Abstract
Background. Chinese herbal medicines are widely used to lower serum uric acid levels. However, no systemic review summarizes and evaluates their efficacies and the underlying mechanisms of action. Objectives. To evaluate the clinical and experimental evidences for the effectiveness and the potential mechanism of Chinese herbal medicines in lowering serum uric acid levels. Methods. Four electronic databases PubMed, Wed of Science, the Cochrane Library and Embase were used to search for Chinese herbal medicines for their effects in lowering serum uric acid levels, dated from 1 January 2009 to 19 August 2020. For clinical trials, randomized controlled trials (RCTs) were included; and for experimental studies, original articles were included. The methodological quality of RCTs was assessed according to the Cochrane criteria. For clinical trials, a meta-analysis of continuous variables was used to obtain pooled effects. For experimental studies, lists were used to summarize and integrate the mechanisms involved. Results. A total of 10 clinical trials and 184 experimental studies were included. Current data showed that Chinese herbal medicines have promising clinical efficacies in patients with elevated serum uric acid levels (SMD: −1.65, 95% CI: −3.09 to −0.22; p = 0.024). There was no significant difference in serum uric acid levels between Chinese herbal medicine treatments and Western medicine treatments (SMD: −0.13, 95% CI: −0.99 to 0.74; p = 0.772). Experimental studies revealed that the mechanistic signaling pathways involved in the serum uric acid lowering effects include uric acid synthesis, uric acid transport, inflammation, renal fibrosis and oxidative stress. Conclusions. The clinical studies indicate that Chinese herbal medicines lower serum uric acid levels. Further studies with sophisticated research design can further demonstrate the efficacy and safety of these Chinese herbal medicines in lowering serum uric acid levels and reveal a comprehensive picture of the underlying mechanisms of action.
Keywords: Chinese herbal medicine, serum uric acid, serum urate, efficacy, mechanism
Introduction
Hyperuricemia refers to an abnormally high concentration of serum uric acid (sUA), typically defined as >7 mg/dL in men and >6 mg/dL in women. The data from the National Health and Nutrition Examination Survey (NHANES) 2007–2016 showed that the prevalence rates of hyperuricemia were 20.2% among men and 20.0% among women between 2015 and 2016 in the United States (Chen-Xu et al., 2019). A cross-sectional survey in China showed that the prevalence of hyperuricemia was 8.4% among Chinese adults from 2009 to 2010 (Liu et al., 2014a). Hyperuricemia and gout remain as a considerable burden, which not only adversely affect patients’ health and quality of life (Burke et al., 2015; Gamala and Jacobs, 2019), but also cast an economic burden in the society (Rai et al., 2015). SUA is associated with cardiovascular diseases, such as hypertension (Kuwabara et al., 2018) and atrial fibrillation (Tang et al., 2014). Elevated sUA can lead to decreased renal function, which in turn reduces the excretion of UA in urine, resulting in an increased risk of hyperuricemia or gout (Sato et al., 2019). Elevated sUA may also contribute to the pathogenesis of metabolic syndrome (Battelli et al., 2018; Battelli et al., 2019), non-alcoholic fatty liver disease (Xu et al., 2015), diabetes (Kim et al., 2015). Urate-lowering therapy is a therapeutic strategy for controlling gout, chronic kidney disease, metabolic syndrome and many other diseases. Current interventions for elevated sUA include xanthine oxidase inhibitors, uricosuric agents and anti-inflammatory drugs. Although both febuxostat and allopurinol are effective in reducing sUA, allopurinol may produce a mild skin rash and severe cutaneous reactions (Strilchuk et al., 2019), while febuxostat has a higher risk of all-cause and cardiovascular mortality (White et al., 2018). Benzbromarone is a uricosuric drug which is widely used, but studies have reported possible complications such as hepatotoxicity (Strilchuk et al., 2019). Although these drugs are clinically used, their efficacies are unsatisfactory, and are usually coupled with adverse side effects in the long-term use (Dalbeth et al., 2016).
Hyperuricemia belongs to the arthromyodynia disease category in traditional Chinese medicine. Chinese herbal medicine has been used to treat hyperuricemia for a long time and has significant clinical efficacy (Lin et al., 2016). Chinese herbal medicines with high efficacy and low incidence of adverse reactions have drawn increasing attention from scholars. Studies have compared Chinese herbal medicine with Western medicine for their efficacies in lowering sUA levels. However, these studies differ in their treatment protocols and evaluation methodologies, which greatly limit their clinical applicability. In addition, the mechanism of Chinese herbal medicine in lowering sUA levels is still being explored. Some herbs such as Phellodendri Chinrnsis Cortex, Atractylodes Lancea (Thunb.)Dc. (Chen et al., 2015b), Smilacis Glabrae Rhixoma (Liu et al., 2015), reduce UA intake and/or increase UA excretion by regulating various physiological and cellular pathways. Some herbs like bergenin (Chen et al., 2020a), alpinia oxyphylla seed extract (Lee et al., 2019b) and rhizoma smilacis glabrae extracts (Liang et al., 2019), promote renal and gut uric acid excretion in hyperuricemia models and also decrease the serum levels of inflammatory cytokines. Most Chinese herbal medicines act on multiple targets to achieve their sUA lowering effects. Therefore, the aim of this systematic review is to evaluate the evidence for the efficacy of Chinese herbal medicines in lowering sUA levels in patients with hyperuricemia, compared to no intervention, placebo or urate-lowering agents, and to comprehensively summarize the mechanisms underlying the sUA lowering effects reported from experimental studies.
Materials and Methods
Data Source and Search Strategy
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009) was used to construct the report of the current study and the completed checklist is provided in Supplementary material, Supplementary Table S1. The electronic databases PubMed, Wed of Science, the Cochrane Library and Embase were systematically searched by three researchers (Liqian Chen, Zhengmao Luo and Ming Wang). For any discrepancies between researchers, consensus was reached through discussion. We reviewed literatures published from 1 January 2009 to 19 August 2020 on elevated sUA that had been treated with Chinese herbal medicines. The following combination of terms were used as search keywords: (Traditional Chinese Medicine OR Herbal Medicine OR Chinese Herbal Drugs OR Chinese Plant Extracts OR (Plants, Medicinal) OR Phytochemicals OR herb* OR natural product) combined with (gout OR pain paralysis OR hyperuricemia OR uric acid). The full search strategy in PubMed was provided in Supplementary material, Supplementary Table S2. The search did not exclude articles based on language. To look for additional relevant studies, references of all potentially relevant articles were also retrieved, and authors of studies that met the inclusion criteria but lacked data would be contacted. Abstracts, meeting proceedings, and personal communications were not used for the purpose of this study.
Study Selection
The articles in this review included clinical trials and in vivo and in vitro studies. When screening clinical trials, the followings are the inclusion criteria:
A). Types of trials: All randomized controlled trials (RCTs) investigating the use of Chinese herbal medicine in lowering sUA will be eligible for inclusion.
B). Types of participants: In accordance with the NHANES-III laboratory definition, hyperuricemia is typically defined as >7 mg/dL in men and >6 mg/dL in women (Choi et al., 2020). All adult patients (18 years and older, no upper age limit) with a diagnosis of hyperuricemia will be considered for this review.
C). Types of interventions: According the General guidelines for methodologies on research and evaluation of traditional medicine from WHO, herbal medicines are defined as materials or products derived from plants that have medical or other beneficial effects on human health, including herbs, herbal materials, herbal products, finished herbal products that contain parts of plants or other plant materials or compositions as active ingredients, as well as materials of inorganic or animal sources. The interventions included in this study include Chinese herbal medicine in various prescriptions, such as herbal formulas, herbal extracts, active ingredients of herbs. Clinical trials comparing Chinese herbal medicine with no intervention, placebo or urate-lowering agents were included in our study. Chinese herbal medicine plus placebo or combined with urate-lowering agents compared to the same medications was also included. We do not limit the formulations or administration of herbal preparations for clinical use.
D). Types of outcome measures: Outcome measures should include at least one essential outcome, such as change in sUA levels after treatment and the overall efficacy.
When screening in vivo or vitro experimental studies using the herbs, the following conditions should be met before inclusion: A) Original article investigating the use of Chinese herbal medicine in lowering sUA will be eligible for inclusion. B) Studies using experimental models of mice, rats, rabbits or cell cultures will be considered. All models used should present corresponding pathological symptoms. C) The model should only be treated with Chinese herbal medicine before or after intervention. If medication other than herbs were being used, both the treatment group and the control group must be administered. D) Outcome measures should include change in sUA after treatment.
Data extraction
The data were extracted by three researchers (Jingru Cheng, Fei Li and Hanqi Lu) independently to obtain the following information: A) the design of study; B) characteristics of trial participants (including sample size, age, period); C) type of intervention (including dose regimen, duration); D) type of outcome measure (including the level of sUA). The reported mean (standard deviation [SD]) or risk estimates and 95% confidence intervals (CIs) of sUA were also extracted.
Quality Assessment
The quality assessment of the studies was performed independently by three researchers (Qiuxing He, Yanting You and Xinghong Zhou), and all discrepancies between researchers were resolved through discussion. The methodological quality of RCTs was assessed according to the Cochrane Handbook for Systematic reviews of Interventions. The scores for each bias domain and the final score of risk of systematic bias were graded as low, high or unclear risk. The overall level of evidence was considered “strong” if there were consistent findings among multiple high quality RCTs, and “moderate” if findings were consistent among multiple low-quality RCTs and/or one high-quality RCT. Level of evidence was “conflicting” if findings were inconsistent across the studies, and “no evidence from trials,” if there were no RCTs.
Statistical Analysis
Statistical analysis was performed with the software STATA version 16.0. The d index and the standard deviation (SDd) values of sUA for each RCT were calculated before using STATA. The d index and SDd values of sUA were continuous, standard mean difference (SMD) with 95% confidence interval (95% CI) was calculated. The overall effect was calculated by a Z-test, and p < 0.05 (2-tailed) was deemed statistically significant. Potential heterogeneity was assessed by I 2 statistics. A fixed effects model was chosen if I 2 ≤ 50%, otherwise, a random effects model was applied. Subgroup analysis was performed to illuminate the heterogeneity according to the study characteristics, such as interventions other than Chinese herbal medicine. A ‘leave-one-out’ sensitivity analysis was carried out to test the reliability of the results. Potential publication bias was evaluated by the Egger’s test and Begg’s test. When the geometric mean and CIs were reported, tools provided in the Cochrane Handbook were used to convert the geometric mean and CIs to arithmetic mean and SD of the raw data.
Results
Literature Flow
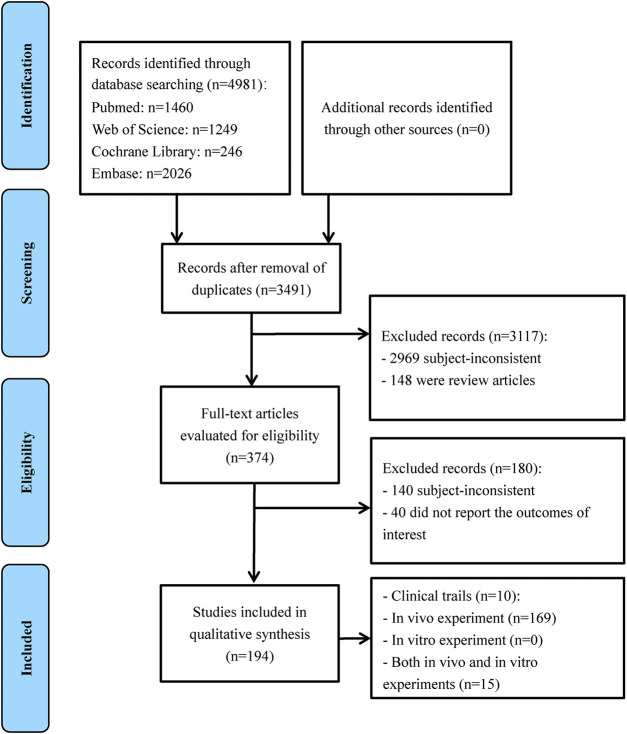
The initial electronic search of the literature yielded 4981 potentially relevant citations. After duplicate removal and title/abstract screening, 374 full-text articles were retrieved for detailed assessment. Of these studies, 180 articles did not meet the inclusion criteria. Finally, 194 articles were included in the review with 10 clinical trials, 169 in vivo experiments, 0 in vitro experiments, and 15 that were a combination of both in vitro and in vivo experiments (Figure 1).
Figure 1.
Flowchart of study selection for the systematic review.
Results of clinical trials
Study Characteristics
The specified characteristics of the included clinical trials as well as their study populations were summarized in Table 1. All clinical trials included were RCTs which mainly explored the effects of Chinese herbal medicine on patients with elevated sUA levels such as hyperuricemia and gout. In order to objectively observe the therapeutic effects of herbs on lowering sUA levels, the changes of sUA levels before and after treatment in 10 clinical trials were listed in Supplementary material, Supplementary Table S3. Of the 10 included trials, nine were treated with herbal formula, and the remaining one was treated with a combination of herbal extracts and active ingredients. In the subgroup analyses of intervention, two studies compared the therapeutic effects of Chinese herbal medicine and placebo (Rozza et al., 2016; Xie et al., 2017), three studies compared Chinese herbal medicine and Western medicine (Zhang et al., 2009; Zhou et al., 2013; Wang et al., 2014), two studies compared Chinese herbal medicine, Western medicine and placebo or no intervention (Zhang et al., 2011; Wang et al., 2019b), one compared two kinds of Chinese herbal medicine and Western medicine (Yu et al., 2018), and two compared Chinese herbal medicine combined Western medicine and Western medicine (Chen et al., 2009; Xiang et al., 2009).
TABLE 1.
Clinical trials for herbs lowering serum uric acid.
| Compounds/formula | Design | Disease | Sample size | T/C | Age (years) | Course of disease | Period | Dose regimen | Duration | Outcome | Side effect | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | |||||||||||
| Chuanhutongfeng mixture | RCT | Chronic gouty arthritis | 165 | 58/55 | 51.10 ± 9.10 | 50.50 ± 8.90 | 9.87 ± 5.50 | 9.91 ± 5.42 | 2014.05–2015.02 | 125 ml, po, bid | 8 weeks | sUA↓ | Diarrhea | Wang et al. (2019b) |
| Yellow-dragon wonderful-seed formula | RCT | Gout | 72 | 24/24 | 45.33 ± 9.86 | 49.21 ± 9.47 | 39.42 ± 29.00 months | 42.96 ± 32.38 months | 2012.03.12–2014.12.15 | 100 ml, po, tid | 4 weeks | sUA↓ | No side effect is found | Yu et al. (2018) |
| Yellow-dragon wonderful-seed formula + Gypsum Fibrosum | RCT | Gout | 72 | 24/24 | 46.13 ± 10.75 | 49.21 ± 9.47 | 55.25 ± 36.58 months | 42.96 ± 32.38 months | 2012.03.12–2014.12.15 | 100 ml, po, tid | 4 weeks | sUA↓ | No side effect is found | Yu et al.(2018) |
| Compound tufuling oral-liquid | RCT | Gout | 210 | 139/71 | 46.00 (21.00) | 49.00 (19.00) | 7.00 (9.00) years | 5.00 (9.00) years | 2012.06.09–2013.05.31 | 250 ml, po, bid | 12 weeks | sUA↓ | Leukopenia | Xie et al. (2017) |
| ZinutriK | RCT | HUA | 16 | 16/16 | 59.00 ± 11.90 | N/A | N/A | N/A | po | 4 weeks | sUA↓ | No side effect is found | Rozza et al. (2016) | |
| The Chuanhu anti-gout mixture | RCT | Acute gouty arthritis | 176 | 88/88 | 51.76 ± 13.21 | 53.82 ± 14.19 | N/A | N/A | 2011.09–2012.09 | 250 ml, po, qd | 10 days | sUA↓ | Diarrhea, nausea | Wang et al. (2014) |
| A series of tongfeng granule | RCT | Gout | 90 | 60/30 | 54.10 ± 13.10 | 49.90 ± 14.20 | 55.30 ± 47.70 months | 53.00 ± 47.30 months | 2010.05–2011.12 | Huzhang tongfeng granule: 12 g, po, bid; Yinlian tongfeng granule: 10 g, po, bid; Jinhuang Ointment: Ad us.ext, qd | 12 weeks | sUA↓ | Indigestion, change in frequency of urination and defecation | Zhou et al. (2013) |
| Xiezhuo chubi recipe | RCT | HUA | 99 | 28/28 | 56.07 ± 17.62 | 53. 18 ± 16. 40 | N/A | N/A | 2009.05.01–2010.03.01 | 0.5 package, po, bid | 20 days | sUA↓ | No side effect is found | Zhang et al. (2011) |
| Retention enema of Chinese herbal medicine | RCT | HUA | 78 | 40/38 | 51.50 ± 3.40 | 51.60 ± 3.30 | 5.40 ± 1.70 years | 5.20 ± 1.90 years | 2006.10–2007.12 | 150 ml for high enema for over 60 min, qd | 6 weeks | sUA↓ | No side effect is found | Chen et al. (2009) |
| Modified sanmiao powder | RCT | Chronic uric acid nephropathy | 94 | 47/47 | 42.36 ± 15.11 | 44.76 ± 14.98 | 13.28 ± 10.63 years | 14.32 ± 12.68 years | 2002.06–2008.06 | 1 package, po, qd | 12 weeks | sUA↓ | ALT↑ | Xiang et al. (2009) |
| Serial gout granules | RCT | Gout | 60 | 30/30 | 52.67 ± 10.59 | 51.10 ± 8.43 | 6.49 ± 4.78 years | 7.02 ± 4.86 years | 2007.03–2008.03 | Huzhang tongfeng granule: 12 g/package, po, bid; Yinlian tongfeng granule: 10 g/package, po,bid | 12 weeks | sUA↓ | Not mentioned | Zhang et al. (2009) |
Data are presented a mean ± SD or a median (QR) for continuous variables and number for categorical variables. T, treatment group; C, control group; N/A: not available; sUA, serum uric acid, HUA, hyperuricemia.
Meta-Analysis
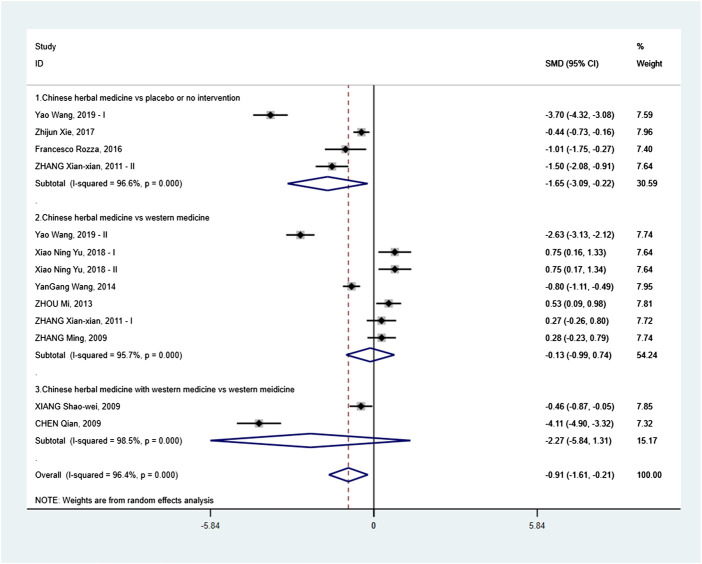
In order to investigate the efficacy of Chinese herbal medicine in lowering sUA, we performed a subgroup analysis based on interventions. A random effects model was used for the analysis because I 2 = 96.4%.
Chinese Herbal Medicine vs Placebo or No Intervention
Four RCTs were analyzed. The subgroup meta-analysis showed that all of them were statistically significant differences between Chinese herbal medicine and placebo or no intervention. The combined SMD was −1.65 with a 95% CI of −3.09 to −0.22 (p = 0.024). Therefore, there was a significant difference between the Chinese herbal medicine and placebo or no intervention in the reduction of sUA (Figure 2).
Figure 2.
Effects of Chinese herbal medicine on serum uric acid in patients with elevated serum uric acid.
Chinese Herbal Medicine vs Western Medicine
Seven RCTs were analyzed. On subgroup meta-analysis, the combined SMD was −0.13 with a 95% CI of −0.99 to 0.74 (p = 0.772), indicating no significant difference between the Chinese herbal medicine and Western medicine in the reduction of sUA (Figure 2).
Chinese Herbal Medicine Plus Western Medicine vs Western Medicine
Two RCTs were analyzed. Subgroup meta-analysis (Figure 2) showed no statistical significance between the Chinese herbal medicine plus Western medicine and Western medicine in the reduction of sUA (SMD −2.27, 95% CI: −5.84 to 1.31, p = 0.214).
Meta-regression Analyses
The meta-regression showed that samples size, duration of treatment, type of diseases and intervention other than Chinese herbal medicine did not influence these results (all p values >0.05) (Supplementary Figure S1).
Quality Assessment
RCT was assessed according to the Cochrane Handbook for Systematic reviews of Interventions (Table 2). Generally, the methodological quality was assessed to be moderate. Most of the studies (8/10, 80%) have details on the random grouping of patients, but only half of the studies (5/10, 50%) fully reported the scheme of concealment allocation. Only four trials (4/10, 40%) had their subjects and investigators blinded during the study, and four trials (4/10, 40%) had all the subjects, investigators and outcome evaluators blinded.
TABLE 2.
Methodological quality assessment of randomized controlled trials according to the Cochrane Handbook.
| Compounds/formula | A | B | C | D | E | F | G | H | References |
|---|---|---|---|---|---|---|---|---|---|
| Chuanhutongfeng mixture | + | + | + | + | + | + | + | ? | Wang et al. (2019b) |
| Yellow-dragon wonderful-seed formula | + | + | − | − | + | + | + | ? | Yu et al. (2018) |
| Compound tufuling oral-liquid | + | + | + | + | + | + | + | ? | Xie et al. (2017) |
| ZinutriK | − | ? | + | + | + | + | + | ? | Rozza et al. (2016) |
| The Chuanhu anti-gout mixture | + | + | + | + | + | + | + | ? | Wang et al. (2014) |
| A series of tongfeng granule | + | ? | − | − | − | + | + | ? | Zhou et al. (2013) |
| Xiezhuo chubi recipe | + | + | − | ? | ? | − | ? | ? | Zhang et al. (2011) |
| Retention enema of Chinese herbal medicine | − | − | − | − | ? | + | + | ? | Chen et al. (2009) |
| Modified sanmiao powder | + | ? | − | − | ? | + | + | ? | Xiang et al. (2009) |
| Serial gout granules | + | ? | − | ? | ? | + | + | ? | Zhang et al. (2009) |
A, Adequate sequence generation; B, Allocation concealment; C, Blinding (patient); D, Blinding (investigator); E, Blinding (assessor); F, Incomplete outcome data addressed; G, Selecting reporting; H, Free of other bias; +, Low risk; -, High risk; ?, Unclear.
Sensitivity Analysis
Sensitivity analysis was conducted to confirm the efficacy Chinese herbal medicine in lowering sUA. The pooled SMDs were repeated by sequentially removing one of the included studies with a random-effects model (Supplementary Figure S2). None of the studies changed the overall effect.
Publication Bias
There was no evidence of publication bias according to the Begg’s test (p = 0.428) and Egger’s test (p = 0.344) for the meta-analysis of Chinese herbal medicine on lowering sUA (Supplementary Figure S3).
Results of experimental studies
We categorized 184 in vivo and in vitro experiments into three groups: 56 active ingredients (Table 3), 78 natural products (Table 4), and 52 herbal formulas (Table 5). Among them, one article described two active ingredients (Lin et al., 2018), and one article (Su et al., 2014) studied one active ingredient and one natural product concurrently. To assure the quality of the studies included, detailed information on herbs (including source, concentration, quality assessment, chemical analysis, and compound purity) was summarized in Supplementary material, Supplementary Tables S4–S6.
TABLE 3.
Active ingredients on lowering serum uric acid based on in vivo and in vitro studies.
| Type | Model | Active ingredients | Inducer | Animal/cell | Major findings | References |
|---|---|---|---|---|---|---|
| In vivo | HUA | Bergenin | Yeast, PO | Mice | SLC2A9, ABCG2, PPAR-γ, IL-6, IL-1β, TNF-α | Chen et al. (2020a) |
| In vivo | HUA | Phloretin | Adenine and PO | Mice | α-SMA, TGF-β, IL-1β, NLRP3, caspase-1, IL-18, XOD, URAT1, GLUT9 | Cui et al. (2020) |
| In vivo | HUA | Polydatin | PO | SD rats | N/A | Han et al. (2020) |
| In vivo | HUA | Total glucosides of herbaceous peony (Paeonia lactiflora Pall.) flower | Adenine, ethambutol | Rats | XOD, URAT1, OAT1, GLUT9 | Kang et al. (2020) |
| In vivo | HUA and hyperlipidemia | Total flavonoids of Mori Cortex | High fat diet, adenine, ethylamine butanol | SD rats | URAT1, IL-6, TNF-α, OAT1 | Dang et al. (2019) |
| In vivo | Gouty arthritis | Pulchinenoside b4 | MSU | SD rats | N/A | Lyu et al. (2019) |
| In vivo | Hyperuricemic nephropathy | Pterostilbene | Adenine and PO | Mice | Fibronectin, collagen I, α-SMA, TGF-β1, Smad3, Src, STAT3 | Pan et al. (2019) |
| In vivo and in vitro | HUA | Liquiritigenin | PO, xanthine | Mice, MDCK-hOAT4, HEK293-hURAT1 | OAT4, URAT1 | Wang et al. (2019e) |
| In vivo | Nonalcoholic fatty liver disease and HUA | Resveratrol | PO and yeast | Rats | FOXO3a, SIRT1, NF-κB | Xu et al. (2019) |
| In vivo | Diabetic nephropathy | Total flavonoids from Oxytropis falcata Bunge | High-fat diet | Mice | MCP-1, NF-κB, IL-6, TGF-β1, JAK 1, STAT3, STAT 4, SOCS-1, SOCS-3 | Yang et al. (2019b) |
| In vivo | Intestinal injury | Ganoderma atrum polysaccharide | Acrylamide | SD rats | MDA, catalase, SOD, glutathione, IL-2, IL-1β, TNF-α, IL-4, IL-10, ALP, endothelin-1 | Yang et al. (2019d) |
| In vivo | HUA and acute gouty arthritis | Luteolin | PO and MSU | Mice | XO, URAT1, GLUT9, IL-1β, TNF-α | Lin et al. (2018) |
| In vivo | HUA and acute gouty arthritis | Luteolin-4′-O-glucoside | PO and MSU | Mice | XO | Lin et al. (2018) |
| In vivo | HUA and gouty arthritis | Hirudin | Hypoxanthine, sodium uric | Mice, wistar rats | XOD, GLUT9 | Liu et al. (2018) |
| In vivo | HUA | Arctiin | Adenine and ethambutanol | Rats | XOD, MCP-1, TNF-α | Louxin et al. (2018) |
| In vivo | HUA | Mangiferin aglycon derivative J99745 | PO | Mice | XO, URAT1, GLUT9, OAT1, ABCG2 | Qin et al. (2018) |
| In vivo | HUA and gouty arthritis | [6]—shogaol | PO, MSU and hypoxanthine | SD rats | IL-1β, TNF-α | Wang et al. (2018) |
| In vivo and in vitro | HUA | Dioscin | PO | Mice, HCT116 cells | URAT1, GLUT9 | Zhang et al. (2018b) |
| In vivo | HUA | Epigallocatechin-3-gallate | PO and yeast | Mice | URAT1, GLUT9, XO | Zhu et al. (2018) |
| In vivo and in vitro | HUA | Taxifolin | Mice: GMP and IMP, cells: Guanosine and inosine | Mice, AML12 cells | XO | Adachi et al. (2017) |
| In vivo | HUA | Vaticaffinol | PO | Mice | XDH, XO, GLUT9, URAT1, OAT1, OCT1, OCT2, OCTN1, NLRP3 | Chen et al. (2017b) |
| In vivo | HUA | Caffeoylquinic acid | PO | Mice | XO, GLUT9, OAT1, URAT1, IL-1β, TNF-α | Jiang et al. (2017) |
| In vivo and in vitro | Gout | Procyanidins | Mice: MSU, cells: LPS and MSU | Mice, raw 264.7 cells | NLRP3, IL-1β, caspase-1, ROS, NR 1, p38, ERK | Liu et al. (2017) |
| In vivo | HUA | Gypenosides | Lipid emulsion | Rats | ADA, XDH, URAT1, GLUT9, OAT1 | Pang et al. (2017) |
| In vivo | Gouty arthritis | Total saponin fraction from dioscorea nipponica makino | MSU | Rats | TLR2, TLR4, IRAK1, TRAF6, NF-κB, IκBα, IKKα, TAK1, IL-1β, IL-6, TNF-α | Zhou et al. (2017) |
| In vivo | HUA | Saponins extracted from dioscorea collettii | Adenine and ethambutol | SD rats | URAT1, GLUT9, OAT1, OAT3 | Zhu et al. (2017) |
| In vivo | HUA | Total saponins from dioscorea septemloba thunb | Adenine | SD rats | OATP1A1 | Chen et al. (2016b) |
| In vivo | HUA with renal dysfunction | Emodinol | PO | Mice | XOD, GLUT9, URAT1, ABCG2, OAT1, OCT1, OCT2, OCTN1, OCTN2, OIT3 | Hui et al. (2016) |
| In vivo and in vitro | HUA | Pallidifloside D | PO | Mice, PC12 | PRPS, HGPRT, PRPPAT | Li et al. (2016) |
| In vivo | HUA | Mangiferin | PO | Mice | XOD | Niu et al. (2016) |
| In vivo | HUA | Salvianolic acid C | PO | Mice | XOD | Tang et al. (2016) |
| In vivo | HUA | Green tea polyphenols | PO | Mice | XO, URAT1, OAT1, OAT3 | Chen et al. (2015a) |
| In vivo | HUA | Flavonoids and phenylethanoid glycosides from Lippia nodiflora | PO and hypoxanthine | SD rats | XO | Cheng et al. (2015) |
| In vivo | HUA | Pallidifloside D | PO | Mice | XO, URAT1, GLUT9, OAT1 | Hou et al. (2015) |
| In vivo | Gout | Lemnalol | MSU | Rats | TGF-β1, MMP-9, cathepsin K, TRAP | Lee et al. (2015) |
| In vivo | HUA and nephropathy | Rhein | Adenine and ethambutol | Mice | IL-1β, TNF-α, PGE2 | Meng et al. (2015) |
| In vivo | HUA | Nuciferine | PO | Mice | URAT1, GLUT9, ABCG2, OAT1, OCT1, OCTN1, OCTN2, IL-1β, TLR2, TLR4, NF-κB, NLRP3, ASC, caspase1 | Wang et al. (2015) |
| In vivo | HUA | Anthocyanins from purple sweet potato | PO | Mice | XO, URAT1, GLUT9, OAT1, OCTN2 | Zhang et al. (2015) |
| In vivo | HUA | Total saponin of dioscorea | PO, ethambutol | SD rats | N/A | Chen et al. (2014b) |
| In vivo | HUA | Chrysanthemum flower oil | PO | Rats | XO | Honda et al. (2014) |
| In vivo | HUA | Exopolysaccharide produced by Cordyceps militaris | PO | Mice | XO | Ma et al. (2014) |
| In vivo | HUA | Dioscin | PO | Mice | OAT1, URAT1, OCT2, XO | Su et al. (2014) |
| In vivo | HUA | Pallidifloside D | PO | Mice | URAT1, GLUT9, OAT1 | Wu et al. (2014a) |
| In vivo | HUA | Smilaxchinoside A, Smilaxchinoside C | PO | Mice | URAT1, GLUT9, OAT1 | Wu et al. (2014b) |
| In vivo | HUA | Riparoside B, timosaponin J | PO | Mice | XO, URAT1, GLUT9, OTA1 | Wu et al. (2014d) |
| In vivo and in vitro | HUA | Total saponins from Discorea nipponica | Mice: PO, cells: IL-1β | Mice, synovial cells | URAT1, GLUT9, OAT1, OAT3 | Zhou et al. (2014) |
| In vivo | Uric acid nephropathy | Quercetin | Adenine and ethambutol | SD rats | NLRP3, ASC, Caspase-1, TLR2, TLR4 | Hu et al. (2013a) |
| In vivo | HUA | Aspalathin | IMP | Mice | XO | Kondo et al. (2013) |
| In vivo | Gouty arthritis | Quercetin | MSU | SD rats | IL–1β, TNF–α, COX-2, PGE2, NO, MDA, SOD, glutathione peroxidase | Huang et al. (2012) |
| In vivo | HUA | Mangiferin | PO | Mice | XDH, XOD | Niu et al. (2012) |
| In vivo | Hyperuricemic nephropathy | Tanshinone IIA | Adenine | SD rats | NF-κB, MCP-1, IL-10 | Wu et al. (2012a) |
| In vivo | Uric acid nephropathy | Tanshinone IIA | Adenine | SD rats | NF-κB, MCP-1, IL-10 | Wu et al. (2012b) |
| In vivo | HUA | Curcumin | Fructose | SD rats | NO, GLUT9, RST, OAT1, MRP4, OCT1, JAK2, STAT3, SOCS3, TGF-β1 | Zhang et al. (2012) |
| In vivo | HUA | Iridoid glycosides of Paederia scandens | PO and adenine | SD rats | MCP-1, α-SMA, NF-κB | Zhu et al. (2012) |
| In vivo | HUA | Mulberroside a | PO | Mice | GLUT9, URAT1, OAT1, OCT1, OCT2, OCTN1, OCTN2 | Wang et al. (2011) |
| In vivo | HUA | Morin | PO | Mice | URAT1, GLUT9, OAT1, OAT3, OCT1, OCT2, OCTN1, OCTN2, | Wang et al. (2010a) |
HUA, hyperuricemia; PO, potassium oxonate; MSU, monosodium urate; SLC2A9, solute carrier family 2, facilitated glucose transporter member; PPAR-γ, peroxisome proliferator-activated receptor γ; IL, interleukin; TNF-α, tumor necrosis factor; α-SMA, alpha-smooth muscle actin; TGF-β, transforming growth factor beta-1; NLRP3, the NOD-like receptor P3 inflammasome; XO/XOD, xanthine oxidase; URAT1, urate transporter one; GLUT9, glucose transporter nine; OAT1/3, organic anion transporter one and three; Smad3, mothers against decapentaplegic homolog three; Src, proto-oncogene tyrosine-protein kinase Src; STAT3, signal transducer and activator of transcription three; FOXO3α, forkhead box O3 alpha; SIRT1, silent information regulator one; NF-κB, nuclear factor-kappa B; MCP-1, monocyte chemoattractant protein-1; JAK2, Janus kinase two; SOCS-1/3, suppressor of cytokine signaling 1/3; MDA, malondialdehyde; SOD, superoxide dismutase; ABCG2, adenosine triphosphate (ATP)-binding cassette transporter two; XDH, xanthine dehydrogenase; OCT1/2, organic cation transporter; ALP: alkaline phosphatase; OCTN1/2, organic cation/carnitine transporter one and two; ROS, reactive oxygen species; NR-1, N-methyl-D-aspartic acid receptor one; p38, phosphorylated p38 mitogen-activated protein kinase; ERK, phosphorylated extracellular regulated protein kinase; ADA, adenosine deaminase; TLRs, toll-like receptors; IRAK, IL-1R-associated kinase; TRAF6, TNF receptor associated factor 6; IκB, inhibitor of NF-κB; IKKα, inhibitor of nuclear factor kappa-B kinase; TAK1, transforming growth factor beta-activated kinase one; OATP1A1, organic anion transporting polypeptide 1A1; OIT3, oncoprotein-induced transcript 3; PRPS, phosphoribosyl pyrophosphate synthetase; HGPRT, hypoxanthine-guanine phosphoribosyl transferase; PRPPAT, phosphoribosyl pyrophosphate amino-transferase; MMP-9, matrix metalloproteinase nine; TRAP, tartrate-resistant acid phosphatase; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; NO, nitric oxide; ASC, the apoptosis-associated speck-like protein containing a caspase recruitment domain; RST, renal-specific transporter; MRP4, multidrug resistance protein 4. N/A, not available.
TABLE 4.
Natural products that lower serum uric acid based on in vivo and in vitro studies.
| Type | Model | Natural product | Inducer | Animal/cell | Major findings | References |
|---|---|---|---|---|---|---|
| In vivo | HUA | Clerodendranthus spicatus | Oteracil potassium | Mice | N/A | Chen et al. (2020b) |
| In vivo | HUA | Sunflower head enzymatic hydrolysate | PO, yeast | Mice | XOD, ADA, URAT1 | Liu et al. (2020) |
| In vivo | HUA | Macroporous Resin extract of Dendrobium candidum leaves | PO, adenine, yeast | SD rats | XOD, ADA, TLR4, NF-κB | Lou et al. (2020) |
| In vivo | Hyperuricemic nephropathy | Ethanol extract of Liriodendron chinense (Hemsl.) Sarg barks | Adenine and PO | Mice | OAT1, OAT3, ABCG2 | Pan et al. (2020) |
| In vivo | HUA | Eucommia ulmoides Oliver | Mice: Oxonic acid potassium salt, rats: hypoxanthine | Mice, rats | URAT1, OAT1, OAT3, GLUT9 | Fang et al. (2019) |
| In vivo | HUA | Smilax glabra extracts | PO | Mice | XOD, OAT1, OCTN2 | Huang et al. (2019b) |
| In vivo and in vitro | HUA | Alpinia oxyphylla seed extract | PO | Rats, oocytes | XO, URAT1, OAT1, IL-1β, IL-6, TNF-α | Lee et al. (2019b) |
| In vivo | Chronic HUA and gout | Rhizoma smilacis glabrae extracts | PO and MSU | Mice | XO, IL-6, IL-12, IL-1β, IL-10, TNF-α | Liang et al. (2019) |
| In vivo | HUA | The extract of O. Sativa | PO | SD rats | XO | Liu et al. (2019) |
| In vivo | Acute gouty arthritis | Ethanolic extract of Polygonum cuspidatum | MSU | Mice | IL-1β, IL-6, TNF-α, NLRP3, ASC, caspase-1 | Ma et al. (2019a) |
| In vivo | Uric acid-induced renal damage | Polygonum cuspidatum | Adenine administering and yeast feeding | SD rats | AMPK, FOXO3α, TLR4, NLRP3, MCP-1 | Ma et al. (2019b) |
| In vivo | Uric acid-induced renal damage | Polygonum cuspidatum | Adenine, yeast | SD rats | AMPK, FOXO3α, TLR4, NLRP3, MCP1 | Ma et al. (2019c) |
| In vivo | HUA | Chrysanthemum extract | PO | SD rats | XOD, ABCG2, URAT1, GLUT9, IL-1β, TNF-α | Peng et al. (2019) |
| In vivo | HUA | The flavonoid-rich fraction from rhizomes of Smilax glabra Roxb. | Yeast pellets and adenine | SD rats | ABCG2, OAT1, OCT2, OCTN2, URAT1, GLUT9 | Wang et al. (2019a) |
| In vivo | HUA | Chicory (Cichorium intybus L.) | Fructose | SD rats | URAT1, GLUT9, OAT1, OAT3 | Wang et al. (2019d) |
| In vivo | HUA and acute gouty arthritis | Terminthia paniculata extract | PO and MSU | Mice | XO | Yang et al. (2019c) |
| In vivo | HUA | Highly acylated anthocyanins from purple sweet potato | PO | Mice | TNF-α, IL-6, IL-1β, TGF-β1, ICAM1, COX-2, NF-κB | Zhang et al. (2019c) |
| In vivo | HUA | Ethanol extract of Dendropanax morbifera leaf | PO | Mice | XO | Cho et al. (2018) |
| In vivo | Diabetic kidney disease | Codonopsis tangshen Oliv. | Unilateral nephrectomy, high fat diet feeding | SD rats | IL-1β, TNF-α, IL-6 | Lu et al. (2018) |
| In vivo | HUA | Ethanol extract of Cudrania tricuspidata leaf | PO | Mice | XO | Song et al. (2018) |
| In vivo | Hypouricemic and nephroprotective | Polyrhachis Vicina Roger | PO | SD rats | IL-1β, IL-6, TNF-α, SOD, MDA, XOD, URAT1, GLUT9, OAT1 | Su et al. (2018) |
| In vivo | HUA | Lagotis brachystachys Maxim extract | PO | Mice | URAT1, GLUT9, OAT1 | Xiong et al. (2018) |
| In vivo | HUA | Toona sinensis leaf extract | PO | Rats | XO | Yuk et al. (2018) |
| In vivo | HUA | Chaenomeles sinensis fruit extract | PO | Mice | URAT1, OAT1 | Zhang et al. (2018a) |
| In vivo | HUA | Fraxini cortex | Hypoxanthine and PO | SD rats | URAT1,GLUT9 | Zhou et al. (2018a) |
| In vivo | HUA | Selaginella tamariscina | Xanthine, PO and MSU | Rats | N/A | Chen et al. (2017a) |
| In vivo | HUA | Rhizoma Polygoni Cuspidati and Ramulus Cinnamomi | Hypoxanthine, ethambutol and PO | SD rats | XO | Han et al. (2017) |
| In vivo | HUA | Salvia plebeia extract | PO | Mice | XO | Kim et al. (2017) |
| In vivo | Gouty arthritis | Mollugo pentaphylla extract | MSU | Mice | TNF-α, IL-1β, NLRP3, ASC, caspase-1, NF-κB | Lee et al. (2017) |
| In vivo | HUA and gout | Sunflower head extract | PO and MSU | Mice | XO | Li et al. (2017a) |
| In vivo | HUA | Aristolochia bracteolata extracts | PO | Rats | XO | Li et al. (2017b) |
| In vivo | HUA | Total flavonoids of Humulus lupulus | PO | Mice | XO | Li et al. (2017c) |
| In vivo | HUA | Chicory | D-fructose | SD rats | ABCG2 | Wang et al. (2017a) |
| In vivo | HUA | Chicory extract | Fructose | SD rats | GLUT9 | Wang et al. (2017b) |
| In vivo | HUA and gout | Cortex fraxini | PO, yeast and adenine | SD rats | N/A | Wang et al. (2017c) |
| In vivo | HUA | Plantago depressa Willd extract | PO | SD rats | XOD | Xia et al. (2017) |
| In vivo | HUA | Ganoderma applanatum extracts | Hypoxanthine and PO | Mice | XO, URAT1, GLUT9, OAT1 | Yong et al. (2017) |
| In vivo | HUA | Quercus acuta Thunb. extract | PO | Mice | XO | Yoon et al. (2017a) |
| In vivo | HUA | Ethanol extracts of Camellia japonica leaf | PO | Mice | XO | Yoon et al. (2017b) |
| In vivo | HUA and acute gouty arthritis | Gnaphalium affine D. Don extract | PO and MSU | Mice | XO, GLUT9, URAT1 | Zhang et al. (2017a) |
| In vivo | HUA | Ginkgo folium | Fructose | SD rats | N/A | Zhang et al. (2017b) |
| In vivo | HUA | Selaginella moellendorffii Hieron. | PO | SD rats | XOD, MDA, SOD, TNF-α, IL-1β | Zhao et al. (2017) |
| In vivo | HUA | Dioscorea tokoro Makino extract | PO | Mice | XOD, URAT1, OAT1 | Fei et al. (2016) |
| In vivo and in vitro | HUA | Mesona procumbens extracts | THP-1 cells: MSU, mice: PO, SD rats: streptozocin | THP-1 cells, mice, rats | XO, OAT1, GLUT9 | Jhang et al. (2016) |
| In vivo | HUA | Ethyl acetate fraction of Dimocarpus longan Lour. extracts | PO | Mice | XO | Sheu et al. (2016) |
| In vivo and in vitro | HUA | Synsepalum dulcificum | Mice: PO, cells: MSU | Mice, RAW264.7 cells | XO | Shi et al. (2016b) |
| In vivo | HUA | Dendropanaxchevalieri extracts | PO | Mice | XOD, URAT1, GLUT9 | Wang et al. (2016a) |
| In vivo | HUA | Tradescantia albiflora Kunth extracts | PO | Rats | XO | Wang et al. (2016c) |
| In vivo | HUA | Cortex fraxini | PO, yeast and adenine | Rats | N/A | Wang et al. (2016e) |
| In vivo | HUA | Cordyceps militaris | PO and hypoxanthine | Mice | XO, URAT1 | Yong et al. (2016) |
| In vivo | HUA | The ethanolic extracts of Corylopsis coreana Uyeki | PO | Mice | XO | Yoon et al. (2016) |
| In vivo | HUA | Leaves of Perilla frutescens | PO | Mice | XO | Huo et al. (2015) |
| In vivo | HUA | Ethanol extracts from Dioscoreae nipponicae rhizoma | Hypoxanthine and PO | Mice | N/A | Shan et al. (2015) |
| In vivo | HUA | Rhododendron oldhamii maxim. Leaf extracts | PO | Mice | N/A | Tung et al. (2015) |
| In vivo | HUA | Smilax riparia | PO | Mice | URAT1, GLUT9, OAT1 | Wu et al. (2015) |
| In vivo | HUA | Lagotis brevituba Maxim. extract | PO | Mice | XOD, OAT1, URAT1, GLUT9 | Zeng et al. (2015) |
| In vivo | HUA | Total saponins from Discorea nipponica makino | Adenine and ethambutol | Rats | OAT1, OAT3, URAT1 | Zhou et al. (2015) |
| In vivo | HUA | Phytochemicals from Davallia formosana | PO | Mice | XO | Chen et al. (2014a) |
| In vivo and in vitro | HUA | Smilacis glabrae rhizoma | PO and uric acid | SD rats, HUVECs | Catalase | Hong et al. (2014) |
| In vivo | HUA | Poecilobdella manillensis | Hypoxanthine | Mice | N/A | Liu et al. (2014b) |
| In vivo | Gouty arthritis | Rhizoma Dioscoreae nipponicae | MSU | Rats | SDF-1, CXCR4, p38, IL-1β, MKK 3/6 | Lu et al. (2014) |
| In vivo | HUA | Rhizoma Dioscoreae septemlobae extracts | PO | Mice | OAT1, URAT1, OCT2, XO | Su et al. (2014) |
| In vivo | HUA | Smilax riparia | PO | Mice | URAT1 | Wu et al. (2014c) |
| In vivo | HUA | Leonurus | PO | SD rats | URAT1, GLUT9, OCT, OCTN | Yan et al. (2014) |
| In vivo and in vitro | Gouty arthritis | Dolichos falcata Klein | MSU | SD rats, raw 264.7 cells | IL-1β, IL-6, TNF-α | Chen et al. (2013b) |
| In vivo | HUA | Ethanol extract of Fructus Gardenia | PO | Mice | URAT1, ABCG2, GLUT9, OAT1, OAT3, OIT3, OCT1, OCT2, OCTN1, OCTN2 | Hu et al. (2013b) |
| In vivo | HUA and gout | The crude extract of Jatropha isabellei | PO and MSU | Rats | XO | Silva et al. (2013) |
| In vivo | HUA | Petroleum ether fraction of Polyrhachis vicina Roger | Hypoxanthine | Mice | XOD | Wei et al. (2013) |
| In vivo | HUA | Rhizoma smilacis glabrae | Hypoxanthine | SD rats | N/A | Xu et al. (2013) |
| In vivo | HUA | Balanophora laxiflora extracts and derived phytochemicals | PO | Mice | XO | Ho et al. (2012) |
| In vivo | HUA | Longan seed extract | PO and hypoxanthine | SD rats | GLUT1, GLUT9, XO | Hou et al. (2012) |
| In vivo | Gouty arthritis | Ethanol extract from Mangifera indica | MSU | SD rats | TNF-α, IL-1β | Jiang et al. (2012) |
| In vivo | HUA | Hibiscus sabdariffa L. extracts | PO | SD rats | XOD | Kuo et al. (2012) |
| In vivo | HUA | Ramulus Mori ethanol extract | PO | Mice | URAT1, GLUT9, OAT1, OCT1, OCT2, OCTN1, OCTN2 | Shi et al. (2012) |
| In vivo | Acute gouty arthritis | Rhizoma Dioscoreae nipponicae | MSU and hypoxanthine | Rats | TRAIL, Neuropilin-2, MMP-13 | Yao et al. (2012) |
| In vivo | HUA | The methanol extract from Prunus mume fruit | PO | Mice | XO | Yi et al. (2012) |
| In vivo | HUA | Casein or soya protein combined with palm or safflower-seed oil | PO and uric acid | Rats | NO, TNF-α, IFN-γ, Nitrotyrosine | Lo et al. (2010) |
| In vivo | Gouty arthritis | Extract of Paederia scandens (LOUR.) MERRILL (Rubiaceae) | MSU | SD rats | TNF-α, IL-1β, NF-κB | Ma et al. (2009) |
Abbreviations can be referred to Table 3. ICAM1, intercellular adhesion molecule one; IFN, interferon; AMPK, adenosine 5′-monophosphate-activated protein kinase; FOXO3α, forkhead box O3 alpha; SDF-1, stromal cell-derived factor 1; CXCR4, SDF-1 receptor; MKK, mitogen-activated kinase; TRAIL, the tumor necrosis factor-related apoptosis-inducing ligand.
TABLE 5.
Herbal formulas that lower serum uric acid based on in vivo and in vitro studies.
| Type | Model | Herbal formula | Inducer | Animal/cell | Major findings | References |
|---|---|---|---|---|---|---|
| In vivo | HUA and hyperlipidemia | Dendrobium officinalis Six Nostrum | PO and hihg-fat sorghum feed | SD rats | XOD, ADA, LPL, FABP1, HPRT1, NLRP3, Caspase-1, TLR4 | Guo et al. (2020) |
| In vivo | Gouty arthritis | Simiao decoction | Yeast, MSU | Mice | NLRP3, ACS, caspase1, XOD, ADA, IL- 1α, IL-6, IL-1β, IL-9, IL-12, granulocyte colony stimulating factor; MCP-1, TNF-α, STAT3, APOB, caspase 8, c-FOS, PPARα, FN1, LPL, MIP-1α, MIP-1β | Lin et al. (2020) |
| In vivo | Acute gouty arthritis with HUA | Tu-Teng-Cao | PO, MSU | SD rats | TNF-α, IL-6, IL-1β | Yao et al. (2020) |
| In vivo | HUA | Erding granules | PO | Mice | N/A | Zuo et al. (2020) |
| In vivo | HUA | TongFengTangSan | PO | SD rats | XOD | Chen et al. (2019b) |
| In vivo | HUA | Alismatis Rhizoma and Rhizoma Smilacis Glabrae decotion | PO, adenine | SD rats | XOD, URAT1 | Cheng et al. (2019) |
| In vivo | HUA | Er Miao wan | Fructose | SD rats | N/A | Huang et al. (2019a) |
| In vivo | Renal stones | Huashi pill | Ethylene glycol, ammonium chloride, calcium gluconate | SD rats | Osteopontin | Yang et al. (2019a) |
| In vivo | HUA | Modified Chuanhu anti-gout mixture | PO | Mice | XOD, URAT1 | You et al. (2019) |
| In vivo | Acute gouty arthritis | Jia-Wei-Si-Miao-Wan | Monosodium urate | Rats | TLR4, NLRP3, ASC, caspase-1, NF-κB, IL-1β, IL-18 | Yuan et al. (2019) |
| In vivo | HUA | Erding granule | PO | Mice, SD rats | GLUT9, OAT1, URAT1 | Zhang et al. (2019a) |
| In vivo and in vitro | HUA and acute gouty arthritis | The Selaginella moellendorffii prescription | PO, adenine, MSU, LPS | Mice, rats, RAW264.7 cells | NO, NF-κB, NLRP3, IL-1β, PGE-2, IL-8 | Zhang et al. (2019b) |
| In vivo | Acute gouty arthritis | Tongfengning capsule | MSU | Rats | TNF-α, IL-1β | Fan et al. (2018) |
| In vivo | T2DM | Spleen-kidney supplementing formula | High-fat diet, low-dose streptozotocin | Wistar rats | TGF-β1, miR-21, PTEN | Tian et al. (2018) |
| In vivo | HUA | Ermiaowan | Xanthine and oxonic acid potassium salt | Rats | N/A | Wei et al. (2018) |
| In vivo | HUA | Compound tufuling granules | Rats | TNF-α, IL-1β | Wu et al. (2018a) | |
| In vivo | HUA | ShiZhiFang | PO | SD rats | XOD, OAT1, OAT3 | Wu et al. (2018b) |
| In vivo | HUA | Erding formula | Hypoxanthine and PO | Mice | URAT1, OAT3 | Zuo et al. (2018) |
| In vivo | HUA | Qi-Zhu-Xie-Zhuo-Fang | Adenine and PO | Rats | XO, E-cadherin, α-SMA | Huijuan et al. (2017) |
| In vivo | HUA | Shizhifang | PO | SD rats | TXNIP, NLRP3, ASC, caspase-1, IL-1β, IL-18, ROS | Wu et al. (2017) |
| In vivo | HUA | Quzhuotongbi decoction | Yeast | SD rats | N/A | Chen et al. (2016a) |
| In vivo | Gouty arthritis | Zisheng Shenqi decoction | MSU | Rats | IL-1β, TNF-α, IκB, NF-κB, NALP1, NALP6, ROS | Han et al. (2016) |
| In vivo | HUA | RuPeng15 powder | PO | SD rats | XO | Kou et al. (2016) |
| In vivo | Gouty arthritis | Xiaofeng granules | MSU | SD rats, rabbits | IL-1β, IL-6, TNF-α, iNOS, ADAMTS-4, TIMP-3 | Shi et al. (2016a) |
| In vivo | HUA | Siwu decoction | PO | Mice | XO, URAT1, GLUT9, OAT1, ABCG2, OCT1, OCT2, OCTN1, OCTN2, NLRP3, ASC, caspase-1, IL-1β | Wang et al. (2016b) |
| In vivo | HUA | Jianpi Huashi decoction | PO | Rats | XO | Wang et al. (2016d) |
| In vivo and in vitro | Gout | Sanmiao formula | Animals: PO and cold bath, cells: MSU | SD rats, rabbits, primary chondrocyte | IL-1β, TNF-α, MMP-3, TIMP-1, ADAMTS-4, TIMP-3 | Zhu et al. (2016) |
| In vivo | HUA | Karapxa decoction | Yeast and PO | Mice | XO | Amat et al. (2015) |
| In vivo | HUA | Ermiao pill | PO | SD rats | XO | Chen et al. (2015b) |
| In vivo | HUA | Si-Wu-Tang and Er-Miao-San | PO and adenine | SD rats | XO, OAT1, OAT3 | Guo et al. (2015) |
| In vivo | HUA | Shuang-Qi gout capsule | PO | Mice | URAT1, OAT1, OCT1, OCT2, ABCG2, OCTN1, OCTN2, GLUT9 | Kodithuwakku et al. (2015) |
| In vivo | Gouty arthritis | RuPeng15 powder | MSU | SD rats | NF-κB, TNF-α, IL-1β, IL-8 | Kou et al. (2015) |
| In vivo | HUA | Compound tufuling granules | Yeast and PO | Mice | GLUT9 | Liu et al. (2015) |
| In vivo | HUA and metabolic syndrome | Simiao pill | Fructose | SD rats | Nephrin, podocin, CD2AP, Sirt1, NF-κB, IL-1β, NLRP3, ACS, caspase1 | Ma et al. (2015) |
| In vivo | HUA | Xie-Zhuo-Chu-Bi-Fang | Adenine and PO | Mice | URAT1, miR-34a | Sun et al. (2015) |
| In vivo | HUA | Wuling san | Fructose | Mice | URAT1, GLUT9, ABCG2, OAT1, OCT1, OCT2 | Yang et al. (2015) |
| In vivo | Urate nephropathy | Compound qingqin liquid | Adenine and PO | SD rats | TLR2, TLR4 | Chen et al. (2014c) |
| In vivo | HUA | Modified Simiao wan | Oxonic acid potassium salt, hypoxanthine | SD rats | XOD | Pan et al. (2014) |
| In vivo | HUA | Compound qingqin liquid | Adenine and PO | SD rats | COX-2 | Shang et al. (2014) |
| In vivo | HUA | Jianpihuashi decoction | PO | SD rats | XO | Chen et al. (2013a) |
| In vivo | HUA | Wuling san | PO | Mice | URAT1, GLUT9, OAT1, OCT1, OCT2, OCTN2 | Ding et al. (2013) |
| In vivo and in vitro | Gout arthritis | Shuang-Qi gout capsule | MSU | Mice, SD rats, HUVECs | TNF-α, IL-1β | Kodithuwakku et al. (2013) |
| In vivo | HUA | Tongfeng granule | Adenine and ethambutol | Rats | N/A | Liu et al. (2013) |
| In vivo | HUA | Jieduxiezhuo decoction | Uric acid | Mice | N/A | Pan et al. (2013) |
| In vivo and in vitro | MSU-induced inflammation | Modified Simiaowan | MSU | Mice, HUVECs | ICAM-1 | Shi et al. (2013) |
| In vivo | HUA | Tongxi powder | Uric acid | Mice | N/A | Yang et al. (2013) |
| In vivo | HUA | Modified Simiao decoction | PO | Mice | XO, URAT1, OAT1, SOD, MDA | Hua et al. (2012) |
| In vivo | Acute gouty arthritis | Compound Shuiniujiao | MSU | SD rats | N/A | Cao et al. (2011) |
| In vivo | HUA | Simiao pill | PO | Mice | URAT1, GLUT9, OAT1, OCT1, OCT2, OCTN1, OCTN2 | Hu et al. (2010) |
| In vivo | HUA | Danggui Buxue Tang | Adenine, ethambutol | Rats | NO, NOS, SOD, MDA | Li et al. (2010) |
| In vivo | HUA | Ermiao pill | Oxygen hydrochloride acid potassium salt | Mice | XOD, URAT1 | Lü et al. (2010) |
| In vivo | HUA | Sanmiao wan | PO | Mice | XOD, URAT1 | Wang et al. (2010b) |
Abbreviations can be referred to Tables 3, 4. FABP1, fatty acid-binding protein; HPRT1, hypoxanthine-guanine phosphoribosyl transferase; APOB, apolipoprotein B; FOS, one subunit of activator protien-1; FN1, fibronectin1; MIP-1α, MIP-1 β, serum proinflammatory cytokines; TXNIP, thioredoxin interacting proteins; PTEN, phosphate and tension homology deleted on chromsome ten; NALP1/6, NACHT, LRR and PYD domains-containing protein one and six; ADAMTs, a family of metalloproteinases with thrombospondin motifs; TIMP, tissue inhibitor of metalloproteinase; CD2AP, CD2-associated protein.
Active Ingredients That Lower Serum Urate in vivo and in vitro Studies
Active ingredient is a single ingredient and studies have shown that it plays an important therapeutic role in reducing sUA. Among the active ingredients with potential of lowering sUA (Table 3), 25 of which act on uric acid synthesis, 28 target uric acid transporter, 19 resolve inflammation, nine possess kidney protective function, and four regulate the oxidative stress.
Natural Products That Lower Serum Urate in vivo and in vitro Studies
Natural products include herbs and relatively complex extracts derived from herbs. Among the natural products with potential of lowering sUA (Table 4), 40 of which act on uric acid synthesis, 29 target uric acid transporter, 17 resolve inflammation, eight possess kidney protective functions, and three regulate the oxidative stress.
Herbal Formulas That Lower Serum Urate in vivo and in vitro Studies
Herbal formula is a combination of a variety of herbs. The composition of the herbal formula included in the current study were listed in Supplementary material, Supplementary Table S7. Among the herbal formula with lowering sUA effects (Table 5), 18 of which act on uric acid synthesis, 15 target uric acid transporter, 15 resolve inflammation, seven possess kidney protective functions, and five regulate the oxidative stress.
Discussion
The efficacies of Chinese herbal medicines lowering Serum uric Acid levels
This systematic review compares the efficacies of the Chinese herbal medicines and the Western medicine in lowering sUA levels by analysing10 RCTs with a total of 1,060 patients. The meta-analysis has three important findings. First, there is a significant difference between the Chinese herbal medicine, placebo or no intervention in the reduction of sUA levels. Second, the efficacies of Chinese herbal medicines in lowering sUA levels are comparable to that of Western medicine. Third, the efficacies of Chinese herbal medicines plus Western medicine in lowering sUA levels are comparable to that of Western medicine. The heterogeneity of the meta-analysis was high. To investigate the source of heterogeneity in our analysis, we conducted a sensitivity analysis, removing one study at one time from the primary analysis did not change the main finding. In addition, our findings were confirmed by the lack of publication bias and effect modifiers according to the Begg’s test, Egger’s test and meta-regression analysis.
The results indicate that the efficacies of Chinese herbal medicines in lowering sUA levels are comparable to that of Western medicine, which is consistent with the analysis result of Lin et al. (Lin et al., 2016). Compared with the study of Lin et al., our study is more comprehensive, because we also compared the sUA lowering efficacy between Chinese herbal medicine and placebo or no intervention, and between Chinese herbal medicine plus western medicine and western medicine. In addition, we have summarized the underlying mechanisms of herbs in lowering sUA. It should be noted that among the 10 RCTs included, a total of six RCTs used one or several components of Simiao Pills, which is a famous formula in traditional Chinese medicine, including Cortex Phellodendri Chinensis, Atractylodes Lancea (Thunb.) DC., Coix lacryma-jobi L.var.ma-yuen (Roman.) Stapf (Yi Yi), Cyathula officinalis Kuan (Xiang et al., 2009; Zhang et al., 2009; Zhang et al., 2011; Zhou et al., 2013; Xie et al., 2017; Yu et al., 2018). The specific doses of herbs in each study have been listed in the Supplementary Tables S4–S6. According to the Pharmacopoeia of the People’s Republic of China revised by the Food and Drug Administration in 2015, except for the use of Cortex Phellodendri Chinensis, Atractylodes Lancea (Thunb.) DC., Cyathula officinalis Kuan in one RCT (Xiang et al., 2009) exceeded the recommended dosage, the dosages of these four herbs in the remaining five RCTs were within the recommended dosage range. Except for the RCT in which the dosage exceeded the recommended dosage (Xiang et al., 2009), the dosage of these four herbs in the remaining five RCTs were all at the high level or even reached the critical value within the recommended range. Despite the high dosage, the three RCTs describing the adverse reactions in the six RCTs showed that the incidence of adverse reactions was either lower than that of the placebo group or was not statistically significant compared with the control group.
In addition to clinical studies, experimental studies have also suggested the urate lowering effects of Simiao Pills, which act on xanthine oxidase (XO), uric acid transport-related proteins urate anion transporter 1 (URAT1) and glucose transporter 9 (GLUT9), and also modulate the inflammation, oxidative stress and other processes (Hu et al., 2010; Hua et al., 2012; Shi et al., 2013; Pan et al., 2014; Ma et al., 2015; Lin et al., 2020). The finding suggests that Chinese herbal medicines are mostly multi-targeted or have interplay with other signaling pathways to lower sUA levels. In contrast, conventional Western medicines used to lower sUA levels include allopurinol, probenecid and benzbromarone, which generally act on specific targets. Allopurinol competitively inhibits xanthine oxidase (Strilchuk et al., 2019). Probenecid and benzbromarone are typical urate-promoting drugs target at URAT1 (Azevedo et al., 2019). These may partly explain why the combination of Chinese and Western medicine can improve the index of renal function while lowering sUA levels in patients with hyperuricemia (Chen et al., 2009; Xiang et al., 2009).
Nevertheless, several limitations of the meta-analysis are worth considering. Due to limited reports, participants with elevated sUA levels in this meta-analysis included patients with hyperuricemia and gout, and most participants were from China. These facts indicate that the current meta-analysis could have potential bias. Further trials need to be carried out in a larger comprehensive population to demonstrate the efficacy of Chinese herbal medicines in lowering sUA. In addition, long-term tracking of sUA is necessary to determine whether Chinese herbal medicine can effectively control sUA. However, long-term follow-ups were not available in the current included studies. The incidence of adverse reactions is an important indicator to compare the efficacy and safety of Chinese herbal medicine and Western medicine in lowering sUA levels. However, of the six included studies comparing Chinese herbal medicine and Western medicine, only 2 has reported the adverse reaction events in details. Thus, we cannot evaluate the safety of Chinese herbal medicine vs. western medicine in lowering sUA levels. Moreover, the standardization of the methodologies and the small number of the included trials may lead to an overestimation of the overall efficacy of Chinese herbal medicine. Therefore, studies with high quality are needed to confirm the efficacy and safety of Chinese herbal medicine in lowering sUA levels.
The mechanisms of Chinese herbal medicines in lowering Serum uric Acid
In current review, 186 in vivo and in vitro experiments with 56 active ingredients (Table 3), 78 natural products (Table 4), and 52 herbal formulas (Table 5) are included to explore the common mechanism of Chinese herbal medicine in lowering sUA levels. According to the summary of the targets of Chinese herbal medicine in lowering sUA, it is clearly revealed that most Chinese herbal medicine lowers sUA by acting on multiple targets or multiple pathways. The therapeutic mechanism of Chinese herbal medicine included in this study mainly involved the UA synthesis, UA transport, inflammation, renal fibrosis and oxidative stress.
Uric acid, produced primarily in the liver, is the final product of diet and endogenous purine metabolism. Problems with key enzymes involved in UA production can cause abnormal UA levels, including phosphoribosyl pyrophosphate (PRPP) synthetase, purine nucleoside phosphorylase, xanthine oxidase, hypoxanthine-guanine phosphoribosyl transferase (HGPRT) (Cammalleri and Malaguarnera, 2007; Maiuolo et al., 2016). In the circulation, UA exists mainly in the form of urate anion under physiologic pH. The saturation level of monosodium urate in human plasma is limited. Hence, UA must be excreted continuously to prevent its accumulation and reduce the toxicity. The primary scavenger of urate is the kidney, which expels about 75% of urate every day (Chaudhary et al., 2013). In recent years, studies have revealed the complex interaction of transporters involved in urate metabolism (Wright et al., 2010). Several transporters involved in urate metabolism have been identified, including URAT1, GLUT9, organic anion transporters (OAT1/3), and adenosine triphosphate (ATP)-binding cassette transporter 2 (ABCG2) (Enomoto et al., 2002; Lipkowitz, 2012; Mandal and Mount, 2015). Previous studies have found that inflammation, oxidative stress, mitochondrial dysfunction and other factors cause abnormalities in these critical proteins, and thus lead to the disorders in UA metabolism (Lanaspa et al., 2012; Zhou et al., 2018b). On the contrary, abnormal UA levels can also lead to the release of some cytokines, such as tumor necrosis factor-α (TNF-α), nuclear factor-kappa B (NF-κΒ), the NOD-like receptor P3 (NLRP3) inflammasome, interleukin-6 (IL-6), interleukin-1β (IL-1β), and so on (Major et al., 2018; Singh et al., 2019).
Herbs Lower Serum Uric Acid by Targeting Uric Acid Synthesis
The substrate for UA synthesis is ribose-5-phosphate which can be converted to PRPP via PRPP synthase and then to inosine monophosphate. This intermediate compound produces adenosine monophosphate and guanosine monophosphate, which subsequently release adenosine and guanosine molecules, respectively. Adenosine deaminase converts adenosine to inosine, while guanosine to free guanine. Inosine is degraded to hypoxanthine via purine nucleoside phosphorylase. Xanthine oxidase, one of the key enzymes involved in UA synthesis, converts hypoxanthine to xanthine which then converts to UA. Guanine is directly converted to xanthine, which subsequently to UA by XO. On the other hand, hypoxanthine and guanine enter a salvage pathway through the activity of HGPRT, which converts these purine into their respective nucleotides (Lipkowitz, 2012; Lima et al., 2015; Maiuolo et al., 2016). Thus, PRPP synthase, XO and HGPRT are all key enzymes that can cause abnormal sUA levels. PRPP gene mutations have been implicated in a number of human diseases. Overexpression of PRPP results in the enhanced activity of phosphoribosyl pyrophosphate synthetase-I, which can lead to excessive production of purine. Patients with active phosphoribosyl pyrophosphate synthetase-I may result in UA over production (Mittal et al., 2015). Lesch-Nyhan disease is caused by a wide variety of mutations in the HGPRT gene and is one of the models of gout caused by the increased production of UA (Fu et al., 2014; Harris, 2018). Lack of HGPRT can lead to Kelley-Seegmiller syndrome, which is characterized by hyperuricemia, hyperuricosuria, gouty arthritis and urolithiasis (Chavarriaga et al., 2019). Among these key enzymes, XO is the most commonly studied enzyme. 3,5,2′,4′-tetrahydroxychalcone significantly inhibits the activities of XO in liver, and the decreased content of PRPP in liver will suppresse the UA production (Niu et al., 2018). In our review, nearly half of the studies (83/186, 45%) confirm that herbs regulate XO synthesis and activity. For example, pallidifloside D, a saponin glycoside constituent from the total saponins of Smilax riparia, enhances the hypouricemic effect of allopurinol by regulating XO activity. The combination of allopurinol and Pallidifloside D significantly up-regulates HGPRT expression and down-regulates the expressions of PRPP in PC12 cells (Li et al., 2016).
Herbs Lower Serum Uric Acid by Targeting Uric Acid Transporter
About two-thirds of UA is excreted by the kidneys, and the remaining is excreted via the gastrointestinal tract. Renal excretion of UA consists of four steps: 1) glomerular filtration, 2) presecretory reabsorption, 3) secretion and 4) post-secretory reabsorption (Maiuolo et al., 2016). Urate is filtered through the glomerulus, so the reabsorption and secretion of UA after glomerulus play an important role in regulating the excretion of UA. Most of the urate (99%) filtered through the glomerulus is reabsorbed in the early S1 segment of the proximal tubule (presecretory reabsorption). Uric acid is then secreted in the S2 segment of the proximal tubule to return approximately 50% of the filtered urate into the tubule lumen. Post-secretory reabsorption occurs primarily in the distal S3 segment of the proximal tubule, followed by about 10% of the secreted urate in the urine (Diamond and Paolino, 1973; Mandal and Mount, 2015). Urate transporters such as URAT1, OAT1, OAT3, GLUT9, ABCG2 play important roles in regulating sUA, and their dysfunction may cause abnormal urate transport. URAT1, the main urate-anion exchanger in the luminal membrane of the proximal tubules, can be inhibited by the uricosuric agents, likes probenecid, benzbromarone and losartan (Enomoto et al., 2002). URAT1 mutations have been found in patients with familial hypouricemia and UA levels below 1 mg/dL, and the UA transport is inactivated when this mutant transporter is expressed in xenopus oocytes (Dinour et al., 2011). Clinical studies have shown that inhibition of URAT1 effectively reduces sUA levels and resolves the gout symptoms (Lee et al., 2019a). URAT1 is a branch of the organic anion transporter (OAT). OAT1 and OAT3 exchange urate with bivalent anions, suggesting that they are suitable for basolateral entry of urate during urate secretion (Mandal and Mount, 2015). Among the 14 members of the GLUT family transport glucose or other monosaccharides, GLUT9 does transport essentially urate (Caulfield et al., 2008). GLUT9 mediates urate transport, which is independent of sodium, chlorine and anions, but voltage-dependent (Anzai et al., 2008). Compared with the mutation in URAT1, the complete loss of GLUT9 results in the net secretion of urate (Preitner et al., 2009). ABCG2 is a multidrug resistance transporter that is also implicated as an important urate transporter. Its gene variation has become the main cause of elevated sUA levels (Dehghan et al., 2008). ABCG2-mediated loss or reduction of renal urate secretion will lead to increased renal urate reabsorption (Woodward et al., 2009).
In our review, more than one-third of the studies (72/186, 39%) show that Chinese herbal medicines target uric acid transporters. Alpinia oxyphylla seed extract enhances UA excretion in the kidney by reducing URAT1 and up-regulating OTA1 (Lee et al., 2019b). Wuling San down-regulates mRNA and protein levels of URAT1 and GLUT9, as well as up-regulates OAT1 in the kidney of hyperuricemic mice. Moreover, Wuling San also up-regulates organic cation/carnitine transporters which are associated with impaired renal function, leading to kidney protection (Ding et al., 2013). Gypenosides, natural saponins extracted from Gynostemma pentaphyllum, significantly lower sUA levels by reducing XO and increasing in urate excretion through regulating URAT1, GLUT9, and OAT1 (Pang et al., 2017).
Herbs Lowering Serum Uric Acid Resolve Inflammation
Uric acid belongs to the damage-associated molecular patterns, altered metabolites of necrotic or stressed cells that the innate immune system sees as an alarm signal (Patel, 2018). Elevated UA levels alters the physiology, boosting the expressions of inflammatory proteins by triggering complex pro-inflammatory cascades that damage cells and tissues (Chen and Lan, 2017). Clinical trials have shown that serum levels of IL-6 and TNF-α are significantly higher in hyperuricemia patients than in healthy people, and that of IL-6 and TNF-α are significantly increased as sUA levels increase (Zhou et al., 2018b). SUA above 9 mg/dl is associated with a gouty arthritis incidence of 4.9%. The accumulation of monosodium urate (MSU) crystals induce a mass of inflammatory cells (such as neutrophils and monocytes) to infiltrate into the site of MSU crystal deposits in patients, resulting in an acute inflammatory response, manifested as acute gout flares (Dalbeth et al., 2016). IL-1 released from these immune cells further triggers the release of various pro-inflammatory cytokines and chemokines, such as IL-8, IL-6 and TNF-α, which can further enhance neutrophil recruitment (El Ridi and Tallima, 2017). Inflammatory cytokines, especially IL-1β, are the key mediators of gouty inflammation. A phase III, international safety study of patients with acute gout arthritis treated with rilonacept (an IL-1 blocker) for 16 weeks shows that rilonacept significantly reduces the risk of gout attacks by 70.3% (Sundy et al., 2014). In experimental studies included in this review, 37 articles (11 active ingredients, 13 natural products, and 13 herbal formulas) describe the effects of these herbs on IL-1. It is worth mentioning that these herbs not only act on IL-1, but also as XO, UA transporter, other inflammatory factors such as IL-6, IL-8, TNF-α, NF-κB, NLRP3, caspase 1, etc.
There are 16 herbs included in this review that interfere with the NLRP3 inflammasome. Consistent with reported studies, elevated UA can be effectively reduced by regulating NLRP3 inflammasome - IL-1 pathway (Dhanasekar and Rasool, 2016; Szekanecz et al., 2019). Acute gout is an inflammatory response to MSU crystals. Innate immune pathways are essential in the pathogenesis of gout, particularly the activation of NLRP3 inflammasome, which leads to the release of IL-1β and other pro-inflammatory cytokines (So and Martinon, 2017). MSU crystals must first be coated with serum proteins and then interact with articular cell’s surface membrane directly or via receptors, to stimulate a cytosolic molecular platform involved in innate immunity and promote the assembly and activation of the NLRP3 inflammasome (El Ridi and Tallima, 2017). NLRP3 inflammasomes are formed by the recruitment of the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and subsequent recruitment of caspase-1. Caspase-1 activates pro-inflammatory cytokines IL-1β and IL-18 by cleaving their respective precursor proteins, pro-IL-1β and pro-IL-18 (So and Martinon, 2017). Neutrophils are recruited and activated in response to the spillover of IL-1, producing ROS, proteolytic enzymes, pro-inflammatory chemokines, cytokines and so on, which recruit and activate macrophages (El Ridi and Tallima, 2017). Thus, the release of IL-1β mediated by inflammasome and the rapid recruitment of neutrophils lead to acute inflammatory episodes in gout patients. It is noteworthy that hyperuricemia may stimulate inflammatory leukocytes through epigenetic modification such as histone methylation, even without MSU crystals, thereby increasing production of IL-1β, IL-6, and TNF-α (Wasilewska et al., 2012; Zhou et al., 2018b).
The activation and maturation of IL-1β in response to endogenous and exogenous stimuli are also involved in the NF-κB pathway (Chen and Lan, 2017). In our review, there are 13 articles focused on NF-κB signaling pathway, showing the inhibitory effect of herbs on NF-κB activation under elevated sUA levels. It has been proved that NF-κB can be activated by UA (Spiga et al., 2017). Its activation transcribes a large number of pro-inflammatory genes. In a resting state, NF-κB combines with IκBα (inhibitor of NF-κB kinase subunit α) form a dimer in the cytoplasm, after IκBα kinase is activated, it phosphorylates IκBα. Subsequently, NF-κB is transferred from the cytoplasm to the nucleus, leading to the transcription and expression of genes related to inflammation. Studies have shown that inhibition of NF-κB restrains inflammation and improves hyperuricemia or gout conditions (Chen et al., 2019a; Wang et al., 2019c). Interestingly, studies focused on NF-κB activity is also related to NLRP3. NF-κB is essential for the initiation, assembly and activation of NLRP3 inflammasome, which is also a key step in the release of inflammatory cytokines in MSU crystal-induced inflammation (So and Martinon, 2017). P38 mitogen-activated protein kinase (MAPK) is also involved in the inflammatory cascade of NF-κB. Uric acid activates NF-κB through MAPK signal pathway, thus leading to the release of inflammatory factors such as TNF-α (Tang et al., 2017).
Herbs Lowering Serum Uric Acid Protect Against Renal Fibrosis
Evidence suggests that UA levels can be used to predict the prognosis of chronic kidney disease and end-stage kidney disease. Elevated sUA level is an independent risk factor for kidney disease and lead to renal fibrosis (Fan et al., 2019). Mice with systemic GLUT9 knockout showed moderate hyperuricemia, excessive hyperaciduria and obstructive nephropathy, along with progressive inflammatory fibrosis (Preitner et al., 2009). Once UA exceeds the maximum amount the kidneys can excrete, it gets deposit in the kidney and firstly causes direct pathological damage to the kidney. Secondly, the deposition of UA in the kidney results in the accumulation of neutrophils and monocytes as well as the release of inflammatory factors, which lead to glomerulosclerosis and interstitial fibrosis and aggravate renal injury (Ye et al., 2018). Prevention and treatment of renal fibrosis is the best treatment for kidney diseases caused by elevated sUA. However, at present, modern medical treatments are not effective in reducing renal fibrosis and preventing the progression of diseases.
A total of 24 studies included in this review are focused on hyperuricemic nephropathy, suggesting that herbal medicines improve renal injury induced by elevated sUA levels by regulating renal fibrosis-related signal pathways. Fibrosis is usually associated with strong inflammatory reactions and immunocyte infiltration. Therefore, inhibition of inflammatory cytokines might be a potential method to prevent fibrosis. Vaticaffinol, one of the herbs included in this review, markedly down-regulates NLRP3, ASC, caspase-1, IL-1β, IL-18, IL-6 and TNF-α in hyperuricemic mice, thus significantly decreases sUA levels and improves kidney function (Chen et al., 2017b). Transforming growth factor-β1/Mothers against decapentaplegic homolog 3 (TGF-β1/Smad3) signaling is the most potent fibrogenic factor in the regulation of renal interstitial fibrosis process (Loeffler and Wolf, 2015). Pterostilbene, also mentioned in this review, suppresses the activation of TGF-β1/Smad3 and proto-oncogene tyrosine-protein kinase Src/Signal transducer and activator of transcription 3 (Src/STAT3) signaling pathway as to decrease sUA level and alleviate renal fibrosis in hyperuricemic mice (Pan et al., 2019). These findings highlight the fact that herbal medicines may be the potential antifibrotic therapeutics for hyperuricemic nephropathy treatment.
Herbs Lowering Serum Uric Acid Modulate Oxidative Stress
In addition to the inflammatory process, oxidative stress is one of the early events related to the elevated sUA. Uric acid entering cells can rapidly induce oxidative stress (Ko et al., 2019; Yin et al., 2019). This state of oxidative stress is governed by the balance between ROS production and their elimination by antioxidants. Since the cell membrane is impermeable to urate anion, cellular concentration of urate depends on the specific transporter of urate and xanthine oxidoreductase (XOR). In mammals, this enzyme exists in two forms, xanthine dehydrogenase (XDH) and XO. XDH transfers electron to NAD+ and generates NADH, and XO transfers electron to O2 and generates oxidative stress (Isaka et al., 2016). In addition, XOR may lead to the production of the superoxide anion and nitric oxide, especially under low pH or hypoxia conditions (Godber et al., 2000; Battelli et al., 2019). It can be seen that the generation of UA mediated by XO is closely related to the production of ROS. On the other hand, antioxidant enzymes that scavenge ROS are ubiquitous, including superoxide dismutase, glutathione peroxidase and catalase, the changes of these enzymatic activities may lead to oxidative stress. However, in inflammatory diseases including hyperuricemia, the production of the superoxide anion is often faster than its removal by superoxide dismutase (Zamudio-Cuevas et al., 2015).
There is an increasing interest in using herbs to resolve the inflammatory conditions, including hyperuricemia, because they play an anti-inflammatory role by inhibiting the production of ROS, such as procyanidins, Shizhifang, Zisheng Shenqi decoction, quercetin, Modified Simiao decoction and other herbs included in this review. Antioxidants like quercetin inhibits inflammation in rat models of chronic MSU-induced arthritis by decreasing inflammatory mediators such as IL-1β (Huang et al., 2012). Other herbs mentioned above also show significant antioxidant effects. For example, Shizhifang effectively suppresses the NLRP3-ASC-caspase-1 axis through accommodating the ROS pathway, thereby alleviates potassium oxonate - induced hyperuricemia (Wu et al., 2017). In summary, the herbs that possess anti-oxidant effects may be developed as anti-inflammatory agents to treat inflammatory diseases such as gout and hyperuricemia.
Limitations and Perspectives From the Experimental Studies
As shown in Tables 3–5, there are fewer studies on the active ingredients of herbs than on the herbal extracts and herbal formulas. Aside from herbal formula, there can be hundreds of active ingredients in a single-flavored herb, making it difficult to identify the ones that actually mediate the therapeutic effects. This partly explains why multiple signaling pathways are involved in herbal treatments. Extraction of components that mediate the therapeutic effects is difficult. In general, safe and effective single-flavored herb can be screened out from literatures or experimental studies, and its effective active ingredients can be separated by pharmacological methods. Then, the mechanism of action of the drug can be explored based on the identified active ingredients from the herbs. In recent years, there are new approaches to identify the active ingredients of herbs and predict their targets, such as Systems Pharmacology. Researchers used Systems Pharmacology as a basis to screen out active ingredients from herbal formulas or herbs, and predict the targets of active ingredients, and finally verify them through in vivo and in vitro experimental studies (Zhou et al., 2016; Hong et al., 2017; Zhao et al., 2019). It really opens up new ideas for the studies of herbs and shortens the time to find out the mechanisms of action underlying the therapeutic effects of the herbs. However, with the increasing number of herbal studies using Systems Pharmacology, we can find that it also has disadvantages, such as limited Systems Pharmacology databases, data not updated, limited prediction results and so on. In view of this, it is still necessary to explore how to efficiently screen out the active ingredients of the herbs and identify their molecular targets.
Conclusion
In conclusion, the results of meta-analysis indicate that Chinese herbal medicines have potent therapeutic effects in lowering sUA levels. The signaling pathways involved in the sUA lowering effects include UA synthesis, UA transport, inflammation, renal fibrosis and oxidative stress. Further studies with sophisticated research design can further demonstrate the efficacy and safety of these Chinese herbal medicines in lowering sUA levels. Identification of the active ingredients and delineation of the underlying mechanisms of action can facilitate the clinical translation and application of these ingredients.
Author Contributions
All the authors contributed sufficiently for their participation in the study, as follows: XZ and LZ conceived, designed, and supervised the study; LC, ZL, MW, JC, FL, and HL conducted the literature search, data extraction, data analysis and interpretation; QH, YY, and XZ appraised the articles; LC, ZL, and MW wrote the paper, HK modified the paper. All authors have read and approved the final manuscript.
Funding
This work was supported by the Key Project of National Natural Science Foundation of China (No. 81830117), the National Science Foundation of China (Nos. 81774212, 81760821, 81703952), the Natural Science Foundation of Guangdong Province, China (Nos. 2017A030313722, 2018A030313375, 2019A1515010400), and the Science & Technical Plan of Guangzhou, Guangdong, China (No.201903010069).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Abbreviations can be referred to Tables 3, 4. FABP1, fatty acid-binding protein; HPRT1, hypoxanthine-guanine phosphoribosyl transferase; APOB, apolipoprotein B; FOS, one subunit of activator protien-1; FN1, fibronectin1; MIP-1α, MIP-1 β, serum proinflammatory cytokines; TXNIP, thioredoxin interacting proteins; PTEN, phosphate and tension homology deleted on chromsome ten; NALP1/6, NACHT, LRR and PYD domains-containing protein one and six; ADAMTs, a family of metalloproteinases with thrombospondin motifs; TIMP, tissue inhibitor of metalloproteinase; CD2AP, CD2-associated protein.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.578318/full#supplementary-material.
References
- Adachi S. I., Nihei K. I., Ishihara Y., Yoshizawa F., Yagasaki K. (2017). Anti-hyperuricemic effect of taxifolin in cultured hepatocytes and model mice. Cytotechnology. 69 (2), 329–336. 10.1007/s10616-016-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat N., Umar A., Hoxur P., Anaydulla M., Imam G., Aziz R., et al. (2015). Traditional Uighur Medicine Karapxa decoction, inhibits liver xanthine oxidase and reduces serum uric acid concentrations in hyperuricemic mice and scavenges free radicals in vitro . BMC Compl. Alternative Med. 15, 131 10.1186/s12906-015-0644-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzai N., Ichida K., Jutabha P., Kimura T., Babu E., Jin C. J., et al. (2008). Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 283 (40), 26834–26838. 10.1074/jbc.C800156200 [DOI] [PubMed] [Google Scholar]
- Azevedo V. F., Kos I. A., Vargas-Santos A. B., da Rocha Castelar Pinheiro G., Dos Santos Paiva E. (2019). Benzbromarone in the treatment of gout. Adv Rheumatol. 59 (1), 37 10.1186/s42358-019-0080-x [DOI] [PubMed] [Google Scholar]
- Battelli M. G., Bortolotti M., Polito L., Bolognesi A. (2018). The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1864 (8), 2557–2565. 10.1016/j.bbadis.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Battelli M. G., Bortolotti M., Polito L., Bolognesi A. (2019). Metabolic syndrome and cancer risk: the role of xanthine oxidoreductase. Redox Biol. 21, 101070 10.1016/j.redox.2018.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B. T., Kottgen A., Law A., Windham B. G., Segev D., Baer A. N., et al. (2015). Physical function, hyperuricemia, and gout in older adults. Arthritis Care Res. 67 (12), 1730–1738. 10.1002/acr.22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri L., Malaguarnera M. (2007). Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int. J. Med. Sci. 4 (2), 83–93. 10.7150/ijms.4.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Xu D., Wu W., Xu T., Li T. (2011). Experimental study on anti-inflammation and analgesic effects of extracts of compound Shuiniujiao. Pharmaceut. Care Res. 11 (2), 103–106. 10.5428/pcar20110209 [DOI] [Google Scholar]
- Caulfield M. J., Munroe P. B., O'Neill D., Witkowska K., Charchar F. J., Doblado M., et al. (2008). SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 5 (10), e197 10.1371/journal.pmed.0050197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary K., Malhotra K., Sowers J., Aroor A. (2013). Uric Acid - key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 3 (3), 208–220. 10.1159/000355405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarriaga J., Ocampo M., Fakih N., Silva Herrera J. (2019). Kelley-seegmiller syndrome: urolithiasis, renal uric acid deposits, and gout: what is the role of the urologist?. Urol. Int. 102 (2), 233–237. 10.1159/000494360 [DOI] [PubMed] [Google Scholar]
- Chen Q., Ma L., Akebaier W. (2009). Clinical study on treatment of hyperuricaemia by retention enema of Chinese herbal medicine combined with allopurinol. Chin. J. Integr. Med. 15 (6), 431–434. 10.1007/s11655-009-0431-2 [DOI] [PubMed] [Google Scholar]
- Chen J. W., Zhou Y., Xue Z. Y., Li C., Guo J., Zhou L. Y., et al. (2013a). [Effect of jianpihuashi decoction on rats with hyperuricemia]. Zhong Yao Cai. 36 (9), 1486–1489 [PubMed] [Google Scholar]
- Chen L., Mola M., Deng X., Mei Z., Huang X., Shu G., et al. (2013b). Dolichos falcata Klein attenuated the inflammation induced by monosodium urate crystals in vivo and in vitro . J. Ethnopharmacol. 150 (2), 545–552. 10.1016/j.jep.2013.08.063 [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Huang C. C., Tsai K. C., Huang W. J., Huang W. C., Hsu Y. C., et al. (2014a). Evaluation of the antihyperuricemic activity of phytochemicals from Davallia formosana by enzyme assay and hyperuricemic mice model. Evid Based Complement Alternat Med. 2014, 873607 10.1155/2014/873607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. L., Wu S., Na S., Li L. (2014b). [Effect of total saponin of dioscorea on uric acid excretion indicators in chronic hyperuricemia rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34 (1), 75–80 [PubMed] [Google Scholar]
- Chen Y., Lu Y., Wang Y. N., Lin Z. C., Gu W., Tan L., et al. (2014c). [Effect of compound qingqin liquid on the expression of toll-like receptor in the renal tissue of rats with urate nephropathy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34 (6), 722–727 [PubMed] [Google Scholar]
- Chen G., Tan M. L., Li K. K., Leung P. C., Ko C. H. (2015a). Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Ethnopharmacol. 175, 14–20. 10.1016/j.jep.2015.08.043 [DOI] [PubMed] [Google Scholar]
- Chen W. J., Wu Y., Xu C., Liu S., Wang W. Z., Song F. R., et al. (2015b). [Study on therapeutic effects of ermiao pill and ermiao pill categorized formula in hyperuricemic rats using spectroscopic methods]. Guang Pu Xue Yu Guang Pu Fen Xi. 35 (4), 956–960 [PubMed] [Google Scholar]
- Chen J., Zhou J., Wei S., Xie Z., Wen C., Xu G. (2016a). Effect of a traditional Chinese medicine prescription Quzhuotongbi decoction on hyperuricemia model rats studied by using serum metabolomics based on gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 1026, 272–278. 10.1016/j.jchromb.2015.10.031 [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen X. L., Xiang T., Sun B. G., Luo H. X., Liu M. T., et al. (2016b). Total saponins from dioscorea septemloba thunb reduce serum uric acid levels in rats with hyperuricemia through OATP1A1 up-regulation. J Huazhong Univ Sci Technolog Med Sci. 36 (2), 237–242. 10.1007/s11596-016-1573-z [DOI] [PubMed] [Google Scholar]
- Chen W. J., Wu Y., Bi R. B., Liu S., Liu Z. Y., Liu Z. Q., et al. (2017a). Therapeutic effects of selaginella tamariscina on the model of acute gout with hyperuricemia in rats based on metabolomics analysis. Chin. J. Chem. 35 (7), 1117–1124. 10.1002/cjoc.201600810 [DOI] [Google Scholar]
- Chen Y. S., Chen C. J., Yan W., Ge H. M., Kong L. D. (2017b). Anti-hyperuricemic and anti-inflammatory actions of vaticaffinol isolated from Dipterocarpus alatus in hyperuricemic mice. Chin. J. Nat. Med. 15 (5), 330–340. 10.1016/s1875-5364(17)30053-5 [DOI] [PubMed] [Google Scholar]
- Chen B., Li H., Ou G., Ren L., Yang X., Zeng M. (2019a). Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IkappaBalpha and blocking mitochondrial damage. Arthritis Res. Ther. 21 (1), 193 10.1186/s13075-019-1974-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-f., Zhang C., Yao Y., Li J.-m., Du W.-d., Li M.-l., et al. (2019b). Study on anti-hyperuricemia effects and active ingredients of traditional Tibetan medicine TongFengTangSan (TFTS) by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Pharmaceut. Biomed. Anal. 165, 213–223. 10.1016/j.jpba.2018.11.038 [DOI] [PubMed] [Google Scholar]
- Chen M., Ye C., Zhu J., Zhang P., Jiang Y., Lu X., et al. (2020a). Bergenin as a novel urate-lowering therapeutic strategy for hyperuricemia. Frontiers in Cell and Developmental Biology. 8 10.3389/fcell.2020.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. D., Zhao Y. L., Sun W. J., He Y. J., Liu Y. P., Jin Q., et al. (2020b). Kidney tea" and its bioactive secondary metabolites for treatment of gout. J. Agric. Food Chem. 10.1021/acs.jafc.0c03848 [DOI] [PubMed] [Google Scholar]
- Chen L., Lan Z. (2017). Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-kappaB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct. 8 (5), 1785–1792. 10.1039/c6fo01561a [DOI] [PubMed] [Google Scholar]
- Chen-Xu M., Yokose C., Rai S. K., Pillinger M. H., Choi H. K. (2019). Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination survey, 2007-2016. Arthritis Rheum 71 (6), 991–999. 10.1002/art.40807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L. C., Murugaiyah V., Chan K. L. (2015). Flavonoids and phenylethanoid glycosides from Lippia nodiflora as promising antihyperuricemic agents and elucidation of their mechanism of action. J. Ethnopharmacol. 176, 485–493. 10.1016/j.jep.2015.11.025 [DOI] [PubMed] [Google Scholar]
- Cheng S., Sun H., Li X., Yan J., Peng Z., You Y., et al. (2019). Effects of alismatis rhizoma and rhizoma smilacis glabrae decoction on hyperuricemia in rats. Evid. base Compl. Alternative Med. 2019, 1–12. 10.1155/2019/4541609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. S., Song S. H., Choi C. Y., Park K. M., Shim J. H., Park D. H. (2018). Optimization of the extraction conditions and biological evaluation of dendropanax morbifera H. Lev as an anti-hyperuricemic source. Molecules. 23 (12), 3313 10.3390/molecules23123313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. K., McCormick N., Lu N., Rai S. K., Yokose C., Zhang Y. (2020). Population impact attributable to modifiable risk factors for hyperuricemia. Arthritis Rheum 72 (1), 157–165. 10.1002/art.41067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D., Liu S., Tang M., Lu Y., Zhao M., Mao R., et al. (2020). Phloretin ameliorates hyperuricemia-induced chronic renal dysfunction through inhibiting NLRP3 inflammasome and uric acid reabsorption. Phytomedicine. 66, 153111 10.1016/j.phymed.2019.153111 [DOI] [PubMed] [Google Scholar]
- Dalbeth N., Merriman T. R., Stamp L. K. (2016). Gout. Lancet. 388 (10055), 2039–2052. 10.1016/s0140-6736(16)00346-9 [DOI] [PubMed] [Google Scholar]
- Dang Y. X., Liang D. L., Zhou X. X., Qin Y., Gao Y., Li W. M. (2019). Protective effect of Mori Cortex on kidney in rats with hyperlipidemia and hyperuricemia based on molecular docking technique. Chin. Tradit. Herb. Drugs. 50 (5), 1175–1181. 10.7501/j.issn.0253-2670.2019.05.022 [DOI] [Google Scholar]
- Dehghan A., Kottgen A., Yang Q., Hwang S. J., Kao W. L., Rivadeneira F., et al. (2008). Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 372 (9654), 1953–1961. 10.1016/s0140-6736(08)61343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekar C., Rasool M. (2016). Morin, a dietary bioflavonol suppresses monosodium urate crystal-induced inflammation in an animal model of acute gouty arthritis with reference to NLRP3 inflammasome, hypo-xanthine phospho-ribosyl transferase, and inflammatory mediators. Eur. J. Pharmacol. 786, 116–127. 10.1016/j.ejphar.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Diamond H. S., Paolino J. S. (1973). Evidence for a postsecretory reabsorptive site for uric acid in man. J. Clin. Invest. 52 (6), 1491–1499. 10.1172/jci107323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. Q., Pan Y., Wang X., Ma Y. X., Kong L. D. (2013). Wuling san ameliorates urate under-excretion and renal dysfunction in hyperuricemic mice. Chin. J. Nat. Med. 11 (3), 214–221. 10.1016/s1875-5364(13)60019-9 [DOI] [PubMed] [Google Scholar]
- Dinour D., Bahn A., Ganon L., Ron R., Geifman-Holtzman O., Knecht A., et al. (2011). URAT1 mutations cause renal hypouricemia type 1 in Iraqi Jews. Nephrol. Dial. Transplant. 26 (7), 2175–2181. 10.1093/ndt/gfq722 [DOI] [PubMed] [Google Scholar]
- El Ridi R., Tallima H. (2017). Physiological functions and pathogenic potential of uric acid: a review. J. Adv. Res. 8 (5), 487–493. 10.1016/j.jare.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A., Kimura H., Chairoungdua A., Shigeta Y., Jutabha P., Cha S. H., et al. (2002). Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 417 (6887), 447–452. 10.1038/nature742 [DOI] [PubMed] [Google Scholar]
- Fan H., Zhao Y., Fu J., Zhang H., Wang D., Gao Y., et al. (2018). Anti-inflammatory effect of Tongfengning Capsule in rats with acute gouty arthritis and its mechanism. J. Jilin Univ. - Med. Ed. 44 (2), 270–274. 10.13481/j.1671-587x.20180212 [DOI] [Google Scholar]
- Fan S., Zhang P., Wang A. Y., Wang X., Wang L., Li G., et al. (2019). Hyperuricemia and its related histopathological features on renal biopsy. BMC Nephrol. 20 (1), 95 10.1186/s12882-019-1275-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C., Chen L., He M., Luo Y., Zhou M., Zhang N., et al. (2019). Molecular mechanistic insight into the anti-hyperuricemic effect of Eucommia ulmoides in mice and rats. Pharm. Biol. 57 (1), 112–119. 10.1080/13880209.2019.1568510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y., Ye D., Fan X. F., Dong F. Q. (2016). Effect of Dioscorea tokoro Makino extract on hyperuricemia in mice. Trop. J. Pharmaceut. Res. 15 (9), 1883–1887. 10.4314/tjpr.v15i9.10 [DOI] [Google Scholar]
- Fu R., Ceballos-Picot I., Torres R. J., Larovere L. E., Yamada Y., Nguyen K. V., et al. (2014). Genotype-phenotype correlations in neurogenetics: lesch-Nyhan disease as a model disorder. Brain. 137 (Pt 5), 1282–1303. 10.1093/brain/awt202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamala M., Jacobs J. W. G. (2019). Gout and hyperuricaemia: a worldwide health issue of joints and beyond. Rheumatology.. 10.1093/rheumatology/kez272 [DOI] [PubMed] [Google Scholar]
- Godber B. L., Doel J. J., Sapkota G. P., Blake D. R., Stevens C. R., Eisenthal R., et al. (2000). Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 275 (11), 7757–7763. 10.1074/jbc.275.11.7757 [DOI] [PubMed] [Google Scholar]
- Guo Y., Jiang Q., Gui D., Wang N. (2015). Chinese herbal formulas Si-Wu-tang and Er-Miao-San synergistically ameliorated hyperuricemia and renal impairment in rats induced by adenine and potassium oxonate. Cell. Physiol. Biochem. 37 (4), 1491–1502. 10.1159/000438517 [DOI] [PubMed] [Google Scholar]
- Guo L. F., Chen X., Lei S. S., Li B., Zhang N. Y., Ge H. Z., et al. (2020). Effects and mechanisms of dendrobium officinalis six nostrum for treatment of hyperuricemia with hyperlipidemia. Evid Based Complement Alternat Med. 2020, 2914019 10.1155/2020/2914019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Xie Y., Sui F., Liu C., Du X., Liu C., et al. (2016). Zisheng Shenqi decoction ameliorates monosodium urate crystal-induced gouty arthritis in rats through anti-inflammatory and anti-oxidative effects. Mol. Med. Rep. 14 (3), 2589–2597. 10.3892/mmr.2016.5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Zhu C. X., Shi W., Huang H. Z., Hu X. G., Zhou X. M., et al. (2017). Effect of Rhizoma Polygoni Cuspidati and Ramulus Cinnamomi compatibility on uric acid metabolism and urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in rats with hyperuricemia. Chin. J. Integr. Med. 23 (7), 535–542. 10.1007/s11655-016-2649-0 [DOI] [PubMed] [Google Scholar]
- Han B., Gong M., Li Z., Qiu Y., Zou Z. (2020). NMR-based metabonomic study reveals intervention effects of polydatin on potassium oxonate-induced hyperuricemia in rats. Oxid Med Cell Longev. 2020, 6943860 10.1155/2020/6943860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. C. (2018). Lesch-Nyhan syndrome and its variants: examining the behavioral and neurocognitive phenotype. Curr. Opin. Psychiatr. 31 (2), 96–102. 10.1097/yco.0000000000000388 [DOI] [PubMed] [Google Scholar]
- Ho S. T., Tung Y. T., Huang C. C., Kuo C. L., Lin C. C., Yang S. C., et al. (2012). The hypouricemic effect of Balanophora laxiflora extracts and derived phytochemicals in hyperuricemic mice. Evid Based Complement Alternat Med. 2012, 910152 10.1155/2012/910152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S., Kawamoto S., Tanaka H., Kishida H., Kitagawa M., Nakai Y., et al. (2014). Administered chrysanthemum flower oil attenuates hyperuricemia: mechanism of action as revealed by DNA microarray analysis. Biosci. Biotechnol. Biochem. 78 (4), 655–661. 10.1080/09168451.2014.890028 [DOI] [PubMed] [Google Scholar]
- Hong Q., Yu S., Mei Y., Lv Y., Chen D., Wang Y., et al. (2014). Smilacis Glabrae Rhizoma reduces oxidative stress caused by hyperuricemia via upregulation of catalase. Cell. Physiol. Biochem. 34 (5), 1675–1685. 10.1159/000366369 [DOI] [PubMed] [Google Scholar]
- Hong M., Li S., Wang N., Tan H. Y., Cheung F., Feng Y. (2017). A biomedical investigation of the hepatoprotective effect of radix salviae miltiorrhizae and network pharmacology-based prediction of the active compounds and molecular targets. Int. J. Mol. Sci. 18 (3). 10.3390/ijms18030620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. W., Lee Y. C., Hung H. F., Fu H. W., Jeng K. C. (2012). Longan seed extract reduces hyperuricemia via modulating urate transporters and suppressing xanthine oxidase activity. Am. J. Chin. Med. 40 (5), 979–991. 10.1142/s0192415x12500723 [DOI] [PubMed] [Google Scholar]
- Hou P. Y., Mi C., He Y., Zhang J., Wang S. Q., Yu F., et al. (2015). Pallidifloside D from Smilax riparia enhanced allopurinol effects in hyperuricemia mice. Fitoterapia. 105, 43–48. 10.1016/j.fitote.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Hu Q. H., Jiao R. Q., Wang X., Lv Y. Z., Kong L. D. (2010). Simiao pill ameliorates urate underexcretion and renal dysfunction in hyperuricemic mice. J. Ethnopharmacol. 128 (3), 685–692. 10.1016/j.jep.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Hua J., Huang P., Zhu C. M., Yuan X., Yu C. H. (2012). Anti-hyperuricemic and nephroprotective effects of Modified Simiao Decoction in hyperuricemic mice. J. Ethnopharmacol. 142 (1), 248–252. 10.1016/j.jep.2012.04.052 [DOI] [PubMed] [Google Scholar]
- Hu Q. H., Miao M. X., Lu G., Ji H. (2013a). Effects of quercetin on expression of renal NLRP3 and TLRs in rats with uric acid nephtopathy. Chin. Tradit. Herb. Drugs. 44 (24), 3496–3502. 10.7501/j.issn.0253-2670.2013.24.013 [DOI] [Google Scholar]
- Hu Q. H., Zhu J. X., Ji J., Wei L. L., Miao M. X., Ji H. (2013b). Fructus Gardenia Extract ameliorates oxonate-induced hyperuricemia with renal dysfunction in mice by regulating organic ion transporters and mOIT3. Molecules. 18 (8), 8976–8993. 10.3390/molecules18088976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhu M., Tao Y., Wang S., Chen J., Sun W., et al. (2012). Therapeutic properties of quercetin on monosodium urate crystal-induced inflammation in rat. J. Pharm. Pharmacol. 64 (8), 1119–1127. 10.1111/j.2042-7158.2012.01504.x [DOI] [PubMed] [Google Scholar]
- Huang B., Hu X., Wang J., Li P., Chen J. (2019a). Study on chemical constituents of herbal formula Er Miao Wan and GC-MS based metabolomics approach to evaluate its therapeutic effects on hyperuricemic rats. J Chromatogr B Analyt Technol Biomed Life Sci. 1118–1119, 101–108. 10.1016/j.jchromb.2019.04.032 [DOI] [PubMed] [Google Scholar]
- Huang L., Deng J., Chen G., Zhou M., Liang J., Yan B., et al. (2019b). The anti-hyperuricemic effect of four astilbin stereoisomers in Smilax glabra on hyperuricemic mice. J. Ethnopharmacol. 238, 111777 10.1016/j.jep.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Hui W., Yongliang Y., Yongde C., Guo L., Li L., Zhonglin Y., et al. (2016). Hypouricemic and nephroprotective effects of emodinol in oxonate-induced hyperuricemic mice are mediated by organic ion transporters and OIT3. Planta Med. 82 (4), 289–297. 10.1055/s-0035-1558212 [DOI] [PubMed] [Google Scholar]
- Huijuan W., Xiaoxu C., Rui S., Xinghui L., Beibei T., Jianchun M. (2017). Qi-Zhu-Xie-Zhuo-Fang reduces serum uric acid levels and ameliorates renal fibrosis in hyperuricemic nephropathy rats. Biomed. Pharmacother. 91, 358–365. 10.1016/j.biopha.2017.04.031 [DOI] [PubMed] [Google Scholar]
- Huo L. N., Wang W., Zhang C. Y., Shi H. B., Liu Y., Liu X. H., et al. (2015). Bioassay-guided isolation and identification of xanthine oxidase inhibitory constituents from the leaves of perilla frutescens. Molecules. 20 (10), 17848–17859. 10.3390/molecules201017848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka Y., Takabatake Y., Takahashi A., Saitoh T., Yoshimori T. (2016). Hyperuricemia-induced inflammasome and kidney diseases. Nephrol. Dial. Transplant. 31 (6), 890–896. 10.1093/ndt/gfv024 [DOI] [PubMed] [Google Scholar]
- Jhang J. J., Ong J. W., Lu C. C., Hsu C. L., Lin J. H., Liao J. W., et al. (2016). Hypouricemic effects of Mesona procumbens Hemsl. through modulating xanthine oxidase activity in vitro and in vivo . Food Funct. 7 (10), 4239–4246. 10.1039/c6fo00822d [DOI] [PubMed] [Google Scholar]
- Jiang Y., You X. Y., Fu K. L., Yin W. L. (2012). Effects of extract from mangifera indica leaf on monosodium urate crystal-induced gouty arthritis in rats. Evid Based Complement Alternat Med. 2012, 967573 10.1155/2012/967573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Lin Y., Hu Y. J., Song X. J., Pan H. H., Zhang H. J. (2017). Caffeoylquinic acid derivatives rich extract from Gnaphalium pensylvanicum willd. Ameliorates hyperuricemia and acute gouty arthritis in animal model. BMC Compl. Alternative Med. 17 (1), 320 10.1186/s12906-017-1834-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L., Miao J. X., Cao L. H., Miao Y. Y., Miao M. S., Liu H. J., et al. (2020). Total glucosides of herbaceous peony (Paeonia lactiflora Pall.) flower attenuate adenine- and ethambutol-induced hyperuricaemia in rats. J. Ethnopharmacol. 261, 113054 10.1016/j.jep.2020.113054 [DOI] [PubMed] [Google Scholar]
- Kim S. C., Liu J., Solomon D. H. (2015). Risk of incident diabetes in patients with gout: a cohort study. Arthritis Rheum 67 (1), 273–280. 10.1002/art.38918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. K., Kim W. J., Hyun J. M., Lee J. S., Kwon J. G., Seo C., et al. (2017). Salvia plebeia extract inhibits xanthine oxidase activity in vitro and reduces serum uric acid in an animal model of hyperuricemia. Planta Med. 83 (17), 1335–1341. 10.1055/s-0043-111012 [DOI] [PubMed] [Google Scholar]
- Ko J., Kang H. J., Kim D. A., Kim M. J., Ryu E. S., Lee S., et al. (2019). Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. Faseb. J., fj201901148R 10.1096/fj.201901148R [DOI] [PubMed] [Google Scholar]
- Kodithuwakku N. D., Pan M., Zhu Y. L., Zhang Y. Y., Feng Y. D., Fang W. R., et al. (2013). Anti-inflammatory and antinociceptive effects of Chinese medicine SQ gout capsules and its modulation of pro-inflammatory cytokines focusing on gout arthritis. J. Ethnopharmacol. 150 (3), 1071–1079. 10.1016/j.jep.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Kodithuwakku N. D., Feng Y. D., Zhang Y. Y., Pan M., Fang W. R., Li Y. M. (2015). The molecular insight into the antihyperuricemic and renoprotective effect of Shuang Qi gout capsule in mice. J. Ethnopharmacol. 163, 278–289. 10.1016/j.jep.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Kondo M., Hirano Y., Nishio M., Furuya Y., Nakamura H., Watanabe T. (2013). Xanthine oxidase inhibitory activity and hypouricemic effect of aspalathin from unfermented rooibos. J. Food Sci. 78 (12), H1935–H1939. 10.1111/1750-3841.12304 [DOI] [PubMed] [Google Scholar]
- Kuo C.-Y., Kao E.-S., Chan K.-C., Lee H.-J., Huang T.-F., Wang C.-J. (2012). Hibiscus sabdariffa L. extracts reduce serum uric acid levels in oxonate-induced rats. Journal of Functional Foods. 4 (1), 375–381. 10.1016/j.jff.2012.01.007 [DOI] [Google Scholar]
- Kou Y. Y., Li Y. F., Xu M., Li W. Y., Yang M., Li R. L. (2015). Effects of RuPeng15 powder (RPP15) on monosodium urate crystal-induced gouty arthritis in rats. Evid Based Complement Alternat Med. 2015, 527019 10.1155/2015/527019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Y., Li Y., Ma H., Li W., Li R., Dang Z. (2016). Uric acid lowering effect of Tibetan Medicine RuPeng15 powder in animal models of hyperuricemia. J. Tradit. Chin. Med. 36 (2), 205–210 [DOI] [PubMed] [Google Scholar]
- Kuwabara M., Hisatome I., Niwa K., Hara S., Roncal-Jimenez C. A., Bjornstad P., et al. (2018). Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension. 71 (1), 78–86. 10.1161/hypertensionaha.117.10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa M. A., Sanchez-Lozada L. G., Choi Y. J., Cicerchi C., Kanbay M., Roncal-Jimenez C. A., et al. (2012). Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287 (48), 40732–40744. 10.1074/jbc.M112.399899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. P., Lin Y. Y., Duh C. Y., Huang S. Y., Wang H. M., Wu S. F., et al. (2015). Lemnalol attenuates mast cell activation and osteoclast activity in a gouty arthritis model. J. Pharm. Pharmacol. 67 (2), 274–285. 10.1111/jphp.12331 [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Shon E. J., Kim O. S., Kim D. S. (2017). Effects of Mollugo pentaphylla extract on monosodium urate crystal-induced gouty arthritis in mice. BMC Compl. Alternative Med. 17 (1), 447 10.1186/s12906-017-1955-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. A., Yu K. S., Park S. I., Yoon S., Onohara M., Ahn Y., et al. (2019a). URC102, a potent and selective inhibitor of hURAT1, reduced serum uric acid in healthy volunteers. Rheumatology.. 10.1093/rheumatology/kez140 [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Sung Y. Y., Yuk H. J., Son E., Lee S., Kim J. S., et al. (2019b). Anti-hyperuricemic effect of Alpinia oxyphylla seed extract by enhancing uric acid excretion in the kidney. Phytomedicine. 62, 152975 10.1016/j.phymed.2019.152975 [DOI] [PubMed] [Google Scholar]
- Li Y. Q., Wei Y. H., Han X. (2010). Effects of Danggui Buxue Tang on heart muscle function in hyperuricemic rats. Chin. Pharmaceut. J. 45 (7), 524–526 [Google Scholar]
- Li H. G., Hou P. Y., Zhang X., He Y., Zhang J., Wang S. Q., et al. (2016). Hypouricemic effect of allopurinol are improved by Pallidifloside D based on the uric acid metabolism enzymes PRPS, HGPRT and PRPPAT. Fitoterapia. 113, 1–5. 10.1016/j.fitote.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Li L., Teng M., Liu Y., Qu Y., Zhang Y., Lin F., et al. (2017a). Anti-Gouty arthritis and antihyperuricemia effects of sunflower (helianthus annuus) head extract in gouty and hyperuricemia animal models. BioMed Res. Int. 2017, 5852076 10.1155/2017/5852076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. P., Wu S., Ran A., Xu D. Y., Wei J. M., Zhao Z. L. (2017b). Aristolochia bracteolate retz. Attenuates hyperuricemia IN a metabolic arthritis rat model. Afr. J. Tradit., Complementary Altern. Med. 14 (4), 180–187. 10.21010/ajtcam.v14i4.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. J., Li Z., Dong X. Y., Lu L. F., Wang C. L. (2017c). Hypouricemic and nephroprotective effects of total flavonoids from the residue of supercritical CO2 extraction of Humulus lupulus in potassium oxonate-induced mice. Pak. J. Pharm. Sci. 30 (2), 493–497 [PubMed] [Google Scholar]
- Liang G., Nie Y., Chang Y., Zeng S., Liang C., Zheng X., et al. (2019). Protective effects of Rhizoma smilacis glabrae extracts on potassium oxonate- and monosodium urate-induced hyperuricemia and gout in mice. Phytomedicine. 59, 152772 10.1016/j.phymed.2018.11.032 [DOI] [PubMed] [Google Scholar]
- Lima W. G., Martins-Santos M. E., Chaves V. E. (2015). Uric acid as a modulator of glucose and lipid metabolism. Biochimie. 116, 17–23. 10.1016/j.biochi.2015.06.025 [DOI] [PubMed] [Google Scholar]
- Lin J., Chen S., Li S., Lu M., Li Y., Su Y. (2016). Efficacy and safety of Chinese medicinal herbs for the treatment of hyperuricemia: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016, 2146204 10.1155/2016/2146204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Liu P. G., Liang W. Q., Hu Y. J., Xu P., Zhou J., et al. (2018). Luteolin-4'-O-glucoside and its aglycone, two major flavones of Gnaphalium affine D. Don, resist hyperuricemia and acute gouty arthritis activity in animal models. Phytomedicine. 41, 54–61. 10.1016/j.phymed.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Lin X., Shao T., Huang L., Wen X., Wang M., Wen C., et al. (2020). Simiao decoction alleviates gouty arthritis by modulating proinflammatory cytokines and the gut ecosystem. Front. Pharmacol. 11, 955 10.3389/fphar.2020.00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz M. S. (2012). Regulation of uric acid excretion by the kidney. Curr. Rheumatol. Rep. 14 (2), 179–188. 10.1007/s11926-012-0240-z [DOI] [PubMed] [Google Scholar]
- Liu J., Xu L. L., Xu Y. (2013). Antigout active fractions in tongfeng granule. Chin. Tradit. Herb. Drugs. 44 (5), 590–594. 10.7501/j.issn.0253-2670.2013.05.018 [DOI] [Google Scholar]
- Liu H., Zhang X. M., Wang Y. L., Liu B. C. (2014a). Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. J. Nephrol. 27 (6), 653–658. 10.1007/s40620-014-0082-z [DOI] [PubMed] [Google Scholar]
- Liu X. H., Huang M. Q., Lin Z. W., Zhen H. S., Liu A. T., Zhang K. Y. (2014b). Anti-gout effect of Poecilobdella manillensis. Chin. Tradit. Herb. Drugs. 45 (12), 1747–1750. 10.7501/j.issn.0253-2670.2014.12.018 [DOI] [Google Scholar]
- Liu Y. W., Sun W. F., Zhang X. X., Li J., Zhang H. H. (2015). Compound Tufuling Granules ([characters: see text]) regulate glucose transporter 9 expression in kidney to influence serum uric acid level in hyperuricemia mice. Chin. J. Integr. Med. 21 (11), 823–829. 10.1007/s11655-015-2052-2 [DOI] [PubMed] [Google Scholar]
- Liu H. J., Pan X. X., Liu B. Q., Gui X., Hu L., Jiang C. Y., et al. (2017). Grape seed-derived procyanidins alleviate gout pain via NLRP3 inflammasome suppression. J. Neuroinflammation. 14 (1), 74 10.1186/s12974-017-0849-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. H., Zhao Y. X., Zhou Y. M., Huang M. Q., Huang S. S., Zhen H. S., et al. (2018). Anti-gout effect of hirudin and its mechanism. Chin. Tradit. Herb. Drugs. 49 (6), 1365–1370. 10.7501/j.issn.0253-2670.2018.06.020 [DOI] [Google Scholar]
- Liu N., Wang Y., Yang M., Bian W., Zeng L., Yin S., et al. (2019). New rice-derived short peptide potently alleviated hyperuricemia induced by potassium oxonate in rats. J. Agric. Food Chem. 67 (1), 220–228. 10.1021/acs.jafc.8b05879 [DOI] [PubMed] [Google Scholar]
- Liu G., Chen X., Lu X., Zhao J., Li X. (2020). Sunflower head enzymatic hydrolysate relives hyperuricemia by inhibiting crucial proteins (xanthine oxidase, adenosine deaminase, uric acid transporter1) and restoring gut microbiota in mice. Journal of Functional Foods. 72 10.1016/j.jff.2020.104055 [DOI] [Google Scholar]
- Lo H. C., Wang Y. H., Chiou H. Y., Lai S. H., Yang Y. (2010). Relative efficacy of casein or soya protein combined with palm or safflower-seed oil on hyperuricaemia in rats. Br. J. Nutr. 104 (1), 67–75. 10.1017/s0007114510000310 [DOI] [PubMed] [Google Scholar]
- Loeffler I., Wolf G. (2015). Epithelial-to-Mesenchymal transition in diabetic nephropathy: fact or fiction?. Cells. 4 (4), 631–652. 10.3390/cells4040631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X. J., Wang Y. Z., Lei S. S., He X., Lu T. T., Zhan L. H., et al. (2020). Beneficial effects of macroporous resin extract of dendrobium candidum leaves in rats with hyperuricemia induced by a high-purine diet. Evid Based Complement Alternat Med. 2020, 3086106 10.1155/2020/3086106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louxin B., Miaoyanyan F., Miaomingsan (2018). Effect of arctiin on rat hyperuricemia model. Acta Med. Mediterr. 34 (6), 1739–1742. 10.19193/0393-6384_2018_6_266 [DOI] [Google Scholar]
- Lü Y. Z., Hu Q. H., Wang X., Ouyang Z., Kong L. D. (2010). Effects of Ermiao Pill water extracts on imbalance of urate levels and its related genes and protein levels in hyperuricemic mice. Chin. Tradit. Herb. Drugs. 41 (3), 418–423 [Google Scholar]
- Lu F., Liu L., Yu D. H., Li X. Z., Zhou Q., Liu S. M. (2014). Therapeutic effect of Rhizoma Dioscoreae Nipponicae on gouty arthritis based on the SDF-1/CXCR 4 and p38 MAPK pathway: an in vivo and in vitro study. Phytother Res. 28 (2), 280–288. 10.1002/ptr.4997 [DOI] [PubMed] [Google Scholar]
- Lu X. Y., Zhou F. H., Dong Y. Q., Gong L. N., Li Q. Y., Tang L., et al. (2018). Codonopsis tangshen oliv. Amelioration effect on diabetic kidney disease rats induced by high fat diet feeding combined with streptozotocin. Nat Prod Bioprospect. 8 (6), 441–451. 10.1007/s13659-018-0187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu S., Ding R., Liu P., OuYang H., Feng Y., Rao Y., et al. (2019). LC-MS analysis of serum for the metabolomic investigation of the effects of pulchinenoside b4 administration in monosodium urate crystal-induced gouty arthritis rat model. Molecules. 24 (17). 10.3390/molecules24173161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhou L. L., Yan H. Y., Liu M. (2009). Effects of extracts from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on MSU crystal-induced rats gouty arthritis. Am. J. Chin. Med. 37 (4), 669–683. 10.1142/s0192415x09007156 [DOI] [PubMed] [Google Scholar]
- Ma L., Zhang S., Yuan Y., Gao J. (2014). Hypouricemic actions of exopolysaccharide produced by Cordyceps militaris in potassium oxonate-induced hyperuricemic mice. Curr. Microbiol. 69 (6), 852–857. 10.1007/s00284-014-0666-9 [DOI] [PubMed] [Google Scholar]
- Ma C. H., Kang L. L., Ren H. M., Zhang D. M., Kong L. D. (2015). Simiao pill ameliorates renal glomerular injury via increasing Sirt1 expression and suppressing NF-κB/NLRP3 inflammasome activation in high fructose-fed rats. J. Ethnopharmacol. 172, 108–117. 10.1016/j.jep.2015.06.015 [DOI] [PubMed] [Google Scholar]
- Ma T. H., Sheng T., Tian C. M., Xing M. Y., Yan L. J., Xia D. Z. (2019a). [Effect of ethanolic extract of Polygonum cuspidatum on acute gouty arthritis in mice through NLRP3/ASC/caspase-1 axis]. Zhongguo Zhongyao Zazhi. 44 (3), 546–552. 10.19540/j.cnki.cjcmm.20180925.001 [DOI] [PubMed] [Google Scholar]
- Ma W. G., Wang J., Bu X. W., Zhang H. H., Zhang J. P., Zhang X. X., et al. (2019b). Effects of polygonum cuspidatum on AMPK-FOXO3alpha signaling pathway in rat model of uric acid-induced renal damage. Chin. J. Integr. Med. 25 (3), 182–189. 10.1007/s11655-017-2979-6 [DOI] [PubMed] [Google Scholar]
- Ma W. G., Wang J., Bu X. W., Zhang H. H., Zhang J. P., Zhang X. X., et al. (2019c). Effects of polygonum cuspidatum on AMPK-FOXO3α signaling pathway in rat model of uric acid-induced renal damage. Chin. J. Integr. Med. 25 (3), 182–189. 10.1007/s11655-017-2979-6 [DOI] [PubMed] [Google Scholar]
- Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. (2016). Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 213, 8–14. 10.1016/j.ijcard.2015.08.109 [DOI] [PubMed] [Google Scholar]
- Major T. J., Dalbeth N., Stahl E. A., Merriman T. R. (2018). An update on the genetics of hyperuricaemia and gout. Nat. Rev. Rheumatol. 14 (6), 341–353. 10.1038/s41584-018-0004-x [DOI] [PubMed] [Google Scholar]
- Mandal A. K., Mount D. B. (2015). The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 77, 323–345. 10.1146/annurev-physiol-021113-170343 [DOI] [PubMed] [Google Scholar]
- Meng Z., Yan Y., Tang Z., Guo C., Li N., Huang W., et al. (2015). Anti-hyperuricemic and nephroprotective effects of rhein in hyperuricemic mice. Planta Med. 81 (4), 279–285. 10.1055/s-0034-1396241 [DOI] [PubMed] [Google Scholar]
- Mittal R., Patel K., Mittal J., Chan B., Yan D., Grati M., et al. (2015). Association of PRPS1 mutations with disease phenotypes. Dis. Markers. 2015, 127013 10.1155/2015/127013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151 (4), 264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Niu Y., Lu W., Gao L., Lin H., Liu X., Li L. (2012). Reducing effect of mangiferin on serum uric acid levels in mice. Pharm. Biol. 50 (9), 1177–1182. 10.3109/13880209.2012.663763 [DOI] [PubMed] [Google Scholar]
- Niu Y., Liu J., Liu H. Y., Gao L. H., Feng G. H., Liu X., et al. (2016). Hypouricaemic action of mangiferin results from metabolite norathyriol via inhibiting xanthine oxidase activity. Pharm. Biol. 54 (9), 1680–1686. 10.3109/13880209.2015.1120322 [DOI] [PubMed] [Google Scholar]
- Niu Y., Zhou Y., Lin H., Gao L. H., Xiong W., Zhu H., et al. (2018). Inhibition of 3,5,2',4'-tetrahydroxychalcone on production of uric acid in hypoxanthine-induced hyperuricemic mice. Biol. Pharm. Bull. 41 (1), 99–105. 10.1248/bpb.b17-00655 [DOI] [PubMed] [Google Scholar]
- Pan Y., Chu Z., Wang W., Yang B. (2013). Pretreatment with Jieduxiezhuo decoction impedes elevations in serum uric acid levels in mice. J. Tradit. Chin. Med. 33 (6), 794–797. 10.1016/s0254-6272(14)60014-5 [DOI] [PubMed] [Google Scholar]
- Pan H. Y., Shi L., Xu L., Yin L., Zeng W. P., Zhang G. J., et al. (2014). Effect of active fractions from modified Simiao Wan on hyperuricemia and its mechanism. Chin. J. Pharmacol. Toxicol. 28 (3), 380–385. 10.3867/j.issn.1000-3002.2014.03.012 [DOI] [Google Scholar]
- Pan J., Shi M., Li L., Liu J., Guo F., Feng Y., et al. (2019). Pterostilbene, a bioactive component of blueberries, alleviates renal fibrosis in a severe mouse model of hyperuricemic nephropathy. Biomed. Pharmacother. 109, 1802–1808. 10.1016/j.biopha.2018.11.022 [DOI] [PubMed] [Google Scholar]
- Pan J., Zhang C., Shi M., Guo F., Liu J., Li L., et al. (2020). Ethanol extract of Liriodendron chinense (Hemsl.) Sarg barks attenuates hyperuricemic nephropathy by inhibiting renal fibrosis and inflammation in mice. J. Ethnopharmacol. 113278 10.1016/j.jep.2020.113278 [DOI] [PubMed] [Google Scholar]
- Pang M., Fang Y., Chen S., Zhu X., Shan C., Su J., et al. (2017). Gypenosides inhibits xanthine oxidoreductase and ameliorates urate excretion in hyperuricemic rats induced by high cholesterol and high fat food (lipid emulsion). Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 23, 1129–1140. 10.12659/msm.903217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. (2018). Danger-Associated molecular patterns (DAMPs): the derivatives and triggers of inflammation. Curr. Allergy Asthma Rep. 18 (11), 63 10.1007/s11882-018-0817-3 [DOI] [PubMed] [Google Scholar]
- Peng A., Lin L., Zhao M., Sun B. (2019). Identifying mechanisms underlying the amelioration effect of Chrysanthemum morifolium Ramat. 'Boju' extract on hyperuricemia using biochemical characterization and UPLC-ESI-QTOF/MS-based metabolomics. Food Funct. 10 (12), 8042–8055. 10.1039/c9fo01821b [DOI] [PubMed] [Google Scholar]
- Preitner F., Bonny O., Laverriere A., Rotman S., Firsov D., Da Costa A., et al. (2009). Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc. Natl. Acad. Sci. U. S. A. 106 (36), 15501–15506. 10.1073/pnas.0904411106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z., Wang S., Lin Y., Zhao Y., Yang S., Song J., et al. (2018). Antihyperuricemic effect of mangiferin aglycon derivative J99745 by inhibiting xanthine oxidase activity and urate transporter 1 expression in mice. Acta Pharm. Sin. B. 8 (2), 306–315. 10.1016/j.apsb.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S. K., Burns L. C., De Vera M. A., Haji A., Giustini D., Choi H. K. (2015). The economic burden of gout: a systematic review. Semin. Arthritis Rheum 45 (1), 75–80. 10.1016/j.semarthrit.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Rozza F., Trimarco V., Izzo R., Grassi D., Ferri C. (2016). Effects of a novel fixed combination of nutraceuticals on serum uric acid concentrations and the lipid profile in asymptomatic hyperuricemic patients : results from the PICONZ-UA study. High Blood Pres. Cardiovasc. Prev. 23 (4), 381–386. 10.1007/s40292-016-0168-x [DOI] [PubMed] [Google Scholar]
- Sato Y., Feig D. I., Stack A. G., Kang D. H., Lanaspa M. A., Ejaz A. A., et al. (2019). The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat. Rev. Nephrol. 10.1038/s41581-019-0174-z [DOI] [PubMed] [Google Scholar]
- Shan H. L., Shan R. P., Fu X. C. (2015). [Hypouricemic effect of ethanol extracts from dioscoreae nipponicae rhizoma]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 44 (1), 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X. Z., Ma W. G., Chen Y., Lu Y., Wang Y. N., Xu Y. M., et al. (2014). [Effect of compound qingqin liquid on the expression levels of ang II and COX-2 mRNA transcription and protein expression in the renal tissue of uric acid nephropathy rats: an experimental study]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34 (7), 819–825 [PubMed] [Google Scholar]
- Sheu S. Y., Fu Y. T., Huang W. D., Chen Y. A., Lei Y. C., Yao C. H., et al. (2016). Evaluation of xanthine oxidase inhibitory potential and in vivo hypouricemic activity of dimocarpus longan lour. Extracts. Phcog. Mag. 12 (Suppl. 2), S206–S212. 10.4103/0973-1296.182176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. W., Wang C. P., Wang X., Zhang Y. L., Liu L., Wang R. W., et al. (2012). Uricosuric and nephroprotective properties of Ramulus Mori ethanol extract in hyperuricemic mice. J. Ethnopharmacol. 143 (3), 896–904. 10.1016/j.jep.2012.08.023 [DOI] [PubMed] [Google Scholar]
- Shi L., Xu L., Yang Y., Song H., Pan H., Yin L. (2013). Suppressive effect of modified Simiaowan on experimental gouty arthritis: an in vivo and in vitro study. J. Ethnopharmacol. 150 (3), 1038–1044. 10.1016/j.jep.2013.10.023 [DOI] [PubMed] [Google Scholar]
- Shi L., Zhao F., Zhu F., Liang Y., Yang F., Zhang G., et al. (2016a). Traditional Chinese Medicine Formula "Xiaofeng granules" suppressed gouty arthritis animal models and inhibited the proteoglycan degradation on chondrocytes induced by monosodium urate. J. Ethnopharmacol. 191, 254–263. 10.1016/j.jep.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Shi Y. C., Lin K. S., Jhai Y. F., Lee B. H., Han Y., Cui Z., et al. (2016b). Miracle fruit (synsepalum dulcificum) exhibits as a novel anti-hyperuricaemia agent. Molecules. 21 (2), 140 10.3390/molecules21020140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. R., Frohlich J. K., Oliveira S. M., Cabreira T. N., Rossato M. F., Trevisan G., et al. (2013). The antinociceptive and anti-inflammatory effects of the crude extract of Jatropha isabellei in a rat gout model. J. Ethnopharmacol. 145 (1), 205–213. 10.1016/j.jep.2012.10.054 [DOI] [PubMed] [Google Scholar]
- Singh A. K., Haque M., O'Sullivan K., Chourasia M., Ouseph M. M., Ahmed S. (2019). Suppression of monosodium urate crystal-induced inflammation by inhibiting TGF-beta-activated kinase 1-dependent signaling: role of the ubiquitin proteasome system. Cell. Mol. Immunol. 10.1038/s41423-019-0284-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A. K., Martinon F. (2017). Inflammation in gout: mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 13 (11), 639–647. 10.1038/nrrheum.2017.155 [DOI] [PubMed] [Google Scholar]
- Song S. H., Park D. H., Bae M. S., Choi C. Y., Shim J. H., Yoon G., et al. (2018). Ethanol extract of cudrania tricuspidata leaf ameliorates hyperuricemia in mice via inhibition of hepatic and serum xanthine oxidase activity. Evid Based Complement Alternat Med. 2018, 8037925 10.1155/2018/8037925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga R., Marini M. A., Mancuso E., Di Fatta C., Fuoco A., Perticone F., et al. (2017). Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-kappaB signaling pathway in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 37 (6), 1241–1249. 10.1161/atvbaha.117.309128 [DOI] [PubMed] [Google Scholar]
- Strilchuk L., Fogacci F., Cicero A. F. (2019). Safety and tolerability of available urate-lowering drugs: a critical review. Expet Opin. Drug Saf. 18 (4), 261–271. 10.1080/14740338.2019.1594771 [DOI] [PubMed] [Google Scholar]
- Su J., Wei Y., Liu M., Liu T., Li J., Ji Y., et al. (2014). Anti-hyperuricemic and nephroprotective effects of Rhizoma Dioscoreae septemlobae extracts and its main component dioscin via regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Arch Pharm. Res. (Seoul). 37 (10), 1336–1344. 10.1007/s12272-014-0413-6 [DOI] [PubMed] [Google Scholar]
- Su Q., Su H., Nong Z., Li D., Wang L., Chu S., et al. (2018). Hypouricemic and nephroprotective effects of an active fraction from polyrhachis vicina roger on potassium oxonate-induced hyperuricemia in rats. Kidney Blood Press. Res. 43 (1), 220–233. 10.1159/000487675 [DOI] [PubMed] [Google Scholar]
- Sun W. F., Zhu M. M., Li J., Zhang X. X., Liu Y. W., Wu X. R., et al. (2015). Effects of Xie-Zhuo-Chu-Bi-Fang on miR-34a and URAT1 and their relationship in hyperuricemic mice. J. Ethnopharmacol. 161, 163–169. 10.1016/j.jep.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Sundy J. S., Schumacher H. R., Kivitz A., Weinstein S. P., Wu R., King-Davis S., et al. (2014). Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: results of RESURGE, a phase III, international safety study. J. Rheumatol. 41 (8), 1703–1711. 10.3899/jrheum.131226 [DOI] [PubMed] [Google Scholar]
- Szekanecz Z., Szamosi S., Kovacs G. E., Kocsis E., Benko S. (2019). The NLRP3 inflammasome - interleukin 1 pathway as a therapeutic target in gout. Arch. Biochem. Biophys. 670, 82–93. 10.1016/j.abb.2019.01.031 [DOI] [PubMed] [Google Scholar]
- Tang R. B., Dong J. Z., Yan X. L., Du X., Kang J. P., Wu J. H., et al. (2014). Serum uric acid and risk of left atrial thrombus in patients with nonvalvular atrial fibrillation. Can. J. Cardiol. 30 (11), 1415–1421. 10.1016/j.cjca.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Tang H., Yang L., Li W., Li J., Chen J. (2016). Exploring the interaction between Salvia miltiorrhiza and xanthine oxidase: insights from computational analysis and experimental studies combined with enzyme channel blocking. RSC Adv. 6 (114), 113527–113537. 10.1039/c6ra24396g [DOI] [Google Scholar]
- Tang L., Xu Y., Wei Y., He X. (2017). Uric acid induces the expression of TNFalpha via the ROSMAPKNFkappaB signaling pathway in rat vascular smooth muscle cells. Mol. Med. Rep. 16 (5), 6928–6933. 10.3892/mmr.2017.7405 [DOI] [PubMed] [Google Scholar]
- Tian C., Wang Y., Chang H., Li J., La X. (2018). Spleen-kidney supplementing formula alleviates renal fibrosis in diabetic rats via TGF-β 1-miR-21-PTEN signaling pathway. Evid. base Compl. Alternative Med. 2018, 1–9. 10.1155/2018/3824357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung Y. T., Lin L. C., Liu Y. L., Ho S. T., Lin C. Y., Chuang H. L., et al. (2015). Antioxidative phytochemicals from Rhododendron oldhamii Maxim. leaf extracts reduce serum uric acid levels in potassium oxonate-induced hyperuricemic mice. BMC Compl. Alternative Med. 15, 423 10.1186/s12906-015-0950-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. P., Wang X., Zhang X., Shi Y. W., Liu L., Kong L. D. (2010a). Morin improves urate excretion and kidney function through regulation of renal organic ion transporters in hyperuricemic mice. J. Pharm. Pharmaceut. Sci. 13 (3), 411–427. 10.18433/j3q30h [DOI] [PubMed] [Google Scholar]
- Wang X., Wang C. P., Hu Q. H., Lv Y. Z., Zhang X., Ouyang Z., et al. (2010b). The dual actions of Sanmiao wan as a hypouricemic agent: down-regulation of hepatic XOD and renal mURAT1 in hyperuricemic mice. J. Ethnopharmacol. 128 (1), 107–115. 10.1016/j.jep.2009.12.035 [DOI] [PubMed] [Google Scholar]
- Wang C. P., Wang Y., Wang X., Zhang X., Ye J. F., Hu L. S., et al. (2011). Mulberroside a possesses potent uricosuric and nephroprotective effects in hyperuricemic mice. Planta Med. 77 (8), 786–794. 10.1055/s-0030-1250599 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang L., Li E., Li Y., Wang Z., Sun X., et al. (2014). Chuanhu anti-gout mixture versus colchicine for acute gouty arthritis: a randomized, double-blind, double-dummy, non-inferiority trial. Int. J. Med. Sci. 11 (9), 880–885. 10.7150/ijms.9165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. X., Liu Y. L., Yang Y., Zhang D. M., Kong L. D. (2015). Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. 747, 59–70. 10.1016/j.ejphar.2014.11.035 [DOI] [PubMed] [Google Scholar]
- Wang J., Xu B. B., Zeng J. X., Zhu J. X., Wang X. Y., Ren G., et al. (2016a). Effect of Dendropanaxchevalieri extracts on uric acid level in hyperuricemic mice and the possible mechanism. Chinese Journal of New Drugs. 25 (3), 334–338 [Google Scholar]
- Wang R., Ma C. H., Zhou F., Kong L. D. (2016b). Siwu decoction attenuates oxonate-induced hyperuricemia and kidney inflammation in mice. Chin. J. Nat. Med. 14 (7), 499–507. 10.1016/s1875-5364(16)30059-0 [DOI] [PubMed] [Google Scholar]
- Wang W. L., Sheu S. Y., Huang W. D., Chuang Y. L., Tseng H. C., Hwang T. S., et al. (2016c). Phytochemicals from tradescantia albiflora kunth extracts reduce serum uric acid levels in oxonate-induced rats. Phcog. Mag. 12 (Suppl. 2), S223–S227. 10.4103/0973-1296.182171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Y., Zhou J. B., Shi B., Guo X. L., Yan Q. C. (2016d). Hypouricemic and nephroprotective effects of Jianpi Huashi decoction in a rat model of hyperuricemia. Int. J. Clin. Exp. Med. 9 (1), 455–465 [Google Scholar]
- Wang Y., Zhao M., Xin Y., Liu J., Wang M., Zhao C. (2016e). (1)H NMR and MS based metabolomics study of the therapeutic effect of Cortex Fraxini on hyperuricemic rats. J. Ethnopharmacol. 185, 272–281. 10.1016/j.jep.2016.03.043 [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin Z., Zhang B., Nie A., Bian M. (2017a). Cichorium intybus L. promotes intestinal uric acid excretion by modulating ABCG2 in experimental hyperuricemia. Nutr. Metab. 14, 38 10.1186/s12986-017-0190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lin Z. J., Nie A. Z., Li L. Y., Zhang B. (2017b). [Effect of Chinese herb chicory extract on expression of renal transporter Glut9 in rats with hyperuricemia]. Zhongguo Zhongyao Zazhi. 42 (5), 958–963. 10.19540/j.cnki.cjcmm.2017.0029 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao M., Ye H., Shao Y., Yu Y., Wang M., et al. (2017c). Comparative pharmacokinetic study of the main components of cortex fraxini after oral administration in normal and hyperuricemic rats. Biomed. Chromatogr. 31 (8), e3934 10.1002/bmc.3934 [DOI] [PubMed] [Google Scholar]
- Wang Q., Yang Q., Cao X., Wei Q., Firempong C. K., Guo M., et al. (2018). Enhanced oral bioavailability and anti-gout activity of [6]-shogaol-loaded solid lipid nanoparticles. Int. J. Pharm. 550 (1-2), 24–34. 10.1016/j.ijpharm.2018.08.028 [DOI] [PubMed] [Google Scholar]
- Wang S., Fang Y., Yu X., Guo L., Zhang X., Xia D. (2019a). The flavonoid-rich fraction from rhizomes of Smilax glabra Roxb. ameliorates renal oxidative stress and inflammation in uric acid nephropathy rats through promoting uric acid excretion. Biomed. Pharmacother. 111, 162–168. 10.1016/j.biopha.2018.12.050 [DOI] [PubMed] [Google Scholar]
- Wang Y., Dong L., Liu P., Chen Y., Jia S., Wang Y. (2019b). A randomized controlled trial of chuanhutongfeng mixture for the treatment of chronic gouty arthritis by regulating miRNAs. Evid Based Complement Alternat Med. 2019, 5917269 10.1155/2019/5917269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lin Z., Zhang B., Jiang Z., Guo F., Yang T. (2019c). Cichorium intybus L. Extract suppresses experimental gout by inhibiting the NF-kappaB and NLRP3 signaling pathways. Int. J. Mol. Sci. 20 (19). 10.3390/ijms20194921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lin Z., Zhang B., Wang X., Chu M. (2019d). Chicory (Cichorium intybus L.) inhibits renal reabsorption by regulating expression of urate transporters in fructose-induced hyperuricemia. Journal of Traditional Chinese Medical Sciences. 6 (1), 84–94. 10.1016/j.jtcms.2019.01.001 [DOI] [Google Scholar]
- Wang Z., Ci X. Y., Cui T., Wei Z. H., Zhang H. B., Liu R., et al. (2019e). Effects of Chinese herb ingredients with different properties on OAT4, URAT1 and serum uric acid level in acute hyperuricemia mice. Chin. Tradit. Herb. Drugs. 50 (5), 1157–1163. 10.7501/j.issn.0253-2670.2019.05.020 [DOI] [Google Scholar]
- Wasilewska A., Tenderenda E., Taranta-Janusz K., Tobolczyk J., Stypulkowska J. (2012). Markers of systemic inflammation in children with hyperuricemia. Acta Paediatr. 101 (5), 497–500. 10.1111/j.1651-2227.2011.02582.x [DOI] [PubMed] [Google Scholar]
- Wei G. N., Su Q. B., He F., Zeng X. B., Ya Q. K., Lü J. H., et al. (2013). Screening and chemical component analysis of anti-hyperuricemic active fraction of ethanol extract from Polyrhachis vicina Roger in hyperuricemia model mice. Chin. J. Pharmacol. Toxicol. 27 (4), 673–677. 10.3867/j.issn.1000-3002.2013.04.012 [DOI] [Google Scholar]
- Wei Z., Xu C., Liu S., Song F., Liu Z., Qu X. (2018). Metabonomics study of the effects of traditional Chinese medicine formula Ermiaowan on hyperuricemic rats. J. Separ. Sci. 41 (2), 560–570. 10.1002/jssc.201700985 [DOI] [PubMed] [Google Scholar]
- White W. B., Saag K. G., Becker M. A., Borer J. S., Gorelick P. B., Whelton A., et al. (2018). Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 378 (13), 1200–1210. 10.1056/NEJMoa1710895 [DOI] [PubMed] [Google Scholar]
- Woodward O. M., Kottgen A., Coresh J., Boerwinkle E., Guggino W. B., Kottgen M. (2009). Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. U. S. A. 106 (25), 10338–10342. 10.1073/pnas.0901249106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. F., Rudan I., Hastie N. D., Campbell H. (2010). A 'complexity' of urate transporters. Kidney Int. 78 (5), 446–452. 10.1038/ki.2010.206 [DOI] [PubMed] [Google Scholar]
- Wu X., Liu L., Xie H., Liao J., Zhou X., Wan J., et al. (2012a). Tanshinone IIA prevents uric acid nephropathy in rats through NF-kappaB inhibition. Planta Med. 78 (9), 866–873. 10.1055/s-0031-1298487 [DOI] [PubMed] [Google Scholar]
- Wu X., Liu L., Xie H., Liao J., Zhou X., Wan J., et al. (2012b). Tanshinone IIA prevents uric acid nephropathy in rats through NF-κB inhibition. Planta Med. 78 (9), 866–873. 10.1055/s-0031-1298487 [DOI] [PubMed] [Google Scholar]
- Wu X. H., Ruan J. L., Zhang J., Wang S. Q., Zhang Y. W. (2014a). Pallidifloside D, a saponin glycoside constituent from Smilax riparia, resist to hyperuricemia based on URAT1 and GLUT9 in hyperuricemic mice. J. Ethnopharmacol. 157, 201–205. 10.1016/j.jep.2014.09.034 [DOI] [PubMed] [Google Scholar]
- Wu X. H., Wang C. Z., Zhang J., Wang S. Q., Han L., Zhang Y. W., et al. (2014b). Effects of Smilaxchinoside A and Smilaxchinoside C, two steroidal glycosides from Smilax riparia, on hyperuricemia in a mouse model. Phytother Res. 28 (12), 1822–1828. 10.1002/ptr.5207 [DOI] [PubMed] [Google Scholar]
- Wu X. H., Yu C. H., Zhang C. F., Anderson S., Zhang Y. W. (2014c). Smilax riparia reduces hyperuricemia in mice as a potential treatment of gout. Am. J. Chin. Med. 42 (1), 257–259. 10.1142/s0192415x14200018 [DOI] [PubMed] [Google Scholar]
- Wu X. H., Zhang J., Wang S. Q., Yang V. C., Anderson S., Zhang Y. W. (2014d). Riparoside B and timosaponin J, two steroidal glycosides from Smilax riparia, resist to hyperuricemia based on URAT1 in hyperuricemic mice. Phytomedicine. 21 (10), 1196–1201. 10.1016/j.phymed.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Wu X. H., Wang C. Z., Wang S. Q., Mi C., He Y., Zhang J., et al. (2015). Anti-hyperuricemia effects of allopurinol are improved by Smilax riparia, a traditional Chinese herbal medicine. J. Ethnopharmacol. 162, 362–368. 10.1016/j.jep.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Wu Y., He F., Li Y., Wang H., Shi L., Wan Q., et al. (2017). Effects of shizhifang on NLRP3 inflammasome activation and renal tubular injury in hyperuricemic rats. Evid Based Complement Alternat Med. 2017, 7674240 10.1155/2017/7674240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Li J., Zhang X., Zeng F., Liu Y., Sun W. (2018a). Study of the treatment effects of compound tufuling granules in hyperuricemic rats using serum metabolomics. Evid Based Complement Alternat Med. 2018, 3458185 10.1155/2018/3458185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang Y., Ou J., Wan Q., Shi L., Li Y., et al. (2018b). Effect and mechanism of ShiZhiFang on uric acid metabolism in hyperuricemic rats. Evid Based Complement Alternat Med. 2018, 6821387 10.1155/2018/6821387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia N., Li B. A., Liu H. J., Fan J. B., Shen W., He X. G. (2017). Anti-hyperuricemic effect of Plantago depressa Willd extract in rats. Trop. J. Pharmaceut. Res. 16 (6), 1365–1368. 10.4314/tjpr.v16i6.21 [DOI] [Google Scholar]
- Xiang S. W., Lai S. C., Meng Y. H. (2009). [Clinical study on modified sanmiao powder in treating chronic uric acid nephropathy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 29 (11), 979–981 [PubMed] [Google Scholar]
- Xie Z., Wu H., Jing X., Li X., Li Y., Han Y., et al. (2017). Hypouricemic and arthritis relapse-reducing effects of compound tufuling oral-liquid in intercritical and chronic gout: a double-blind, placebo-controlled, multicenter randomized trial. Medicine (Baltim.). 96 (11), e6315 10.1097/MD.0000000000006315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W. W., Zhang H. Y., Wen L., Wang X. Y., Zhong G. Y., Shi Y. F., et al. (2018). Effect of Lagotis brachystachys Maxim extract on xanthine oxidase and renal urate transporters in hyperuricemia mice. Chinese Journal of New Drugs. 27 (13), 1538–1543 [Google Scholar]
- Xu W. A., Yin L., Pan H. Y., Shi L., Xu L., Zhang X., et al. (2013). Study on the correlation between constituents detected in serum from Rhizoma Smilacis Glabrae and the reduction of uric acid levels in hyperuricemia. J. Ethnopharmacol. 150 (2), 747–754. 10.1016/j.jep.2013.09.024 [DOI] [PubMed] [Google Scholar]
- Xu C., Wan X., Xu L., Weng H., Yan M., Miao M., et al. (2015). Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: one stone hits two birds. J. Hepatol. 62 (6), 1412–1419. 10.1016/j.jhep.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Xu K., Liu S., Zhao X., Zhang X., Fu X., Zhou Y., et al. (2019). Treating hyperuricemia related non-alcoholic fatty liver disease in rats with resveratrol. Biomed. Pharmacother. 110, 844–849. 10.1016/j.biopha.2018.12.039 [DOI] [PubMed] [Google Scholar]
- Yan M., An Y. T., Li J., Wu Z. Z., Wang T. (2014). [Regulatory effect of leonurus extracts on hyperuricemia in rats]. Zhongguo Zhongyao Zazhi. 39 (24), 4856–4859 [PubMed] [Google Scholar]
- Yang H. X., Su J. X., Jiang G. Q., Chen X. P. (2013). Effect of Tongxi Powder on blood uric acid of mice with acute hyperuricemia. Chin. Tradit. Herb. Drugs. 44 (12), 1635–1637. 10.7501/j.issn.0253-2670.2013.12.021 [DOI] [Google Scholar]
- Yang Y., Zhang D. M., Liu J. H., Hu L. S., Xue Q. C., Ding X. Q., et al. (2015). Wuling San protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. J. Ethnopharmacol. 169, 49–59. 10.1016/j.jep.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Yang A., Guo H., Fu M., Liu M. (2019a). Inhibitive effects of huashi pill on formation of renal stones by modulating urine biochemical indexes and osteopontin in renal stone rat models. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 25, 8335–8344. 10.12659/msm.916247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. X., Xue J. J., Meng X. Y., Wang Y. S., Wu L. L., Lv C. Y., et al. (2019b). Effects of total flavonoids from Oxytropis falcata Bunge on the SOCS/JAK/STAT inflammatory signaling pathway in the kidneys of diabetic nephropathy model mice. Eur. J. Inflamm. 17 10.1177/2058739219861877 [DOI] [Google Scholar]
- Yang T. H., Yan D. X., Huang X. Y., Hou B., Ma Y. B., Peng H., et al. (2019c). Termipaniculatones A-F, chalcone-flavonone heterodimers from Terminthia paniculata, and their protective effects on hyperuricemia and acute gouty arthritis. Phytochemistry. 164, 228–235. 10.1016/j.phytochem.2019.05.019 [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhang L., Jiang G., Lei A., Yu Q., Xie J., et al. (2019d). Evaluation of the protective effects of Ganoderma atrum polysaccharide on acrylamide-induced injury in small intestine tissue of rats. Food Funct. 10 (9), 5863–5872. 10.1039/c9fo01452g [DOI] [PubMed] [Google Scholar]
- Yao L., Dong W., Lu F., Liu S. (2012). An improved acute gouty arthritis rat model and therapeutic effect of rhizoma Dioscoreae nipponicae on acute gouty arthritis based on the protein-chip methods. Am. J. Chin. Med. 40 (1), 121–134. 10.1142/s0192415x12500103 [DOI] [PubMed] [Google Scholar]
- Yao R., Geng Z., Mao X., Bao Y., Guo S., Bao L., et al. (2020). Tu-teng-cao extract alleviates monosodium urate-induced acute gouty arthritis in rats by inhibiting uric acid and inflammation. Evid Based Complement Alternat Med. 2020, 3095624 10.1155/2020/3095624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Zhang Y., Wang B., Walana W., Wei J., Gordon J. R., et al. (2018). CXCR1/CXCR2 antagonist G31P inhibits nephritis in a mouse model of uric acid nephropathy. Biomed. Pharmacother. 107, 1142–1150. 10.1016/j.biopha.2018.07.077 [DOI] [PubMed] [Google Scholar]
- Yi L. T., Li J., Su D. X., Dong J. F., Li C. F. (2012). Hypouricemic effect of the methanol extract from Prunus mume fruit in mice. Pharm. Biol. 50 (11), 1423–1427. 10.3109/13880209.2012.683115 [DOI] [PubMed] [Google Scholar]
- Yin W., Zhou Q. L., OuYang S. X., Chen Y., Gong Y. T., Liang Y. M. (2019). Uric acid regulates NLRP3/IL-1beta signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K(+) efflux. BMC Nephrol. 20 (1), 319 10.1186/s12882-019-1506-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong T., Zhang M., Chen D., Shuai O., Chen S., Su J., et al. (2016). Actions of water extract from Cordyceps militaris in hyperuricemic mice induced by potassium oxonate combined with hypoxanthine. J. Ethnopharmacol. 194, 403–411. 10.1016/j.jep.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Yong T., Chen S., Xie Y., Chen D., Su J., Shuai O., et al. (2017). Hypouricemic effects of ganoderma applanatum in hyperuricemia mice through OAT1 and GLUT9. Front. Pharmacol. 8, 996 10.3389/fphar.2017.00996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon I. S., Park D. H., Ki S. H., Cho S. S. (2016). Effects of extracts from Corylopsis coreana Uyeki (Hamamelidaceae) flos on xanthine oxidase activity and hyperuricemia. J. Pharm. Pharmacol. 68 (12), 1597–1603. 10.1111/jphp.12626 [DOI] [PubMed] [Google Scholar]
- Yoon I. S., Park D. H., Bae M. S., Oh D. S., Kwon N. H., Kim J. E., et al. (2017a). In Vitro and in vivo studies on quercus acuta thunb. (Fagaceae) extract: active constituents, serum uric acid suppression, and xanthine oxidase inhibitory activity, Evid. Based Complement. Alternat. Med. 2017, 4097195 10.1155/2017/4097195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon I. S., Park D. H., Kim J. E., Yoo J. C., Bae M. S., Oh D. S., et al. (2017b). Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo . Int. J. Mol. Med. 39 (6), 1613–1620. 10.3892/ijmm.2017.2973 [DOI] [PubMed] [Google Scholar]
- You W., Wang J., Zou Y., Che K., Hou X., Fei H., et al. (2019). Modified Chuanhu anti-gout mixture, a traditional Chinese medicine, protects against potassium oxonate-induced hyperuricemia and renal dysfunction in mice. J. Int. Med. Res. 47 (5), 1927–1935. 10.1177/0300060519831182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. N., Wu H. Y., Deng Y. P., Zhuang G. T., Tan B. H., Huang Y. Z., et al. (2018). Yellow-dragon Wonderful-seed Formula" for hyperuricemia in gout patients with dampness-heat pouring downward pattern: a pilot randomized controlled trial. Trials. 19 (1), 551 10.1186/s13063-018-2917-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Fan Y. S., Xu L., Xie G. Q., Feng X. H., Qian K. (2019). Jia-Wei-Si-Miao-Wan alleviates acute gouty arthritis by targeting NLRP3 inflammasome. J. Biol. Regul. Homeost. Agents. 33 (1), 63–71 [PubMed] [Google Scholar]
- Yuk H. J., Lee Y. S., Ryu H. W., Kim S. H., Kim D. S. (2018). Effects of toona sinensis leaf extract and its chemical constituents on xanthine oxidase activity and serum uric acid levels in potassium oxonate-induced hyperuricemic rats. Molecules. 23 (12). 10.3390/molecules23123254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio-Cuevas Y., Hernandez-Diaz C., Pineda C., Reginato A. M., Cerna-Cortes J. F., Ventura-Rios L., et al. (2015). Molecular basis of oxidative stress in gouty arthropathy. Clin. Rheumatol. 34 (10), 1667–1672. 10.1007/s10067-015-2933-y [DOI] [PubMed] [Google Scholar]
- Zeng J. X., Xu B. B., Li M., Li X. W., Zhu J. X., Wang X. Y., et al. (2015). Effect of Lagotis brevituba Maxim. extract in reducing uric acid level in hyperuricemia mice and it's mechanism. Chinese Journal of New Drugs. 24 (21), 2489–2493 [Google Scholar]
- Zhang M., Wang J. F., Wang Y. F. (2009). [Effects of serial gout granules on insulin-resistance in primary gout patients]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 29 (12), 1068–1072 [PubMed] [Google Scholar]
- Zhang X. X., Sun W. F., Xu W. (2011). [Assessment on the clinical efficacy and safety of xiezhuo chubi recipe in treating hyperuricemia]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 31 (9), 1216–1219 [PubMed] [Google Scholar]
- Zhang D. M., Li Y. C., Xu D., Ding X. Q., Kong L. D. (2012). Protection of curcumin against fructose-induced hyperuricaemia and renal endothelial dysfunction involves NO-mediated JAK-STAT signalling in rats. Food Chem. 134 (4), 2184–2193. 10.1016/j.foodchem.2012.04.026 [DOI] [PubMed] [Google Scholar]
- Zhang Z. C., Su G. H., Luo C. L., Pang Y. L., Wang L., Li X., et al. (2015). Effects of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) on the serum uric acid level and xanthine oxidase activity in hyperuricemic mice. Food Funct. 6 (9), 3045–3055. 10.1039/c5fo00499c [DOI] [PubMed] [Google Scholar]
- Zhang H. J., Li L. N., Zhou J., Yang Q. Q., Liu P. G., Xu P., et al. (2017a). Effects of Gnaphalium affine D. Don on hyperuricemia and acute gouty arthritis. J. Ethnopharmacol. 203, 304–311. 10.1016/j.jep.2017.03.057 [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhuang J., Yue G., Wang Y., Liu M., Zhang B., et al. (2017b). Lipidomics to investigate the pharmacologic mechanisms of ginkgo folium in the hyperuricemic rat model. J Chromatogr B Analyt Technol Biomed Life Sci. 1060, 407–415. 10.1016/j.jchromb.2017.06.037 [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhan S., Li S., Zhu Z., He J., Lorenzo J. M., et al. (2018a). Anti-hyperuricemic and nephroprotective effects of extracts from Chaenomeles sinensis (Thouin) Koehne in hyperuricemic mice. Food Funct. 9 (11), 5778–5790. 10.1039/c8fo01480a [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jin L., Liu J., Wang W., Yu H., Li J., et al. (2018b). Effect and mechanism of dioscin from Dioscorea spongiosa on uric acid excretion in animal model of hyperuricemia. J. Ethnopharmacol. 214, 29–36. 10.1016/j.jep.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Zhang W., Du W., Li G., Zhang C., Yang W., Yang S., et al. (2019a). Constituents and anti-hyperuricemia mechanism of traditional Chinese herbal formulae erding granule. Molecules. 24 (18). 10.3390/molecules24183248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y., Cheng J., Zhao P., Chen K. L., Li J. (2019b). Screening the best compatibility of selaginella moellendorffii prescription on hyperuricemia and gouty arthritis and its mechanism. Evid Based Complement Alternat Med. 2019, 7263034 10.1155/2019/7263034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. C., Zhou Q., Yang Y., Wang Y., Zhang J. L. (2019c). Highly acylated anthocyanins from purple sweet potato ( Ipomoea batatas L.) alleviate hyperuricemia and kidney inflammation in hyperuricemic mice: possible attenuation effects on allopurinol. J. Agric. Food Chem. 67 (22), 6202–6211. 10.1021/acs.jafc.9b01810 [DOI] [PubMed] [Google Scholar]
- Zhao P., Chen K. L., Zhang G. L., Deng G. R., Li J. (2017). Pharmacological basis for use of selaginella moellendorffii in gouty arthritis: antihyperuricemic, anti-inflammatory, and xanthine oxidase inhibition. Evid Based Complement Alternat Med. 2017, 2103254 10.1155/2017/2103254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Lv C., Wu Q., Zeng H., Guo X., Yang J., et al. (2019). Computational systems pharmacology reveals an antiplatelet and neuroprotective mechanism of Deng-Zhan-Xi-Xin injection in the treatment of ischemic stroke. Pharmacol. Res. 147, 104365 10.1016/j.phrs.2019.104365 [DOI] [PubMed] [Google Scholar]
- Zhou M., Wang Y. F., Zhou R., Zhang M., Li B. (2013). [Treatment of gouty arthritis in different phases by a series of tongfeng granule: an efficacy observation]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 33 (12), 1603–1607 [PubMed] [Google Scholar]
- Zhou Q., Yu D. H., Zhang C., Liu S. M., Lu F. (2014). Total saponins from Discorea nipponica ameliorate urate excretion in hyperuricemic mice. Planta Med. 80 (15), 1259–1268. 10.1055/s-0034-1383048 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Yu D. H., Liu S. M., Liu Y. (2015). Total saponins from Discorea nipponica makino ameliorate urate excretion in hyperuricemic rats. Phcog. Mag. 11 (43), 567–573. 10.4103/0973-1296.160442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Wang J., Wu Z., Huang C., Lu A., Wang Y. (2016). Systems pharmacology exploration of botanic drug pairs reveals the mechanism for treating different diseases. Sci. Rep. 6, 36985 10.1038/srep36985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Lin F. F., Liu S. M., Sui X. F. (2017). Influence of the total saponin fraction from Dioscorea nipponica Makino on TLR2/4-IL1R receptor singnal pathway in rats of gouty arthritis. J. Ethnopharmacol. 206, 274–282. 10.1016/j.jep.2017.04.024 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhang X., Li C., Yuan X., Han L., Li Z., et al. (2018a). Research on the pharmacodynamics and mechanism of Fraxini Cortex on hyperuricemia based on the regulation of URAT1 and GLUT9. Biomed. Pharmacother. 106, 434–442. 10.1016/j.biopha.2018.06.163 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhao M., Pu Z., Xu G., Li X. (2018b). Relationship between oxidative stress and inflammation in hyperuricemia: analysis based on asymptomatic young patients with primary hyperuricemia. Medicine (Baltim.). 97 (49), e13108 10.1097/md.0000000000013108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Pang M., Dong L., Huang X., Wang S., Zhou L. (2012). Anti-inflammatory and immunomodulatory effects of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on uric acid nephropathy rats. Life Sci. 91 (11-12), 369–376. 10.1016/j.lfs.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Zhu F., Yin L., Ji L., Yang F., Zhang G., Shi L., et al. (2016). Suppressive effect of Sanmiao formula on experimental gouty arthritis by inhibiting cartilage matrix degradation: an in vivo and in vitro study. Int. Immunopharm. 30, 36–42. 10.1016/j.intimp.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Zhu L., Dong Y., Na S., Han R., Wei C., Chen G. (2017). Saponins extracted from Dioscorea collettii rhizomes regulate the expression of urate transporters in chronic hyperuricemia rats. Biomed. Pharmacother. 93, 88–94. 10.1016/j.biopha.2017.06.022 [DOI] [PubMed] [Google Scholar]
- Zhu C., Xu Y., Liu Z. H., Wan X. C., Li D. X., Tai L. L. (2018). The anti-hyperuricemic effect of epigallocatechin-3-gallate (EGCG) on hyperuricemic mice. Biomed. Pharmacother. 97, 168–173. 10.1016/j.biopha.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Zuo J., He H., Zuo Z., Bou-Chacra N., Lobenberg R. (2018). Erding Formula in hyperuricaemia treatment: unfolding traditional Chinese herbal compatibility using modern pharmaceutical approaches. J. Pharm. Pharmacol. 70 (1), 124–132. 10.1111/jphp.12840 [DOI] [PubMed] [Google Scholar]
- Zuo J., Zhang W., Jian H., Bou-Chacra N., Löbenberg R. (2020). Esculetin as bioactive marker: towards a rational scientific approach for the treatment of hyperuricemia using traditional Chinese medicine. Brazilian Journal of Pharmaceutical Sciences. 56 10.1590/s2175-97902019000417827 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.