Abstract
Study Design:
This study presents a case-control study of 33 patients who underwent secondary orbital reconstruction, evaluating techniques and outcome.
Objective:
Adequate functional and aesthetical appearance are main goals for secondary orbital reconstruction. Insufficient premorbid orbital reconstruction can result in hypoglobus, enophthalmos, and diplopia. Computer-assisted surgery and the use of patient-specific implants (PSIs) is widely described in the literature. The authors evaluate the use of selective laser-melted PSIs and hypothesize that PSIs are an excellent option for secondary orbital reconstruction.
Methods:
The sample was composed of 33 patients, previously treated with primary orbital reconstruction, presenting themselves with indications for secondary reconstruction (i.e. enophthalmos, diplopia, or limited eye motility). Computed tomography and/or cone beam data sets were assessed before and after secondary reconstruction comparing intraorbital volumes, infraorbital angles, and clinical symptoms. Clinical outcomes were assessed using a standardized protocol.
Results:
Results show a significant change in intraorbital volumes and a reduction of clinical symptoms after secondary reconstruction.
Conclusions:
Outcomes of this study suggest that secondary orbital reconstruction can be performed routinely using selective laser-melted PSIs and titanium spacers.
Keywords: orbital reconstruction, selective laser melting, patient-specific implant, three-dimensional mesh, enophthalmos, diplopia, computer-assisted surgery
Introduction
Primary orbital reconstruction after trauma or ablative surgery can be extremely challenging.1 Reconstructive orbital procedures primarily aim to reconstruct premorbid bony anatomy in order to restore functionality.2 Computed tomography (CT) is helpful for diagnosis and treatment planning.1,3 However, a significant minority of patients with, for instance, complex two-wall injuries will have postoperative enophthalmos, hypoglobus, and diplopia, requiring secondary orbital reconstruction.4 Patients with both functional (diplopia) and esthetic (globe malposition) deformities will benefit from early revision surgery to precisely reconstruct the premorbid orbital contour.5,6 To our knowledge, there is no existing workflow to correct secondary orbital deformities. Frequently secondary reconstruction is solemnly based on surgeons’ experience in orbital reconstruction.7 Usually clinical symptoms occur due to changes in intraorbital volume. Experimental alternatives to adjust the orbital volume are, that is, bioabsorbable sheets (polylactic acid, polyglycolic acid, etc.),8,9 the use of expanders,10 titanium meshes, glass-bioceramic implants,11 and fat augmentation.12 Recent advances in computer-aided 3D printing allows for the use of selective laser melting for fabrication of patient-specific titanium implants. Benefits of patient-specific implants (PSIs) are extremely accurate shape, biocompatibility, structural stability, radio-opacity, and ease of insertion/stabilization.13
Aim of this study was to describe a solution for simulation of the globe position and the restoration of bony orbital boundaries. By using virtual planning techniques such as mirroring of the unaffected side and the smart shaper tool for overcorrection, an adequate secondary reconstruction of complex orbital fractures could be performed.
Materials and Methods
After approval by the Ethics Committees of the University of Hannover School of Medicine and the Medical School of Heinrich-Heine University of Duesseldorf, 33 consecutive patients with previously treated, unilateral orbital or orbito-zygomatic injuries were identified. Indications for secondary orbital reconstruction included significant enophthalmos (>2 mm), significant exophthalmos (>2 mm), limited eye motility, and persistent diplopia over a period of at least 6 months. Thin cut (0.75-1.25 mm) preoperative CT data were uploaded into presurgical planning software (iPlan 3.0.5, BrainLab), which was used to evaluate each orbital injury.
The unaffected bony contour of the contralateral side was segmented into an isolated virtual object (i.e. virtual orbit). The virtual object was then mirrored across the midline and positioned over the injured side. Uninjured landmarks at the periphery of the injury were used to align with the virtual orbit, generating a virtual plan, which was then used for surgical reconstruction. The virtual plan was then exported as an STL file and used as a template for planning the PSI. In order to adjust intraorbital volumes, the smart shaper tool was used (iPlan 3.0.5, BrainLab). In cases of great intraorbital volume adjustments, additional titanium spacers were used (Figures 1 to 3; the indication for additional spacers was a greater volume in comparison to the unaffected side in combination with clinical symptoms and an interoperative lack of volume due to changes in soft tissue). If the orbital volume had to be increased, a re-osteotomy and zygomatic repositioning was performed. In collaboration with engineers (KLS-Martin), a virtual implant was fabricated, covering the orbital injury and resting on stable bone. Once a final implant contour was approved, the PSI was fabricated using selective laser melting. Fabrication time was around 5 days. The patient was then taken to the operating room and the injured orbit was exposed using a transconjunctival (retroseptal incision without lateral canthotomy, n = 32) or an infraorbital (n = 1) approach. Previous hardware, that is, titanium meshes or previous PSIs, were removed if these were hindering the secondary reconstruction, infection or if it appeared they were causing the clinical symptoms (objectified by clinical assessment in combination with 3D imaging). The implant was initially positioned to cover the area of injury using peripheral anatomic landmarks for alignment. Precise implant position was confirmed (to within 1 mm) by comparison to the planned virtual reconstruction using intraoperative navigation (Kick, BraiLab). Confirmation points included the infraorbital rim, lateral rim, orbital floor, medial/lateral orbital walls, posteromedial bulge, posterior shelf, and globe projection. The implant was then fixated with 1.3-mm microscrews (DePuy, Synthes-Compact) and the incision was in all cases a retroseptal transconjunctival approach. All operations were performed by the same surgeon. Postoperative cone beam (NewTom DVT 9000, Deutschland AG) or intraoperative fan beam scans (Siemens Arcadis Orbic 3D) were obtained in all patients. If the insertion of the PSI did not create the desired changes in soft tissue, additional spacers were added. Their use was an intraoperative, subjective decision by the head surgeon and made by trial and error. In order not to dislocate or hinder eye movement, spacers were placed in periosteal pockets on the lateral or medial orbital wall depending on globe dislocation. There were no typical intraoperative complications. The postoperative images were then superimposed onto the preoperative plan. Any differences of the actual implant position and the planned implant position were documented. All patients were evaluated for postoperative enophthalmos, diplopia, visual acuity, and hypoesthesia. Times of follow-up imaging ranged from 2 to 14 days. Clinical follow-up was performed weekly over a period of 1 month and again after 3 months.
Figure 1.

Example of four sizes of titanium spacers (20 mm = 456.4431 mm3, 25 mm = 584.0979 mm3) used in this study in order to add volume and correct the eyeball position.
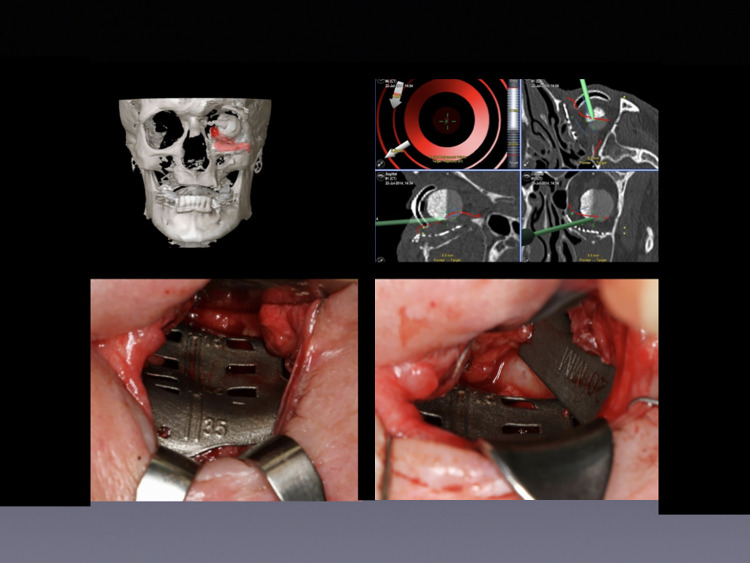
Figure 2.
Implant and spacer insertion for treatment of exophthalmia using a transconjunctival approach. Upper left: 3D model of the placed implant involving the left orbital floor. Upper right: Intraoperative navigation using previously planned trajectories. Lower left: Intraoperative view of computer-assisted patient-specific SLM implant placement via transconjunctival approach. Lower right: Placement of a 20-mm titanium spacer via transconjunctival approach to augment volume.
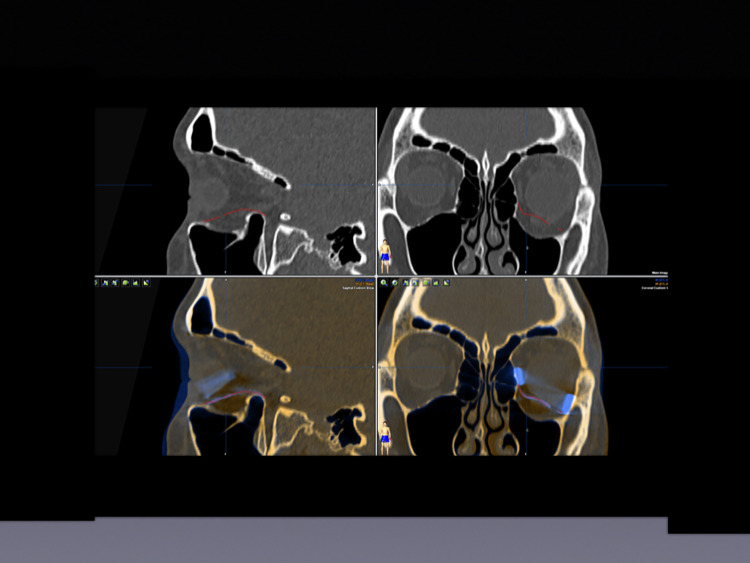
Figure 3.
Planning of implant placement and volume augmentation using titanium spacers. Upper left: Sagittal view—SLM implant (red) placed to reconstruct the orbital floor. Upper right: Axial view of the placed SLM implant (red). Lower left: Sagittal view of the SLM implant (red) with an additional titanium spacer (blue) used to augment volume. Lower right: Coronal view of the midface. SLM implant (red) placed on the left orbital floor and two titanium spacers placed at the lateral and the medial lower orbital walls to add volume.
Postoperatively, all data sets were aligned following the lower orbital rim to the external ear canal and the medial–sagittal axis. Furthermore, all patients were examined by an ophthalmologist. Primary outcome measurements were:
Orbital Volume: Pre- and postoperative orbital CT volumes were measured (iPlan CMF 3.0.5, BrainLab).14 The preoperative volume of the affected exe was compared to the volume of the unaffected side and to the postoperative volume. Analysis was performed by the primary surgeon (M.R.) to assure intra-observer repeatability. To calculate volumes, the orbital cavities were segmented automatically and in case of remaining defects or reduction of the orbital cavity due to titanium meshes, extensive scaring, bone fragments, and so on were manually adjusted using the smart shaper tool.
Intraorbital angles (anterior, medial, and posterior angles between the medial orbital wall and the orbital floor): Intraorbital angles were compared at three points (anterior, middle, and posterior) in both the virtual plan and postoperative CT scan. They were measured in the coronal layers. The measurements were made using the anterior orbital rim, the posterior ledge and the exact in-between as fixed reference points in sagittal layers.
Diplopia: Diplopia was documented as either negative or positive. If positive, it was documented as being either in primary gaze or with globe excursion (including the direction of excursion that resulted in diplopia).
Visual acuity: Visual acuity was graded as either normal (0) or reduced (1). It was determined via finger perimetry.
Enophthalmos/Exophthalmos: Pre- and postoperative globe position was documented using a Naugle Exophthalmometer (Oculus).15,16 Persistent enophthalmos/exophthalmos was defined as a difference of >2 mm between sides.
Motility: Extraocular movement was checked having the patient follow the examiner’s finger moving across their full range of horizontal and vertical eye movement.
Hypesthesia: Reduced sensitivity in V2 areas was checked by clinical evaluation (sharp–dull, hot–cold, and 2-point discrimination using the Nerve Evaluation Protocol of 2014 by the California Association of Oral and Maxillofacial Surgeons, classified by the MRC Scale).
In this prospective study, data were analyzed using IBM SPSS for Mac, Version 22.0 (IBM Corp.). Each study variable was computed by descriptive statistics. The Shapiro–Wilk test was used for evaluation of normal distribution. For testing differences between planned versus achieved orbital volume and three angles (anterior, medial, posterior), a matched pair t-test was used to assess the differences. The level of statistical significance was set at P ≤ 0.05. All P values were two-sided. Because of the small number of the sample, means and ranges were used as descriptive statistics.
Results
Fourteen women and 19 men were included in the study, with an age range of 17-74 years (mean 33 years). Indications for surgery were enophthalmos (n = 4), diplopia (n = 2), limited motility (n = 1), hypoglobe (n = 1), and a combination of symptoms (additionally including hypoesthesia and exophthalmos) (n = 25). The anteroposterior dimension of the disrupted orbital floor measured on average 19.1 × 24.6 mm (axial × sagittal). For previous orbital reconstruction, poly-p-dioxanone (PDS) foil (n = 15), titanium-dynamic mesh (n = 10), PDS foil and titanium-dynamic mesh in combination (n = 2), and open reduction and fixation only (n = 6) had been used.
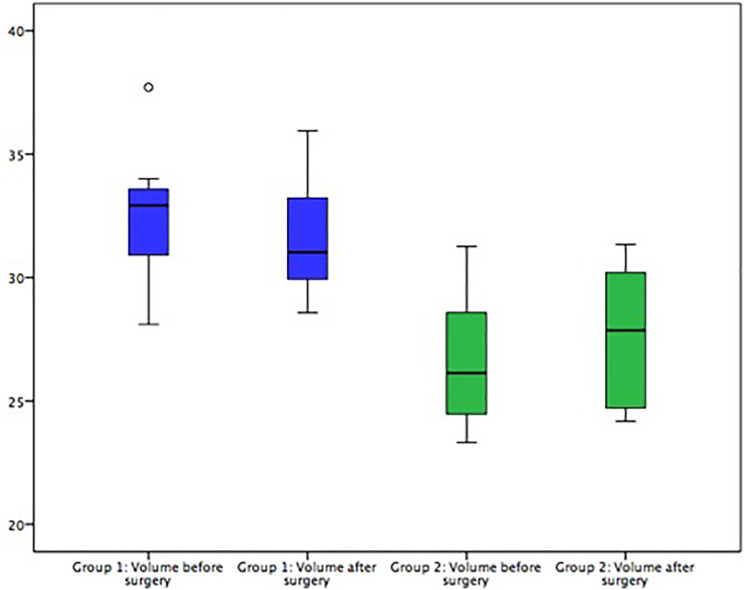
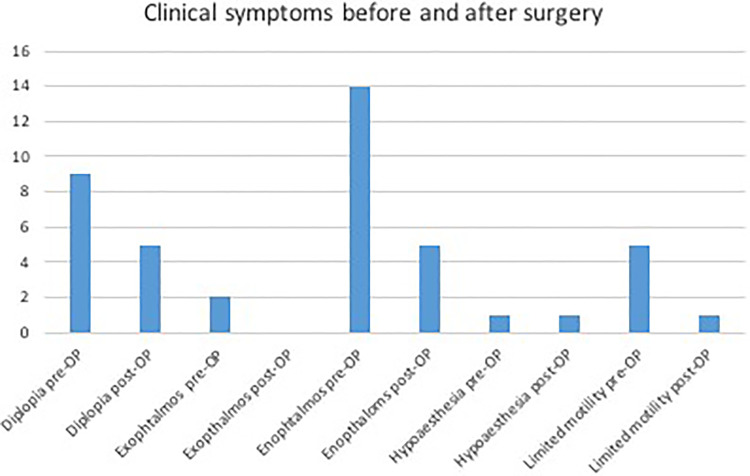
Intraorbital volumes were evaluated before and after secondary reconstructive surgery. Cases were divided into two groups. Group 1: The orbital volume was bigger than the volume of the unaffected side (i.e. due to insufficient fracture reposition etc.), causing clinical symptoms (n = 18). Group 2: The orbital volume was smaller than of the unaffected other orbit (i.e. due to mispositioned titanium meshes, extensive scaring, etc.), causing clinical symptoms (n = 8). For group 1, the mean volume was 28.35 cm3 (SD ± 4.44 cm3) before secondary surgery and 27.28 cm3 (SD ± 4.46 cm3) after secondary surgery. There was a significant difference in infraorbital volumes pre- and postoperatively (–1.07 cm3, P ≤ 0.001). In group 2, a mean volume of 26.44 cm3 (SD ± 2.92 cm3) before surgery and 27.35 cm3 after surgery (SD ± 2.94 cm3) was evaluated, creating a statistically significant difference of +0.91 cm3 (P = 0.034) (Figure 4). Additionally, differences in volume before and after surgery were compared to those of the unaffected mirrored side. The presurgical mean of differences was 1.95 cm3 (SD ± 2.11 cm3), the postsurgically average difference in comparison to the volume of the healthy side was 1.88 cm3 (SD ± 2.28 cm3). The infraorbital angles were measured pre- and postoperatively. There were no statistically significant changes in angle sizes (values of the two groups mentioned above were tested separately but results showed no further statistical benefit). Furthermore, clinical symptoms consecutively leading to surgical revision (i.e. diplopia, enophthalmos, etc.) were assessed and reevaluated after secondary reconstruction. In general, clinical symptoms decreased. Diplopia could be reduced from occurrence in nine cases down to five cases and motility could be improved from five cases down to only one (Figure 5). One case of hypoesthesia was reported (classified as grade S0 on the MRC Scale), which did not change after surgery.
Figure 4.
Results of the intraorbital volume measurements. In group 1, volume had to be reduced in order to reconstruct the original orbit. Results show that the volume is significantly reduced after surgery (blue boxes). In group 2, volume had to be added in order to reconstruct the original orbit. Results show a significant addition in volume (green boxes).
Figure 5.
Clinical symptoms before and after surgery.
Discussion
Complex orbital fractures are frequently associated with complications, such as persistent diplopia, infraorbital and optical nerve injuries, enophthalmos, facial disproportion, and hypoglobe.17 Exact reconstruction cannot always be achieved, requiring secondary surgery.17 Accuracy of secondary orbital reconstruction is even harder to obtain and cannot always fully restore the original condition.18 Computer-assisted navigated surgery has many advantages compared to conventional surgical techniques.19 Virtual modeling and planning provides large benefits for the surgeon: better preparation and a shorter duration of the operation itself.20 When it comes to secondary reconstruction, cases are predominantly more challenging because the orbital anatomy is harmed and landmarks are hard to find.21 Nkenke et al. compared prefabricated implants with titanium meshes for the correction of post-traumatic enophthalmos and concluded that titanium meshes should be favored.11 However, the use of preplanned PSIs allows accurate intraoperative navigation and verifies correct positioning of the implant. In the past years, many studies and case reports have proven the advantages of digital planning together with customized orbital implants.6,19,22–25 A recent study by Chen et al. combined intraoperative navigation with endoscopic assistance giving even greater intraoperative overview.26 When using PSIs, size and thickness are variable providing solutions for difficult anatomic situations as in secondary orbital reconstruction. In addition, titanium spacers can be used for intraorbital volume adjustments (Figures 1 to 3). Different techniques for secondary reconstruction are currently under development. Gaffrée et al. for example, presented a case in which a titanium mesh in combination with a buccal fat pad graft has successfully been used.27 Results of this study show a significant change in intraorbital volume. PSIs were created mirroring the unaffected bony side. The correction was planned true to original. A change in volume might alleviate clinical symptoms. Due to the nature of the operation with primarily reconstructed defects, extensive changes in infraorbital angles or volume modifications to meet the exact volume of the healthy side could not be expected. Since changes in soft tissue and contractions by scar tissue currently cannot be predicted by planning software, desired changes in soft tissue were corrected intraoperatively by trial and error using additional spacers. There is no protocol to follow for the use of theses spacers yet. It will be subject to further research. A potential side effect of the use of titanium spacers might be dislocation due to incorrect spacer placement. In addition, one might think that the spacer itself or extensive scar formation might create an obstacle for eye motility over time. So far no complication of this kind has been reported. Recurring clinical checkups will hopefully uncover yet unknown side effects. Furthermore, when comparing results of this study, changes in volumes remained low. Unpredictable shifts in soft tissue might be the reason for a possible alleviation of symptoms not the volume change itself. Unfortunately, no correlation between the volume discrepancy and degree of enophthalmos could be calculated because although using a Naugle Exophthalmometer the exact degree of exophthalmos was not examined. This presents a limitation of this study and constraints its informative value.
A well-planned PSI appears important, selective laser melting seems to be a suitable technique. In comparison to other materials, selective laser-melted implants are still pricey to produce. Moreover, the navigation system is expensive to obtain.1 Furthermore, bioresorbable materials have recently proven to be an option for secondary reconstruction. Pan et al. could improve diplopia, exophthalmos, and ocular motility in 16 cases using Rapidsorb implants.28 However, long-term results still have to follow and an ideal solution for secondary reconstruction has not yet been found. This innovative approach might be another step to provide best functional care and esthetic appearance for patients.
In conclusion, the use of PSIs appears to be a possible solution for delayed secondary orbital reconstruction. This technique is a valuable tool for optimization and assessment regarding the bony structures after reestablishing correct anatomical conditions. The results obtained from this study may be summarized as follows:
Secondary orbital reconstruction can be performed using PSIs.
In two-thirds of all cases, clinical symptoms can be eliminated or reduced when treated with customized intraorbital volume changes through the use of PSIs and titanium spacers.
Considering the advantages, these techniques provide a valuable tool for optimization of secondary reconstruction of bony structures. The effect on facial soft tissues is still insufficient and has to be taken into account when preforming surgery.
Acknowledgements
The authors thank Merve Karahisarlioglu and Prof. Dr Norbert R. Kübler for their advice, constructive critics, and support.
Authors’ Note: D.D.S. and L.S. contributed equally to the work.
Data Availability Statement: The data sets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All procedures performed in the presented study involving human participants were in accordance with the ethical standards of ethic commission of Hannover Medical School, Germany (no. 2281-2014) and the ethic commission of Duesseldorf Medical School (no. 2018-250-Zweitvotum) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was registered at the German Clinical Trials Register (DRKS-ID: DRKS00006549). The article does not comprise personal details of patients.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lara Schorn  https://orcid.org/0000-0002-4301-0693
https://orcid.org/0000-0002-4301-0693
References
- 1. Gellrich NC, Schramm A, Hammer B, et al. Computer-assisted secondary reconstruction of unilateral posttraumatic orbital deformity. Plast Reconstr Surg. 2002;110(6):1417–1429. [DOI] [PubMed] [Google Scholar]
- 2. Rana M, Chui CHK, Wagner M, Zimmerer R, Rana M, Gellrich N-C. Increasing the accuracy of orbital reconstruction with selective laser-melted patient-specific implants combined with intraoperative navigation. J Oral Maxillofac Surg. 2015;73(6):1113–1118. [DOI] [PubMed] [Google Scholar]
- 3. Manson PN, Markowitz B, Mirvis S, Dunham M, Yaremchuk M. Toward CT-based facial fracture treatment. Plast Reconstr Surg. 1990;85(2):202–212; discussion 213-214. [PubMed] [Google Scholar]
- 4. Biesman BS, Hornblass A, Lisman R, Kazlas M. Diplopia after surgical repair of orbital floor fractures. Ophthal Plast Reconstr Surg. 1996;12(1):9–16; discussion 17. [DOI] [PubMed] [Google Scholar]
- 5. Burnstine MA. Clinical recommendations for repair of isolated orbital floor fractures: an evidence-based analysis. Ophthalmology. 2002;109(7):1207–1210; discussion 1210-1211; quiz 1212-1213. [DOI] [PubMed] [Google Scholar]
- 6. Bell RB, Markiewicz MR. Computer-assisted planning, stereolithographic modeling, and intraoperative navigation for complex orbital reconstruction: a descriptive study in a preliminary cohort. J Oral Maxillofac Surg. 2009;67(12):2559–2570. [DOI] [PubMed] [Google Scholar]
- 7. Whitaker LA, Yaremchuk MJ. Secondary reconstruction of posttraumatic orbital deformities. Ann Plast Surg. 1990;25(6):440–449. [DOI] [PubMed] [Google Scholar]
- 8. Villarreal PM, Monje F, Morillo AJ, Junquera LM, González C, Barbón JJ. Porous polyethylene implants in orbital floor reconstruction. Plast Reconstr Surg. 2002;109(3):877–885; discussion 886-887. [DOI] [PubMed] [Google Scholar]
- 9. Rubin PA, Bilyk JR, Shore JW. Orbital reconstruction using porous polyethylene sheets. Ophthalmology. 1994;101(10):1697–1708. [DOI] [PubMed] [Google Scholar]
- 10. Schittkowski MP, Gundlach KK, Guthoff RF. Treatment of congenital clinical anophthalmos with high hydrophilic hydrogel expanders. Ophthalmologe. 2003;100(7):525–534. [DOI] [PubMed] [Google Scholar]
- 11. Nkenke E, Vairaktaris E, Spitzer M, et al. Secondary reconstruction of posttraumatic enophthalmos: prefabricated implants vs titanium mesh. Arch Facial Plast Surg. 2011;13(4):271–277. [DOI] [PubMed] [Google Scholar]
- 12. Baum SH, Schmeling C, Pförtner R, Mohr C. Autologous dermis—fat grafts as primary and secondary orbital transplants before rehabilitation with artificial eyes. J Craniomaxillofac Surg. 2018;46(1):90–97. [DOI] [PubMed] [Google Scholar]
- 13. Mantripragada VP, Lecka-Czernik B, Ebraheim NA, Jayasuriya AC. An overview of recent advances in designing orthopedic and craniofacial implants. J Biomed Mater Res A. 2013;101(11):3349–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rana M, Essig H, Rücker M, Gellrich N-C. Development and demonstration of a novel computer planning solution for predefined correction of enophthalmos in anophthalmic patients using prebended 3D titanium-meshes—a technical note. J Oral Maxillofac Surg. 2012;70(11):e631–e638. [DOI] [PubMed] [Google Scholar]
- 15. Ramli N, Kala S, Samsudin A, Rahmat K, Abidin ZZ. Proptosis—correlation and agreement between Hertel exophthalmometry and computed tomography. Orbit. 2015;34(5):257–262. [DOI] [PubMed] [Google Scholar]
- 16. Jeon HB, Kang DH, Oh SA, Gu JH. Comparative study of Naugle and Hertel exophthalmometry in orbitozygomatic fracture. J Craniofac Surg. 2016;27(1):142–144. [DOI] [PubMed] [Google Scholar]
- 17. Wolfe SA, Ghurani R, Podda S, Ward J. An examination of posttraumatic, postsurgical orbital deformities: conclusions drawn for improvement of primary treatment. Plast Reconstr Surg. 2008;122(6):1870–1881. [DOI] [PubMed] [Google Scholar]
- 18. Hammer B, Kunz C, Schramm A, DeRoche R, Prein J. Repair of complex orbital fractures: technical problems, state-of-the-art solutions and future perspectives. Ann Acad Med Singapore. 1999;28(5):687–691. [PubMed] [Google Scholar]
- 19. Bartling SH, Leinung M, Graute J, et al. Increase of accuracy in intraoperative navigation through high-resolution flat-panel volume computed tomography: experimental comparison with multislice computed tomography-based navigation. Otol Neurotol. 2007;28(1):129–134. [DOI] [PubMed] [Google Scholar]
- 20. Rana M, Essig H, Eckardt AM, et al. Advances and innovations in computer-assisted head and neck oncologic surgery. J Craniofac Surg. 2012;23(1):272–278. [DOI] [PubMed] [Google Scholar]
- 21. Stoetzer M, Rana M, von See C, Eckardt AM, Gellrich N-C. Reconstruction of defects of maxillary sinus wall after removal of a huge odontogenic lesion using prebended 3D titanium-mesh and CAD/CAM technique. Head Face Med. 2011;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bly RA, Chang S-H, Cudejkova M, Liu JJ, Moe KS. Computer-guided orbital reconstruction to improve outcomes. JAMA Facial Plast Surg. 2013;15(2):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Metzger MC, Schön R, Zizelmann C, Weyer N, Gutwald R, Schmelzeisen R. Semiautomatic procedure for individual preforming of titanium meshes for orbital fractures. Plast Reconstr Surg. 2007;119(3):969–976. [DOI] [PubMed] [Google Scholar]
- 24. Tabakovic SZ, Konstantinović VS, Radosavljević R, Movrin D, Hadžistević M, Hatab N. Application of computer-aided designing and rapid prototyping technologies in reconstruction of blowout fractures of the orbital floor. J Craniofac Surg. 2015;26(5):1558–1563. [DOI] [PubMed] [Google Scholar]
- 25. Baumann A, Sinko K, Dorner G. Late reconstruction of the orbit with patient-specific implants using computer-aided planning and navigation. J Oral Maxillofac Surg. 2015;73(12 Suppl):S101–106. [DOI] [PubMed] [Google Scholar]
- 26. Chen CT, Pan C-H, Chen C-H, Shyu V, et al. Clinical outcomes for minimally invasive primary and secondary orbital reconstruction using an advanced synergistic combination of navigation and endoscopy. J Plast Reconstr Aesthet Surg. 2018;71(1):90–100. [DOI] [PubMed] [Google Scholar]
- 27. Gaffrée G, Santos R, Cupello V, Polinati JM, Paredes L. Secondary reconstruction of posttraumatic enophthalmos with titanium mesh and buccal fat pad graft: case report. Surg J (N Y). 2017;3(3):e101–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan H, Zhang Z, Tang W, LI W, Deng Y. Bioresorbable material in secondary orbital reconstruction surgery. J Ophthalmol. 2019;2019:8715314. [DOI] [PMC free article] [PubMed] [Google Scholar]