Description
A 42-year-old woman presented to the emergency department with progressive dyspnoea. The patient’s medical history comprises of hypertension, hyperlipidaemia, transient ischaemic attack, chronic tubular interstitial disease post-renal transplant in 2003. Five years prior, she developed delayed graft rejection with a repeat renal transplant. The patient received a kidney from a 5-year-old who died of a motor vehicle accident. The donor anatomy was notable for a single transplant renal artery (TRA) anastomosed to the external iliac artery (EIA) with good postprocedure reperfusion and an insignificant postoperative course.
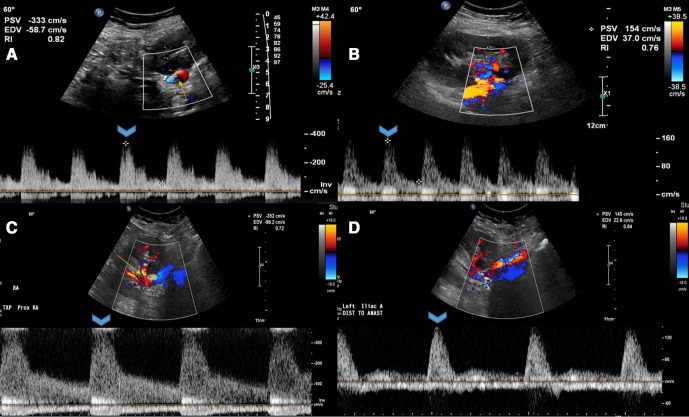
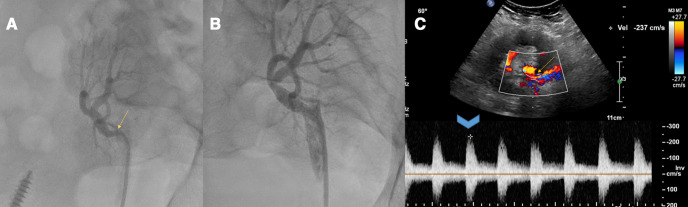
The patient’s current immunosuppressant regimen comprised of maintenance tacrolimus, mycophenolate and prednisone. On presentation, the patient had elevated blood pressure. Physical examination revealed mild respiratory distress, an ECG was normal and a chest X-ray showed pulmonary congestion. Significant laboratory findings were an elevated serum creatinine at 2 mg/dL (baseline for patient was 1.1–1.4 mg/dL). Transthoracic echocardiogram and pulmonary function testing were normal. A screening ultrasound of the renal allograft showed a sharp bend in the renal artery with a significant increase in peak systolic velocities in the TRA just beyond the anastomosis to approximately 350 cm/s (figure 1A) compared with 154 cm/s in the previous ultrasound with relatively unchanged intraparenchymal resistive indices (figure 1B). Duplex ultrasonography of renal arterial circulation revealed a normal kidney size, peak velocities of 440 cm/s (figure 1C) in the TRA and 122 cm/s in the EIA (figure 1D) with a renal/iliac ratio of 3.1, normal parenchymal blood flow with an unchanged resistive index. Possible differential diagnoses included tortuosity of the renal artery, true stenosis and thrombus. A renal angiogram confirmed severe stenosis of the origin of the TRA with a recorded pressure gradient of 80 mm Hg (figure 2A). The stenosis was crossed with a guidewire and a drug-eluting stent was placed followed by postdilation with satisfactory angiographic results (figure 2B). The patient was placed on dual antiplatelet therapy (DAPT) with aspirin and clopidogrel, and showed symptomatic improvement with reduction in high blood pressure and a gradual baseline return of renal function on subsequent follow-up visits. A follow-up ultrasound of kidney allograft showed patent main renal artery with normalised peak velocities (figure 2C).
Figure 1.
Ultrasonographic features of transplant renal artery stenosis with (A) sharp bend at the origin of anastomosis, (B) prior ultrasound with normal peak velocities, (C) duplex study showing increased peak velocities at the stenosis and (D) preserved peak velocities at external iliac artery.
Figure 2.
Invasive angiography with (A) severe stenosis at the origin of transplant renal artery, (B) stenting and postdilation with satisfactory angiographic results and (C) follow-up ultrasound at 3 months with normalisation of peak velocities of transplant renal artery.
TRA stenosis (TRAS) is a recognised complication post-transplantation with a reported incidence of 1%–23%.1 It occurs mostly within 3 months to 2 years after renal transplantation, but it may present anytime. Early-onset TRAS may be associated with mechanical lesions of the blood vessels during organ recruitment or the surgical procedure. Our case demonstrated late-onset TRAS after 5 years, likely due to underlying progressive atherosclerotic disease in the recipient with traditional cardiovascular risk factors.1 Common presenting manifestations of TRAS include worsening or refractory hypertension, fluid retention or graft dysfunction without evidence of rejection.1 Donor artery atherosclerosis, suture techniques, traumatic or immunogenic vascular damage to donor or recipient arteries are identified as potential causes. Duplex ultrasonography is a safe, non-invasive initial test. Elevated peak systolic velocity in the TRA >200–300 cm/s and a ratio of peak systolic velocity in TRA: EIA >1.8 has a high sensitivity for the detection of TRAS.1 2 Invasive angiography provides a definitive diagnosis of TRAS; however, as it is an invasive procedure, with a risk of contrast-induced acute kidney injury, it should ideally be performed in symptomatic patients or patients with high-risk sonographic features.1 Endovascular intervention should be considered as the therapy for TRAS despite the inherent procedural risks. Although percutaneous transluminal angioplasty does have favourable success rate with improvement in blood pressure control and creatinine levels, stent placement offers further benefits of sealing of dissections, reduced risk of abrupt vessel closure and reduced immediate residual stenosis and a comparable reduced restenosis rates of 10%–30%.3 Newer generation drug-eluting stents have improved designs and thinner struts for comfortable placements in tortuous anatomy. Drug-eluting stents have shown reductions in restenosis and target vessel revascularisation compared with bare-metal stents. The occurrence of delayed stent thrombosis remains a concern, which can be reduced by the use of DAPT.3
Learning points.
Transplant renal artery stenosis (TRAS) is a recognised complication, occurring mostly within 3 months to 2 years, but potentially anytime post renal transplantation.
TRAS may present as worsening/refractory hypertension, fluid retention and graft failure.
Awareness of this complication is critical and use of non-invasive ultrasonography can help detect virtually all haemodynamically significant stenosis.
Invasive angiography can provide definitive diagnosis and treatment, but carries a risk of contrast exposure to the graft and is reserved for high-risk patients.
Endovascular therapy with percutaneous transluminal angioplasty and drug-eluting stent placement results in improved blood pressure control and renal function.
Footnotes
Twitter: @TahaAhmedMD
Contributors: TA designed the study, performed the literature review and drafted the manuscript. SHL performed the literature review and suggested pertinent modifications.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Chen W, Kayler LK, Zand MS, et al. Transplant renal artery stenosis: clinical manifestations, diagnosis and therapy. Clin Kidney J 2015;8:71–8. 10.1093/ckj/sfu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fananapazir G, McGahan JP, Corwin MT, et al. Screening for transplant renal artery stenosis: Ultrasound-based stenosis probability stratification. AJR Am J Roentgenol 2017;209:1064–73. 10.2214/AJR.17.17913 [DOI] [PubMed] [Google Scholar]
- 3.Abate MT, Kaur J, Suh H, et al. The use of drug-eluting stents in the management of transplant renal artery stenosis. Am J Transplant 2011;11:2235–41. 10.1111/j.1600-6143.2011.03652.x [DOI] [PubMed] [Google Scholar]