that targeting the JAK/STAT pathway with tofacitinib was the best approach to achieve a swift response without the risk of reactivation of tumour cells in this patient.
ICIs have caused a paradigm shift in oncology outcomes, although significant irAE may occur. Given ICI-induced inflammatory arthritis is a novel condition, there is currently a paucity of data on the underlying immunological responses and optimal treatment. Recent studies suggest that ICI-induced arthritis can become chronic,20 studies of the cellular infiltrate at the site of inflammation will inform much needed future treatment regimes.
To our knowledge, this is the first case of a patient with ICI-induced inflammatory arthritis treated successfully with tofacitinib. We provide evidence that tofacitinib decreased polyfunctional cytokine expressing T cells, which we and others have shown to be highly pathogenic.17 18 In addition, we report a marked T-cell dominant synovial infiltrate with highly polyfunctional CD4+ T cells and aberrant proinflammatory cytokine expression. The possibility of tumour recurrence led us away from targeting T cells directly; however, we have shown that inhibition of the JAK/STAT pathway reduces the frequency of pathogenic polyfunctional T cells, providing a profound clinical response for the patient. Given the role of JAK/STAT in regulation of PD-1 ligand and IFN-γ signalling in tumours, there is a theoretical risk of inducing tumour anti-PD-1 resistance.21 To date we have not observed tumour recurrence, or indeed, significant adverse effects from targeting the JAK/STAT pathway in this patient. Based on these data tofacitinib could be a promising option for the swift treatment and resolution of ICI-induced irAE.
Patient’s perspective.
In January 2018, I was on holiday in Austria with my wife for a week enjoying the beautiful scenery, fresh air and good food after a hectic Christmas…life was good and my wife and I were both enjoying our lives with very few worries. During our holiday we decided to take a day trip to Salzburg and took a coach to the city that morning little did I know that on this day my whole life would change forever.
On the morning of arriving to Salzburg I began to get a numbing sensation on my right side and thankfully my wife got me to a hospital pretty quickly, I was taken for a MRI scan of the brain thinking I might be having a stroke but when the results came back I was diagnosed with a cancerous tumour in the brain and the doctors wanted to know where the cancer started and with some more tests it showed up in the right lung. I was diagnosed with non-small cell carcinoma of the right lung with brain metastases, a very frightening prognosis for me. I returned to Ireland and was under the guidance of the oncology team. I started radiotherapy to the tumour in the brain and right lung in early March of 2018 and after a biopsy of the lung I got the good news that I was eligible to receive the new immunotherapy treatment - Pembrolizumab, this news gave me a lot of hope during these frightening times as I was aware of the good results that were coming from patients on it. I started this treatment in May 2018 and over the next few months things were going really well for me, I was responding to the treatment really well and thankfully the cancer was not spreading.
After several immunotherapy treatments, however I began to notice my right knee swelling up with fluid and causing me acute pain. As time went on I had to get my right knee drained a few times to relieve the discomfort and not long after my left knee started to swell as well and the pain was so bad that at times I was unable to walk. I felt really low during this time, I noticed soon after, the joints in my hands and elbows were stiffening and swelling and I found it hard to bend them, the pain was indescribable, usually I have a strong resistance to pain but this really affected me deeply and I felt I was losing the fight. It was at this point that my oncologist stopped all treatments for the cancer and referred me to a and his to a rheumatologist to do a more in-depth study of the side effects from Pembrolizumab. The rheumatology team did a biopsy to my right knee and I was diagnosed with Inflammatory Arthritis. I felt a glimmer of hope knowing I was under a superb team. With this diagnosis I started on methotrexate, and tofacitinib and my progress was monitored closely. Over the next few months on these drugs I was responding really well, swelling was reducing, movement was returning and I could walk again without an aid. The methotrexate was reduced substantially and I am now on only a small dose along with tofacitinib. I can happily say that over the last few months my life has returned to a form of normality that I probably could have only dreamed of in early 2018 but thanks to the amazing immunotherapy drug Pembrolizumab that has put my cancer under control and thankfully I have not received treatment since mid 2019 and of course the rheumatoid drugs, I can exercise again, work again and enjoy my life with my wife again.
I cannot thank my consultants enough, they have given me hope and a new lease of life that I cherish so much now. Thank you to all the amazing medical staff I owe my life to.
Learning points.
Immune checkpoint inhibitors (ICIs) have transformed the outcomes of many cancers.
Inflammatory adverse reactions including arthritis do occur and may be debilitating in some patients.
Conventional therapies, including steroids, may not provide significant benefit.
Small molecular inhibitors of the JAK/STAT pathway such as tofacitinib, as in this case, may provide swift and sustained remission from inflammatory arthritis associated with ICI treatment.
Footnotes
Twitter: @dougveale
Contributors: KM, AF (Aurelie Fabre, Dept of Pathology, UCD), JC (John Crown, Dept. of Oncology, UCD), DV were involved in the care of the patient. AF and UF performed and analysed the flow cytometry. CM (Ciara Murray, Dept of Pathology, UCD) and AF performed the immunohistochemical analysis. KM, AF, CM, AF, JC, UF and DV were involved in drafting the manuscript. The authors were solely responsible for final review and approval of the report. The corresponding author had final responsibility for the decision to submit for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 2.Weber JS, Hodi FS, Wolchok JD, et al. . Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–92. 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 4.Wang DY, Salem J-E, Cohen JV, et al. . Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischmann R, Kremer J, Cush J, et al. . Placebo-Controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. 10.1056/NEJMoa1109071 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy A, Ng CT, Chang TC, et al. . Tumor necrosis factor blocking therapy alters joint inflammation and hypoxia. Arthritis Rheum 2011;63:923–32. 10.1002/art.30221 [DOI] [PubMed] [Google Scholar]
- 7.Basdeo SA, Cluxton D, Sulaimani J, et al. . Ex-Th17 (nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J.i. 2017;198:2249–59. 10.4049/jimmunol.1600737 [DOI] [PubMed] [Google Scholar]
- 8.Keir ME, Latchman YE, Freeman GJ, et al. . Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol 2005;175:7372–9. 10.4049/jimmunol.175.11.7372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauken KE, Nelson CE, Martinov T, et al. . Cutting edge: identification of autoreactive CD4+ and CD8+ T cell subsets resistant to PD-1 pathway blockade. J Immunol 2015;194:3551–5. 10.4049/jimmunol.1402262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Walsh AM, Canavan M, et al. . Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One 2018;13:e0192704. 10.1371/journal.pone.0192704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappelli LC, Gutierrez AK, Baer AN, et al. . Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. 10.1136/annrheumdis-2016-209595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, et al. . Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76:2061–4. 10.1136/annrheumdis-2017-211560 [DOI] [PubMed] [Google Scholar]
- 14.Kostine M, Finckh A, Bingham CO, et al. . EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis 2021;80:36–48. 10.1136/annrheumdis-2020-217139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGarry T, Orr C, Wade S, et al. . Jak/Stat blockade alters synovial bioenergetics, mitochondrial function, and proinflammatory mediators in rheumatoid arthritis. Arthritis Rheumatol 2018;70:1959–70. 10.1002/art.40569 [DOI] [PubMed] [Google Scholar]
- 16.Rosengren S, Corr M, Firestein GS, et al. . The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis 2012;71:440–7. 10.1136/ard.2011.150284 [DOI] [PubMed] [Google Scholar]
- 17.Basdeo SA, Cluxton D, Sulaimani J, et al. . Ex-Th17 (nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J Immunol 2017;198:2249–59. 10.4049/jimmunol.1600737 [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Fanok MH, Mediero-Munoz A, et al. . Augmented Th17 differentiation leads to cutaneous and Synovio-Entheseal inflammation in a novel model of psoriatic arthritis. Arthritis Rheumatol 2018;70:855–67. 10.1002/art.40447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade SM, Canavan M, McGarry T, et al. . Association of synovial tissue polyfunctional T-cells with DAPSA in psoriatic arthritis. Ann Rheum Dis 2019;78:350–4. 10.1136/annrheumdis-2018-214138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braaten TJ, Brahmer JR, Forde PM, et al. . Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 2020;79:332–8. 10.1136/annrheumdis-2019-216109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. . Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016;375:819–29. 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]