Abstract
Comorbidity between diabetes mellitus (DM) and depression, two chronic and devastating diseases spreading worldwide, has been confirmed by a large body of epidemiological and clinical studies. Due to the bidirectional relationship between DM and depression, this comorbidity leads to poorer outcomes in both conditions. Given the adverse effects and limited effectiveness of the existing therapies for depression associated with diabetes, the development of novel therapeutic drugs with more potency and fewer side effects is still the most important goal. Hence, many researchers have made great efforts to investigate the potential usefulness of traditional Chinese medicine (TCM) and natural products, including natural extracts and purified compounds, in the treatment of comorbid depression in diabetes. Here, we reviewed the related literature on TCM and natural products that can remedy the comorbidity of diabetes and depression and presented them on the basis of their mechanism of action, focusing on shared risk factors, including insulin resistance, oxidative stress and inflammation, and nervous disturbances. In short, this review suggests that TCM and natural products could expand the therapeutic alternatives to ameliorate the association between DM and depressive disorders.
Keywords: depression, diabetes, comorbidity, natural products, TCM
Introduction
Diabetes mellitus (DM) is a prevalent chronic metabolic disease characterized by high blood glucose levels, whereas depression is a mood disorder that is marked by a loss of interest, constant sadness, feelings of guilt or low self-worth, tiredness, disturbed sleep or appetite, and poor concentration (Koye et al., 2018; Lim et al., 2018; Lu et al., 2019). Statistics from the International Diabetes Federation (IDF) revealed that 451 million people lived with DM worldwide in 2017, and these figures are expected to reach 700 million by 2045 (Cho et al., 2018). Moreover, the accelerated pace of life and increased social pressure further lead to a fast-growing incidence of depression. The World Health Organization (WHO) projected that by the year 2030, diabetes would become the seventh leading cause of death worldwide and depression would rank first in global disease burdens (Lepine and Briley, 2011; Roshan and Stanton, 2013).
Considerable evidence supporting a close association between DM and depression has been published. On the one hand, pre-existing depression increases the risk of DM. As early as the 17th century, Thomas Willis, a famous British doctor and anatomist, hypothesized that long-term grief could sometimes cause diabetes. Strong epidemiological evidence provided by longitudinal studies has also indicated a high incidence of DM in individuals who previously developed depression (Knol et al., 2006; Engum, 2007; Golden et al., 2008). In terms of mechanism, the physiological changes elicited by depression (e.g., the release of counter-regulatory hormones, stimulation of sympathetic nervous system release, and altered inflammation state) can induce peripheral insulin resistance, which appears to be the main responsible for DM development (Champaneri et al., 2010). Moreover, poor behavioral factors associated with depression, such as lack of physical inactivity and high caloric intake, can partially contribute to the development of diabetes (Strine et al., 2008).
On the other hand, DM appears to increase the risk of incident or recurrent depression. For example, continuous hyperglycemia in diabetic patients can result in the activation of the hypothalamic-pituitary-adrenal (HPA) axis, which leads to endocrine disorders, characterized by the excess release of cortisol, a hormone that plays an important role in depression (Chiodini et al., 2006; Yi et al., 2010). In addition, several studies have suggested that diagnosed but not undiagnosed diabetes is significantly associated with depression, indicating that the burden of diabetes and/or the psychological stress experienced while coping with diabetes might relate to these depressive symptoms (Knol et al., 2007; Icks et al., 2008; Li et al., 2016). Likewise, epidemiologic studies have demonstrated that the incidence of depression in diabetic patients is two times higher than in the general population (Anderson et al., 2001). Although the relationship between DM and depression has been confirmed, compared with some widely studied complications associated with DM, including retinopathies and atherosclerosis, the comorbidity of diabetes and depression is greatly underestimated due to some circumstances, such as stealthiness, different syndrome types, and difficulties in the assessment of depression.
It should be noted that depression and diabetes each increases the risk of the other in a bidirectional interaction. A few systematic reviews and meta-analysis have estimated that depression accounts for a 60% increase in diabetes risk, and diabetes was estimated to account for a 24% increased risk of depression (Mezuk et al., 2008; Rubin et al., 2008; Yu et al., 2015; Khaledi et al., 2019; Nouwen et al., 2019). The comorbidity of DM and depression thus leads to higher medical care costs, worse prognosis, increased severity, decline in quality of life, and increased treatment resistance and mortality for both diseases (Pradeepa and Mohan, 2017).
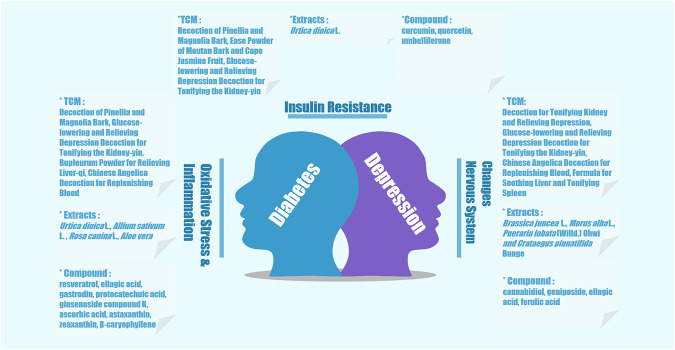
In case of comorbid depression with diabetes, a key therapeutic aim is to relieve depression and improve blood glucose levels (Zhang and Feng, 2016). Clinically, it is generally considered that comorbid diabetes and depression should be treated with a combination of hypoglycemic drugs with antidepressants (Li et al., 2018). Although antidepressants are employed for pharmacological interventions in DM, their effectiveness in the improvement of depressive symptoms is moderate (Petrak et al., 2015). Apart from the documented adverse reactions like cardiometabolic effects, a large number of antidepressant agents, such as mirtazapine, can increase appetite in patients and result in a high risk of weight gain, which is not conducive to the control of blood sugar (Gill et al., 2020). Therefore, it is urgent to explore and discover novel agents with better efficacy and fewer adverse reactions. Given the multicomponent and multitarget characteristics of traditional Chinese medicine (TCM) as well as the value of natural products (natural extracts and single isolated compounds) for drug discovery, a growing body of research has investigated and established therapeutic effects and related mechanisms of multiple TCM interventions and natural products on diabetic depression (Zheng et al., 2020). We summarized these TCM and natural products and their effects against the comorbidity of diabetes and depression (Tables 1–3 and Figure 1) and classified them according to their mechanism of action, as outlined below.
TABLE 1.
TCM formulae with effects on depression associated with diabetes.
| TCM-preparation | Botanical drugs included | Species | Action | References |
|---|---|---|---|---|
| Decoction of Pinellia and Magnolia Bark | Pinelliae, officinal magnolia bark, Poria cocos, fresh ginger, Folium Perillae | Pinellia ternate (Thunb.) Makino, Magnolia officinalis Rehd. et Wils., Smilax glabra Roxb., Perilla frutescens (L.) Britt., Zingiber officinale Rosc. | 1) Suppresses NLRP3 inflammasome activation. | Jia et al. (2017) |
| 2) Improves peripheral insulin signaling impairment in the liver and brain in CUMS rats. | ||||
| Bupleurum Powder for Relieving Liver-qi | Pericarpium Citri Reticulatae, Radix Bupleuri, Rhizoma Ligustici Chuanxiong, Rhizoma Cyperi, Fructus Aurantii, Paeonia lactiflora, Radix Glycyrrhizae. | Bupleurum chinense DC., Citrus reticulata Blanco, Ligusticum striatum DC., Cyperus rotundus L., Citrus aurantiumL., Paeonia lactiflora Pall., Glycyrrhiza glabra L. | 1) Suppresses the TLR4/MyD88/NF-κB pathway and NLRP3 inflammasome activation, and improves insulin signaling in CUMS rats. | Jia et al. (2018) |
| Chinese Angelica Decoction for Replenishing Blood | Radix Angelicae Sinensis, Radix Astragali | Angelica sinensis (Oliv.) Dielss, Astragalus mongholicus Bunge | 1) Inhibits systemic inflammatory response by reducing serum levels of inflammatory factors such as TNF-α, CRP, IL-6 and IL-8 | Wang et al. (2018), Wang et al. (2020a), Wang et al. (2020b) |
| 2) Improves the depression-like behavior of Goto-Kakizaki rats, achieves hippocampal protection, and its antidepressant mechanism involves the CREB/BDNE/TrkB pathway. | ||||
| Ease Powder of Moutan Bark and Cape Jasmine Fruit | Radix Bupleuri, Radix Angelicae Sinensis, Paeonia lactiflora, Poria cocos, Rhizoma Atractylodis Macrocephalae, Radix Glycyrrhizae, Moutan Bark, Cape Jasmine | Bupleurum chinense DC., Angelica sinensis (Oliv.) Dielss, Paeonia lactiflora Pall, Smilax glabra Roxb., Atractylodes lancea (Thunb.) DC., Glycyrrhiza glabra L., Paeonia suffruticosa Andr., Gardenia jasminoides Ellis | 1) Regulates the IRS2-PI3K signaling pathway in liver tissues. | Wang et al. (2013), Zhang et al. (2017) |
| Formula for Soothing Liver and Tonifying Spleen | Radix Bupleuri, Poria cocos, Radix Astragali, Rhizoma Acori Tatarinowii, Radix Salviae Miltiorrhizae, Huai wheat | Bupleurum chinense DC., Smilax glabra Roxb., Astragalus mongholicus Bunge, Acorus calamus var. angustatus Besser, Salvia miltiorrhiza Bunge, Triticum aestivum L. | 1) Upregulates the expression of BDNF mRNA and protein levels to improve the depressive symptoms associated with diabetes mellitus | Lei et al. (2017) |
| Decoction for Tonifying Kidney and Relieving Depression | Radix Rehmanniae Preparata, Fructus Corni, Fructus Schisandrae Chinensis, Radix Bupleuri, Radix Paeoniae Alba, Rhizoma Acori Tatarinowii, Semen Ziziphi Spinosae. | Rehmannia glutinosa (Gaertn.) DC., Cornus officinalis Siebold & Zucc., Schisandra chinensis (Turcz.) Baill., Bupleurum chinense DC., Paeonia lactiflora Pall., Acorus tatarinowii Schott, Ziziphus jujuba Mill. | 1) Decreases cortisol level and improves dysfunction of the HPA axis in patients with type 2 diabetes and depression. | Lu (2012) |
| Glucose-lowering and Relieving Depression Decoction for Tonifying the Kidney-yin | Radix Astragali, Radix Rehmanniae Preparata, Fructus Corni, Barbary Wolfberry, Semen Cuscutae, Cortex Eucommiae, Radix Salviae Miltiorrhizae, Cortex Moutan, Radix Achyranthis Bidentatae, Rhizoma Curcumae Longae, Fructus Forsythiae | Astragalus mongholicus Bunge, Rehmannia glutinosa (Gaertn.) DC., Cornus officinalis Siebold & Zucc., Lycium barbarum L., Cuscuta chinensis Lam, Eucommia ulmoides Oliv., Salvia miltiorrhiza Bunge, Paeonia suffruticosa Andr., Achyrantes bidentata Bl., Curcuma longa L., Hypericum perforatum L. | 1) Improves insulin resistance. | Wang et al. (2014), Wang et al. (2015), Yang et al. (2018), Yang et al. (2019), Yang et al. (2020) |
| 2) Decreases the expression of IL-1β and TNF-α. | ||||
| 3) Reduces hippocampal CORT expression by inhibiting the expression of 11𝛽-HSD1 and increasing the levels of GR in the hippocampus. |
TABLE 3.
Natural compounds with effects on depression associated with diabetes.
| Natural compounds | Plant source | Action | References |
|---|---|---|---|
| Ascorbic acid | Citrus sinensis, Actinidia chinensis Planch. | 1) Ameliorates oxidative stress and inflammatory response by reducing catalase levels, increasing SOD content and CAT activity and IL-10 levels in the prefrontal cortex of diabetic rats. | Shivavedi et al. (2019) |
| Astaxanthin | Haematococcus pluvialis, Oncorhynchus tschawytscha | 1) Downregulates the expression of IL-6, IL-1β, and COX-2 in the hippocampus. | Zhou et al. (2017) |
| β-Caryophyllene | Syzygium aromaticum (L.) Merr. and L.M. Perry, Piper nigrum L., Salvia rosmarinus Spenn. | 1) Inhibits of inflammation/cytokines such as IL-1β, TNF-α, and IL-6 | Aguilar-Avila et al. (2019) |
| Cannabidiol | Cannabis sativa L. | 1) Activates the serotonergic system through 5-HT1A receptors | de Morais et al. (2018); Jesus et al. (2019) |
| Curcumin | Curcuma longa L. | 1) Improves insulin sensitivity, upregulates the phosphorylation of IRS-1 and AKT, and inhibits GSK-3β, PEPCK and glucose 6-phosphatase. | Shen et al. (2017) |
| Ellagic acid | Fragaia ananassa (Duchesne ex Weston) | 1) Modulates inflammation status and BDNF expression. | Farbood et al. (2019) |
| Gastrodin | Gastrodia elata Bl. | 1) Inhibits ER stress and NLRP3 inflammasome activation in db/db mice. | Ye et al. (2018) |
| Geniposide | Gardenia jasminoides J.Ellis | 1) Enhances BDNF expression in the hippocampus of streptozotocin-evoked diabetic mice | Wang et al. (2016) |
| Ginsenoside compound K | Panax ginseng C. A. Mey. | 1) Inhibits ER stress and the NLRP3 inflammasome pathway | Li et al. (2020) |
| Protocatechuic acid | Salvia miltiorrhiza Bunge | 1) Suppresses oxidative damage, inflammation, and the activities of caspase-3 and acetylcholinesterase in diabetic rats. | Adedara et al. (2019) |
| Quercetin | Euonymus alatus (Thunb.) Siebold, Rosa canina L., Allium cepa L. et al. | 1) Upregulates GLUT4 expression. | Anjaneyulu et al. (2003) |
| Resveratrol | Polygonum cuspidatum Sieb. and Zucc., Paeonia lactiflora Pall., Vatica rassak (Korth.) Blume | 1) Inhibits oxidative stress. | Jing et al. (2013); Sahin et al. (2019) |
| 2) Reduces astrocytic activation as well as TNF-α and IL-6 transcripts in the hippocampus of diabetic rats. | |||
| Umbelliferone | Daucus carota L., Coronilla varia L., Ruta graveolens L. | 1) Attenuates CUMS-induced-insulin resistance in rats | Su et al. (2016) |
| Zeaxanthin | Spinacia oleracea L., Brassica oleracea L. | 1) Reduces the excessive production of IL-6, IL-1β, and TNF-α | Zhou et al. (2018) |
FIGURE 1.
Schematic of TCM and natural products against comorbid depression with diabetes and their related mechanism.
TCM and Natural Products with Effects on Insulin Resistance
Insulin Resistance and Comorbid Depression with Diabetes
Insulin resistance caused by the decrease in insulin sensitivity disrupts the entry of glucose into muscle, liver, and adipose tissue, which eventually leads to diabetes. Physiologically, the interaction of insulin with the insulin receptor (InsR) on the cell membrane activates an intrinsic tyrosine protein kinase, which autophosphorylates the receptor as well as downstream insulin receptor substrates (IRSs) (Jung and Choi, 2014). The phosphorylation of IRS proteins on tyrosine residues activates downstream PI3K-AKT signaling, which alters the activity or expression of several key factors involved in glucose metabolism and glycogen/lipid/protein synthesis, such as glycogen synthase kinase (GSK)-3β, regulatory element-binding protein-1c (SREBP1-c), and glucose transporter (GLUT) translocation, especially GLUT4 (Beale, 2013). The insulin-mediated IRS-PI3K-AKT signaling pathway is therefore a key pathway affecting the metabolic effects of insulin and is usually dysregulated in people with diabetes (Yaribeygi et al., 2019). Moreover, impairment of insulin signaling is also present in high-fat diet (HFD)-induced diabetic rats with depressive-like behavior (Papazoglou et al., 2015). Furthermore, a meta-analysis of 21 studies found a statistically significant association between depression and insulin resistance, and this positive association suggests a biological link between depression and DM (Kan et al., 2013). Insulin receptors are expressed throughout the brain, and several studies have indicated the ability of insulin to affect the nervous system (Porte et al., 2002; Banks et al., 2012). The knockdown of insulin receptors in the hypothalamus of rats triggers depressive behaviors (Kleinridders et al., 2015). Consistently, insulin administration improves working memory in both human and animal studies (Gupta et al., 2014a; Zhao et al., 2019), confirming that insulin is involved in hippocampal neurogenesis linked with depression.
Considering that insulin resistance is a shared metabolic abnormality among many individuals with DM and depression, repurposing therapies approved for treating insulin resistance might be useful in the treatment of comorbid DM and depression (Hamer et al., 2019). A clinical study of 488 patients with major depression found that selective agonists of the nuclear transcription factor peroxisome proliferator-activated receptor-γ (PPAR-γ), which are used for the treatment of insulin resistance-related diabetes, exhibit significant antidepressant properties by virtue of their ability to ameliorate insulin resistance and glucose tolerance (Colle et al., 2017). Further, the classic antidiabetic drug metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice (Zemdegs et al., 2019), suggesting that the management of depression-associated diabetes through targeting insulin resistance is effective and practicable.
TCM and Natural Products for Treating Insulin Resistance Associated with Comorbid Depression and Diabetes
Decoction of Pinellia and Magnolia Bark (Banxia-Houpu decoction) is a well-known formula of TCM consisting of Pinellia ternata (Thunb.) Makino, Magnolia officinalis Rehd. et Wils. , Smilax glabra Roxb., Perilla frutescens (L.) Britt., and Zingiber officinale Rosc. In chronic unpredictable mild stress (CUMS) rats, decoction of Pinellia and Magnolia Bark has been found to regulate InsR/IRS1/AKT insulin signaling, to decrease the level of serum corticosterone (CORT), and to improve glucose tolerance in both peripheral liver and brain regions (Jia et al., 2017). Clinical trials have showed that Ease Powder of Moutan Bark and Cape Jasmine Fruit (Danzhi Xiaoyao powder, DZXYP) has the ability to regulate glucose and improve the symptoms of depression and insomnia (Zhang and Xiao, 2008). Likewise, a meta-analysis of DZXYP for type 2 DM complicating depression suggested that treatment with DZXYP improved fasting blood glucose, HbA1c, Hamilton depression scale score, and self-rating depression scale score (Zhang et al., 2017). Mechanism research revealed that DZXYP alleviates diabetes-associated depression through the IRS2-PI3K signaling pathway in the liver tissues of rats (Wang et al., 2013). Two other studies verified the hypoglycemic and antidepressant effects of Glucose-Lowering and Relieving Depression Decoction for Tonifying the Kidney-yin (Zuogui Jiangtang Jieyu prescription, ZGJTJY) (Wang et al., 2014; Yang et al., 2020), showing that ZGJTJY can effectively regulate the insulin signaling pathway and improve insulin resistance in the hippocampus of rats with diabetes-related depression (Yang et al., 2020). In line with these observations, the hydroalcoholic extract of Urtica dioica L. leaves was found to reverse the decline in hippocampal GLUT4 expression and plasma CORT induced by dexamethasone, which correlated with its ability to improve hyperglycemia and associated depressive-like behavior (Patel and Udayabanu, 2014) (Tables 1, 2; Figure 1).
TABLE 2.
Natural extracts with effects on depression associated with diabetes.
| Medicinal plant | Active parts | Action | References |
|---|---|---|---|
| Allium sativum L. | Homogenate | 1) Attenuates brain MDA levels and increases SOD and GPx activities. | Rahmani et al. (2020) |
| Aloe vera | Mucilaginous | 1) Increases activities of hippocampal SOD and CAT and reduces MDA levels in the hippocampus tissue of STZ-induced diabetic rats | Tabatabaei et al. (2017) |
| Brassica juncea L. | Leave | 1) Increases dopamine, norepinephrine, and serotonin in the brain. | Thakur et al. (2014) |
| Morus alba L. | Root bark | 1) Restores BDNF levels in the prefrontal cortex through ERK and Akt signaling. | Ye et al. (2017) |
| Pueraria lobata (Willd.) Ohwi and Crataegus pinnatifida Bunge | Whole | 1) Promotes BDNF expression and ERK activation to prevent depression in a diabetic rat model. | Luo et al. (2016) |
| Rosa canina L. | Fruits | 1) Attenuates impairment of recognition memory and depressive-like behavior through the modulation of oxidative stress in the STZ model of diabetes in the mouse brain. | Farajpour et al. (2017) |
| Urtica dioica L. | Leaves | 1) Improves hippocampal GLUT4 mRNA expression. | Patel and Udayabanu (2014); Patel et al. (2018) |
| 2) Upregulates BDNF, TrKB, Cyclin D1, Bcl2, and autophagy, downregulates iNOS mRNA expression in the hippocampus of diabetic mice, and decreases the expression of TNF-α in hippocampus of diabetic mice. |
A large number of monomeric compounds possess hypoglycemic and antidepressant properties via different mechanisms. Among them, curcumin and quercetin are representative purified compounds capable of improving insulin resistance. Curcumin has been reported to enhance insulin sensitivity and increase the hepatic glycogen content by upregulating the phosphorylation of IRS-1 and AKT and inhibiting GSK-3β in rats with depression complicated by insulin resistance using a 12-week exposure to chronic mild stress, which were conducive to reversing the metabolic abnormalities and depressive-like behaviors (Shen et al., 2017). In the Porsolt forced swimming-induced behavioral despair test, quercetin treatment was found to reduce the immobility period and to reverse depressive-like behavior in a dose-dependent manner in streptozotocin (STZ)-induced diabetic mice (Anjaneyulu et al., 2003). Further study has indicated that quercetin administration in male Swiss albino mice can significantly attenuate insulin resistance and elevate hippocampal GLUT4 levels (Mehta et al., 2017). Similarly, umbelliferone treatment (20 and 40 mg/kg, for 3 weeks) in CUMS-induced rats effectively improved depression symptoms and maintained blood glucose balance. Western blot analysis demonstrated that umbelliferone administration markedly increases the phosphorylation of InsR, IRS-1, AKT, PI3K, and GSK-3β, suggesting an ameliorative effect of umbelliferone against insulin resistance (Su et al., 2016) (Table 3; Figure 1).
TCM and Natural Products Linked with Oxidative Stress and Inflammation
The Role of Oxidative Stress and Inflammation in Comorbid Depression with DM
Oxidative stress and inflammation interact in the pathophysiology of both DM and depression. For instance, the elevated release of ROS, a characteristic of oxidative stress, not only causes direct damage to the insulin-producing pancreatic β-cells but also results in inflammatory processes, immune activation, increased oxidation of monoaminergic neurotransmitters, and lipid peroxidation, all of which correlate with DM and depression (Gerber and Rutter, 2017; Forrester et al., 2018; Reus et al., 2019). Similarly, increased concentrations of cytokines resulting from inflammatory processes also lead to the apoptosis of pancreatic β-cells and insulin resistance as well as enhanced oxidative stress in the brain and activation of the HPA axis and the tryptophan-kynurenine pathway, contributing to the risk of coexistence of diabetes and depression symptoms (Moulton et al., 2015). There is evidence that patients with diabetes or depression have significantly increased levels of oxidative stress markers including malondialdehyde (MDA), F2-isoprostanes, and 8-OH 2-deoxyguanosine (8-OHdG), as well as proinflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). In addition, decrease in the content or activity of important antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT), has also been observed (Myint et al., 2005; Ballak et al., 2015; Lindqvist et al., 2017; Luc et al., 2019).
Moreover, injection of cytokines in animals and humans can induce depression-like symptoms and diabetes (Horikawa et al., 2003; Sauter et al., 2015; Abdul Aziz et al., 2016). Consistent with these findings, clinical trials have indicated that nonsteroidal anti-inflammatory drugs (NSAIDs) and cytokine inhibitors may yield antidepressant effects (Kohler et al., 2016). Treatment with antioxidants including N-acetylcysteine and deferoxamine ameliorates diabetes-induced depressive-like behavior (Reus et al., 2016). Thus, blockade of oxidative stress and targeting the proinflammatory signaling pathway might be potential therapeutic strategies for comorbid depression in diabetes.
TCM and Natural Products with Antioxidative Stress and Inflammation Effects against Comorbid Depression and Diabetes
The antioxidative stress and anti-inflammatory activities of TCM and natural extracts and compounds have been widely explored. In this sense, decoction of Pinellia and Magnolia Bark was found to inhibit NLRP3 inflammasome activation in a number of tissues, such as the liver, hypothalamus, hippocampus, and prefrontal cortex, which contributes to the prevention of hyperglycemia and depressive-like behavior in CUMS rats (Jia et al., 2017). The ZGJTJY treatment mentioned above was found to reduce IL-1β and TNF-α levels in the hippocampus in the rat model of diabetes complicated by depression induced by high-fat emulsion, injection of STZ via coccygeal vein, and chronic stress (Yang et al., 2018). Inspired by a meta-analysis showing the effectiveness and safety of Bupleurum Powder for Relieving Liver-qi (Chaihu-Shugan San, CHSGS) in the clinical treatment of depression (Wang et al., 2012b), a recent study by Jia et al. further confirmed that CHSGS improves glucose tolerance in the rat model of depression. Briefly, this formula was found to improve insulin signaling and to suppress both the TLR4/MyD88/NF-κB pathway and the activation of NLRP3 inflammasome, thereby reducing blood glucose levels and ameliorating depressive-like behaviors through inhibiting liver-brain inflammation axis (Jia et al., 2018). The previous study established a relatively precise effect of the Chinese Angelica Decoction for Replenishing Blood (Danggui-Buxue decoction, DBD) on DM with depression (Wang et al., 2018), and investigation of the mechanisms showed that DBD reduces serum levels of inflammatory factors such as TNF-α, CRP, IL-6, and IL-8, thereby inhibiting the systemic inflammatory response and achieving the positive regulation of DM complicated with depression (Wang et al., 2020a) (Table 1; Figure 1).
In the same way, hydroalcoholic extracts of Rosa canina L. fruit, an Iranian traditional medicinal herb with antioxidant activities, were found to enhance the attenuation of depressive-like behavior and recognition memory impairment through regulation of the oxidative stress marker MDA and total antioxidant capacity (TAC) in diabetic mice (Sadigh-Eteghad et al., 2011; Farajpour et al., 2017). Likewise, Aloe vera gel was found to increase activities of hippocampal SOD and CAT and to reduce MDA levels in the hippocampus tissue of STZ-induced diabetic rats, thus improving oxidative status and behavioral deficits (Tabatabaei et al., 2017). In STZ-induced diabetic rats, garlic (Allium sativum L.) (0.5 g/kg, gavage, 10 days) treatment also decreased the total duration of immobility, attenuated MDA levels, and increased SOD and glutathione peroxidase (GSH-Px) activities, indicating that garlic alleviates depression-related behaviors partly by attenuating brain oxidative stress (Rahmani et al., 2020). Additionally, extracts of Urtica dioica L. were proven to be effective for anxiety- and depressive-like behavior mediated by DM via decreasing the expression of TNF-α in the hippocampal area (Patel et al., 2018) (Table 2; Figure 1).
Numerous phytochemical molecules with various pharmacological effects exhibit antidepressant activity and improve glucose tolerance through the modulation of oxidative stress and/or inflammatory response. For instance, the anxiolytic-like and neuroprotective effects of resveratrol, a stilbenoid isolated from multiple medicinal herbs, correlate with its ability to strengthen the antioxidant action of SOD enzymes and to attenuate the expression of TNF-α, IL-6, and NF-κB in the hippocampus of diabetic rats (Jing et al., 2013; Sahin et al., 2019). Two phenolic compounds, ellagic acid and protocatechuic acid, were able to suppress inflammatory biomarkers and ameliorate diabetes-induced cognitive deficits reflected by the improvement of locomotor activity (Adedara et al., 2019; Farbood et al., 2019). In diabetic db/db mice, gastrodin administration and treatment with ginsenoside compound K were found to significantly attenuate blood glucose levels and to reverse behavioral impairment and cognitive dysfunction due to the inhibitory effects of these compounds on endoplasmic reticulum (ER) stress and NLRP3 inflammasome activation in the hippocampus (Ye et al., 2018; Li et al., 2020). Similar outcomes also have been observed for several molecules from medicinal and edible plants. Naveen Shivaved et al. revealed that ascorbic acid, which is abundant in fresh fruits and vegetables, ameliorates oxidative stress and inflammatory response by reducing catalase levels and increasing SOD content, CAT activity, and IL-10 levels in the prefrontal cortex of diabetic rats, indicating the therapeutic potential of ascorbic acid against diabetes comorbid depression (Shivavedi et al., 2019). In addition, studies by Zhou et al. showed that carotenoids, including astaxanthin and zeaxanthin, downregulate IL-6 and IL-1β in the hippocampus and protect neurons from hyperglycemic damage, implicating that depression can be prevented by astaxanthin and zeaxanthin through the inhibition of hippocampal inflammation in diabetic mice (Zhou et al., 2017; Zhou et al., 2018). Finally, β-caryophyllene, a natural sesquiterpene found in some food condiments, was proved to attenuate the expression of cytokines and depressive-like behavior in experimental diabetic mice subjected to the marble test, forced swim test, and tail suspension test (Aguilar-Avila et al., 2019) (Table 3; Figure 1).
TCM and Natural Products with Effects on Changes in Nervous System
Nervous System Changes and Comorbid Depression with Diabetes
In patients with DM and depressive disorders, a wide variety of disturbances may affect the central nervous systems, including the overactivation of the HPA axis, decreased monoamine neurotransmitters, and dysfunctional brain-derived neurotrophic factor (BDNF) (Donato, 2012; Biessels and Reijmer, 2014). Firstly, patients with diabetic depression present significant HPA axis dysfunction as well as higher levels of cortisol than control patients (Joseph and Golden, 2017). Hyperactivity of the HPA axis results in elevated levels of cortisol, which impairs the ability of insulin to transfer the intracellular glucose transporter GLUT4 to cell membrane and hampers neurogenesis in the hippocampus (Coderre et al., 1996; Moulton et al., 2015; Shirazi et al., 2015). Therefore, the chronic exposure to excess cortisol eventually leads to insulin resistance and concomitant metabolic syndromes including type 2 diabetes as well as mental disorders including depression (Rhyu et al., 2019). Furthermore, the decreased or insufficient secretion of monoamine transmitters in the brain, such as norepinephrine (NE), dopamine (DA), γ-aminobutyric (GABA), and serotonin (5-HT), is also a major cause of comorbid diabetic depression (Hashim et al., 2016; Ma et al., 2019; Mukherjee and Chaturvedi, 2019; Zhang et al., 2020). Experimental studies have revealed that impairment of 5-HT causes nerve cell damage and decreases neurogenesis, leading to anxiety and depression (Mahar et al., 2014; Yohn et al., 2017). In addition to acting as a neurotransmitter that regulates the function of the central nervous system, 5-HT also exerts multiple physiological functions in the periphery, and several studies have revealed the relationship between the peripheral 5-HT system and some diseases including type 2 diabetes (Fu et al., 2018). Two studies by Gupta et al. showed the counteractive effect of 5HT3 receptor antagonists against diabetes-induced depressive phenotypes in STZ-induced diabetic mice, further revealing the implication of the serotonergic system in diabetic depression (Gupta et al., 2014b; Gupta et al., 2016). As a neurotrophic factor, BDNF promotes the proliferation, remodeling, and regeneration of nerve cells and thus regulates neuroplasticity. Serum BDNF concentrations have been found to be decreased in patients with depression and diabetes (Karege et al., 2005; Krabbe et al., 2007). Convincing evidence supports the view that BDNF plays an important role in the pathogenesis of depression and diabetes.
Briefly, BDNF may improve glucose metabolism and reduce food consumption by modulating the production of insulin, leptin, ghrelin, neurotransmitters/neuropeptides, and proinflammatory cytokines associated with energy homeostasis. Clinical studies have shown that depressive states could be reversed by administration of BDNF (Wang et al., 2012a). Moreover, BDNF is positively regulated by the ER chaperon sigma-1 receptor (S1R), and studies have shown that fluvoxamine, a S1R agonist, ameliorates depression-like behaviors in DM-induced depression (Lenart et al., 2016). In short, the repair of the nervous system serves as a therapeutic regimen for the prevention of DM-associated depression.
TCM and Natural Products Targeting Nervous System Changes Associated with Comorbid Depression and Diabetes
As mentioned above, changes in the nervous system are critical for the development of diabetic depression. From this perspective, Decoction for Tonifying Kidney and Relieving Depression (Yishen Jieyu decoction) improves HPA axis function and decreases the cortisol level in patients with DM-related depression and TCM syndrome, which is diagnostic of kidney deficiency and liver-qi stagnation (Lu, 2012). HPA axis disorder also causes an abnormal increase in CORT in rodents, which is closely related to two essential proteins expressed in the hippocampus, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), and glucocorticoids (GR) (Wang et al., 2015; Meijer et al., 2018). The aforementioned ZGJTJY (10.26 g/kg) also increases motor activities and improves cognitive ability in rats with diabetes-related depression by increasing the expression of GR and decreasing 11β-HSD1 (Wang et al., 2015; Yang et al., 2019). Taking into consideration the observed effectiveness of Formula for Soothing Liver and Tonifying Spleen (Shugan Jianpi formula, SGJP) in the treatment of diabetes-related depression (Wang, 2008; Li, 2017), a recent study by Lei et al. indicated that SGJP improves depressive symptoms in CUMS and STZ-induced rats by upregulating the expression of BDNF mRNA and protein (Lei et al., 2017). Similarly, BDNF elevation is yet another mechanism of DBD against comorbid depression and diabetes. In CUMS-induced spontaneous diabetic rats, DBD (4.0 g/kg) and its main active ingredient ferulic acid (1.36 mg/kg) could increase serum and hippocampal BDNF levels to improve depression-like behavior, an effect that might relate to the activation of the CREB/TrkB signaling pathway (Wang et al., 2020b) (Table 1; Figure 1).
Likewise, the methanolic extract of Brassica juncea leaves (100, 200, and 400 mg/kg/day, p.o.) has been found to display antidepressant activity in alloxan monohydrate-induced diabetic rodents and to compensate for the low levels of DA, NE, and 5-HT in the brain of diabetic rats (Thakur et al., 2014). Cannabidiol (CBD), a non-psychotomimetic compound derived from Cannabis sativa, has been identified as a promising small molecule for the treatment of psychiatric disorders. It is reported that CBD might be effective in the treatment of diabetic depression, and this effect seems to be mediated by the activation of the serotonergic system through the 5-HT1A receptor (de Morais et al., 2018; Jesus et al., 2019). In the diabetic rat model induced by high-fat diet and low-dose STZ, treatment of Morus alba L. and the combination of Pueraria lobata (Willd.) Ohwi and Crataegus pinnatifida Bunge could reduce blood glucose levels and attenuate depressive-like behaviors, which might be partially ascribed to the upregulated BDNF expression in the prefrontal cortex (Luo et al., 2016; Ye et al., 2017). In addition, restoration of BDNF is also involved in the geniposide- and ellagic acid-mediated alleviation of depression-like behavior in STZ-evoked diabetic mice (Wang et al., 2016; Farbood et al., 2019) (Tables 2, 3; Figure 1).
Conclusion
Recently, some hospital-based cross-sectional studies have further clarified the comorbidity between DM and depression (Khassawneh et al., 2020; Tusa et al., 2020). In this review, we summarized TCM formulae and natural products with antidiabetic depression activities as well as several mechanisms related to their activities. Although the TCM and natural bioactive products discussed here greatly expand the spectrum of potential candidates for the prevention and treatment of the comorbidity of depression with DM, their efficacy should be further evaluated in randomized, double-blinded, and placebo-controlled clinical trials. Moreover, the active components in TCM or natural extracts, such as ZGJTJY and hydroalcoholic extract of Urtica dioica leaves, remain unclear in the literature. More importantly, the underlying mechanisms in most studies were only partially understood and mainly focused on changes in signaling pathways (Jia et al., 2018), cytokines and neurotransmitters content (Thakur et al., 2014; Aguilar-Avila et al., 2019; Farbood et al., 2019), and antioxidant enzyme activity (Rahmani et al., 2020). The target(s) with which the bioactive compounds interact and how the changes are triggered should be studied in depth. For example, omics technologies and network pharmacology might be helpful for the identification or prediction of the target of purified compounds or active ingredients in TCM or natural extracts, which may in turn enhance our understanding of the molecular mechanisms underlying the comorbidity between DM and depression. In addition, some studies on natural extracts and purified compounds from medicinal and edible plants suggest the potential of diet or nutritional therapy as an adjuvant for treating depression commonly associated with diabetes (Shen et al., 2017; Shivavedi et al., 2019; Rahmani et al., 2020). Overall, this review provides an important theoretical basis for therapeutic alternatives and potential intervention strategies to ameliorate comorbid DM and depression and broadens the scope of knowledge to guide future studies on this subject.
Author Contributions
YL, TA, and KM were involved in the development of the subject matter, drafting of the article, design of the figure, critical revision of the article, and final approval of the version to be published; HT and XG were involved in critical revision of the article and final approval of the version to be published; SW and FW were involved in the development of the subject matter, drafting of the article, critical revision of the article, and final approval of the version to be published. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by the Shandong Provincial Natural Science Foundation (ZR2020QH329, ZR2019BH027 and ZR2019ZD23), Shandong Province Universities’ Development Plan for Youth Innovation Teams (2019-9-202, 2019-201, and 2019KJK013), National Nature Science Foundation of China (81903948), and Shandong Province University Scientific Research Project (J18KZ014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Z Zhang for a thoughtful review of the content related to Chinese medicine.
Glossary
- 11β-HSD1
11β-hydroxysteroid dehydrogenase type 1
- 5-HT
serotonin
- 8-OHdG
8-OH 2-deoxyguanosine
- BDNF
brain-derived neurotrophic factor
- CAT
catalase
- NSAIDs
nonsteroidal anti-inflammatory drugs
- CBD
cannabidiol
- CHSGS
Chaihu-Shugan San or Bupleurum Powder for Relieving Liver-qi
- CORT
corticosterone
- CUMS
chronic unpredictable mild stress;
- DA
dopamine
- DBD
Danggui-Buxue decoction or Chinese Angelica Decoction for Replenishing Blood
- DM
diabetes mellitus
- DZXYP
Danzhi Xiaoyao powder or Ease Powder of Moutan Bark and Cape Jasmine Fruit
- ER
endoplasmic reticulum
- GLUT
glucose transporter
- GR
glucocorticoid
- GSH-Px
glutathione peroxidase
- GSK
glycogen synthase kinase
- HFD
high-fat diet
- HPA
hypothalamic-pituitary-adrenal
- IDF
International Diabetes Federation
- IL-1
interleukin-1
- IL-6
interleukin-6
- InsR
insulin receptor
- IRS
insulin receptor substrates
- MDA
malondialdehyde
- NE
norepinephrine
- PPAR-γ
peroxisome proliferator-activated receptor-gamma;
- S1R
sigma-1 receptor
- SGJP
Shugan Jianpi formula or Formula for Soothing Liver and Tonifying Spleen
- SOD
superoxide dismutase
- SREBP1-c
sterol regulatory element-binding protein-1c
- STZ
streptozotocin
- TAC
total antioxidant capacity
- TCM
traditional Chinese medicine
- TNF-α
tumor necrosis factor-alpha
- WHO
World Health Organization
- ZGJTJY
Zuogui Jiangtang Jieyu prescription
References
- Abdul Aziz S. H., John C. M., Mohamed Yusof N. I., Nordin M., Ramasamy R., Adam A., et al. (2016). Animal model of gestational diabetes mellitus with pathophysiological resemblance to the human condition induced by multiple factors (nutritional, pharmacological, and stress) in rats. BioMed Res. Int. 2016, 9704607 10.1155/2016/9704607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adedara I. A., Fasina O. B., Ayeni M. F., Ajayi O. M., Farombi E. O. (2019). Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem. Toxicol. 125, 170–181. 10.1016/j.fct.2018.12.040 [DOI] [PubMed] [Google Scholar]
- Aguilar-Avila D. S., Flores-Soto M. E., Tapia-Vazquez C., Pastor-Zarandona O. A., Lopez-Roa R. I., Viveros-Paredes J. M. (2019). Beta-caryophyllene, a natural sesquiterpene, attenuates neuropathic pain and depressive-like behavior in experimental diabetic mice. J. Med. Food. 22 (5), 460–468. 10.1089/jmf.2018.0157 [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Freedland K. E., Clouse R. E., Lustman P. J. (2001). The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 24 (6), 1069–1078. 10.2337/diacare.24.6.1069 [DOI] [PubMed] [Google Scholar]
- Anjaneyulu M., Chopra K., Kaur I. (2003). Antidepressant activity of quercetin, a bioflavonoid, in streptozotocin-induced diabetic mice. J. Med. Food. 6 (4), 391–395. 10.1089/109662003772519976 [DOI] [PubMed] [Google Scholar]
- Ballak D. B., Stienstra R., Tack C. J., Dinarello C. A., van Diepen J. A. (2015). IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 75 (2), 280–290. 10.1016/j.cyto.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W. A., Owen J. B., Erickson M. A. (2012). Insulin in the brain: there and back again. Pharmacol. Ther. 136 (1), 82–93. 10.1016/j.pharmthera.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale E. G. (2013). Insulin signaling and insulin resistance. J. Invest. Med. 61 (1), 11–14. 10.2310/JIM.0b013e3182746f95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels G. J., Reijmer Y. D. (2014). Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI?. Diabetes. 63 (7), 2244–2252. 10.2337/db14-0348 [DOI] [PubMed] [Google Scholar]
- Champaneri S., Wand G. S., Malhotra S. S., Casagrande S. S., Golden S. H. (2010). Biological basis of depression in adults with diabetes. Curr. Diabetes Rep. 10 (6), 396–405. 10.1007/s11892-010-0148-9 [DOI] [PubMed] [Google Scholar]
- Chiodini I., Di Lembo S., Morelli V., Epaminonda P., Coletti F., Masserini B., et al. (2006). Hypothalamic-pituitary-adrenal activity in type 2 diabetes mellitus: role of autonomic imbalance. Metabolism. 55 (8), 1135–1140. 10.1016/j.metabol.2006.04.010 [DOI] [PubMed] [Google Scholar]
- Cho N. H., Shaw J. E., Karuranga S., Huang Y., da Rocha Fernandes J. D., Ohlrogge A. W., et al. (2018). IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281. 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- Coderre L., Vallega G. A., Pilch P. F., Chipkin S. R. (1996). In vivo effects of dexamethasone and sucrose on glucose transport (GLUT-4) protein tissue distribution. Am. J. Physiol. 271 (4), E643–E648. 10.1152/ajpendo.1996.271.4.E643 [DOI] [PubMed] [Google Scholar]
- Colle R., de Larminat D., Rotenberg S., Hozer F., Hardy P., Verstuyft C., et al. (2017). PPAR-gamma agonists for the treatment of major depression: a review. Pharmacopsychiatry. 50 (2), 49–55. 10.1055/s-0042-120120 [DOI] [PubMed] [Google Scholar]
- de Morais H., Chaves Y. C., Waltrick A. P. F., Jesus C. H. A., Genaro K., Crippa J. A., et al. (2018). Sub-chronic treatment with cannabidiol but not with URB597 induced a mild antidepressant-like effect in diabetic rats. Neurosci. Lett. 682, 62–68. 10.1016/j.neulet.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Donato J., Jr. (2012). The central nervous system as a promising target to treat diabetes mellitus. Curr. Top. Med. Chem. 12 (19), 2070–2081. 10.2174/156802612804910214 [DOI] [PubMed] [Google Scholar]
- Engum A. (2007). The role of depression and anxiety in onset of diabetes in a large population-based study. J. Psychosom. Res. 62 (1), 31–38. 10.1016/j.jpsychores.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Farajpour R., Sadigh-Eteghad S., Ahmadian N., Farzipour M., Mahmoudi J., Majdi A. (2017). Chronic administration of Rosa canina hydro-alcoholic extract attenuates depressive-like behavior and recognition memory impairment in diabetic mice: a possible role of oxidative stress. Med. Princ. Pract. 26 (3), 245–250. 10.1159/000464364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbood Y., Rashno M., Ghaderi S., Khoshnam S. E., Sarkaki A., Rashidi K., et al. (2019). Ellagic acid protects against diabetes-associated behavioral deficits in rats: possible involved mechanisms. Life Sci. 225, 8–19. 10.1016/j.lfs.2019.03.078 [DOI] [PubMed] [Google Scholar]
- Forrester S. J., Kikuchi D. S., Hernandes M. S., Xu Q., Griendling K. K. (2018). Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122 (6), 877–902. 10.1161/CIRCRESAHA.117.311401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Li C., Zhang G., Tong X., Zhang H., Ding J., et al. (2018). Crucial roles of 5-HT and 5-HT2 receptor in diabetes-related lipid accumulation and pro-inflammatory cytokine generation in hepatocytes. Cell. Physiol. Biochem. 48 (6), 2409–2428. 10.1159/000492656 [DOI] [PubMed] [Google Scholar]
- Gerber P. A., Rutter G. A. (2017). The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxidants Redox Signal. 26 (10), 501–518. 10.1089/ars.2016.6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H., Gill B., El-Halabi S., Chen-Li D., Lipsitz O., Rosenblat J. D., et al. (2020). Antidepressant medications and weight change: a narrative review. Obesity. 28 (11), 2064–2072. 10.1002/oby.22969 [DOI] [PubMed] [Google Scholar]
- Golden S. H., Lazo M., Carnethon M., Bertoni A. G., Schreiner P. J., Diez Roux A. V., et al. (2008). Examining a bidirectional association between depressive symptoms and diabetes. J. Am. Med. Assoc. 299 (23), 2751–2759. 10.1001/jama.299.23.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D., Kurhe Y., Radhakrishnan M. (2014a). Antidepressant effects of insulin in streptozotocin induced diabetic mice: modulation of brain serotonin system. Physiol. Behav. 129, 73–78. 10.1016/j.physbeh.2014.02.036 [DOI] [PubMed] [Google Scholar]
- Gupta D., Radhakrishnan M., Kurhe Y. (2014b). Ondansetron, a 5HT3 receptor antagonist reverses depression and anxiety-like behavior in streptozotocin-induced diabetic mice: possible implication of serotonergic system. Eur. J. Pharmacol. 744, 59–66. 10.1016/j.ejphar.2014.09.041 [DOI] [PubMed] [Google Scholar]
- Gupta D., Thangaraj D., Radhakrishnan M. (2016). A novel 5HT3 antagonist 4i (N-(3-chloro-2-methylphenyl)quinoxalin-2-carboxamide) prevents diabetes-induced depressive phenotypes in mice: modulation of serotonergic system. Behav. Brain Res. 297, 41–50. 10.1016/j.bbr.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Hamer J. A., Testani D., Mansur R. B., Lee Y., Subramaniapillai M., McIntyre R. S. (2019). Brain insulin resistance: a treatment target for cognitive impairment and anhedonia in depression. Exp. Neurol. 315, 1–8. 10.1016/j.expneurol.2019.01.016 [DOI] [PubMed] [Google Scholar]
- Hashim N. A., Ariaratnam S., Salleh M. R., Said M. A., Sulaiman A. H. (2016). Depression and associated factors in patients with type 2 diabetes mellitus. East Asian Arch. Psychiatry. 26 (2), 77–82 [PubMed] [Google Scholar]
- Horikawa N., Yamazaki T., Izumi N., Uchihara M. (2003). Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective study. Gen. Hosp. Psychiatr. 25 (1), 34–38. 10.1016/s0163-8343(02)00239-6 [DOI] [PubMed] [Google Scholar]
- Icks A., Kruse J., Dragano N., Broecker-Preuss M., Slomiany U., Mann K., et al. (2008). Are symptoms of depression more common in diabetes? Results from the Heinz Nixdorf Recall study. Diabet. Med. 25 (11), 1330–1336. 10.1111/j.1464-5491.2008.02585.x [DOI] [PubMed] [Google Scholar]
- Jesus C. H. A., Redivo D. D. B., Gasparin A. T., Sotomaior B. B., de Carvalho M. C., Genaro K., et al. (2019). Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res. 1715, 156–164. 10.1016/j.brainres.2019.03.014 [DOI] [PubMed] [Google Scholar]
- Jia K. K., Pan S. M., Ding H., Liu J. H., Zheng Y. J., Wang S. J., et al. (2018). Chaihu-shugan san inhibits inflammatory response to improve insulin signaling in liver and prefrontal cortex of CUMS rats with glucose intolerance. Biomed. Pharmacother. 103, 1415–1428. 10.1016/j.biopha.2018.04.171 [DOI] [PubMed] [Google Scholar]
- Jia K. K., Zheng Y. J., Zhang Y. X., Liu J. H., Jiao R. Q., Pan Y., et al. (2017). Banxia-houpu decoction restores glucose intolerance in CUMS rats through improvement of insulin signaling and suppression of NLRP3 inflammasome activation in liver and brain. J. Ethnopharmacol. 209, 219–229. 10.1016/j.jep.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Jing Y. H., Chen K. H., Kuo P. C., Pao C. C., Chen J. K. (2013). Neurodegeneration in streptozotocin-induced diabetic rats is attenuated by treatment with resveratrol. Neuroendocrinology. 98 (2), 116–127. 10.1159/000350435 [DOI] [PubMed] [Google Scholar]
- Joseph J. J., Golden S. H. (2017). Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 1391 (1), 20–34. 10.1111/nyas.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung U. J., Choi M. S. (2014). Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15 (4), 6184–6223. 10.3390/ijms15046184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan C., Silva N., Golden S. H., Rajala U., Timonen M., Stahl D., et al. (2013). A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 36 (2), 480–489. 10.2337/dc12-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F., Bondolfi G., Gervasoni N., Schwald M., Aubry J. M., Bertschy G. (2005). Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatr. 57 (9), 1068–1072. 10.1016/j.biopsych.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Khaledi M., Haghighatdoost F., Feizi A., Aminorroaya A. (2019). The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. 56 (6), 631–650. 10.1007/s00592-019-01295-9 [DOI] [PubMed] [Google Scholar]
- Khassawneh A. H., Alzoubi A., Khasawneh A. G., Abdo N., Abu-Naser D., Al-Mistarehi A. H., et al. (2020). The relationship between depression and metabolic control parameters in type 2 diabetic patients: a cross-sectional and feasibility interventional study. Int. J. Clin. Pract. e13777 10.1111/ijcp.13777 [DOI] [PubMed] [Google Scholar]
- Kleinridders A., Cai W., Cappellucci L., Ghazarian A., Collins W. R., Vienberg S. G., et al. (2015). Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. United States. 112 (11), 3463–3468. 10.1073/pnas.1500877112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol M. J., Heerdink E. R., Egberts A. C., Geerlings M. I., Gorter K. J., Numans M. E., et al. (2007). Depressive symptoms in subjects with diagnosed and undiagnosed type 2 diabetes. Psychosom. Med. 69 (4), 300–305. 10.1097/PSY.0b013e31805f48b9 [DOI] [PubMed] [Google Scholar]
- Knol M. J., Twisk J. W., Beekman A. T., Heine R. J., Snoek F. J., Pouwer F. (2006). Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 49 (5), 837–845. 10.1007/s00125-006-0159-x [DOI] [PubMed] [Google Scholar]
- Kohler O., Krogh J., Mors O., Benros M. E. (2016). Inflammation in depression and the potential for anti-inflammatory treatment. Curr. Neuropharmacol. 14 (7), 732–742. 10.2174/1570159x14666151208113700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koye D. N., Magliano D. J., Nelson R. G., Pavkov M. E. (2018). The global epidemiology of diabetes and kidney disease. Adv. Chron. Kidney Dis. 25 (2), 121–132. 10.1053/j.ackd.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe K. S., Nielsen A. R., Krogh-Madsen R., Plomgaard P., Rasmussen P., Erikstrup C., et al. (2007). Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 50 (2), 431–438. 10.1007/s00125-006-0537-4 [DOI] [PubMed] [Google Scholar]
- Lei Y., Xu R., Li H. (2017). Effect of shugan jianpi prescription on the expression of BDNF mRNA and protein levels in the rats with diabetic depression. J. Liaoning Univ. Tradit. Chin. Med. 19 (1), 31–34. 10.13194/j.issn.1673-842x.2017.01.009 [DOI] [Google Scholar]
- Lenart L., Hodrea J., Hosszu A., Koszegi S., Zelena D., Balogh D., et al. (2016). The role of sigma-1 receptor and brain-derived neurotrophic factor in the development of diabetes and comorbid depression in streptozotocin-induced diabetic rats. Psychopharmacology (Berl). 233 (7), 1269–1278. 10.1007/s00213-016-4209-x [DOI] [PubMed] [Google Scholar]
- Lepine J. P., Briley M. (2011). The increasing burden of depression. Neuropsychiatric Dis. Treat. 7 (1), 3–7. 10.2147/NDT.S19617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. W., Deng M. Z., Gao Z. J., Dang Y. Y., Zheng G. D., Yang X. J., et al. (2020). Effects of compound K, a metabolite of ginsenosides, on memory and cognitive dysfunction in db/db mice involve the inhibition of ER stress and the NLRP3 inflammasome pathway. Food Funct. 11 (5), 4416–4427. 10.1039/c9fo02602a [DOI] [PubMed] [Google Scholar]
- Li D., Chen W., Zhu X., Liu Y. (2018). Research progress of diabetes mellitus complicated with depression. Med. Information. 31 (14), 20–23. 10.3969/j.issn.1006-1959.2018.14.008 [DOI] [Google Scholar]
- Li X. (2017). Clinical experience of shugan jianpi therapy in diabetes combined with depression. China’s Naturopathy. 25 (7), 47–48. 10.19621/j.cnki.11-3555/r.2017.07.036 [DOI] [Google Scholar]
- Li Z., Guo X., Jiang H., Sun G., Sun Y., Abraham M. R. (2016). Diagnosed but not undiagnosed diabetes is associated with depression in rural areas. Int. J. Environ. Res. Publ. Health. 13 (11). 10.3390/ijerph13111136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G. Y., Tam W. W., Lu Y., Ho C. S., Zhang M. W., Ho R. C. (2018). Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 8 (1), 2861 10.1038/s41598-018-21243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D., Dhabhar F. S., James S. J., Hough C. M., Jain F. A., Bersani F. S., et al. (2017). Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 76, 197–205. 10.1016/j.psyneuen.2016.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W. (2012). Research on the Symptomatology of Diabetes with Depression and Clinical Evaluation of Yishen Jieyu Decoction Treatment. Beijing, China: Beijing University of Chinese Medicine; [Google Scholar]
- Lu Y., Ma X., Kong Q., Xu Y., Hu J., Wang F., et al. (2019). Novel dual-color drug screening model for GLUT4 translocation in adipocytes. Mol. Cell. Probes. 43, 6–12. 10.1016/j.mcp.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Luc K., Schramm-Luc A., Guzik T. J., Mikolajczyk T. P. (2019). Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 70 (6), 809–824. 10.26402/jpp.2019.6.01 [DOI] [PubMed] [Google Scholar]
- Luo C., Ke Y., Yuan Y., Zhao M., Wang F., Zhang Y., et al. (2016). A novel herbal treatment reduces depressive-like behaviors and increases brain-derived neurotrophic factor levels in the brain of type 2 diabetic rats. Neuropsychiatric Dis. Treat. 12, 3051–3059. 10.2147/NDT.S117337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Zhang H., Wang S., Wang H., Wang Y., Liu J., et al. (2019). The molecular mechanism underlying GABAergic dysfunction in nucleus accumbens of depression-like behaviours in mice. J. Cell Mol. Med. 23 (10), 7021–7028. 10.1111/jcmm.14596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I., Bambico F. R., Mechawar N., Nobrega J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 38, 173–192. 10.1016/j.neubiorev.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Mehta V., Parashar A., Sharma A., Singh T. R., Udayabanu M. (2017). Quercetin ameliorates chronic unpredicted stress-mediated memory dysfunction in male Swiss albino mice by attenuating insulin resistance and elevating hippocampal GLUT4 levels independent of insulin receptor expression. Horm. Behav. 89, 13–22. 10.1016/j.yhbeh.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Meijer O. C., Koorneef L. L., Kroon J. (2018). Glucocorticoid receptor modulators. Ann. Endocrinol. 79 (3), 107–111. 10.1016/j.ando.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Mezuk B., Eaton W. W., Albrecht S., Golden S. H. (2008). Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 31 (12), 2383–2390. 10.2337/dc08-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton C. D., Pickup J. C., Ismail K. (2015). The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 3 (6), 461–471. 10.1016/S2213-8587(15)00134-5 [DOI] [PubMed] [Google Scholar]
- Mukherjee N., Chaturvedi S. K. (2019). Depressive symptoms and disorders in type 2 diabetes mellitus. Curr. Opin. Psychiatr. 32 (5), 416–421. 10.1097/YCO.0000000000000528 [DOI] [PubMed] [Google Scholar]
- Myint A. M., Leonard B. E., Steinbusch H. W., Kim Y. K. (2005). Th1, Th2, and Th3 cytokine alterations in major depression. J. Affect. Disord. 88 (2), 167–173. 10.1016/j.jad.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Nouwen A., Adriaanse M. C., van Dam K., Iversen M. M., Viechtbauer W., Peyrot M., et al. (2019). Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet. Med. 36 (12), 1562–1572. 10.1111/dme.14054 [DOI] [PubMed] [Google Scholar]
- Papazoglou I. K., Jean A., Gertler A., Taouis M., Vacher C. M. (2015). Hippocampal GSK3beta as a molecular link between obesity and depression. Mol. Neurobiol. 52 (1), 363–374. 10.1007/s12035-014-8863-x [DOI] [PubMed] [Google Scholar]
- Patel S. S., Ray R. S., Sharma A., Mehta V., Katyal A., Udayabanu M. (2018). Antidepressant and anxiolytic like effects of Urtica dioica leaves in streptozotocin induced diabetic mice. Metab. Brain Dis. 33 (4), 1281–1292. 10.1007/s11011-018-0243-1 [DOI] [PubMed] [Google Scholar]
- Patel S. S., Udayabanu M. (2014). Urtica dioica extract attenuates depressive like behavior and associative memory dysfunction in dexamethasone induced diabetic mice. Metab. Brain Dis. 29 (1), 121–130. 10.1007/s11011-014-9480-0 [DOI] [PubMed] [Google Scholar]
- Petrak F., Baumeister H., Skinner T. C., Brown A., Holt R. I. G. (2015). Depression and diabetes: treatment and health-care delivery. Lancet Diabetes Endocrinol. 3 (6), 472–485. 10.1016/S2213-8587(15)00045-5 [DOI] [PubMed] [Google Scholar]
- Porte D., Jr., Baskin D. G., Schwartz M. W. (2002). Leptin and insulin action in the central nervous system. Nutr. Rev. 60(10), S20–S29. 10.1301/002966402320634797 [DOI] [PubMed] [Google Scholar]
- Pradeepa R., Mohan V. (2017). Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. Eur. J. Clin. Nutr. 71 (7), 816–824. 10.1038/ejcn.2017.40 [DOI] [PubMed] [Google Scholar]
- Rahmani G., Farajdokht F., Mohaddes G., Babri S., Ebrahimi V., Ebrahimi H. (2020). Garlic (Allium sativum) improves anxiety- and depressive-related behaviors and brain oxidative stress in diabetic rats. Arch. Physiol. Biochem. 126 (2), 95–100. 10.1080/13813455.2018.1494746 [DOI] [PubMed] [Google Scholar]
- Reus G. Z., Carlessi A. S., Silva R. H., Ceretta L. B., Quevedo J. (2019). Relationship of oxidative stress as a link between diabetes mellitus and major depressive disorder. Oxid. Med. Cell Longev. 2019, 8637970 10.1155/2019/8637970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus G. Z., Dos Santos M. A., Abelaira H. M., Titus S. E., Carlessi A. S., Matias B. I., et al. (2016). Antioxidant treatment ameliorates experimental diabetes-induced depressive-like behaviour and reduces oxidative stress in brain and pancreas. Diabetes Metab. Res. Rev. 32 (3), 278–288. 10.1002/dmrr.2732 [DOI] [PubMed] [Google Scholar]
- Rhyu Y. A., Jang J. Y., Park S., An J. H., Kim D. L., Kim S. K., et al. (2019). Impaired cortisol and growth hormone counterregulatory responses among severe hypoglycemic patients with type 2 diabetes mellitus. Endocrinol Metab. (Seoul). 34 (2), 187–194. 10.3803/EnM.2019.34.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan B., Stanton R. C. (2013). A story of microalbuminuria and diabetic nephropathy. J. Nephropathol. 2 (4), 234–240. 10.12860/JNP.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. R., Ma Y., Marrero D. G., Peyrot M., Barrett-Connor E. L., Kahn S. E., et al. (2008). Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care. 31 (3), 420–426. 10.2337/dc07-1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadigh-Eteghad S., Tayefi-Nasrabadi H., Aghdam Z., Zarredar H., Shanehbandi D., Khayyat L., et al. (2011). Rosa canina L. Fruit hydro-alcoholic extract effects on some immunological and biochemical parameters in rats. Bioimpacts. 1 (4), 219–224. 10.5681/bi.2011.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin T. D., Gocmez S. S., Eraldemir F. C., Utkan T. (2019). Anxiolytic-like and antidepressant-like effects of resveratrol in streptozotocin-induced diabetic rats. Noro Psikiyatr Ars. 56 (2), 144–149. 10.29399/npa.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter N. S., Thienel C., Plutino Y., Kampe K., Dror E., Traub S., et al. (2015). Angiotensin II induces interleukin-1beta-mediated islet inflammation and beta-cell dysfunction independently of vasoconstrictive effects. Diabetes. 64 (4), 1273–1283. 10.2337/db14-1282 [DOI] [PubMed] [Google Scholar]
- Shen J. D., Wei Y., Li Y. J., Qiao J. Y., Li Y. C. (2017). Curcumin reverses the depressive-like behavior and insulin resistance induced by chronic mild stress. Metab. Brain Dis. 32 (4), 1163–1172. 10.1007/s11011-017-0017-1 [DOI] [PubMed] [Google Scholar]
- Shirazi S. N., Friedman A. R., Kaufer D., Sakhai S. A. (2015). Glucocorticoids and the brain: neural mechanisms regulating the stress response. Adv. Exp. Med. Biol. 872, 235–252. 10.1007/978-1-4939-2895-8_10 [DOI] [PubMed] [Google Scholar]
- Shivavedi N., Charan Tej G. N. V., Neogi K., Nayak P. K. (2019). Ascorbic acid therapy: a potential strategy against comorbid depression-like behavior in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 109, 351–359. 10.1016/j.biopha.2018.10.070 [DOI] [PubMed] [Google Scholar]
- Strine T. W., Mokdad A. H., Dube S. R., Balluz L. S., Gonzalez O., Berry J. T., et al. (2008). The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen. Hosp. Psychiatr. 30 (2), 127–137. 10.1016/j.genhosppsych.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Su Q., Tao W., Wang H., Chen Y., Huang H., Chen G. (2016). Umbelliferone attenuates unpredictable chronic mild stress induced-insulin resistance in rats. IUBMB Life. 68 (5), 403–409. 10.1002/iub.1496 [DOI] [PubMed] [Google Scholar]
- Tabatabaei S. R. F., Ghaderi S., Bahrami-Tapehebur M., Farbood Y., Rashno M. (2017). Aloe vera gel improves behavioral deficits and oxidative status in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 96, 279–290. 10.1016/j.biopha.2017.09.146 [DOI] [PubMed] [Google Scholar]
- Thakur A. K., Chatterjee S. S., Kumar V. (2014). Antidepressant-like effects of Brassica juncea L. leaves in diabetic rodents. Indian J. Exp. Biol. 52 (6), 613–622 [PubMed] [Google Scholar]
- Tusa B. S., Alemayehu M., Weldesenbet A. B., Kebede S. A., Dagne G. A. (2020). Prevalence of depression and associated factors among diabetes patients in east shewa, Ethiopia: bayesian approach. Depress Res Treat. 2020, 4071575 10.1155/2020/4071575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Duan P., Cui Y., Li Q., Shi Y. (2016). Geniposide alleviates depression-like behavior via enhancing BDNF expression in hippocampus of streptozotocin-evoked mice. Metab. Brain Dis. 31 (5), 1113–1122. 10.1007/s11011-016-9856-4 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhao X., He M. (2012a). Is BDNF biological link between depression and type 2 diabetes mellitus?. Med. Hypotheses. 79 (2), 255–258. 10.1016/j.mehy.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Wang Y., Fan R., Huang X. (2012b). Meta-analysis of the clinical effectiveness of traditional Chinese medicine formula Chaihu-Shugan-San in depression. J. Ethnopharmacol. 141 (2), 571–577. 10.1016/j.jep.2011.08.079 [DOI] [PubMed] [Google Scholar]
- Wang R. (2008). Clinical Study on Treating Diabetes Mellitus Combined with Depression with Shu Gan Jian Pi Jie Yu Formular. Jinan, China: Shandong University of Traditional Chinese Medicine; [Google Scholar]
- Wang W., Sun Y., Zhong Q., Liu Y., Xue M. (2020a). Interventional effect of danggui buxue decoction on serum inflammatory factors in diabetic model rats. Acta Chinese Medicine. 6 (35), 1258–1261. 10.16368/j.issn.1674-8999.2020.06.284 [DOI] [Google Scholar]
- Wang W., Zhang W., Sun Y., Zhong Q., Xue M. (2020b). Effects of danggui buxue decoction and its main active ingredient ferulic acid on CUMS model GK rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 31 (6), 649–654. 10.19378/j.issn.1003-9783.2020.06.005 [DOI] [Google Scholar]
- Wang W., Zhang L., Sun Y., Xue M. (2018). Research progress of treatment of danggui buxue decoction on diabetes mellitus with depression. World Sci. Tech./Modernization of Tradit. Chin. Med. Materia Medica. 20 (12), 2191–2195. 10.11842/wst.2018.12.016 [DOI] [Google Scholar]
- Wang X., Lou Y., Gao Z. (2013). Effects of danzhi xiaoyao power on IRS-2-PI3K signaling pathway in liver tissues on diabetes mellitus rats with depression. Chin. Archives of Tradit. Chin. Med. 31 (11), 2450–2452. 10.13193/j.issn.1673-7717.2013.11.052 [DOI] [Google Scholar]
- Wang Y. H., Yin L. T., Yang H., Li X. L., Wu K. G. (2014). Hypoglycemic and anti-depressant effects of Zuogui Jiangtang Jieyu formulation in a model of unpredictable chronic mild stress in rats with diabetes mellitus. Exp. Ther. Med. 8 (1), 281–285. 10.3892/etm.2014.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang H., Li W., Meng P., Han Y., Zhang X., et al. (2015). Zuogui jiangtang jieyu formulation prevents hyperglycaemia and depressive-like behaviour in rats by reducing the glucocorticoid level in plasma and Hippocampus. Evid. Based Complement Alternat. Med. 2015, 158361 10.1155/2015/158361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Han Y., Liu Z., Meng P., Du Q., Zhao H., et al. (2019). Effect of zuogui jiangtang jieyu formulation on signaling pathway in hypothalamus of diabetic rats with depression. Chin. J. Modern Applied Pharmacy. 36 (11), 1333–1337. 10.13748/j.cnki.issn1007-7693.2019.11.004 [DOI] [Google Scholar]
- Yang H., Liu J., Tang L., Lin X., Luo W., Han Y., et al. (2020). Effects of Zuogui Jiangtang Jieyu Formulation on insulin resistance in hipppocampus of rats with diabetes-related depression. Chin. Tradit. Herb. Drugs. 52 (11), 3013–3020. 10.7501/j.issn.0253-2670.2020.11.020 [DOI] [Google Scholar]
- Yang H., Wang Y., Meng P., Liu Z., Liu J., Yang Q., et al. (2018). Effects of zuogui jiangtang jieyu decoction on hippocampal chemokine CX3C receptor 1 and neuroinflammatory factors in Hippocampus of diabetic rats with depression. J. Tradit. Chin. Med. 59 (20), 1783–1787. 10.13288/j.11-2166/r.2018.20.015 [DOI] [Google Scholar]
- Yaribeygi H., Farrokhi F. R., Butler A. E., Sahebkar A. (2019). Insulin resistance: review of the underlying molecular mechanisms. J. Cell. Physiol. 234 (6), 8152–8161. 10.1002/jcp.27603 [DOI] [PubMed] [Google Scholar]
- Ye M., Ke Y., Liu B., Yuan Y., Wang F., Bu S., et al. (2017). Root bark of Morus alba ameliorates the depressive-like behaviors in diabetic rats. Neurosci. Lett. 637, 136–141. 10.1016/j.neulet.2016.11.036 [DOI] [PubMed] [Google Scholar]
- Ye T., Meng X., Wang R., Zhang C., He S., Sun G., et al. (2018). Gastrodin alleviates cognitive dysfunction and depressive-like behaviors by inhibiting ER stress and NLRP3 inflammasome activation in db/db mice. Int. J. Mol. Sci. 19 (12). 10.3390/ijms19123977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S. S., Hwang I. K., Shin J. H., Choi J. H., Lee C. H., Kim I. Y., et al. (2010). Regulatory mechanism of hypothalamo-pituitary-adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J. Chem. Neuroanat. 40 (2), 130–139. 10.1016/j.jchemneu.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Yohn C. N., Gergues M. M., Samuels B. A. (2017). The role of 5-HT receptors in depression. Mol. Brain. 10 (1), 28 10.1186/s13041-017-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Zhang X., Lu F., Fang L. (2015). Depression and risk for diabetes: a meta-analysis. Can. J. Diabetes. 39 (4), 266–272. 10.1016/j.jcjd.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Zemdegs J., Martin H., Pintana H., Bullich S., Manta S., Marques M. A., et al. (2019). Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J. Neurosci. 39 (30), 5935–5948. 10.1523/JNEUROSCI.2904-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chi X., Pan W., Wang S., Zhang Z., Zhao H., et al. (2020). Antidepressant mechanism of classical herbal formula lily bulb and Rehmannia decoction: insights from gene expression profile of medial prefrontal cortex of mice with stress-induced depression-like behavior. Gene Brain Behav. 19 (5), e12649 10.1111/gbb.12649 [DOI] [PubMed] [Google Scholar]
- Zhang M., Xiao W. (2008). Clinical observation on the treatment of diabetes and depression with modified danzhi xiaoyao powder. J. Liaoning Univ. Tradi. Chin. Med. 10 (2), 108–109. 10.13194/j.jlunivtcm.2008.02.110.zhangm.053 [DOI] [Google Scholar]
- Zhang W., Cheng H., Feng X. (2017). Danzhi xiaoyao powder (丹栀逍遥散) adjustment on the treatment of type 2 diabetes mellitus complicating depression: a meta-analysis. Guiding J. Tradi. Chin. Med. Pharmacology. 23 (23), 63–67. 10.13862/j.cnki.cn43-1446/r.2017.23.018 [DOI] [Google Scholar]
- Zhang W., Feng X. (2016). Summary of traditional Chinese medicine on the treatment of diabetes mellitus complicating depression. Chi. J. Tradi. Chin. Med. Pharmacy. 31 (4), 1374–1376 [Google Scholar]
- Zhao F., Siu J. J., Huang W., Askwith C., Cao L. (2019). Insulin modulates excitatory synaptic transmission and synaptic plasticity in the mouse Hippocampus. Neuroscience. 411, 237–254. 10.1016/j.neuroscience.2019.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Wang G., Zhang Z., Wang Z., Ma K. (2020). Research progress on classical traditional Chinese medicine formula Liuwei Dihuang pills in the treatment of type 2 diabetes. Biomed. Pharmacother. 121, 109564 10.1016/j.biopha.2019.109564 [DOI] [PubMed] [Google Scholar]
- Zhou X., Gan T., Fang G., Wang S., Mao Y., Ying C. (2018). Zeaxanthin improved diabetes-induced anxiety and depression through inhibiting inflammation in hippocampus. Metab. Brain Dis. 33 (3), 705–711. 10.1007/s11011-017-0179-x [DOI] [PubMed] [Google Scholar]
- Zhou X. Y., Zhang F., Hu X. T., Chen J., Tang R. X., Zheng K. Y., et al. (2017). Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res. 1657, 262–268. 10.1016/j.brainres.2016.12.018 [DOI] [PubMed] [Google Scholar]