Abstract
Objectives: Two pilot studies aimed to determine the effects of individual computer engagement on behavioral health outcomes in individuals with dementia. The focus was on participants’ mental health, challenging behaviors, antipsychotic medications, and professional caregiver stress. Methods: Two pilot randomized control trials were conducted. First trial involved residents with advanced dementia in a long-term care facility. The second trial involved residents with mild dementia in an assisted living setting. The participants in the experimental group in both studies were provided with guided iN2L computer engagement followed by unrestricted use. Results: Statistically reliable improvements were found in both studies for participants’ emotional well-being and professional caregiver stress. Reliable improvements in cognition and depression were found in the mild dementia study, but not in the advanced dementia study. No statistically reliable changes were observed for antipsychotic medications or challenging behaviors. Discussion: Computer engagement was associated with improvements in participants’ emotional well-being and with a reduction in professional caregiver stress. Results should be interpreted with caution in the context of high attrition. Future studies may build upon these pilot findings and examine effects of technology use on mood and cognition in larger samples of older adults across a wider range of outcome measures.
Keywords: computer engagement, technology, dementia, mental health, professional caregiver stress
The National Partnership to Improve Dementia Care in Nursing Homes (Bakerjian, 2014) has stressed the need for more non-pharmacological approaches to enhance quality of life in nursing home residents. Particularly, there has been a considerable focus in the recent literature on non-pharmacological interventions that may improve psychological well-being and reduce agitation in older adults with cognitive impairment. For example, in a recent systematic review of 19 studies, Kim and Park (2017) reported that person-centered care interventions were shown to improve quality of life and reduce agitation, neuropsychiatric symptoms, and depression in patients with dementia. Similarly, Livingston et al. (2014) concluded that person-centered care, sensory interventions, and music therapy may lead to a decrease in agitation in this population. In another study spanning 30 nursing homes over a 12-month period, Fitzler et al. (2016) demonstrated quality improvement in the management of challenging behaviors through habilitation therapy techniques. Thus, extant literature suggests that non-pharmacologic interventions for dementia may result in improved behavioral health outcomes.
Furthermore, published research indicates that non-pharmacological interventions may be associated with improved cognitive outcomes as well. Specifically, cognitive stimulation has been identified as one of the most promising non-pharmacological interventions associated with improved cognition in individuals with mild cognitive impairment (Gates et al., 2011) and dementia (Quayhagen et al., 2000). For example, in one study, participants who were exposed to cognitive stimulation over a period of 3 months showed improvement in cognitive outcomes and their professional caregivers showed a decrease in depressive symptoms (Quayhagen et al., 2000). Moreover, results of a meta-analysis conducted by Aguirre et al. (2013) revealed that cognitive stimulation interventions benefited cognitive function and well-being in individuals with dementia. These authors concluded that cognitive stimulation should be made more widely available in dementia care (Aguirre et al., 2013). Finally, published studies even suggest that engagement in mentally stimulating activities may reduce one’s risk of dementia (Valenzuela & Sachdev, 2006; Wilson et al., 2002).
Although mental engagement has proven to be beneficial for those with cognitive impairment, access to this type of stimulation has been restrained by many factors including time-consuming individualized creative behavioral interventions. Access to in-room computer engagement could be a possible solution to these barriers. For instance, Vahia et al. (2017) in their study of 36 inpatients at a geriatric psychiatry unit reported that tablet use appears to be feasible and safe as a nonpharmacologic intervention for agitation in older adults, including those with severe dementia. In that study participants were provided with tablets when they were agitated, and they were able to use various applications (apps) related to communication, games, music, web browser, and photography. All participants, regardless of dementia severity, were able to use apps and were rated by staff to have benefitted clinically from tablet use. Further, Leng et al. (2014) compared responses of patients with dementia to traditional activities and iPad-based activities. They concluded that iPad activities have the potential to be as effective and engaging as other conventional activities in achieving patients’ well-being. A number of other studies indicate that older adults with dementia are capable of using a touch-screen computer system and can find it engaging and enjoyable (e.g., Astell et al., 2010, 2016).
In fact, there is a rising interest in the published literature on the use of various computer technologies for patients with cognitive impairment, including smartphones (De Leo et al., 2011), tablets (Lim et al., 2013), ALADDIN platform for dementia and care providers (Torkamani et al., 2014), touch screen game Bubble Xplode (Astell et al., 2016), virtual reality desktop computers (Zucchella et al., 2014), nature scenes and music for challenging behaviors (Eggert et al., 2015), tailored computer interventions (Tak et al., 2015), technology-aided verbal reminiscence (Lancioni et al., 2014), and computer-based creativity promoting touch e-pad (Leuty et al., 2013). However, to the authors’ knowledge, guided individualized computer engagement in the elderly population has not been previously studied in randomized control trials.
The present study aimed to address this gap in the published literature. It involved a recently developed touch-screen computer platform “It’s Never Too Late” (iN2L, Colorado, CO, USA). Screens of iN2L computers are larger than screens of most commercially marketed tablets, allowing for larger icons and pictures to appear to the screen. This is especially user-friendly for older adults, since many have age-related problems with eyesight. The iN2L system provides the opportunity to personalize computer applications to enhance social connection, facilitate entertainment, and implement cognitive training through various brain fitness programs.
The present study was conducted within a life plan community with residents who had been diagnosed with advanced and mild all-cause dementia residing in skilled nursing and assisted living settings. The primary objectives of the present study were to evaluate the effects of iN2L computer engagement on participants’ mental health, challenging behaviors, antipsychotic medications, as well as on professional caregiver stress. This project involved an innovative collaboration between a life plan community and multiple academic and community resources in a naturalistic setting.
Method
This study was approved by the local medical school’s Internal Review Board with subsequent amendments to include Study 1 (advanced dementia) and Study 2 (mild dementia). Informed consent was given by the Legal Authoritative Representatives (LAR) of the individuals with dementia and by the participating professional caregivers.
Study 1: Advanced Dementia
Inclusion criterion was a score ≤24 on the Montreal Cognitive Assessment (MoCA) for those residing within the skilled nursing unit. Initially, 62 individuals were identified and randomized into two groups: Experimental (n = 31) and Control (n = 31). The Experimental Group had iN2L computers at their sides 24 hours a day, 7 days a week for 12 weeks. Therapeutic Recreation interns and medical students assisted each resident in the Experimental Group with individual guided computer engagement using 10 specific applications (e.g., big band music, laughing babies) 1 hour per day, 5 hours per week, for 12 weeks. Following the 1 hour per day guided engagement, residents were free to engage in any applications they wished for unlimited access every day for 12 weeks with or without the assistance of Certified Nurse Assistants (CNAs). Residents in the Control Group did not have personalized computer access. However, both Experimental and Control Groups had limited exposure to weekly group computer activity sessions as part of the facility’s standard of care. Participants were assessed prior to the start of the study and at the end of the study.
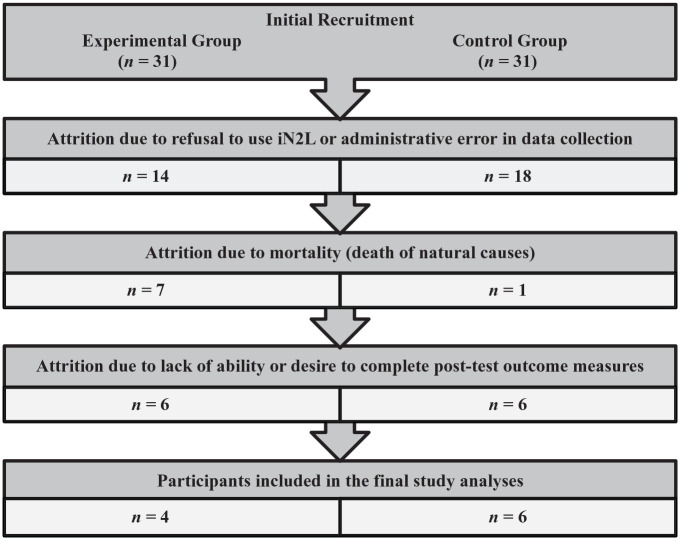
Although there were initially 62 participants significant attrition resulted in only four individuals in the final Experimental Group (total all-cause attrition was 87%) and six individuals in the Control Group (total all-cause attrition was 81%). Within the Experimental Group 14 participants refused to use the iN2L, and seven died of natural causes unrelated to the study. Out of the remaining 10, 6 were unable to complete the outcome measures due to increasing dementia and sensory changes. Within the Control Group, there was a total all-cause attrition of 25 participants (81%) leaving six participants at the end of the study. Eighteen participants were accidently moved out of the Control Group and placed into the Experimental Group; however, once this was discovered, all data for those participants were removed from the study. There was one participant in the Control Group who died of natural causes unrelated to the study, and three participants were removed because they did not wish to participate any longer. Out of the remaining nine participants, three were unable to complete all required assessments secondary to increasing dementia and sensory changes. Attrition process for Study 1 is illustrated in Figure 1. The final participant characteristics were as follows: control group n = 6, experimental group n = 4, mean age of 93, mean MoCA score of 10, 8 of 10 participants were female. All participants were Caucasian. Professional caregivers (CNAs) (n = 30) also consented to participate and were assessed at baseline and at the end of the study.
Figure 1.
Attrition process in study 1 (advanced dementia).
Study 2: Mild Dementia
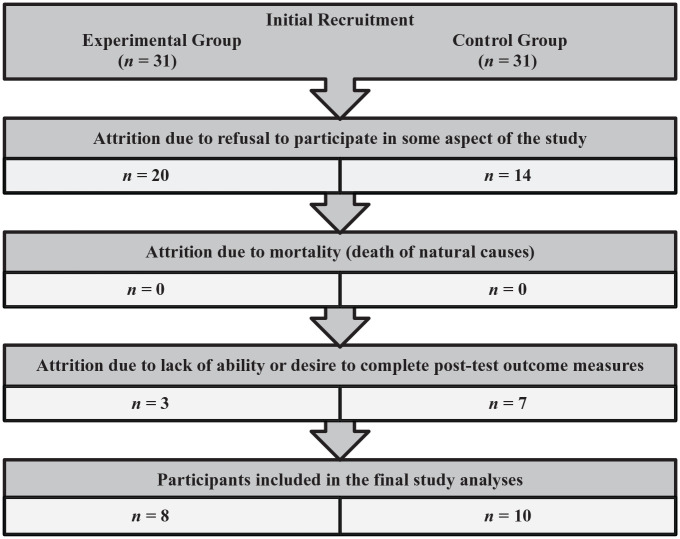
The methodology was similar to the Advanced Dementia Study. However, due to extenuating circumstances in the preparation and training of staff, there was a 6-week baseline period. The intervention then occurred for 6 weeks, with no cross-over. The inclusionary criteria for the Mild Dementia Study were that the participant resided in an assisted living unit and had a MoCA of ≤24. Initially, there were 62 eligible individuals. They were randomized into an Experimental Group (n = 31) and a Control Group (n = 31). However, as in the first study, significant attrition resulted in eight individuals in the final Experimental Group and 10 in the final Control Group (Experimental Group total all-cause attrition was 74%; Control Group total all-cause attrition was 68%). Specifically, there were 11 residents in the Experimental Group of the Mild Dementia Study who had consented to participate through their LAR, but when the study started, the residents did not want to participate. Therefore, only 20 willing participants remained in the Experimental Group of the Mild Dementia Study on Day 1. Throughout the duration of the Mild Dementia Study, nine more participants dropped out of the Experimental Group refusing to continue for various reasons, which resulted in only 11 participants left in the Experimental Group at the end of the study. Out of these 11 participants, 3 participants did not complete the post-experimental psychometric measures for various reasons (e.g., being in a hospital, unavailable or unwilling to complete the assessments). Therefore, the second study only had complete data on eight participants in the Experimental Group at the end of Mild Dementia Study. Within the Control Group, 14 residents dropped out of the experiment secondary to refusing to participate at some point in the study. Additionally, seven participants consented by their LAR refused to take the required outcome assessments. This left 10 residents in the Control Group at the end of the study. Attrition process for Study 2 is illustrated in Figure 2. The final participant characteristics were as follows: control group n = 10, experimental group n = 8, mean age of 87, mean MoCA score of 17, 16 of 18 participants were female. All participants were Caucasian. Professional caregivers (CNAs) (n = 9) also consented to participate in this study and were assessed at baseline and at the end of the study.
Figure 2.
Attrition process in study 2 (mild dementia).
Outcome Measures for Participants with Dementia
Clinical information
Blood pressure readings were recorded from the residents’ medical charts. Psychotropic medications were reviewed in the resident’s medical chart to assess changes across time prior to the intervention and at the end of the intervention. Frequency counts of challenging behaviors, documented in the medical chart, were collected prior to intervention and at the end of the intervention to assess changes across time. Demographic information from residents’ charts was also collected.
Affect Balance Scale (ABS)
The Affect Balance Scale (ABS; Bradburn, 1969), is a 10-item instrument designed to measure psychological well-being, especially mood state or happiness. The ABS is an extensively studied scale with excellent data on its applicability in a broad range of situations and cultures. The current version yields scores on two distinct conceptual dimensions, positive affect (items 1, 3, 5, 7, and 9) and negative affect (items 2, 4, 6, 8, and 10). This measure is in the public domain.
Geriatric Depression Scale (GDS)
The Geriatric Depression Scale (GDS; Yesavage et al., 1982), has been tested and used extensively with an older adult population. The GDS Long Form is a 30-item questionnaire in which participants are asked to respond by answering “Yes” or “No” in reference to how they felt over the past week. The GDS Short Form consists of 15 questions and was developed in 1986. The GDS Short Form was used in this study, and permission to use the scale was granted as long as it was not used for profit and The Hartford Institute for Geriatric Nursing, New York University College of Nursing was cited as the source.
The Montreal Cognitive Assessment Scale (MoCA)
The Montreal Cognitive Assessment (MoCA) was developed as a quick screening tool for individuals with Mild Cognitive Impairment and early Alzheimer’s dementia (Nasreddine et al., 2005). The test evaluates attention and concentration, executive functions, memory, language, conceptual thinking, calculations, and orientation. The total possible score is 30 points with a score of 26 points or more usually considered normal. This cognitive measure is routinely used within the participating Life Plan Community to evaluate levels of cognitive function in all residents in the assisted living and dementia units, and the current score in the resident’s chart at the beginning of the study was used to determine inclusion or exclusion to participate. Raw MoCA scores were used in both studies.
Outcome Measure for Professional Caregivers
Perceived Stress Scale (PSS)
The Perceived Stress Scale (PSS; Cohen et al., 1983), is a widely used psychological instrument for measuring the perception of stress, consisting of 10 items with a five-point Likert Scale rating. It is a measure of the degree to which situations in one’s life are appraised as stressful. The questions are of a general nature and hence are relatively free of content specific to any sub-population group. The questions in the PSS ask about feelings and thoughts during the last month. This measure is in the public domain.
Analytic Procedures
For all resident outcome measures mixed-design 2 (experimental vs. control) × 2 (pre- and post-measures) ANCOVAs were conducted, with MoCA scores added as a covariate. Post-hoc pairwise comparisons were then performed. Professional caregiver responses on the PSS were analyzed using repeated-measures ANOVA. Pre-measures regardless of subject treatment condition were collected at the beginning of the respective studies and post-measurers were obtained at the end. The results were interpreted at a significance level of alpha < 0.05.
Results
Study 1: Advanced Dementia
There was a reliable difference in ABS scores between the Experimental Group and the Control Group (interaction effect F[1, 7] = 6.74, p = .036). Post-hoc pairwise comparisons revealed that emotional well-being in the Experimental Group significantly improved, whereas no reliable change was found in the Control Group. Effect size was large (partial eta squared = 0.49). There also was a significant difference in systolic blood pressure between the groups over a 12-week period (interaction effect F[1, 11] = 5.12, p = .045). Specifically, post-hoc pairwise comparisons indicated that systolic blood pressure in the Experimental Group significantly decreased over the course of 12 weeks, whereas systolic blood pressure of participants in the Control Group appeared to increase. Effect size was large (partial eta squared = 0.32). Results for Study 1 are summarized in Table 1. No statistically significant differences were observed between the Experimental and Control Group on the other outcome measures during the Advanced Dementia Study including challenging behaviors or the use of antipsychotic medications. Finally, results revealed a statistically significant decrease in professional caregiver stress (F[1, 29] = 6.06, p = .02) with a large effect size (Cohen’s d = 1.33). Table 3 presents changes in Perceived Stress Scale scores for professional caregivers in both studies.
Table 1.
Pre-Test and Post-Test Outcome Measures for Study 1 (Advanced Dementia).
| Outcome measures | Experimental group | Control group | ||
|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | |
| M (SE) | M (SE) | M (SE) | M (SE) | |
| Symptom measures for participants with dementia | ||||
| Affect balance scale | 46.33 (1.89) | 52.90 (3.10) | 52.45 (2.79) | 51.57 (1.51) |
| Geriatric depression scale | 5.00 (1.47) | 6.75 (1.93) | 4.17 (0.98) | 5.50 (2.25) |
| Systolic blood pressure | 123.25 (4.36) | 114.25 (5.89) | 115.17 (2.38) | 126.67 (2.64) |
| Diastolic blood pressure | 66.50 (3.13) | 69.25 (4.15) | 71.67 (2.28) | 71.00 (3.59) |
Note. Bold font indicates statistically reliable change between pre-test and post-test (p < .05).
Table 3.
Perceived Stress Scale Scores for Professional Caregivers in Studies 1 and 2.
| Outcome measure for professional caregivers | Study 1 (n = 30) | Study 2 (n = 9) | ||
|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | |
| M (SE) | M (SE) | M (SE) | M (SE) | |
| Perceived stress scale | 13.87 (1.24) | 11.03 (1.23) | 9.22 (0.88) | 4.89 (0.86) |
Note. Bold font indicates statistically reliable change between pre-test and post-test (p < .05).
Study 2: Mild Dementia
There was a statistically significant difference in ABS scores between the Experimental Group and the Control Group (interaction effect F[1, 16] = 9.36, p = .007). Post-hoc pairwise comparisons revealed that ABS scores in the Experimental Group significantly increased, indicating improved emotional well-being, whereas ABS scores in the Control Group decreased, indicating a decline in emotional well-being. Effect size was large (partial eta squared = 0.37). This finding remained significant with and without MoCA added as a covariate. A follow-up analysis revealed that this effect was attributed to a significant increase in Positive Affect. Unlike the Advanced Dementia Study, there was a statistically significant difference in the abbreviated GDS scores between the Experimental and Control Groups (interaction effect F[1, 13] = 6.82, p = .022). Specifically, pairwise comparisons indicated that GDS score in the Experimental Group significantly decreased, while GDS scores in the Control Group increased. Effect size again was large (partial eta squared = 0.34). There were also reliable MoCA score differences when it was analyzed as the dependent variable and age was added as a covariate in a 2 × 2 ANCOVA (interaction effect, F[1, 11] = 5.41, p = .04; partial eta squared = 0.33). Pairwise comparisons demonstrated that MoCA scores in the Control Group decreased, while MoCA scores in the Experimental Group remained approximately the same. Results for Study 2 are summarized in Table 2. Finally, there was a significant reduction in perceived professional caregiver stress (PSS) across the second study as well (F[1, 8] = 7.77, p = .024), with a large effect size (Cohen’s d = 1.66). Table 3 presents changes in Perceived Stress Scale scores for professional caregivers in both studies.
Table 2.
Pre-Test and Post-Test Outcome Measures for Study 2 (Mild Dementia).
| Outcome measures | Experimental group | Control group | ||
|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | |
| M (SE) | M (SE) | M (SE) | M (SE) | |
| Symptom measures for participants with dementia | ||||
| Affect balance scale | 3.50 (0.71) | 23.62 (5.81) | 32.60 (5.68) | 15.80 (7.06) |
| Geriatric depression scale | 5.43 (1.20) | 2.43 (0.48) | 2.00 (0.29) | 4.00 (0.97) |
| MoCA | 22.57 (0.52) | 24.14 (0.77) | 19.14 (2.07) | 15.14 (1.66) |
Note. Bold font indicates statistically reliable change between pre-test and post-test (p < .05). MoCA = montreal cognitive assessment.
Discussion
There is a substantial and growing interest in the use of computer engagement strategies for cognitively impaired older adults. This study included two randomized control trials that evaluated the effects of computer engagement on older individuals with advanced and mild dementia within a naturalistic setting. Results of this investigation support the use of computer engagement in these populations. Overall, results indicate that in both studies emotional well-being scores reliably increased in the experimental group. There were no reliable increases in the control group. Additionally, professional caregiver perceived stress was reliably reduced across the interventions in both studies. This investigation also found that individual guided computer engagement combined with unrestricted access in the Mild Dementia Study, but not the Advanced Dementia Study, had a positive benefit on depression and cognition. Statistically reliable improvements were found in depression scores within the experimental group, while these improvements did not occur in the control group. Cognition (MoCA scores) remained stable in the experimental group over the course of the Mild Dementia Study, in marked contrast to the control group where it declined.
The lack of a beneficial mood effect in the Advanced Dementia Study compared to the Mild Dementia Study may be attributed to impaired awareness in the Advanced Dementia Group compared to the Mild Dementia Group, as well as to the particular scale used. Specifically, the GDS requires a certain level of self-awareness to complete it accurately. Individuals who are unaware of the extent of their cognitive impairment may not be able to evaluate their problems fully, causing their responses on mood assessments to be relatively uniform without much variance; whereas, individuals with mild dementia would be more aware of cognitive changes, resulting in greater variance of their depression scores.
Contrary to a prior report by Chidester et al. (2016), there were no statistically reliable or significant effects in either study on challenging behaviors or antipsychotic use. The failure to reduce antipsychotic use may have been due to the small number of participants in this study who were prescribed those medications. They were residents in a Medicare five-star rated facility that makes great efforts to avoid using antipsychotics for challenging behaviors in individuals with cognitive impairments. Future randomized control studies are needed with larger numbers of individuals on antipsychotic medications to address this issue.
One of the most compelling outcomes of this study was the demonstration of a productive collaboration between academic institutions and a long-term care/assisted-living facility involving faculty from a medical school, three universities, medical students, doctoral psychology students, undergraduate recreational therapy students, advanced high school students, and practitioners working together. The benefits of this collaboration cannot be understated with regard to generating interest in future careers, inter-professionalism, and enriching the lives of all involved, including participants, family members, nurses, trainees, therapists, administrators, and investigators. This has led to cross-fertilization of future careers and research plans.
The present study is not free from limitations. The most notable limitation is the high attrition rate and the resulting small sample size of the analyzed data. High attrition rate is inherent in naturalistic settings involving older adults with dementia, and given that the average age of participants in the advanced dementia study was 93, mortality from natural causes (as well as progressing dementia, confusion, and refusal to complete the study) presented significant challenges in this project. Thus, statistical significance and meaning of findings have to be interpreted with caution in this context. Overall, the present study serves as a description of two preliminary pilot trials that demonstrated that computer engagement may in fact be beneficial for mood and cognition in older adults with dementia, but these findings need to be replicated in larger samples using more robust methodology. The present pilot studies provided further “proof of concept” evidence that technology use in individuals with dementia in a naturalistic setting is feasible, yet quite challenging. We hope that our findings may be of use as a starting point to future investigators examining technology use in the elderly, who may learn from our experience and challenges when designing their prospective studies. We recommend that future studies plan for at least 75% attrition rate or higher and design contingency plans to address high drop-out rates due to mortality, illness, and progressing dementia. Overall, the present study underscores the importance of additional computer intervention studies to address the challenges of working with persons diagnosed with dementia.
Additionally, it is not clear if the observed benefits were due to computer engagement alone, to the increased person-centered interactions related to guided sessions (as well as professional caregivers assisting with computers at other times), or to the specific functions of the iN2L computer platform. Further studies may consider alternative research methodologies to better differentiate these competing explanations. Future research should also aim to utilize measures with robust psychometric properties and demographically-adjusted norms (e.g., education-adjusted norms for the MoCA) and consider including other validated measures for the assessment of behaviors characteristic of older adults in a long-term care setting (e.g., Neuropsychiatric Inventory for Nursing Homes).
Finally, we recommend that future studies involve an on-going assent process with participants who have dementia, as well as include a staff education component. Although formal informed consent had been provided by legally authorized representatives for all study participants prior to study enrollment, participants with dementia may forget the exact nature and purpose of the study over time, especially if it lasts for several weeks or months. Therefore, an on-going process of building rapport between study personnel and participants is essential for any future studies that may wish to replicate our findings. Continuous education of staff on how to build such rapport may also be beneficial. Providing additional explanations regarding the nature of the study and obtaining assent from participants on a regular basis (not only at the beginning of the study) may help with reducing attrition. We hope that our study provides a first step in the formal investigation of technology use in older adults with varying levels of dementia using randomized control trials.
Clinical and Practical Implications
Prior research has consistently demonstrated that cognitively stimulating activities (Akbaraly et al., 2009; Rebok et al., 2014) and social engagement (Jao et al., 2018; Litwin & Shaul, 2019; Litwin & Stoeckel, 2016; Sharifian et al., 2020) have been linked to improved cognition and behavioral health outcomes in older adults. When older adults are faced with situations in which they are required to socially isolate (e.g., due to medical conditions or during pandemics, such as COVID-19 outbreak in 2019–2020), adequate social engagement, and cognitive stimulation may become rather limited, as the elderly constitute a vulnerable group during outbreaks of infectious disease and are often required to self-isolate to protect their health. This may lead to loneliness and an accompanying array of negative mental health outcomes, including depression (Liu et al., 2016; Taylor et al., 2018). Therefore, examining various ways in which the elderly can stay engaged and connected with the outside world even during periods of social isolation is of critical importance to their mental health.
Personal computers and electronic devices afford multiple opportunities for seniors to remain socially connected as long as they have access to internet. A plethora of free and readily available messaging and videoconferencing applications currently exist on the market and are often pre-programmed in various devices or can be easily downloaded and installed (e.g., instant messaging, FaceTime, Skype, Zoom, etc.). These applications may allow older adults to socially engage with their families and friends without increasing the risk of transmission of an infectious disease. Given the benefits of continued social engagement to older adults’ mental health and cognition (e.g., Jao et al., 2018; Sharifian et al., 2020), it is recommended that they regularly engage with loved ones through technology in times of social distancing.
Additionally, technology allows older adults to remain mentally active by engaging in interactive brain games, sudoku, crossword puzzles, playing word games and trivia with others, or through formal brain training programs that are available online. Various cognitive training programs have been associated with improved mood and cognition in middle-aged and older adults (e.g., McLaughlin et al., 2018; O’Shea et al., 2019). While remaining in the confines of their homes, seniors can also listen to audio books on their personal electronic devices to remain mentally active, as listening to audio books has been related to improved mental health in older adults (Ameri et al., 2017).
Furthermore, personal electronic devices allow users to engage in mood-boosting activities, such as listening to music and watching light entertainment (comedy shows, sitcoms, entertaining videos online). In the present study, listening to music and watching old sitcoms (such as “I Love Lucy”) were reported among the most popular and enjoyable activities by study participants. And in fact, these findings have scientific support, as previous research has linked humor and music to improved psychological wellbeing (Kaufmann et al., 2018; Sarkamo, 2018; Szabo, 2003); specifically, older adults who often listen to music have been reported to also engage in cognitive, physical, social, and spiritual activities (Kaufmann et al., 2018).
As far as spiritual activities are concerned, many religious organizations and churches resorted to broadcasting their services online during a recent COVID-19 pandemic to protect their congregations and to reduce the spread of coronavirus. Older adults who have access to a personal computer, a tablet, or a smartphone at home may continue to participate in spiritual and religious activities (e.g., streaming church services online, engaging in small groups, and prayer activities through video conferencing applications) even during periods of social distancing. Continued engagement in such activities may further contribute to positive health outcomes in this population (Agli et al., 2015).
Finally, physical activity has also been connected with improved cognition and mood in older adults (Barha et al., 2017; de Souto Barreto et al., 2016; Erickson et al., 2019; Ludyga et al., 2020; Northey et al., 2018). Access to a personal computer or a smart TV allows older adults to retain at least a certain level of physical activity when they are unable to access a gym or exercise outside. For example, various gaming platforms that integrate play with physical activity (such as Nintendo® Wii™ Sports) may improve depression and cognitive functioning in older adults (Rosenberg et al., 2010). In addition, multitude of exercise, yoga, and stretching videos exist online, and many are designed specifically for senior citizens. Of course, such activities should first be approved by one’s healthcare provider, but technology allows remote access to healthcare as well: seniors may consult with their healthcare providers through telehealth services that are becoming increasingly available.
In summary, technology platform utilized in this study (iN2L) and other commercially available technologies provide versatile opportunities for older adults to remain mentally, socially, and physically active during times of medically necessary social isolation. Although technology use is often seen as a feature of younger generations, emerging research indicates that older adults (including adults with cognitive impairments) can and do enjoy using technology. Published literature reveals that older adults with dementia use various mobile health applications and that the use of technology has been correlated with improvements in physical, mental, and social health in this population (Brown & O’Connor, 2020). However, available applications for patients with dementia do not yet meet the complex needs of this growing group (Guo et al., 2020). Therefore, based on the results of the present study and review of published literature, further investigations of various technologies and ways to adapt these technologies for comfortable use by the elderly are paramount at this time.
Conclusion
Presented studies evaluated the effects of computer engagement on mental health, challenging behaviors, antipsychotic medications, and professional caregiver stress in patients with advanced and mild dementia. No statistically reliable changes were observed for antipsychotic medications or challenging behaviors. Improvements were found in both studies for participants’ emotional well-being and professional caregiver stress. Additional reliable improvements in cognition and depression were found in the mild dementia study, but not in the advanced dementia study. Findings are limited by high attrition rate and the resulting small sample size; therefore, results should be interpreted with caution in this context. Thus, further research focusing on benefits of computer engagement in older adults with cognitive impairments is warranted in larger samples across a wider range of behavioral health outcomes.
Acknowledgments
This research was supported by faculty and students from Eastern Virginia Medical School, Virginia Wesleyan University, Regent University, Norfolk State University, Bayside High School Health Sciences Academy, and Westminster-Canterbury on Chesapeake Bay. We are thankful to our colleagues Laura Mock, Christy Kyrus, Mary Werber, Dr. Wayne Pollock, Dr. Abdinur Ali, Jacob Phillips, and Rebekah Kintzing who provided expertise that greatly assisted the research. Additionally, we would like to thank Dr. Robert Taylor, Mara Li Hart, and Trevor Wolterstorff for reviewing and providing editing expertise for this publication. Portions of this research have been presented to the International Alzheimer’s Conference in Toronto, Canada 2016 and the Society for Neuroscience Washington, DC 2017. We are also grateful to Ben Unkle, CEO of Westminster-Canterbury on Chesapeake Bay, for bringing together the various components in making this vision a reality and for providing the community at Westminster-Canterbury on Chesapeake Bay as a research site. Our deepest appreciation and gratitude are expressed to Sue and George Birdsong for providing the funding for this study. It could not have been possible without their generous donation.
Footnotes
Author’s Note: The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official position, policy or decision of the Eastern Virginia Medical School, Regent University, Westminster Canterbury on Chesapeake Bay, US Department of Veterans Affairs or US Department of Defense, unless so designated by other official documentation.
Writing of this manuscript was supported in part by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Research & Academic Affairs Service Line, Salisbury VA Medical Center, Salisbury, NC, and the Department of Veterans Affairs Mid-Atlantic (VISN 6) Mental Illness Research, Education, and Clinical Center (MIRECC).
This study was approved by Internal Review Board at Eastern Virginia Medical School with subsequent amendments (IRB # 15-05-EX-0104) to include Study 1 (advanced dementia) and Study 2 (mild dementia).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Birdsong Foundation and the Westminster-Canterbury on Chesapeake Bay.
ORCID iD: Anna S. Ord  https://orcid.org/0000-0003-3373-2016
https://orcid.org/0000-0003-3373-2016
References
- Agli O., Bailly N., Ferrand C. (2015). Spirituality and religion in older adults with dementia: A systematic review. International Psychogeriatrics, 27(5), 715–725. 10.1017/s1041610214001665 [DOI] [PubMed] [Google Scholar]
- Akbaraly T. N., Portet F., Fustinoni S., Dartigues J. F., Artero S., Rouaud O., Touchon J., Ritchie K., Berr C. (2009). Leisure activities and the risk of dementia in the elderly: Results from the Three-City Study. Neurology, 73(11), 854–861. 10.1212/WNL.0b013e3181b7849b [DOI] [PubMed] [Google Scholar]
- Ameri F., Vazifeshenas N., Haghparast A. (2017). The impact of audio book on the elderly mental health. Basic and Clinical Neuroscience, 8(5), 361–370. 10.18869/nirp.bcn.8.5.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell A. J., Ellis M. P., Bernardi L., Alm N., Dye R., Gowans G., Campbell J. (2010). Using a touch screen computer to support relationships between people with dementia and caregivers. Interacting with Computers, 22(4), 267–275. 10.1016/j.intcom.2010.03.003 [DOI] [Google Scholar]
- Astell A. J., Joddrell P., Groenewoud H., Lange J. D., Goumans M., Cordia A., Schikhof Y. (2016). Does familiarity affect the enjoyment of touchscreen games for people with dementia? International Journal of Medical Informatics, 91, 1–8. 10.1016/j.ijmedinf.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Aguirre E., Woods R. T., Spector A., Orrell M. (2013). Cognitive stimulation for dementia: A systematic review of the evidence of effectiveness from randomized controlled trials. Ageing Research Reviews, 12(1), 253–262. [DOI] [PubMed] [Google Scholar]
- Bakerjian D. (2014). CMS national partnership to improve dementia care in nursing homes. Geriatric Nursing, 35, 77–79. [Google Scholar]
- Barha C. K., Davis J. C., Falck R. S., Nagamatsu L. S., Liu-Ambrose T. (2017). Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Frontiers in Neuroendocrinology, 46, 71–85. 10.1016/j.yfrne.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Bradburn N. (1969). The structure of psychological well-being. Aldine. [Google Scholar]
- Brown A., O’Connor S. (2020). Mobile health applications for people with dementia: a systematic review and synthesis of qualitative studies. Informatics for Health and Social Care, 45(4), 343–359. [DOI] [PubMed] [Google Scholar]
- Chidester A. S., Sautter S. W., Aravich P., Ord A. S. (2016). Transforming dementia care and quality of life using innovating touch screen computer engagement: A research study – The Birdsong Initiative [Paper presentation]. Annual Alzheimer’s Association International Conference, Toronto, Canada. 10.1016/j.jalz.2016.06.465 [DOI]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- De Leo G., Brivio E., Sautter S. W. (2011). Supporting autobiographical memory in patients with Alzheimer’s disease using smart phones. Applied Neuropsychology, 18(1), 69–76. 10.1080/09084282.2011.545730 [DOI] [PubMed] [Google Scholar]
- De Souto Barreto P., Delrieu J., Andrieu S., Vellas B., Rolland Y. (2016). Physical activity and cognitive function in middle-aged and older adults: An analysis of 104,909 people from 20 Countries. Mayo Clinic Proceedings, 91(11), 1515–1524. 10.1016/j.mayocp.2016.06.032 [DOI] [PubMed] [Google Scholar]
- Eggert J., Dye C. J., Vincent E., Parker V., Daily S. B., Pham H., Watson A. T., Summey H., Roy T. (2015). Effects of viewing a preferred nature image and hearing preferred music on engagement, agitation, and mental status in persons with dementia. SAGE Open Medicine, 3, 2050312115602579. 10.1177/2050312115602579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Hillman C., Stillman C. M., Ballard R. M., Bloodgood B., Conroy D. E., Macko R., Marquez D. X., Petruzzello S. J., Powell K. E. (2019). Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Medicine and Science in Sports and Exercise, 51(6), 1242–1251. 10.1249/mss.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzler S., Raia P., Buckley F. O., Wang M. (2016). Does nursing facility use of habilitation therapy improve performance on quality measures? American Journal of Alzheimer’s Disease & Other Dementias, 31(8), 687–692. 10.1177/1533317516662335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates N. J., Sachdev P. S., Fiatarone Singh M. A., Valenzuela M. (2011). Cognitive and memory training in adults at risk of dementia: A systematic review. BMC Geriatrics, 11(1), 55 10.1186/1471-2318-11-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Yang F., Hu F., Li W., Ruggiano N., Lee H. Y. (2020). Existing mobile phone apps for self-care management of people with Alzheimer’s disease and related dementias: Systematic analysis. Journal of Medical Internet Research: Aging, 3(1), e15290. 10.2196/15290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao Y. L., Loken E., MacAndrew M., Van Haitsma K., Kolanowski A. (2018). Association between social interaction and affect in nursing home residents with dementia. Aging and Mental Health, 22(6), 778–783. 10.1080/13607863.2017.1304526 [DOI] [PubMed] [Google Scholar]
- Kaufmann C. N., Montross-Thomas L. P., Griser S. (2018). Increased engagement with life: Differences in the cognitive, physical, social, and spiritual activities of older adult music listeners. Gerontologist, 58(2), 270–277. 10.1093/geront/gnw192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Park M. (2017). Effectiveness of person-centered care on people with dementia: A systematic review and meta-analysis. Clinical Interventions in Aging, 12, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancioni G. E., O’Reilly M. F., Singh N. N., Sigafoos J., Alberti G., Boccasini A., Oliva D., Lang R. (2014). Technology-aided programs to enable persons with multiple disabilities to move through sequences of occupational activities independently. Journal of Developmental and Physical Disabilities, 26(6), 703–715. 10.1007/s10882-014-9390-4 [DOI] [Google Scholar]
- Leng F. Y., Yeo D., George S., Barr C. (2014). Comparison of iPad applications with traditional activities using person-centered care approach: Impact on well-being for persons with dementia. Dementia, 13(2), 265–273. 10.1177/1471301213494514 [DOI] [PubMed] [Google Scholar]
- Leuty V., Boger J., Young L., Hoey J., Mihailidis A. (2013). Engaging older adults with dementia in creative occupations using artificially intelligent assistive technology. Assistive Technology, 25(2), 72–79. 10.1080/10400435.2012.715113 [DOI] [PubMed] [Google Scholar]
- Lim F. S., Wallace T., Luszcz M. A., Reynolds K. J. (2013). Usability of tablet computers by people with early-stage dementia. Gerontology, 59(2), 174–182. 10.1159/000343986 [DOI] [PubMed] [Google Scholar]
- Litwin H., Shaul A. (2019). The effect of social network on the physical activity-cognitive function nexus in late life. International Psychogeriatrics, 31(5), 713–722. 10.1017/s1041610218001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin H., Stoeckel K. J. (2016). Social network, activity participation, and cognition: A complex relationship. Research on Aging, 38(1), 76–97. 10.1177/0164027515581422 [DOI] [PubMed] [Google Scholar]
- Liu L., Gou Z., Zuo J. (2016). Social support mediates loneliness and depression in elderly people. Journal of Health Psychology, 21(5), 750–758. 10.1177/1359105314536941 [DOI] [PubMed] [Google Scholar]
- Livingston G., Kelly L., Lewis-Holmes E., Baio G., Morris S., Patel N., Omar R. Z., Katona C., Cooper C. (2014). Non-pharmacological interventions for agitation in dementia: Systematic review of randomised controlled trials. The British Journal of Psychiatry, 205(6), 436–442. 10.1192/bjp.bp.113.141119 [DOI] [PubMed] [Google Scholar]
- Ludyga S., Gerber M., Puhse U., Looser V. N., Kamijo K. (2020). Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nature Human Behavior. Advance online publication. 10.1038/s41562-020-0851-8 [DOI] [PubMed]
- McLaughlin P. M., Curtis A. F., Branscombe-Caird L. M., Comrie J. K., Murtha S. J. E. (2018). The feasibility and potential impact of brain training games on cognitive and emotional functioning in middle-aged adults. Games for Health Journal, 7(1), 67–74. 10.1089/g4h.2017.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J. L., Chertkow H. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Northey J. M., Cherbuin N., Pumpa K. L., Smee D. J., Rattray B. (2018). Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. British Journal of Sports Medicine, 52(3), 154–160. 10.1136/bjsports-2016-096587 [DOI] [PubMed] [Google Scholar]
- O’Shea D. M., De Wit L., Smith G. E. (2019). Doctor, should I use computer games to prevent dementia? Clinical Gerontologist, 42(1), 3–16. 10.1080/07317115.2017.1370057 [DOI] [PubMed] [Google Scholar]
- Quayhagen M. P., Quayhagen M., Corbeil R. R., Hendrix R. C., Jackson J. E., Snyder L., Bower D. (2000). Coping with dementia: Evaluation of four nonpharmacologic interventions. International Psychogeriatrics, 12(2), 249–265. 10.1017/s1041610200006360 [DOI] [PubMed] [Google Scholar]
- Rebok G. W., Ball K., Guey L. T., Jones R. N., Kim H. Y., King J. W., Marsiske M., Morris J.N., Tennstedt S.L., Unverzagt F.W., Willis S.L.; ACTIVE Study Group. (2014). Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62(1), 16–24. 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D., Depp C. A., Vahia I. V., Reichstadt J., Palmer B. W., Kerr J., Norman G., Jeste D. V. (2010). Exergames for subsyndromal depression in older adults: A pilot study of a novel intervention. The American Journal of Geriatric Psychiatry, 18(3), 221–226. 10.1097/JGP.0b013e3181c534b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkamo T. (2018). Cognitive, emotional, and neural benefits of musical leisure activities in aging and neurological rehabilitation: A critical review. Annals of Physical and Rehabilitation Medicine, 61(6), 414–418. 10.1016/j.rehab.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Sharifian N., Kraal A. Z., Zaheed A. B., Sol K., Zahodne L. B. (2020). Longitudinal associations between contact frequency with friends and with family, activity engagement, and cognitive functioning. Journal of the International Neuropsychological Society, 26(8), 815 10.1017/s1355617720000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A. (2003). The acute effects of humor and exercise on mood and anxiety. Journal of Leisure Research, 35(2), 152–162. [Google Scholar]
- Tak S. H., Zhang H., Patel H., Hong S. H. (2015). Computer activities for persons with dementia. The Gerontologist, 55(Suppl. 1), S40–S49. 10.1093/geront/gnv003 [DOI] [PubMed]
- Taylor H. O., Taylor R. J., Nguyen A. W., Chatters L. (2018). Social isolation, depression, and psychological distress among older adults. Journal of Aging and Health, 30(2), 229–246. 10.1177/0898264316673511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamani M., McDonald L., Saez Aguayo I., Kanios C., Katsanou M. N., Madeley L., Limousin P. D., Lees A. J., Haritou M., Jahanshahi M. (2014). A randomized controlled pilot study to evaluate a technology platform for the assisted living of people with dementia and their carers. Journal of Alzheimer’s Disease, 41(2), 515–523. [DOI] [PubMed] [Google Scholar]
- Vahia I. V., Kamat R., Vang C., Posada C., Ross L., Oreck S., Bhatt A., Depp C., Jeste D. V., Sewell D. D. (2017). Use of tablet devices in the management of agitation among inpatients with dementia: An open-label study. The American Journal of Geriatric Psychiatry, 25(8), 860–864. 10.1016/j.jagp.2016.07.011 [DOI] [PubMed] [Google Scholar]
- Valenzuela M., Sachdev P. (2006). Brain reserve and dementia: A systematic review. Psychological Medicine, 36(4), 441–454. 10.1017/S0033291705006264 [DOI] [PubMed] [Google Scholar]
- Wilson R. S., Mendes De, Leon C. F., Barnes L. L., Schneider J. A., Bienias J. L., Evans D. A., Bennett D. A. (2002). Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA, 287(6), 742–748. [DOI] [PubMed] [Google Scholar]
- Yesavage J. A., Brink T., Rose T. L., Lum O., Huang V., Adey M., Leirer V. O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Zucchella C., Sinforiani E., Tassorelli C., Cavallini E., Tost-Pardell D., Grau S., Pazzi S., Puricelli S., Bernini S., Bottiroli S., Vecchi T., Sandrini G., Nappi G. (2014). Serious games for screening pre-dementia conditions: From virtuality to reality? A pilot project. Functional Neurology, 29(3), 153–158. [PMC free article] [PubMed] [Google Scholar]