Abstract
A method for the extraction and quantification of pretomanid in 40 μl of human plasma, by high performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) detection was developed and validated. Samples were prepared using liquid-liquid extraction and chromatographic separation was achieved on an Agilent Poroshell C18 column using an isocratic elution at a flow rate of 400 μl/min. Electrospray ionization with mass detection at unit resolution in the multiple reaction monitoring (MRM) mode on an AB Sciex API 3200 mass spectrometer was used. Over the validation period, accuracy, precision, selectivity, sensitivity, recovery and stability were assessed. The calibration range was 10 – 10 000 ng/ml. Inter- and intra-day precision, expressed as the coefficient of variation (%CV), was shown to be lower than 9% at all concentrations tested with accuracies between 95.2 and 110%. The recovery was 72.4% overall and reproducible at the low, medium and high end of the calibration range. The method was shown to be specific for pretomanid with no significant matrix effects observed. The validated method facilitated the analysis of pretomanid in plasma collected from adults with pulmonary TB as part of a clinical pharmacokinetic study.
Keywords: tuberculosis (TB), pretomanid, liquid-liquid extraction, LC-MS/MS, validation
1. Introduction
Tuberculosis (TB) remains a primary health threat despite there being more than 20 drugs developed for its treatment [1,2]. TB control efforts are hampered by the presence of multidrug resistant isolates of M. tuberculosis (M.tb.) as well as long treatment duration, and challenges with adherence, amongst other factors [3]. Drug resistant isolates are poorly responsive to the current treatment and the situation is exacerbated by the fact that many current second line TB drugs are toxic, poorly tolerated, difficult to administer and require a very long treatment timeline [4].
To eliminate the resistant isolates of M.tb. as well as shorten the duration of treatment, a new drug called pretomanid has been developed. Pretomanid (previously PA-824) is a bicyclic nitroimidazole which consists of the nitroimidazole pyran A/B rings, an ether link as well as a hydrophobic side chain (presented in Figure 1a). The hydrophobic side chain is important for drug efficacy [5]. Pretomanid targets actively replicating and nonreplicating bacteria as well as resistant strains of M.tb, via two main mechanisms [5,6]. Like isoniazid, pretomanid requires activation by the bacterium, via the deazaflavin-dependant nitroreductase enzyme [5]. It has been shown that the activation of pretomanid leads to the formation of a metabolite, des-nitroimidazole, which is associated with the generation of reactive nitrogen species, including nitric oxide. The generation of the nitrogen species causes damage to the intracellular macromolecules which likely accounts for its activity against M.tb in anaerobic environments [7]. Pretomanid also eliminates the bacteria by causing the accumulation of hydroxymycolic acid, thus inhibiting the formation of the cell wall precursor ketomycolate [6].
Figure 1:
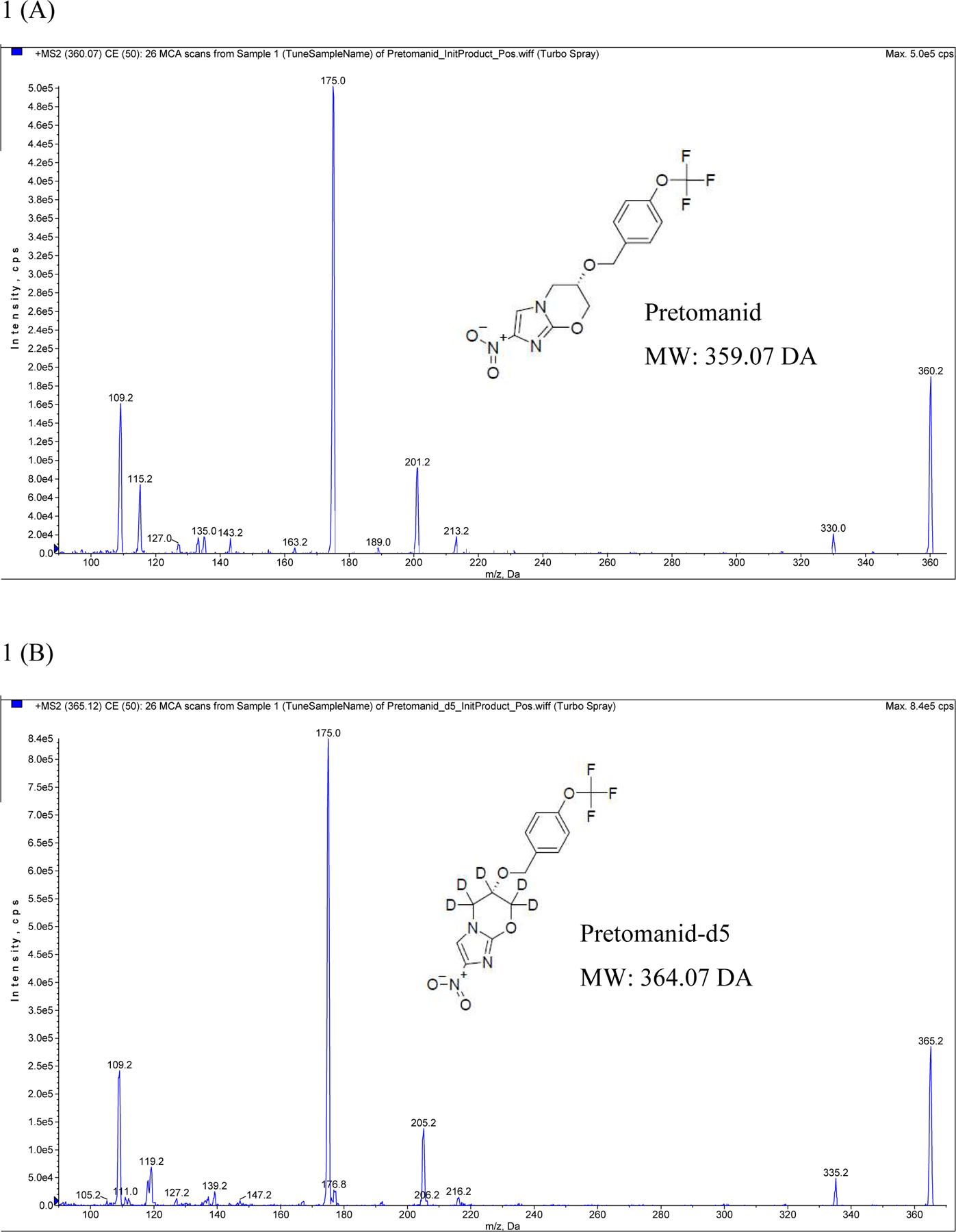
Initial product ion mass spectra of (A) pretomanid and (B) pretomanid- d5
The maximum doses given in clinical studies involving healthy volunteers was 1500 mg for a single dose and 600 mg for multiple doses. The average maximal blood concentration (Cmax) of the drug was approximately 2.9 μg/ml when given as a 1500 mg single dose, whereas it was 1.8 μg/ml when the drug was dosed at 600 mg daily. The average half-life for the drug is about 16–18 hours [7].
In patients with sputum smear positive TB pretomanid monotherapy was administered at dosages of 200 mg, 600 mg, 1000 mg or 1200 mg once a day. All the tested dosages showed clinically significant and equivalent early bacterial activity, over the test period, with maximum efficacy achieved even at the lowest dose [8]. Due to lack of early bacterial activity dose response, lower doses of 50 mg, 100 mg, 150 mg and 200 mg were investigated [8,9]. The lower dose investigation resulted in an average Cmax range of 465.3 – 1 183 ng/ml (50 mg – 200 mg) with higher dose dependent bacterial activity seen at dosages 100 mg, 150 mg and 200 mg. Therefore, doses of 100 mg to 200 mg were selected for further investigation in longer-term efficacy studies [9].
Several studies have been designed to determine the mechanism of action, pharmacokinetic and pharmacodynamic properties of pretomanid. It has been shown to be a promising drug for the treatment of both drug sensitive and drug resistant TB. Various authors have published methods for the quantification of pretomanid, including validated methods from rat plasma [10,11,12], lung and brain tissue [12], a method for determination from pharmaceutical formulations [13], and a brief summary of a method used for the determination of pretomanid from human plasma [14]. Pretomanid has shown promising outcomes in clinical studies and further studies have been undertaken to optimize its use in multi-drug regimens, hence a validated method for its quantification in human plasma was required.
In this article, we describe a specific, sensitive and robust method for quantification of pretomanid that performs in line with the requirements of the US Food and Drug Administration [15] and the European Medicines Agency [16, 17] bioanalytical method validation guidance. A method for the determination of pretomanid using 40 μl human plasma, liquid-liquid extraction and LC-MS/MS analysis is described here.
2. Experimental
2.1. Collection and storage of plasma samples
Drug free plasma, supplied by the South African National Blood Service, was used during method development, for the preparation of calibration standards (STDs) and quality control samples (QCs), and for validation experiments. Clinical samples were collected from adults with drug-sensitive sputum smear positive pulmonary TB. Blood was drawn into 4 ml K3EDTA tubes, centrifuged and plasma stored in 1.8 ml cryovials at ~−80°C until analysis.
2.2. Chemicals
Pretomanid (99.7% purity) and PA-824-d5 (used as internal standard) (98.4% purity) were donated by RTI International (Research Triangle Park, North Carolina), sourced from Mayne pharma (Greenville, NC, USA) and Synthese AptoChem Inc. (Montreal, Canada) respectively with the assistance of The Global Alliance for TB Drug Development.
Ethyl acetate (Pro-Analysis grade) was purchased from Sigma-Aldrich (Darmstadt, Germany). Acetonitrile and methanol (LC-MS grade) were purchased from Honeywell (Burdick and Jackson, Muskegon, Michigan). Formic acid (LC-MS grade) was purchased from Merck (Darmstadt, Germany). LC-MS grade water was produced in-house (Merck Millipore, Darmstadt, Germany).
2.3. Pretomanid extraction procedure
Plasma samples, including STD and QC samples, were thawed at room temperature. The thawed samples were sonicated for approximately 5 minutes at full power. A volume of 40 μl of water was aliquoted into 1.5 ml microcentrifuge tubes followed by aliquots of 40 μl of each plasma sample, STD or QC. Thereafter 300 μl of internal standard extraction solution was added (ethyl acetate containing 500 ng/ml of PA-824-d5). The double blank was extracted with 300 μl of internal standard-free extraction solution. The samples were vortexed for approximately 1 minute, then centrifuged at approximately 16 000 g for 5 minutes and the aqueous layer frozen on a cold plate for approximately 5 minutes at ~−30°C. Thereafter, the supernatant was decanted into glass tubes and evaporated to dryness under a gentle stream of nitrogen at ~40°C for approximately 30 minutes. Following this, 200 μl of reconstitution solution (acetonitrile: water: formic acid; 80:20:0.1, v/v/v) was added and the samples were vortexed for approximately 30 seconds. Samples were then transferred to a 96 well plate and 10 μl was injected onto the HPLC column.
2.4. Instrumentation and liquid chromatography/tandem mass spectrometry
Chromatography was performed on a Shimadzu HS602 High Performance Liquid Chromatography system (Kyoto, Japan). Detection was performed on an AB Sciex API 3200 Q trap Mass Spectrometer (AB Sciex™, Germany).
Separation was achieved using reversed-phase chromatography with a Poroshell 120 EC-C18 column, 50 mm × 4.6 mm, 2.7 μm (Agilent, Santa Clara, California) at ~30°C in the column oven. The aqueous mobile phase (mobile phase A) contained 0.1% formic acid in LC/MS grade water, while the organic mobile phase (mobile phase B) contained 0.1% formic acid in acetonitrile. Both mobile phases were degassed with helium and sonicated prior to use. A sample volume of 10 μl was injected and the temperature of the autosampler was set to ~8°C. Pretomanid and the internal standard eluted isocratically with mobile phase A at 15% and mobile phase B at 85%. The run time was 4 minutes at a flow rate of 400 μl/min.
Electrospray ionization was performed in the positive ion mode using nitrogen as the nebulizing, turbo spray and curtain gas with the optimum values set at 40, 50 and 20 (arbitrary unit), respectively. The heated nebulizer temperature was set at 550°C. The ion spray voltage, declustering potential, entrance potential, collision energy and collision cell exit potential were set to 5500 V, 70 V, 16 V, 41 eV and 4 V, respectively. The product ion mass spectra for pretomanid and internal standard, shown in Figure 1a and 1b respectively, were obtained by infusing a solution of each compound dissolved in mobile phase at a constant flow of 10 μl/min. The AB Sciex API 3200 Q Trap mass spectrometer was operated at unit resolution in the MRM mode, monitoring the transition of the protonated molecular ion m/z 360.2 to the product ions at m/z 175.0 for pretomanid, and the protonated molecular ion m/z 365.2 to the product ions m/z 175.0 for PA-824-d5. The pause time was set at 5 ms, the dwell time at 150 ms and the collision gas (N2) was set to low (arbitrary value). The instrument was interfaced with a computer running Analyst® version 1.6.2 software (AB Sciex™, Germany).
2.5. Method validation
2.5.1. Preparation of calibration standards, quality controls and dilution quality controls
The STD and QC samples were prepared volumetrically in blank human plasma (anticoagulant K3EDTA) at room temperature (~22°C). Stock solutions were prepared at 1000 μg/ml in methanol and these stock solutions were used to prepare working solutions which were in turn used to spike blank plasma as required. These working solutions were used to spike K3EDTA plasma for each respective STD to obtain a total of nine calibration standards (10 000, 7000, 2500, 1000, 400, 150, 60, 20 and 10 ng/ml) [7]. A similar methodology was applied for the preparation of QCs (QC-Dil at 20 000, QCH at 8000, QCM at 4000, QCL at 25 and LLOQ at 10 ng/ml). Thereafter, 100 μl aliquots of each standard and quality control were stored in individual 1.5 ml polypropylene tubes at ~−80°C to allow for duplicate 40 μl STD or QC sample preparation from each tube.
The calibration curve for pretomanid was validated over the range 10 – 10 000 ng/ml by analysing the QC samples in six-fold at high, medium, low and at the lowest level of quantification (LLOQ) concentrations (8000, 4000, 25 and 10 ng/ml respectively) over a period of 3 days to determine the intra- and inter-day accuracy and precision. An additional QC, QC dilute, was prepared at double the QCH concentration to test whether samples above the ULOQ can be diluted (1:4, v/v) and analysed. Calibration curves were constructed using a quadratic regression weighted 1/x2 (x = concentration) of the peak-area ratio of pretomanid to its internal standard versus nominal concentration.
2.5.2. Stock solution stability
Stock solution stability for pretomanid and the internal standard in methanol was evaluated for ~4 hours at room temperature. In addition, the stability of pretomanid was evaluated for ~4 months and 23 days in methanol at ~−80°C. Stock solution stability was evaluated by diluting the test and reference samples from 1 mg/ml to 10 μg/ml in injection solution and the peak heights of six separate samples were compared by means of HPLC (same conditions as described in section 2.4 and ultraviolet (UV) detection at a wavelength of 330 nm.
2.5.3. Working solution stability
The short-term stability of the working solutions was evaluated over ~72 hours at ~−20°C, and for 2 hours at RT. Long-term stability was evaluated for ~77 days at ~−80°C. The reference stocks were stored at ~−80°C for 3 days. Working solution stability was evaluated by diluting (1:25) of the test and reference working solutions in injection solution containing the internal standard. The analyte to internal standard ratios of six separate samples were compared using LC-MS/MS.
2.5.4. On-instrument stability and reinjection reproducibility
To evaluate reinjection reproducibility, the samples extracted in validation 1 remained in the autosampler at ~8°C for the following 48 hours. The analytical run was reinjected in its entirety after ~48 hours. In order to assess autosampler stability, the reinjected low (25 ng/ml) and high (8000 ng/ml) control peak area ratios (in six fold) were compared to those obtained during the first injection. This provided an estimation of absolute autosampler stability over ~48 hours.
2.5.5. Stability in matrix
To evaluate pretomanid short-term stability in matrix, low (25 ng/ml) and high (8000 ng/ml) QCs were stored at ~−80°C and ~−20°C for ~9 days. Additionally, long-term matrix stability was evaluated at ~−80°C over ~203 days and then analysed against a fresh calibration curve. The samples were analysed in six fold against a freshly prepared calibration curve and compared to the nominal concentration.
2.5.6. Freeze-thaw stability
To ascertain freeze-thaw stability, low (25 ng/ml) and high (8000 ng/ml) QCs were frozen at ~−80°C and subjected to three consecutive freeze and thaw cycles. Sample aliquots were prepared and frozen for at least 24 hours prior to starting this experiment. Each cycle consisted of sufficient thawing time at room temperature (~22°C) followed by 12 – 24 hours freezing time. These test samples were analysed, in six fold, against a freshly prepared valid calibration curve and assessed for accuracy against the nominal QC concentrations.
2.5.7. Benchtop stability
To evaluate benchtop stability, low (25 ng/ml) and high (8000 ng/ml) QCs were frozen at ~−80°C and subsequently left on bench for approximately 14.5 hours. These test samples were analysed, in six fold, against a freshly prepared calibration curve.
2.5.8. Recovery
Recovery was evaluated at high, medium and low concentrations (8000, 4000 and 25 ng/ml respectively). Reference samples were prepared by spiking blank samples post-extraction at the expected QC concentrations. The QC samples at the high, medium and low levels were used as the test samples. Recoveries were calculated by comparing the peak area ratios of the extracted samples (in six fold) to the reference samples.
2.5.9. Process efficiency
To evaluate process efficiency the pre-extraction spiked samples were prepared in six different lots of matrix at the high, medium and low concentration level (8000, 4000 and 25 ng/ml respectively). The neat, un-extracted samples were prepared in the injection solution (ACN: water: formic acid; 80:20:0.1, v/v/v) at theoretical QC concentrations with adjustments made for dilution during the extraction process, in triplicate (no matrix present). The internal standard was spiked at the working concentration of the method. The analyte peak areas observed after extraction are compared to the peak areas of the neat samples and expressed as percentage process efficiency.
2.5.10. Matrix effects evaluation
The evaluation of matrix effects was conducted using the method described by Matuszewski [18, 19]. Blank plasma from six different sources was extracted and spiked with pretomanid at QC concentrations with adjustments made for dilution during the extraction process and at a single internal standard concentration (500 ng/ml). The peak area ratios of the analyte/internal standard for each level in each source were used to generate regressions for each individual matrix.
2.5.11. Haemolysis evaluation
The effect of the presence of haemolysed blood was assessed using six plasma samples containing 2% haemolysed blood at low (25 ng/ml) and high (8000 ng/ml) QC concentrations. The peak area ratios for these test samples were compared with six reference low and high QCs.
2.5.12. Whole blood stability
Whole blood stability was evaluated over ~2 hours at room temperature (~22°C). Fresh whole blood was spiked at low (25 ng/ml) and high (8000 ng/ml) QC concentrations. The reference samples were immediately centrifuged following spiking to collect and store plasma at ~−80°C. After ~2 hours at room temperature the test samples were centrifuged, and plasma was collected and stored at (~−80°C) until analysis. The peak area ratios for these test samples were compared with the reference samples (in six fold).
2.5.13. Effect of concomitant medication
To evaluate the influence of concomitant medications on the quantification of pretomanid, first line TB drugs (ethambutol, isoniazid, pyrazinamide and rifampicin) and rifabutin were added to low (25 ng/ml) and high (8000 ng/ml) QCs already spiked with pretomanid as per the analytical method. Concentrations of the concomitant medications were tested at their expected Cmax concentrations in plasma [20]. The peak area ratios of pretomanid and the internal standard of the test samples were compared with that of the reference samples (in six fold) to calculate the overall percentage difference.
2.5.14. Specificity and carryover
Six blank plasma samples were extracted without internal standard to ensure that the bioanalytical method does not detect an endogenous matrix component with the same transition as pretomanid in the blank. A double blank sample was positioned in the injection sequence immediately after the highest calibration standard in order to assess possible carry-over effects.
2.6. Application to a Clinical Study
The assay was used for the analysis of clinical samples generated during a phase 2 randomised, open label trial of pretomanid-containing regimens (dose of 200 mg) for drug-sensitive sputum smear positive pulmonary TB in adults (NCT02256696). Population characteristics included participants that had no prior history of tuberculosis disease or tuberculosis treatment, age ≥ 18 years, weight ≥ 40 kg and ≤ 80 kg, HIV negative, or positive with CD4 ≥350 cells/cu mm and not currently taking or planning to take combination antiretroviral therapy for HIV during the experimental phase of the treatment, ability to adhere with study follow-up and signed informed consent. Participants had both semi-intensive pharmacokinetic sampling (performed immediately prior to administration of study medications and then at 1, 2, 5, 8 and 24 hours post dose) and sparse pharmacokinetic sampling (performed pre-dose within 2 hours of scheduled dosing and then 2–4 hours after an observed dose) done. The University of Cape Town Faculty of Health Science Research Ethics Committee and the Institutional Review Board of Johns Hopkins University gave approval to conduct this study.
3. Results and Discussion
Method validation
The method performed well during the validation period with the calibration standards having percentage accuracy in the range of 95.2% – 102.9% (%CV all < 4%) and the quality controls with an accuracy range of 98.5% – 110.7% (%CV all < 7%). The calibration curve was shown to fit a quadratic (weighted by 1/x2, x = concentration) regression over the range 10.0 – 10 000 ng/ml. The summary of the combined accuracy and precision data from validation days 1, 2 and 3 are presented in Table 1.
Table 1:
Summary of the validation test results based on accuracy and precision
| Validation experiment | Sample tested | n | Precision CV(%) | Accuracy (%Nom) |
|---|---|---|---|---|
| Day 1+2+3 | QC LLOQ | 19 | 6.3 | 102.0 |
| QCL | 18 | 4.6 | 98.5 | |
| QCM | 18 | 6.6 | 109.9 | |
| QCH | 18 | 2.6 | 108.6 | |
| QC DIL | 6 | 2.2 | 110.7 | |
| Matrix stability | QCL at ~80°C for 9 days | 6 | 5.2 | 98.1 |
| QCH at ~80°C for 9 days | 6 | 1.8 | 106.3 | |
| QCL at ~−20°C for 9 days | 6 | 5.4 | 95.8 | |
| QCH at ~−20°C for 9 days | 6 | 3.0 | 103.3 | |
| QCL at ~−80°C for ~203 days | 6 | 1.5 | 106.6 | |
| QCH at ~−80°C for ~203 days | 6 | 1.2 | 105.2 | |
| Freeze and thaw stability | QCL | 6 | 3.9 | 105.6 |
| QCH | 6 | 1.2 | 107.5 | |
| Benchtop stability | QCL | 6 | 12.4 | 107.9 |
| QCH | 6 | 2.6 | 104.3 | |
| Concomitant medication | QCL | 6 | 2.2 | 91.3 |
| QCH | 6 | 1.5 | 92.3 |
Concentrations (ng/ml): QC DIL= 20 000, QCH = 8 000, QCM= 4000, QCL= 25, QC LLOQ= 10
Stability assessment data are presented in Tables 1 and 2. Stock solution stability of both pretomanid and internal standard in methanol tested at 1 mg/ml on bench at room temperature for 4 hours, had percentage differences and %CVs below 9% when compared to the freshly prepared reference stock solution, demonstrating short term stock stability. Long-term stock stability for pretomanid was demonstrated at ~−80°C for up to ~4 months and 23 days in methanol.
Table 2:
Summary of the validation test results based on precision and % difference
| Validation experiment | Sample tested | n | Precision CV(%) | %Difference |
|---|---|---|---|---|
| Stock solution stability | room temperature for 4 h | 6 | 8.9 | 7.8 |
| ~4 months and 23 days | 6 | 0.9 | 1.6 | |
| Working solution stability | *0.200 μg/ml at ~−20°C for ~72 h | 6 | 2.0 | −4.2 |
| **160 μg/ml at ~−20°C for ~72 h | 6 | 0.9 | 0.1 | |
| *0.200 μg/ml at ~4°C for ~72 h | 6 | 3.0 | 9.5 | |
| **160 μg/ml at ~4°C for ~72 h | 6 | 1.5 | 18.6 | |
| *0.200 μg/ml at RT for ~2 h | 6 | 2.2 | −5.4 | |
| ****160 μg/ml at RT for ~2 h | 6 | 1.4 | 1.9 | |
| Whole blood stability | QCL | 6 | 3.0 | −0.5 |
| QCH | 6 | 1.5 | −1.7 | |
| 2% Haemolysis | QCL | 6 | 1.6 | 3.1 |
| QCH | 6 | 2.1 | −5.8 |
Lowest working solution concentration: 0.200 μg/ml;
Highest working solution concentration: 160 μg/ml
Short-term working solution stability was demonstrated for ~72 hours at ~−20°C and for 2 hours at room temperature at the high working solution concentration (QCH level) and the lowest working solution (QC LLOQ). Long-term stability was demonstrated at ~−80°C for up to ~ 77 days in methanol, with %CVs all less than 5% and percentage differences less than 10%.
Absolute on-instrument stability and reinjection reproducibility for pretomanid was indicated for at least 48 hours. The precision for both on-instrument stability and reinjection reproducibility was less than 7% and the percentage difference for pretomanid peak area ratios were all less than 6%. These results indicate that an entire batch may be reinjected after 48 hours when stored at ~8°C in the autosampler.
Short term matrix stability results indicate stability of pretomanid when stored at ~−80°C and ~−20°C for at least 9 days. Additionally, long term matrix stability was demonstrated over ~203 days at ~−80°C. This means samples may initially be stored at ~−20°C if a ~−80°C freezer is not immediately available; however, samples should be transferred from the ~−20°C freezer to a ~−80°C freezer within 9 days.
Freeze-thaw stability was demonstrated for three cycles at ~−80°C only. The observed concentrations of the test QCs were all within 8% of the nominal concentrations. Similarly, bench top stability was shown with percentage differences across high and low concentrations of less than 8% when compared to the nominal concentrations, indicating stability in plasma at room temperature for ~14.5 hours.
The average percentage recovery over high, medium and low concentrations of pretomanid was determined to be 72.4% (%CV = 6.5) with an average process efficiency of 68.4% (%CV = 9.8). The method was shown to be reliable and selective with no significant endogenous matrix effects using plasma originating from six different plasma sources, according to the criteria outlined by Matuszewski [18, 19] (Table 3). In addition, pretomanid was shown to be stable in whole blood for up to 2 hours at room temperature, and its quantification in plasma was not significantly affected by the presence of haemolysed blood (up to 2%). Similarly, no significant effect on analyte quantification was observed in the presence of other drugs likely to be co-administered with pretomanid. The percentage difference between the test sample containing the five drugs tested (ethambutol at 6 μg/ml, isoniazid at 6 μg/ml, pyrazinamide at 50 μg/ml, rifabutin at 1.03 μg/ml and rifampicin at 24 μg/ml; n = 6) and reference samples at the high QC concentration (8000 ng/ml) was 5.1% and at the low QC concentration (25 ng/ml) was 5.4%.
Table 3:
Assessment of matrix effects of six different plasma sources
| High Conc. | Medium Conc. | Low Conc. | Area Ratio | |
|---|---|---|---|---|
| 8000 ng/ml | 4000 ng/ml | 25 ng/ml | v Conc. | |
| Peak Area Ratio | Peak Area Ratio | Peak Area Ratio | Regression Slope | |
| Average | 4.33 | 2.41 | 0.0124 | 0.000542 |
| STDEV | 0.0528 | 0.0228 | 0.000421 | 0.00000664 |
| % CV | 1.2 | 0.9 | 3.4 | 1.2 |
The signal- to- noise ratio at LLOQ was above the minimum accepted criteria of 5 [13, 14]. The percentage accuracy of the LLOQ over the three validation days (n = 19) was 102% (CV%= 6.3), well within acceptable limits. No carry over was observed. LC-MS/MS chromatograms for the analysis of pretomanid in plasma are shown in Figure 2.
Fig. 2.
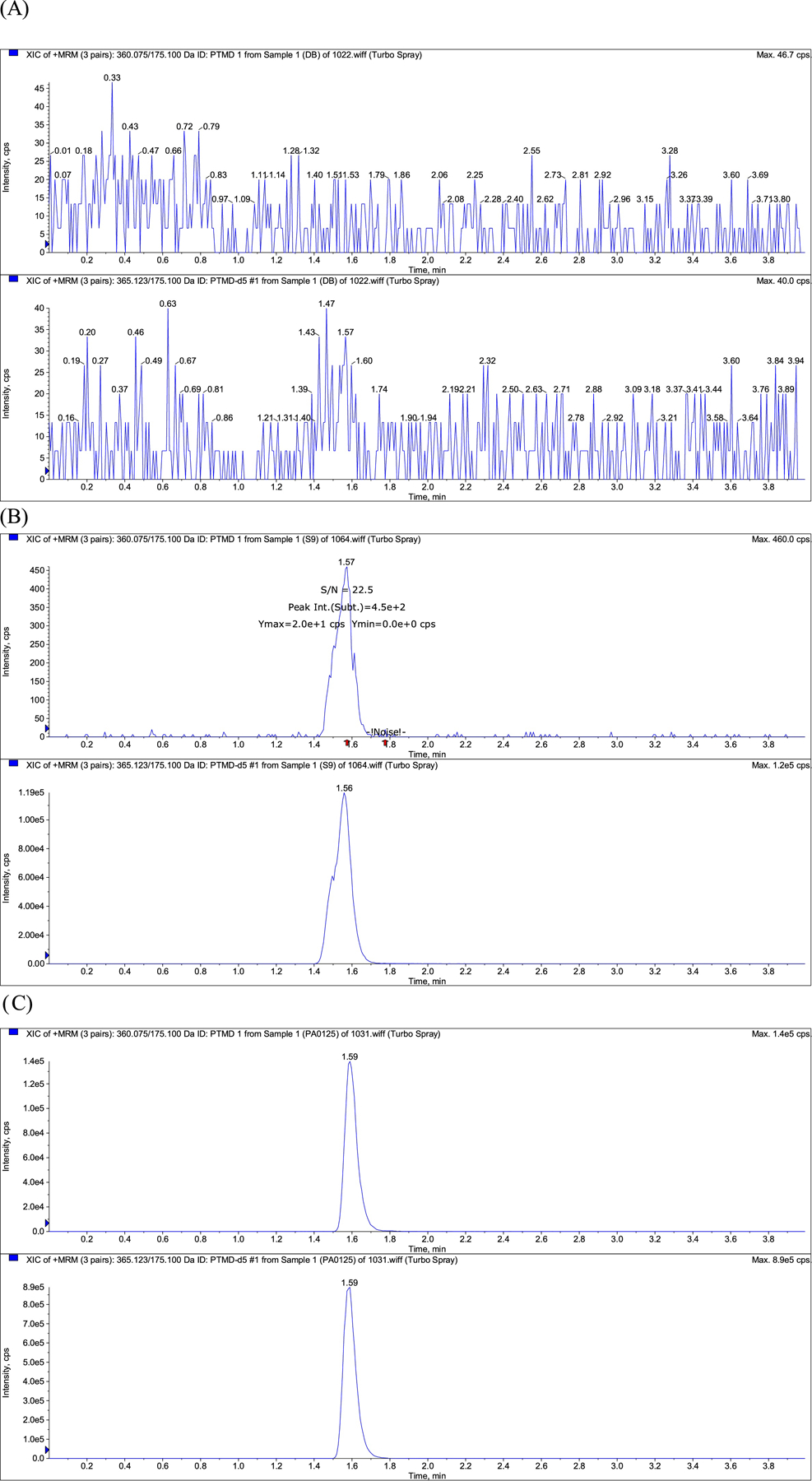
Raw LC-MS/MS chromatograms for the analysis of pretomanid in plasma: (A) blank (pretomanid free) plasma; (B) blank plasma spiked with pretomanid at the LLOQ (10ng/mL) with S/N ratio; (C) Plasma sample from a TB patient on a pretomanid based regimen.
4. Application to a clinical pharmacokinetic study
The assay was used to determine pretomanid concentrations in human plasma samples in the presence of other TB drugs. The time to reach maximum plasma concentrations was approximately 5 hours, which is similar to a pharmacokinetic study of pretomanid in smear positive TB patients [8]. The maximum plasma concentration for pretomanid was between 2000 and 4000 ng/ml, which is slightly higher than reported maximum concentrations in previous studies [9, 21]. Concentration versus time profiles for ten patients are presented in Figure 3. The validated method performed well for the analysis of clinical samples, generated during a phase 2 randomised, open label trial of pretomanid-containing regimens for drug-sensitive sputum smear positive pulmonary TB in adults. The primary objectives of the study were to compare the efficacy, safety and tolerability of the different test regimens against the standard treatment (PMID 33229425). During sample analysis, quality controls were used to monitor the accuracy and precision of the assay, resulting in average accuracies of 98.3%, 96.0% and 97.3% and precision of 2.3%, 1.4% and 4.1% at high (8000 ng/ml), medium (4000 ng/ml) and low (25 ng/ml) QC concentrations respectively.
Fig. 3.
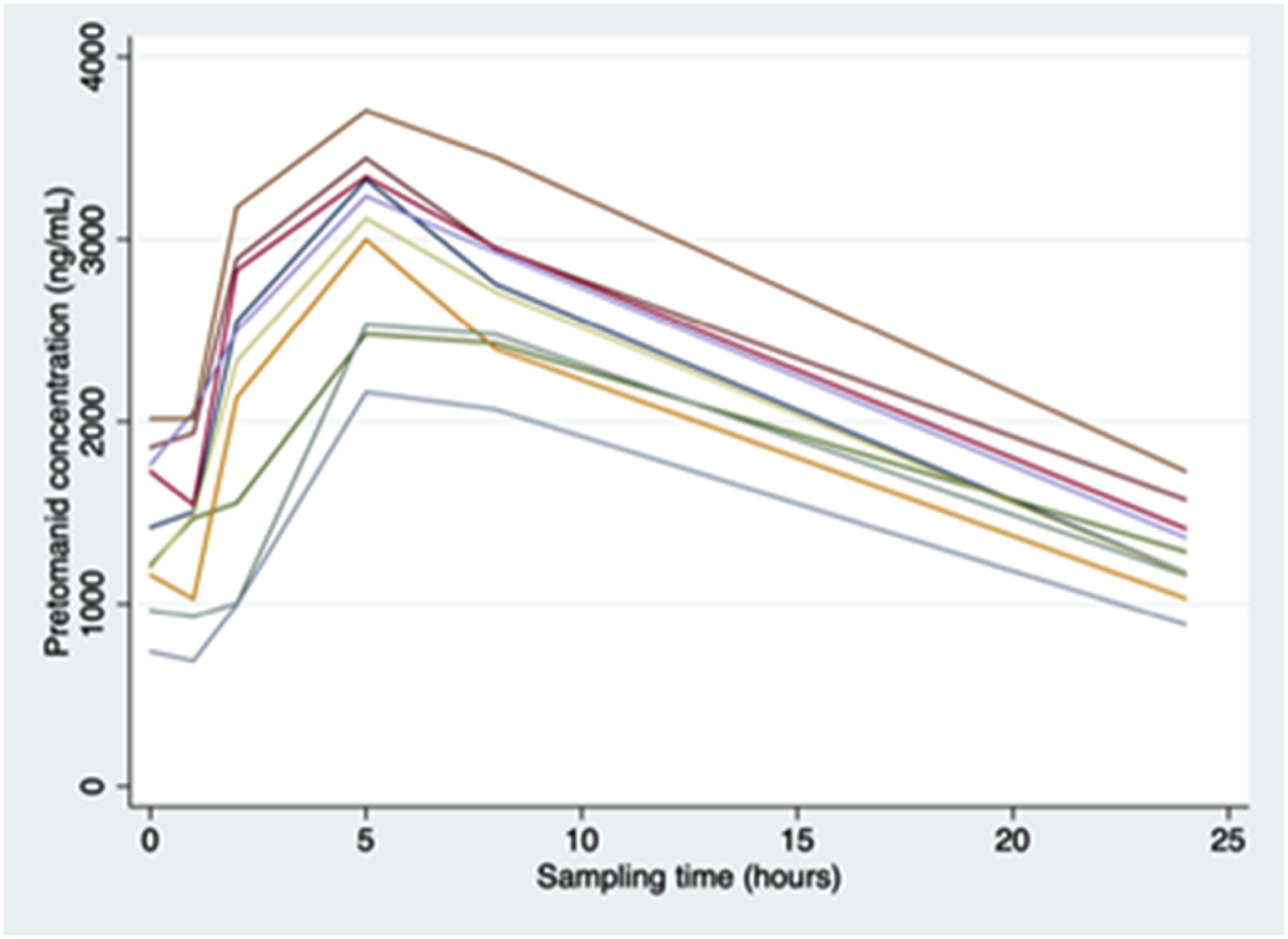
Concentration versus time profiles for pretomanid (N = 10).
5. Conclusion
A method for the extraction and quantification of pretomanid in human plasma was developed and fully validated according to FDA and EMA guidelines over the concentration range 10 to 10 000 ng/ml. The assay requires only 40 μl of plasma which would make analysis of patient samples possible even when a small volume of sample is available, as would be the case in paediatric patients. The method has been shown to be robust and reproducible when applied to pharmacokinetic sample analysis in a clinical trial of patients with TB. Pretomanid will be a key component in future TB regimens and thus the development of this method is an important addition to the literature.
Highlights.
An LC–MS/MS assay was developed and validated for pretomanid in human plasma
Patient plasma should be collected within 2 hours of blood withdrawal
Pretomanid is stable in plasma at ~−80°C for at least 203 days
Analysis of clinical trial samples showed method to be robust and reliable
Acknowledgements
The development, validation and sample analysis were supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (UM1 AI068634, UM1 AI068636 and UM1 AI106701) and the FDA orphan drugs grant (R01FD004794), and the National Institute of Mental Health (AI068632). Kelly E. Dooley is supported by K24AI150349, and Elisa H. Ignatius is supported by T32 GM066691. The Global Alliance for TB Drug Development (TB Alliance) provided reference standards for pretomanid and PA-824-d5 for this study. The work is also based on the research supported in part by the National Research Foundation of South Africa (HBG171031277340).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Organization WH. Global tuberculosis report 2017: World Health Organization; 2017. Report No: 9241565055. 2018. [Google Scholar]
- [2].Kanabus A Information about Tuberculosis: TB Statistics-Global, Regional and High Burden. Global Health Education (GHE) www tbfacts org. 2016. [Google Scholar]
- [3].Kaufmann SH, van Embden JD. Tubercolosis: a neglected disease strikes back. Trends in microbiology. 1993;1(1):2–5. [DOI] [PubMed] [Google Scholar]
- [4].Organization WH. Global tuberculosis report 2013: World Health Organization; 2013. [Google Scholar]
- [5].Rakesh Bruhn DF, Scherman MS, Singh AP, Yang L, Liu J, et al. Synthesis and evaluation of pretomanid (PA-824) oxazolidinone hybrids. Bioorganic & Medicinal Chemistry Letters. 2016;26(2):388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405(6789):962. [DOI] [PubMed] [Google Scholar]
- [7].Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrobial agents and chemotherapy. 2009;53(9):3720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrobial agents and chemotherapy. 2010;54(8):3402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, et al. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrobial agents and chemotherapy. 2012;56(6):3027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang L, Xu Y, Liang L, Diao C, Liu X, Zhang J, et al. LC–MS/MS method for the simultaneous determination of PA-824, moxifloxacin and pyrazinamide in rat plasma and its application to pharmacokinetic study. Journal of pharmaceutical and biomedical analysis. 2014;97:1–8. [DOI] [PubMed] [Google Scholar]
- [11].Wang L, Ma Y, Duan H, Yao J, Liang L, Zhang R, et al. Pharmacokinetics and tissue distribution study of PA-824 in rats by LC–MS/MS. Journal of Chromatography B. 2015;1006:194–200. [DOI] [PubMed] [Google Scholar]
- [12].Bratkowska D, Shobo A, Singh S, Bester LA, Kruger HG, Maguire GE, et al. Determination of the antitubercular drug PA-824 in rat plasma, lung and brain tissues by liquid chromatography tandem mass spectrometry: Application to a pharmacokinetic study. Journal of Chromatography B. 2015;988:187–94. [DOI] [PubMed] [Google Scholar]
- [13].Momin MA, Thien SJ, Krittaphol W, Das SC. Simultaneous HPLC assay for pretomanid (PA-824), moxifloxacin and pyrazinamide in an inhaler formulation for drug-resistant tuberculosis. Journal of pharmaceutical and biomedical analysis. 2017;135:133–9. [DOI] [PubMed] [Google Scholar]
- [14].Dooley KE, Luetkemeyer AF, Park J-G, Allen R, Cramer Y, Murray S, et al. Phase I safety, pharmacokinetics, and pharmacogenetics study of the antituberculosis drug PA-824 with concomitant lopinavir-ritonavir, efavirenz, or rifampin. Antimicrobial agents and chemotherapy. 2014;58(9):5245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Food and Drug Administration. Draft guidance: guidance for industry: bioanalytical method validation. Rockville, MD, USA: Federal Food and Drug Administration; 2013. [Google Scholar]
- [16].European Medicines Agency. Guideline on bioanalytical method validation. London: UK; 2012. [DOI] [PubMed] [Google Scholar]
- [17].European Medicines Agency. Reflection paper for laboratories that perform the analysis or evaluation of clinical trial samples. London: UK; 2012. [Google Scholar]
- [18].Matuszewski B, Constanzer M, Chavez-Eng C. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC− MS/MS. Analytical chemistry. 2003;75(13):3019–30. [DOI] [PubMed] [Google Scholar]
- [19].Matuszewski B Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. Journal of Chromatography B. 2006;830(2):293–300. [DOI] [PubMed] [Google Scholar]
- [20].Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62(15):2169–83. [DOI] [PubMed] [Google Scholar]
- [21].Keam SJ. Pretomanid: first approval. Drugs. 2019;79(16):1797–803. [DOI] [PubMed] [Google Scholar]


