Abstract
Study Design:
Retrospective comparative interventional series of all patients who had undergone orbital fracture repair by 2 senior orbital surgeons in a single tertiary trauma center from January 2005 to December 2014.
Objective:
To compare the outcomes of different implants used for various types of orbital fractures.
Methods:
Patients were evaluated by age, gender, etiology of fracture, clinical findings, type of fractures, and implant used. Main outcome measures included restoration of premorbid state without morbidity and complications including enophthalmos, diplopia, infraorbital hypoesthesia, and ocular motility restriction 1 year after fracture repair. Implant-related complications were collected for analysis.
Results:
There were a total of 274 patients with 307 orbits reconstructed. Thirty-three (12.0%) patients sustained bilateral injuries; 58.0% (n = 178) of orbits had simple fractures (isolated orbital floor, medial wall, or combined floor and medial wall). The distribution of implants used were bioresorbable (n = 117, 38.1%) and prefabricated titanium plates (n = 98, 31.9%) depending upon the nature of fracture. Bioresorbables, titanium plate, and porous polyethylene were used significantly more than titanium mesh for simple fractures, and prefabricated anatomic titanium implants were used significantly more than the other implants for complex fractures. There was a statistically significant improvement in diplopia, enophthalmos, ocular motility, and infraorbital hypoesthesia (p-value < 0.001) 1 year following orbital fracture reconstruction.
Conclusions:
When used appropriately, diverse alloplastic materials used in orbital fracture repair tailored to the indication aid orbital reconstruction outcomes with each material having its own unique characteristics.
Keywords: orbital fractures, orbital implants, bioresorbable implants, blow out fractures, orbitofacial fractures, orbital reconstruction, prefabricated anatomical titanium implants
Introduction
Orbital wall fractures are commonly encountered in the context of facial trauma and are becoming more frequent because of the increasing number of traffic accidents, industrial accidents, sport-related injuries, and physical assaults.1 They occur as a result of energy transmitted directly to the wall(s), indirectly from increased orbital pressure or a combination of the above. Acute mechanical orbital injuries may result in orbital rim and/or orbital wall defects, with periosteal dehiscence and herniation of extraocular muscles. This may result in entrapment of extraocular muscles and/or intermuscular septum and loss of orbital volume with resultant diplopia and enophthalmos respectively. Other possible complications include optic nerve injury, infra or supraorbital nerve injury, injury to anterior, posterior ethmoidal, infraorbital or supraorbital vessels, and injury to the lacrimal drainage system.2
We classified orbital fractures based on the framework provided by the Practical Classification of Orbital and Orbitofacial Fractures.3 Simple orbital fractures most commonly included blowout fractures (either single walled—orbital floor or medial wall, or combined orbital floor and medial wall) while complex orbital fractures included zygomaticomaxillary complex (ZMC) fractures and other orbito-facial fractures (ie, naso-orbito-ethmoidal (NOE) complex fractures, cranio-orbital, orbito-facial, and panfacial fractures). Patients with complex fractures tended to have bilateral fractures in view of the high impact and force involved. The orbital fractures may be approached through varied incisions and can be reconstructed using various implant materials.2,4–6 Complete reduction of orbital contents with anatomically correct reconstruction is necessary to avoid functional deficits and for restoration of anatomical relations and cosmesis. Goals of orbital reconstruction include atraumatic release of herniated or prolapsed orbital soft tissue and complete orbital soft tissue reduction, accurate and anatomic reconstruction of the orbital wall(s), restoration of premorbid orbital volume, avoidance of damage to vital structures, for example, extraocular muscles, intermuscular septum, motor and optic nerves, thereby preserving vision and minimizing or preventing diplopia and to avoid both short- and long-term implant-related complications. While the choice of material may be less important in repair of small orbital defects, it may contribute significantly to the long-term success of medium and large size defects. While there have been several reviews on the various types of implants available for orbital fractures,2,7 there is little in the literature on the spectrum of implants for varied types and severity of orbital and orbito-facial fractures. We conducted a study to determine the indications for repair, types of orbital implants, outcomes, and implant-related complications of all orbital and orbito-facial fractures treated in a single tertiary institution over a 10-year period.
Material and Methods
We performed a retrospective 10-year review of all patients who had undergone orbital fracture repair by 2 senior orbital surgeons in a single tertiary trauma center from January 2005 to December 2014 (Figure 1). Alloplastic implants used were a mixture of both bioresorbable implants and permanent implants such as prefabricated titanium plate, titanium mesh, titanium crab-plate, porous polyethylene (Medpor), and other implants. The study was conducted in accordance with the tenets of the Declaration of Helsinki as revised in 1989. Institutional Review Board (IRB) approval was obtained (DSRB 2013/00751).
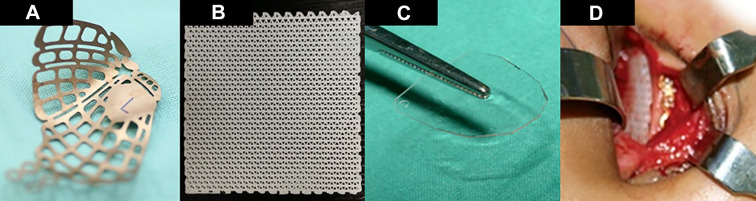
Figure 1.
Various types of orbital implants: Prefabricated titanium implant for left orbit (A); Osteomesh (polycaprolactone) bioresorbable implant (B); PolyMax (P[L/DL]LA) 70/30 implant that has been cut to the shape of the orbital floor (C); intraoperative view of a cut Osteomesh implant placed along the orbital floor (D).
The main outcome measures included restoration of premorbid condition with improvement in clinical findings of ocular motility/diplopia, infraorbital hypoesthesia, and enophthalmos. We also recorded the mechanism of injury and associated injuries such as intracranial injury, nasolacrimal duct injury, optic neuropathy, globe rupture, orbital compartment syndrome, and adverse outcomes if any.
Orbital fractures were classified into simple and complex orbital fractures. Simple orbital fractures included 1 wall and 2 wall blowout fractures and blow-in fractures. Complex orbital fractures include ZMC fractures, NOE fractures, cranio-orbital, orbito-facial, and panfacial fractures with orbital involvement. Incisions for the orbital fractures depended upon the type of fracture. Access to the orbital floor and medial wall was gained through a lower eyelid forniceal transconjunctival incision with (“swinging eyelid” approach) or without a lateral canthotomy and cantholysis. Combined orbital floor and medial wall fractures were approached either through a swinging eyelid approach with or without a retrocaruncular extension. Spectrum of the incisions included a contiguous inferior and medial transconjunctival approach with disinsertion of inferior oblique tendon subperiosteally, separate inferior transconjunctival and medial transconjunctival approach with preservation of inferior oblique origin or direct inferior transconjunctival approach alone. ZMC fractures were accessed either through the swinging eyelid approach with an upper blepharoplasty incision alone (Type I), the above with an oral supragingival incision (Type II), or the above along with transoral and coronal incision (for comminuted displaced Type III fracture with zygomatic arch involvement). NOE fractures were accessed through a coronal incision. Other fractures (cranio-orbital, orbito-facial, and panfacial fractures involving the orbit) were accessed based on the exposure required, usually through varied combinations of the above incisions.
For orbital floor fractures, following an inferior transconjunctival incision, a periosteal incision was made along the anterior edge of the infraorbital rim and elevated with subperiosteal dissection to expose the orbital fracture with its herniated contents. Entrapped tissue was freed from the fracture site and elevated from the maxillary sinus cavity into the orbit. Care was taken to avoid damage to the infraorbital neurovascular bundle and the anterior or posterior ethmoidal neurovascular bundles for medial wall fractures. Additional exposure of the orbital floor for large comminuted fractures was obtained by releasing the contents of the inferior orbital fissure. The medial, lateral, and posterior edges of the fracture site were well visualized, and the posterior ledge identified and confirmed prior to the placement of the implant. This was vital to ensure that the implant was placed over the fracture site with proper support to prevent postoperative prolapse of the implant and tissues into the sinus. Combined orbital floor and medial wall fractures were repaired either by prebending prefabricated anatomical titanium implants based on the contralateral angle of inferomedial orbital strut prior to insertion or prebending the titanium plate over 3D printed models based on mirrored pretreatment DICOM data. In complex fractures, following fracture reduction the rims were secured with miniplates prior to reconstructing the orbital walls. Indications for the use of intraoperative navigation included, large, complex, and severely displaced fractures and revision orbital and orbito-facial fracture repairs. Forced duction test was performed preoperatively, after complete reduction of orbital contents, and after placement and verification of placement of orbital implant and finally prior to wound closure to confirm any entrapment. Intraoperatively, we performed constant pupil monitoring to monitor optic nerve function. After complete reduction of orbital contents confirmed by forced duction tests, the periosteum and conjunctiva were closed with 6-0 vicryl suture. Postoperatively, all patients were admitted for overnight monitoring of optic nerve function. Comminuted and extensive fractures with surgeries lasting more than 2 hours received perioperative systemic antibiotics while isolated orbital blowout fractures did not receive any. Patients were followed up 1 day, 1 week, 1 month, 3 months, 6 months, and 15-24 months postoperatively. Patients with complex orbital fractures had early postoperative imaging for implant position verification and those with bioresorbable implants had imaging 12-15 months postoperatively.
We compared the type of fractures and the type of implant used. The Bonferroni correction was applied to all the P values when the pairwise comparisons were conducted. Cochran’s Q test was used to determine whether there were any differences between preoperative and postoperative clinical findings (ocular motility limitation, diplopia, enophthalmos, and infraorbital hypoesthesia). A significance level of 0.05 was used and statistical analysis was performed in SPSS Statistics 19.0 (IBM, New York, USA).
Results
Two hundred seventy-four patients underwent orbital fracture repair by 2 oculoplastic surgeons over a 10-year period, a total of 307 orbits were repaired. Complex orbital fractures were co-managed with the craniomaxillofacial team. Patient demographics and fracture patterns are listed in Table 1. The majority of patients were male (83.6%, n = 229) and the most common mechanisms of injury was motorcycle injuries (22.6%), followed by assault (19.0%). Simple fractures (58.0%, n = 178) were more common than complex fractures. The most common associated injury was intracranial injury (15.0%) followed by frontal sinus injury (11.3%). Intraoperative navigation was used in 55 orbits (17.9%). Indications for orbital fracture repair included medium to large fractures of one or more walls, comminuted or non-comminuted displaced orbital rims, ZMC, NOE, or other orbito-facial fractures. Relative contraindications included minimally displaced fractures without entrapment, high-risk patients (uncontrolled diabetes mellitus, hypertension, intracranial injury, elderly patients), and unwilling patients.
Table 1.
Demographics of orbital fracture patients in the study.
| Demographics (n = 274) | |
|---|---|
| Gender (%) | |
| Male | 229 (83.6) |
| Female | 45 (16.4) |
| Race (%) | |
| Chinese | 169 (61.7) |
| Malay | 41 (15.0) |
| Indian | 28 (10.2) |
| Caucasian | 4 (1.5) |
| Eurasian | 4 (1.5) |
| Others | 28 (10.2) |
| Age (years) | |
| Mean ± SD | 33.7 ± 13.5 |
| Median | 31.0 |
| Range | 5-72 |
| Laterality (%) | |
| Unilateral | 241 (88.0) |
| Bilateral | 33 (12.0) |
| Total number of orbits | 307 |
| Mechanism of injury (%) | |
| Motorcycle accident | 62 (22.6) |
| Assault | 52 (19.0) |
| Sport-related trauma | 36 (13.1) |
| Fall on level ground | 32 (11.7) |
| Other road traffic accident | 30 (11.0) |
| Blunt trauma sustained at work | 29 (10.6) |
| Fall from height | 12 (4.4) |
| Gunshot/explosion | 1 (0.4) |
| Others | 20 (7.3) |
The most common implants used in our study were bioresorbable implants (n = 117, 38.1%) which included implants such as poly-L/DL-lactide implants (P[L/DL]LA) 85/15 (Rapidsorb; Synthes, Oberdorf, Switzerland), polycaprolactone mesh implant (Osteomesh, Osteopore International, Singapore) and poly-L/DL-lactide implants (P[L/DL]LA) 70/30 (PolyMax; Synthes, Oberdorf, Switzerland) (Figure 1C and 1D) and (MacroPore; Biosurgery, Inc., San Diego, USA). This was followed by permanent implants which included prefabricated titanium orbital implants (n = 98, 31.9%), titanium mesh implants (n = 46, 15.0%), titanium “crab-plate” implants (n = 21, 6.8%), and porous polyethylene (Medpor, Stryker, USA) (n = 20, 6.5%).
Table 2 shows the preoperative and postoperative clinical outcomes. There was significant improvement in ocular motility, diplopia, infraorbital hypoesthesia and significant enophthalmos postoperatively for all fractures that were operated on (P < 0.001). Enophthalmos was defined as backward displacement of more than 2 mm compared to normal side in unilateral cases and 16 mm and below in bilateral cases by exophthalmometric readings. This improvement was sustained with postoperative follow-up. Comparison of postoperative outcomes among the fracture types (simple and complex) and type of implants (bioresorbables, prefabricated titanium plate, titanium mesh, titanium crab-plate and porous polyethylene) showed no significant difference in outcomes at month 1, 3, 6, and 12 (P > 0.05).
Table 2.
Pre- and postoperative clinical outcomes.
| Pre-op | POM1 | POM3 | POM6 | POM12 | P value | |
|---|---|---|---|---|---|---|
| Present (%) | ||||||
| Diplopia | 122 (44.5) | 81 (29.6) | 27 (9.9) | 16 (5.8) | 15 (5.5) | <0.001 |
| Enophthalmos | 101 (36.9) | 30 (10.9) | 14 (5.1) | 6 (2.2) | 6 (2.2) | <0.001 |
| Limitation in ocular motility | 69 (25.2) | 47 (17.2) | 8 (2.9) | 6 (2.2) | 6 (2.2) | <0.001 |
| Infraorbital hypoesthesia | 72 (26.3) | 29 (10.6) | 8 (2.9) | 2 (0.7) | 2 (0.7) | <0.001 |
Table 3 illustrates the type of fracture and type of implant used (Figure 2). When comparing the different types of fractures and the types of implants used, there was a significant difference in the types of implants used depending on the type of fracture (P < 0.001). In addition, after the Bonferroni correction was applied for comparison across multiple groups, bioresorbables, titanium plate, and porous polyethylene were used significantly more than titanium mesh for simple fractures. Porous polyethylene was used significantly more than prefabricated titanium plate. For complex fractures, titanium mesh implants were used significantly more than bioresorbables, titanium plate, and porous polyethylene. In addition, for complex fracture, prefabricated titanium plate was used significantly more than porous polyethylene.
Table 3.
Types of fractures and implants used.
| Type of implants | ||||||
|---|---|---|---|---|---|---|
| Fractures | Bioresorbables | Prefabricated titanium plate | Titanium mesh | Titan plate | Medpor porous polyethylene | Others (nonbioresorbable) |
| No. (%) | ||||||
| Simple | 79 (67.5) | 48 (49.0) | 16 (34.8) | 15 (71.4) | 16 (80.0) | 4 (80.0) |
| Orbital floor | 53 (45.3) | 13 (13.3) | 10 (21.7) | 8 (38.1) | 13 (65.0) | 2 (40.0) |
| Medial wall | 5 (4.3) | 2 (2.0) | 1 (2.2) | 1 (4.8) | 1 (5.0) | 0 (0.0) |
| Combined floor and medial wall | 21 (17.9) | 33 (33.7) | 5 (10.9) | 6 (28.6) | 2 (10.0) | 2 (40.0) |
| Complex | 38 (32.5) | 50 (51.0) | 30 (65.2) | 6 (28.6) | 4 (20.0) | 1 (20.0) |
| Zygomaticomaxillary complex | 29 (24.8) | 45 (45.9) | 26 (56.5) | 4 (19.0) | 2 (10.0) | 1 (20.0) |
| Others | 9 (7.7) | 5 (5.1) | 4 (8.7) | 2 (9.5) | 2 (10.0) | 0 (0.0) |
| Total | 117 (100) | 98 (100) | 46 (100) | 21 (100) | 20 (100) | 5 (100) |
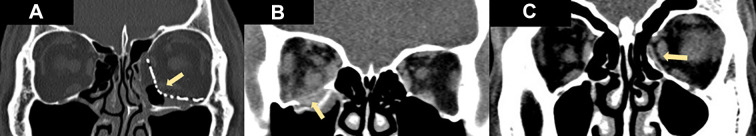
Figure 2.
Various types of implants used for different types of fractures: Prefabricated anatomical titanium orbital implant for left combined floor and medial wall fracture (A); bioresorbable implant for right orbital floor blowout fracture (B); bioresorbable implant for left orbital medial wall blowout fracture (C). The arrows point to the implants.
We classified complications into minor and major (requiring repeat operation) and also as early, intermediate, and late postoperative complications. Of the 307 orbits, 15 orbits (4.9%) required a repeat operation. The indications for reoperation were as follows: implant-related complications (n = 6, 2.0%), lid-related complications (n = 4, 1.3%), strabismus (n = 3, 1.0%), and residual enophthalmos (n = 2, 0.7%). Implant-related complications requiring a repeat operation are elaborated upon in Table 4.
Table 4.
Implant-related complications requiring reoperation.
| Type of fracture and implant used | Complication | |
|---|---|---|
| 1. | Zygomaticomaxillary fracture repaired using titanium mesh implant (complex fracture) | Protrusion of screws into lacrimal sac causing nasolacrimal duct obstruction. |
| 2. | Zygomaticomaxillary fracture repaired using a titanium mesh implant (complex fracture) | Orbital adherence syndrome. |
| 3. | Zygomaticomaxillary fracture repaired using a bioresorbable implant (complex fracture) | Implant malposition with fascial tethering of right inferior rectus. |
| 4. | Medial wall fracture repaired using a prefabricated titanium plate (simple fracture) | Incomplete correction of medial wall fracture. |
| 5. | Orbital floor fracture repaired using titan plate (simple fracture) | Malposition of titanium implant that was noted on the CT scan at postoperative day 1. The medial aspect of the implant was inferior to the orbital wall. |
| 6. | Orbital floor fracture repaired using a prefabricated titanium plate (simple fracture) | Palpable implant requiring removal of loose screw. |
Complications noted within 1-month postoperative period included lid-related complications (n = 13, 4.2%) such as ptosis and ectropion, implant-related complications (n = 3, 1.0%), and wound infection (n = 1, 0.3%). Most lid-related complications were managed conservatively and subsequently resolved with only 1 orbit requiring correction of his ectropion. Three orbits with preoperative ptosis underwent surgical correction after 6 months of stability.
Discussion
Orbital wall fractures are commonly encountered in facial trauma. In more than 40% of all facial fractures, parts of the orbital rim and/or the internal orbit are injured showing various fracture patterns.8 Commonly, multiple portions of the orbit are involved. The framework provided by the Practical Classification of Orbital and Orbitofacial Fractures3 that was used to classify orbital fractures in this article is an orbit centric and practical classification system that also helped guide clinical decision-making. Long bones are routinely weight and impact bearing. However, orbital bones are not and hence have different healing characteristics requiring adequate support during the process of healing. Even in simple fracture patterns, such as the common single-wall “blowout” fracture, reconstruction should not be underestimated, as the orbit is a complex 3D structure with soft tissue implications. Hence all orbital fractures require precise reconstruction after traumatic derangement to avoid complications in structure and function such as visual loss, diplopia, and enophthalmos.8–10 The fractured bone elements typically are unable to be reduced and so must be augmented with alloplastic material to restore the normal internal volume. In addition to volume, the complex contours of the orbital walls and variations in patient anatomy must be considered to accurately reconstruct the orbital shape which, in turn, determines the final globe’s position. The process of manually forming, fitting, and aligning orbital implants for anatomically accurate reconstruction can be challenging.
Orbital fractures can be reconstructed by using different techniques and implant materials. Currently, autologous and alloplastic implants are available for orbital reconstruction.11–14 Autologous bone grafts are well tolerated by the host but may have significant donor-site morbidity, varying degrees of resorption, and may be difficult to shape in multicontoured areas.15,16 Alloplastic implant materials such as titanium, porous polyethylene (Medpor), or silicone elastomers provide good tensile strength, are readily available, easy to handle, and undergo minimal or no resorption.17,18 However, they are permanent “foreign bodies” and hence may be susceptible to infection, migration, palpability, and exposure over time.19–21 Moreover, quite a few of these are not “anatomically” matched to the complex contours of the floor and medial walls of the orbits. Bioresorbable implants offer a useful alternative in the reconstruction of small to medium size defects in simple or complex orbito-cranial deformities. Depending on the type implant, the advantages of bioresorbable implants over permanent implants include (1) ability to contour (polylactides), (2) provide mechanical integrity (polycaprolactone for small fractures, thermolabile polylactides for medium-large fractures) while the polymer resorbs, (3) without donor-site morbidity,22 and (4) avoids long-term complications of permanent implants such as palpability, exposure, and orbital adherence syndrome. Hence, bioresorbable alloplastic materials have been gaining popularity for reconstruction of varied orbital defects.22–25
The ideal implant should be adaptable to regional anatomy, be easy to shape and possess initial strength to meet biomechanical demand, and be able to retain its structural integrity during the healing process. An ideal implant should not cause complications which may require medical or surgical intervention. Factors that determined choice of orbital implant included availability of implants, economic considerations, nature and size of the defect, surgeon and patient’s preference, feasibility of long-term follow-up, and the predisposition of the patient to repeat injuries (eg, athletes). In general, while the nature of implant is immaterial for small fractures (<1/3 of a single orbital wall), it becomes important for medium (>½ of a single orbital wall) and large or complex fractures (>½ of a single orbital wall or multiple wall defects), which often require a supportive bioresorbable implant (polylactide) or permanent implant (titanium implants). Moreover some implants have the advantage of minimizing postoperative orbital compartment syndrome because of their porosity, for example, osteopore, titanium mesh, prefabricated titanium implants. Implants that are relatively high risk for compartment syndrome include nonporous implants such as porous polypropylene implants with barrier sheet and combined porous polypropylene-titanium implants (Titan, Synpor).
Our study is one of the few large series studying the use of various implants in simple and complex orbital fracture repairs. While there was an almost equal distribution for the most commonly used implants—bioresorbable and prefabricated titanium implants, there were different indications for the individual implants. This is related to different properties of the implants that make them suitable for particular fracture types.
Simple orbital fractures were the most common type of fractures in our series. For simple fractures, we found that bioresorbable (Figure 2B), porous polyethylene (Medpor) and titanium plate implants were used significantly more than titanium meshes. The amount of empirical support for individual materials used for orbital floor fracture reconstruction differs and no definite conclusion has been reached regarding the best material for orbital floor fracture repair. There is a lack of standardized guideline or consensus with different surgeons having different preferences and practices that are each supported by varying amounts of research.7,26 Both bioresorbable and porous polyethylene (Medpor) implants have been shown to be stable, biocompatible, easily shaped two-dimensionally and allow tissue incorporation, with no additional donor site required.22–25,27–29 These properties make them easy to use and suitable for simple 1-walled small to medium and large sized blowout fractures.29 The degradable nature of bioresorbable implants also make them an attractive option for simple blowout fractures, providing enough mechanical strength during the initial bone healing process while eventually hydrolyzed, hence avoiding all the potential long-term complications of permanent implants.
In contrast, prefabricated anatomic titanium orbital implants were used significantly more than other implants for complex fractures (Figure 2A). This is likely related to anodized titanium implants’ long track record in craniofacial reconstruction. It has the following advantages: easy to use, has stood the test of time, possesses good mechanical strength, has rigid fixation, and is well-seen on imaging.30,31 Titanium mesh has demonstrated excellent biocompatibility and provides long-lasting stability of the internal orbital reconstruction.32 Although titanium mesh is easily manipulated intraoperatively, the complex internal orbital shape required is often difficult to accurately recreate. Fortunately, despite its complexity, the orbital shape varies only a small amount between individuals and commercially available preformed anatomic orbital implants have been developed to streamline this process.33,34 The latter hence allows the distinct orbital anatomical contours to be addressed, particularly the orbital strut between the medial wall and floor, and the S-shaped bulge of the posterior orbit which is often ill addressed by sheet implants.
A limitation of our study is its retrospective nonrandomized nature, hence some bias owing to the progressive experience and evolution of implants available over the study period. Surgeon preferences and patient expectations also influenced the choice of implants used with a varied number of various types of orbital fractures. In addition, while the authors follow general guidelines for choice of certain types of implants, there is no fixed protocol to the type of implant used, and the final choice often depended on a combination of patient, surgeon, and fracture factors. Despite the shortcomings, we believe that the high volume of orbital fractures repaired and the low incidence of complications adds important information to the ongoing debate on the ideal implant for orbito-facial reconstruction.
Conclusion
In conclusion, our 10-year series showed that the diverse alloplastic materials used in orbital fracture repair are all successful to variable degrees in terms of the recorded outcome measures with each material having its own unique characteristics. However, given the absence of difference in outcomes and the potential reported long-term complications of permanent implants, bioresorbable implants are a reasonable option in the management of orbital wall reconstruction.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Stephanie Young, FRCOphth  https://orcid.org/0000-0001-8223-342X
https://orcid.org/0000-0001-8223-342X
References
- 1. Shin JW, Lim JS, Yoo G, Byeon JH. An analysis of pure blowout fractures and associated ocular symptoms. J Craniofac Surg. 2013;24(3):703–707. [DOI] [PubMed] [Google Scholar]
- 2. Mok D, Lessard L, Cordoba C, Harris PG, Nikolis A. A review of materials currently used in orbital floor reconstruction. Can J Plast Surg. 2004;12(3):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sundar G. Classification of orbital & orbitofacial fractures In: Sundar G. (Ed). Orbital Fractures – Principles, Concepts & Management. USA: Imaging Science Today; 2018. ISBN: 978-0-9977819-2-2. [Google Scholar]
- 4. Bartoli D, Fadda MT, Battisti A, et al. Retrospective analysis of 301 patients with orbital floor fracture. J Craniomaxillofac Surg. 2015;43(2):244–247. [DOI] [PubMed] [Google Scholar]
- 5. Holtmann H, Eren H, Sander K, Kubler NR, Handschel J. Orbital floor fractures—short- and intermediate-term complications depending on treatment procedures. Head Face Med. 2016;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tong L, Bauer RJ, Buchman SR. A current 10-year retrospective survey of 199 surgically treated orbital floor fractures in a nonurban tertiary care center. Plast Reconstr Surg. 2001;108(3):612–621. [DOI] [PubMed] [Google Scholar]
- 7. Gunarajah DR, Samman N. Biomaterials for repair of orbital floor blowout fractures: a systematic review. J Oral Maxillofac Surg. 2013;71(3):550–570. [DOI] [PubMed] [Google Scholar]
- 8. Prein J, Ehrenfeld M, Manson PN. Principles of Internal Fixation of the Craniomaxillofacial Skeleton. Thieme; 2012. [Google Scholar]
- 9. Hammer B, Prein J. Correction of post-traumatic orbital deformities: operative techniques and review of 26 patients. J Craniomaxillofac Surg. 1995;23(2):81–90. [DOI] [PubMed] [Google Scholar]
- 10. Ramieri G, Spada MC, Bianchi SD, Berrone S. Dimensions and volumes of the orbit and orbital fat in posttraumatic enophthalmos. Dentomaxillofac Radiol. 2000;29(5):302–311. [DOI] [PubMed] [Google Scholar]
- 11. Cho YR, Gosain AK. Biomaterials in craniofacial reconstruction. Clin Plast Surg. 2004;31(3):377–385. [DOI] [PubMed] [Google Scholar]
- 12. Karesh JW. Biomaterials in ophthalmic plastic and reconstructive surgery. Curr Opin Ophthalmol. 1998;9(5):66–74. [DOI] [PubMed] [Google Scholar]
- 13. Morrison AD, Sanderson RC, Moos KF. The use of silastic as an orbital implant for reconstruction of orbital wall defects: review of 311 cases treated over 20 years. J Oral Maxillofac Surg. 1995;53(4):412–417. [DOI] [PubMed] [Google Scholar]
- 14. Converse JM, Smith B, Obear MF, Wood-Smith D. Orbital blowout fractures: a ten-year survey. Plast Reconstr Surg. 1967;39(1):20–36. [DOI] [PubMed] [Google Scholar]
- 15. Ilankovan V, Jackson IT. Experience in the use of calvarial bone grafts in orbital reconstruction. Br J Oral Maxillofac Surg. 1992;30(2):92–96. [DOI] [PubMed] [Google Scholar]
- 16. Martinez-Lage JL. Bony reconstruction in the orbital region. Ann Plast Surg. 1981;7(6):464–479. [DOI] [PubMed] [Google Scholar]
- 17. Meyer DR. Alloplastic materials for orbital surgery. Curr Opin Ophthalmol. 1995;6(5):43–52. [DOI] [PubMed] [Google Scholar]
- 18. Chowdhury K, Krause GE. Selection of materials for orbital floor reconstruction. Arch Otolaryngol Head Neck Surg. 1998;124(12):1398–1401. [DOI] [PubMed] [Google Scholar]
- 19. Punja KG, Kikkawa DO, Morrison VL, Pornpanich K, Holmes RE, Cohen SR. Reconstruction of complex orbitocranial deformities using bioresorbable mesh, sterilized orbital models, and in situ contouring. Ophthalmic Plast Reconstr Surg. 2006;22(1):20–24. [DOI] [PubMed] [Google Scholar]
- 20. Yaremchuk MJ, Gruss JS, Manson PN. Rigid Fixation of the Craniomaxillofacial Skeleton. Butterworth-Heinemann; 1992. [Google Scholar]
- 21. Brown AE, Banks P. Late extrusion of alloplastic orbital floor implants. Br J Oral Maxillofac Surg. 1993;31(3):154–157. [DOI] [PubMed] [Google Scholar]
- 22. Al-Sukhun J, Tornwall J, Lindqvist C, Kontio R. Bioresorbable poly-L/DL-lactide (P[L/DL]LA 70/30) plates are reliable for repairing large inferior orbital wall bony defects: a pilot study. J Oral Maxillofac Surg. 2006;64(1):47–55. [DOI] [PubMed] [Google Scholar]
- 23. Landes CA, Ballon A, Roth C. In-patient versus in vitro degradation of P(L/DL)LA and PLGA. J Biomed Mater Res B Appl Biomater. 2006;76(2):403–411. [DOI] [PubMed] [Google Scholar]
- 24. Kontio R, Suuronen R, Salonen O, Paukku P, Konttinen YT, Lindqvist C. Effectiveness of operative treatment of internal orbital wall fracture with polydioxanone implant. Int J Oral Maxillofac Surg. 2001;30(4):278–285. [DOI] [PubMed] [Google Scholar]
- 25. Lieger O, Schaller B, Zix J, Kellner F, Iizuka T. Repair of orbital floor fractures using bioresorbable poly-L/DL-lactide plates. Arch Facial Plast Surg. 2010;12(6):399–404. [DOI] [PubMed] [Google Scholar]
- 26. Courtney DJ, Thomas S, Whitfield PH. Isolated orbital blowout fractures: survey and review. Br J Oral Maxillofac Surg. 2000;38(5):496–504. [DOI] [PubMed] [Google Scholar]
- 27. Rubin PA, Bilyk JR, Shore JW. Orbital reconstruction using porous polyethylene sheets. Ophthalmology. 1994;101(10):1697–1708. [DOI] [PubMed] [Google Scholar]
- 28. Romano JJ, Iliff NT, Manson PN. Use of Medpor porous polyethylene implants in 140 patients with facial fractures. J Craniofac Surg. 1993;4(3):142–147. [DOI] [PubMed] [Google Scholar]
- 29. Seen S, Young SM, Teo SJ, et al. Permanent versus bioresorbable implants in orbital floor blowout fractures. Ophthalmic Plast Reconstr Surg. 2018;34(6):536–543. [DOI] [PubMed] [Google Scholar]
- 30. Kirby EJ, Turner JB, Davenport DL, Vasconez HC. Orbital floor fractures: outcomes of reconstruction. Ann Plast Surg. 2011;66(5):508–512. [DOI] [PubMed] [Google Scholar]
- 31. Banica B, Ene P, Vranceanu D, Ene R. Titanium preformed implants in orbital floor reconstruction—case presentation, review of literature. Maedica. 2013;8(1):34–39. [PMC free article] [PubMed] [Google Scholar]
- 32. Potter JK, Malmquist M, Ellis E., 3rd Biomaterials for reconstruction of the internal orbit. Oral Maxillofac Surg Clin North Am. 2012;24(4):609–627. [DOI] [PubMed] [Google Scholar]
- 33. Metzger MC, Schon R, Weyer N, et al. Anatomical 3-dimensional pre-bent titanium implant for orbital floor fractures. Ophthalmology. 2006;113(10):1863–1868. [DOI] [PubMed] [Google Scholar]
- 34. Strong EB, Fuller SC, Wiley DF, Zumbansen J, Wilson MD, Metzger MC. Preformed vs intraoperative bending of titanium mesh for orbital reconstruction. Otolaryngol Head Neck Surg. 2013;149(1):60–66. [DOI] [PubMed] [Google Scholar]