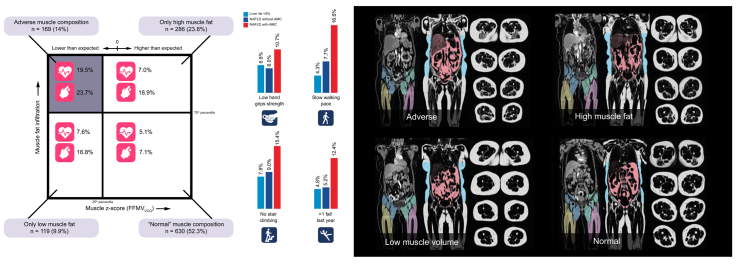
Abstract
Background & Aims
Sarcopenia and frailty are recognised as important factors in later stages of liver disease. However, their role in non-alcoholic fatty liver disease (NAFLD) is not yet fully understood. In this study we investigate the associations of MRI-measured adverse muscle composition (AMC: low muscle volume and high muscle fat) with poor function, sarcopenia, and metabolic comorbidity within NAFLD in the large UK Biobank imaging study.
Methods
A total of 9,545 participants were included. Liver fat, fat-tissue free muscle volume, and muscle fat infiltration were quantified using a rapid MRI protocol and automated image analysis (AMRA® Researcher). For each participant, a personalised muscle volume z-score (sex- and body size-specific) was calculated and combined with muscle fat infiltration for AMC detection. The following outcomes were investigated: functional performance (hand grip strength, walking pace, stair climbing, falls) and metabolic comorbidities (coronary heart disease, type 2 diabetes). Sarcopenia was detected by combining MRI thresholds for low muscle quantity and low hand grip strength according to the European working group definition.
Results
The prevalence of sarcopenia in NAFLD (1.6%) was significantly lower (p <0.05) compared with controls without fatty liver (3.4%), whereas the prevalence of poor function and metabolic comorbidity was similar or higher. Of the 1,204 participants with NAFLD, 169 (14%) had AMC and showed 1.7–2.4× higher prevalence of poor function (all p <0.05) as well as 2.1× and 3.3× higher prevalence of type 2 diabetes and coronary heart disease (p <0.001), respectively, compared with those without AMC.
Conclusions
AMC is a prevalent and highly vulnerable NAFLD phenotype displaying poor function and high prevalence of metabolic comorbidity. Sarcopenia guidelines can be strengthened by including cut-offs for muscle fat, enabling AMC detection.
Lay summary
Today, it is hard to predict whether a patient with fatty liver disease will progress to more severe liver disease. This study shows that measuring muscle health (the patient's muscle volume and how much fat they have in their muscles) could help identify the more vulnerable patients and enable early prevention of severe liver disease.
Keywords: Magnetic resonance imaging, Fatty liver, Non-alcoholic steatohepatitis, Skeletal muscle, Myosteatosis, Cardiovascular disease, Diabetes mellitus, Sarcopenia
Abbreviations: AMC, adverse muscle composition; CHD, coronary heart disease; DXA, dual-energy x-ray absorptiometry; FFMV, fat-tissue free muscle volume; FIB-4, fibrosis-4; HbA1c, glycated haemoglobin; MFI, muscle fat infiltration; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PDFF, proton density fat fraction; T2D, type 2 diabetes; VCG, virtual control group
Graphical abstract
Highlights
-
•
The role of sarcopenia and frailty in NAFLD is not yet fully understood.
-
•
Magnetic resonance imaging enables quantification of muscle composition.
-
•
Myosteatosis in combination with low muscle volume characterises an adverse muscle composition.
-
•
Adverse muscle composition is a novel NAFLD phenotype associated with poor function and metabolic comorbidity.
-
•
Sarcopenia guidelines can be strengthened by including cut-offs for muscle fat.
Introduction
As part of the obesity epidemic, non-alcoholic fatty liver disease (NAFLD) is recognised as the leading cause of chronic liver disease.1 Patients with NAFLD, especially with concurring inflammation (non-alcoholic steatohepatitis [NASH]), can progress to advanced liver disease, cirrhosis, hepatocellular carcinoma, and end-stage liver disease requiring liver transplant.2 NAFLD is prevalent, in the USA and worldwide, with prevalence estimated at 34% and 25.2%, respectively, indicating that the NAFLD population is highly heterogeneous.[3], [4], [5] Only a minority of NAFLD patients progress to end-stage liver disease, and the progression of fibrosis is a silent and slow process that can last for decades. It is therefore imperative to identify major drivers of disease to improve patient care.
Sarcopenia (progressive and generalised loss of skeletal muscle mass, strength, and/or physical performance) is strongly associated with cirrhosis and commonly observed in end-stage liver disease.6,7 Although reported associations between sarcopenia and severity of liver disease are consistent, reported associations between sarcopenia and NAFLD/NASH are less uniform.[8], [9], [10], [11] A challenge in understanding sarcopenia in the context of liver disease is the lack of consensus on how to diagnose and quantitatively assess the disease. A recently published expert opinion statement on sarcopenia in liver transplantation recommends the lone use of skeletal muscle index as assessed by tomographic imaging.12 However, there is no consensus on how to assess sarcopenia within NAFLD/NASH and sarcopenia consensus groups instead agree that a combination of a functional test and a measure of muscle quantity/quality is needed.7,13 The reason why such combination is needed is that if a patient presents with poor function, the physician cannot know whether the aetiology is muscle-related (probable sarcopenia) or caused by other problems or underlying conditions. For example, poor function could be attributed to arthritis, pain, low overall fitness, or neurological disorders. Thus, to confirm sarcopenia, a muscle-specific measurement needs to be taken (where muscle mass is currently recommended).
Previous studies on sarcopenia lack clear (or consistent) clinically meaningful associations for muscle-specific measurements, such as muscle mass, which further complicates this picture. A recent study utilising MRI data from over 10,000 UK Biobank participants showed that muscle volume alone inadequately detected individuals with poor function and increased hospitalisation.14 However, a personalised muscle volume z-score (sex and body size-specific) was significantly associated with these outcomes, and the relationship was strengthened when combining the muscle volume z-score with muscle fat infiltration. These results indicate that assessing muscle volume is clinically relevant and that the lack of proper body size-normalisations have obscured this relationship. Investigations of different body-size normalisations for muscle quantity showed that division of height2 and weight resulted in strong correlations with BMI but with inverse directions of association. A study on the NHANES III data further showed that these 2 ways of normalising skeletal muscle index (SMI) as measured by bioelectrical impedance (BIA; resulting in SMI/height2 and SMI/weight) for sarcopenia detection also resulted in inversely directional associations with NAFLD.15 The association between poor function and muscle quantity has also potentially been further obscured by most studies on sarcopenia using techniques lacking proper standardisation, such as BIA or dual-energy x-ray absorptiometry (DXA), which are also confounded by factors such as hydration status and body thickness.16,17 The study based on UK Biobank also showed that muscle fat infiltration has an important role to play in muscle health assessment, in line with the growing body of evidence around myosteatosis.18
In this work, using data from the UK Biobank, we estimate the prevalence of poor function and sarcopenia7 within NAFLD. We further propose a standardised muscle-specific metric combining MRI-measured fat-tissue free muscle volume (FFMV) and muscle fat infiltration (MFI) to describe how adverse muscle composition (AMC) relates to functional performance and metabolic comorbidity within NAFLD.
Participants and methods
This study was based on the first 10,019 participants in the UK Biobank imaging study.19 The UK Biobank study is a long-term study following 500,000 volunteers aged 40–69 years at recruitment in 2006–2010.20 As a sub-study, 100,000 participants are being re-called for a detailed imaging assessment including repeat of baseline assessment.
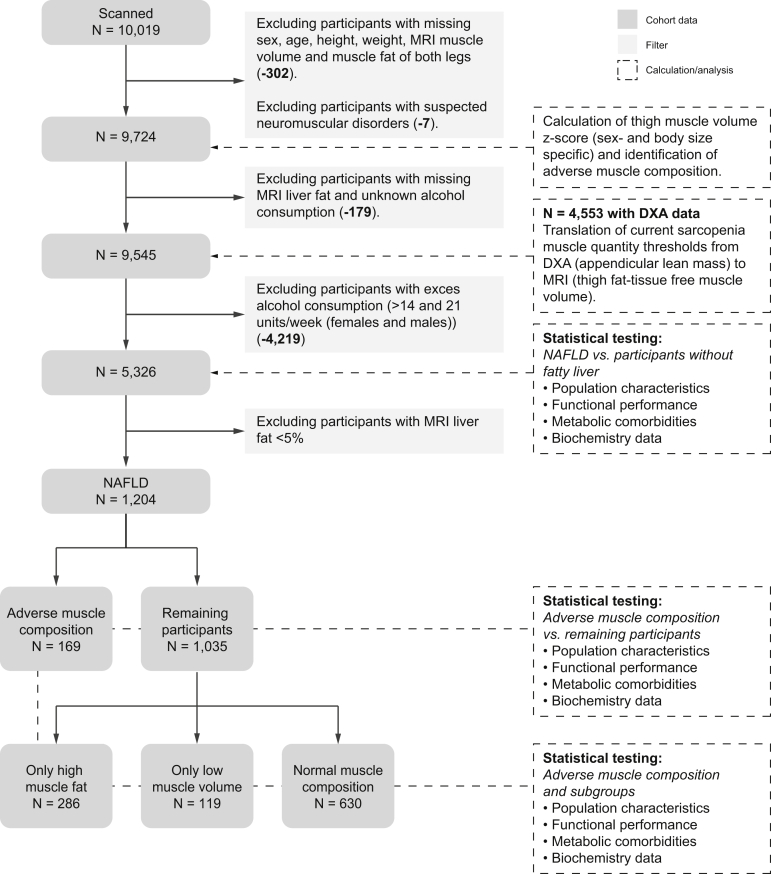
This research was conducted using the UK Biobank resource, project ID 6569. The study was approved by the North West Multicenter Research Ethics Committee, UK. Written informed consent was obtained before study entry. A schematic overview of the data and analysis performed in this study is provided in Fig. 1. Details of inclusion, data, and analyses follows.
Fig. 1.
Schematic overview of study data, calculations and analyses.
DXA, dual-energy x-ray absorptiometry; NAFLD, non-alcoholic fatty liver disease.
Inclusion
For muscle composition assessment, participants were required to have known sex, age, weight, height, and complete description of muscle composition (FFMV and MFI of at least 1 leg) (−302). Seven participants with suggestive neuromuscular disorders (following visual inspection of participants with high MFI or left/right asymmetry) were excluded (−7) resulting in 9,724 participants.
For investigations of NAFLD, participants were further required to have non-missing values for MRI liver proton density fat fraction (PDFF), known alcohol consumption (−179) and low alcohol consumption (<14/21 units/week [females/males]21) (−4,219), resulting in 5,326 participants.
MRI measurements
The participants were scanned in a Siemens MAGNETOM Aera 1.5-T MRI scanner (Siemens Healthineers, Erlangen, Germany) using a 6-min dual-echo Dixon Vibe protocol, providing a water and fat separated volumetric data set covering neck to knees, and a single-slice multiecho Dixon acquisition for liver PDFF assessment. For body composition profiling,22 acquired image data were analysed for liver PDFF, thigh FFMV, anterior thigh MFI, and visceral and subcutaneous adipose tissue volume. Briefly, the image analysis consisted of (1) image calibration, (2) fusion of image stacks, (3) image segmentation, and (4) quantification of fat and muscle volumes[23], [24], [25], [26], [27] and included manual quality control by a trained operator. Body composition analyses were performed using AMRA® Researcher (AMRA Medical AB, Linköping, Sweden). Details of in vivo acquisitions and analysis are provided in the Supplementary material. The MRI measurements utilised in this study are publicly available through UK Biobank (Category 149, Abdominal composition):
-
•
Liver PDFF, defined as the average PDFF of 9 regions of interest, placed while avoiding any inhomogeneities, major vessels, and bile ducts.22 Supplementary material provides further details.
-
•
FFMV, defined as the volume of all voxels with fat fraction <50% (‘viable muscle tissue’) in the thighs. This is to be differentiated from lean muscle volume (sometimes called ‘contractile muscle volume’) calculated by subtracting the fat volume from the entire muscle volume.23,24 If data were missing for 1 leg, the total thigh muscle volume was estimated through multiplication by 2 of the other leg.
-
•
Muscle volume z-score (FFMVVCG): For each participant, a matched virtual control group (VCG) was created to calculate a personalised z-score based on thigh FFMV (sex- and body size-specific) – measuring how many standard deviations each participant is from the mean thigh muscle volume of their matched group with same sex and body size. This data-driven measurement has been previously described and associated with poor function and increased hospitalisation.14
-
•
MFI, defined as the mean fat fraction in the ‘viable muscle tissue’ (FFMV) of the right and left anterior thighs.23,24 If data were missing for 1 leg, the mean MFI was estimated by the MFI of the other leg.
Definitions
NAFLD, defined by MRI liver PDFF >5% and lack of excess alcohol consumption (<14/21 units/week [females/males]28). MRI liver PDFF correlates strongly with histopathological hepatic triglyceride content and, although the literature on optimal threshold for defining steatosis is conflicting, cut-off values of 5% and 5.56% are frequently used.29,30 NAFLD stratification was made directly from the UK Biobank imaging participants (community volunteers, not selected owing to abnormal liver function tests). No exclusions were made based on rarer forms of liver disease or medications.
Sarcopenia, defined according to the recently published recommendation by the European working group7 requiring low hand grip strength (<16/27 kg [females/males]) and low muscle quantity (DXA-based appendicular lean mass/height2 <6.0/7.0 kg/m2 [females/males]). Identification of low muscle quantity was based on MRI (thresholds translated from DXA to MRI) to utilise the full dataset for sarcopenia detection (only 48% of the participants had DXA data). The Supplementary material provides details of threshold translation.
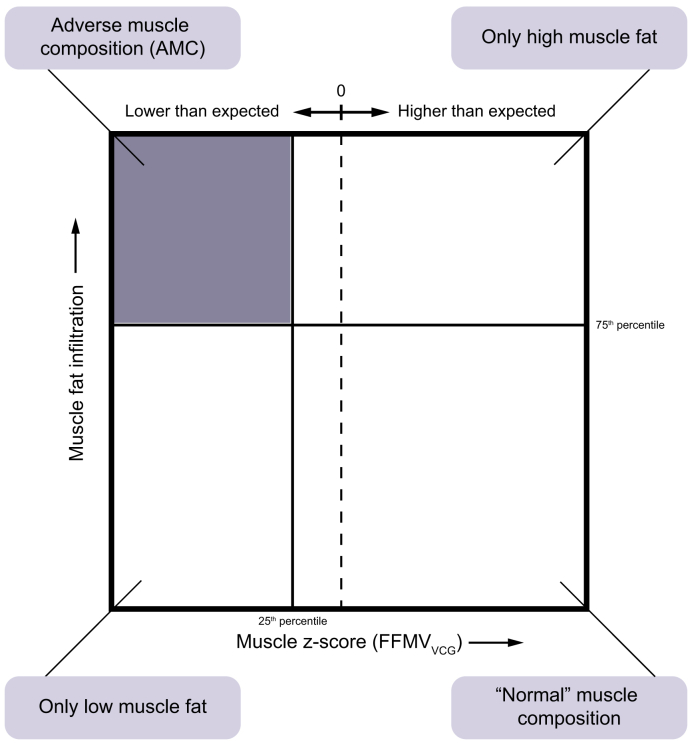
AMC, defined by low thigh muscle volume (FFMVVCG) coupled with high muscle fat (MFI). ‘Low muscle volume’ was defined as <25th percentile of the whole cohort (FFMVVCG = −0.68 [both females and males]), and ‘high muscle fat’ as >75th percentile (sex-specific) of the whole cohort (MFI = 8.88 and 7.69% [females/males]). Resulting muscle composition groups are shown in Fig 2.
Fig. 2.
Visualisation of muscle composition groups.
Adverse muscle composition (AMC) cut-offs: muscle volume z-score (FFMVVCG), −0.68 (both females and males), muscle fat infiltration, 8.88% and 7.69% (females and males, respectively). FFMV, fat-tissue free muscle volume (thigh); VCG, virtual control group.
Outcomes (functional performance)
-
•
Low hand grip strength, below 16/27 kg (females/males)7 based on the dominant hand. If information of handedness was missing or a participant reported using both hands, the mean of the right and left hand was used; N = 9,516 (29 with missing data).
-
•
Slow walking pace, defined by participants self-reporting ‘Slow pace’ compared with ‘Steady average pace’ or ‘Brisk pace’; N = 9,535 (10 with missing data).
-
•
No stair climbing, defined by participants self-reporting climbing no flights of stairs/day (approximately 10 steps) compared with 1 or more flights of stairs/day in the past 4 weeks; N = 9,524 (21 with missing data).
-
•
More than 1 fall in the past year, defined by participants self-reporting more than 1 fall within the past year compared with only 1 or no falls; N = 9,540 (5 with missing data).
Outcomes (metabolic comorbidity)
-
•
Coronary heart disease (CHD), defined by ICD-10 codes I20–I25, Z95.1. Controls excluded participants with these codes and those with self-reported history of heart attack, angina, or other heart/cardiac problems (N = 82 excluded). Analyses included both prevalent and incident CHD.
-
•
Type 2 diabetes (T2D), diagnosed by a doctor and with age at diagnosis above 30 years. Controls excluded type 1 diabetes and/or gestational diabetes (N = 39 excluded). Analyses included only prevalent T2D.
Data collection UK Biobank
Height was recorded using a Seca Height measure (Seca GMBH, Hamburg, Germany), weight with a Tanita BC418ma (Tanita Europe, Amsterdam, The Netherlands), and hand grip strength using a Jamar J00105 hydraulic hand dynamometer (Lafayette Instrument, Lafayette, IN, USA) (protocol in Supplementary material). Handedness, usual walking pace, frequency of stair climbing, and number of falls in the past year were acquired through touchscreen questionnaires. Whole body DXA was performed using a GE-Lunar iDXA (Madison, WI, USA) and appendicular lean mass was calculated by summarising lean mass for arms and legs (UK Biobank Field IDs 23275, 23258). Diagnosis for CHD and T2D was based on electronic health care records (accessed May 2019, available from April 1992 through March 2017) in combination with self-reported information collected via interviews with trained nurses. Medications (statins and insulin treatment) were self-reported by brand via interviews (listed in the Supplementary material). The blood biochemistry data31 were available only from the baseline assessment (years 2006–2010) and used to calculate the aspartate transaminase/alanine transaminase (AST:ALT) ratio, fibrosis-4 (FIB-4) and NAFLD fibrosis score. Alcohol consumption was assessed through touchscreen questionnaires about frequency of intake and average intake of specific beverages (‘red wine’, ‘champagne plus white wine’, ‘beer plus cider’, ‘spirits’, ‘fortified wines’, and ‘other alcoholic drinks’). Number of alcohol units per week was calculated using the Drinkaware definition.32
Statistical analysis
Differences between muscle composition groups were tested using the 2-proportions z-test (binary variables), Wilcoxon signed-rank test (continuous variables), and logistic/linear regression modelling. All models were adjusted for (1) sex, age, BMI, and liver fat and (2) sex, age, BMI, liver fat, and medications (statins and insulin) without correction for multiple comparisons.
Explorative, post hoc analysis
To investigate the separate contribution of muscle volume and muscle fat to the increased prevalence of poor function and metabolic comorbidities within AMC, participants with NAFLD were partitioned into 4 groups: (1) AMC; (2) ‘only low muscle volume’ (FFMVVCG); (3) ‘only high muscle fat’ (MFI); and (4) ‘normal muscle composition’ (Fig. 2). Participants with NAFLD and ‘normal muscle composition’ were also compared with the whole cohort. In addition, CHD incidence was compared between groups.
Results were independently replicated by an experienced statistician. Computations were performed using R version 3.4.4 (The R Foundation, Vienna, Austria). Associations were considered significant for α <0.05.
Results
Population characteristics
The population characteristics are presented in Table 1. The NAFLD population (prevalence 22.6%) was of similar age compared with those without fatty liver but had fewer females and showed a high prevalence of overweight (88.2%). AMC was more common within the NAFLD population (14.0%) compared with those without fatty liver (9.4%).
Table 1.
Cohort characteristics.
| Liver fat ≤5% and low alcohol consumption∗ | NAFLD | p value | p value (adjusted) | |
|---|---|---|---|---|
| Participants, n | 4,122 | 1,204 | – | – |
| Females, % | 58.69 | 46.51 | <0.001 | 1.000 |
| Age, years | 62.58 (7.68) | 62.90 (7.48) | 0.202 | – |
| Weight, kg | 71.88 (13.14) | 86.32 (15.68) | <0.001 | 0.224 |
| BMI, kg/m2 | 25.35 (3.88) | 30.14 (4.76) | <0.001 | – |
| Overweight (BMI >25 kg/m2), % | 48.30 | 88.21 | <0.001 | 0.990 |
| Visceral adipose tissue volume, L | 2.96 (1.81) | 5.67 (2.12) | <0.001 | <0.001 |
| Abdominal subcutaneous adipose tissue volume, L | 6.58 (2.99) | 9.29 (3.76) | <0.001 | 0.023 |
| Appendicular lean mass/height2, kg/m2 | 7.11 (1.14) | 7.94 (1.29) | <0.001 | 0.001 |
| Liver fat, % | 1.79 (1.27–2.67) | 8.92 (6.51–13.50) | <0.001 | – |
| Muscle composition | ||||
| Fat-tissue free muscle volume (FFMV), L | 9.89 (2.47) | 10.99 (2.56) | <0.001 | 0.846 |
| Muscle volume z-score (FFMVVCG), SD | −0.05 (1.02) | −0.02 (0.94) | 0.385 | 0.292 |
| Muscle fat infiltration (MFI), % | 7.21 (1.82) | 8.03 (2.08) | <0.001 | 0.453 |
| Adverse muscle composition, % | 9.39 | 14.04 | <0.001 | 0.657 |
| Only high muscle fat, % | 10.55 | 23.75 | <0.001 | 0.185 |
| Only low muscle volume, % | 18.32 | 9.88 | <0.001 | 0.382 |
| Normal muscle composition, % | 61.74 | 52.33 | <0.001 | 0.870 |
| Functional performance and metabolic comorbidity | ||||
| Sarcopenia, % | 3.44 | 1.58 | 0.001 | 0.874 |
| Low hand grip strength, % | 6.79 | 6.64 | 0.874 | 0.898 |
| Slow walking pace, % | 4.25 | 8.97 | <0.001 | 0.811 |
| No stair climbing, % | 7.84 | 9.88 | 0.024 | 0.542 |
| More than 1 fall in the past year, % | 4.83 | 6.23 | 0.055 | 0.906 |
| Coronary heart disease (prevalent), % | 4.29 | 7.81 | <0.001 | 0.164 |
| Coronary heart disease (incident), % | 1.48 | 2.24 | 0.055 | 0.787 |
| Type 2 diabetes, % | 3.30 | 13.21 | <0.001 | <0.001 |
Values are presented as mean (SD); for liver fat data are presented as median (IQR). The p values represent comparison between non-alcoholic fatty liver disease and participants without fatty liver and excess alcohol consumption tested using two-proportions z-test (binary variables), Wilcoxon signed-rank test (continous variables) and logistic/linear regression adjusted for sex, age and BMI. The p values >0.05 are presented as n.s. (non-significant).
NAFLD, non-alcoholic fatty liver disease; VCG, virtual control group adjusted.
Low alcohol consumption defined as <14/21 units/week (females/males).19
Sarcopenia and NAFLD
Only 19 participants with NAFLD had sarcopenia (prevalence 1.6%), which was 2.2 times more prevalent among participants without fatty liver (prevalence 3.4%). However, the prevalence of poor function was either similar or higher in the NAFLD population: low hand grip strength 6.6% vs. 6.8% (p = 0.874), slow walking pace 9.0% vs. 4.3% (p <0.001), no stair climbing 9.9% vs. 7.8% (p = 0.024), and more than 1 fall in the past year 6.2% vs. 4.8% (p = 0.055).
MRI thresholds for sarcopenia detection were 3.0 and 3.6 L/m2 for thigh FFMV/height2 (females/males). Sensitivity and specificity compared with DXA were 0.93 and 0.99, respectively. The Supplementary material contains details on threshold translation.
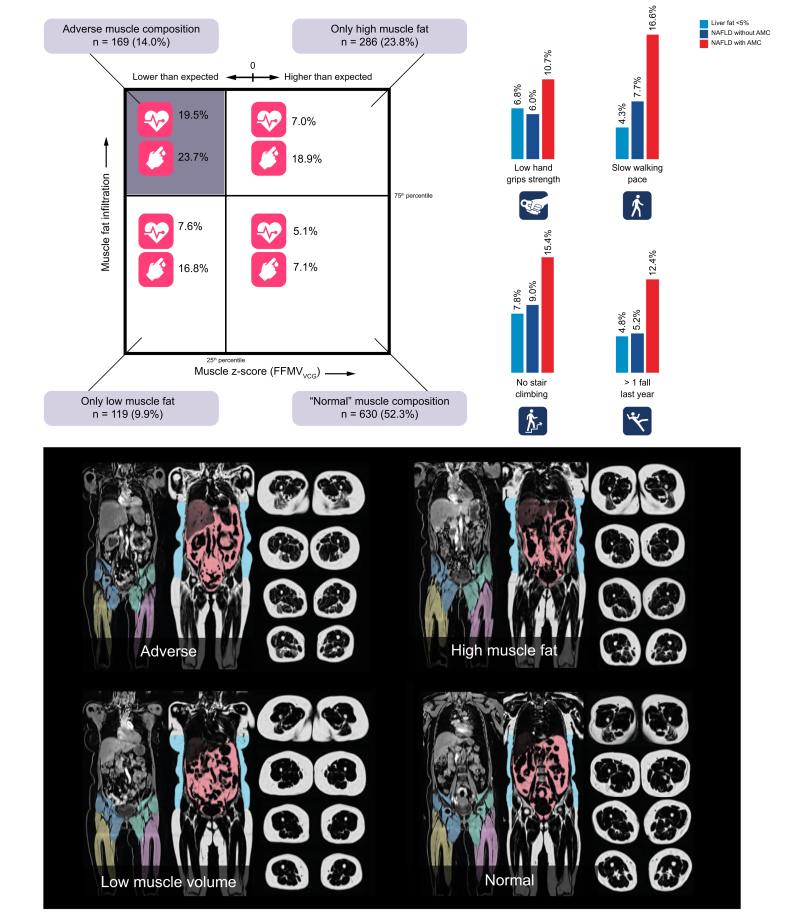
AMC within NAFLD
A total of 169 (14.0%) out of 1,204 participants with NAFLD had AMC (low muscle volume coupled with high muscle fat). Participants with AMC had significantly higher prevalence of poor function (low hand grip strength [1.8 times higher, p = 0.028], slow walking pace [2.1 times higher, p <0.001], no stair climbing [1.7 times higher, p = 0.011], more than 1 fall in the past year [2.4 times higher, p <0.001]) and metabolic comorbidities (CHD [3.3 times higher, p <0.001], T2D [2.1 times higher, p <0.001]) compared with those without AMC. Following adjustment for sex, age, BMI, and liver fat, all associations remained significant except low hand grip strength (p = 0.079) and no stair climbing (p = 0.193). Although muscle fat infiltration and visceral fat was significantly higher in AMC (p <0.001), no significant difference in liver fat between the groups was detected (p = 0.783).
Following imaging, 88 new cases of CHD has been recorded. Twenty-seven (30.7%) out of these 88 patients were within NAFLD. Among participants with NAFLD and AMC, 4.1% out of 169 suffered from a CHD event post imaging – more than twice as high incidence compared with those without AMC (1.9% out of 1,035 participants) (borderline significant difference p = 0.053).
The biochemistry data revealed participants with NAFLD and AMC had significantly higher glycated haemoglobin (HbA1c), with similar results in the adjusted model. FIB-4 was significantly higher in those with AMC only after adjustment for sex, age, BMI, and liver fat. Both statins and insulin medication use were more common in those with AMC. However, further adjustment for these medications did not change the level of association with measured outcomes. A summary of the results is presented in Table 2.
Table 2.
Comparison of characteristics for participants with NAFLD with/without adverse muscle composition.
| NAFLD and adverse muscle composition | NAFLD without adverse muscle composition | p value | p value (adjusted) | |
|---|---|---|---|---|
| Participants, n | 169 | 1,035 | – | – |
| Females, % | 41.42 | 47.34 | 0.153 | 1.000 |
| Age, years | 66.09 (6.75) | 62.38 (7.47) | <0.001 | – |
| Weight, kg | 88.30 (14.53) | 85.99 (15.85) | 0.077 | 0.610 |
| BMI, kg/m2 | 30.87 (4.58) | 30.02 (4.78) | 0.032 | – |
| Overweight (BMI>25 kg/m2), % | 95.27 | 87.05 | 0.003 | 0.998 |
| Visceral adipose tissue volume, L | 6.72 (2.44) | 5.50 (2.01) | <0.001 | <0.001 |
| Abdominal subcutaneous adipose tissue volume, L | 10.04 (3.56) | 9.17 (3.78) | 0.005 | <0.001 |
| Appendicular lean mass/height2, kg/m2 | 7.48 (1.04) | 8.02 (1.32) | <0.001 | <0.001 |
| Liver fat, % | 8.40 (6.41–12.89) | 8.95 (6.51–13.61) | 0.783 | – |
| Muscle composition | ||||
| Fat-tissue free muscle volume (FFMV), L | 9.80 (1.96) | 11.19 (2.60) | <0.001 | <0.001 |
| Muscle volume z-score (FFMVVCG), SD | −1.30 (0.47) | 0.19 (0.83) | <0.001 | <0.001 |
| Muscle fat infiltration, % | 10.10 (2.11) | 7.70 (1.87) | <0.001 | <0.001 |
| Adverse muscle composition, % | 100.00 | 0.00 | – | – |
| Only high muscle fat, % | 0.00 | 27.63 | – | – |
| Only low muscle volume, % | 0.00 | 11.50 | – | – |
| Normal muscle composition, % | 0.00 | 60.87 | – | – |
| Functional performance & metabolic comorbidity | ||||
| Sarcopenia, % | 5.92 | 0.87 | <0.001 | <0.001 |
| Low hand grip strength, % | 10.65 | 5.99 | 0.028 | 0.079 |
| Slow walking pace, % | 16.57 | 7.73 | <0.001 | 0.004 |
| No stair climbing, % | 15.38 | 8.99 | 0.011 | 0.193 |
| More than one fall in the past year, % | 12.43 | 5.22 | <0.001 | 0.001 |
| Coronary heart disease (prevalent), % | 19.53 | 5.89 | <0.001 | <0.001 |
| Coronary heart disease (incident), % | 4.14 | 1.93 | 0.053 | 0.184 |
| Type 2 diabetes, % | 23.67 | 11.50 | <0.001 | 0.001 |
| Biomarker panel∗ | ||||
| Glycated haemoglobin (HbA1c), mmol/mol | 38.83 (6.69) | 36.88 (6.55) | 0.001 | 0.036 |
| Glucose, mmol/L | 5.48 (1.68) | 5.21 (1.32) | 0.028 | 0.174 |
| Albumin, g/L | 45.09 (2.37) | 45.49 (2.43) | 0.060 | 0.247 |
| Direct bilirubin, μmol/L | 1.83 (0.76) | 1.81 (0.75) | 0.691 | 0.848 |
| Total bilirubin, μmol/L | 9.07 (3.99) | 9.24 (4.65) | 0.669 | 0.553 |
| Gamma glutamyltransferase, U/L | 42.57 (37.07) | 38.14 (35.62) | 0.150 | 0.276 |
| Alanine aminotransferase (ALT), U/L | 27.95 (14.38) | 29.41 (16.36) | 0.292 | 0.198 |
| Aspartate aminotransferase (AST), U/L | 27.06 (8.61) | 27.79 (10.37) | 0.404 | 0.121 |
| Cholesterol, mmol/L | 5.63 (1.30) | 5.70 (1.11) | 0.503 | 0.915 |
| HDL cholesterol, mmol/L | 1.25 (0.30) | 1.23 (0.27) | 0.456 | 0.251 |
| LDL direct, mmol/L | 3.58 (0.97) | 3.67 (0.84) | 0.223 | 0.505 |
| Triglycerides, mmol/L | 2.32 (1.33) | 2.22 (1.11) | 0.350 | 0.314 |
| C-reactive protein, mg/L | 3.38 (3.09) | 2.93 (3.95) | 0.173 | 0.231 |
| AST:ALT | 1.10 (0.41) | 1.06 (0.35) | 0.178 | 0.200 |
| Fibrosis-4 | 1.26 (0.51) | 1.23 (0.48) | 0.448 | 0.033 |
| NAFLD fibrosis score | −1.87 (1.08) | −2.07 (1.06) | 0.048 | 0.189 |
Adverse muscle composition: low thigh muscle volume (FFMVVCG) and high muscle fat infiltration. Values are presented as mean (SD); liver fat data are presented as median (IQR). The p values represent comparison between NAFLD with/without adverse muscle composition tested using two-propotions z-test (binary variables), Wilcoxon signed-rank test (continuous variables) and logist/linear regression adjusted for sex, age and BMI. The p values are shown for unadjusted and adjusted (sex, age, BMI, liver fat) modelling.
NAFLD, non-alcoholic fatty liver disease; VCG, virtual control group adjusted.
Data extracted from baseline assessment (years 2006–2010).
Details and results for using muscle volume z-score (FFMVVCG) and MFI as continuous variables investigating functional performance and metabolic comorbidity within NAFLD can be found in the Supplementary material.
Muscle composition within NAFLD
Metabolic comorbidity
Characteristics of the NAFLD population divided into the 4 muscle composition groups are presented in Table 3. The prevalence of metabolic comorbidities in each muscle composition groups is shown in Fig. 3. The difference in T2D prevalence was non-significant comparing ‘only low muscle volume’ and ‘only high muscle fat’ with AMC. However, T2D was significantly more common in all abnormal muscle composition groups as compared with ‘normal muscle composition’ (all p <0.01). Prevalence of CHD did not differ significantly between ‘normal muscle composition’ and ‘only low muscle volume’ or ‘only high muscle fat’. However, CHD was significantly more common in AMC compared with all other muscle composition groups (all p <0.01).
Table 3.
Comparison between 4 muscle composition groups within NAFLD.
| NAFLD and adverse muscle composition | NAFLD and only low muscle volume (FFMVVCG) | NAFLD and only high muscle fat (MFI) | NAFLD and normal muscle composition | |
|---|---|---|---|---|
| Participants, n (%) | 169 (14.0) | 119 (9.9) | 286 (23.8) | 630 (52.3) |
| % Females | 41.42 | 41.18 | 60.84 | 42.38 |
| Age, years | 66.09 (6.75) | 63.36 (7.21) | 63.98 (7.12) | 61.47 (7.54) |
| Weight, kg | 88.30 (14.53) | 78.61 (14.57) | 92.03 (17.63) | 84.65 (14.26) |
| BMI, kg/m2 | 30.87 (4.58) | 27.47 (4.04) | 32.78 (5.46) | 29.25 (3.97) |
| Overweight (BMI>25 kg/m2), % | 95.27 | 69.75 | 93.71 | 87.30 |
| Visceral adipose tissue volume, L | 6.72 (2.44) | 5.30 (2.01) | 6.11 (2.11) | 5.26 (1.91) |
| Abdominal subcutaneous adipose tissue volume, L | 10.04 (3.56) | 8.26 (3.39) | 11.16 (4.19) | 8.44 (3.31) |
| Appendicular lean mass/height2, kg/m2 | 7.48 (1.04) | 7.12 (1.10) | 8.35 (1.28) | 8.07 (1.28) |
| Liver fat, % | 8.40 (6.41–12.89) | 8.19 (6.09–11.85) | 10.25 (6.84–16.09) | 8.74 (6.48–13.19) |
| Muscle composition | ||||
| Fat-tissue free muscle volume (FFMV), L | 9.80 (1.96) | 9.61 (2.08) | 10.92 (2.42) | 11.60 (2.64) |
| Muscle volume z-score (FFMVVCG), SD | −1.30 (0.47) | −1.10 (0.35) | 0.20 (0.68) | 0.43 (0.73) |
| Muscle fat infiltration (MFI), % | 10.10 (2.11) | 7.13 (0.93) | 9.95 (1.69) | 6.78 (1.05) |
| Functional performance & metabolic comorbidity | ||||
| Sarcopenia, % | 5.92 | 6.72 | 0.00 | 0.16 |
| Low hand grip strength, % | 10.65 | 8.40 | 7.69 | 4.76 |
| Slow walking pace, % | 16.57 | 7.56 | 15.38 | 4.29 |
| No stair climbing, % | 15.38 | 9.24 | 10.49 | 8.25 |
| More than one fall in the past year, % | 12.43 | 3.36 | 7.34 | 4.60 |
| Coronary heart disease (prevalent), % | 19.53 | 7.56 | 6.99 | 5.08 |
| Coronary heart disease (incident), % | 4.14 | 0.84 | 1.75 | 2.22 |
| Type 2 diabetes, % | 23.67 | 16.81 | 18.88 | 7.14 |
| Biomarker panel∗ | ||||
| Glycated haemoglobin (HbA1c), mmol/mol | 38.83 (6.69) | 37.3 (7.94) | 38.48 (8.29) | 36.07 (5.06) |
| Glucose mmol/L | 5.48 (1.68) | 5.23 (1.23) | 5.47 (1.52) | 5.09 (1.22) |
| Albumin, g/L | 45.09 (2.37) | 45.91 (2.28) | 44.64 (2.38) | 45.81 (2.39) |
| Direct bilirubin, μmol/L | 1.83 (0.76) | 1.76 (0.65) | 1.74 (0.76) | 1.84 (0.76) |
| Total bilirubin, μmol/L | 9.07 (3.99) | 8.72 (3.94) | 8.77 (4.18) | 9.54 (4.93) |
| Gamma glutamyltransferase, U/L | 42.57 (37.07) | 38.56 (30.85) | 41.08 (44.20) | 36.75 (31.92) |
| Alanine aminotransferase (ALT), U/L | 27.95 (14.38) | 26.47 (11.97) | 29.70 (16.44) | 29.81 (16.97) |
| Aspartate aminotransferase (AST), U/L | 27.06 (8.61) | 25.95 (7.28) | 28.55 (13.28) | 27.79 (9.29) |
| Cholesterol, mmol/L | 5.63 (1.30) | 5.63 (1.21) | 5.66 (1.16) | 5.73 (1.07) |
| HDL-cholesterol, mmol/L | 1.25 (0.30) | 1.25 (0.29) | 1.25 (0.27) | 1.22 (0.26) |
| LDL direct, mmol/L | 3.58 (0.97) | 3.58 (0.92) | 3.65 (0.90) | 3.70 (0.80) |
| Triglycerides, mmol/L | 2.32 (1.33) | 2.20 (1.30) | 2.2 (1.01) | 2.24 (1.12) |
| C-reactive protein, mg/L | 3.38 (3.09) | 2.78 (3.62) | 3.76 (3.97) | 2.59 (3.95) |
| AST:ALT | 1.10 (0.41) | 1.08 (0.32) | 1.06 (0.33) | 1.06 (0.37) |
| Fibrosis-4 | 1.26 (0.51) | 1.17 (0.44) | 1.27 (0.54) | 1.22 (0.46) |
| NAFLD fibrosis score (NFS) | −1.87 (1.08) | −2.35 (1.05) | −1.71 (1.06) | −2.18 (1.02) |
Values are presented as mean (SD) unless otherwise indicated; for liver fat data are presented as median (IQR).
NAFLD, non-alcoholic fatty liver disease; VCG, virtual control group adjusted.
Data extracted from baseline assessment (years 2006–2010). Supplementary material contains levels of significance for all comparisons.
Fig. 3.
NAFLD and adverse muscle composition.
Adverse muscle composition (AMC): low muscle volume coupled with high muscle fat. Square muscle composition plot includes prevalence of coronary heart disease and type 2 diabetes. Bar plots show prevalence of poor function. Images are coronal and transversal magnetic resonance images of individuals representative for each of the muscle composition groups. AMC cut-offs: Muscle volume z-score (FFMVVCG), −0.68 (both females and males), muscle fat infiltration, 8.88% and 7.69% (females and males, respectively). FFMV, fat-tissue free muscle volume; NAFLD, non-alcoholic fatty liver disease; VCG, virtual control group.
Functional performance
Categorising the NAFLD population into the 4 muscle composition groups (Fig. 2) showed that poor function was significantly more prevalent within AMC as compared with ‘normal muscle composition’ for all outcome variables (all p <0.008). Comparing ‘normal muscle composition’ to having ‘only low muscle volume’ or ‘only high muscle fat’, showed few statistically significant differences for functional performance. Details of results on statistical testing between groups are presented in Table S3.
NAFLD and ‘normal muscle composition’
Although participants with NAFLD and ‘normal muscle composition’ showed significantly more prominent obesity characteristics as compared with the whole cohort (mean weight 84.7 vs. 75.5 kg, BMI 29.3 vs. 26.3 kg/m2, waist circumference 95.0 vs. 87.3 cm, visceral fat volume 5.3 vs. 3.7 L, and median liver fat 8.7 vs. 2.4), the prevalence of poor function and metabolic comorbidity (with exception of T2D prevalence) were comparable between these groups: low hand grip strength, factor 0.8 (p = 0.14); slow walking pace, factor 1.0 (p = 0.96); no stair climbing, factor 1.1 (p = 0.77); more than 1 fall in the past year, factor 1.0 (p = 0.86); CHD, factor 1.1 (0.71); T2D, factor 1.6 (p <0.01).
Discussion
This study reports results on NAFLD and muscle health based on approximately 10,000 individuals from the largest population-based imaging study to date. Main findings include (1) adverse muscle composition (AMC) is common within NAFLD (14.0%) and identifies individuals exhibiting poor function and high prevalence of metabolic comorbidities using muscle-specific imaging biomarkers alone, and (2) the AMC phenotype, associated with poor muscle health, is overlooked by current sarcopenia assessments, which do not include reference values for muscle fat.
Sarcopenia and frailty are recognised as important factors in understanding disease progression in later stages of liver disease. This study provides MRI-based thresholds for sarcopenia detection according to current definitions enabling investigations of sarcopenia using MRI with the added benefits of acquiring high-precision assessment of liver fat, muscle fat, and abdominal fat distribution (visceral and subcutaneous fat). This allows metabolic sub-phenotyping and risk assessment based on ectopic fat accumulation and fat distribution.33,34 However, this study also showed that current ways of defining sarcopenia underestimates muscle-related problems within NAFLD; contrary to poor function being more prevalent in the NAFLD population, the sarcopenia prevalence was almost twice as high in those without fatty liver. Several studies have, conversely, reported a positive association between NAFLD and current sarcopenia definitions.8 However, the conclusion of whether there is an association or not seems to greatly depend on different ways of defining sarcopenia and normalising muscle quantity for body size. A recent study showed that previous ways of adjusting muscle quantity for body size has been ineffective,14 and another study showed that using division by height2 or weight to normalise muscle quantity resulted in completely different conclusions about the association between NAFLD and sarcopenia.15 A proper body-size normalisation for sarcopenia assessment is especially important when considering sarcopaenic obesity and highly relevant for NAFLD as obesity is a common comorbidity. The hypertrophic effect of increased weight on ALM and thigh FFMV – both consisting mostly of weight-bearing muscles – further motivates the importance of understanding this issue. A previous study showed that the normalisation applied to calculate muscle volume z-score (FFMVVCG) properly adjusted thigh FFMV for body size and resulted in significant associations with functional performance and hospitalisation.14 Adding other muscles (such as psoas or spinal erectors), that may be less affected by the sedentary lifestyle associated with obesity, could provide an even greater understanding of the overall muscle health for the individual patient by capturing different aspects of disease processes, everyday functional fitness, and resulting quality of life. Different studies on sarcopenia and NAFLD do utilise different muscle groups, which could be a reason why results differentiate. Another aspect to consider is the liver disease demographics in different studies. It seems the link between sarcopenia and more severe liver disease, even with NASH, is stronger as compared with that with NAFLD. UK Biobank is not specifically designed to study liver disease and our study investigates NAFLD in a setting that is close to NAFLD screening in the general UK population. A potential lower prevalence of NASH and more advanced liver disease is probably lowering the prevalence of sarcopenia. The sarcopenia field is in a great need of standardisation of assessment and the availability of muscle-specific, high-precision biomarkers with strong links to function opens up the possibility of objective and close tracking of muscle health.
AMC is a highly vulnerable phenotype within NAFLD associated with high prevalence of metabolic comorbidity and poor function. Although the AMC phenotype was shown to be highly vulnerable, indicating the identification of an extreme group within NAFLD, as many as 14.0% had AMC within NAFLD. These individuals might be more prone to progress from NAFLD to liver disease and could be a population of interest for preventive interventions. The fact that only 5.9% of individuals with NAFLD and AMC had sarcopenia shows that this phenotype, clearly associated with poor muscle health, is overlooked by current sarcopenia assessments. Further investigations of NAFLD without AMC were performed by grouping participants based on muscle composition (‘only high muscle fat’, ‘only low muscle volume’, and ‘normal muscle composition’) and revealed that it is important to assess both muscle volume and muscle fat to identify the more vulnerable group of patients. It remains unknown why muscle composition varies between individuals, but the higher prevalence of T2D for ‘only high muscle fat’ or ‘only low muscle volume’ indicates these phenotypes are associated with metabolic dysfunction and might require medical attention. Also, the mechanisms causing differences in muscle composition phenotype could differ, and consequently, could require different treatment and prevention strategies. The intersect between muscle health and metabolic disorders such as NAFLD and diabetes is complex. Whether sarcopenia and frailty accelerate the progression of metabolic disorders, or the other way around, is not yet fully understood. For example, individuals with diabetes commonly show an accelerated ageing process and more rapid decline of muscle strength and function. However, lower muscle mass will lower the glucose uptake, leading to higher insulin secretion and insulin resistance.35 Alternatively, it may be that one does not cause the other and the effect on liver and muscle is concurrent. A rapid (6–10 min) MRI-protocol coupled with automatic segmentation and quantification of fat distribution (liver, visceral, and subcutaneous) as well as muscle composition (fat-tissue free muscle volume and muscle fat infiltration) could be an effective tool in future investigations of interactions between metabolic disorders and sarcopenia-related disease.
Overall, the results indicate that assessing muscle composition provides a clinically meaningful description of the NAFLD heterogeneity. Interestingly, participants with NAFLD displaying ‘normal muscle composition’ (accounting for approximately 50% of the NAFLD population), showed significantly more prominent obesity characteristics, but did not express a phenotype more vulnerable than the general population (with exception of higher T2D prevalence). Thus, assessing muscle composition in NAFLD could facilitate identification of patients with a potentially good prognosis who could forego extensive medical care. Muscle composition assessment could also be used to enrich NAFLD populations for inclusion in clinical trials, lowering the heterogeneity of the study population, and potentially increasing the probability of proving efficacy.
There are some limitations; the biochemistry data from the imaging visit is not available and therefore, the baseline data were used. Thus, associations between muscle composition and biochemistry data within NAFLD can only be viewed as indicative. However, the results indicate that AMC cannot be detected using circulating biomarkers. Statistical significance was only detected for HbA1c and FIB-4 (both higher in NAFLD with AMC). Further research can elucidate whether AMC is predictive of fibrosis development.
Although hand grip strength was recorded using reference standard methods, remaining variables describing functional performance were self-reported. The quality of walking pace is likely low, as the perception of ‘slow’, ‘steady/average’, and ‘brisk’ pace could differ, whereas the quality of stair climbing and falls should mainly be affected by memory and misreporting. These results should be replicated using other commonly used functional measurements (e.g. recorded gait speed and timed up and go test) and mobility information gathered through motion sensors.
CHD cases were identified through electronic health care records, providing highly reliable data. T2D cases were identified through interviews with trained nurses. Participants were required to report time of diagnosis and medications related to their condition. Although the reliability of these data is somewhat lower, many T2D diagnoses are recorded in the primary care data, which were not available at the time of this study. In addition, this study was cross-sectional and detected associations requires replication in prospective studies. A test for future CHD events (post imaging) were included and showed a borderline significant association with AMC in NAFLD. Although these results indicate that assessing muscle composition within NAFLD has predictive value, cases were too few to separate first events from recurring events.
Lastly, the NAFLD population in this study is not directly comparable with those seen in clinical care. However, this study does include interesting results from the perspective of NAFLD in a general Caucasian population. Although there is evidence that UK Biobank exhibits a healthy volunteer bias,36 the prevalence of fatty liver in those reporting low alcohol consumption (22.6%) was comparable with the estimated worldwide prevalence. Prevalence of AMC in the NAFLD population seen in clinical care might be higher than estimated by this study and other thresholds for identifying AMC could be more effective. In this work, the muscle volume z-score was sex- and body-size-adjusted, and sex-specific thresholds were used to identify individuals with high muscle fat. There is a slight overweight of males within NAFLD (47% females) in this cohort, and in spite of AMC thresholds being sex-adjusted, this is more pronounced in all muscle composition groups (∼42% females) except the ‘only high muscle fat’ group (61% females). That females and males seem to express different muscle composition phenotypes as well as potential sex-related metabolic and functional differences might affect the study results. However, results from the sex-adjusted modelling was not notably different from originally observed associations. More importantly, although statistical modelling also included age adjustment, the UK Biobank consists of a relatively young population from a sarcopenia perspective and larger deficits in function usually appears later in life. Age might influence what is an optimal threshold for identifying vulnerable NAFLD patients, especially for muscle fat infiltration.13,37 Lastly, the discussion of what should be considered low drinking and how this interacts with what is defined as NAFLD is still ongoing. Different cut-offs for low alcohol consumption will affect the characteristics of the population studied and as a consequence potentially affect study results.
A large study like UK Biobank is very useful for first investigations of emerging techniques such as the MRI-based muscle composition described in this work. With positive indicative results on the utility of this technique to assess muscle-related problems within NAFLD, there is motivation to design prospective studies to further investigate its predictive power as well as focused studies in more clinically relevant and ethnically diverse populations.
Conclusions
The NAFLD phenotype with adverse muscle composition (low muscle volume z-score and high muscle fat) is prevalent within NAFLD and represents a highly vulnerable subpopulation (high prevalence of metabolic comorbidity and poor function). This phenotype, associated with poor muscle health, is not described in current literature and is not captured by current sarcopenia assessments. Sarcopenia guidelines can be strengthened by including cut-offs for muscle fat enabling AMC detection.
Financial support
Financial support was gratefully received from Pfizer Inc.
Authors' contributions
Conceptualization: J.L., O.D.L. Formal analysis: J.L. Investigation: J.L., O.D.L. Methodology: J.L., O.D.L. Visualization: J.L. Writing – original draft: J.L. Writing – review and editing: all authors.
Conflicts of interest
JL and ODL are employees and stockholders of AMRA Medical AB.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2020.100197.
Data availability
This research was conducted using the UK Biobank resource, project ID 6569. UK Biobank data are available through the procedure described at http://www.ukbiobank.ac.uk/using-the-resource/.
Supplementary data
References
- 1.Loomba R., Sanyal A.J. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Kim D., Kim W.R., Kim H.J., Therneau T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Bhanji R.A., Carey E.J., Yang L., Watt K.D. The long winding road to transplant: how sarcopenia and debility impact morbidity and mortality on the waitlist. Clin Gastroenterol Hepatol. 2017;15:1492–1497. doi: 10.1016/j.cgh.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Fré C.H., De Fré M.A., Kwanten W.J., Op de Beeck B.J., Van Gaal L.F., Francque S.M. Sarcopenia in patients with non-alcoholic fatty liver disease: is it a clinically significant entity? Obes Rev. 2019;20:353–363. doi: 10.1111/obr.12776. [DOI] [PubMed] [Google Scholar]
- 9.Yu R., Shi Q., Liu L., Chen L. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: a meta-analysis. BMC Gastroenterol. 2018;18:51. doi: 10.1186/s12876-018-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan X., Han Y., Zou T., Zhu G., Xu K., Zheng J. Sarcopenia contributes to the progression of nonalcoholic fatty liver disease- related fibrosis: a meta-analysis. Dig Dis. 2018;36:427–436. doi: 10.1159/000491015. [DOI] [PubMed] [Google Scholar]
- 11.Wijarnpreecha K., Panjawatanan P., Thongprayoon C., Jaruvongvanich V., Ungprasert P. Sarcopenia and risk of nonalcoholic fatty liver disease: a meta-analysis. Saudi J Gastroenterol. 2018;24:12–17. doi: 10.4103/sjg.SJG_237_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey E.J., Lai J.C., Sonnenday C., Tapper E.B., Tandon P., Duarte-Rojo A. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70:1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B. The FNIH sarcopenia project, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linge J., Heymsfield S.B., Dahlqvist Leinhard O. On the definition of sarcopenia in the presence of aging and obesity – initial results from UK Biobank. J Gerontol A Biol A Med Sci. 2020;75:1309–1316. doi: 10.1093/gerona/glz229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng T.C., Wu L.W., Chen W.L., Liaw F.Y., Chang Y.W., Kao T.W. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): the importance of sarcopenia definition. Clin Nutr. 2019;38:422–428. doi: 10.1016/j.clnu.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Prado C.M., Heymsfield S.B. Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enteral Nutr. 2014;38:940–953. doi: 10.1177/0148607114550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckinx F., Landi F., Cesari M., Fielding R.A., Visser M., Engelke K. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9:269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachit M., Leclercq I.A. Emerging awareness on the importance of skeletal muscle in liver diseases: time to dig deeper into mechanisms! Clin Sci (Lond) 2019;133:465–481. doi: 10.1042/CS20180421. [DOI] [PubMed] [Google Scholar]
- 19.UK Biobank imaging study. https://imaging.ukbiobank.ac.uk Available at:
- 20.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. Plos Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 22.Linge J., Borga M., West J., Tuthill T., Miller M.R., Dumitriu A. Body composition profiling in the UK Biobank imaging study. Obesity (Silver Spring) 2018;26:1758–1795. doi: 10.1002/oby.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson A., Rosander J., Romu T., Tallberg J., Grönqvist A., Borga M. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J Magn Reson Imaging. 2015;41:1558–1569. doi: 10.1002/jmri.24726. [DOI] [PubMed] [Google Scholar]
- 24.West J., Romu T., Thorell S., Lindblom H., Berin E., Holm A.S. Precision of MRI-based body composition measurements of postmenopausal women. PLoS One. 2018;13:e0192495. doi: 10.1371/journal.pone.0192495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West J., Dahlqvist Leinhard O., Romu T., Collins R., Garratt S., Bell J.D. Feasibility of MR-based body composition analysis in large scale population studies. PLoS One. 2016;11:e0163332. doi: 10.1371/journal.pone.0163332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borga M., Thomas E.L., Romu T., Rosander J., Fitzpatrick J., Dahlqvist Leinhard O. Validation of a fast method for quantification of intra-abdominal and subcutaneous adipose tissue for large scale human studies. NMR Biomed. 2015;28:1747–1753. doi: 10.1002/nbm.3432. [DOI] [PubMed] [Google Scholar]
- 27.Dahlqvist Leinhard O, Rydell J, Smedby O, Nystrom FH, Lundberg P, Borga M, et al. Quantitative abdominal fat estimation using MRI. Presented at the 19th International Conference on Pattern Recognition, Tampa, FL, 2008. doi:10.1109/ICPR.2008.4761764.
- 28.Leoni S., Tovoli F., Napoli L., Serio I., Ferri S., Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroentorol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasr P., Forsgren M.F., Ignatova S., Dahlström N., Cedersund G., Dahlqvist Leinhard O. Using a 3% proton density fat fraction as a cut-off value increases sensitivity of detection of hepatic steatosis, based on results from histopathology analysis. Gastroenterology. 2017;153:53–55.e7. doi: 10.1053/j.gastro.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 31.UK Biobank Biomarker assay quality procedures. http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/biomarker_issues.pdf Available at:
- 32.Drinkaware What is an alcohol unit? https://www.drinkaware.co.uk/alcohol-facts/alcoholic-drinks-units/what-is-an-alcohol-unit/ Available at:
- 33.Linge J., Whitcher B., Borga M., Dahlqvist Leinhard O. Sub-phenotyping metabolic disorders using body composition: an individualized, nonparametric approach utilizing large data sets. Obesity (Silver Spring) 2019;27:1190–1199. doi: 10.1002/oby.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neeland I.J., Ross R., Després J.P., Matsuzawa Y., Yamashita S., Shai I. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 35.Perkisas S., Vandewoude M. Where frailty meets diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):261–267. doi: 10.1002/dmrr.2743. [DOI] [PubMed] [Google Scholar]
- 36.Fry A., Littlejohns T.J., Sudlow C., Doherty N., Adamska L., Sprosen T. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcus R.L., Addison O., Kidde J.P., Dibble L.E., Lastayo P.C. Skeletal muscle fat infiltration: impact of age inactivity, and exercise. J Nutr Health Aging. 2010;14:362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research was conducted using the UK Biobank resource, project ID 6569. UK Biobank data are available through the procedure described at http://www.ukbiobank.ac.uk/using-the-resource/.