Summary
Mouse models are essential for studying pain neurobiology and testing pain therapeutics. The reliance on assays that only measure the presence, absence, or frequency of a reflex have limited the reliability of preclinical pain studies. Our high-speed videography protocol overcomes this by projecting the discrete sub-second kinematic behavioral features induced by hind paw stimulation onto a “mouse pain scale.” This provides a more objective and robust pain measurement in mice by quantifying the quality of the stimulus-induced hind paw reflex.
For complete details on the use and execution of this protocol, please refer to Abdus-Saboor et al. (2019).
Subject areas: Model organisms, Neuroscience
Graphical Abstract
Highlights
-
•
High-speed imaging of paw withdrawal reflex enhances resolution of mouse pain assessment
-
•
Four sub-second features distinguish withdrawals induced by noxious vs innocuous stimuli
-
•
Principal component analysis projects behavioral features onto a single axis pain scale
-
•
Protocol only requires 500–1,000 fps camera and standard preclinical pain-related stimuli
Mouse models are essential for studying pain neurobiology and testing pain therapeutics. The reliance on assays that only measure the presence, absence, or frequency of a reflex have limited the reliability of preclinical pain studies. Our high-speed videography protocol overcomes this by projecting the discrete sub-second kinematic behavioral features induced by hind paw stimulation onto a “mouse pain scale.” This provides a more objective and robust pain measurement in mice by quantifying the quality of the stimulus-induced hind paw reflex.
Before you begin
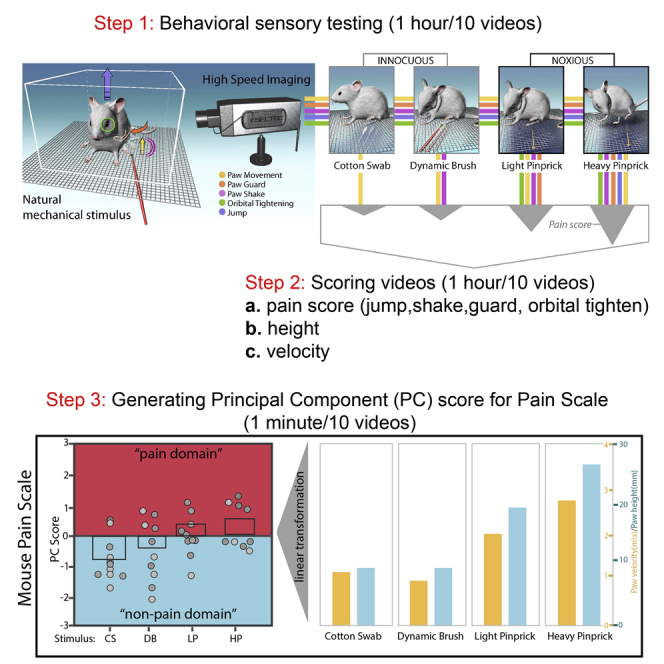
This pain scale measures six sub-second behavioral features of the hind paw withdrawal movement (paw height, paw velocity, orbital tightening, paw guarding, paw shaking, and jumping) following mechanical stimulation of the hind paw plantar surface. For each movement, these parameters are projected onto a “mouse pain scale” via a principle component analysis that combines these six features into a single axis through a dimension-reduction transformation. This transformation is a simple linear equation where each movement parameter (i.e., height, velocity, etc.) can be easily plugged into variables that are weighted with values produced by a principle component analysis.
This equation was developed from a “response library” used to generate the weighted eigenvalues that represent the range of these six movement features (cotton swab at the lower end and heavy pinprick at the upper end) for CD1 and C57 male and female mice. Thus, the equation we provide within this manuscript allows for the experimenter to determine how similar their observed paw withdrawal movement is to either an innocuous cotton swab response or a noxious heavy pinprick response in these genotypes/sexes. While we have seen great similarities between both sexes of other genotypes and this initial “response library” (Abdus-Saboor et al., 2019; Jones et al., 2020), it may be useful to develop an “in-house response library” that reflects the genotype of interest being investigated in a given lab. For example, in a recent study from our group, we found baseline differences in mechanical nociception in the SJL strain with this strain behaving as an outlier compared to more canonically used strains (Jones et al., 2020). Details on either using our response library equation or creating a local response library can be seen within this protocol.
Prior to using this protocol, it is essential to obtain appropriate stimuli/restrainers and habituate mice sufficiently to the testing environment. Since each experimenter may apply each stimulus in slightly different ways, we recommend having a single experimenter apply a particular stimulus within a particular experiment. While we recommend the below stimuli, any other stimulus can be used but should be validated for consistency.
This protocol includes the following steps: 1) habituation, 2) sensory testing, 3) scoring behavioral features, and 4) statistical mapping onto the mouse pain scale with our response library. If creating a local response library, step 4 will use a different linear equation that is generated from the experimenter’s own response library.
Habituation
Timing: 5 days
Proper habituation to equipment is essential to reducing variability when using this method and should be performed for every new group of animals. If mice are purchased from a commercial vendor and not bred in-house, allow mice at least two weeks of undisturbed habituation in an animal facility prior to beginning this experimental habituation step.
-
1.Establish a dedicated testing environment that has consistent temperature, light, noise, and humidity. While no behavior room is perfect, limiting any variability is helpful to provide consistent behavior.
-
a.Choose a testing room isolated from external stimuli (noise, light, etc.), preferably one that does not require moving mice far from their housing.
-
b.Use a sturdy and level table large enough to hold the testing platform with room around it to move and set up a camera. Camera and lighting angles may need to be modified so plenty of room is preferred.
-
a.
-
2.Habituate mice in testing environment for 5 days, 1 h a day, before testing. This habituation environment should mirror the experimental olfactory, auditory, and visual environment.
-
a.Set up testing platform on table, with paper towels or diaper pad on table below to catch waste and make for easier clean up.
-
b.Place mice in testing chambers on platform (Figure 1A).
-
i.Multiple chambers can be placed on the testing platform at once (we place anywhere between 1 and 8 chambers at once on our 20 inch × 15 inch platform), but it is important that the mice cannot see each other to prevent the influence of visual cues. We simply place a piece of paper between the chambers. Mice on the platform should be cage mates and of the same sex; unfamiliar animals or the opposite sex can potentially affect the experimental animal’s behavior.
-
ii.Place a weight on each mouse-containing chamber to prevent the mouse from escaping and limit movement. Tape can also be used but should not obscure the line of sight for the camera. Even slight movements of the experimenter or animal can cause unwanted movement of the entire testing platform.
-
i.
-
c.Turn on camera and place it near the cages as if recording for the experiment. Ensure all of those who will be present during testing are present for habituation to acclimate the animal to the camera’s noise and experimenters’ scent/presence. Be sure any other equipment that may be used for other purposes is present and on.
-
d.Allow an hour to pass.
-
e.Remove mice from chambers one at a time and place them back in their home container.
-
f.Clean chambers and platform with ethanol and paper towels to remove all olfactory cues. Take special care to clean the grated experimental platform as feces or other residues can easily be missed within the grates.
-
g.Repeat b through f until all mice are habituated, before disposing of paper towels/diaper-pad, cleaning the table, and finishing for the day.
-
a.
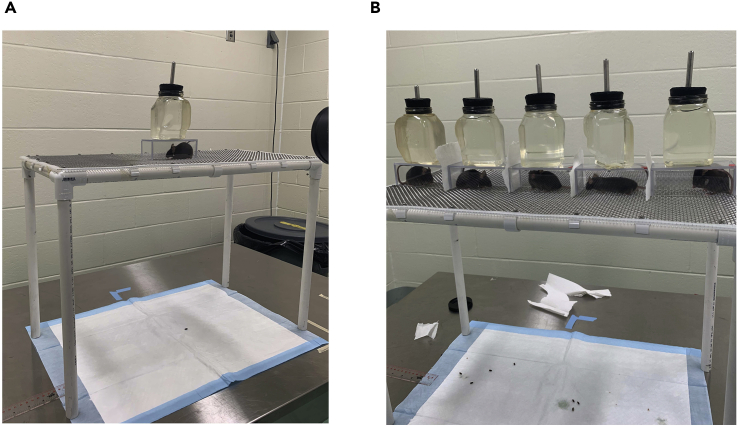
Figure 1.
Restrained mice during acclimation
(A) Single mouse setup with mouse in chamber on platform held down with weighted water bottle.
(B) Five mouse setup with paper towel pieces preventing them from seeing each other.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| PennBox | This paper | https://upenn.box.com/s/oro330k43pnqskss4prdvdzvdtubuin5 |
| Experimental models: organisms/strains | ||
| Mouse: C57BL6/J (8-week-old males and females) | Jackson Laboratory | 000664; RRID: IMSR_JAX:000664 |
| Mouse: CD-1 (8-week-old males and females) | Charles River | 022; RRID: IMSR_CRL:22 |
| Software and algorithms | ||
| Photron Software Package 4.0.3.2 | Photron | N/A |
| Statistical Analytical System (SAS) | SAS Software | N/A |
| Other | ||
| FastCAM UX100 high-speed camera | Photron | 800K-M-4GB |
| von Frey hairs | Stoelting Company | 58011 |
| Concealer makeup brush | e.l.f.TM, CVS | N/A |
| Insect pins | Austerlitz | N/A |
| Cotton swab | Q-Tip | N/A |
| Mesh mouse holding platform | McMaster-Carr | 1337T93 |
| Red or infrared light | CMVision IP65 | N/A |
Materials and equipment
Recommended materials and stimuli
| Item | Specifications | Purpose | Source |
|---|---|---|---|
| Mesh-top platform | The mesh should contain small holes around a half centimeter in width to allow for stimulus access. We used super-corrosion resistant stainless steel purchased from McMaster-Carr (product no: 1337T93). The platform should be high enough (~12–16 inches) to allow for the experimenter to have access from below | Mice will be placed on this platform to gain access to their paws from underneath | Machine Shop (custom made) |
| 5-sided rectangular plexiglass mouse chamber | Container should measure ~4.25 cm tall × 4.5 cm deep × 11.5 cm long. The thickness of the plexiglass used was 0.25 cm. It is essential for this restrainer to be rectangular because it positions the rodent’s paw perpendicular to the camera when recording as opposed to circular restrainers which allow the rodent to face any direction | Individual mice will be placed in this restrainer during experimentation | Machine Shop (custom made) |
| High-speed camera w/ tripod | There is a range of high-speed cameras on the market. We recommend a camera that can record 2–3 s of 500–2,000 fps. We have successfully resolved all behaviors at 500 fps. To determine whether a particular camera is suitable for this method, we recommend recording a video and simply confirming whether all behavioral features can be resolved both temporally and spatially | Camera for recording high-speed videos of mouse reactions | We use the FastCAM UX100 800K-M-4GB - Monochrome 800K with 4 GB memory for our recordings, but other cameras, even low-cost cameras, are available and should be sufficient. We recommend testing any camera to confirm all sub-second behavioral features can be resolves |
| Red or infrared light | If using infrared light, be sure the camera used is IR-sensitive | To decrease the disturbance of rodents, we recommend lighting the behavior room with red or infrared light | CMVision IP65 |
| Recommended stimuli | |||
| Cotton swab | To simulate an innocuous “static touch” stimulus, we use a cone-shaped cotton swab (do not use a pointed cotton swab) | This stimulus should be applied to the plantar surface of the hind paw through the mesh briefly (<1 s). Ensure sufficient pressure is placed to allow the cotton swab to move past the mesh and make contact with the rodent’s paw, only part of the Q-tip will pass the mesh and touch the paw | Q-Tip Cotton Swabs that can be purchased from any pharmacy store |
| Makeup brush | To simulate an innocuous “dynamic touch” stimulus, we use a makeup brush | Gently brush the plantar surface of the hind paw in a proximal to distal (i.e., heel to toe) direction for the length of the hind paw with the brush. The brush should be moved at a moderate speed (<1 s) and should be consistent between animals | e.l.f. Foundation Makeup Brush purchased at CVS |
| Metal needle | To simulate a noxious stimulus, we apply a small metal needle to the mouse’s foot | Apply the needle to the plantar surface of the hind paw at two different speeds/forces (light & heavy pinprick). Both pinprick forces should not push the rodent’s paw upward and should instead stop at or close to the mesh surface. Pushing the needle through too far can injure the animal or obscure the height measurement during analysis. This can be confirmed in the video. To simulate the “light pinprick,” gently raise the needle until it comes into contact with and puts pressure on the paw and then remove the stimulus. To simulate “heavy pinprick,” quickly and forcefully poke the paw | Austerlitz Insect Pins (size 6, 38 mm length, 0.65 mm diameter) |
| von Frey Hairs | To simulate a range of mechanical forces, we have used VFHs | From our analysis, we have found that 4 g induces a movement similar to that which is seen with heavy pinprick. 1.4 g induces a movement similar to that which is seen with light pinprick and 0.6 induces a movement similar to that which is seen with dynamic brush (i.e., 4 g likely induces pain, 1.4 g border-line pain, and 0.6 g non-painful) | Von Frey Hairs, Stoelting Company, 58011 |
Step-by-step method details
Sensory testing
Timing: 2 h
We recommend using a cotton swab, dynamic brush, and/or a 0.6 g VFH or lower to measure innocuous mechanical touch and the metal needles or 4 g VFH or higher to measure noxious mechanical pain. These instructions assume that the researcher is using a photron camera and the accompanying software, some specifics will vary depending on the setup. Consult the makers of the camera/software used for more info.
Note: For each mouse group, this will take 30 min for experimental day habituation of all mice on the restrainer and then 5 min for each mouse sensory testing with a single stimulus.
-
1.Set up the testing environment by placing testing platform on the table, with paper towels or diaper pad underneath.
-
a.Place testing chambers on the platform so the long side of the chamber can be seen perpendicularly by the camera. Additional chambers should be placed side by side (Figure 1B).
-
b.Set up camera on stand in front of the table so its lens is level and parallel with the side of the testing chamber (Figures 2A and 2B).
-
c.Camera setup.
-
i.Turn on the camera, remove the lens cap, ensuring that the power cable is plugged in and its ethernet cable is plugged into the laptop.
-
ii.Open photron fastcam software (obtainable free from their website) on the laptop and ensure that the camera is functional. Note, this software is simply a useful tool available with our high-speed camera, but other software may be used. The software used should simply have the ability to play the recorded videos and perform accurate measurements of paw height and velocity.
-
iii.Set camera to record at 500–1,000 fps with the spatial resolution and framing sufficient to resolve the full body of the mouse.
-
iv.We recommend setting the camera to “stop recording” when a trigger is pressed since the movement is short and camera memory generally only allows for a few seconds of recording. The behaviors are complete within 1,000 ms, and a second researcher or a single researcher with a trigger can assist in this.
-
v.(optional) Depending on lighting and camera, it may be necessary to utilize infrared lights to ensure a clear image so that the movement of the paw and the eye are clearly visible. Placement of these lights must ensure that reflection of light on the plastic restrainer does not obstruct the image. Though some trial and error might be necessary to get an optimal image, placement of the light above the camera has previously been effective (Figure 2C).
-
i.
-
a.
-
2.
Place mice in their chambers disturbing them as little as possible, making certain that all limbs and tail are contained within the chamber before placing the weight on the top.
-
3.
Line the chambers with their long sides perpendicular to the camera and place a piece of paper towel or other obstruction between each to prevent visual cues from neighboring mice.
-
4.
Allow 15–30 min to pass for experimental day habituation. It is imperative that mice are completely still but awake before applying stimuli to the paw.
-
5.Apply stimulus to hind paw while recording.
-
a.Move camera to ensure entire side of mouse is within frame and the eye and paw are within focus.
-
b.Ensure that tail is not on the side being recorded as it will make proper analysis impossible with an obstructed view of the paw. Mice will turn every so often, so if one mouse is not in the proper position, the experimenter can move onto a different mouse until the previous has moved appropriately.
-
c.Hold stimulus below the mesh and apply to hind paw plantar surface, as described in Materials and equipment. We direct the readers to the sample videos in the training data to get an idea of how sensory stimuli are delivered, as well as typical mouse responses.
-
i.In the past, we measured “time to response” but found with a factor analysis that it did not provide any additional information to distinguish between noxious and innocuous stimuli so we have concluded that it is not important to see the exact moment the stimulus touches the paw. However, it is essential to confirm the stimulus came in contact with the paw and not a different part of the body such as the belly.
-
i.
-
d.Trigger the camera to stop recording after stimulation and behavior is complete. Generally, the full behavior is complete within 1000 ms, but we ensure 2–3 s of recording from the point of stimulation to capture the full behavior.
-
e.Confirm all behavioral features can be resolved in the recorded video.
-
i.Ensure that reaction occurred and was fully recorded, from the stimulus application to the timepoint where the mouse returns its paw to the mesh.
-
ii.Ensure that the stimulus made the desired contact with the paw, while not being obstructed by the mesh or touching other body parts. It is common that experimenters accidentally touch other parts of the body or parts of the mesh so confirming contact was made is essential.
-
iii.Ensure that the paw and eye are unobstructed so that all behavioral features can be measured.
-
iv.If the video is not viable for recording, wait 5 min before repeating application of the stimulus.
-
i.
-
a.
-
6.
Repeat step 5 for another stimulus or move the camera to a new mouse and repeat step 5, waiting at least 10–15 min between testing the same mouse. We recommend performing stimulus application to at least an innocuous stimulus and a noxious stimulus.
CRITICAL: There are instances where a stimulus makes contact with the hind paw, but the mouse does not react. In these instances, the recording obviously cannot be used, but it is best to keep track of reactions and non-reactions to report response rate.
CRITICAL: Previous experiments have found that for the most consistent results, it is best for some more intense stimuli to only apply one stimulus a day, waiting about 24 h after each stimulus before applying the next. Applying one stimulus per day to a given mouse also avoids potential sensitization, which could cause altered responses with repetitive stimulation, making it difficult for trial-by-trial comparisons.
CRITICAL: Whether the stimuli are applied on the same day or on separate days, it is recommended that stimuli are applied in order from least intense to most intense to avoid sensitization.
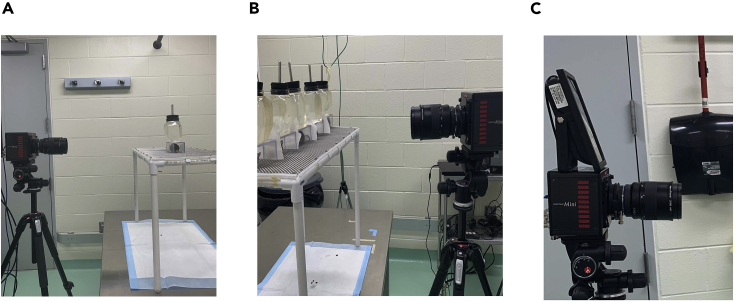
Figure 2.
High-speed videography behavioral setup with camera set level with the platform and perpendicular to the chamber
(A) Single mouse set up, (B) multiple mouse setup focused on center mouse, and (C) camera setup with infrared light on top.
 Pause Point: A pause can be taken after each day of experiments, though if a mouse is not habituated or tested for over 2 weeks, it may be necessary to repeat habituation again.
Pause Point: A pause can be taken after each day of experiments, though if a mouse is not habituated or tested for over 2 weeks, it may be necessary to repeat habituation again.
Scoring behavioral features
Timing: 10–20 min
We used Photron fastcam viewer (open-source, but only available for windows. Other software such as ImageJ can also be used) to score each video for three variables: paw height, paw velocity, and pain behavior score. After setting a scale based on a known length in the video, a researcher can score these variables. To limit variability in scoring, we suggest the same scorer is used for all videos in an experiment, especially if the researchers scoring videos are inexperienced in scoring this type of data.
-
7.
Calibrating Length and Frame Rate. To score paw height and velocity accurately, the video’s frame rate and a scale must be set within the software. For most recording software, each video recording will be a series of frames without an inherent frame rate or scale assigned to the file. In Photron fastcam, it will ask for the frame rate when opening the video. By entering the frame rate used for recording, the time will be set appropriately for measuring velocity. For measuring length accurately, it is important to set a scale to a known distance within the video. In Photron fastcam, this can be done by clicking “Dimensions” > “Calibration” and then choosing the “Manual” radio button. Choose “calibrate by length between two points” and enter the length. Finally, move the red “measurement line” in the video to the known distance and click “apply.” The grid or the sides of the chamber can be good targets for this.
Note: The grid view of the cursor in Photron fastcam can obscure measurement. To remove it, go to “Menu” > “Configuration” > “Preference” > “Zoom Ratio” > 100%.
Note: When calibrating the length, recognize that although the video recorded has depth, the video itself is two-dimensional. We suggest having a clear object that is aligned to either the y or x axis for more accurate calibration. The PFV manual that is included with the download has more detailed instructions on software use. The manual can be downloaded here: https://photron.com/software-downloads/
-
8.
Measuring paw height. After calibrating the video length, the paw height (mm) can be measured. Paw height should be measured as the distance from the apex of the first upward movement to the point directly below it on the mesh (Figures 3A and 3B). This can be done in Photron fastcam by clicking “Dimensions” > “Measurements” and choosing “Two points.” Play through the entire movement of the paw to identify the point at which the paw is at its apex during its first upward movement. Choose an easily identifiable region of the paw (i.e., middle of plantar surface, the point where a digit intersects with the paw, heel, etc.) and click it to set that as the initial point. Rewind the video to identify where that region was prior to stimulation and choose the second point at that location along the y axis. Importantly, ensure the line measurement is parallel to the y axis and not at an angle. The height will appear on the top left of the screen.
CRITICAL: It is important to play through the entire paw movement to identify the apex of the first upward movement. In some behaviors, especially painful ones, a paw shake may lead a mouse to raise its paw initially, move it back down, and then raise it even higher. In this scenario, the apex of the first upward movement should be used to measure the paw height. Do not use the second upward movement as that is a measurement of the paw shake instead.
-
9.
Measuring paw velocity. After calibrating the video length and frame rate, the paw velocity (m/s) can be measured. This can be done in Photron fastcam by clicking “Dimensions” > “Measurements” and choosing “Two points.” Play through the entire movement of the paw to identify the frames in which the paw is experiencing its first upward movement. Paw velocity is measured by taking two points at different frames during this movement (Figures 3C and 3D). Similar to a pendulum swinging, the paw’s upward movement will begin slowly, speed up, and then decrease its velocity near the middle of this movement. To measure the velocity of the movement, it is important to choose frames during the middle of the movement. We recommend choosing the starting frame as 1–2 frames after the paw has completely lifted off the mesh and the last frame a few frames later (5–10 depending on the movement) to capture the paw velocity. Select an easily identifiable region of the paw (i.e., middle of plantar surface, the point where a digit intersects with the paw, heel, etc.) in the starting and ending frame. For velocity measurements, the measurement line will likely be at an angle (unlike the height measurement). If using Photron fastcam, the height will appear on the top left of the screen. The velocity can also be calculated manually by simply dividing the distance by the number of frames or time between the start and end frames.
CRITICAL: It is recommended not to use the toe for velocity measurements since in some movements (primarily noxious), the toe can act as a cantilever and may feature much higher velocities than the center region of the plantar surface that are not accurate estimations of the paw’s velocity.
CRITICAL: Velocity will show the most variability in scoring with even the same scorer having the potential to come up with slightly different numbers when they rescore data. If properly done though with consistent standards for measurement, this variability should not exceed +/- 0.05 m/s.
-
10.
Measuring pain score. The pain score is a combination of the presence of four behaviors that typically only occur with noxious-stimuli (orbital tightening, jumping, paw shaking, paw guarding). If three of the four behaviors occur within a video, the behavior will be scored as a 3.
Note: We define the presence of jumping if all paws leave the mesh and at least three paws are lifted off the mesh surface at the same time.
Note: We define the presence of shaking when the stimulated hind paw’s movement includes at least one up-down-up movement (i.e., the hind paw is lifted up, moves downward, and lifts back up again). Although paw shaking generally includes the paw moving up and down several times, just one shake is sufficient to be scored as occurring.
Note: We define the presence of orbital tightening when the eyelid either slightly or extremely narrows during the movement. This is a component of the Mouse Grimace Scale and for our pain score, we would count either a level 1 or level 2 orbital tightening. We direct readers to the sample videos in the training data set for examples of orbital tightening. Those scoring videos must be careful that they do not mistake the apparent narrowing of a mouse’s eye due to the angled turning of its head for grimacing. The return to the eyes original level of openness normally indicates it is a true grimace.
Note: We define presence of paw guarding when the paw does not immediately return to the mesh surface following stimulation or returns abnormally to the surface such as on its toes or other twisted paw configurations.
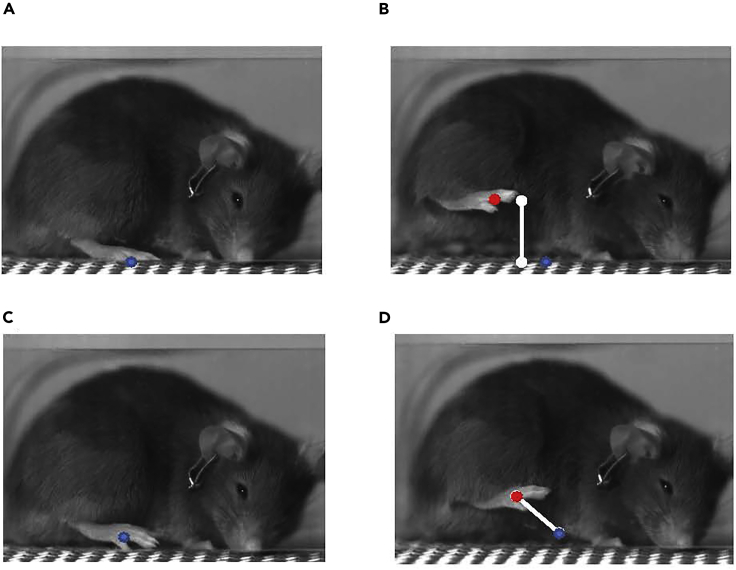
Figure 3.
How to score height and velocity measurements
Height is taken by recording the location of the paw on the mesh before stimulus (A) and the location of the paw at its apex (B). The difference in distance in the Y direction is then taken to get the height (B). Velocity is measured by picking a point shortly after the paw rises from the mesh (C) and a point shortly before it reaches its apex (D) and measuring the distance between those two points (D) and dividing by the difference in time.
Statistical mapping onto the mouse pain scale with CD1/C57 response library
To perform dimension reduction of these data (i.e., combining paw height, velocity, and pain score into a single number for comparison between videos), a Principle Component Analysis (PCA) is used. In our original paper (Abdus-Saboor et al., 2019), we used the eigenvalues of the first principle component from this PCA that was developed from a CD1/C57 male and female response library. The averages, standard deviations, and eigenvalue weights were therefore developed across both these genotypes and sexes. Since we saw limited variability between each genotype and sex, we can use and recommend using these aggregated averages, standard deviations, and eigenvalue weights (Table 1). However, it is also appropriate to use the variables from a specific strain and sex.
These variables represent the range of measurements (from both noxious and innocuous stimuli) to be expected in these genotypes/sexes. For this calculation, each measurement is first converted into a z score with a “z score transformation” using the average and standard deviation of that behavioral feature. The aggregated z scores of all three parameters are then combined using a weighted eigenvalue projection.
As an example, if a particular video had a velocity of 1,427.51 mm/s, height of 11.14 mm, and pain score of 2, these would first be transformed into the following z score equation and subsequently weighted with an eigenvalue projection with the CD1/C57 M/F response library:
- Z score Transformation
- Velocity: (1,427.51 mm/s - 1,106.372 mm/s) / 645.624 = 0.49741
- Height: (11.14 mm − 11.167 mm) / 6.552 = −0.00412
- Pain Score: (2 − 1.04)/1.15 = 0.83478
- Weighted Eigenvalue Projection
- (0.49741 × 0.582674) + (−0.00412 × 0.580398) + (0.83478 × 0.568885) = 0.76233
Thus, this example video would be given a “0.76233” on the Mouse Pain Scale (Table 1).
Table 1.
CD1/C57 M/F response library values for z-score transformation and weighted eigenvalue projection
| Z-score transformation |
Weighted Eigenvalue projection |
|||||
|---|---|---|---|---|---|---|
| paw velocity | paw height | pain score | paw velocity | paw height | pain score | |
| Average | 1106.372 | 11.167 | 1.040 | 0.582674 | 0.580398 | 0.568885 |
| STD | 645.624 | 6.552 | 1.150 | |||
| Average | 951.739 | 12.214 | 1.126 | 0.574310 | 0.591051 | 0.566416 |
| STD | 493.958 | 7.382 | 1.072 | |||
| Average | 1123.477 | 10.100 | 0.950 | 0.594993 | 0.570744 | 0.565892 |
| STD | 666.112 | 5.791 | 1.300 | |||
| Average | 1149.993 | 12.223 | 0.929 | 0.577430 | 0.605828 | 0.547319 |
| STD | 765.829 | 7.499 | 1.013 | |||
| Average | 1209.275 | 10.012 | 1.171 | 0.584451 | 0.560678 | 0.586564 |
| STD | 616.786 | 4.898 | 1.224 | |||
Creation of in-house response library
If using a different genotype/sex combination or if considered that responses to stimuli may be different in a new researcher’s hands under new experimental conditions, it may be important to generate a new “in-house response library.” However, since we saw little variability between our genotypes/sexes, this may not be necessary. We thus recommend using our response library values.
However, to generate response library values, a researcher should record videos for each genotype and sex being used in their studies in response to static touch, dynamic touch, light pinprick, and heavy pinprick as to capture the full range of possible behavioral features. We recommend capturing videos of 10 different mice per stimulus per group (genotype and sex). Thus, if using all four stimuli for males of genotype X, 40 videos would be recorded to generate the response library. If using both male and female, 80 videos would be recorded.
To generate the variables for z score transformation, simply calculate the average and standard deviation of each behavioral feature (velocity, height, pain score) for each group (i.e., male of genotype X) across ALL stimuli (static touch, cotton swab, light pinprick, heavy pinprick). If the researcher finds no difference between males and females or cross-genotype, we recommend finding an overall average and standard deviation across all groups to capture the full range of possible behavioral feature measurements.
Next, the researcher will need to perform a principle component analysis (PCA) with SAS or other software to obtain the first principle component. This will produce a weighted eigenvalue for each measurement that can then be used to transform your three-dimensional z scores into a single number. It is recommended that the researcher only use the first principle component as we found it accounts reliably for most of the variance within this system and it projects in an easily-interpretable single dimension. To see how we performed this PCA with our initial videos, please see (Abdus-Saboor et al., 2019).
Expected outcomes
The final calculation for the pain score will be on a scale from −3 to 3 and can be used to gauge the comparable intensity reactions to a given stimulus. Scores below 0 indicate the behavior is more comparable to that caused by a cotton swab (i.e., innocuous) while scores above 0 are more comparable to that prompted by a heavy pinprick (i.e., maximally noxious).
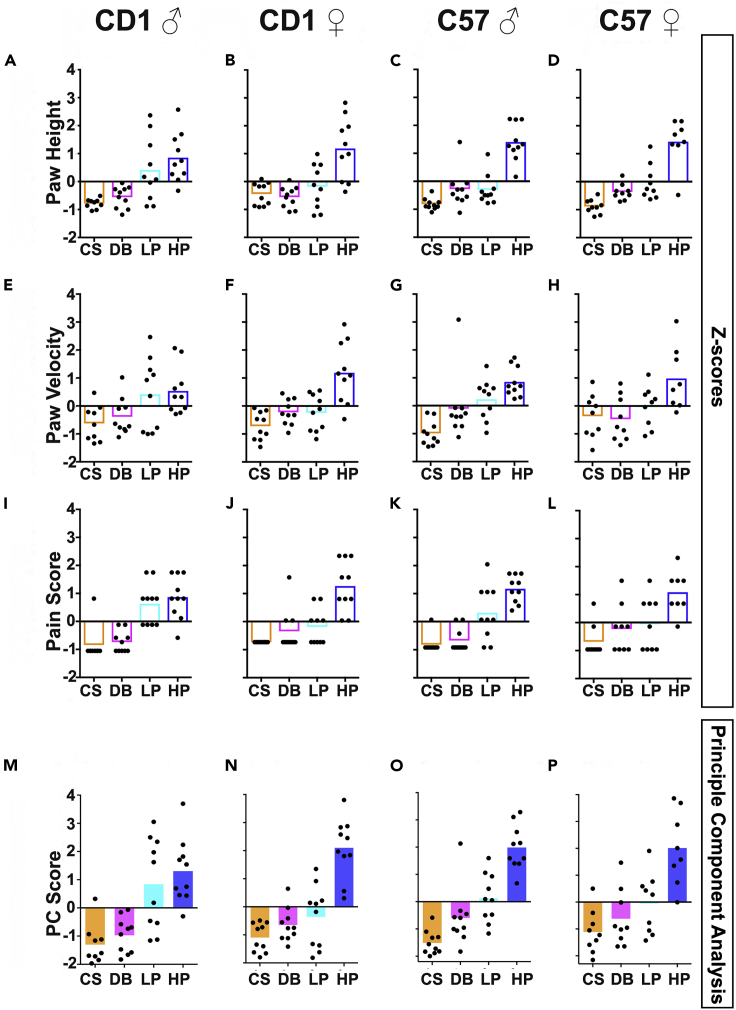
Mice are expected to show more extreme reactions as the stimulus gets more intense. There are a multitude of factors in their environment which can affect a particular reaction so only mice from similar environments and the same testing conditions should be compared. A graphical representation of the range of individual measurements can be seen in Figures 4A–4L and a graphical representation of the range for each behavioral feature’s z score and overall PC Score (i.e., pain scale score) can be seen in Figures 5A–5P.
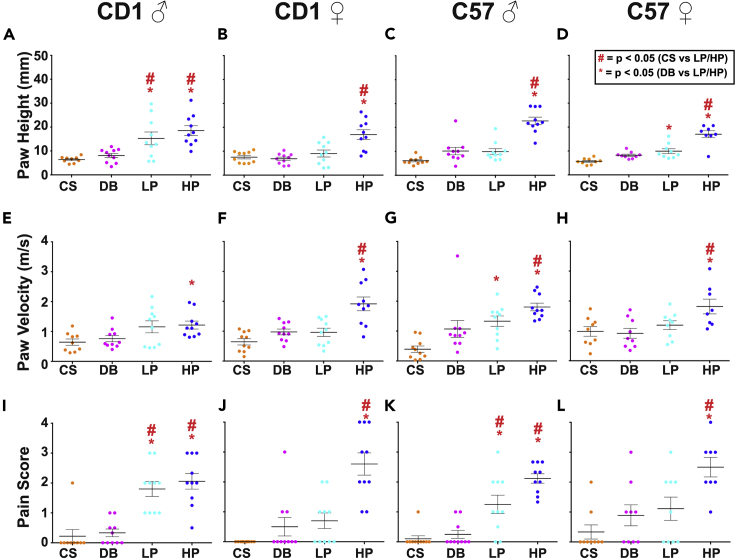
Figure 4.
Raw data from published studies on pain behavioral features
Paw height (A–D), paw velocity (E–H), and pain behavior scores (I–L) across males and females from CD1 and C57 mouse lines. Some light pinprick data and almost all heavy pinprick data is found to have significantly higher values compared to cotton swab and dynamic brush data. Images were adapted from Abdus-Saboor et al. (2019) with permission from the publisher Elsevier.
Figure 5.
Z scores from published studies generated from raw data
Data for paw height (A–D), paw velocity (E–H), and pain behavior scores (I–L) across males and females from CD1 and C57 mouse lines. These are used to produce Principle Component (PC) Scores for each data point. PC scores (M–P) increase with the intensity of the stimuli. Images were adapted from Abdus-Saboor et al. (2019) with permission from the publisher Elsevier.
If creating an in-house response library, similar values are likely to be produced. However, response library values may be rather different depending on the acclimation or when using other genotypes. Importantly, no matter which response library is used, if all videos are converted to a pain score using the same response library values, meaningful comparisons can still be made between their experimental mice and their own robust controls.
Training data
We also include a range of videos demonstrating the different types of responses to be expected from each stimulus. We have calculated the height, velocity, and pain score for each of these videos and converted them into a final PC score on the mouse pain scale in an excel spreadsheet included. Researchers may score these videos themselves and then compare them to scores generated by our lab as part of training and learning how to score videos. The videos and data can be found at (https://drive.google.com/file/d/1AI5Ol529nwV6wiMipoM_0txzhV5Mi49f/view?usp=drivesdk).
In conclusion, we present the details on how to use a platform that measures mechanical nociception in rodents at millisecond resolution with a high-speed camera. For researchers who currently perform related experiments in rodents, the only addition to your experimental setup is the use of a high-speed camera. In terms of why a researcher should adopt this protocol instead of, or in addition to, canonical mechanical nociception assays and measurements, it is because more information about the animal’s response to a given sensory stimulus is provided. Therefore, the likelihood about accurately predicting the animal’s internal sensory state is higher. For example, we have seen that paw withdrawal frequency, which is a traditional measurement for pain sensitivity, can be just as high for an innocuous stimulus as a noxious stimulus (Abdus-Saboor et al., 2019; Jones et al., 2020). In scenarios such as these, assigning the valence of the response can be challenging. If we aim to derive maximal translational benefits from our preclinical pain models, we will need robust tools that approximate the animal’s pain state.
Limitations
This pain scale is sufficient to detect pain intensity of mechanical stimuli. Other sensory modalities (thermal or chemical pain) have not been tested. The platform has difficulty detecting mechanical hypersensitivity following 20 microliter injection of 100% CFA (Complete Freund’s Adjuvant) (Jones et al., 2020), which is a commonly used inflammatory agent in the pain research field (Le Bars et al., 2001). In these cases, the nature of the chronic condition should be considered. Inflammatory agents or neuropathic injury may lead mice to not withdraw their paws as quickly to avoid further pain from the movement. In cases of where transdermal optogenetic stimulation is used to activate non-peptidergic nociceptors in the hind paw (Abdus-Saboor et al., 2019), hypersensitivity can be detected with CFA, but natural mechanical stimuli are currently limited in their ability to consistently detect this hypersensitivity.
Troubleshooting
Problem 1
Mice overexcited and not settling for testing (step 4).
Potential solution
The primary causes of this problem are distractions in the testing environment and/or not enough habituation time. Where possible, researchers should provide consistent environments to eliminate stress. If all reasonable steps have been taken to ensure this and the problem persists, additional habituation time can be added until mice are calm enough for testing. Even in a perfect environment, some strains require more time than others to habituate.
Problem 2
It is unclear what factor is stressing the mice out and/or how to eliminate it (step 4).
Potential solution
In some cases, the animal housing environment and/or the transportation of the mice to the testing environment can lead to increased stress in mice. Specifically cleaning routines and loud noise in the mouse facility can potentially cause increased stress. Where possible disturbances to mouse sleep patterns should be eliminated and cleaning procedures should be designed to disturb mice as little as possible. It has been observed that the farther mice need to be transported from housing to the testing facility the longer it will take for them to settle down for testing. In addition, taking mice into elevators should be avoided as it also appears to increase the time it takes for mice to settle and the variability in their behavior. Finally, the time at which mice are tested can sometimes have an effect so if data appears to be abnormally variable do your best to test at the same time each day, and avoid testing when the mice are likely to sleep.
Problem 3
Baseline testing data does not distinguish between noxious and innocuous stimuli (step 5).
Potential solution
If the inbred line being used does not have abnormalities in pain sensation, then the cause/s of this problem could fall into two categories. First, the mice could be under increased stress leading to abnormal pain behaviors. This stress is likely a result of how the mice are being handled or housed, and investigation may be needed to isolate the factor/s responsible. Second, experimenter error in applying stimuli could lead to inconsistent data. The same researcher should apply the stimuli throughout, and time should be taken to practice application to ensure consistency. Refer to the Step-by-step method details section and accompanying sample test videos for best practices.
Problem 4
It is unclear where to measure the height (step 8).
Potential solution
The paw height should be measured from the apex height of the first paw rise to the point directly below it. The first rise is the period from when the paw rises after the stimulus is applied to the point where it goes down again. If the paw goes down after rising, the first rise has ended.
Problem 5
The eye or paws are obscured in a way that it makes it impossible to determine pain behavior score, paw velocity or height (step 10).
Potential solution
If a particular measurement results in an “na,” this video cannot be used for final calculations since a missing measurement will ultimately decrease the final weighted eigenvalue PC score on the mouse pain scale, artificially lowering the response. It is highly recommended to review each recorded video immediately to determine if it is a video that can be used.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Dr. Ishmail Abdus-Saboor (ishmail@sas.upenn.edu).
Materials availability
Mouse lines used in this study are commercially available at the Jackson Laboratories and Charles River (022; RRID:IMSR_CRL:22, 000664; RRID:IMSR_JAX:000664).
Data and code availability
Datasets and code generated or analyzed in this study can be found in (Abdus-Saboor et al., 2019) and https://upenn.box.com/s/oro330k43pnqskss4prdvdzvdtubuin5.
Acknowledgments
The authors begin by acknowledging Dr. Wenqin Luo at the University of Pennsylvania, in whose lab this research was initially conceived and performed. The authors acknowledge Janet Sinn-Hanlon for the illustrations in the graphical abstract. J.B. and I.A.-S. are supported by startup funds from the University of Pennsylvania and by a grant from the National Institute of Dental and Craniofacial Research (NIDCR, R00-DE026807). N.T.F. is supported by the Rutgers Camden Provost Fund for Research Catalyst Grant.
Author contributions
All authors contributed equally in writing this protocol.
Declaration of interests
The authors declare no competing interests.
References
- Abdus-Saboor I., Fried N.T., Lay M., Burdge J., Swanson K., Fischer R., Jones J., Dong P., Cai W., Guo X. Development of a mouse pain scale using sub-second behavioral mapping and statistical modeling. Cell Rep. 2019;28:1623–1634.e4. doi: 10.1016/j.celrep.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.M., Foster W., Twomey C.R., Burdge J., Ahmed O.M., Pereira T.D., Wojick J.A., Corder G., Plotkin J.B., Abdus-Saboor I. A machine-vision approach for automated pain measurement at millisecond timescales. eLife. 2020;9:e57258. doi: 10.7554/eLife.57258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D., Gozariu M., Cadden S.W. Animal models of nociception. Pharmacol. Rev. 2001;53:597–652. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets and code generated or analyzed in this study can be found in (Abdus-Saboor et al., 2019) and https://upenn.box.com/s/oro330k43pnqskss4prdvdzvdtubuin5.