Abstract
Acute cerebellar ataxia is a rare primary manifestation of neuropsychiatric systemic lupus erythematosus (NPSLE). We report a case of a 22-year-old woman who presented with gait instability, behavioural changes and new-onset seizures. The tempo of disease progression was explained by an autoimmune cause, eventually fulfilling the criteria for systemic lupus erythematosus. The patient’s neurological symptoms improved markedly following administration of steroids and immunomodulators. A review of literature on cerebellar ataxia in NPSLE and a summary of all reported cases to date are also presented.
Keywords: neurology, epilepsy and seizures, brain stem / cerebellum, rheumatology, systemic lupus erythematosus
Background
Cerebellar ataxia as a manifestation of neuropsychiatric systemic lupus erythematosus (NPSLE) is rarely described. Less than 2% of patients with systemic lupus erythematosus (SLE) present with cerebellar involvement.1 Neurological symptoms attributed to SLE require thorough clinical evaluation, relevant laboratory testing and imaging studies. Establishing the diagnosis of NPSLE is vital since it carries significant morbidity if left untreated. Administering corticosteroids in the early stages of the disease is beneficial to patients with NPSLE by improving cerebellar signs.2
Case presentation
A 22-year-old, right-handed woman presented to the emergency department with gait instability. Prior to admission, she had a 1-month history of unsteady gait associated with easy fatigability. The patient also complained of persistent dull holocranial headache, visual hallucinations and disorientation for a week. This was followed by paraesthesias and clumsiness in manipulating objects. She had convulsive episodes with facial twitching and bruxism lasting for minutes, recurring every hour. The patient had no previous nor current symptoms of dysarthria, emotional or cognitive changes, rash, hair loss, mouth sores, chest pain, fatigue, joint pain or haematuria. There was no personal nor familial history of epilepsy or autoimmune disease.
On examination, the patient had no cranial nerve deficits, motor or sensory dysfunction nor signs of meningeal irritation. Further testing showed cerebellar signs of bilateral dysmetria, dysdiadochokinesia and leg dystaxia. She had an ataxic, wide-based gait and was unable to perform tandem walk.
Investigations
Initial blood tests including complete blood count, kidney function test, liver function test, hepatitis profile and human immunodeficiency virus (HIV) were unremarkable. Urinalysis was normal with no presence of protein, glucose, red blood cells, white cell count or cellular casts. Cranial computed tomography (CT) and chest radiograph did not show any abnormalities. An electroencephalogram revealed frequent multifocal epileptiform discharges in the frontocentrotemporal regions suggestive of multifocal irritative cerebral dysfunction (figure 1).
Figure 1.
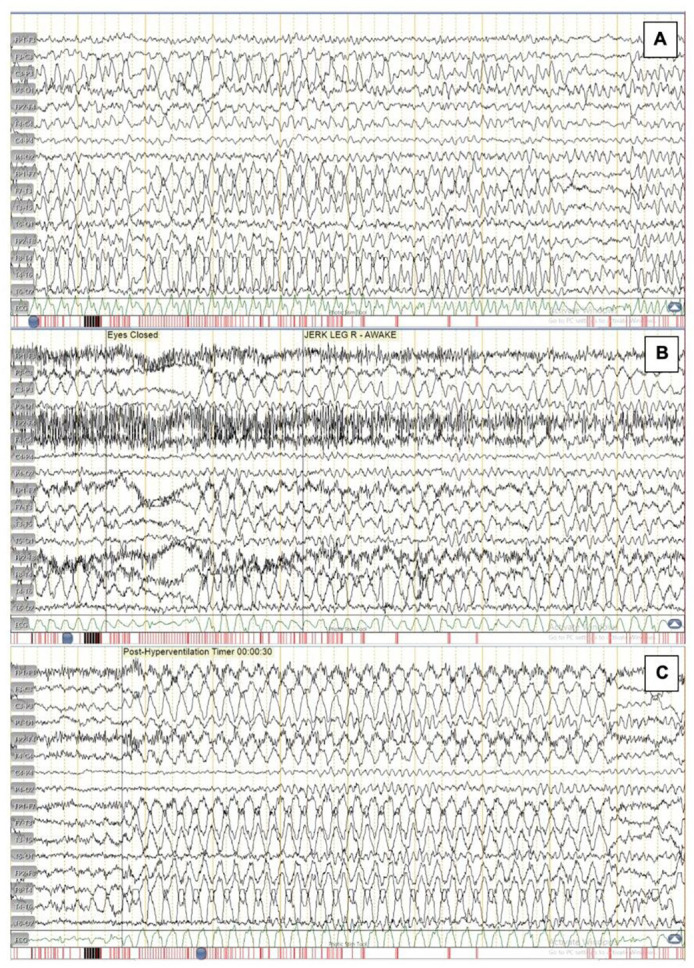
Electroencephalogram findings of a 22-year-old woman with (A) frequent polysharps occurring in runs in both the frontal and centrotemporal regions lasting for 7–10 s. (B) Jerking motion of the right leg was recorded associated with polysharps in the left frontotemporal region lasting for 1–2 s. (C) During and immediately after hyperventilation, there were runs of polysharps in the left frontocentrotemporal and right frontotemporal regions.
Cerebrospinal fluid (CSF) studies revealed normal opening pressure and cell counts. CSF Gram stain and culture, cryptococcal antigen test, tuberculosis panel, rapid plasma reagin and herpes simplex virus PCR all revealed unremarkable results. Serum HIV antigen/antibody was negative. Cranial magnetic resonance imaging (MRI) with gadolinium ruled out acute ischaemic infarct, parenchymal haemorrhage, mass lesion and demyelinating process. More specifically, the cranial MRI failed to reveal any lesion particularly in the posterior fossa that could support the findings of acute cerebellar ataxia. Ideally, a positron emission tomography or a functional MRI could be done to demonstrate areas of dysfunction.
An electromyography and a nerve conduction study were done to assess possible nerve damage and myopathy. The motor amplitudes and sensory potentials of the nerves were all normal, with no focal or widespread myopathic pattern.
In the absence of an infectious or vascular cause, the work-up for an autoimmune disease was pursued. Autoimmune encephalitis was considered; however, CSF anti-N-methyl-D-aspartate receptor antibody assay and serum anti-voltage gated potassium channel-complex were negative. The diagnosis of SLE was made based on history of ataxia and seizure, positive antibody antinuclear antibody (ANA) titre (1:160), positive anti-double-stranded DNA (anti-dsDNA) of 24.50 IU/mL, low C3 value of 851 mg/L and low C4 value of 4 mg/dL. Other immunological tests such as antiphospholipid antibody and extractable nuclear antigen (ENA) serologies were not performed. The patient thus fulfilled four of the 11 Systemic Lupus International Collaborating Clinics Classification (SLICC) criteria.3
Treatment
On establishing a working impression of NPSLE, prednisone was given at 50 mg/day. The patient was then sent home with hydroxychloroquine 200 mg/day and levetiracetam 1 g/day, under close monitoring.
Outcome and follow-up
On follow-up, the convulsions and cerebellar symptoms gradually subsided, but she continued to have intermittent headache until her last visit.
Discussion
The estimated prevalence of neuropsychiatric signs in SLE is between 12% and 95%.4 According to the 1999 American College of Rheumatology consensus statement for NPSLE, 19 neuropsychiatric syndromes have been defined. Further classification included diffuse psychiatric or neuropsychological manifestations and focal neurological syndromes. Localised central nervous system (CNS) involvement is represented by focal NPSLE.5 However, cerebellar symptoms or ataxia, such as those seen in our patient, has not been described previously as manifestations in the NPSLE spectrum.
The CNS is considered immunologically unique due to the presence of tightly regulated and highly restrictive blood–brain and blood–CSF barrier. Interestingly, the two brain regions preferentially targeted by autoimmunity are the limbic system and the cerebellum. Numerous immune-mediated diseases affect the cerebellum and the mechanisms for each differ. These may include paraneoplastic cerebellar degeneration, Miller Fisher syndrome, postinfectious cerebellitis and cerebellar ataxia associated with connective tissue diseases.5 Interestingly, cerebellar involvement in SLE occurs only in less than 2% of reported cases.1 6 Possible causes of cerebellar ataxia related to SLE are cerebral ischaemia, vasogenic oedema and antibody-mediated dysfunction.6 Because specific markers of cerebellar involvement in SLE have not been validated, a response to therapy can be accepted as a surrogate marker of immunopathogenesis.7
To our knowledge, the evaluation of pooled information regarding patients with NPSLE presenting as cerebellar ataxia has not been done in the literature. However, multiple case reports on the subject have been cited since 1988.8 The patients described are female and between 14 and 41 years old. These individuals were previously diagnosed with SLE, eventually developing cerebellar ataxia as a neuropsychiatric manifestation of the disease.1 8 9 Majority had imaging evidence, revealing a cerebellar infarct, solitary lesions in the junction between the pons and the medulla, or cerebellar atrophy. Most reports of cerebellar ataxia in SLE demonstrated response to immunosuppression with high-dose corticosteroid. Immunomodulators such as azathioprine, cyclophosphamide or hydroxychloroquine were also beneficial. Consequently, majority of patients had good outcomes with improvement of neurological symptoms after treatment.1 8 9
This is a rare case of a patient initially presenting with cerebellar ataxia without the classic symptoms of SLE who subsequently tested positive for ANA and anti-dsDNA. An ENA panel is recommended following a positive ANA test to diagnose an autoimmune disorder. A patient with SLE may have a positive anti-Smith, anti-SS-A (Ro), anti-SS-B (La) or ribonucleoprotein antibody.10 The patient eventually fulfilled the SLICC criteria and responded to treatment with prednisone and hydroxychloroquine.
Patients presenting with cerebellar dysfunction with no obvious cause should be investigated to consider SLE. We report a unique case of a 22-year-old woman who initially presented with symptoms of ataxia and new-onset seizures. A diagnosis of SLE was established after eliminating a vascular, infectious or other autoimmune causes. The patient had good clinical response after treatment with oral steroids and immunomodulators.
Learning points.
Neuropsychiatric systemic lupus erythematosus (NPSLE) presents as a broad range of neurological and psychiatric manifestations.
Cerebellar ataxia is a rare manifestation of NPSLE.
Ataxia in lupus may be caused by cerebral ischaemia, vasogenic oedema and antibody-mediated dysfunction.
Treatment with immunosuppression favours good clinical outcomes.
Footnotes
Twitter: @charmysy, @g_racaza
Contributors: MCCS conceived the idea and wrote the initial drafts and revisions of the manuscript. NGDR, GTZ and MLLF made substantial contributions to the revisions of the manuscript for intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Iwasaki Y, Okamoto A, Shoda H, et al. . Subacute cerebellar ataxia and atrophy developed in a young woman with systemic lupus erythematosus whose cerebrospinal fluid was positive for antineuronal cell antibody. Lupus 2012;21:324–8. 10.1177/0961203311418270 [DOI] [PubMed] [Google Scholar]
- 2.Mitoma H, Adhikari K, Aeschlimann D, et al. . Consensus paper: neuroimmune mechanisms of cerebellar ataxias. Cerebellum 2016;15:213–32. 10.1007/s12311-015-0664-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petri M, Orbai A-M, Alarcón GS, et al. . Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 2019;15:137–52. 10.1038/s41584-018-0156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlasson S, Wiseman S, Wardlaw J. Neurological disease in lupus: toward a personalized medicine approach. Front Immunol 2018:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baizabal-Carvallo JF, Bonnet C, Jankovic J. Movement disorders in systemic lupus erythematosus and the antiphospholipid syndrome. J Neural Transm 2013;120:1579–89. 10.1007/s00702-013-1023-z [DOI] [PubMed] [Google Scholar]
- 7.Tomita M, Holman BJ, Williams LS, et al. . Cerebellar dysfunction is associated with overexpression of proinflammatory cytokine genes in lupus. J Neurosci Res 2001;64:26–33. 10.1002/jnr.1050 [DOI] [PubMed] [Google Scholar]
- 8.Singh RR, Prasad K, Kumar A, et al. . Cerebellar ataxia in systemic lupus erythematosus: three case reports. Ann Rheum Dis 1988;47:954–6. 10.1136/ard.47.11.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RW, Ellison DW, Jenkins EA, et al. . Cerebellum and brainstem vasculopathy in systemic lupus erythematosus: two clinico-pathological cases. Ann Rheum Dis 1994;53:327–30. 10.1136/ard.53.5.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banhuk FW, Pahim BC, Jorge AS, et al. . Relationships among antibodies against extractable nuclear antigens, antinuclear antibodies, and autoimmune diseases in a Brazilian public hospital. Autoimmune Dis 2018;2018:1–8. 10.1155/2018/9856910 [DOI] [PMC free article] [PubMed] [Google Scholar]


